Integrating testing for sexually transmissible infections into annual health assessments for Aboriginal and Torres Strait Islander young people: a cross-sectional analysis
Heather McCormack A B * , Handan Wand A , Christopher Bourne A B C , James Ward D , Clare Bradley D , Donna Mak E F and Rebecca Guy A
A B * , Handan Wand A , Christopher Bourne A B C , James Ward D , Clare Bradley D , Donna Mak E F and Rebecca Guy A
A
B
C
D
E
F
Abstract
In the context of an expanding syphilis epidemic, we assessed the integration of sexually transmissible infection (STI) testing within annual health assessments for Aboriginal and Torres Strait Islander young people aged 16–29 years in Aboriginal Community Controlled Health Services between 2018 and 2020.
Using routinely collected electronic medical record data from a national sentinel surveillance system (ATLAS), we performed a cross-sectional analysis to calculate the proportion of assessments that integrated any or all of the tests for chlamydia, gonorrhoea, syphilis, and HIV. We used logistic regression to identify correlates of integration of any STI test.
Of the13 892 assessments, 23.8% (95% CI 23.1, 24.6) integrated a test for any STI and 11.5% (95% CI 10.9, 12.0) included all four STIs. Of assessments that included a chlamydia/gonorrhoea test, 66.9% concurrently included a syphilis test. Integration of any STI test was associated with patients aged 20–24 years (OR 1.2, 95% CI 1.1–1.4) and 25–29 years (OR 1.1, 95% CI 1.0–1.2) compared to 16–19 years and patients residing in very remote (OR 4.2, 95% CI 3.7–4.8), remote (OR 2.4, 95% CI 2.1–2.8), and regional areas (OR 2.5, 95% CI 2.2–2.8) compared to metropolitan areas. There was no association with patient sex.
Integration of STI testing into annual health assessments for Aboriginal and Torres Strait Islander young people was higher in remote areas where disease burden is greatest. Integration is similar in men and women, which contrasts with most studies that have found higher testing in women.
Keywords: epidemiology, health promotion, health services, HIV prevention, interventions, Medicare Benefits Schedule Item 715, prevention, sexually transmissible infections, social determinants, statistics.
Sexually transmissible infections (STIs) predominantly affect adolescents and young adults aged 16–29 years,1 and if untreated, they can result in complications including pelvic inflammatory disease, ectopic pregnancy, and infertility.2,3 Untreated syphilis during pregnancy can have severe outcomes for the foetus.4 STIs are also associated with shame, stigma, reputation damage, relationship breakdowns, and negative health outcomes.5
Adolescents and young Aboriginal and/or Torres Strait Islander people are identified as a priority population in Australian STI and HIV strategies,6–8 particularly those residing in remote and very remote regions, where prevalence of chlamydia and gonorrhoea is much higher and there is a sustained syphilis outbreak.9 Infectious syphilis has also more recently spread into metropolitan areas, demonstrating the need to increase syphilis testing among all young Aboriginal and/or Torres Strait Islander people.10
In Australia and other countries primary care services for adolescents and young adults play a significant role in STI prevention and management, as up to 90% of young people attend primary care at least once annually.11 To facilitate inclusion of sexual health in primary care, clinical guidelines in Australia and other countries recommend regularly screening for STIs in routine primary care consultations,12 recognising that adolescents and young adults may not proactively request STI tests and may not disclose risk behaviours to health care providers unless prompted.13 Qualitative research conducted in mainstream primary care with clinicians14 and young women15 strongly supports normalisation of age-based STI screening to reduce the stigma associated with clinician risk assessment. In Australia, Aboriginal and Torres Strait Islander people can access primary care via either mainstream general practice or Aboriginal Community Controlled Health Services (ACCHS), which are Indigenous-specific primary health care providers managed by and for Aboriginal and Torres Strait Islander communities.16 The provision of culturally safe sexual healthcare through Indigenous-specific primary health care services such as ACCHS has been recognised in Australia and other Western countries with a history of colonisation as a solution for health disparities that is underpinned by self-determination.17 Aboriginal and Torres Strait Islander young people are more likely to access STI testing at ACCHS than other primary health care settings.18
An opportunity to normalise age-based STI screening occurs by offering it as part of a broader health check. Routine health assessments for Aboriginal and Torres Strait Islander people in primary care settings are reimbursed and incentivised by Australia’s universal health insurance scheme, Medicare. This is the only routine health assessment with an associated Medicare rebate for delivery in Australian primary care to include young people within its target age range of 15–54 years. STI testing is a recommended inclusion within the health assessment, and guidelines specifically emphasise STI testing for those aged under 35 years, providing an opportunity to support access to STI testing for a priority population.19 Routine adolescent health assessments have been implemented in other countries20–22 and recommended as appropriate for low- and middle-income countries23 but, to our knowledge, without an equivalent rebate as in Australia. As such, there is only one previous study in ACCHS in Australia that explored the association between participating in an incentivised health assessment and receiving an STI test and found a strong association at the health service level,24 but this study did not explore completeness of STI testing or correlated patient socio-demographics.
A national sentinel surveillance network involving 34 ACCHS was established in 2016, providing a unique opportunity to determine the integration of STI testing for adolescents and young adults within health assessments in the ACCHS setting. The establishment and operation of the network has been described elsewhere.25 In this analysis, we aimed to assess what proportion of health assessments conducted in ACCHS with Aboriginal and Torres Strait Islander young people aged 16–29 integrated STI testing and identified factors associated with integration.
Methods
Strengths-based approach
This study explicitly adopts a strengths-based approach to its subject matter and analysis. Commonly used deficit-focused epidemiological framing in Indigenous health research results in stigma that itself contributes to poor health.26 Strengths-based approaches can reveal alternative strategies to address health issues27 as well as identify statistical methodologies that better reflect Indigenous knowledge and values.28
Study design and data source
We performed a cross-sectional analysis of routinely collected electronic medical record (EMR) data for all patients aged 16–29 years who had a health assessment between 2018 and 2020 at 1 of 31 ACCHS in the ATLAS network that were able to provide retrospective data for the entire study period. ATLAS includes ACCHS clustered in five clinical hubs across four Australian states and territories.
The Medicare Benefits Schedule Item 715 (MBS 715) annual health assessment
The focus of the analysis is integration of STI testing into health assessments. These health assessments have been a central component in the Australian Government’s Closing the Gap strategy to reduce health disparities affecting Aboriginal and Torres Strait Islander populations.29 National guidelines19 recommend that a health assessment include assessment of lifestyle factors; strengths-based assessment of social and emotional wellbeing; review and update of vaccination status; assessment of eye health, cardiovascular risk, diabetes, and kidney disease; and screening for STIs for young people aged under 35 years. Although a health assessment can be conducted in any primary care setting, Aboriginal and Torres Strait Islander young people report accessing health assessments at ACCHS more frequently than in mainstream general practices.30
Data collection
Using the ATLAS data, we obtained de-identified line-listed data on all health assessments completed for all individuals aged 16–29 years. The process for identifying health assessments in the datasets is outlined in Fig. 1. Other information collected included Aboriginal and Torres Strait Islander status, age, sex, postcode, and date of test/consultation.
Process for identification of MBS 715 Adult Health Assessment in ATLAS data extracted from an ACCHS EMR.
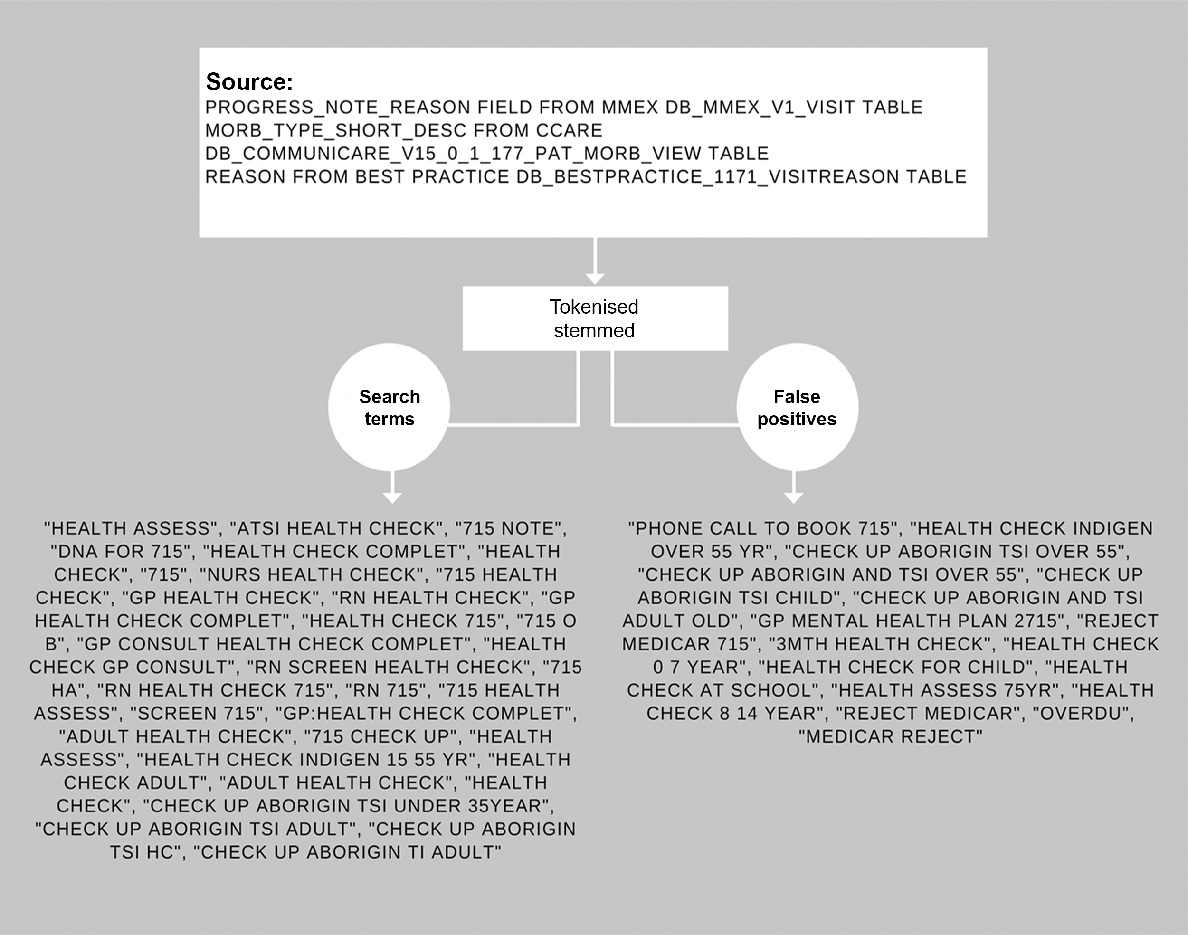
Participants’ ages were categorised in the following age groups: 16–19, 20–24, and 25–29 years. Area of residence was assigned by postcode using the Modified Monash Model,31 and we collapsed inner and outer regional areas into a singular regional category. Socio-Economic Indexes for Areas (SEIFA) and Index of Relative Socio-Economic Advantage and Disadvantage (IRSAD)32 were also created using the postcodes and categorised using quintiles. The first quintile represented the most relatively disadvantaged areas, whereas the fifth quintile represented the most relatively advantaged areas. To avoid sparseness of the data, we further collapsed quintiles 1 and 2 into one group and quintiles 3 and 4 into another.
Chlamydia, gonorrhoea, syphilis, and/or HIV tests were included in this analysis. Chlamydia and gonorrhoea (CT/NG) were assessed as a single variable, as these STIs are routinely combined in a duplex test using nucleic acid amplification technology, and even if only one test is requested, the other is conducted automatically. Testing for Trichomonas vaginalis was excluded from our analysis, as guidelines only recommend testing for this infection in high prevalence areas. Syphilis and HIV were assessed as single and separate variables, as both are conducted using the same blood sample but need to be individually requested by the clinician and involve different tests. At the time of data collection, guidelines recommended a CT/NG test annually for all Aboriginal and Torres Strait Islander young people and a syphilis and/or HIV test for those at increased risk or following a positive CT/NG result.19
Primary outcome
The primary outcome was the proportion of Aboriginal and/or Torres Strait Islander young people who had a health assessment who received a test for any STI within 1 day of their health assessment, which was calculated by taking the difference between the health assessment consultation and STI test request date. We allowed for 1 day as some investigations attached to a health assessment may be conducted on subsequent days to the initial consultation.
Statistical analysis
We conducted a descriptive analysis and presented frequencies of the categorical variables by the primary outcome measure (i.e. the proportion of health assessments that included an STI test within 1 day).
We conducted a univariate logistic regression analysis to identify the correlates of being tested for STIs within 1 day of the health assessment. A backward-selection technique was used to finalise the multivariable model. Participants’ age, sex, area of residence, SEIFA and year of consultation were all considered as potential correlates of the primary outcome. Unadjusted and adjusted odds ratios (OR) and 95% confidence intervals (95% CI) were presented. Factors with P < 0.05 were considered statistically significant. All analyses were conducted using STATA 14.0 software (College Station, TX, USA).
Ethical approval
Ethics approval for the ATLAS network has been granted by the Aboriginal Health Research Ethics Committee (EC00185, approval 04-17-732); the Aboriginal Health and Medical Research Ethics Committee (EC00342, approval 1300/17); the Western Australian Aboriginal Health Ethics Committee (EC00292, approval 805); the Far North Queensland Human Research Ethics Committee (EC00157, approval HREC/17/QCH/102-1173); and the University of Queensland Human Research Ethics Committees (EC00456 and EC00457, Clearance Number: 2020000740/04-17-732).
Results
During the study period, there were 13 892 individuals who had at least one episode of a health assessment annually recorded as a clinical encounter in EMR; this was lower in 2020 (n = 1597) compared to 2019 (n = 6443) and 2018 (n = 5852). These health assessments were conducted among 10 641 unique Aboriginal and Torres Strait Islander patients, of whom 41% were men and 59% women with a median age of 22 years (interquartile range: 19–26). Of the 10 641 patients, 13% resided in very remote areas, 14% in remote areas, 44% in outer/inner regional areas, and 29% in a major city. Just under half (43%) of the patients lived in postcodes with SEIFA scores of 1 or 2 (most disadvantaged), 18% resided in postcodes with SEIFA scores of 3 or 4, and 39% resided in postcodes with SEIFA scores of 5 or higher.
Integration of CT/NG testing within the health assessment
Overall, 20% (2804/13 892) of the unique health assessments included a CT/NG test; this was slightly higher in the 20–24-year age group (21.9%) compared to 16–19 years (19.1%) or 25–29 years (19.5%) and slightly higher in males (21.1%) than females (19.6%). The highest proportion of health assessments that included CT/NG testing was among patients residing in very remote regions (33.8%) and in regions with SEIFA scores of 3–4 (27.2%), whereas the lowest proportion was in metropolitan regions (10.8%) and lower SEIFA 1–2 (16.0%) regions. The proportion of health assessments completed that included CT/NG was higher in 2018 (21.6%) and 2019 (20.6%) and lower in 2020 (13.3%).
Integration of any STI test within the health assessment
The proportion of health assessments that included any STI test (any one or more of CT, NG, syphilis, or HIV) was 23.8% overall (3312/13 892), and the proportion that included complete STI testing (CT, NG, syphilis and HIV) was 11.5% overall. Socio-demographic and year differences for both outcomes were similar compared with CT/NG tests only (Table 1). The proportion of health assessments that included all four STIs was also higher in 2018 (12.1%) and 2019 (11.9%) and lower in 2020 (7.5%).
| Category | Breakdown | Proportion of MBS 715s with a CT/NG test within 1 day | Proportion of MBS 715, with any STI test (CT, NG, syphilis or HIV) within 1 day | Proportion of MBS 715, that included CT/NG and syphilis and HIV within 1 day | |
|---|---|---|---|---|---|
| Overall | 2804/13 892 (20.18%) | 3312/13 892 (23.84%) | 1596/13 892 (11.47%) | ||
| Year of MBS 715 request | 2018 | 1266/5852 (21.63%) | 1438/5852 (24.57%) | 707/5852 (12.08%) | |
| 2019 | 1326/6443 (20.58%) | 1586/6443 (24.62%) | 767/6443 (11.90%) | ||
| 2020 | 212/1597 (13.27%) | 288/1597 (18.03%) | 120/1597 (7.51%) | ||
| Age group (years) | 16–19 | 797/4170 (19.11%) | 904/4170 (21.68%) | 407/4170 (9.76%) | |
| 20–24 | 1051/4810 (21.85%) | 1237/4810 (25.72%) | 612/4810 (12.72%) | ||
| 25–29 | 956/4912 (19.46%) | 1171/4912 (23.84%) | 575/4912 (11.71%) | ||
| Sex | Male | 1191/5651 (21.08%) | 1392/5651 (24.63%) | 1022/5651 (18.09%) | |
| Female | 1613/8241 (19.57%) | 1920/8241 (23.30%) | 1301/8241 (15.79%) | ||
| Patient area of residence | Very remote | 602/1782 (33.78%) | 668/1782 (37.49%) | 357/1782 (20.03%) | |
| Remote | 443/1973 (22.45%) | 513/1973 (26.00%) | 216/1973 (10.95%) | ||
| Inner/outer regional | 1331/6159 (21.61%) | 1631/6159 (26.48%) | 778/6159 (12.63%) | ||
| Metropolitan | 428/3978 (10.76%) | 500/3978 (12.57%) | 243/3978 (6.11%) | ||
| Patient SEIFA – IRSAD | 1–2 | 728/4557 (15.98%) | 858/4557 (18.83%) | 382/4557 (8.38%) | |
| 3–4 | 1052/3862 (27.24%) | 1219/3862 (31.56%) | 598/3862 (15.48%) | ||
| 5+ | 1024/5473 (18.71%) | 1235/5473 (22.57%) | 614/5473 (11.22%) |
Concurrent syphilis and HIV testing when a CT/NG test was done within the health assessment
Of the unique health assessments that included a CT/NG test, the proportion that also included a concurrent syphilis test (Table 2) was 66.9% overall (1877/2804); this was highest in the 25–29 years age group (71.6%) compared to the 16–19 years (60.5%) and 20–24 years age group (67.6%); males (70.8%) compared to females (64.1%); patients residing in very remote areas (80.9%) compared to remote, (56.2%), regional (66.6%), and metropolitan areas (59.4%); and in SEIFA 3–4 (70%) regions. It was lowest in SEIFA 1–2 (63.1%) regions. By year, the proportions were similar overall (2018: 65.4%, 2019: 68.5%, 2020: 66.5%) with variations by area of residence increasing in very remote areas between 2018 and 2019 with no significant change in proportions in remote, regional, or metropolitan areas in the same period (Table 3).
| Category | Breakdown | Proportion of MBS 715 inclusive of CT/NG that also included syphilis | Proportion of MBS 715 inclusive of CT/NG that also included HIV | Proportion of MBS 715 inclusive of CT/NG, that also included syphilis and HIV | |
|---|---|---|---|---|---|
| Overall | 1877/2804 (66.94%) | 1670/2804 (59.56%) | 1596/2804 (56.92%) | ||
| Year of MBS 715 request | 2018 | 828/1266 (65.40%) | 746/1266 (58.93%) | 707/1266 (55.85%) | |
| 2019 | 908/1326 (68.48%) | 797/1326 (60.11%) | 769/1326 (57.99%) | ||
| 2020 | 141/212 (66.51%) | 127/212 (59.91%) | 129/212 (56.60%) | ||
| Age group (years) | 16–19 | 482/797 (60.48%) | 429/797 (53.83%) | 408/797 (51.19%) | |
| 20–24 | 710/1051 (67.55%) | 639/1051 (60.8%) | 612/1051 (58.23%) | ||
| 25–29 | 685/956 (71.65%) | 602/956 (62.97%) | 576/956 (60.25%) | ||
| Sex | Male | 843/1191 (70.78%) | 749/1191 (62.89%) | 726/1191 (60.96%) | |
| Female | 1034/1613 (64.10%) | 921/1613 (57.1%) | 870/1613 (53.94%) | ||
| Patient area of residence | Very remote | 487/602 (80.90%) | 362/602 (60.13%) | 357/602 (59.30%) | |
| Remote | 249/443 (56.21%) | 219/443 (49.44%) | 216/443 (48.76%) | ||
| Inner/outer regional | 887/1331 (66.64%) | 837/1331 (62.89%) | 780/1331 (56.78%) | ||
| Metropolitan | 254/428 (59.35%) | 252/428 (58.88%) | 243/428 (56.78%) | ||
| Patient SEIFA – IRSAD | 1–2 | 450/728 (63.05%) | 408/728 (56.04%) | 384/728 (52.75%) | |
| 3–4 | 736/1052 (69.96%) | 614/1052 (58.37%) | 592/1052 (56.84%) | ||
| 5+ | 682/1024 (66.60%) | 648/1024 (63.28%) | 614/1024 (59.96%) |
| Category | Breakdown | Proportion of MBS 715 inclusive of CT/NG that also included syphilis | |||
|---|---|---|---|---|---|
| 2018 (n = 1266) | 2019 (n = 1326) | 2020 (n = 212) | |||
| Patient area of residence | Very remote | 242/315 (76.83%) (95% CI: 72%, 81%) | 213/248 (85.89%) (95% CI: 81%, 90%) | 32/39 (82.05%) (95% CI: 67%, 91%) | |
| Remote | 143/238 (60.08%) (95% CI: 54%, 66%) | 103/197 (52.28%) (95% CI: 45%, 59%) | 3/8 (37.50%) (95% CI: 14%, 69%) | ||
| Inner/outer regional | 336/536 (62.69%) (95% CI:58%, 67%) | 470/673 (69.84%) (95% CI: 66%, 73%) | 81/122 (66.39%) (95% CI: 58%, 74%) | ||
| Metropolitan | 107/177 (60.45%) (95% CI: 53%, 67%) | 122/208 (58.65%) (95% CI: 52%, 65%) | 25/43 (58.14%) (95% CI: 43%, 72%) | ||
Of health assessments that included a CT/NG test, the proportion that also included a HIV test was 59.6% overall (1670/2804) with a similar age group and sex breakdown as syphilis testing (Table 2). Integration of a HIV test was highest in patients residing in regional areas (62.9%) compared to very remote (60.1%), remote (49.4%), and metropolitan areas (58.9%); highest in SEIFA 5+ (63.2%) regions; and lowest in SEIFA 1–2 (56%) regions. By year, the proportion was similar (2018: 58.9%, 2019: 60.1%, 2020: 59.9%).
Correlates of the integration of any STI testing in the MBS 715 health assessment
In the univariate analysis, integration of any STI test in a health assessment was more likely in those aged 20–24 years (OR 1.2 (95% CI: 1.1–1.4)) and 25–29 years (OR 1.1 (95% CI: 1.0–1.2)), compared to those aged 16–29 years; those residing in very remote areas (OR 4.2 (95% CI: 3.7–4.8)), remote (OR 2.4 (95% CI: 2.1–2.8)), and regional areas (OR 2.5 (95% CI: 2.2–2.8)) compared to metropolitan area; those living in areas with a SEIFA index of 3–4 (OR 1.99 (95% CI: 1.8–2.2)) and 5 or more (OR 1.3 95% CI: 1.1–1.4) compared to 1–2; and less likely in 2020 (OR 0.7 (95% CI: 0.6–0.8) compared to 2018. In the multivariate analysis, all variables remained statistically significant and had similar strengths in the associations (Table 4).
| Category | Breakdown | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|---|
| Odds ratio (95% CI) | P-value | Odds ratio (95% CI) | P-value | |||
| Year of MBS 715 | 2018 | 1 | 1 | |||
| 2019 | 1.00 (0.92, 1.09) | 0.956 | 1.02 (0.94, 1.11) | 0.621 | ||
| 2020 | 0.68 (0.59, 0.78) | <0.001 | 0.72 (0.62, 0.83) | <0.001 | ||
| Age group (years) | 16–19 | 1 | 1 | |||
| 20–24 | 1.25 (1.13, 1.38) | <0.001 | 1.29 (1.17, 1.43) | <0.001 | ||
| 25–29 | 1.13 (1.02, 1.25) | 0.015 | 1.15 (1.04, 1.28) | 0.007 | ||
| Sex | Male | 1.08 (1.0, 1.16) | 0.070 | – | ||
| Female | 1 | – | ||||
| Patient area of residence | Very remote | 4.17 (3.65, 4.77) | <0.001 | 4.37 (3.81, 5.03) | <0.001 | |
| Remote | 2.44 (2.13, 2.80) | <0.001 | 2.12 (1.84, 2.44) | <0.001 | ||
| Inner/outer regional | 2.51 (2.15, 2.80) | <0.001 | 2.67 (2.39, 2.98) | <0.001 | ||
| Metropolitan | 1 | 1 | ||||
| SEIFA – IRSAD | 1–2 | 1 | 1 | |||
| 3–4 | 1.99 (1.80, 2.20) | <0.001 | 2.10 (1.88, 2.32) | <0.001 | ||
| 5+ | 1.26 (1.14, 1.39) | <0.001 | 1.50 (1.35, 1.66) | <0.001 | ||
Discussion
Among a large national sample of 16–29-year-old Aboriginal and/or Torres Strait Islander people attending ACCHS between 2018 and 2020, we found 23.9% of health assessments integrated a test for any STI and 11.5% included tests for all four STIs. When chlamydia/gonorrhoea testing was integrated, 67% also included a syphilis test, which was highest in very remote areas (80.9%). Although fewer than a quarter of total health assessments included an STI test, this was in part attributable to low uptake in metropolitan areas, as a considerably higher proportion (37.6%) included an STI test in very remote regions where the highest STI notification rates have historically been recorded.9 Correlates of integration of any STI test included patients aged 20–24 and 25–29 years compared to 16–19 years and patients residing in very remote, remote, and regional areas.
The very high rate of integration of syphilis testing into CT/NG tests done as part of the health assessment is encouraging, as the infectious syphilis notification rate for Aboriginal and Torres Strait Islander people is highest in remote and very remote regions and has increased most rapidly among 20–29-year-olds.33 Higher uptake of testing in very remote regions could reflect the impact of recent health promotion programs34 and clinical initiatives35 conducted in response to the ongoing syphilis outbreak in remote regions across northern Australia,10,36 indicating that interventions to increase syphilis testing have been accurately targeted to those most at risk. However, lower levels of integration for syphilis in urban and regional areas and for HIV in urban areas is of concern, as syphilis notifications are increasing in these regions and HIV prevalence in Australia is greatest in metropolitan areas.33
We found no statistical association with receiving an STI test as part of a health assessment and gender, indicating that using the health assessment may have achieved gender parity, which is in contrast with past studies that have consistently shown that young Aboriginal and Torres Strait Islander men have lower uptake of STI testing in this setting.9,18,24,37–40 Another surprising finding was when the multivariate analysis found that receiving any STI test as part of a health assessment was most strongly correlated with residing in SEIFA – IRSAD 3–4 areas, not 1–2 areas (representing the areas of lowest socio-economic disadvantage) as anticipated. This may reflect unmeasured factors, such as greater health seeking practices or enhanced STI programs in the clinics in the SEIFA – IRSAD 3–4 areas, or the SEIFA of the community may be different to the SEIFA of those attending the health services due to the impacts of industry in these post codes.41,42 We also identified that both the total number of health assessments and the proportion of health assessments that included an STI test decreased in 2020 compared to both 2018 and 2019, which may reflect the impact of coronavirus disease 2019 (COVID-19) on ACCHS service delivery.
Although uptake of STI testing among Aboriginal and/or Torres Strait Islander young people is substantially higher in settings where STI testing is a central focus, such as publicly funded sexual health clinics, previous research has found much lower testing uptake in both ACCHS and mainstream general practice settings,40 reflecting that the majority of consultations in the primary care setting are sought for purposes other than an STI test. Previous research that examined STI testing in general ACCHS consultations outside of the health assessment have found annual testing rates under 15%.39,43 Limited data is available on the uptake of STI testing among Aboriginal and/or Torres Strait Islander young people attending mainstream general practices, but one study has found an annual STI testing rate of 8.9% among a national sample of 16–29-year-olds (inclusive of Indigenous and non-Indigenous young people).44 In line with our findings, this study found higher testing rates in non-metropolitan regions compared to urban areas. Within the context of the primary care setting, the MBS 715 health assessment presents an opportunity for an annual STI screen to be offered to all sexually active Aboriginal and/or Torres Strait Islander young people in line with clinical guidelines, and we recommend investment into further research to identify strategies to both increase the proportion of health assessments that integrate a comprehensive STI test and increase the proportion of Aboriginal and/or Torres Strait Islander young people who receive a health assessment annually.
However, although a comprehensive STI is a recommended inclusion of a health assessment for young people, clinician discretion to exclude an STI test from a young person’s health assessment may sometimes be warranted. This may include when the young person is not sexually active, or has recently received an STI test outside the health assessment, or when a clinician of a specific gender is preferred for sexual and reproductive health consultations for cultural reasons.45 Previous research has also identified that many Aboriginal and/or Torres Strait Islander young people may choose to access sexual healthcare at non-Indigenous-specific health services, separate to their general healthcare, due to concerns about confidentiality in small communities.46–48 We are unable to estimate the proportion of our sample who have accessed a different service for their sexual healthcare vs their routine annual health assessment, but this should be considered when assessing the completeness of STI testing within the health assessment.
The strength of our analyses lies in the use of data collected from patient medical records, rather than from self-reporting. The Medicare rebate attached to the MBS 715 health assessment provides a unique opportunity to measure uptake, as it must be recorded in the patient management system of a primary care practice as a specific consultation type. There are also some limitations to consider when interpreting these findings. First, as routinely collected data were used, we cannot be certain if some MBS items or STI tests were not recorded in clinic systems; however, findings are broadly consistent with other studies that have reported an uptake of STI testing in young people.40 Further, we applied SEIFA – IRSAD at a postcode level, which may mask an individual’s socio-economic status. Finally, our findings include data collected from a national surveillance network of ACCHS across four jurisdictions with broad geographical coverage of metropolitan, regional, remote, and very remote regions nationally, although with slight under-representation from regional communities compared to actual geographic distribution of ACCHS.25
In conclusion, this study has assessed the integration of testing for STIs into routine annual health assessments in Aboriginal and Torres Strait Islander young people aged 16–29 years attending ACCHS across Australia. The MBS 715 health assessment provides a unique opportunity for routine STI screening of this population in both young men and women and has the potential to lead to timely treatment, in turn reducing the morbidities associated with STIs and HIV. Further research is recommended to identify effective strategies to increase STI testing within the health assessment and culturally appropriate strategies to ensure all Indigenous young people receive comprehensive HIV/STI screening.
Data availability
The data that support the findings of this study remain the property of the participating ACCHS and are not publicly available. However, data may be available upon reasonable request and with explicit permission of the participating ACCHS. Please contact the corresponding author for further information.
Declaration of funding
The ATLAS Indigenous Primary Care Surveillance Network is funded through a National Health and Medical Research Council Partnerships Grant, GNT2006987 and a MRFF Primary Healthcare Research Data Infrastructure Grant, PHRDI000054. Neither the National Health and Medical Research Council or the Medical Research Future Fund had a role in the study design; extraction, analysis and interpretation of data; the writing of this manuscript; or the decision to submit the manuscript for publication.
Author contributions
HM, RG and CB contributed to study conceptualisation. HM designed the data analysis plan. HW conducted the statistical analysis. CB verified the data analysis. JW and CB contributed to funding acquisition and CB contributed to project administration. HM wrote and revised the manuscript. All authors reviewed and edited the final manuscript.
Acknowledgements
The authors gratefully acknowledge the contribution and support of the organisations forming the CRE-ASH clinical hubs, as well as the individual health services participating in the ATLAS network.
References
1 Kirby Institute. HIV, viral hepatitis and sexually transmissible infections in Australia: annual surveillance report 2018. UNSW; 2018. Available at https://www.kirby.unsw.edu.au/research/reports/asr2018
2 Reekie J, Donovan B, Guy R, Hocking JS, Jorm L, Kaldor JM, et al. Hospitalisations for pelvic inflammatory disease temporally related to a diagnosis of chlamydia or gonorrhoea: a retrospective cohort study. PLoS One 2014; 9(4): e94361.
| Crossref | Google Scholar |
3 Reekie J, Donovan B, Guy R, Hocking JS, Kaldor JM, Mak D, et al. Risk of ectopic pregnancy and tubal infertility following gonorrhea and chlamydia infections. Clin Infect Dis 2019; 69(9): 1621-3.
| Crossref | Google Scholar |
4 Wijesooriya NS, Rochat RW, Kamb ML, Turlapati P, Temmerman M, Broutet N, et al. Global burden of maternal and congenital syphilis in 2008 and 2012: a health systems modelling study. Lancet Glob Health 2016; 4(8): e525-e33.
| Google Scholar |
5 Bell S, Aggleton P, Ward J, Maher L. Sexual agency, risk and vulnerability: a scoping review of young Indigenous Australians’ sexual health. J Youth Stud 2017; 20(9): 1208-24.
| Crossref | Google Scholar |
9 Graham S, Smith LW, Fairley CK, Hocking J. Prevalence of chlamydia, gonorrhoea, syphilis and trichomonas in Aboriginal and Torres Strait Islander Australians: a systematic review and meta-analysis. Sex Health 2016; 13(2): 99-113.
| Crossref | Google Scholar |
10 Nattabi B, Girgis S, Matthews V, Bailie R, Ward JE. Clinic predictors of better syphilis testing in Aboriginal primary healthcare: a promising opportunity for primary healthcare service managers. Aust J Prim Health 2018;
| Crossref | Google Scholar | PubMed |
11 Webb MJ, Kauer SD, Ozer EM, Haller DM, Sanci LA. Does screening for and intervening with multiple health compromising behaviours and mental health disorders amongst young people attending primary care improve health outcomes? A systematic review. BMC Fam Pract 2016; 17: 104.
| Crossref | Google Scholar | PubMed |
12 The Royal Australian College of General Practitioners. Guidelines for preventive activities in general practice. East Melbourne: RACGP; 2016. Available at https://www.racgp.org.au/download/Documents/Guidelines/Redbook9/17048-Red-Book-9th-Edition.pdf
13 Sanci L, Webb M, Hocking J. Risk-taking behaviour in adolescents. Aust J Gen Pract 2018; 47(12): 829-34.
| Crossref | Google Scholar |
14 Hocking JS, Parker RM, Pavlin N, Fairley CK, Gunn JM. What needs to change to increase chlamydia screening in general practice in Australia? The views of general practitioners. BMC Public Health 2008; 8: 425.
| Crossref | Google Scholar | PubMed |
15 Pavlin NL, Parker R, Fairley CK, Gunn JM, Hocking J. Take the sex out of STI screening! Views of young women on implementing chlamydia screening in general practice. BMC Infect Dis 2008; 8: 62.
| Crossref | Google Scholar | PubMed |
16 Harfield SG, Davy C, McArthur A, Munn Z, Brown A, Brown N. Characteristics of Indigenous primary health care service delivery models: a systematic scoping review. Global Health 2018; 14(1): 12.
| Crossref | Google Scholar |
18 Ward J, Bryant J, Worth H, Hull P, Solar S, Bailey S. Use of health services for sexually transmitted and blood-borne viral infections by young Aboriginal people in New South Wales. Aust J Prim Health 2013; 19(1): 81-6.
| Crossref | Google Scholar | PubMed |
20 Nikander K, Hermanson E, Vahlberg T, Kaila M, Sannisto T, Kosola S. Associations between study questionnaire-assessed need and school doctor-evaluated benefit of routine health checks: an observational study. BMC Pediatr 2021; 21(1): 346.
| Crossref | Google Scholar |
21 Rieck T, Feig M, Delere Y, Wichmann O. Utilization of administrative data to assess the association of an adolescent health check-up with human papillomavirus vaccine uptake in Germany. Vaccine 2014; 32(43): 5564-9.
| Google Scholar |
22 Coker TR, Sareen HG, Chung PJ, Kennedy DP, Weidmer BA, Schuster MA. Improving access to and utilization of adolescent preventive health care: the perspectives of adolescents and parents. J Adolesc Health 2010; 47(2): 133-42.
| Crossref | Google Scholar | PubMed |
23 Chingono RMS, Mackworth-Young CRS, Ross DA, Tshuma M, Chiweshe T, Nyamayaro C, et al. Designing routine health checkups for adolescents in Zimbabwe. J Adolesc Health 2021; 69(6): 940-7.
| Crossref | Google Scholar |
24 Nattabi B, Matthews V, Bailie J, Rumbold A, Scrimgeour D, Schierhout G, et al. Wide variation in sexually transmitted infection testing and counselling at Aboriginal primary health care centres in Australia: analysis of longitudinal continuous quality improvement data. BMC Infect Dis 2017; 17(1): 148.
| Crossref | Google Scholar |
25 Bradley C, Hengel B, Crawford K, Elliott S, Donovan B, Mak DB, et al. Establishment of a sentinel surveillance network for sexually transmissible infections and blood borne viruses in Aboriginal primary care services across Australia: the ATLAS project. BMC Health Serv Res 2020; 20(1): 769.
| Crossref | Google Scholar |
26 Thurber KA, Thandrayen J, Banks E, Doery K, Sedgwick M, Lovett R. Strengths-based approaches for quantitative data analysis: a case study using the australian Longitudinal Study of Indigenous Children. SSM - Population Health 2020; 12: 100637.
| Crossref | Google Scholar |
27 Fogarty W, Lovell M, Langenberg J, Heron M-J. Deficit Discourse and Strengths-based Approaches: Changing the Narrative of Aboriginal and Torres Strait Islander Health and Wellbeing. The Lowitja Institute; 2018. Available at https://www.lowitja.org.au/page/services/resources/Cultural-and-social-determinants/racism/deficit-discourse-strengths-based
29 Bailie J, Laycock A, Matthews V, Peiris D, Bailie R. Emerging evidence of the value of health assessments for Aboriginal and Torres Strait Islander people in the primary healthcare setting. Aust J Prim Health 2019; 25(1): 1-5.
| Crossref | Google Scholar | PubMed |
34 D’Costa B, Lobo R, Thomas J, Ward JS. Evaluation of the young deadly free peer education training program: early results, methodological challenges, and learnings for future evaluations. Front Public Health 2019; 7: 74.
| Crossref | Google Scholar |
35 Ward J, Guy RJ, Rumbold AR, McGregor S, Wand H, McManus H, et al. Strategies to improve control of sexually transmissible infections in remote Australian Aboriginal communities: a stepped-wedge, cluster-randomised trial. Lancet Glob Health 2019; 7(11): e1553-63.
| Crossref | Google Scholar |
36 Bright A, Dups J. Infectious and congenital syphilis notifications associated with an ongoing outbreak in Northern Australia; 2016. Available at https://www1.health.gov.au/internet/main/publishing.nsf/Content/cda-cdi4001b.htm
37 Bell S, Aggleton P, Ward J, Murray W, Silver B, Lockyer A, et al. Young Aboriginal people’s engagement with STI testing in the Northern Territory, Australia. BMC Public Health 2020; 20(1): 459.
| Crossref | Google Scholar | PubMed |
38 Harrod ME, Couzos S, Ward J, Saunders M, Donovan B, Hammond B, et al. Gonorrhoea testing and positivity in non-remote Aboriginal Community Controlled Health Services. Sex Health 2017; 14(4): 320-4.
| Crossref | Google Scholar |
39 Graham S, Wand HC, Ward JS, Knox J, McCowen D, Bullen P, et al. Attendance patterns and chlamydia and gonorrhoea testing among young people in Aboriginal primary health centres in New South Wales, Australia. Sex Health 2015; 12(5): 445-52.
| Crossref | Google Scholar |
40 Ward J, Goller J, Ali H, Bowring A, Couzos S, Saunders M, et al. Chlamydia among Australian Aboriginal and/or Torres Strait Islander people attending sexual health services, general practices and Aboriginal community controlled health services. BMC Health Services Research 2014; 14(1): 285.
| Crossref | Google Scholar |
41 Kotey B. Demographic and economic changes in remote Australia. Aust Geogr 2015; 46(2): 183-201.
| Crossref | Google Scholar |
42 Reeson AF, Measham TG, Hosking K. Mining activity, income inequality and gender in regional Australia*. Aust J Agric Resour Econ 2012; 56(2): 302-13.
| Crossref | Google Scholar |
43 Goller JL, Ward J, Saunders M, Couzos S, Kaldor J, Hellard MA, et al. Chlamydia sentinel surveillance in Aboriginal Community Controlled Health Services finds higher testing and positivity rates among younger people. Aust N Z J Public Health 2012; 36(6): 577-581.
| Crossref | Google Scholar |
44 Kong FYS, Guy RJ, Hocking JS, Merritt T, Pirotta M, Heal C, et al. Australian general practitioner chlamydia testing rates among young people. Med J Aust 2011; 194(5): 249-252.
| Crossref | Google Scholar |
45 Hengel B, Guy R, Garton L, Ward J, Rumbold A, Taylor-Thomson D, et al. Barriers and facilitators of sexually transmissible infection testing in remote Australian Aboriginal communities: results from the Sexually Transmitted Infections in Remote Communities, Improved and Enhanced Primary Health Care (STRIVE) Study. Sex Health 2015; 12(1): 4-12.
| Crossref | Google Scholar |
46 Ubrihien A, Lewis DA, Rambaldini B, Kirwan M, Gwynne K. Clinicians’ perspectives on why young Aboriginal people are not testing for sexually transmissible infections in Western Sydney. Int J STD AIDS 2023;; 9564624231179766.
| Crossref | Google Scholar |
47 Graham S, Martin K, Gardner K, Beadman M, Doyle MF, Bolt R, et al. Aboriginal young people’s perspectives and experiences of accessing sexual health services and sex education in Australia: A qualitative study. Glob Public Health 2023; 18(1): 2196561.
| Crossref | Google Scholar |
48 Spurway K, Sullivan C, Leha J, Trewlynn W, Briskman L, Soldatic K. “I felt invisible”: First nations LGBTIQSB+ young people’s experiences with health service provision in Australia. J Gay Lesbian Soc Serv 2022; 35(1): 68-91.
| Crossref | Google Scholar |


