Feasibility and economic costs of syphilis self-testing to expand test uptake among gay, bisexual and transgender men: results from a randomised controlled trial in Zimbabwe
Definate Nhamo A B * , Collin Mangenah C D , Gwendoline Chapwanya A , Takudzwa Mamvuto A , Imelda Mahaka A , Clarisse Sri-Pathmanathan E , Rashida A. Ferrand E F , Katharina Kranzer E F , Fern Terris-Prestholt
A B * , Collin Mangenah C D , Gwendoline Chapwanya A , Takudzwa Mamvuto A , Imelda Mahaka A , Clarisse Sri-Pathmanathan E , Rashida A. Ferrand E F , Katharina Kranzer E F , Fern Terris-Prestholt  G H , Michael Marks E and Joseph D. Tucker
G H , Michael Marks E and Joseph D. Tucker  E I
E I
A Pangaea Zimbabwe AIDS Trust (PZAT), Harare, Zimbabwe.
B Department of Nursing and Public Health, University of KwaZulu Natal (UKZN), Durban, South Africa.
C Centre for Sexual Health and HIV Research (CeSHHAR), Harare, Zimbabwe.
D Department of International Global Public Health, Liverpool School of Tropical Medicine (LSTM), Liverpool, UK.
E Department of Clinical Research, London School of Hygiene and Tropical Medicine (LSHTM), London, UK.
F Biomedical Research and Training Institute, Harare, Zimbabwe.
G Department of Global Health and Development, London School of Hygiene and Tropical Medicine (LSHTM), London, UK.
H UNAIDS, Joint United Nations Programme on HIV/AIDS, Geneva, Switzerland.
I Institute of Global Health and Infectious Diseases, University of North Carolina at Chapel Hill (UNC), Chapel Hill, NC, USA.
Abstract
Access to syphilis testing and treatment is frequently limited for men who have sex with men (MSM). A two-armed randomised controlled trial compared feasibility and costs of facility-based syphilis testing with self-testing among MSM in Zimbabwe.
This randomised controlled trial was conducted in Harare, with participants randomised 1:1. Syphilis self-testing was offered in community-based settings. The primary outcome was the relative proportion of individuals taking up testing. Total incremental economic provider and user costs, and cost per client tested, diagnosed and treated were assessed using ingredients-based costing in 2020 US$.
A total of 100 men were enrolled. The two groups were similar in demographics. The mean age was 26 years. Overall, 58% (29/50) and 74% (37/50) of facility- and self-testing arm participants, respectively, completed syphilis testing. A total of 28% of facility arm participants had a reactive test, with 50% of them returning for confirmatory testing yielding 28% reactivity. In the self-testing arm, 67% returned for confirmatory testing, with a reactivity of 16%. Total provider costs were US$859 and US$736, and cost per test US$30 and US$15 for respective arms. Cost per reactive test was US$107 and US$123, and per client treated US$215 and US$184, respectively. The syphilis test kit was the largest cost component. Total user cost per client per visit was US$9.
Syphilis self-testing may increase test uptake among MSM in Zimbabwe. However, some barriers limit uptake including lack of self-testing and poor service access. Bringing syphilis testing services to communities, simplifying service delivery and increasing self-testing access through community-based organisations are useful strategies to promote health-seeking behaviours among MSM.
Keywords: costs of syphilis testing, facility-based syphilis testing, hidden populations, men who have sex with men, MSM, self-care products, sexually transmitted infections, syphilis, syphilis self-testing, syphilis testing barriers, Zimbabwe.
Background
Delayed diagnosis of syphilis and ongoing transmission is an urgent global public health problem, it is not only responsible for high rates of neonatal deaths, but predisposes many to HIV infection. Men who have sex with men (MSM) are particularly affected by this delayed diagnosis of syphilis.1–3 A systematic review of the prevalence of syphilis among MSM found a global pooled prevalence of 7.5% (95% CI 7.0–8.0). A 2020 study found high rates of syphilis among MSM in two of Zimbabwe’s largest cities, Harare and Bulawayo (5.5% and 5.6%, respectively).4 A 2020 study found high rates of syphilis among MSM in two of Zimbabwe’s largest cities, Harare and Bulawayo (5.5% and 5.6%, respectively).4
Despite the high prevalence of syphilis among MSM, they remain largely neglected in sexual health research in low- and middle-income countries.5
Access to health care by MSM is frequently limited by cultural taboos, stigma and discrimination.6 In Zimbabwe, same-sex acts are punishable by 1 year in prison.7 MSM in Zimbabwe often suffer from violence, public outing, police harassment and blackmail because of their sexual orientation.5 MSM also experience cultural, religious and social stigma from health care professionals.7 Access to appropriate sexual health services is therefore limited, with the recent COVID-19 pandemic making facility-based care even more difficult.4
Regular testing is key to controlling syphilis transmission, and reducing morbidity through timely diagnosis and treatment.8 Although pregnant women are routinely tested for syphilis, there are no public programs in Zimbabwe focused on syphilis control among MSM despite the high rates of syphilis. Physicians do not routinely ask about same-sex behaviour or recommend syphilis testing for MSM.9 Syphilis self-testing may be an effective way to increase test uptake among MSM communities by providing out-of-facility testing opportunities.10 Self-testing involves individuals collecting their own blood specimen, performing the test using a disposable device and interpreting the results in private. The syphilis self-test kit is a rapid test that detects treponemal antibodies in under 30 min.11 These point-of-care testing devices for syphilis are readily available in the private sector. The tests are currently used as rapid syphilis tests and can be adapted for individual, home-based use.12
However, although regular syphilis self-testing testing may be good in increasing access to testing, treponemal-only rapid tests will only be able to detect new syphilis infections, and fail to detect syphilis infections in those previously treated for syphilis.
We assessed context-specific facilitators and barriers, and evaluated the usability of syphilis self-testing in MSM.13 In our formative research, we found that MSM were willing to use self-test kits, and that this method provided privacy, convenience, autonomy, and reduced the potential for social and health care provider stigma.13 There is extensive evidence on the effectiveness and acceptability of HIV self-testing in low- and middle-income countries, but data on syphilis self-testing are limited.14 As a result of this research gap, the World Health Organization has only given a conditional recommendation for the use of self-testing for syphilis diagnosis, even though it could potentially help to curb the syphilis epidemic in low-resource settings.15
In a two-arm randomised controlled trial (RCT), we assessed the effectiveness of syphilis self-testing uptake compared with facility-based syphilis testing among MSM in Zimbabwe. Alongside the RCT, we undertook an analysis of the economic costs of syphilis self-testing both from provider and client perspectives.
Methods
Study design
This RCT, conducted between October 2019 and July 2020, compared syphilis self-testing with facility-based testing. Participants were randomised 1:1 to either the syphilis self-testing or facility-based testing group. The study was conducted in Harare, Zimbabwe, by Pangaea Zimbabwe AIDS Trust (a Zimbabwean local registered non-profit organisation working to improve the health and well-being of people in Zimbabwe).16 The organisation works with MSM among other population groups.
Syphilis testing in Zimbabwe
Facility-based syphilis testing used a lateral flow point-of-care blood-based assay to detect treponemal antibodies. Syphilis self-testing was not available at facilities during the RCT. For the RCT, the study team used rapid treponemal syphilis tests manufactured by SD Biosensor. Syphilis tests were individually repackaged in a ziplock bag, and included capillary pipettes, lancets, alcohol swab and testing device (see Supplementary material S1).
Study procedures
Recruitment
Eligible participants were enrolled in Harare between 1 October and 19 December 2019. Pre-screening was conducted on the phone, with actual recruitment conducted in person. Participants had to: be aged 16 years or older; be residing in Harare and planning to remain there for the next 6 months; be born biologically male; have no history of syphilis testing in the past 12 months; and have at least one sexual risk factor (defined as condomless anal sex with a man in the past 3 months), be in a non-monogamous relationship with a man, have more than three male sexual partners in the past 3 months, be previously diagnosed with a sexually transmitted infection (including syphilis) or currently taking oral pre-exposure prophylaxis willing to provide a mobile phone number (for follow-up) and to give informed consent. Fliers outlining the study and the eligibility criteria were distributed to community-based organisations working with MSM. Interested men contacted study staff via dedicated phone numbers.
Randomisation
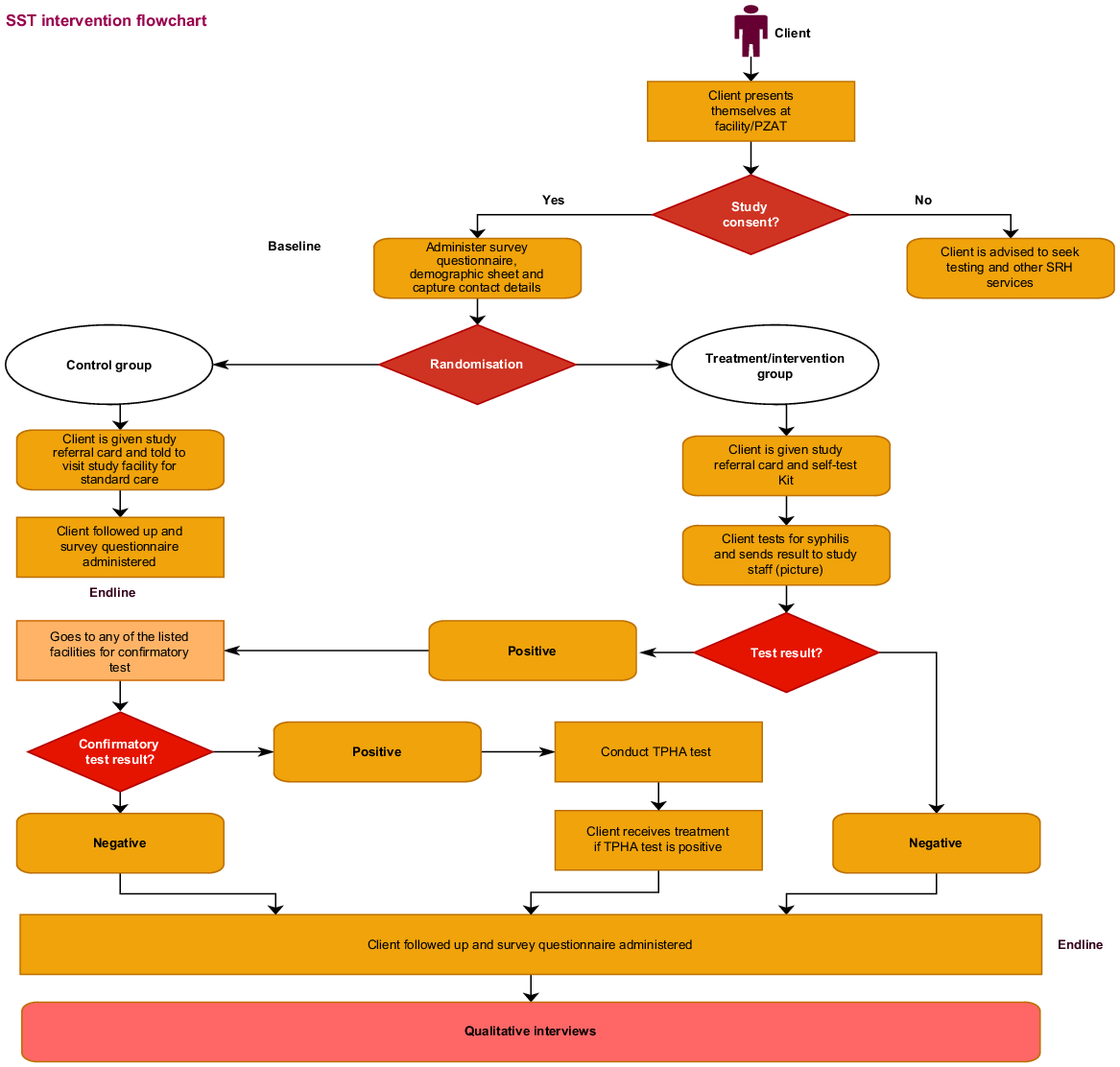
An individual randomisation sequence was computer generated. Each number was assigned to either facility-based testing or syphilis self-testing. The number and assigned arm were inserted in envelopes and sealed. The envelopes were stacked, with participants asked to pick an envelope that contained printed cards assigning them to either of the study arms. Participants assigned to the self-testing arm were given a syphilis self-test kit and general information sheet (Supplement 1) on syphilis. In addition, participants could access an instructional video via WhatsApp on their personal devices or program tablets if they did not have a smart phone. Self-testing arm participants needing assistance with the test could elect to self-test at the community-based organisation site in a private area, but with a program officer on hand to assist if needed. Participants assigned to the facility-testing arm were given a referral slip to take to the facility for testing (Fig. 1). Facility health care providers had been sensitised and received specific training on provision of syphilis testing. Participants in both arms received US$10 for time spent completing the enrolment process. Enrolment in the study occurred at a local non-profit organisation in Harare that typically works with the MSM community. Staff also knew the individuals, which removed the possibility of participants enrolling twice.
Data collection
At baseline, we collected data on demographics, sexual behaviour, and previous HIV and syphilis testing. This included age, sexual orientation, marital status, educational attainment, living arrangements, disclosure of sexual orientation to a health care provider and/or family member, sexual roles, number of sexual partners in the past 6 months, relationship type (long-term, short-term) with each sexual partner, condom use, history of STIs, group sex, and substance use. Data was captured electronically on tablets using Open Data Kit.
Follow up
Data logs were created for nurses at the facility to capture facility-testing arm participant data and those who tested positive. Data were compiled on a weekly basis from the facility, capturing identification numbers of participants who came to the facility and their syphilis test results. Self-testing arm participants were asked to send a picture of their test results via WhatsApp or bring their test kits to the program office. Participants in both the self-testing and facility-testing arms with reactive syphilis test results were followed up by the study team and referred back to the health facility for further blood samples to test for Treponema pallidum haemagglutination and rapid plasma reagin tests. Following enrolment, blood samples were processed at Multi-tech Diagnostics, a commercial pathology laboratory. Participants with positive results for both Treponema pallidum haemagglutination and rapid plasma reagin tests had treatment administered according to the STI.17,18 In both arms, participants with reactive tests were followed up at 48 h, and then at 3 and 6 months. Part of the follow up took place in the context of COVID-19 (beginning March 2020), layered with lock-down restrictions on movement. Laboratory samples for those with reactive tests were scheduled based on specific laboratory sample pick up days, and therefore not always feasible to have on the day of the reactive test.
Lost to follow up
Participants who enrolled, but failed to either self-test or attend facility testing, were followed up by telephone beginning at 48 h and then weekly thereafter up to three times, and if not-contactable, were then considered lost to follow up. If a participant responded positively to a follow-up call, then the normal process, as described above, would be followed.
Statistical analysis
We report descriptive statistics for demographic characteristics, substance use, sexual behaviour, and history of HIV and syphilis testing. The primary outcome was the difference in the proportion of individuals who undertook a syphilis test between study arms between October 2019 and July 2020. Syphilis test uptake was assessed by photoverification (among those with a smartphone), receipt of the completed test kit or telephone call. Secondary outcomes included the proportion of individuals who tested positive by either self-testing or facility-based testing, associated costs of delivery and those who reported engaging in condomless sex. We used an intention-to-treat approach to analyse the primary and secondary outcomes. The analysis was conducted using Stata version 15 (StataCorp, College Station, TX, USA).
Economic cost analysis
An ingredients-based costing exercise estimated the incremental economic cost of syphilis self-test kit distribution (see Appendix 1 in Supplementary material). Standard costing guidelines were used to estimate the total cost, and cost per client tested for syphilis, per syphilis diagnosis, and per person treated in the facility-based and self-test arms.19–21 Consistent with other self-testing studies,22–24 costs of training and start-up (venue and reimbursement expenses), staffing and supplies for demand creation, self-testing, initial facility and confirmatory tests (including laboratory tests), and treatment were estimated. We also administered a short exit/telephone interview to 10 clients who had accessed syphilis testing at the facility to measure user costs in terms of productivity losses (lost income) and other out-of-pocket expenses incurred seeking syphilis testing. Out-of-pocket expenses included transportation for the self-test kit collection, initial facility test and confirmatory testing, food, and or other payments incurred. Costs are presented in 2020 US$. The US$ has been part of the official basket of currencies in use in Zimbabwe since 2009.
Ethical approval
The study received ethical approval from the Medical Research Council of Zimbabwe (MRCZ/2533) and the ethics committee at the London School of Hygiene & Tropical Medicine (LSHTM). All participants provided written informed consent. The study was registered on clinicaltrials.gov (NCT04480749).
Results
Of 104 MSM approached and screened, 100 MSM enrolled, with 50 participants assigned to each arm. The four individuals who screened out were ineligible based on age and not planning to stay in Harare for the next 6 months. Of the100 men, 66 had primary outcome data available at 6 months, and 34 of 100 were lost to follow up. Potential participants were identified and referred to the Pangaea Zimbabwe AIDS Trust by community-based organisations working with MSM. The mean age was 26 years (s.d. 5.72). Overall, 40% of participants reported taking HIV pre-exposure prophylaxis at the time of enrolment. Most of the participants (81%) had never been married, whereas 13% were separated or divorced and 6% engaged or married. Just under one-third of the participants (30%) reported having achieved a tertiary level of education, whereas 48% had completed secondary education. Most participants identified as gay (49%), followed by bisexual (31%) and transgender (19%; Table 1). The primary outcome (syphilis testing uptake) was assessed by photoverification for 33 individuals, receipt of test kit for four individuals and clinic records for 29 individuals.
| Total (n = 100) (%) | Facility-testing arm (n = 50) (%) | Self-testing arm (n = 50) (%) | ||
|---|---|---|---|---|
| Age (years) | ||||
| >24 | 37 (37) | 14 (28) | 23 (46) | |
| ≥24 and <30 | 39 (39) | 22 (44) | 17 (34) | |
| ≥30 and <40 | 20 (20) | 12 (24) | 8 (16) | |
| ≥40 and <50 | 4 (4) | 2 (4) | 2 (4) | |
| Mean (s.d.) | ||||
| Marital status | ||||
| Engaged or married | 6 (6) | 3 (6) | 3 (6) | |
| Never married | 81 (81) | 42 (84) | 39 (78) | |
| Separated or divorced | 13 (13) | 5 (10) | 8 (16) | |
| Highest education | ||||
| High school or below | 22 (22) | 9 (18) | 13 (26) | |
| Secondary school | 48 (48) | 23 (46) | 25 (50) | |
| Tertiary or beyond | 30 (30) | 18 (36) | 12 (24) | |
| Spoken to family member about sexual orientation | ||||
| No | 43 (43) | 24 (48) | 19 (38) | |
| Yes | 57 (57) | 26 (52) | 31 (62) | |
| Spoken to a physician about sexual orientation | ||||
| No | 42 (42) | 22 (44) | 20 (40) | |
| Yes | 58 (58) | 28 (56) | 30 (60) | |
| Ever tested for HIV | ||||
| No | 2 (2) | 0 (0) | 2 (4) | |
| Yes | 98 (98) | 50 (100) | 48 (96) | |
| Ever used an HIV self-test | ||||
| No | 32 (32) | 15 (30) | 17 (34) | |
| Yes | 66 (66) | 35/50 (70) | 31 (62) | |
| 2 (2) | 0 | 2 (4) | ||
| Ever tested for syphilis | ||||
| No | 75 (75) | 37 (74) | 38 (76) | |
| Yes | 25 (25) | 13 (26) | 12 (24) | |
| Primary identification | ||||
| Bisexual | 31 (31) | 16 (32) | 15 (30) | |
| Gay | 49 (49) | 26 (52) | 23 (46) | |
| Transgender | 19 (19) | 7 (14) | 12 (24) | |
| Unsure, other | 1 (1) | 1 (2) | 0 | |
| Spoken to a physician – sexual orientation | ||||
| No | 42/100 (42) | 22/50 (44) | 20 (40) | |
| Yes | 58/100 (58) | 28/50 (56) | 30 (60) | |
| Spoken to a family member – sexual orientation | ||||
| No | 43 (43) | 24 (48) | 19 (38) | |
| Yes | 57 (57) | 26 (52) | 31 (62) | |
Two-thirds of participants (68%) were living with family; only 8% were living with an intimate male partner. Just over half of the participants (58%) had spoken to a physician and/or a family member about their sexual orientation. A total of 98 participants reported ever testing for HIV (98%), and 66 had previously performed an unsupervised HIV self-test (66%). Only 25% of the participants had ever tested for syphilis, most commonly through a community-based organisation. One individual had tested for syphilis using a self-test kit prior to this study. Among those who reported ever testing for syphilis, six had a reactive test and had been treated before. Based on their serological data and clinical history, the clinician decided that further syphilis treatment was not necessary for these six individuals (Table 2).
| Total (n = 100) | Facility-testing arm (n = 50) | Self-testing arm (n = 50) | ||
|---|---|---|---|---|
| Tested for syphilis | 66/100 (66%) | 29/50 (58%) | 37/50 (74%) | |
| Had a reactive syphilis test | 14/66 (21%) | 8/29 (28%) | 6/37 (16%) | |
| Treated for syphilis | 8/14 (57%) | 4/8 (50%) | 4/6 (67%) |
In the self-testing arm, 37 of 50 MSM (74%) completed a syphilis self-test (see Fig. 2). Among individuals in the control arm, 29 participants (58%) accessed facility-based syphilis testing (absolute difference 16%, 95% CI −4% to +36%, P = 0.14). Six participants in the self-test arm and eight in the facility-testing arm had a reactive syphilis test result. Of these, four in each arm returned for confirmatory testing. None of the participants reported having received prior syphilis treatment.
One participant reported that they had been pressured to take a syphilis test by their regular male sexual partner. Two participants had difficulties conducting the syphilis self-test alone and sought assistance from a staff member. After additional support, both participants were able to use the self-test kit. There were no adverse events experienced because of MSM participating in the study.
Economic cost results
Total provider program costs were US$736 for the self-testing intervention, and US$859 for facility-based syphilis testing (Table 3). The cost per self-test was US$15 compared with US$30 per facility-based test, reflecting the impact of freeing up health providers’ time, as clients test themselves. Cost per reactive client was US$123 compared with US$107 per facility-based test due to the lower number of syphilis-positive clients. Cost results per client treated were US$183 versus US$215 in the self-testing and facility arms, respectively. The largest cost component for each of the arms was the initial screening test (31% for self-testing arm vs 33% in facility). In the self-testing arm, confirmatory testing contributed 27% (personnel 7% + supplies 20%), demand creation 26% (personnel 17% + supplies 9%), and training and start-up 15%. Confirmatory testing contributed 30% (personnel 8% + supplies 22%) and demand creation 28% (personnel 15% + supplies 8%) for the facility test. Training and start-up costs were 11% of total costs.
| Input categories | Facility testing | % | Self-testing | % | |
|---|---|---|---|---|---|
| Training and start-up costs | $110 | 13 | $110 | 15 | |
| Demand creation – personnel | $125 | 15 | $125 | 17 | |
| Demand creation – supplies | $67 | 8 | $67 | 9 | |
| Initial test – personnel | $166 | 19 | $- | 0 | |
| Initial syphilis test – supplies | $121 | 14 | $229 | 31 | |
| Confirmatory testing – personnel | $69 | 8 | $51 | 7 | |
| Confirmatory syphilis test (including laboratory tests) – supplies | $192 | 22 | $144 | 20 | |
| Treatment – personnel | $8 | 1 | $8 | 1 | |
| Treatment – supplies | $2 | 0 | $2 | 0 | |
| Total program cost | $859 | 100 | $736 | 100 | |
| People tested/no. of kits distributed | 29 | 50 | |||
| Cost per kit distributed | $29.61 | $14.71 | |||
| No. of positive/reactive client | 8 | 6 | |||
| Cost per positive/reactive client | $107.35 | $122.60 | |||
| No. of clients treated | 4 | 4 | |||
| Cost per client treated | $214.69 | $183.90 |
Self-reported user costs were collected through exit interviews with 10 men. Transport costs contributed nearly half of the user costs (52% in self-testing vs 44% in the facility arm, respectively). On average, transport cost was US$1.90 each way for self-test kit collection and the initial facility test. Clients returned for confirmatory testing and upon a confirmed positive result for a single dose of treatment; that is, men needed to take three trips to receive treatment. Food and other expenses averaged US$2.12 per visit. We also assessed productivity losses incurred while seeking syphilis testing, with average self-reported lost income estimated at US$0.76 per hour. This was multiplied by the estimated time for travel (79 min one-way) and at the facility (56 min). In total, each visit incurred an average lost income of US$3.32. The total user cost per client per visit was US$9 for self-testing and US$12 for facility testing. Attending the three visits required to receive syphilis treatment would consume almost 92% of the client’s weekly income (average weekly earnings US$30.30).
Discussion
Our study found that promoting syphilis self-testing among MSM through community-based organisation distribution may increase syphilis test uptake compared with offering facility-based syphilis testing. This finding, where syphilis self-testing was seen to be more acceptable and accessible to MSM, is consistent with studies reporting results of HIV11,25 and hepatitis C virus self-testing.13 Syphilis self-testing may decrease the stigma associated with testing and increase the ease of use compared with facility-based testing. Finally, syphilis self-testing allows the tester to decide when, where and with whom they test. The higher uptake of syphilis testing in the self-testing arm is consistent with our previous qualitative study suggesting that facility-based services may be avoided by some MSM13 due to stigma and discrimination, as well as cultural taboos of being a MSM.26 A key consideration for the current study is that facility testing for syphilis among MSM is not standard of care (n Zimbabwe, and is only routinely provided to pregnant women. Thus, facility-based testing did not represent a true ‘control’ group within the context of this study, as it was only feasible due to extensive training and sensitisation.
We observed poor rates of linkage to care after a positive syphilis self-test in both arms of the study. This finding of poor linkage to care among MSM is in line with other studies2 that showed poor linkage to care for syphilis treatment among MSM. Linkage to care has also been shown to be problematic, especially for diseases where patients are asymptomatic. This may be related to stigma associated with hospitals, homophobic clinical environments and related linkage costs. Future studies may need to focus on how to strengthen linkages to syphilis care as a critical public health intervention.
Syphilis prevalence among MSM in the study was high at 21% (14/66), higher than that reported in a global syphilis systematic review (7.5%) and in the Zimbabwe 2020 study (5.5%).4
Costs of syphilis self-testing in the community appear to be lower than for facility testing. This is in part due to reductions in the cost of personnel, as clients test themselves, reducing the high costs of health providers’ time. Although the numbers are small, we saw that men who used a self-test kit could find their way to the facility for confirmatory testing and treatment. Further analysis of the linkage to care in larger samples is important to fully ascertain the cost-effectiveness of self-testing.
Clients, however, incur substantial costs in accessing testing services related to transport and productivity losses, as well as food and other expenses, particularly due to the need for multiple visits. Client costs have been shown to discourage care-seeking most critically among low-income earners, impeding continuity of treatment and care, even where services are provided at no user fee, and can consign already poor clients to deeper levels of poverty.6,15 Future distribution should consider having the initial screening done in communities, so that user costs can be significantly reduced with only those with a reactive test needing to visit facilities. This would both widen access to testing and reduce user costs and pressures on facility-based services. Furthermore, as the self-testing intervention develops into a fully-fledged community-based distribution model, higher numbers of kits delivered where they are needed most may potentially result in even greater cost reductions. More generally, facility testing algorithms should aim for same day testing and treatment, to ensure treatment of all those with a positive test and reduce user costs.
Our study had several limitations. First, we did not anticipate the high rates of syphilis testing observed in the facility arm. This may have been related to the intensive health care worker training provided to health care workers and the buy-in from the facility, and free travel to the facilities. As a result of this, our study was not adequately powered to detect a difference in the two arms. Second, a key consideration for the current study was that facility testing for syphilis among MSM is not standard of care in Zimbabwe. However, we did not feel it was ethical to recruit patients into a syphilis testing study and offer no route to testing for participants. In addition, COVID-19 restrictions during the study period impacted on the ability of men to travel to the non-profit organisation or the facility. COVID-19 concerns may have made it less likely for people to attend the facility and decreased linkage to care in both arms.
There are challenges associated with potential use of treponemal-only rapid tests as the self-tests, as these would no longer be helpful for those with previously treated syphilis. Thus, the use of dual and non-treponemal tests may need to be developed into self-tests.
In conclusion, this RCT demonstrates the feasibility and potential to increase syphilis testing uptake among MSM through a community-based organisation-delivered self-testing model. Such efforts need to be augmented with broader policies supporting MSM to screen and test for STIs in low- and middle-income country settings. Bringing health services to the people and communities, most importantly to those who need them the most, simplifying service delivery, increasing self-care products, and increasing service access through community-based organisations can be a useful strategy to promote health seeking behaviours among hidden populations, such as MSM.
Data availability
The data that support this study are available in the article and accompanying online supplementary material. If any additional data is required, data that support this study will be shared upon reasonable request to the corresponding author.
Conflicts of interest
Joseph D. Tucker is an Editor-in-Chief of Sexual Health and Michael Marks is an Associate Editor of Sexual Health, to mitigate this potential conflict of interest they were blinded from the review process.
Acknowledgements
The authors acknowledge the support from the Ministry of Health and Child Care, City of Harare, GALZ, Hands of Hope and Zimbabwe Rainbow Community who were instrumental in the research. The authors acknowledge support from Dr Hilda Bara and Ms Anna Machiha in particular. The authors also thank all the men who volunteered their time to participate in the study.
References
1 Hawkes SJ, Gomez GB, Broutet N. Early antenatal care: does it make a difference to outcomes of pregnancy associated with syphilis? A systematic review and meta-analysis. PLoS ONE 2013; 8: e56713.
| Crossref | Google Scholar |
2 Peeling RW, Mabey D, Kamb ML, Chen X-S, Radolf JD, Benzaken AS. Syphilis. Nat Rev Dis Primers 2017; 3(1): 17073.
| Crossref | Google Scholar |
3 Nyitray AG, Carvalho da Silva RJ, Baggio ML, Lu B, Smith D, Abrahamsen M, et al. Age-specific prevalence of and risk factors for Anal Human Papillomavirus (HPV) among men who have sex with women and men who have sex with men: the HPV in men (HIM) study. J Infect Dis 2011; 203(1): 49-57.
| Crossref | Google Scholar |
6 Bien-Gund CH, Zhao P, Cao B, Tang W, Ong JJ, Baral SD, et al. Providing competent, comprehensive and inclusive sexual health services for men who have sex with men in low- and middle-income countries: a scoping review. Sex Health 2019; 16(4): 320-31.
| Crossref | Google Scholar | PubMed |
7 Tsang EY-H, Qiao S, Wilkinson JS, Fung AL-C, Lipeleke F, Li X. Multilayered stigma and vulnerabilities for HIV infection and transmission: a qualitative study on male sex workers in Zimbabwe. Am J Men’s Health 2019; 13(1): 1557988318823883.
| Crossref | Google Scholar |
8 Chow EPF, Callander D, Fairley CK, Zhang L, Donovan B, Guy R, et al. Increased syphilis testing of men who have sex with men: greater detection of asymptomatic early syphilis and relative reduction in secondary syphilis. Clin Infect Dis 2017; 65(3): 389-95.
| Crossref | Google Scholar | PubMed |
9 ZIMPHIA. Zimbabwe population-based HIV impact assessment ZIMPHIA 2020 key findings. 2020. Available at https://phia.icap.columbia.edu/wp-content/uploads/2020/11/ZIMPHIA-2020-Summary-Sheet_Web.pdf [cited 23 March 2021]
10 Ong JJ, Fu H, Smith MK, Tucker JD. Expanding syphilis testing: a scoping review of syphilis testing interventions among key populations. Expert Rev Anti Infect Ther 2018; 16(5): 423-32.
| Crossref | Google Scholar |
11 Wang C, Cheng W, Li C, Tang W, Ong JJ, Smith MK, et al. Syphilis self-testing: a nationwide pragmatic study among men who have sex with men in China. Clin Infect Dis 2020; 70(10): 2178-86.
| Crossref | Google Scholar |
12 Marks M, Mabey DCW. The introduction of syphilis point of care tests in resource limited settings. Expert Rev Mol Diagn 2017; 17(4): 321-5.
| Crossref | Google Scholar | PubMed |
13 Sri-Pathmanathan C, Nhamo D, Mamvuto T, Chapwanya G, Terris-Prestholt F, Mahaka I, et al. Syphilis self-testing to expand test uptake among men who have sex with men: a theoretically informed mixed methods study in Zimbabwe. Sex Transm Infect 2022; 98(3): 197-202.
| Crossref | Google Scholar |
14 Rivera AS, Hernandez R, Mag-usara R, Sy KN, Ulitin AR, O’Dwyer LC, et al. Implementation outcomes of HIV self-testing in low- and middle- income countries: a scoping review. PLoS ONE 2021; 16(5): e0250434.
| Crossref | Google Scholar | PubMed |
15 World Health Organization. WHO consolidated guideline on self-care interventions for health. Available at https://apps.who.int/iris/bitstream/handle/10665/325480/9789241550550-eng.pdf.
| Google Scholar |
16 Pangaea Zimbabwe Aids Trust. Advocates for evidence-driven strategies to expand quality health services; 2022. Available at https://www.pzat.org/ [cited 10 February 2022]
17 Walensky RP, Jernigan DB, Bunnell R, Layden J, Kent CK, Gottardy AJ, et al. Morbidity and mortality weekly report. Sexually transmitted infections treatment guidelines, 2021. 2021; Centers for Disease Control and Prevention, MMWR Editorial and Production Staff (Serials) MMWR Editorial Board. Available at https://www.cdc.gov/std/treatment-guidelines/STI-Guidelines-2021.pdf [cited 2 February 2022]
18 Ministry of Health and Child Care. Guidelines for antiretroviral therapy for the prevention and treatment of HIV in Zimbabwe. 2016. Available at https://depts.washington.edu/edgh/zw/vl/project-resources/ZIM_ART_Guidelines_2016_-_review_final.pdf [cited 15 July 2020]
19 Vassall A, Sweeney S, Kahn JG, Gomez G, Bollinger L, Marseille E, et al. Reference case for estimating the costs of global health services and interventions. 2018. Available at https://researchonline.lshtm.ac.uk/id/eprint/4653001/1/vassall_etal_2018_reference_case_for_estimating_costs_global_health_services.pdf [cited 23 October 2021]
20 Wilkinson T, Sculpher MJ, Claxton K, Revill P, Briggs A, Cairns JA, et al. The international decision support initiative reference case for economic evaluation: an aid to thought. Value Health 2016; 19(8): 921-8.
| Crossref | Google Scholar |
21 Fern T-P, Andreia S, Sedona S, Lilani K. The rapid syphilis test toolkit: implementation 1: guidelines for cost effectiveness analysis for syphilis screening strategies. 2011. Available at https://media.tghn.org/articles/IMPLEMENTATION_1.pdf [cited 23 October 2021]
22 Sande L, Maheswaran H, Mangenah C, Mwenge L, Indravudh P, Mkandawire P, et al. Costs of accessing HIV testing services among rural Malawi communities. AIDS Care 2018; 30(sup3): 27-36.
| Crossref | Google Scholar |
23 Mwenge L, Sande L, Mangenah C, Ahmed N, Kanema S, d’Elbée M, et al. Costs of facility-based HIV testing in Malawi, Zambia and Zimbabwe. PLoS ONE 2017; 12(10): e0185740.
| Crossref | Google Scholar |
24 Mangenah C, Mwenge L, Sande L, Ahmed N, d’Elbée M, Chiwawa P, et al. Economic cost analysis of door-to-door community-based distribution of HIV self-test kits in Malawi, Zambia and Zimbabwe. J Int AIDS Soc 2019; 22(S1): e25255.
| Crossref | Google Scholar |
25 Bil JP, Prins M, Stolte IG, Dijkshoorn H, Heijman T, Snijder MB, et al. Usage of purchased self-tests for HIV and sexually transmitted infections in Amsterdam, the Netherlands: results of population-based and serial cross-sectional studies among the general population and sexual risk groups. BMJ Open 2017; 7(9): e016609.
| Crossref | Google Scholar |
26 Ndione AG, Procureur F, Senne J-N, Cornaglia F, Gueye K, Ndour CT, et al. Sexuality-based stigma and access to care: intersecting perspectives between healthcare providers and men who have sex with men in HIV care centres in Senegal. Health Policy Plan 2022; 37(5): 587-96.
| Crossref | Google Scholar |