Socioeconomic, behavioural and health factors associated with chlamydia testing in sexually active young women: an Australian observational cohort study
Louise Forsyth Wilson A * , Annette Jane Dobson A , Jenny Doust A and Gita Devi Mishra A
A * , Annette Jane Dobson A , Jenny Doust A and Gita Devi Mishra A
A The University of Queensland, NHMRC Centre for Research Excellence on Women and Non-communicable Diseases (CREWaND), School of Public Health, Herston Road, Herston, Qld, Australia.
Sexual Health 19(2) 112-121 https://doi.org/10.1071/SH21230
Submitted: 18 November 2021 Accepted: 10 March 2022 Published: 28 April 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Background: Chlamydia trachomatis is the most frequently notified sexually transmitted infection in Australia. Untreated infections in women can cause health problems. Professional guidelines encourage opportunistic testing of young people. To increase understanding of who is being tested, we investigated factors associated with testing in a population of young women.
Methods: In total, 14 002 sexually active women, aged 18–23 years at baseline (2013), from the Australian Longitudinal Study on Women’s Health, were included. We used random intercepts, mixed-effects binary logistic regression with robust standard errors to assess associations between socioeconomic, health and behavioural factors and chlamydia testing.
Results: Associations between chlamydia testing and partner status varied by a woman’s body mass index (BMI). Compared to women with a stable partner/BMI <25 kg/m2, women with a stable partner/BMI ≥25 kg/m2 were less likely to be tested (adjusted odds ratios [AOR] = 0.79, 95% CI: 0.71–0.88). In contrast, although women without a partner were more likely to be tested irrespective of BMI, the odds were higher for those with a BMI <25 kg/m2 (AOR = 2.68, 95% CI: 2.44–2.94) than a BMI ≥25 kg/m2 (AOR = 1.65, 95% CI: 1.48–1.84). Women who reported a prior chlamydia infection were also more likely to be tested (AOR = 2.01, 95% CI: 1.83–2.20), as were women engaging in any combination of cannabis use and/or heavy episodic drinking compared to doing neither of these activities.
Conclusions: Women without a partner, women with a prior chlamydia infection and those engaging in risk-taking behaviours are more likely to have chlamydia testing. Additional research is needed to understand whether there are deficits in testing among overweight/obese women.
Keywords: Australia, binge drinking, cannabis use, Chlamydia trachomatis, cohort study, obesity, testing, women.
Introduction
Chlamydia trachomatis is the most frequently notified sexually transmitted infection (STI) in several countries, including Australia (100 775 notifications in 2017).1–4 Most infections occur in people aged 15–29 years, with 79% of notifications in Australia in this age group.1 Although the percent of notifications in Australia in 2017 were similar for men (48%) and women (52%), the impacts of chlamydia infection are potentially greater for women as they are more likely to be asymptomatic and, left untreated, infection can lead to long-term impacts including pelvic inflammatory disease, infertility, ectopic pregnancy, spontaneous abortion and stillbirth.5,6
The Fourth National Sexually Transmissible Infections Strategy 2018–22 for Australia has targets to reduce the prevalence of chlamydia infection and increase STI testing coverage in priority populations, including young people aged 15–29 years.7 The Royal Australian College of General Practitioners recommends opportunistically offering screening for chlamydia infection in sexually active young people.8 Testing for chlamydia in Australia is available in a variety of settings, including sexual health clinics and general practice,7 and testing is provided free-of-charge to the patient. The majority of tests are funded through the Medicare program, the universal health insurance system that subsidises use of medical and hospital services for all Australian citizens, permanent residents, and certain categories of visitors to Australia.9 State and Territory governments fund a proportion of tests done in sexual health clinics, including for people who do not hold a Medicare card.10
To gauge the effectiveness of current strategies to encourage chlamydia testing, it is important to understand the characteristics of people who do or do not undergo testing. Few studies have looked at factors associated with chlamydia testing, and none have been longitudinal. Previous studies in other countries were cross-sectional and relied on self-report of testing.11–13
The disproportionate burden of chlamydia infection in young people may be due to their engagement in greater levels of sexual behaviour that are higher risk for example, multiple partners, and unprotected sex.7 Research suggests that illicit drug use and risky alcohol consumption, separately and in combination, have direct effects on sexual decisions and have been associated with a greater likelihood of high-risk sexual contact and STI transmission.14 It is unclear, however, whether young people who engage in one or more of these behaviours are more or less likely to be tested. To our knowledge, no studies have investigated the associations between combinations of these behaviours and chlamydia testing, focussing instead on the associations between testing and risky sexual practices rather than the behaviours that precede (and potentially precipitate) these practices.
Therefore, the aim of our study was to investigate the socioeconomic, behavioural and health factors associated with chlamydia testing (ascertained through linked administrative data) in a population of young women born in 1989–95 over repeated surveys, with a particular focus on the associations between behaviours (i.e. binge drinking and cannabis use) known to be associated with risky sexual behaviour and chlamydia infection.
Materials and methods
Study design
This is an observational cohort study using self-reported and linked administrative data from the Australian Longitudinal Study on Women’s Health (ALSWH).
Study population
The ALSWH is a national longitudinal study established in 1996 to explore factors contributing to women’s health and wellbeing and their use of health services across key life stages. Three cohorts of women born in 1921–26, 1946–51 and 1973–78 were initially included in the study.15 To provide contemporary health information about women in early adulthood, a fourth cohort of women born in 1989–95 was recruited in 2012–13 through a range of conventional and online recruitment methods.16 The main avenues for recruitment were targeted advertising through Facebook (70%), a recruitment campaign coordinated by a contracted marketing company (13%), and word-of-mouth, (e.g. by study staff members, professional bodies, and already enrolled participants (7%)).17 Women in the fourth cohort were eligible to participate in the study if they were living in Australia, held a valid Medicare number and consented to linkage of survey data with administrative health data.16 Only 17 women have opted-out of data linkage since the first survey.18 Further details on survey methodology and response rates can be found at www.alswh.org.au. The women were surveyed annually between 2013 and 2017 (Surveys 1–5) and in 2019 (Survey 6). We used data from Surveys 1, 2, 3, and 5 in our analyses. We did not include Survey 4 because several covariates of interest (questions about recent cannabis use, vaginal symptoms, urinary symptoms, prior chlamydia infection and other STI infection) were not asked at this Survey. We did not include Survey 6 because the most recent chlamydia testing data available were to 30 June 2020 and many women had not returned Survey 6 by this date. At each survey, women were asked if they had ever had vaginal sex. Women were only included in the analysis if they responded yes to this question.
Chlamydia testing data
We sourced information on chlamydia testing through data linkage with the Medicare database. Medicare Personal Identifier Numbers (PINs) for ALSWH participants were validated by Medicare Australia on enrolment to the Study. As the primary Accredited Integrating Authority for national health data, the Australian Institute of Health and Welfare conducts annual deterministic data linkage of ALSWH cohorts, using the Medicare PINs. Checks are also undertaken periodically to investigate any apparent discrepancies. Therefore, the sensitivity of matching is considered extremely high. Researchers only have access to de-identified participant data.
Chlamydia tests funded under Medicare are listed in the Medical Benefits Schedule (MBS). These tests are usually requested by general practitioners and processed in private pathology laboratories. Tests funded by States and Territories (done in sexual health clinics and processed in public pathology laboratories) were not included. The MBS items used in the analysis were: #69316, #69317 and #69319 (full descriptions are available at www.mbsonline.gov.au). We created a time-varying chlamydia test variable, whereby a woman was considered to have had a test at a survey if she: (1) responded to that survey; and (2) had a record of a chlamydia test within 12 months after returning the survey. A woman could have more than one chlamydia test over the study period; however, if she had more than one chlamydia test within 12 months of returning a survey, only the first test was counted in the analysis.
Covariates
Covariates were identified from the literature on factors associated with either chlamydia testing or infection.11–13,19–22 We also included body mass index (BMI) as obese women are less likely to undergo screening for cervical cancer,23 and we hypothesised that there may be a similar association with chlamydia testing. All covariates were measured by self-report and were time-varying, except for language spoken at home, which was only measured at Survey 1. Categories for each variable are included in Table 1.
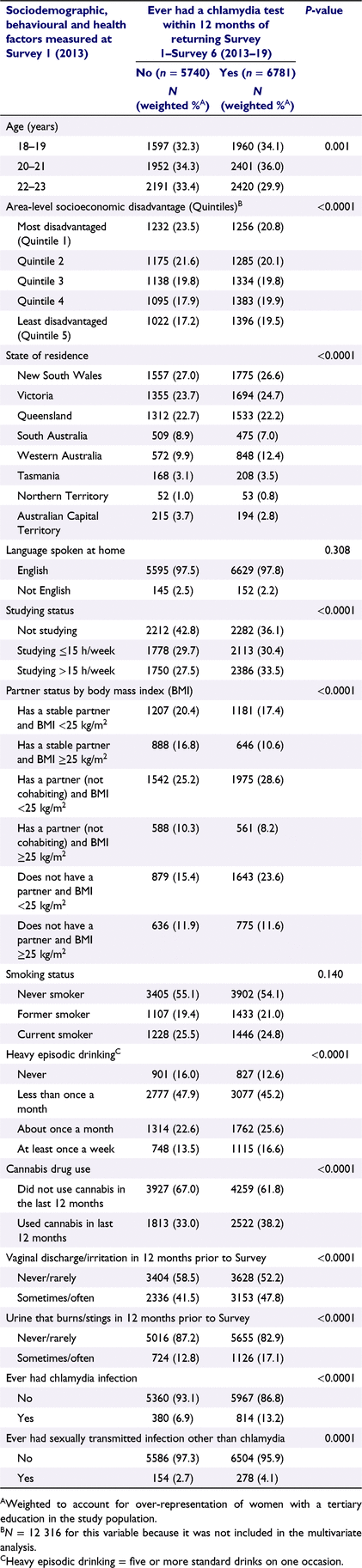
|
The age of women was included as a continuous variable. We included State and Territory of residence as there are differences in the number and location of sexual health clinics funded by each jurisdiction. Other sociodemographic variables included area-level socioeconomic disadvantage (categorised in sample-specific quintiles),24 highest attained educational qualification, whether a woman was currently studying (categorised according to the number of hours per week studying: none, ≤15 h/week, >15 h/week), and whether she spoke a language other than English at home. Women were asked about their current relationship status; we grouped the seven response options to this question into three categories: living with a partner, engaged, married = ‘has a stable partner’; living together = ‘has a partner (not cohabiting)’, and single, divorced, separated = ‘does not have a partner’.
Behavioural variables were smoking status, recent cannabis use (in the last 12 months), and frequency of heavy episodic drinking (HED) defined as five or more standard drinks of alcohol on one occasion.
Health factors included body mass index (BMI), calculated using self-reported height and weight.25 Women were asked if they had experienced vaginal discharge or irritation or urine that burns or stings in the last 12 months with response options of never, rarely, sometimes, or often. We created separate dichotomous variables for vaginal symptoms and urinary symptoms (never/rarely and sometimes/often). Women were also asked if they had ever been diagnosed or treated for chlamydia, gonorrhoea, genital herpes, or genital warts (HPV). We created two dichotomous variables: ever had chlamydia infection (yes/no) and ever had a STI other than chlamydia (yes/no).
Statistical analysis
Baseline characteristics (Survey 1, 2013) of women were compared by chlamydia testing status (ever/never having a test within 12 months after the date of return of any survey). Differences between women who did and did not have a chlamydia test were assessed by using chi-squared tests. Percentages were weighted to account for over-representation of women with a tertiary education in the study population compared to the Australian female population aged 18–23 years at the 2011 Australian Census (see Supplementary Material Appendix S1 for details). We assessed the impact of missing data on our analysis by comparing the differences at Survey 1 (study baseline) of those included in the analysis (n = 14 002) and those excluded due to missing information (n = 2249). Differences between groups were assessed by using chi-squared tests.
We estimated odds ratios (OR) and 95% confidence intervals (CI) for the associations between the variables of interest (described above) and chlamydia testing using random intercepts, mixed effects binary logistic regression with robust standard errors. Age- and survey-adjusted ORs were calculated for each variable; the fully adjusted model included all variables considered, except for area-level socioeconomic disadvantage, because this variable was strongly associated with the other sociodemographic measures. Sample weights were not used, but educational qualification was included as a covariate.
As well as examining the additive effects of the included factors on chlamydia tests, we considered plausible multiplicative interactions between selected covariates (cannabis use × HED, studying × HED, studying × cannabis use, partner status × BMI). Where these were statistically significant (Pinteraction < 0.05), we created a new variable with categories for combinations of the variables (with some collapsing of categories where effect estimates were similar and confidence intervals overlapped).
Because we did not include Survey 4 in our primary analysis (as questions for some key covariates were not asked at this Survey), in sensitivity analyses, we included Survey 4 by: (1) carrying forward the values from Survey 3 for those covariates missing at Survey 4; and (2) carrying backward the values from Survey 5 for the missing covariates. The data analysis for this paper was performed using SAS software, version 9.4 of the SAS system for Windows Copyright 2002–12 by SAS Institute Inc (SAS Institute Inc., Cary, NC, USA).
Ethics approval
The ALSWH has been granted ethics clearance by the University of Newcastle (ethics approval H0760795) and the University of Queensland (ethics approval 2004000224). All participants provided informed consent at each survey.
Results
Our analysis included 14 002 women with complete information at one or more surveys. Of the 17 010 women who completed Survey 1, 17 were excluded a priori because they did not consent to data linkage with the Medicare database. Fig. 1 shows the number of women included at each survey, and those excluded because they reported never being sexually active or had missing data on one or more covariates. Overall, compared to the women included in the analysis, the women with missing data were more likely to be younger, not speak English at home, have only a high school education, or were studying more than 15 h/week. They were less likely to have a partner, or be current or former smokers, heavy episodic drinkers, or recent cannabis users. They were also less likely to report frequent vaginal or urinary symptoms or a previous chlamydia or other STI infection (Table S1).
|
|
Descriptive characteristics of the women included in our analysis at study baseline (Survey 1) are summarised in Table 1. The interactions for cannabis use × HED, studying × HED and studying × cannabis use were not statistically significant (Pinteraction = 0.359, 0.653 and 0.792 respectively). The interaction between partner status and BMI was statistically significant (Pinteraction < 0.0001), so we created a new variable with six categories and included this in our primary analysis (see Table 1 for categories). Compared to women who did not have a chlamydia test over the study period, women who had a chlamydia test were more likely to: be younger, not have a partner in combination with a BMI of <25 kg/m2, studying >15 h/week, engage in heavy episodic drinking at least once a month, be recent cannabis users, experience more frequent urinary or vaginal symptoms, or report a previous chlamydia or other STI infection. They were less likely to live in the most disadvantaged areas or have a stable partner in combination with a BMI of ≥25 kg/m2 (Table 1). In the 12-month period following the return of each survey, between 27 and 30% of women had a chlamydia test (Table S2). In the fully adjusted model (Table 2), compared to women with a stable partner/BMI <25 kg/m2, women with a stable partner/BMI ≥25 kg/m2 were less likely to be tested (adjusted odds ratios [AOR] = 0.79, 95% CI: 0.71–0.88). In contrast, compared to women with a stable partner/BMI <25 kg/m2, women with a partner (not cohabiting) and women without a partner were more likely to have a chlamydia test irrespective of BMI category. However, among women without a partner, the odds of being tested were higher for those with a BMI <25 kg/m2 (AOR = 2.68, 95% CI: 2.44–2.94) than those with a BMI ≥25 kg/m2 (AOR = 1.65, 95% CI: 1.48–1.84). Chlamydia testing was also more likely to be undertaken by women who reported having a prior chlamydia infection (AOR 2.01, 95% CI: 1.83–2.20), or who engaged in HED once a month (AOR 1.37, 95% CI; 1.24–1.51) or at least once a week (AOR 1.38, 95% CI: 1.24–1.55) compared to women who never engaged in HED. Living in Western Australia or the Northern Territory (compared to New South Wales); currently studying >15 h/week; being a current smoker or a recent cannabis user; having vaginal or urinary symptoms; or a previous sexually transmitted infection (other than chlamydia) were also associated with chlamydia testing (Table 2). In contrast, women who lived in South Australia or the Australian Capital Territory were less likely to be tested (Table 2).
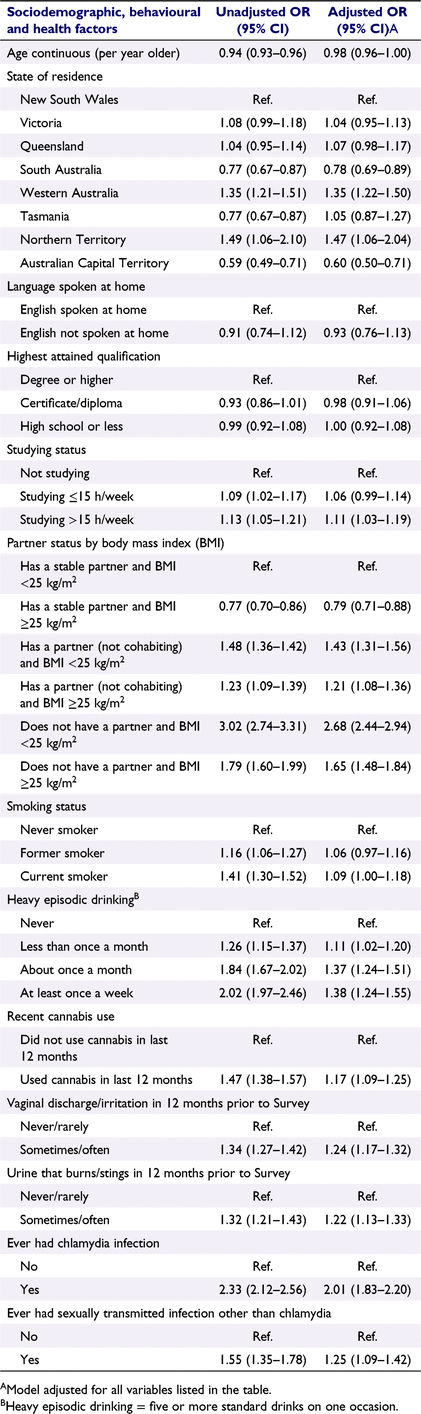
|
Although the interaction between cannabis use and HED was not statistically significant, because these covariates were of particular interest, for illustrative purposes, we also modelled a six-category variable reflecting different behaviour combinations. Women who engaged in any combination of recent cannabis use and/or HED were more likely to be tested for chlamydia compared to women who never did either of these activities. The highest odds ratios were seen in women who reported both recent cannabis use (i.e. in the 12 months prior to completing a survey) and HED at least once per month (AOR 1.61, 95% CI: 1.44–1.79) (Fig. 2).
In our sensitivity analyses where we included Survey 4, the estimates were essentially unchanged (results not shown).
Discussion
In this study of young, sexually active women, chlamydia testing was most likely in women without a partner (especially if they had a BMI <25 kg/m2), as well as women who reported a history of chlamydia infection, or recent cannabis use and heavy episodic drinking at least once a month. In contrast, chlamydia testing was less likely in women who were in a stable relationship who were overweight or obese.
Like Australia, New Zealand recommends opportunistic screening of all sexually active people aged < 30 years. A 2014–15 New Zealand health survey found that 27% of women aged 16–29 years reported having a chlamydia test in the previous year,11 similar to the annual testing prevalences seen in our study (27–30%). Lower estimates were seen in an Australian study conducted in 2007–08 (12.5% for sexually active females aged 16–29 years26); however, increases in chlamydia testing rates in the intervening period are likely to explain this difference.1 As expected, reported annual testing rates are higher in the UK (54% of women aged 16–24 years13), which has a National Chlamydia Screening Programme recommending annual screening in women aged <25 years, in the US (38% among sexually active women aged 15–25 years12) where the Centers for Disease Control and Prevention recommend annual screening of sexually active women aged ≤25 years.
Few studies have looked at factors associated with chlamydia testing. We found that compared to women who had a stable partner, testing was higher in both women who had a partner but were not cohabiting and women without a partner. These associations may be due to differences between the partner groups in the likelihood of having multiple sex partners, with women who are engaged, married, or living with their partner (i.e. in a stable relationship) more likely to be monogamous.27 We could not test this, however, as women in our study were not asked about the number of sexual partners they had. Although no other studies have looked at relationship status and chlamydia testing, three studies from New Zealand,11 the UK13,28 and the US12 have all reported that testing is associated with having multiple sex partners.
We also found that the associations between partner status and chlamydia testing differed according to a woman’s BMI. Among both women with a stable partner and women without a partner, those who were overweight or obese had lower odds of testing than women who were underweight or a healthy weight in the same partner group. The associations between overweight/obesity and chlamydia testing have also not previously been studied. A US study looking at body appreciation and protective sexual behaviours did not find an association between BMI and any type of STI testing; however, the sample size was small (n = 285 women) with a wide age range (18–61 years).29 One reason for the lower likelihood of testing in overweight/obese women (particularly those without a partner) may be that the prevalence of risky sexual behaviours (which may lead to the need for testing) is lower; however, the evidence for this is mixed. In a French National Survey of sexual behaviours (n = 5535 women), obese women were less likely to report having a sexual partner in the previous 12 months than normal weight women,30 and a US study using data from the 1999–2000 National Health and Nutrition Examination Survey (NHANES, n = 1250 women) found that obese women reported fewer sexual partners.31 In contrast, another US study using data from the 2002 National Survey of Family Growth (NSFG, n = 6690 women) found no differences between BMI groups in the number of sexual partners in the previous 12 months, number of current partners or age at first intercourse.32
Another explanation may be that overweight/obese women are more reluctant to undergo testing. This would be congruent with findings that overweight and obese women are less likely to participate in prevention programs such as cervical cancer screening,23 potentially due to factors such as embarrassment, inadequate facilities or perceived disrespectful treatment.33 Further research is needed to understand these associations, particularly whether deficits in testing are occurring in at-risk groups of women.
Women who reported having a prior chlamydia infection or urinary or vaginal symptoms were also more likely to have a chlamydia test; this is consistent with other studies that have reported prior infection34 or symptoms11,13 as reasons for testing.
To our knowledge, ours is the first study to look at combinations of cannabis use and HED behaviour and chlamydia testing. A UK study found that women (aged 16–24 years) who engaged in binge drinking (number of drinks not defined) at least weekly were not more likely to have a chlamydia test in the adjusted analysis;13 however, the reference group included never binge drinkers as well as women who engaged in binge drinking less than once a month. In addition, as the UK has a population screening program for chlamydia, there may be smaller differences in testing rates between groups at lower and higher risk of infection. Although not directly comparable (as we only had consistent information across surveys on cannabis use and not injecting drug use), an Australian study looking at the correlates of STI testing (i.e. broader than just chlamydia testing) in women aged 16–44 years found that injecting drug users were more likely to have had an STI test. Our finding that women who engaged in any combination of recent cannabis use and/or HED had higher testing rates than women who did neither of these behaviours is encouraging as it potentially demonstrates an awareness of the links between these risky behaviours and chlamydia infection. The women in our study were more highly educated and therefore may be more health literate than other population groups. However, evidence that successful awareness-raising of the links between alcohol and drug use and STI infection can also extend to more disadvantaged populations, which was demonstrated in a US cluster randomised clinical trial of adolescents in the juvenile justice system (n = 460), where an intervention that included content on both sexual risk reduction as well as alcohol and cannabis use was more successful at reducing rates of STI infection than an intervention that only included content on sexual risk reduction.35
Strengths of our study include the longitudinal design and large community-based sample of young women. The socioeconomic, health and behavioural factors were consistently measured using the same questions at each included survey. We objectively ascertained chlamydia testing using linked administrative data. Although the validity of self-report of chlamydia testing has not been formally assessed, other studies have shown that many women have an incorrect understanding of whether they have had an STI test (often confusing cervical screening for testing) or are unclear about which STI they have been tested for,36,37 indicating that self-report may not accurately reflect testing history. In addition, the recency of the linked data (2013–19) used in our analysis reflects contemporary trends in testing.
Limitations are that all survey information was collected by self-report, which may be subject to the biases associated with this method of data collection, including recall and social desirability bias. We only had chlamydia testing data retrieved from the Medicare database, so some under-counting of testing in our study population will have occurred. Although we were unable to quantify the extent of undercounting in our study population of young women, it is estimated that a large majority (82%) of chlamydia tests (for both men and women of all ages) are requested by general practitioners and processed by private pathology laboratories and claimed through Medicare.38 The remaining proportion of tests are mostly funded by State/Territory governments and processed by public pathology laboratories (with testing done in sexual health clinics or hospitals). There is also a small, but growing, private market providing online STI testing services that are paid for by the user.39 The Medicare database only includes information on whether a woman had a chlamydia test; it does not include the reason for the test (e.g. symptom-driven, partner notification or annual screening), so we could not explore whether factors associated with testing may differ by the motivation for testing. The database also does not include information on the results of tests or information on antibiotic prescriptions (which may indicate a positive test result). Finally, compared to the Australian population, our study population were more highly educated and predominantly of white, Anglo-Celtic descent; therefore, our results may be less generalisable to women with lower levels of education levels or diverse cultural backgrounds.
In conclusion, women with a history of chlamydia infection, those who were not in a relationship, and those who reported alcohol and cannabis use were the most likely to undergo a chlamydia test in our study. This is encouraging as it potentially indicates that general practitioners may be promoting testing and these young women are responsive to this suggestion, or the women themselves may be seeking testing because they are aware of the implications of their risky behaviour. However, additional research is required to understand whether there are deficits in testing by women who are overweight/obese.
Supplementary material
Supplementary material is available online.
Data availability
The ALSWH survey data are owned by the Australian Government Department of Health and due to the personal nature of the data collected, release by the ALSWH is subject to strict contractual and ethical restrictions. Ethical review of the ALSWH is by the Human Research Ethics Committees at The University of Queensland and The University of Newcastle. De-identified data are available to collaborating researchers where a formal request to make use of the material has been approved by the ALSWH Data Access Committee. The committee is receptive of requests for datasets required to replicate results. Information on applying for ALSWH data is available from https://alswh.org.au/for-data-users/applying-for-data/. In addition, linked administrative data have been provided by the following third party [Australian Institute of Health and Welfare HREC (EC00103), Protocol EO2020/3/1115]. In order for these linked data to be accessed through the ALSWH, every data user must be added to the applicable Data Use Agreements and Human Research Ethics Committee protocols.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
The ALSWH is funded by the Australian Government Department of Health. LFW was supported by an Australian National Health and Medical Research Council (NHMRC) Centres for Research Excellence grant (APP1153420) and GDM was supported by an NHMRC Principal Research Fellowship (APP1121844). The funding bodies played no role in the design; in the collection, analysis, or interpretation of data; in the writing of the manuscript; or in the decision to submit the manuscript for publication.
Acknowledgements
The research on which this paper is based was conducted as part of the Australian Longitudinal Study on Women’s Health by the University of Queensland and the University of Newcastle. We are grateful to the Australian Government Department of Health for funding and to the women who provided the survey data. The authors acknowledge the Australian Government Department of Health for providing the Medical Benefits Schedule (MBS) data, and the Australian Institute of Health and Welfare (AIHW) as the integrating authority.
References
[1] Kirby Institute. HIV, viral hepatitis and sexually transmissible infections in Australia: annual surveillance and monitoring report 2018. Sydney: Kirby Institute, UNSW Sydney; 2018.[2] Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2019. Atlanda, USA: Department of Health and Human Services; 2021.
[3] Mitchell H, Allen H, Sonubi T, Kuyumdzhieva G, Harb A, Shah A, et al. Sexually transmitted infections and screening for chlamydia in England, 2019. London: Public Health England; 2020.
[4] The Institute of Environmental Science and Research Ltd (ESR). Sexually transmitted infections in New Zealand: annual surveillance report 2016. Porirua, New Zealand: ESR; 2019.
[5] Price MJ, Ades AE, De Angelis D, Welton NJ, Macleod J, Soldan K, et al. Risk of pelvic inflammatory disease following Chlamydia trachomatis infection: analysis of prospective studies with a multistate model. Am J Epidemiol 2013; 178 484–92.
| Risk of pelvic inflammatory disease following Chlamydia trachomatis infection: analysis of prospective studies with a multistate model.Crossref | GoogleScholarGoogle Scholar | 23813703PubMed |
[6] Tang W, Mao J, Li KT, Walker JS, Chou R, Fu R, et al. Pregnancy and fertility-related adverse outcomes associated with Chlamydia trachomatis infection: a global systematic review and meta-analysis. Sex Transm Infect 2020; 96 322–29.
| Pregnancy and fertility-related adverse outcomes associated with Chlamydia trachomatis infection: a global systematic review and meta-analysis.Crossref | GoogleScholarGoogle Scholar | 31836678PubMed |
[7] Australian Government Department of Health. Fourth National Sexually Transmissible Infections Strategy 2018–2022. Canberra: Commonwealth of Australia; 2018.
[8] The Royal Australian College of General Practitioners. Guidelines for preventive activities in general practice. 9th edn. East Melbourne, Vic.: RACGP; 2018.
[9] Australian Government Department of Health. Medicare benefits schedule book. Canberra: Commonwealth of Australia; 2019.
[10] Communicable Diseases Network Australia (CDNA). National blood-borne viruses and sexually transmissible infections surveillance and monitoring 2018–2022, accessed 31 August 2020. CDNA; 2020. Available at https://www1.health.gov.au/internet/main/publishing.nsf/Content/AE05C032DDCB7533CA257BF00020AAC4/$File/Surveil-Monit-Plan-2018-2022-Nat-BBV-STI.pdf
[11] Righarts A, Gray AR, Morgan J, Saxton PJ, Green JA, Connor JL, et al. Chlamydia testing in New Zealand: analysis of the 2014/15 National Health Survey. Sex Transm Dis 2021; 48 493–8.
| Chlamydia testing in New Zealand: analysis of the 2014/15 National Health Survey.Crossref | GoogleScholarGoogle Scholar | 33264263PubMed |
[12] Tao G, Hoover KW, Leichliter JS, Peterman TA, Kent CK. Self-reported Chlamydia testing rates of sexually active women aged 15–25 years in the United States, 2006–2008. Sex Transm Dis 2012; 39 605–7.
| Self-reported Chlamydia testing rates of sexually active women aged 15–25 years in the United States, 2006–2008.Crossref | GoogleScholarGoogle Scholar | 22801342PubMed |
[13] Woodhall SC, Soldan K, Sonnenberg P, Mercer CH, Clifton S, Saunders P, et al. Is chlamydia screening and testing in Britain reaching young adults at risk of infection? Findings from the third National Survey of Sexual Attitudes and Lifestyles (Natsal-3). Sex Transm Infect 2016; 92 218–27.
| Is chlamydia screening and testing in Britain reaching young adults at risk of infection? Findings from the third National Survey of Sexual Attitudes and Lifestyles (Natsal-3).Crossref | GoogleScholarGoogle Scholar | 26290483PubMed |
[14] Bellis MA, Hughes K, Calafat A, Juan M, Ramon A, Rodriguez JA, et al. Sexual uses of alcohol and drugs and the associated health risks: a cross sectional study of young people in nine European cities. BMC Public Health 2008; 8 155
| Sexual uses of alcohol and drugs and the associated health risks: a cross sectional study of young people in nine European cities.Crossref | GoogleScholarGoogle Scholar | 18471281PubMed |
[15] Dobson AJ, Hockey R, Brown WJ, Byles JE, Loxton DJ, McLaughlin D, et al. Cohort profile update: Australian Longitudinal Study on Women’s Health. Int J Epidemiol 2015; 44 1547–f.
| Cohort profile update: Australian Longitudinal Study on Women’s Health.Crossref | GoogleScholarGoogle Scholar | 26130741PubMed |
[16] Loxton D, Tooth L, Harris ML, Forder PM, Dobson A, Powers J, et al. Cohort profile: the Australian Longitudinal Study on Women’s Health (ALSWH) 1989–95 cohort. Int J Epidemiol 2018; 47 391–2e.
| Cohort profile: the Australian Longitudinal Study on Women’s Health (ALSWH) 1989–95 cohort.Crossref | GoogleScholarGoogle Scholar | 29025118PubMed |
[17] Mishra GD, Hockey R, Powers J, Loxton D, Tooth L, Rowlands I, et al. Recruitment via the internet and social networking sites: the 1989–1995 cohort of the Australian Longitudinal Study on Women’s Health. J Med Internet Res 2014; 16 e279
| Recruitment via the internet and social networking sites: the 1989–1995 cohort of the Australian Longitudinal Study on Women’s Health.Crossref | GoogleScholarGoogle Scholar | 25514159PubMed |
[18] Australian Longitudinal Study on Women’s Health (ALSWH). Health record linkage opt-outs (as at June 2021), accessed 9 September 2021. ALSWH; 2021. Available at https://alswh.org.au/for-data-users/linked-data-overview/opt-outs/
[19] Ali H, Guy RJ, Fairley CK, Wand H, Chen MY, Dickson B, et al. Understanding trends in genital Chlamydia trachomatis can benefit from enhanced surveillance: findings from Australia. Sex Transm Infect 2012; 88 552–7.
| Understanding trends in genital Chlamydia trachomatis can benefit from enhanced surveillance: findings from Australia.Crossref | GoogleScholarGoogle Scholar | 22645390PubMed |
[20] Crichton J, Hickman M, Campbell R, Batista-Ferrer H, Macleod J. Socioeconomic factors and other sources of variation in the prevalence of genital chlamydia infections: a systematic review and meta-analysis. BMC Public Health 2015; 15 729
| Socioeconomic factors and other sources of variation in the prevalence of genital chlamydia infections: a systematic review and meta-analysis.Crossref | GoogleScholarGoogle Scholar | 26224062PubMed |
[21] Gravningen K, Braaten T, Schirmer H. Self-perceived risk and prevalent chlamydia infection among adolescents in Norway: a population-based cross-sectional study. Sex Transm Infect 2016; 92 91–6.
| Self-perceived risk and prevalent chlamydia infection among adolescents in Norway: a population-based cross-sectional study.Crossref | GoogleScholarGoogle Scholar | 26275416PubMed |
[22] Martin-Smith HA, Okpo EA, Bull ER. Exploring psychosocial predictors of STI testing in University students. BMC Public Health 2018; 18 664
| Exploring psychosocial predictors of STI testing in University students.Crossref | GoogleScholarGoogle Scholar | 29843658PubMed |
[23] Maruthur NM, Bolen SD, Brancati FL, Clark JM. The association of obesity and cervical cancer screening: a systematic review and meta-analysis. Obesity 2009; 17 375–81.
| The association of obesity and cervical cancer screening: a systematic review and meta-analysis.Crossref | GoogleScholarGoogle Scholar | 18997682PubMed |
[24] Pink B (Australian Statistician). Technical Paper. Socio-Economic Indexes for areas (SEIFA) 2011. ABS Catalogue no. 2033.0.55.001. Canberra: Australian Bureau of Statistics; 2013.
[25] World Health Organization Consultation on Obesity. Obesity: preventing and managing the global epidemic: report of a WHO consultation. Geneva: WHO; 1999.
[26] Kong FY, Guy RJ, Hocking JS, Merritt T, Pirotta M, Heal C, et al. Australian general practitioner chlamydia testing rates among young people. Med J Aus 2011; 194 249–52.
| Australian general practitioner chlamydia testing rates among young people.Crossref | GoogleScholarGoogle Scholar |
[27] Kost K, Forrest JD. American women’s sexual behavior and exposure to risk of sexually transmitted diseases. Fam Plann Perspect 1992; 24 244–54.
| American women’s sexual behavior and exposure to risk of sexually transmitted diseases.Crossref | GoogleScholarGoogle Scholar | 1483527PubMed |
[28] Clifton S, Mercer CH, Sonnenberg P, Tanton C, Field N, Gravningen K, et al. STI risk perception in the British population and how it relates to sexual behaviour and STI healthcare use: findings from a cross-sectional survey (Natsal-3). eClinicalMedicine 2018; 2–3 29–36.
| STI risk perception in the British population and how it relates to sexual behaviour and STI healthcare use: findings from a cross-sectional survey (Natsal-3).Crossref | GoogleScholarGoogle Scholar | 30320305PubMed |
[29] Winter VR, Satinsky S. Body appreciation, sexual relationship status, and protective sexual behaviors in women. Body Image 2014; 11 36–42.
| Body appreciation, sexual relationship status, and protective sexual behaviors in women.Crossref | GoogleScholarGoogle Scholar | 24075832PubMed |
[30] Bajos N, Wellings K, Laborde C, Moreau C. Sexuality and obesity, a gender perspective: results from French national random probability survey of sexual behaviours. BMJ 2010; 340 c2573
| Sexuality and obesity, a gender perspective: results from French national random probability survey of sexual behaviours.Crossref | GoogleScholarGoogle Scholar | 20551118PubMed |
[31] Nagelkerke NJ, Bernsen RM, Sgaier SK, Jha P. Body mass index, sexual behaviour, and sexually transmitted infections: an analysis using the NHANES 1999–2000 data. BMC Public Health 2006; 6 199
| Body mass index, sexual behaviour, and sexually transmitted infections: an analysis using the NHANES 1999–2000 data.Crossref | GoogleScholarGoogle Scholar | 16884541PubMed |
[32] Kaneshiro B, Jensen JT, Carlson NE, Harvey SM, Nichols MD, Edelman AB. Body mass index and sexual behavior. Obstet Gynecol 2008; 112 586–92.
| Body mass index and sexual behavior.Crossref | GoogleScholarGoogle Scholar | 18757656PubMed |
[33] Amy NK, Aalborg A, Lyons P, Keranen L. Barriers to routine gynecological cancer screening for White and African-American obese women. Int J Obesity 2006; 30 147–55.
| Barriers to routine gynecological cancer screening for White and African-American obese women.Crossref | GoogleScholarGoogle Scholar |
[34] Grulich AE, de Visser RO, Badcock PB, Smith AMA, Richters J, Rissel C, et al. Knowledge about and experience of sexually transmissible infections in a representative sample of adults: the Second Australian Study of Health and Relationships. Sex Health 2014; 11 481–94.
| Knowledge about and experience of sexually transmissible infections in a representative sample of adults: the Second Australian Study of Health and Relationships.Crossref | GoogleScholarGoogle Scholar | 25377001PubMed |
[35] Bryan AD, Magnan RE, Gillman AS, Yeater EA, Feldstein Ewing SW, Kong AS, et al. Effect of including alcohol and cannabis content in a sexual risk-reduction intervention on the incidence of sexually transmitted infections in adolescents: a cluster randomized clinical trial. JAMA Pediatr 2018; 172 e175621
| Effect of including alcohol and cannabis content in a sexual risk-reduction intervention on the incidence of sexually transmitted infections in adolescents: a cluster randomized clinical trial.Crossref | GoogleScholarGoogle Scholar | 29435591PubMed |
[36] Head SK, Crosby RA, Shrier LA, Moore GR. Young women’s misperceptions about sexually transmissible infection testing: a ‘clean and clear’ misunderstanding. Sex Health 2007; 4 273–5.
| Young women’s misperceptions about sexually transmissible infection testing: a ‘clean and clear’ misunderstanding.Crossref | GoogleScholarGoogle Scholar | 18082072PubMed |
[37] Royer HR, Falk EC, Heidrich SM. Sexually transmitted disease testing misconceptions threaten the validity of self-reported testing history. Public Health Nurs 2013; 30 117–27.
| Sexually transmitted disease testing misconceptions threaten the validity of self-reported testing history.Crossref | GoogleScholarGoogle Scholar | 23452106PubMed |
[38] Ali H, Cameron E, Drovandi CC, McCaw JM, Guy RJ, Middleton M, et al. A new approach to estimating trends in chlamydia incidence. Sex Transm Infect 2015; 91 513–9.
| A new approach to estimating trends in chlamydia incidence.Crossref | GoogleScholarGoogle Scholar | 25564675PubMed |
[39] Groos A, Peardon-Freeman S, McFarlane K, Braithwaite S, Gajjar D, Murch P, et al. Free online chlamydia and gonorrhoea urine test request in Queensland, Australia: convenience of home sample collection versus pathology collection centre attendance for faster results. Sex Health 2021; 18 254–9.
| Free online chlamydia and gonorrhoea urine test request in Queensland, Australia: convenience of home sample collection versus pathology collection centre attendance for faster results.Crossref | GoogleScholarGoogle Scholar | 34148563PubMed |


