Sex film viewing, but not hypersexual concerns, are associated with more sexual arousal in anticipation of an intimate partner experience
Nicole Prause A * and Greg Siegle B
A * and Greg Siegle B
A University of California, 405 Hilgard Avenue Box 951405, Los Angeles, CA 90095-1405, USA.
B University of Pittsburgh, School of Medicine, 3811 O’Hara Street, Pittsburgh, PA 15213, USA.
Sexual Health 19(2) 79-91 https://doi.org/10.1071/SH21219
Submitted: 5 November 2021 Accepted: 19 February 2022 Published: 26 April 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Background: Hypersexual behaviours could reflect psychopathology, in part, because they impair interactions with intimate partners.
Methods: Hypersexual concerns were measured as: (1) concern about inability to control one’s own sexual behaviours; and (2) sexual films viewed. The outcome, sexual arousal, was measured using two indicators: (1) self-reported sexual arousal (before/after); and (2) skin conductance response from the person stimulated (continuously). Two-hundred and fifty participants completed Orgasmic Meditation (OM), a coupled, structured, 15-min manual-genital stroking.
Results: Reported difficulty controlling their own sexual behaviours was not related to sexual arousal reports. Participants who viewed more sexual films reported more sexual arousal before starting OM than participants who viewed less sexual films. Strokers who viewed more sexual films were associated with a higher skin conductance response in the stroked partner.
Conclusions: Despite statistical power and pre-registration, hypersexual concerns did not predict sexual responses with a partner. Sex film viewing may increase sexual responsiveness in individuals and their partners.
Keywords: addiction, compulsive sexual behaviour disorder, compulsivity, International Classification of Disorders, media, Orgasmic Meditation, pornography, sexuality.
Introduction
Frequent sexual urges or behaviours can cause distress, but the cause of this distress is debated. Identifying the best model of hypersexual concerns is necessary to develop effective interventions to reduce those concerns. For this project, ‘hypersexuality’ is conceptualised as an unspecified model, simply describing this distress about sexual behaviours or feelings that are felt to be too intense or frequent. One way to understand this distress is as a part of a compulsivity model. Compulsive behaviours have been conceptualised as rigid behaviours that are resistant to extinction and performed to reduce negative consequences, including negative affect.1 In disorders of compulsivity, the behaviours become unwanted and cause functional impairment.2 Although models of compulsivity can vary, these tend to be their core, proposed features. Consistent with other compulsivity models from non-sexual domains,3 the compulsivity model of sexual behaviours has been described as sexual ‘behaviour that is driven by anxiety reduction rather than sexual desire’.4 A sexual ‘compulsivity’ model, thus, suggests that frequent, distressing sexual behaviours are a function of reducing negative, rather than increasing positive, affect. That is, in the sexual compulsivity model, an affected person might be motivated to engage in sexual behaviour by sexual desire, but is always motivated by negative affect (not necessarily related to sex); a non-affected person engages in sexual behaviour primarily from sexual desire, although they might also use sex to regulate negative affect and not feel concern about this strategy.5
The current study helps test support for competing models of ‘out of control’, frequent sexual behaviours such as impulsive and compulsive models,6 as well as normal variation that is not clinically relevant5 in a non-patient sample. Compulsivity is a transdiagnostic construct describing a habit of problematic decision-making that has become separated from behavioural goals.7 Those with compulsivity have been characterised by enhanced autonomic responses to stressors of all types, which are slow to resolve.8
Disambiguating compulsivity and impulsivity models
One claim of the sexual compulsivity model is functional impairment. Specifically, those who are sexually compulsive should struggle to experience sexual arousal with a human partner.9 Relatedly, others have suggested that sexual compulsivity negatively affects an individual’s relationships.10 In the sexual compulsivity model, this arousal impairment is hypothesised to occur because compulsivity is characterised by constantly high levels of negative affect that the sexual behaviours are used to reduce.11
Impulsivity models of behaviour are heterogeneous, but generally describe rapid decisions to act with little forethought.12 Specifically, impulsivity is characterised by a tendency to value immediate, smaller rewards over later rewards and inability to inhibit responses.13 Impulsive behaviours have been described as characterised by feelings of urgency, lack of premeditation, low perseverance, and high sensation/excitement-seeking.14 People with impulsive problems appear to have lower conditional associative learning abilities, which results in poorer inhibition during decision-making.15 With respect to pleasure, impulsivity shares a mesolimbic pathway with anhednoia, characterised by lower dopamine reactivity.16 Sexual impulsivity, then, is a tendency to engage in sexual behaviours quickly or without fully thinking through the consequences.17 Impulsivity is correlated with a variety of risky sexual behaviours.18,19
An extensive literature disambiguating compulsivity from impulsivity exists. Persistence has been proposed as a key distinguishing feature, where compulsive behaviours are uniquely difficult to stop.20 Berlin and Hollander21 highlight a number of differences in impulsivity and compulsivity with respect to pathology. Impulsive behaviours are not well thought out when performed, whereas compulsions are understood as harmful, perseverative rituals that one feels emotionally compelled to perform. Impulsive behaviours tend to occur with feelings of pleasure (ego-syntonic), where compulsions are often performed to reduce tension (ego-dystonic). Further, impulsive behaviours are characterised by action without foresight, whereas compulsive behaviours are planned, ruminative, rigid, and stereotypic. These constructs also have differentiable neuroanatomy.22
Addiction and compulsivity models
A third popular pathology model is the sexual addiction model, which the current data do not attempt to distinguish from compulsivity, but which also features prominently in the literature. Although addictions are characterised by varying elevations in compulsivity and impulsivity, the addiction model adds unique predictions of behaviours. Time-course is thought to be a defining feature, where the behaviour initially is pleasurable and liked, but becomes craved and experienced with less pleasure.23,24 Further, tolerance and withdrawal tend to be uniquely predicted by addiction models.25 For example, addictive sexual behaviours are thought to be characterised by a lesser sexual response to the same stimulus over time.26 Debates exist concerning whether substance addiction criteria can or should be applied to proposed models of behavioural addictions,27 which we will not attempt to resolve here.
The claim that compulsivity could lead to sexual difficulties with a partner is consistent with other pathology models, such as that of anxiety mediating erectile dysfunction. Anxiety is well-characterised as a predictor of sexual arousal difficulties, especially erectile difficulties.28 Although the relationship between anxiety and sexual arousal is sometimes complex,29 very high levels of anxiety appear to consistently diminish feelings of sexual arousal.30 It may be that no separable ‘compulsive sex’ pathology exists, but people who believe they have this problem are simply more anxious about sex. None of these predictions have been tested with real-world sexual behaviours, which is the first step to determine whether sexual compulsivity is useful to assess in patient populations.
Ecological context: orgasmic meditation
Much of the laboratory research regarding out-of-control, frequent sexual behaviours has regarded a single individual interacting with computer-based material. This is in stark contrast to the partnered activities, which allegedly confer real-life dangers to individuals. As such, this study employed a partnered context. We needed a safe, predictable partnered sexual interaction protocol. This means a protocol that is explicitly consensual, standardised, and not a disease risk. A protocol ensuring continual consent should minimise volunteer bias.31 Although such safeguards can disrupt sexual experiences,32 they were necessary for safely testing in a laboratory environment. Sexual anxiety was minimised by not demanding erections28 or orgasms.33 Orgasmic Meditation (OM) increases closeness between partners in a laboratory setting34 and appears to provide a positive and safe sexual arousal experience for individuals who have experienced sexual trauma.35
OM was a form of partnered meditation that met study requirements. Further, 15 min of the practice was indirect, manual stimulation of the clitoral shaft, which is the preferred area for female genital stimulation.36 Human clitoral touch afferents also are active in roles of social touch.37
Current study
Different models of ‘out-of-control’ sexual behaviours make different predictions about sexual arousal experienced with a partner.38,39 A compulsivity model predicts that behaviours are engaged habitually and become dissociated from goals. For sex, we interpret compulsivity as predicting less sexual arousal following partnered sexual stimulation. An impulsivity model predicts that behaviours occur without delay to reap rewards sooner rather than later. Considering sex, we interpret impulsivity as predicting stronger sexual arousal preceding a sexual interaction, a response one may struggled to inhibit. An addiction model predicts that craving for stimuli drives behaviour, not pleasurable liking, and tolerance develops to the stimulus over time. Some have described individuals having ‘impairments of sexual arousal…in intimate relationships’.40 For sex, we interpret that the addiction model predicts high sexual arousal prior to engaging in sexual activity, with significantly less/no increase in sexual arousal following partner intimacy due to tolerance to such a repeated, standard intimate experience. We note that this prediction shares some predictions with other models. Thus, the pattern of results may not be able to rule out certain models. In summary, distress about sexual behaviours that feel out of control may have been associated with lower sexual arousal following the sexual interaction (compulsivity), higher sexual arousal preceeding the stimulation (impulsivity), or sexual arousal that may decrease or not change after stimulation (addiction). A normative response would be that sexual arousal is initated during sexual stimulation, and is not related to distress. For these reasons, our tests were two-tailed. We test the relationship between sexual arousal experienced before and after OM as predicted by these individual differences. Consistent with best practices, we registered our primary hypotheses (H) through the Open Science Framework (https://osf.io/zf4vd/register/564d31db8c5e4a7c9694b 2be).
(H1) Feeling that one’s sexual behaviours are out-of-control would predict sexual arousal experienced with a partner after controlling the sexual arousal experienced in anticipation of the partner stimulation.
-
Compulsivity and addiction models predict that the association with sexual arousal after partnered stimulation is negative.
-
Impulsivity models predict that the association with sexual arousal before partnered stimulation is positive.
(H2) The amount of sex film viewing would predict sexual arousal experienced with a partner after entering controlling the sexual arousal experienced in anticipation of the partner stimulation.
-
Compulsivity and addiction models predict that the association with sexual arousal after partnered stimulation is negative.
-
Impulsivity models predict that the association with sexual arousal before partnered stimulation is positive.
All three models depend on the idea that general arousal is directly linked to hypersexuality. To test this basic claim, we measured general arousal from the strokee using skin conductance response (SCR). SCR is known to be sensitive, but not specific, to sexual arousal.41 Thus, SCR was included only as a secondary measure of general arousal. We expected SCR would follow the same predictions as for sexual arousal, because it is an index of arousal broadly. These were not included in the preregistration:
(H3) Feeling that one’s sexual behaviours are out-of-control and sex film viewing would be associated with SCR change.
Methods
Participants
Participants comprised 125 couples (N = 250) who had practised OM at least 10 times in their lifetime, so that they had a general familiarity with the practice (Table 1). They were recruited through both social media advertisements (Twitter, Facebook) and by word-of-mouth, including sharing on OM listservs. Participants had to be aged ≥18 years, free from a history of neuropsychiatric diagnosis (e.g. stroke, multiple sclerosis), have normal or corrected-to-normal vision, and have a regular OM partner who also would qualify and participate. Partners were not required to be romantic partners. Each participant agreed to abstain from any alcohol or recreational drug use in the 24 h prior to participating. The laboratory was mobile (see below), so participants were tested in private settings that included apartments, offices, and event spaces in Los Angeles, San Francisco, and New York, so long as privacy could be protected.
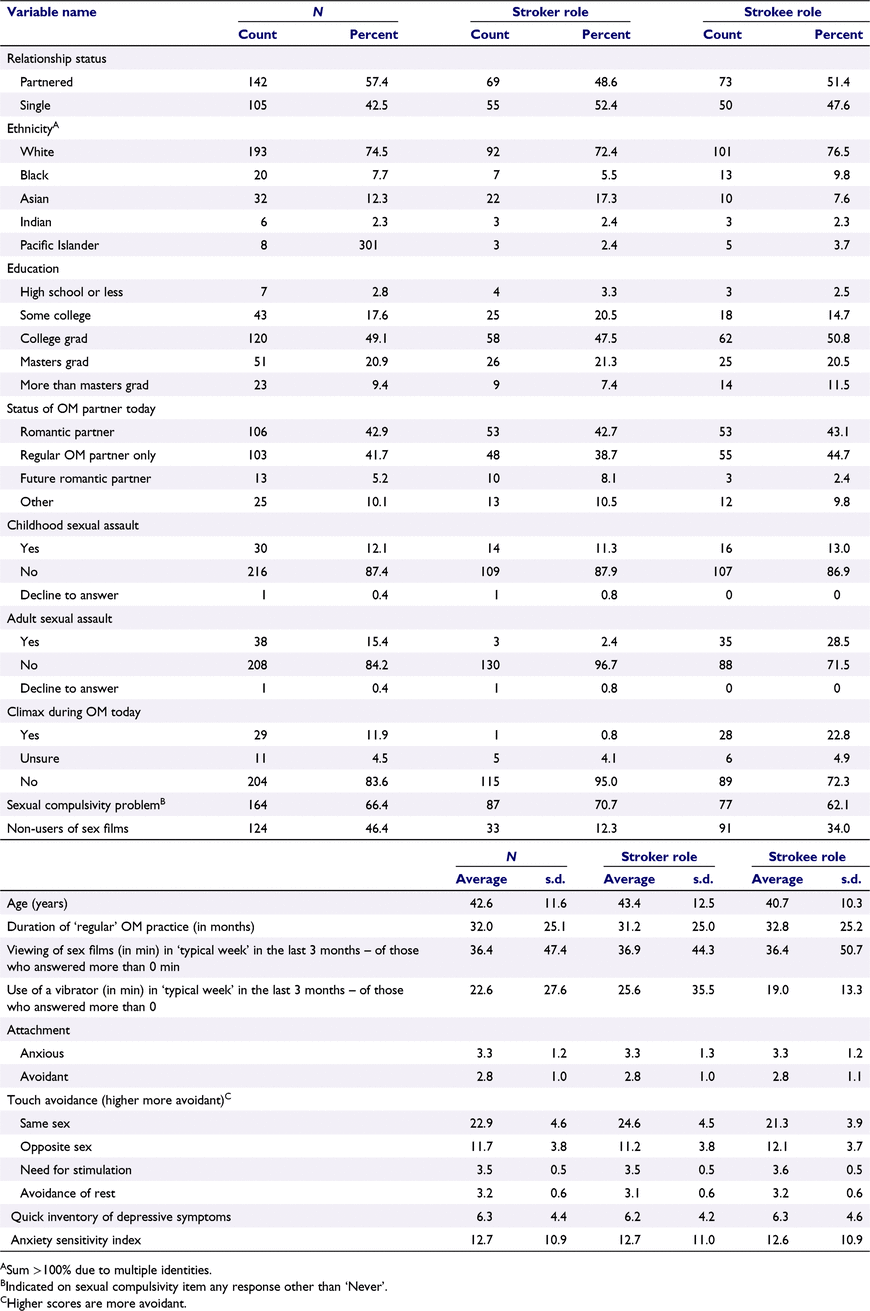
|
Questionnaire measures
Individual characteristics
Background information was collected on participants’ levels of sensation seeking, touch avoidance, pathology symptoms, and sexual assault history. These are used to characterise the sample. The Need Inventory of Sensation Seeking42 measures the need to seek varied, novel, complex and intense sensations and experiences. It is a 17-item list of statements that can be endorsed as feeling each one on a scale of 1 (‘almost never’) to 5 (‘almost always’) in the last 6 months (range 17–85). Higher scores indicate a greater need for sensation seeking. The scale is commonly broken into subscales of ‘Need for Stimulation’ and ‘Avoidance of Rest’, each ranging from 1 to 5.43
Depressive symptoms were assessed using the 16-item Quick Inventory of Depressive Symptoms.44 The scale asks about feelings in the last 7 days, including thoughts of death or suicide and decreased appetite. The scale score ranged from 0 to 27, with scores above six considered consistent with depression.44 This Inventory converges with other measures of depression.45 One-month test–retest reliability was moderate (r = 0.54)46 and discriminant validity is less well-established.47 The measure was sensitive to clinical improvement with treatment.48
The 16-item Anxiety Sensitivity Inventory49 was used to quantify threatening beliefs regarding feelings of arousal. Respondents were asked to rate a series of statements by the degree to which they agreed using a five-point Likert-like scale ranging from 0 (not at all) to 4 (very much). This results in a score range of 0–64. Some have suggested that the scale contains two underlying factors, but these may be hierarchical.50 Thus, we use a single score for this scale. The scale has reasonable test–retest reliability over 5 weeks in a non-clinical sample (r = 0.65).51 It was moderately related with measures of generalised anxiety disorder and obsessive compulsive disorder.52 Psychometric properties appear stable across age ranges.53
Finally, participants were asked two questions about their history of sexual abuse and assault: ‘Have you had sex (oral, anal, genital) BEFORE age 16 years when you did not want to because someone forced you in some way or threatened to harm you if you did not?’ and ‘Have you had sex (oral, anal, genital) AFTER or when you were 16 years when you did not want to because someone forced you in some way or threatened to harm you if you did not?’.54
Hypersexual problem question
Consistent with previous, independently replicated research,55,56 hypersexual problems were quantified as a dimensional, individual difference construct. To understand whether sexual compulsivity is best thought of as one or more constructs, we subjected the widely used Hypersexual Behavior Inventory57 and used a graded response model (see Appendix A in Supplementary material). This approach identified that a single item had the highest discriminability (d = 4.28) of all 19 items. Further, a receiver operator curve analysis showed this item alone predicted diagnosis of hypersexual problems by an experienced, trained clinician (AUC = 0.91) with good specificity (88%) and reasonable sensitivity (75%). This item also showed convergent validity and replication with previous studies of romantic attachment, and was moderately correlated with both anxious attachment and avoidant attachment. In summary, an extensive analysis supported the use of this single item in this study (see Appendix A in Supplementary material). The item was: ‘Even though I promised myself I would not repeat a sexual behaviour, I find myself returning to it over and over again’. Response options ranged from 1 (‘Never’) to 5 (‘Very often’).
Patients were not sought for this study. A non-pathological sample offered several advantages. First, a continuous measure of hypersexual problems allows for a more powerful statistical ‘dose-response’ test rather than comparing categories (patient vs non-patient). Second, using a categorical approach risks recreating a symptom cluster actually created due to inclusion/exclusion criteria. Third, there is currently no agreed symptom cluster for hypersexual disorders. Focusing on a single, primary symptom across models of different symptom clusters appeared to be a fairer test of the proposed models. Hypersexual disorder was rejected for inclusion in the latest Diagnostic and Statistical Manual, and was added without any field testing to the International Classification of Disorder 11 as a compulsivity disorder. There appeared to be no consensus that would allow a reasonable categorical approach to test these models. Finally, data have shown these concepts structurally are continuous, not categorical, in nature.56 For these reasons, a continuous approach was more desirable than a categorical approach for this study.
Sex film viewing question
Participants were instructed ‘Think of a typical week in the last 3 months. How many minutes did you view adult sex films (‘porn’) in this week?’. Response options ranged from ‘0’ to ‘More than 10 h’ in 10 min increments (62 choices). Each hour was noted alongside the minutes to aid estimations. This approach is consistent with previous studies to estimate current levels of consumption.58 Although attempts have been made to create composite measures of sex film involvement, such as including the age of regular viewing initiation, these measures face the same challenges raised by sexual risk composite measures. We follow the advice for sexual risk measures.59 Briefly, this means reporting on sexual behaviours individually rather than attempting to create a composite risk index.
Skin conductance response
Skin conductance response is thought to index sympathetic tone. Sympathetic nervous system activity reliably facilitates sexual arousal,60 including a skin conductance response.61,62 Although SCR is sensitive to sexual arousal, it is not specific to sexual arousal.41 SCR also increases during other emotional experiences,63 making SCR less desirable than genital measures for sexual arousal assessments when affective states are mixed.41,64 SCR was recorded from the strokee throughout the 15 min of genital stroking in a single OM session in 100 strokees. These data have been shown to be monotonic, and change (increase or decrease) in SCR related, as expected, to state measures experienced following genital stroking, including feeling anxious or close to others.34 As objective and subjective measures of arousal frequently diverge, SCR was examined to test whether objectively measured arousal was related to measures of sexual compulsivity or sex film viewing. The SCR analysis was not pre-registered for this purpose, as SCR was being collected primarily for a different study. Given the divergence of self-report and physical indicators of arousal in sexuality studies, we decided SCR was worthwhile to add as an exploratory approach to complement the pre-registered analyses.
Of the N = 125 participants on whom SCR data were collected, data loss (data file corrupt, N = 5; physiological data not stored, N = 3; timing markers not stored, N = 5; recording terminated early, N = 3; non-reactive SCR, N = 12) left N = 93 couples (of 125 couples) available for analysis.
Task
Instructions were presented on a monitor on the ground, placed to be visible to the stroking partner, and included brief auditory alerts during each OM transition. The stroker was instructed by the experimenter to press any key after completing each stage. During ‘Stroking’ and ‘Stroking 2 min’, the computer timed for 13 min and 2 min, respectively, advancing automatically. These reflect the stages practised in OM (see above).
Sexual arousal rating
Immediately before and after the OM, participants rated the level of ‘sexual arousal’ (0 – ‘Not at all’, 7 – ‘Extremely’) that they felt. Rated sexual arousal was the primary dependent variable.
This rating approach was first used by Heiman and Rowland65 and is a common approach used to study felt sexual response in men and women.66 Rated sexual arousal tends to converge strongly with genital response in men.67 Sexual arousal ratings are uniquely elevated to sexual stimuli (e.g. as compared to exciting films68). Physiological genital arousal was not measured. With the hand over the vulva and thumb over the vaginal os (opening), we felt large, irregular artifacts were very likely to render many possible measures impossible to process. Also, felt sexual arousal is prioritised over physiological measures in making clinical judgments of sexual problems, especially for women.69 Also, the presence of the thumb over the introitus would make vaginal and vulvar measures impossible without inventing new instrumentation for the purpose (e.g. robust to thumb movements, not confounded by heat from the hand, etc.). Finally, physiological sexual arousal that does not reach conscious awareness through attention is not expected to influence sexual function or behaviours. Given that the goal of the study was to characterise responsiveness to, and regulation of, sexual responses that are expected to affect problematic urges to behave sexually, self-reported sexual arousal ratings were the primary dependent variable.
Procedure
All study procedures were continuously approved by the University of Pittsburgh, Institutional Review Board. Volunteers were contacted by phone and screened for inclusion criteria (see Participants). Each identified their intended partner by name, who volunteered independently, and also provided that person’s name. They were scheduled for one, 3-h session in a private environment. Informed consent in writing was obtained from all individual participants included in the study. After providing informed consent, they completed a series of questionnaires assessing demographics, sexual history, experience with OM, mental health, emotional attachment, and current feelings of closeness to their OM partner, closeness to others, and emotions. They then donned equipment for assessment of electroencephalography. They completed a series of three computer tasks, one assessing their emotional responsivity, a persistent vigilance task, and a paced serial addition task. Results of the other physiology and tasks will be reported elsewhere.
Orgasmic meditation
Afterwards, they completed one OM in a private room while their biological signals were monitored using additional physiological monitoring equipment. OM has been described in detail in prior publications.34,35 Although the provance of this OM protocol is unclear, many who practice OM reference a book published that described the practice in 2011 as having originated from a Buddhist tradition.70 Many free guides, both video71 and written,72 provide instruction for couples interested in learning the practice. The couple set up their space as desired for the OM, including pillows, blankets, and yoga mats for comfort. The person to be stroked laid flat on their back with their feet together and legs butterflied open. The experimenter attached biological recording devices designed to not restrict movement, then left the room and shut a door between the test and experimenter rooms. The strokee removed their pants or raised their skirt to expose their vulva, thus only those with a vulva could be a strokee. The person to provide the stroking sat beside with their legs comfortably around the strokee (see Appendix B in Supplementary material). The stroker could be any gender. No parallel practice currently exists for stroking the penis. The stroker advanced the OM by pressing a key beside him or her. The largest portion of the OM, and focus of study, was a 15-min period of slow, clitoral stroking with a goal only ‘to feel’.
After the OM, participants completed the same computer tasks again. They then answered questions about their response during the OM and their current feelings. Participants were offered the opportunity to ask any questions that they had. Each participant received US$25 cash. Data were anonymised by eliminating all links with their Informed Consent. No one withdrew during the session.
Data analysis
The primary tests used the hypersexuality measures to predict sexual arousal responses in the laboratory. Specifically, the pre-registered approach used regression, where hypersexuality was a predictor of self-reported sexual arousal after the genital stroking practice. Although we did not expect that individuals would report sexual arousal prior to engaging in the stimulation practice, we entered self-reported sexual arousal prior to stimulation first in the regression to address this possibility.
Self-reported sexual arousal prior to the genital stroking practice was entered first in stepwise regression. The hypersexuality item was slightly skewed (skew = 0.6, z = 3.9, P < 0.001), so we used tests that did not make normality assumptions. Also, three participants did not answer this question. Regular sex film viewing was denied by 50% of participants (N = 125 of 250), requiring an approach accounting for zero-inflation. This approach analyses the viewing variable as continuous in those who endorsed any viewing. The R library PSCL73 zero-inflation was used with log link distribution and binomial family, which uses the partial Wald tests of coefficients.74 In both analyses, sexual arousal reported before the OM was entered first as a control.
Finally, SCR was transformed from raw to relative units (see above for details75).
Power analyses were conducted using the R library ‘pwr’.76 Planned analyses were powered to detect a small effect size. In the power analysis for the self-reported sexual arousal data, we assumed 1 − β = 0.8, d.f. = 2, P = 0.05 and f2 = 0.10. This required 98 participants per analysis. Although this hypothesis applied to the entire sample, we wanted to be able to check whether the pattern was consistent for strokers and strokees. Thus, 98 strokers and 98 strokees were needed to test the hypothesis in each subsample individually. As the GSR data were collected only in the strokee and are the sole predictor (d.f. = 1), n = 80 were required to power that analysis.
Results
In questionnaires, participants did not show evidence of pathology consistent with depression or anxiety (Table 1).
Participants reported a range of distress about engaging in unintended sexual behaviours (Table 1). Specifically, a response rating of 2 (n = 96) and 3 (n = 55) were frequently endorsed, whereas some also endorsed 4 (n = 11). The highest response rating, 5 (‘Very often’), was rarely endorsed (n = 5). The proportion endorsing concern did not differ significantly by their role in the OM.
Self-reported sexual arousal increased significantly from before OM to after OM with a moderately large effect size (t(243) = –8.7, P < 0.001, CI = −0.8 to −0.5, gHedges = 0.59). The average increase was 0.7.
Self-reported sexual arousal
Distress about hypersexuality was not associated with self-reported sexual arousal after OM for either strokers (hypersexuality coefficient P > 0.05, 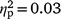 ) or strokees (hypersexuality coefficient P > 0.05,
) or strokees (hypersexuality coefficient P > 0.05,  ) after entering self-reported sexual arousal before OM.A To test whether effects might emerge given a much larger sample, a counternull coefficient77 was calculated based on the non-significant correlation values for self-reported sexual arousal before OM (r = 0.03) and self-reported sexual arousal after OM (r = 0.07). Counternull coefficients for self-reported sexual arousal before OM (r-counternull = 0.06) and after OM (r-counternull = 0.14) remained small. These indicate that the non-significant effects we reported are as likely to contain zero as a small effect.
) after entering self-reported sexual arousal before OM.A To test whether effects might emerge given a much larger sample, a counternull coefficient77 was calculated based on the non-significant correlation values for self-reported sexual arousal before OM (r = 0.03) and self-reported sexual arousal after OM (r = 0.07). Counternull coefficients for self-reported sexual arousal before OM (r-counternull = 0.06) and after OM (r-counternull = 0.14) remained small. These indicate that the non-significant effects we reported are as likely to contain zero as a small effect.
The amount of sex film viewing was related to self-reported sexual arousal before starting the OM (poisson with log link coefficient = 0.11, s.e. = 0.04, z = 2.6, P = 0.009),B but was not related to self-reported sexual arousal after the OM (Fig. 1). People who view sex films report more sexual arousal before starting an intimate interaction the more minutes per week they viewed.
Skin conductance response
Skin conductance response of the strokee was unrelated to measures of sexual compulsivity (r’s< 0.005). Similarly, the relationship of sex film viewing and SCR in the strokee was not significant (r = −0.08, P = 0.46). However, the strokee’s SCR was positively related to the strokers viewing of sex films with a small-to-moderate effect size (ρ = 0.23, P = 0.03).
Given that SCR does not, itself, distinguish positive and negative emotions, we conducted an exploratory follow-up analysis. The relationship of the sex film viewing by the stroker, and change in affect reported by the strokee (sexual arousal, happy, amused, anxious, angry) were examined. We used an ordinal analysis and simple difference scores for affect (emotion rated after OM – emotion rated before OM). The only significant association was between porn viewed by the stroker and self-reported sexual arousal by the strokee (ρ = 0.25, P = 0.006). Strokers who reported viewing more porn were more likely to provoke more sexual arousal in their partner.
Discussion
Concern about hypersexual behaviours was not significantly related to the experience of sexual arousal with a partner, despite the study being sufficiently statistically powered to detect small effects (Hypothesis 1a and 1b). Those who viewed more sex films reported higher sexual arousal before starting OM, and sex film viewing was not associated with sexual arousal after OM (Hypothesis 2a and 2b). The more time the stroker spent viewing sex films, the greater the autonomic arousal exhibited by the strokee. Concern about hypersexual behaviours and sex film viewing did not impede sexual arousal with a partner, as measured in this study. In fact, viewing sex films was associated with two indicators of higher sexual arousal.
The different models made different predictions about how feeling sexually out-of-control would affect sexual arousal with a partner. Despite having sufficient variance and power, feeling out-of-control was not predictive of sexual arousal or general arousal during this intimate partnered experience. However, sex film viewing predicted sexual arousal experienced and physiological general arousal. Experiencing greater sexual arousal in anticipation of partner intimacy would be consistent with addiction or normophilic models of sex film viewing; however, addiction and compulsivity models also predict a diminished sexual response to partners, which did not occur. These results appear most consistent with a normophilic model of sex film viewing. This is consistent with previously published cross-sectional and longitudinal studies (described above), which relied on retroactive recall of partner experiences using questionnaires.
Limitations, concerns, and their implications for future research
Volunteer bias is always a concern with laboratory studies. Those who volunteer for psychophysiological studies of sexual response do not consistently differ in many domains,78 with the exception of generally being higher sensation seeking and more sex positive.79 The present study participants who practice OM resembled the ethnic diversity of the country well. The rates of adult sexual assault appeared higher in OM participants than the general USA population. Specifically, 28.5% of women in the sample versus 11.5% in the general population, and 2.4% of men in the sample versus 1% in the general population reported adult sexual assault.80 Relatedly, childhood sexual abuse was reported by 13.0% of women in the sample versus 7% in the general population, and 11.3% of men in the sample versus 2.0% of men in the general population. This may reflect a more victimised sample, or that the question wording in the nationally representative sample was much more restrictive (e.g. required anal or vaginal penetration or attempted penetration). Participants in this study reported levels of anxiety81 and depression symptoms that were inconsistent with pathology. Study participants also reported a ‘Need for Stimulation’ similar to another convenience sample for a study of sexual behaviours, but their ‘Avoidance of Rest’ was higher (M = 2.6).43 Recruitment was minimally restrictive to avoid ‘super-normal’ samples.82 However, those who practice OM or volunteered for the study may be found to differ in some other systematic way in future studies. This sample may experience hypersexual distress differently than those persons who do not practice OM or chose not to volunteer for this study. A future approach to support stronger external validity could provide instructions to intimate couples in increasingly ‘usual’ settings that are explicitly sexual.
One other limitation of the study is that the current sample was not recruited for hypersexual problems. Individuals with more extreme experiences of hypersexual problems or more frequent viewing of sex films might produce different observations than those reported in this study. The potential differences from a treatment-seeking sample could even lead to different conclusions regarding the best-fitting model. For example, depression rates typically are higher in those who seek treatment for hypersexual behaviours.83 Of course, this raises a serious question about whether hypersexual behaviours are really distinct from depression. Ignoring that concern for a moment, those who are depressed certainly experience avolition and lower pleasure, on average, than those without depression. That should affect the sexual arousal experience of a treatment-seeking sample in a protocol like this. Any study also could be subject to demand characteristics. It is not clear what a participant might assume represents a ‘desirable’ response, offering some reassurance that this risk may be low.
A fair proportion of the current sample also denied recent porn viewing (Table 1) when a common method of measuring pornography viewing was used.84 A different, nationally representative sample of US adults was recently reported concerning the viewing of free pornography on the Internet in the last year. Their response options varied from 1 = never to 5 = daily.85 In that study, the average frequency was 1.6 (s.d. = 0.9) for women and 2.7 (s.d. = 1.4) for men. Other national data from the Netherlands documented ‘In 2006, 80% of all men sometimes use one or several forms of pornography, 66% at least once a month. […] Women use pornography to a lesser extent than men do; still, 40% of the women report using it sometimes, and 18% use pornography at least once a month’.86 The mean frequency of online sex activities, including porn viewing, was between ‘seldom’ and ‘sometimes’ ratings (2.5 on a five-point scale) in a representative German sample.87 In a representative study in Australia, 72.1% of respondents reported having viewed pornography in the last year.88 Pornography viewing in the current sample does not appear inconsistent with these other estimates of viewing pornography. Certainly, there are limits to assessments of pornography viewing that rely on self-report.84 One alternative could have been to examine acute effects, such as participants’ viewing sexual or non-sexual films just before practising OM.
Relatedly, one might be reasonably concerned about whether these results will generalise to those who are not engaged in OM. Of the many demographic measures, low ‘touch avoidance’, stood out as potentially unique to a group comfortable with non-romantic partners touching their genitals or touching others’ genitals. Studies of volunteer bias for sexual psychophysiological studies, independent of OM, suggest volunteers tend to be more sex positive and more sexually experienced,89 but do not differ from non-volunteers in a number of other tested domains like personality90 or attendance at religious services.79 Of course, generalisability issues are a concern with all laboratory studies and require continued monitoring.
Also, sexual response might fail in actual partnered sex, which differs from OM. The relationship between self-reported sexual arousal and genital response is often very high in men (e.g. r = 0.85).91 suggesting it might be reasonable to assume strokers’ judgments of sexual arousal reflect actual erectile responses. The same is not true for women, who sometimes judge their own sexual arousal almost independently of their level of vaginal engorgement.67 Typically, the coherence is low because women exhibit genital responses, but do not report feeling sexually aroused. Given this direction of women’s response bias, it is unlikely that low coherence in women explains observations in the current data. Nevertheless, it remains unclear how OM may reflect naturally occuring sexual behaviours or decision-making. As laboratory protocols continue to be developed for partnered intimate activity, they could consider the breadth of sexual behaviours made available, modeling reciprocal sexual stimulation, and allowing decisions to limit engagement in more (e.g. intercourse) or less (e.g. kissing) intense sexual behaviours.
The design does not allow attribution of variance due to gender, because women were always receiving the genital stimulation. It may be that aspects of the protocol are more beneficial for men or women, or would be more beneficial if roles (stroker, strokee) were reversed. Similar clinical speculations have been made about Sensate Focus exercises, which shares features of safety and predictability with OM, as the cognitive component of Sensate Focus appeared less helpful for women than men.92,93 OM ordinarily is only taught with females being stimulated.
The mechanism relating the strokers’ sex film viewing and the strokees’ SCR is unclear. As more sex film viewing has been related to higher sex drive, it may be that the strokers had a higher sex drive that they shared with the strokee. For example, the stroking pattern provided by those who view more sex films might be more likely to evoke sexual arousal to share that emotional experience. Emotional contagion are well-documented in romantic couples.94 Sexual urges also might be shared in this non-verbal setting. Monitoring the SCR of the stroker (for covariance with the SCR of the strokee) would allow one test of this possibility.
Use of ecological sexual behaviours
These data represent an important step to test models of sexual compulsivity using actual, partnered, intimate behaviours. Sex films represent a secondary (cue for primary) reward. Those who view sex films overwhelmingly describe using them in a solo setting for masturbation.95–97 In this scenario, genital stimulation is a primary, unconditioned stimulus (UCS) and visual erotica is the conditioned stimulus (CS).
Some have argued that sex films are primary unconditioned stimuli no different than actual sex. For example, passively viewing sexual images and experiencing sexual arousal was described as a ‘primary sexual reward’98 or argued that the inability to self-stimulate in the laboratory makes sexual images alone primary rewards.99 Of course, images of cocaine also induce cravings for cocaine, which are not modelled as the actual cocaine reward,100 and participants in sex film studies report engaging in sexual activities (masturbation, intercourse, partnered sexual activity) immediately after leaving the lab.101 Sex films also appear to manifest very differently in the brain than actual sexual stimulation; viewing sex films involves focused effortful processing and genital stimulation, an opposing, ‘letting-go’ neural signature.5
The presence of a partner also creates different demands from less social practices such as viewing films.69,102 Human touch also appears unique, such as a hand touch without a glove rated as more pleasant with greater evoked blood oxygen dependent level (BOLD) in insula (left and right) and pregenual anterior cingulate cortex (pgACC) than hand touch in a glove.103 Holding a person’s hand also decreases the pain threat response in proportion to the closeness and quality of the personal relationship.104 Of note, one partnered protocol using manual penile stimulation was conducted,105 but did not measure experienced affect, and was not powered to investigate individual differences. Sex film models are inadequate to test predictions related to partnered sex, and the OM protocol offers one potential method to examine partnered intimate interactions.
Implications
Sexual compulsivity has been included in the International Classification of Disorders-11. These data tested one core hypothesis of the compulsivity model of sexual behaviour. Specifically, ‘compulsivity’ describes being motivated to repeat a behaviour despite intentions to stop. We did not find evidence that our non-clinical participants complaining of difficulty controlling their sexual urges actually experienced more (or less) motivation to engage in sexual behaviours following OM. There was evidence that those who viewed sex films at all were more sexually aroused before the OM the more sex films they viewed. This appears more consistent with a high sex drive, where those who seek out more sexual stimulation in films also are more motivated to engage with a partner.
The pathology models of feeling sexual behaviours are out-of-control did not predict experiences of sexual arousal with a partner in the lab. Sex film viewing has been proposed to primarily function as a sign of masturbation and sexual desire,106,107 which may be why those who viewed more sex films continued to be very sexually aroused prior to this intimate experience with a partner. It may be that a construct like sex drive, or personal experiences that influence romantic attachment difficulties, will prove sufficient to explain variation in partnered intimate experiences.
Supplementary material
Supplementary material is available online.
Data availability
Analyses for this study were pre-registered, except as noted in the manuscript. De-identified data are available to any qualified statistician on request through the pre-registration site https://osf.io/zf4vd/register/564d31db8c5e4a7c9694b2be.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
This research was funded by the Institute of OM foundation, which had no input in the preparation of this manuscript.
References
[1] Fineberg NA, Chamberlain SR, Goudriaan AE, Stein DJ, Vanderschuren LJ, Gillan CM, et al. New developments in human neurocognition: clinical, genetic, and brain imaging correlates of impulsivity and compulsivity. CNS Spectr 2014; 19 69–89.| New developments in human neurocognition: clinical, genetic, and brain imaging correlates of impulsivity and compulsivity.Crossref | GoogleScholarGoogle Scholar | 24512640PubMed |
[2] Grant JE, Kim SW. Brain circuitry of compulsivity and impulsivity. CNS Spectr 2014; 19 21–7.
| Brain circuitry of compulsivity and impulsivity.Crossref | GoogleScholarGoogle Scholar | 23659364PubMed |
[3] Najmi S, Kuckertz JM, Amir N. Automatic avoidance tendencies in individuals with contamination-related obsessive-compulsive symptoms. Behav Res Ther 2010; 48 1058–62.
| Automatic avoidance tendencies in individuals with contamination-related obsessive-compulsive symptoms.Crossref | GoogleScholarGoogle Scholar | 20650448PubMed |
[4] Coleman E. The obsessive-compulsive model for describing compulsive sexual behavior. Am J Prev Psychiatr Neurol 1990; 2 9–14.
[5] Prause N. Evaluate models of high-frequency sexual behaviors already. Arch Sex Behav 2017; 46 2269–74.
| Evaluate models of high-frequency sexual behaviors already.Crossref | GoogleScholarGoogle Scholar | 28940038PubMed |
[6] Ciocca G, Solano C, D’Antuono L, Longo L, Limoncin E, Bianciardi E, et al. Hypersexuality: the controversial mismatch of the psychiatric diagnosis. J Psychopathol Behav Assess 2018; 24 187–91. https://art.torvergata.it/handle/2108/256910
[7] Tracy JA, Ghose SS, Stecher T, McFall RM, Steinmetz JE. Classical conditioning in a nonclinical obsessive-compulsive population. Psychol Sci 1999; 10 9–13.
| Classical conditioning in a nonclinical obsessive-compulsive population.Crossref | GoogleScholarGoogle Scholar |
[8] Hoehn-Saric R, McLeod DR, Zimmerli WD, Hipsley PA. Symptoms and physiologic manifestations in obsessive compulsive patients before and after treatment with clomipramine. J Clin Psychiatry 1993; 54 272–6. https://www.ncbi.nlm.nih.gov/pubmed/8335655
| 8335655PubMed |
[9] Blais-Lecours S, Vaillancourt-Morel M-P, Sabourin S, Godbout N. Cyberpornography: time use, perceived addiction, sexual functioning, and sexual satisfaction. Cyberpsychol Behav Soc Netw 2016; 19 649–55.
| Cyberpornography: time use, perceived addiction, sexual functioning, and sexual satisfaction.Crossref | GoogleScholarGoogle Scholar | 27831753PubMed |
[10] Skegg K, Nada-Raja S, Dickson N, Paul C. Perceived “out of control” sexual behavior in a cohort of young adults from the dunedin multidisciplinary health and development study. Arch Sex Behav 2010; 39 968–78.
| Perceived “out of control” sexual behavior in a cohort of young adults from the dunedin multidisciplinary health and development study.Crossref | GoogleScholarGoogle Scholar | 19421850PubMed |
[11] Bancroft J, Vukadinovic Z. Sexual addiction, sexual compulsivity, sexual impulsivity, or what? Toward a theoretical model. J Sex Res 2004; 41 225–34.
| Sexual addiction, sexual compulsivity, sexual impulsivity, or what? Toward a theoretical model.Crossref | GoogleScholarGoogle Scholar | 15497051PubMed |
[12] Evenden JL. Varieties of impulsivity. Psychopharmacology 1999; 146 348–61.
| Varieties of impulsivity.Crossref | GoogleScholarGoogle Scholar | 10550486PubMed |
[13] Dalley JW, Everitt BJ, Robbins TW. Impulsivity, compulsivity, and top-down cognitive control. Neuron 2011; 69 680–94.
| Impulsivity, compulsivity, and top-down cognitive control.Crossref | GoogleScholarGoogle Scholar | 21338879PubMed |
[14] Whiteside SP, Lynam DR. The five factor model and impulsivity: using a structural model of personality to understand impulsivity. Pers Individ Dif 2001; 30 669–89.
| The five factor model and impulsivity: using a structural model of personality to understand impulsivity.Crossref | GoogleScholarGoogle Scholar |
[15] Finn PR, Justus A, Mazas C, Steinmetz JE. Working memory, executive processes and the effects of alcohol on Go/No-Go learning: testing a model of behavioral regulation and impulsivity. Psychopharmacology 1999; 146 465–72.
| Working memory, executive processes and the effects of alcohol on Go/No-Go learning: testing a model of behavioral regulation and impulsivity.Crossref | GoogleScholarGoogle Scholar | 10550497PubMed |
[16] Zisner A, Beauchaine TP. Neural substrates of trait impulsivity, anhedonia, and irritability: mechanisms of heterotypic comorbidity between externalizing disorders and unipolar depression. Dev Psychopathol 2016; 28 1177–208.
| Neural substrates of trait impulsivity, anhedonia, and irritability: mechanisms of heterotypic comorbidity between externalizing disorders and unipolar depression.Crossref | GoogleScholarGoogle Scholar | 27739396PubMed |
[17] Erez G, Pilver CE, Potenza MN. Gender-related differences in the associations between sexual impulsivity and psychiatric disorders. J Psychiatr Res 2014; 55 117–25.
| Gender-related differences in the associations between sexual impulsivity and psychiatric disorders.Crossref | GoogleScholarGoogle Scholar | 24793538PubMed |
[18] Black RA, Serowik KL, Rosen MI. Associations between impulsivity and high risk sexual behaviors in dually diagnosed outpatients. Am J Drug Alcohol Abuse 2009; 35 325–28.
| Associations between impulsivity and high risk sexual behaviors in dually diagnosed outpatients.Crossref | GoogleScholarGoogle Scholar | 20180659PubMed |
[19] McCoul MD, Haslam N. Predicting high risk sexual behaviour in heterosexual and homosexual men: the roles of impulsivity and sensation seeking. Pers Individ Dif 2001; 31 1303–10.
| Predicting high risk sexual behaviour in heterosexual and homosexual men: the roles of impulsivity and sensation seeking.Crossref | GoogleScholarGoogle Scholar |
[20] Robbins T, Curran H, de Wit H. Special issue on impulsivity and compulsivity. Psychopharmacology 2012; 219 251–2.
| Special issue on impulsivity and compulsivity.Crossref | GoogleScholarGoogle Scholar | 22124671PubMed |
[21] Berlin GS, Hollander E. Compulsivity, impulsivity, and the DSM-5 process. CNS Spectr 2014; 19 62–8.
| Compulsivity, impulsivity, and the DSM-5 process.Crossref | GoogleScholarGoogle Scholar | 24229702PubMed |
[22] Robbins TW, Gillan CM, Smith DG, de Wit S, Ersche KD. Neurocognitive endophenotypes of impulsivity and compulsivity: towards dimensional psychiatry. Trends Cogn Sci 2012; 16 81–91.
| Neurocognitive endophenotypes of impulsivity and compulsivity: towards dimensional psychiatry.Crossref | GoogleScholarGoogle Scholar | 22155014PubMed |
[23] Robinson T. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Rev 1993; 18 247–91.
| The neural basis of drug craving: an incentive-sensitization theory of addiction.Crossref | GoogleScholarGoogle Scholar | 8401595PubMed |
[24] Kalivas PW, O’Brien C. Drug addiction as a pathology of staged neuroplasticity. Neuropsychopharmacology 2008; 33 166–80.
| Drug addiction as a pathology of staged neuroplasticity.Crossref | GoogleScholarGoogle Scholar | 17805308PubMed |
[25] Koob GF, Le Moal M. Addiction and the brain antireward system. Annu Rev Psychol 2008; 59 29–53.
| Addiction and the brain antireward system.Crossref | GoogleScholarGoogle Scholar | 18154498PubMed |
[26] Zapf JL, Greiner J, Carroll J. Attachment styles and male sex addiction. Sex Addict Compulsivity 2008; 15 158–75.
| Attachment styles and male sex addiction.Crossref | GoogleScholarGoogle Scholar |
[27] Grant JE, Potenza MN, Weinstein A, Gorelick DA. Introduction to behavioral addictions. Am J Drug Alcohol Abuse 2010; 36 233–41.
| Introduction to behavioral addictions.Crossref | GoogleScholarGoogle Scholar | 20560821PubMed |
[28] Barlow DH. Causes of sexual dysfunction: the role of anxiety and cognitive interference. J Consult Clin Psychol 1986; 54 140–8.
| Causes of sexual dysfunction: the role of anxiety and cognitive interference.Crossref | GoogleScholarGoogle Scholar | 3700800PubMed |
[29] Barlow DH, Sakheim DK, Beck JG. Anxiety increases sexual arousal. J Abnorm Psychol 1983; 92 49–54.
| Anxiety increases sexual arousal.Crossref | GoogleScholarGoogle Scholar | 6833633PubMed |
[30] Bradford A, Meston CM. The impact of anxiety on sexual arousal in women. Behav Res Ther 2006; 44 1067–77.
| The impact of anxiety on sexual arousal in women.Crossref | GoogleScholarGoogle Scholar | 16199003PubMed |
[31] Rogge RD, Cobb RJ, Story LB, Johnson MD, Lawrence EE, Rothman AD, et al. Recruitment and selection of couples for intervention research: achieving developmental homogeneity at the cost of demographic diversity. J Consult Clin Psychol 2006; 74 777–84.
| Recruitment and selection of couples for intervention research: achieving developmental homogeneity at the cost of demographic diversity.Crossref | GoogleScholarGoogle Scholar | 16881785PubMed |
[32] Crosby R, Milhausen R, Yarber WL, Sanders SA, Graham CA. Condom ‘turn offs’ among adults: an exploratory study. Int J STD AIDS 2008; 19 590–4.
| Condom ‘turn offs’ among adults: an exploratory study.Crossref | GoogleScholarGoogle Scholar | 18725548PubMed |
[33] Chadwick SB, van Anders SM. Do women’s orgasms function as a masculinity achievement for men? J Sex Res 2017; 54 1141–52.
| Do women’s orgasms function as a masculinity achievement for men?Crossref | GoogleScholarGoogle Scholar | 28276934PubMed |
[34] Prause N, Siegle G, Coan J. Partner intimate touch is associated with increased interpersonal closeness, especially in non-romantic partners. PLoS One 2021; 16 e0246065
| Partner intimate touch is associated with increased interpersonal closeness, especially in non-romantic partners.Crossref | GoogleScholarGoogle Scholar | 33690603PubMed |
[35] Prause N, Cohen H, Siegle GJ. Effects of adverse childhood experiences on partnered sexual arousal appear context dependent. Sex Relation Ther 2021;
| Effects of adverse childhood experiences on partnered sexual arousal appear context dependent.Crossref | GoogleScholarGoogle Scholar |
[36] Schober JM, Meyer-Bahlburg HFL, Ransley PG. Self-assessment of genital anatomy, sexual sensitivity and function in women: implications for genitoplasty. BJU Int 2004; 94 589–94.
| Self-assessment of genital anatomy, sexual sensitivity and function in women: implications for genitoplasty.Crossref | GoogleScholarGoogle Scholar | 15329118PubMed |
[37] Georgiadis JR, Kringelbach ML. Intimacy and the brain: lessons from genital and sexual touch. In: Olausson H, Wessberg J, Morrison I, McGlone F, editors. Affective touch and the neurophysiology of CT afferents. Springer Science + Business Media; 2016. pp. 301–321.
| Crossref |
[38] Walton MT, Cantor JM, Bhullar N, Lykins AD. Hypersexuality: a critical review and introduction to the ‘sexhavior cycle’. Arch Sex Behav 2017; 46 2231–51.
| Hypersexuality: a critical review and introduction to the ‘sexhavior cycle’.Crossref | GoogleScholarGoogle Scholar | 28687897PubMed |
[39] Sassover E, Weinstein A. Should compulsive sexual behavior (CSB) be considered as a behavioral addiction? A debate paper presenting the opposing view. J Behav Addict 2020;
| Should compulsive sexual behavior (CSB) be considered as a behavioral addiction? A debate paper presenting the opposing view.Crossref | GoogleScholarGoogle Scholar | 32997646PubMed |
[40] Voon V, Mole TB, Banca P, Porter L, Morris L, Mitchell S, et al. Neural correlates of sexual cue reactivity in individuals with and without compulsive sexual behaviours. PLoS One 2014; 9 e102419
| Neural correlates of sexual cue reactivity in individuals with and without compulsive sexual behaviours.Crossref | GoogleScholarGoogle Scholar | 25013940PubMed |
[41] Zuckerman M. Physiological measures of sexual arousal in the human. Psychol Bull 1971; 75 297–329.
| Physiological measures of sexual arousal in the human.Crossref | GoogleScholarGoogle Scholar | 4931713PubMed |
[42] Roth M, Hammelstein P. The need inventory of sensation seeking (NISS). Eur J Psychol Assess 2012; 28 11–8.
| The need inventory of sensation seeking (NISS).Crossref | GoogleScholarGoogle Scholar |
[43] Marker C, Schneider FM. Further evidence for the validity of the need inventory of sensation seeking. Pers Individ Dif 2015; 77 41–4.
| Further evidence for the validity of the need inventory of sensation seeking.Crossref | GoogleScholarGoogle Scholar |
[44] Rush JA, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, et al. The 16-item quick inventory of depressive symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry 2003; 54 573–83.
| The 16-item quick inventory of depressive symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression.Crossref | GoogleScholarGoogle Scholar |
[45] Rush AJ, Bernstein IH, Trivedi MH, Carmody TJ, Wisniewski S, Mundt JC, et al. An evaluation of the quick inventory of depressive symptomatology and the hamilton rating scale for depression: a sequenced treatment alternatives to relieve depression trial report. Biol Psychiatry 2006; 59 493–501.
| An evaluation of the quick inventory of depressive symptomatology and the hamilton rating scale for depression: a sequenced treatment alternatives to relieve depression trial report.Crossref | GoogleScholarGoogle Scholar | 16199008PubMed |
[46] Geschwind N, van Teffelen M, Hammarberg E, Arntz A, Huibers MJH, Renner F. Impact of measurement frequency on self-reported depressive symptoms: an experimental study in a clinical setting. J Affect Disord Rep 2021; 5 100168
| Impact of measurement frequency on self-reported depressive symptoms: an experimental study in a clinical setting.Crossref | GoogleScholarGoogle Scholar |
[47] Reilly TJ, MacGillivray SA, Reid IC, Cameron IM. Psychometric properties of the 16-item quick inventory of depressive symptomatology: a systematic review and meta-analysis. J Psychiatr Res 2015; 60 132–40.
| Psychometric properties of the 16-item quick inventory of depressive symptomatology: a systematic review and meta-analysis.Crossref | GoogleScholarGoogle Scholar | 25300442PubMed |
[48] Geschwind N, Arntz A, Bannink F, Peeters F. Positive cognitive behavior therapy in the treatment of depression: a randomized order within-subject comparison with traditional cognitive behavior therapy. Behav Res Ther 2019; 116 119–30.
| Positive cognitive behavior therapy in the treatment of depression: a randomized order within-subject comparison with traditional cognitive behavior therapy.Crossref | GoogleScholarGoogle Scholar | 30897464PubMed |
[49] Reiss S, Peterson RA, Gursky DM, McNally RJ. Anxiety sensitivity, anxiety frequency and the prediction of fearfulness. Behav Res Ther 1986; 24 1–8.
| Anxiety sensitivity, anxiety frequency and the prediction of fearfulness.Crossref | GoogleScholarGoogle Scholar | 3947307PubMed |
[50] Schmidt NB, Joiner TE. Structure of the anxiety sensitivity index psychometrics and factor structure in a community sample. J Anxiety Disord 2002; 16 33–49.
| Structure of the anxiety sensitivity index psychometrics and factor structure in a community sample.Crossref | GoogleScholarGoogle Scholar | 12171212PubMed |
[51] Schmidt NB, Lerew DR, Joiner TE. Anxiety sensitivity and the pathogenesis of anxiety and depression: evidence for symptom specificity. Behav Res Ther 1998; 36 165–77.
| Anxiety sensitivity and the pathogenesis of anxiety and depression: evidence for symptom specificity.Crossref | GoogleScholarGoogle Scholar | 9613023PubMed |
[52] Allan NP, Korte KJ, Capron DW, Raines AM, Schmidt NB. Factor mixture modeling of anxiety sensitivity: a three-class structure. Psychol Assess 2014; 26 1184–95.
| Factor mixture modeling of anxiety sensitivity: a three-class structure.Crossref | GoogleScholarGoogle Scholar | 25068913PubMed |
[53] Taylor S, Zvolensky MJ, Cox BJ, Deacon B, Heimberg RG, Ledley DR, et al. Robust dimensions of anxiety sensitivity: development and initial validation of the anxiety sensitivity index-3. Psychol Assess 2007; 19 176–188.
| Robust dimensions of anxiety sensitivity: development and initial validation of the anxiety sensitivity index-3.Crossref | GoogleScholarGoogle Scholar | 17563199PubMed |
[54] Rellini A, Meston C. Sexual function and satisfaction in adults based on the definition of child sexual abuse. J Sex Med 2007; 4 1312–21.
| Sexual function and satisfaction in adults based on the definition of child sexual abuse.Crossref | GoogleScholarGoogle Scholar | 17727351PubMed |
[55] Graham FJ, Walters GD, Harris DA, Knight RA. Is hypersexuality dimensional or categorical? Evidence from male and female college samples. J Sex Res 2016; 53 224–38.
| Is hypersexuality dimensional or categorical? Evidence from male and female college samples.Crossref | GoogleScholarGoogle Scholar | 26169176PubMed |
[56] Walters GD, Knight RA, Långström N. Is hypersexuality dimensional? Evidence for the DSM-5 from general population and clinical samples. Arch Sex Behav 2011; 40 1309–21.
| Is hypersexuality dimensional? Evidence for the DSM-5 from general population and clinical samples.Crossref | GoogleScholarGoogle Scholar | 21290258PubMed |
[57] Reid RC, Garos S, Carpenter BN. Reliability, Validity, and psychometric development of the hypersexual behavior inventory in an outpatient sample of men. Sex Addict Compulsivity 2011; 18 30–51.
| Reliability, Validity, and psychometric development of the hypersexual behavior inventory in an outpatient sample of men.Crossref | GoogleScholarGoogle Scholar |
[58] Prause N, Steele VR, Staley C, Sabatinelli D, Hajcak G. Modulation of late positive potentials by sexual images in problem users and controls inconsistent with ‘porn addiction’. Biol Psychol 2015; 109 192–9.
| Modulation of late positive potentials by sexual images in problem users and controls inconsistent with ‘porn addiction’.Crossref | GoogleScholarGoogle Scholar | 26095441PubMed |
[59] Catania JA, Gibson DR, Chitwood DD, Coates TJ. Methodological problems in AIDS behavioral research: influences on measurement error and participation bias in studies of sexual behavior. Psychol Bull 1990; 108 339–62.
| Methodological problems in AIDS behavioral research: influences on measurement error and participation bias in studies of sexual behavior.Crossref | GoogleScholarGoogle Scholar | 2270232PubMed |
[60] Meston CM. Sympathetic nervous system activity and female sexual arousal. Am J Cardiol 2000; 86 30F–34F.
| Sympathetic nervous system activity and female sexual arousal.Crossref | GoogleScholarGoogle Scholar | 10899275PubMed |
[61] Wenger MA, Averill J, Smith DDB. Autonomic activity during sexual arousal. Psychophysiology 1968; 4 468–78.
| Autonomic activity during sexual arousal.Crossref | GoogleScholarGoogle Scholar | 5662817PubMed |
[62] Laan E, Everaerd W, Evers A. Assessment of female sexual arousal: response specificity and construct validity. Psychophysiology 1995; 32 476–85.
| Assessment of female sexual arousal: response specificity and construct validity.Crossref | GoogleScholarGoogle Scholar | 7568642PubMed |
[63] Dawson ME, Schell AM, Filion DL. The electrodermal system. In: Cacioppo JT, Tassinary LG, Berntson GG, editors. Handbook of psychophysiology. Cambridge University Press; 2007. vol. 2. pp. 200–223.
[64] Hain JD, Linton PH. Physiological response to visual sexual stimuli. J Sex Res 1969; 5 292–302.
| Physiological response to visual sexual stimuli.Crossref | GoogleScholarGoogle Scholar |
[65] Heiman JR, Rowland DL. Affective and physiological sexual response patterns: the effects of instructions on sexually functional and dysfunctional men. J Psychosom Res 1983; 27 105–16.
| Affective and physiological sexual response patterns: the effects of instructions on sexually functional and dysfunctional men.Crossref | GoogleScholarGoogle Scholar | 6683315PubMed |
[66] Handy AB, Stanton AM, Meston CM. Understanding women’s subjective sexual arousal within the laboratory: definition, measurement, and manipulation. Sex Med Rev 2018; 6 201–16.
| Understanding women’s subjective sexual arousal within the laboratory: definition, measurement, and manipulation.Crossref | GoogleScholarGoogle Scholar | 29339116PubMed |
[67] Chivers ML, Seto MC, Lalumière ML, Laan E, Grimbos T. Agreement of self-reported and genital measures of sexual arousal in men and women: a meta-analysis. Arch Sex Behav 2010; 39 5–56.
| Agreement of self-reported and genital measures of sexual arousal in men and women: a meta-analysis.Crossref | GoogleScholarGoogle Scholar | 20049519PubMed |
[68] Staley C, Prause N. Erotica viewing effects on intimate relationships and self/partner evaluations. Arch Sex Behav 2013; 42 615–24.
| Erotica viewing effects on intimate relationships and self/partner evaluations.Crossref | GoogleScholarGoogle Scholar | 23224749PubMed |
[69] Bancroft J, Loftus J, Long JS. Distress about sex: a national survey of women in heterosexual relationships. Arch Sex Behav 2003; 32 193–208.
| Distress about sex: a national survey of women in heterosexual relationships.Crossref | GoogleScholarGoogle Scholar | 12807292PubMed |
[70] Daedone N. Slow sex: the art and craft of the female orgasm. Grand Central Life & Style; 2011.
[71] OneTaste. Orgasmic meditation – step by step instructions. Oliver Rivers; 2013. Available at https://www.youtube.com/watch?v=-86ZJvBBnNU
[72] Institute of OM. The container and form of orgasmic meditation. Vol. 1.4. OneTaste; 2015. Available at https://static1.squarespace.com/static/5c2a6e7670e8020ac2e6beca/t/5dcdf8de72f1a7543c9c61a3/1573779678477/container.pdf
[73] Zeileis A, Kleiber C, Jackman S. Regression models for count data in R. J Stat Softw 2008; 27 1–25. http://edoc.unibas.ch/dok/A5252903
[74] Engle RF. Chapter 13 Wald, likelihood ratio, and Lagrange multiplier tests in econometrics. In: Durlauf SN, Hansen LP, Heckman JJ, Matzkin RL, editors. Handbook of econometrics. Zoe Kruze; 1984. pp. 775–826.
| Crossref |
[75] Boucsein W, Fowles DC, Grimnes S, Ben-Shakhar G, Roth WT, Dawson ME, et al. Publication recommendations for electrodermal measurements. Psychophysiology 2012; 49 1017–34.
| Publication recommendations for electrodermal measurements.Crossref | GoogleScholarGoogle Scholar | 22680988PubMed |
[76] Champely S. pwr: Basic functions for power analysis. R package version. 2015; 1(1). Available at https://cran.r-project.org/web/packages/pwr/
[77] Rosenthal R, Rubin DB. The counternull value of an effect size: a new statistic. Psychol Sci 1994; 5 329–34.
| The counternull value of an effect size: a new statistic.Crossref | GoogleScholarGoogle Scholar |
[78] Woo JST, Brotto LA, Yule MA. Do East Asian and Euro-Canadian women differ in sexual psychophysiology research participation? J Sex Res 2010; 47 345–54.
| Do East Asian and Euro-Canadian women differ in sexual psychophysiology research participation?Crossref | GoogleScholarGoogle Scholar |
[79] Plaud JJ, Gaither GA, Hegstad HJ, Rowan L, Devitt MK. Volunteer bias in human psychophysiological sexual arousal research: to whom do our research results apply? J Sex Res 1999; 36 171–9.
| Volunteer bias in human psychophysiological sexual arousal research: to whom do our research results apply?Crossref | GoogleScholarGoogle Scholar |
[80] Desai S, Arias I, Thompson MP, Basile KC. Childhood victimization and subsequent adult revictimization assessed in a nationally representative sample of women and men. Violence Vict 2002; 17 639–53.
| Childhood victimization and subsequent adult revictimization assessed in a nationally representative sample of women and men.Crossref | GoogleScholarGoogle Scholar | 12680680PubMed |
[81] Bravo IM, Silverman WK. Anxiety sensitivity, anxiety, and depression in older patients and their relation to hypochondriacal concerns and medical illnesses. Aging Ment Health 2001; 5 349–57.
| Anxiety sensitivity, anxiety, and depression in older patients and their relation to hypochondriacal concerns and medical illnesses.Crossref | GoogleScholarGoogle Scholar | 11767983PubMed |
[82] Kendler KS. The super-normal control group in psychiatric genetics. Psychiatr Genet 1990; 1 45–53.
| The super-normal control group in psychiatric genetics.Crossref | GoogleScholarGoogle Scholar |
[83] Wéry A, Vogelaere K, Challet-Bouju G, Poudat F-X, Caillon J, Lever D, et al. Characteristics of self-identified sexual addicts in a behavioral addiction outpatient clinic. Journal of Behavioral Addictions 2016; 5 623–30.
| Characteristics of self-identified sexual addicts in a behavioral addiction outpatient clinic.Crossref | GoogleScholarGoogle Scholar | 27774812PubMed |
[84] Kohut T, Balzarini RN, Fisher WA, Grubbs JB, Campbell L, Prause N. Surveying pornography use: a shaky science resting on poor measurement foundations. J Sex Res 2020; 57 722–42.
| Surveying pornography use: a shaky science resting on poor measurement foundations.Crossref | GoogleScholarGoogle Scholar | 31821049PubMed |
[85] Herbenick D, Fu T-C, Wright P, Paul B, Gradus R, Bauer J, et al. Diverse sexual behaviors and pornography use: findings from a nationally representative probability survey of americans aged 18 to 60 years. J Sex Med 2020; 17 623–33.
| Diverse sexual behaviors and pornography use: findings from a nationally representative probability survey of americans aged 18 to 60 years.Crossref | GoogleScholarGoogle Scholar | 32081698PubMed |
[86] Vanwesenbeeck I, Bakker F, Gesell S. Sexual health in the Netherlands: main results of a population survey among dutch adults. Int J Sex Health 2010; 22 55–71.
| Sexual health in the Netherlands: main results of a population survey among dutch adults.Crossref | GoogleScholarGoogle Scholar |
[87] Döring N, Rohangis Mohseni M. Are online sexual activities and sexting good for adults’ sexual well-being? Results from a national online survey. Int J Sex Health 2018; 30 250–63.
| Are online sexual activities and sexting good for adults’ sexual well-being? Results from a national online survey.Crossref | GoogleScholarGoogle Scholar |
[88] Rissel C, Richters J, de Visser RO, McKee A, Yeung A, Caruana T. A profile of pornography users in Australia: findings from the second Australian study of health and relationships. J Sex Res 2017; 54 227–40.
| A profile of pornography users in Australia: findings from the second Australian study of health and relationships.Crossref | GoogleScholarGoogle Scholar | 27419739PubMed |
[89] Dawson SJ, Huberman JS, Bouchard KN, McInnis MK, Pukall CF, Chivers ML. Effects of individual difference variables, gender, and exclusivity of sexual attraction on volunteer bias in sexuality research. Arch Sex Behav 2019; 48 2403–17.
| Effects of individual difference variables, gender, and exclusivity of sexual attraction on volunteer bias in sexuality research.Crossref | GoogleScholarGoogle Scholar | 31011994PubMed |
[90] Bouchard KN, Stewart JG, Boyer SC, Holden RR, Pukall CF. Sexuality and personality correlates of willingness to participate in sex research. Can J Hum Sex 2019; 28 26–37.
| Sexuality and personality correlates of willingness to participate in sex research.Crossref | GoogleScholarGoogle Scholar |
[91] Sakheim DK, Barlow DH, Gayle Beck J, Abrahamson DJ. A comparison of male heterosexual and male homosexual patterns of sexual arousal. J Sex Res 1985; 21 183–98.
| A comparison of male heterosexual and male homosexual patterns of sexual arousal.Crossref | GoogleScholarGoogle Scholar |
[92] Dove NL, Wiederman MW. Cognitive distraction and women’s sexual functioning. J Sex Marital Ther 2000; 26 67–78.
| Cognitive distraction and women’s sexual functioning.Crossref | GoogleScholarGoogle Scholar | 10693117PubMed |
[93] Ferraro KF. Women’s fear of victimization: shadow of sexual assault? Soc Forces 1996; 75 667–90.
| Women’s fear of victimization: shadow of sexual assault?Crossref | GoogleScholarGoogle Scholar |
[94] Saxbe D, Repetti RL. For better or worse? Coregulation of couples’ cortisol levels and mood states. J Pers Soc Psychol 2010; 98 92–103.
| For better or worse? Coregulation of couples’ cortisol levels and mood states.Crossref | GoogleScholarGoogle Scholar | 20053034PubMed |
[95] Baćak a V, Štulhofer A. Masturbation among sexually active young women in croatia: associations with religiosity and pornography use. Int J Sex Health 2011; 23 248–57.
| Masturbation among sexually active young women in croatia: associations with religiosity and pornography use.Crossref | GoogleScholarGoogle Scholar |
[96] Carvalheira A, Træen B, Stulhofer A. Masturbation and pornography use among coupled heterosexual men with decreased sexual desire: how many roles of masturbation? J Sex Marital Ther 2015; 41 626–35.
| Masturbation and pornography use among coupled heterosexual men with decreased sexual desire: how many roles of masturbation?Crossref | GoogleScholarGoogle Scholar | 25189834PubMed |
[97] Downing MJ, Schrimshaw EW, Scheinmann R, Antebi-Gruszka N, Hirshfield S. Sexually explicit media use by sexual identity: a comparative analysis of gay, bisexual, and heterosexual men in the United States. Arch Sex Behav 2017; 46 1763–76.
| Sexually explicit media use by sexual identity: a comparative analysis of gay, bisexual, and heterosexual men in the United States.Crossref | GoogleScholarGoogle Scholar | 27709363PubMed |
[98] Creswell JD, Pacilio LE, Denson TF, Satyshur M. The effect of a primary sexual reward manipulation on cortisol responses to psychosocial stress in men. Psychosom Med 2013; 75 397–403.
| The effect of a primary sexual reward manipulation on cortisol responses to psychosocial stress in men.Crossref | GoogleScholarGoogle Scholar | 23576768PubMed |
[99] Gola M, Wordecha M, Marchewka A, Sescousse G. Visual sexual stimuli—cue or reward? A perspective for interpreting brain imaging findings on human sexual behaviors. Front Hum Neurosci 2016; 10 402
| Visual sexual stimuli—cue or reward? A perspective for interpreting brain imaging findings on human sexual behaviors.Crossref | GoogleScholarGoogle Scholar | 27574507PubMed |
[100] Garavan H, Pankiewicz J, Bloom A, Cho JK, Sperry L, Ross TJ, et al. Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. Am J Psychiatry 2000; 157 1789–98.
| Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli.Crossref | GoogleScholarGoogle Scholar | 11058476PubMed |
[101] Both S, Spiering M, Everaerd W, Laan E. Sexual behavior and responsiveness to sexual stimuli following laboratory-induced sexual arousal. J Sex Res 2004; 41 242–58.
| Sexual behavior and responsiveness to sexual stimuli following laboratory-induced sexual arousal.Crossref | GoogleScholarGoogle Scholar | 15497053PubMed |
[102] Willoughby BJ, Vitas J. Sexual desire discrepancy: the effect of individual differences in desired and actual sexual frequency on dating couples. Arch Sex Behav 2012; 41 477–486.
| Sexual desire discrepancy: the effect of individual differences in desired and actual sexual frequency on dating couples.Crossref | GoogleScholarGoogle Scholar | 21573707PubMed |
[103] Lindgren L, Westling G, Brulin C, Lehtipalo S, Andersson M, Nyberg L. Pleasant human touch is represented in pregenual anterior cingulate cortex. Neuroimage 2012; 59 3427–32.
| Pleasant human touch is represented in pregenual anterior cingulate cortex.Crossref | GoogleScholarGoogle Scholar | 22100768PubMed |
[104] Coan JA, Schaefer HS, Davidson RJ. Lending a hand: social regulation of the neural response to threat. Psychol Sci 2006; 17 1032–9.
| Lending a hand: social regulation of the neural response to threat.Crossref | GoogleScholarGoogle Scholar | 17201784PubMed |
[105] Georgiadis JR, Farrell MJ, Boessen R, Denton DA, Gavrilescu M, Kortekaas R, et al. Dynamic subcortical blood flow during male sexual activity with ecological validity: a perfusion fMRI study. Neuroimage 2010; 50 208–16.
| Dynamic subcortical blood flow during male sexual activity with ecological validity: a perfusion fMRI study.Crossref | GoogleScholarGoogle Scholar | 20006720PubMed |
[106] Perry SL. Is the link between pornography use and relational happiness really more about masturbation? Results from two national surveys. J Sex Res 2020; 57 64–76.
| Is the link between pornography use and relational happiness really more about masturbation? Results from two national surveys.Crossref | GoogleScholarGoogle Scholar | 30633584PubMed |
[107] Prause N. Porn is for masturbation. 2019; 48 2271–2277.
| Porn is for masturbation.Crossref | GoogleScholarGoogle Scholar | 30847758PubMed |
A The hypersexuality coefficient also was not a significant predictor collapsing across role (hypersexuality coefficient P > 0.05, partial-eta squared = 0.02). Sexual arousal reported before the OM was correlated with the sexual arousal reported after the OM (r = 0.46), raising the possibility that the effects of hypersexual concerns might be obscured by our statistical approach. We also tested hypersexual concerns and sexual arousal reported before the OM as an interaction term, as well as sexual arousal reported before the OM as a mediator of a relationship between hypersexual concerns and sexual arousal reported after the OM. In no analysis, including the direct relationship of hypersexual concerns to arousal after the OM, not accounting for arousal before the OM, were hypersexual concerns a significant predictor of sexual arousal reported after the OM.
B We also removed the four women who served as strokers, and the pattern of this effect did not change.


