Trends in high-risk human papillomavirus infection and cervical cytology of women in Karamay City, 2012–2021
Xiaoping Jia A , Min Jiang B , Jing Zhou C and Cailing Ma D *
D *
A
B
C
D
Abstract
To describe the changes in cervical lesions prevalence and high-risk human papillomavirus (HR-HPV) infections from 2012 to 2021, which have never been reported before, to provide direction for the effective implementation of cervical cancer prevention measures.
This retrospective study included women aged >25 years who received either organised or opportunistic cervical HR-HPV screening from January 2012 to December 2021 in Karamay Central Hospital, Karamay, China. The patients were split into four groups according to age 25–35, 36–45, 46–55 and >55 years, respectively. The Joinpoint Regression Program was used to analyse the trends of HR-HPV infection and the detection of cervical lesions.
Data from 85,429 women revealed a decline in HR-HPV infection rates across all age groups from 2012 to 2021. Although HR-HPV infection rates decreased, cervical lesion detection rates increased, although the proportion of cervical cancer in these lesions declined, likely due to enhanced awareness and HPV vaccination in Karamay. From 2012 to 2021, the prevalence of low-grade squamous intraepithelial lesions was 9.70%, and high-grade squamous intraepithelial lesions was 5.85%. HR-HPV infections were highest in the ≥55 years age group, with HPV52 (20.96%) being the most prevalent type.
In the past 10 years, the prevalence of HR-HPV infection has shown a decreasing trend, whereas the detection prevalence of cervical lesions has shown an upward trend among women in Karamay City. Importantly, particular emphasis should be placed on cervical cancer screening in women aged >55 years.
Keywords: cervical cancer, cervical lesions, high-grade squamous intraepithelial lesions, high-risk human papilloma virus, infection, Karamay, low-grade squamous intraepithelial lesions, prevalence.
Introduction
Cervical cancer (CC) is one of the most common malignant tumours that threatens the health of women worldwide. In 2020, there were 342,000 deaths and 604,000 new cases from CC worldwide, of which 59,060 deaths and 109,741 new cases occurred in China.1 China has a huge burden on those who underwent CC radiotherapy and chemotherapy.2 The main cause of CC and precancerous cervical lesions (CLs) was infection with high-risk human papillomavirus (HR-HPV).3–5 HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66 and 68 are associated with CC prevalence.6 Screening for CC and vaccination for HPV is available in most regions, and awareness of these services has been increasing due to proactive promotion from physicians and public media.7,8 Regional differences exist in infection rates (IRs) and common HR-HPV types.9–12 Cervical HR-HPV infection widely varies across China.13–17
Cervical low-grade squamous intraepithelial lesions (LSIL) and high-grade squamous intraepithelial lesions (HSIL) are precancerous lesions that can develop into CC. Nearly 48% of LSIL resolved spontaneously, 21% can progress to HSIL and 0.15% of LSIL can progress to invasive CC.18,19 Approximately 20–30% of cervical HSILs can progress to CC within 10 years.20 Long progression from asymptomatic HPV infection to cervical precancerous lesions and ultimately to CC is well identified.21 Therefore, early identification of HR-HPV infection, LSIL and HSIL is important in preventing CC from developing.22 In Karamay, China, the guidelines for CC screening in China are adhered to.23 The HPV IR was 17. 51% from 2010 to 2019 in Karamay.24 The effectiveness and reliability of HR-HPV testing are significantly improved in comparison with the cytology, and virus testing is one of the most important methods used to screen for CC.25 To decrease the prevalence of CC, it is of great importance that as many women as possible are screened for HR-HPV infection.26 As an increasing number of people receive regular CC screening, the prevalence of cervical HR-HPV infection, associated LSIL and HSIL, and the prevalence of CC have changed in different periods and ages. Therefore, an updated epidemiological study is warranted to describe the regional prevalence of CLs and HR-HPV infections. In this report, we describe the changes in CLs prevalence and HR-HPV infections from 2012 to 2021, to provide direction for effective implementation of CC prevention measures.
Patients and methods
Study participants
Women aged >25 years who received either organised or opportunistic cervical HR-HPV screening from January 2012 to December 2021, in Karamay Central Hospital, were retrospectively analysed for this study. Those who had a history of cervical conisation, total hysterectomy or radiotherapy and chemotherapy for CC were not selected for this research. The number of cervical HR-HPV tests in each year from 2012 to 2021 are shown in Table 1. According to age, the screened participants were split into 25–35 years (18,015, 21.09%), 36–45 years (35,077, 41.06%), 46–55 years (29,948, 35.06%) and >55 years (2389, 2.80%). HR-HPV testing can be used in combination with cytology for CC in women aged >25 years, as well as for triage of women aged 21–25 years whose initial cytology screening was mildly abnormal. HR-HPV testing was performed when cytology was atypical squamous cells of unspecified significance, with colposcopy for positive cases and cytology in 12 months for negative cases. HR-HPV testing was also used as primary screening for CC in women aged >25 years, with cytological triage for positives and routine follow up for negatives. The CLs (LSIL, HSIL and CC) were identified by biopsy under colposcopy in HR-HPV-positive patients. The work described was performed in line with The Code of Ethics of the World Medical Association. The Ethics Committee of the Karamay Central Hospital approved this study.
| Calendar year | Screening (n) | HR-HPV(+), n (%) | LSIL+a, n (%) | HSIL+b, n (%) | CC, n (%) | |
|---|---|---|---|---|---|---|
| 2012 | 8391 | 1376 (16.40) | 54 (3.92) | 34 (2.47) | 11 (20.37) | |
| 2013 | 7106 | 1479 (20.81) | 107 (7.23) | 71 (4.80) | 14 (13.08) | |
| 2014 | 5842 | 1472 (25.20) | 81 (5.50) | 58 (3.94) | 9 (11.11) | |
| 2015 | 9895 | 1950 (19.71) | 109 (5.59) | 64 (3.28) | 11 (10.09) | |
| 2016 | 9153 | 1263 (13.80) | 101 (8.00) | 69 (5.46) | 14 (13.86) | |
| 2017 | 7528 | 1010 (13.42) | 109 (10.79) | 73 (7.23) | 13 (11.92) | |
| 2018 | 7864 | 1021 (12.98) | 105 (10.28) | 70 (6.86) | 10 (9.52) | |
| 2019 | 8119 | 1044 (12.86) | 124 (11.88) | 73 (6.99) | 9 (7.26) | |
| 2020 | 10048 | 1153 (11.47) | 166 (14.40) | 94 (8.15) | 11 (6.63) | |
| 2021 | 11,483 | 1268 (11.04) | 309 (24.37) | 156 (12.30) | 12 (3.88) | |
| Total | 85,429 | 13,036 (15.26) | 1265 (9.70) | 762 (5.85) | 114 (9.08) |
LSIL+a: LSIL, HSIL and cervical cancer; HSIL+b: HSIL and cervical cancer.
HR-HPV(+), high-risk human papillomaviruses infection; LSIL, low-grade squamous intraepithelial lesion or higher; HSIL, high-grade squamous intraepithelial lesion or higher; CC, cervical cancer.
HR-HPV testing
Vaginal scrubbing, vaginal medication and sexual activity were prohibited within 72 h before sampling. Cervical exfoliated cells were collected into ThinPrep preservation solution for HR-HPV testing. From 2012 to 2015, quantitative detection of HPV-DNA was performed using the HC2 kit (Qiagen); in 2016, the HC2 assay was replaced by the Cobas 4800 (Roche) HPV DNA test kit. A total of 14 carcinogenic HR-HPV genotypes including HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66 and 68 can be identified using the Cobas 4800 detection kit. HPV16 and HPV18 were highlighted, and the remainder were marked as ‘others.’ From 2020 to 2021, 1641 HR-HPV-positive women were randomly selected, and 28 HPV types were detected by HPV genotyping (HEAS) kit including 15 HR-HPV types: 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68 and 82. Testing was performed according to supplier instructions. Testing laboratories and personnel were properly trained and qualified.
CLs identification
Colposcopy would be performed in those who tested positive for HR-HPV with cytological atypical squamous cells of unspecified significance or higher lesions, and those who were positive for HPV types 16/18. A total of 2979 women underwent colposcopy, and 10,057 did not. Colposcopy was performed by specially trained physicians who followed a standard protocol. Specifically, areas of suspected CLs were evaluated using acetic acid solution (3% to 5%) and Lugol’s iodine solution, and multipoint biopsies were collected and subjected to pathology examination. Pathologists classified the examinations as normal or inflammatory cervix, LSIL, HSIL and CC based on the 2020 WHO classification.27 LSIL+ lesions in the current study included HSIL, LSIL and CC, whereas HSIL+ lesions included HSIL and CC.
Statistical analyses
A database was established with Microsoft Excel® (version 2013), and analyses were conducted with the Joinpoint Regression Program (version 4.9.0.1; US National Cancer Institute). Descriptive statistical analysis was performed to describe the age groups of HR-HPV testing, and the frequency and rate were used for the HR-HPV genotype distribution. The Joinpoint Regression Program was used to analyse the rates of the trend of HR-HPV infection and the detection of CLs. Joinpoint regression models were used to identify the best-fitting point, where a statistically significant change (called the ‘joinpoint’) had occurred, and to determine the trends between joinpoints.28 The results were expressed as the annual percentage change (APC). The existing node represented the junction point of an upward trend and downward trend, whereby APC >0 indicated an upward trend, and APC <0 referred to a downward trend. Statistical significance was defined as P < 0.05. Application R4.0.3 was used to draw compound line charts, stacked bar charts and histograms.
Results
Overall rates of HR-HPV infection and CLs detection
From 2012 to 2021, the overall cervical HR-HPV IR was 15.26%. Among HR-HPV-positive cases, 9.70% had LSIL+ and 5.85% had HSIL+. Additionally, 9.08% of LSIL+ cases progressed to CC. Detailed data is shown in Table 1.
From 2012 to 2021, two stages were identified in the annual HR-HPV IRs. From 2012 to 2014, the rates had upward trends from 16.40% to 25.20% (APC 12.55%), although the annual change did not reach statistical significance (P > 0.05). From 2014 to 2021, HR-HPV IRs had downward trends from 19.71% to 11.04% (APC −10.49%), with statistically significant annual change (P < 0.05). From 2012 to 2021, the overall HR-HPV IR showed a downward trend (APC −7.34%, P < 0.05; Table 1 and Fig. 1).
The trends of HR-HPV infection and detection of cervical lesions from 2012 to 2021. HR-HPV(+), high-risk human papillomaviruses infection, LSIL+, low-grade squamous intraepithelial lesion or higher; HSIL+, high-grade squamous intraepithelial lesion or higher; CC, cervical cancer. LSIL+ is defined as: LSIL, HSIL and cervical cancer; HSIL+ is defined as: HSIL and cervical cancer. The trends of HR-HPV infection and detection of cervical lesions were separately described from 2012 to 2021, the HR-HPV infection rate showed a general decreasing trend, the detection rates of cervical HSIL+ and LSIL+ showed an increasing trend; however, the proportion of cervical cancer in cervical lesions showed a downward trend.
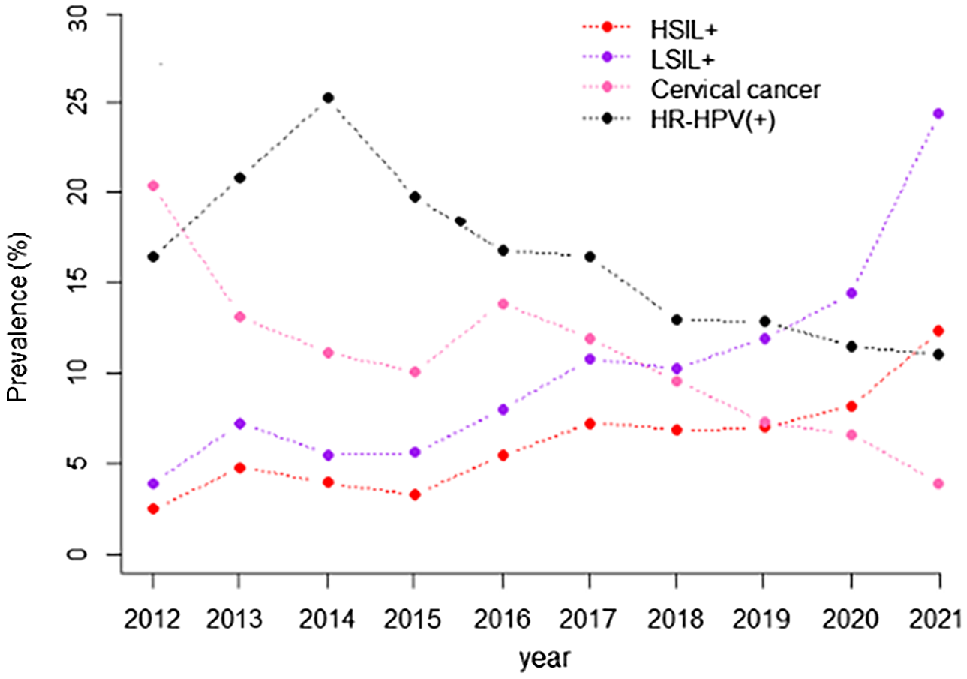
From 2012 to 2021, the prevalence of LSIL+ increased from 3.92% to 24.37%, and the prevalence of HSIL+ increased from 2.47% to 12.30%. Time trend analysis showed that the prevalence of cervical HSIL+ and LSIL+ increased from 2012 to 2021 (APC 15.30%, APC 19.36%, P < 0.05). However, the proportion of CC in CLs decreased from 20.37% to 3.88% gradually over the prior 10 years, following a downward trend (APC −12.65%, P < 0.05; Table 1, Fig. 1).
Changes in HR-HPV infection and CLs by age group
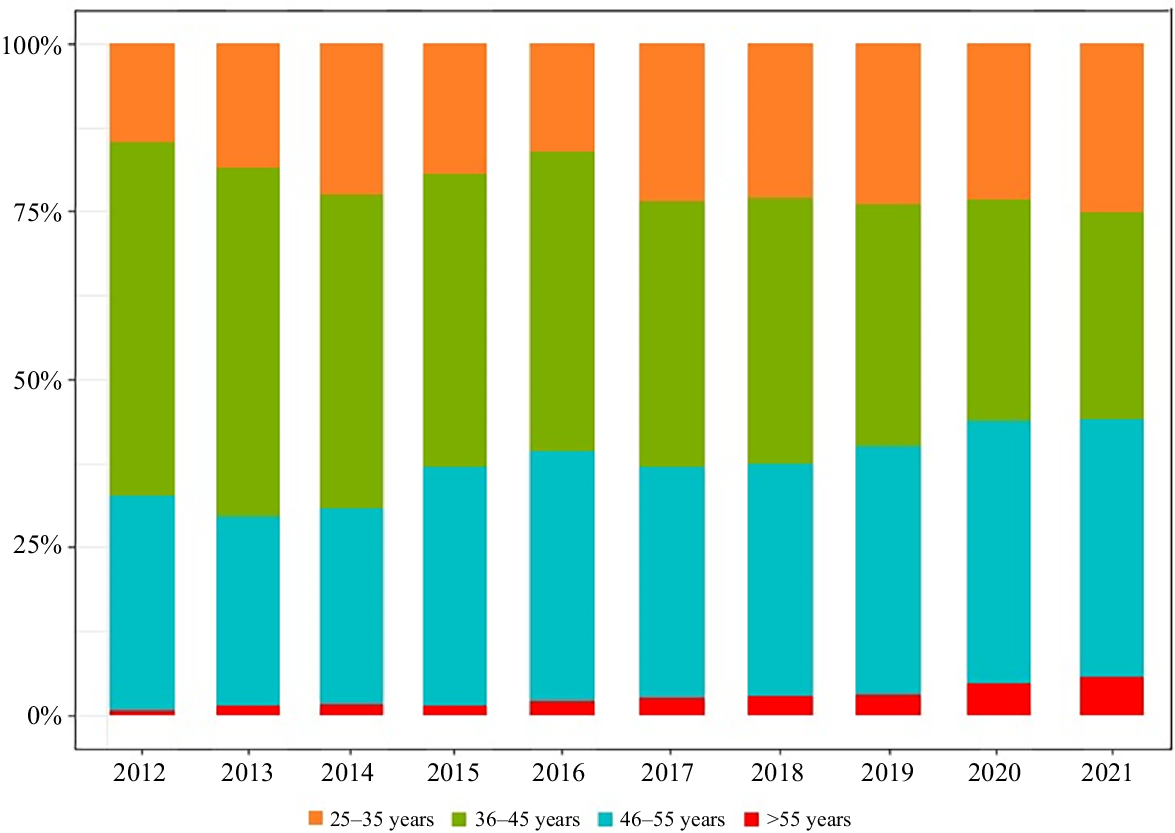
From 2012 to 2021, more individuals in the 36–45 years age group and 46–55 years age group received HR-HPV testing compared with individuals in the 25–35 years and >55 years age groups. The HR-HPV cervical screening rate for people aged >55 years was the lowest in every year (Table 2 and Fig. 2).
| Calendar year | 25–35 years (n) | 36–45 years (n) | 46–55 years (n) | >55 years (n) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Screening | HR-HPV+ | LSIL+ | HSIL+ | Screening | HR-HPV+ | LSIL+ | HSIL+ | Screening | HR-HPV+ | LSIL+ | HSIL+ | Screening | HR-HPV+ | LSIL+ | HSIL+ | ||
| 2012 | 1241 | 184 | 4 | 3 | 4412 | 718 | 35 | 20 | 2682 | 459 | 9 | 5 | 56 | 15 | 6 | 6 | |
| 2013 | 1317 | 287 | 19 | 10 | 3686 | 723 | 57 | 41 | 2009 | 428 | 28 | 7 | 94 | 41 | 3 | 3 | |
| 2014 | 1316 | 352 | 21 | 12 | 2722 | 688 | 36 | 30 | 1706 | 386 | 17 | 9 | 98 | 46 | 7 | 7 | |
| 2015 | 1915 | 409 | 22 | 14 | 4313 | 807 | 52 | 32 | 3527 | 688 | 32 | 15 | 140 | 46 | 3 | 3 | |
| 2016 | 1471 | 263 | 21 | 11 | 4082 | 502 | 39 | 26 | 3408 | 455 | 36 | 28 | 192 | 43 | 5 | 4 | |
| 2017 | 1769 | 305 | 22 | 10 | 2984 | 338 | 39 | 25 | 2590 | 327 | 38 | 30 | 185 | 40 | 10 | 8 | |
| 2018 | 1810 | 241 | 21 | 11 | 3115 | 399 | 33 | 24 | 2709 | 334 | 37 | 21 | 230 | 47 | 14 | 14 | |
| 2019 | 1949 | 260 | 32 | 17 | 2920 | 365 | 38 | 18 | 3000 | 370 | 35 | 23 | 250 | 49 | 19 | 15 | |
| 2020 | 2344 | 334 | 52 | 28 | 3306 | 343 | 47 | 27 | 3914 | 396 | 46 | 23 | 484 | 80 | 21 | 16 | |
| 2021 | 2883 | 369 | 79 | 44 | 3537 | 362 | 101 | 51 | 4403 | 435 | 95 | 42 | 660 | 102 | 34 | 19 | |
HR-HPV(+), high-risk human papillomaviruses infection; LSIL+, low-grade squamous intraepithelial lesion or higher; HSIL+, high-grade squamous intraepithelial lesion or higher.
The distribution of HR-HPV testing in different age groups from 2012 to 2021. The distribution of HR-HPV testing in different age groups from 2012 to 2021, which the testing rate of HR-HPV was the lowest in women aged >55 years.
Similar to the general population, the IR of HR-HPV in all age groups increased during 2012 to 2014, with APCs of 22.52% (25–35 years group), 12.55% (36–45 years group), 7.27% (46–55 years group) and 5.55% (>55 years group), respectively. However, we did not identify any significant difference in annual change trend (P > 0.05). During 2014 to 2021, the IR showed a downward trend, with APC of −9.67% (25–35 years group), −11.63% (36–45 years group), −11.03% (46–55 years group) and −13.03% (>55 years group), respectively, and the annual change trend reached statistical significance (P < 0.05). The IR of HR-HPV was the highest among women aged >55 years, followed by women aged 25–35 years (Table 2 and Fig. 3).
The trends of HR-HPV infection and detection of cervical lesions in each group from 2012 to 2021. Abbreviation: HR-HPV, high-risk human papillomaviruses, LSIL+, low-grade squamous intraepithelial lesion or higher; HSIL+, high-grade squamous intraepithelial lesion or higher. LSIL+ is defined as: LSIL, HSIL and cervical cancer; HSIL+ is defined as: HSIL and cervical cancer. The trends of HR-HPV infection and detection of cervical lesions in each group were separately described from 2012 to 2021, the HR-HPV infection rate showed a decreasing trend (a), the detection rates of cervical LSIL+ showed an increasing trend (b), the detection rates of cervical HSIL+ showed an increasing trend (c).
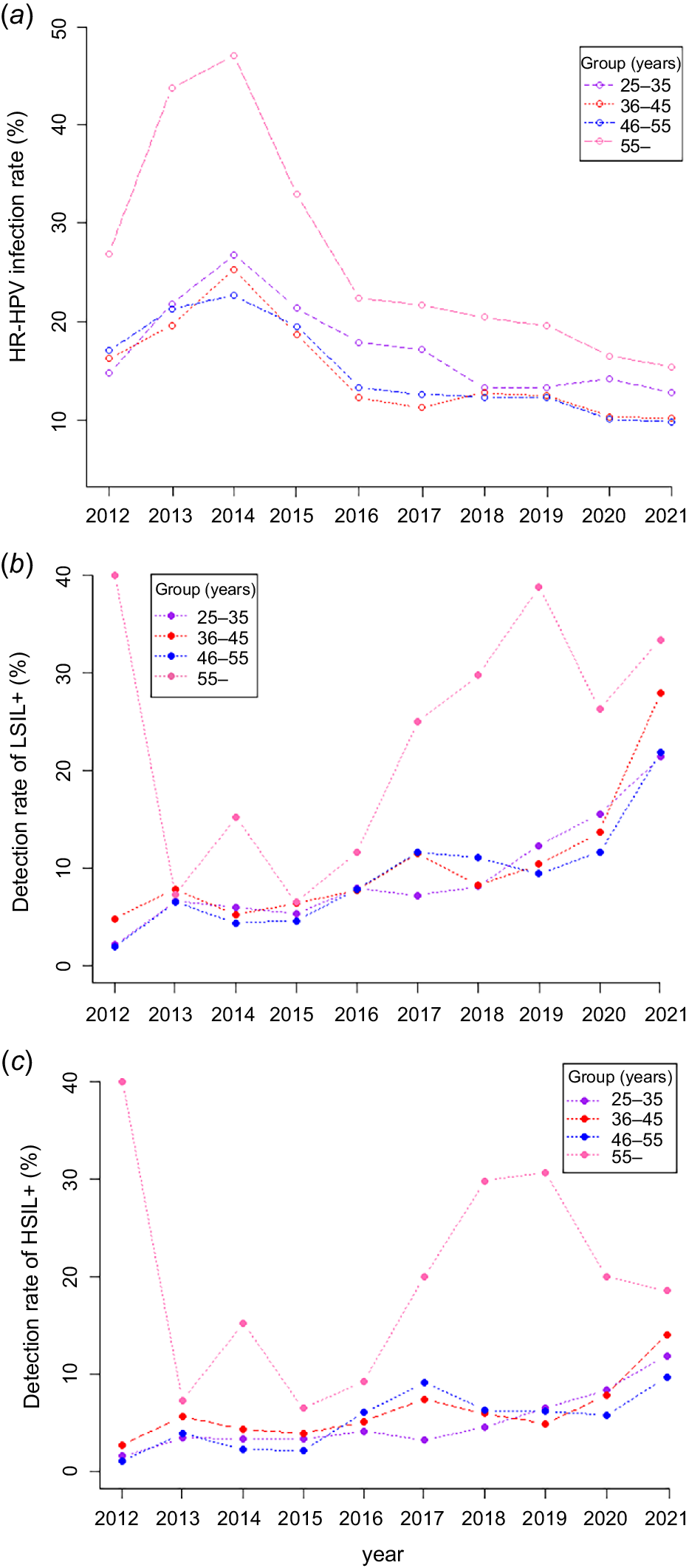
The annual detection rates of LSIL+ and HSIL+ were the highest among women in the >55 years group, and we did not identify any obvious trend from 2012 to 2021 (APC of 2.35% and 8.73%, P > 0.05). Prevalence of HSIL+ and LSIL+ in the 25–35, 36–45 and 46–55 years groups showed upward trends from 2012 to 2021, with APC of 19.74% and 20.16%, 13.28% and 18.59%, and 15.13% and 20.35%, respectively, and the annual changes reached statistically significance (P < 0.05; Table 2 and Fig. 3).
Occurrence of HR-HPV types
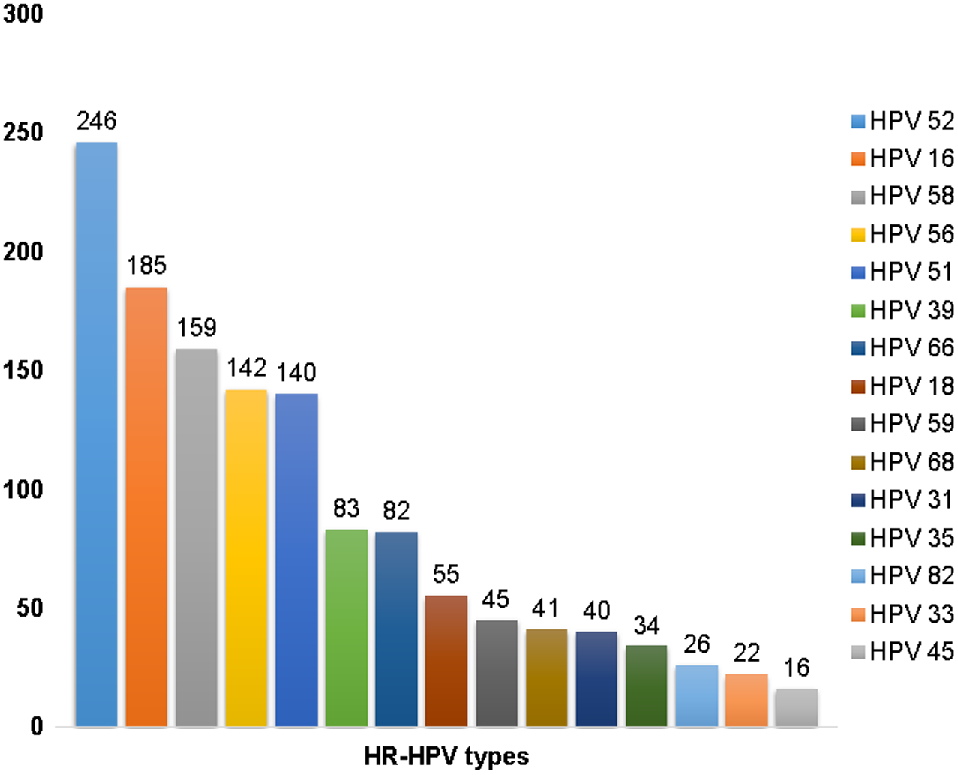
We examined 1641 occurrences of HR-HPV infection with specific typing from 2019 to 2021. Single infection (1316, 80.20%) served as one of the most common infection types in the normal population, although multiple infections (325, 19.80%) also occurred. The 10 most common HPV infection types were as follows: HPV31 (4.39%), HPV68 (4.69%), HPV18 (5.36%), HPV39 (6.95%), HPV66 (7.86%), HPV51 (12.31%), HPV56 (13.04%), HPV58 (13.95%), HPV16 (16.70%) and HPV52 (20.96%). Notably, HPV 52, 58 and 16 were the most common types in Karamay City, along with HPV56 and 51 (Fig. 4).
Discussion
The process of cervical HR-HPV screening and HPV vaccine implementation is similar in many cities in China. The overall HR-HPV IR in this study was 15.26%, slightly higher than the 14.02% reported by Wang et al.29 in Xinjiang, China, and the differences may be associated with population composition and period.30 The total IR of HR-HPV was 13.5% in Xi’an, China, from 2018 to 2020,31 similar to the rate that we found. HC2 was mainly used to detect HR-HPV in Karamay City before 2015, and Cobas 4800 was used after 2016. We found an increase in the rate of HR-HPV infection during 2012–2014, but the overall IR and the rates for each age group showed downward trends from 2014 to 2021. This change indicated that the IR of HR-HPV in this study had little to do with the detection method. The IR of HR-HPV began to decrease in 2014 and continued to decline from 2015 to 2021. This decrease may be due to an increase in awareness of preventing cervical HPV and the availability of an HPV vaccine in the city of Karamay region.7,32 The HPV vaccine bivalent was started in Karamay in 2016, followed by the quadrivalent and nine-valent HPV vaccines in the region.7,32 The HPV IR was 17.51% from 2010 to 2019 in Karamay.24 The decrease in HR-HPV IR may be related to vaccination. The decline of HR-HPV IR demonstrates that the prevention of cervical HPV was effective.
Previous studies indicated that the prevalence of HPV infection varied by age,33 and the prevalence was higher in young women and women aged ≥45 years.34 However, many recent reports suggest that the prevalence of cervical HR-HPV infection is high in women aged ≥50.35–37 We found that IR of HR-HPV was highest for women aged ≥55, followed by women in the 25–35 years range, similar to previous studies.36,38
CC exhibits a prolonged precancerous phase that can last decades without presenting any symptoms. Screening is a valid approach to detect precancerous lesions before they progress to invasive cancer.23 Papillomavirus testing demonstrates greater sensitivity in detecting HSIL+ disease than cytology testing.39 In this study, the initial referral reason for colposcopy was cytological abnormalities, followed by identification of high-risk HPV16 and 18, and continuous HR-HPV positivity. We found that the prevalence of HSIL+ increased, whereas the prevalence of CC decreased during the prior 10 years. These trends showed that we successfully identified more patients in the precancerous phase compared with previous screening. The highest prevalence of cervical LSIL+ and HSIL+ in this study was also women aged >55 years. In addition, we found that the prevalence of LSIL+ and HSIL+ displayed upward trends in the 25–35, 36–45 and 46–55 years age groups during the previous few years. Fortunately, no significant upward trend was identified for women aged >55 years.
Many studies showed that cervical HR-HPV is dominated by a single infection,31,35,37,40 as we observed in the present study. It has been controversial whether multiple HR-HPV infections is more prone to progressing into precancerous cervical lesions and CC.41–44 This study was not powered to examine this important question. The regional and national distribution of HR-HPV types is variable.45,46 The prevalent high-risk HPV types worldwide comprise HPV16, 18, 31, 52 and 58,47 whereas HPV16, 52, 58, 18 and 56 stand out as the most common HR-HPV types in Asia.48,49 The prevalence of the most frequent HR-HPV types in China differs regionally.50,51 The prevalent types identified from this study were HPV52, 16, 58, 56 and 51. The prevalent HR-HPV types reported in Xinjiang in 2019 comprised HPV16, 52, 58, 53 and 31.29 The decrease of HPV16 IR may be related to more women receiving bivalent HPV vaccine in this region.52 Our findings show the current common HR-HPV infection, and provide a reference for regional proper HPV vaccination.
In the current study, the IR of cervical HR-HPV had decreased in the past 10 years (2012–2021). According to the national routine immunisation report, total HPV vaccine doses in China were 3.417 million, 6.753 million and 12.279 million doses for the years 2018, 2019 and 2020, respectively.53 The health education on HPV and CC for both sexes, condom use, regular and effective CC screening strategies, and CC vaccine application may be crucial measures for CC prevention and control. More comprehensive strategies for CC prevention and control should be developed to reduce the occurrence of CC.
Limitations of our research merit attention. Primarily, being a hospital-centric survey, it pertains solely to a confined demographic, failing to mirror the wider female populace in Karamay. Additionally, the research did not consider vaccination prevalence, thereby impeding an evaluation of the vaccine’s precise influence on HPV incidence rates. Finally, the insufficiency of data regarding lifestyle patterns, reproductive records, economic status and sexual conduct obstructs the formulation of effective HPV infection mitigation strategies.
Conclusion
In summary, it was observed that the cervical HR-HPV IR decreased from 2012 to 2021. Although the prevalence of CLs showed an upward trend, the prevalence of CC displayed a downward trend, which indicated that the current preventive measures and screening methods effectively protected women at risk. However, more effort should be devoted to enhancing HR-HPV screening among women aged >55 years. The prevalent HR-HPV strains comprised HPV52, 16, 58, 56 and 51 in this area, which shed light on the future direction of cervical HPV vaccination and can more effectively prevent CC.
Consent for publication
This study utilised data from a pre-existing dataset collected with the prior consent of participants. At the time of data collection, participants had given consent for their data to be used in future research.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Declaration of funding
This study was supported by the National Natural Science Foundation of China (NSFC) under Grant No. 82260613, and the Natural Science Foundation of Xinjiang Uygur Autonomous Region (No. 2021D01A21).
Author contributions
Xiaoping Jia, Cailing Ma, conceiving and designing the study; Xiaoping Jia, Min Jiang, Jing Zhou, collecting the data; Xiaoping Jia, Min Jiang, Jing Zhou, analysing and interpreting the data; Xiaoping Jia, writing the manuscript; Cailing Ma, providing critical revisions that are important for the intellectual content; Xiaoping Jia, Min Jiang, Jing Zhou, Cailing Ma, approving the final version of the manuscript.
Acknowledgements
Yinshuai Zheng performed statistical analysis of the data and drew the bar charts and histogram. This work used data that had been provided and collected by the Pathology Department of Karamay Central Hospital. The authors thank AiMi Academic Services (www.aimieditor.com) for the English language editing and review services.
References
1 Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021; 71(3): 209-49.
| Crossref | Google Scholar |
2 Duan R, Qiao Y, Clifford G, Zhao F. Cancer burden attributable to human papillomavirus infection by sex, cancer site, age, and geographical area in China. Cancer Med 2020; 9(1): 374-84.
| Crossref | Google Scholar | PubMed |
3 Arbyn M, Weiderpass E, Bruni L, de Sanjosé S, Saraiya M, Ferlay J, et al. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Glob Health 2020; 8(2): e191-e203.
| Crossref | Google Scholar |
4 Crosbie EJ, Einstein MH, Franceschi S, Kitchener HC. Human papillomavirus and cervical cancer. Lancet 2013; 382(9895): 889-99.
| Crossref | Google Scholar | PubMed |
5 Liverani CA, Di Giuseppe J, Giannella L, Delli Carpini G, Ciavattini A. Cervical cancer screening guidelines in the postvaccination era: review of the literature. J Oncol 2020; 2020: 8887672.
| Crossref | Google Scholar |
6 Arbyn M, de Sanjosé S, Saraiya M, Sideri M, Palefsky J, Lacey C, et al. EUROGIN 2011 roadmap on prevention and treatment of HPV-related disease. Int J Cancer 2012; 131(9): 1969-82.
| Crossref | Google Scholar | PubMed |
7 Zhao C, Zhao Y, Li J, Li M, Shi Y, Wei L. Opportunities and challenges for human papillomavirus vaccination in China. Hum Vaccin Immunother 2024; 20(1): 2329450.
| Crossref | Google Scholar | PubMed |
8 Zhao XL, Hu SY, Hu JW, Wang HH, Wen TM, Feng YS, et al. Tackling barriers to scale up human papillomavirus vaccination in China: progress and the way forward. Infect Dis Poverty 2023; 12(1): 86.
| Crossref | Google Scholar | PubMed |
9 Wheeler CM, Hunt WC, Cuzick J, Langsfeld E, Pearse A, Montoya GD, et al. A population-based study of human papillomavirus genotype prevalence in the United States: baseline measures prior to mass human papillomavirus vaccination. Int J Cancer 2013; 132(1): 198-207.
| Crossref | Google Scholar | PubMed |
10 Dareng EO, Ma B, Famooto AO, Adebamowo SN, Offiong RA, Olaniyan O, et al. Prevalent high-risk HPV infection and vaginal microbiota in Nigerian women. Epidemiol Infect 2016; 144(1): 123-137.
| Crossref | Google Scholar | PubMed |
11 Teka B, Gizaw M, Ruddies F, Addissie A, Chanyalew Z, Skof AS, et al. Population-based human papillomavirus infection and genotype distribution among women in rural areas of South Central Ethiopia. Int J Cancer 2021; 148(3): 723-730.
| Crossref | Google Scholar | PubMed |
12 Zhu Y, Qian F, Zou W, Wu X, Liu C, Shen G, et al. Prevalence and genotype distribution of human papillomavirus infection in Huzhou City, eastern China, 2018–2019. Trans R Soc Trop Med Hyg 2021; 115(1): 30-37.
| Crossref | Google Scholar | PubMed |
13 Yang J, Wang W, Wang Z, Wang Z, Wang Y, Wang J, et al. Prevalence, genotype distribution and risk factors of cervical HPV infection in Yangqu, China: a population-based survey of 10086 women. Hum Vaccin Immunother 2020; 16(7): 1645-52.
| Crossref | Google Scholar | PubMed |
14 Jiang L, Tian X, Peng D, Zhang L, Xie F, Bi C, et al. HPV prevalence and genotype distribution among women in Shandong Province, China: Analysis of 94,489 HPV genotyping results from Shandong’s largest independent pathology laboratory. PLoS ONE 2019; 14(1): e0210311.
| Crossref | Google Scholar | PubMed |
15 Zhang C, Cheng W, Liu Q, Guan Q, Zhang Q. Distribution of human papillomavirus infection: a population-based study of cervical samples from Jiangsu Province. Virol J 2019; 16(1): 67.
| Crossref | Google Scholar | PubMed |
16 Mai Q, Yang X, Cheng H, Wu G, Wu Z. Prevalence and genotype distribution of human papillomavirus among women with cervical lesions in Shenzhen city, China. Hum Vaccin Immunother 2021; 17(4): 965-71.
| Crossref | Google Scholar | PubMed |
17 Hao S, Wang C, Liu S, He J, Jiang Y. HPV genotypic spectrum in Jilin province, China, where non-vaccine-covered HPV53 and 51 are prevalent, exhibits a bimodal age-specific pattern. PLoS ONE 2020; 15(3): e0230640.
| Crossref | Google Scholar | PubMed |
18 Melnikow J, Nuovo J, Willan AR, Chan BK, Howell LP. Natural history of cervical squamous intraepithelial lesions: a meta-analysis. Obstet Gynecol 1998; 92(4 Pt 2): 727-35.
| Crossref | Google Scholar | PubMed |
19 Perkins RB, Wentzensen N, Guido RS, Schiffman M. Cervical cancer screening: a review. JAMA 2023; 330(6): 547-58.
| Crossref | Google Scholar | PubMed |
20 Tainio K, Athanasiou A, Tikkinen KAO, Aaltonen R, Cárdenas J, Hernándes, et al. Clinical course of untreated cervical intraepithelial neoplasia grade 2 under active surveillance: systematic review and meta-analysis. BMJ 2018; 360: k499.
| Crossref | Google Scholar | PubMed |
21 Wu W, Song L, Yang Y, Wang J, Liu H, Zhang L. Exploring the dynamics and interplay of human papillomavirus and cervical tumorigenesis by integrating biological data into a mathematical model. BMC Bioinformat 2020; 21(Suppl 7): 152.
| Crossref | Google Scholar |
22 Demarco M, Cheung LC, Kinney WK, Wentzensen N, Lorey TS, Fetterman B, et al. Low risk of cervical cancer/precancer among most women under surveillance postcolposcopy. J Low Genit Tract Dis 2018; 22(2): 97-103.
| Crossref | Google Scholar | PubMed |
23 Li M, Wei L, Sui L, Ma D, Kong B, Wu X, et al. Guidelines for cervical cancer screening in China. Gynecol Obstet Clin Med 2023; 3(4): 189-94.
| Crossref | Google Scholar |
24 Nie Y-W, Ma Y-H, Wang C-C, Tian M-Z, Jiang M, Yang Z, et al. Analysis of HPV infection characteristics and trend of cohort population in Karamay City, Xinjiang, 2010–2019 based on Joinpoint and APC Model. Mod Prevent Med 2022; 49(11): 1921-5.
| Google Scholar |
25 Ronco G, Giorgi-Rossi P, Carozzi F, Confortini M, Dalla Palma P, Del Mistro A, et al. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomised controlled trial. Lancet Oncol 2010; 11(3): 249-57.
| Crossref | Google Scholar | PubMed |
26 Brisson M, Drolet M. Global elimination of cervical cancer as a public health problem. Lancet Oncol 2019; 20(3): 319-21.
| Crossref | Google Scholar | PubMed |
27 McCluggage WG, Singh N, Gilks CB. Key changes to the World Health Organization (WHO) classification of female genital tumours introduced in the 5th edition (2020). Histopathology 2022; 80(5): 762-78.
| Crossref | Google Scholar |
28 Ilic M, Ilic I. Cancer mortality in Serbia, 1991–2015: an age-period-cohort and joinpoint regression analysis. Cancer Commun 2018; 38(1): 10.
| Crossref | Google Scholar |
29 Wang J, Tang D, Wang K, Wang J, Zhang Z, Chen Y, et al. HPV genotype prevalence and distribution during 2009–2018 in Xinjiang, China: baseline surveys prior to mass HPV vaccination. BMC Women’s Health 2019; 19(1): 90.
| Crossref | Google Scholar |
30 Cohen PA, Jhingran A, Oaknin A, Denny L. Cervical cancer. Lancet 2019; 393(10167): 169-82.
| Crossref | Google Scholar |
31 Han X, Song G, Li Y, Dong Z, Yan X, Wang S, et al. Prevalence and genotype distribution of human papillomavirus infection among women aged 30–65 years in Xi’an, China: a population-based study of 14,655 women. Human Vaccin & Immunotherap 2021; 17(12): 5439-46.
| Crossref | Google Scholar |
32 Pan XF, Li R, Pan A, Larson H. Human papillomavirus vaccine approval in China: a major step forward but challenges ahead. Lancet Infect Dis 2016; 16(12): 1322-23.
| Crossref | Google Scholar | PubMed |
33 Franceschi S, Herrero R, Clifford GM, Snijders PJ, Arslan A, Anh PT, et al. Variations in the age-specific curves of human papillomavirus prevalence in women worldwide. Int J Cancer 2006; 119(11): 2677-84.
| Crossref | Google Scholar | PubMed |
34 de Sanjosé S, Diaz M, Castellsagué X, Clifford G, Bruni L, Muñoz N, et al. Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: a meta-analysis. Lancet Infect Dis 2007; 7(7): 453-59.
| Crossref | Google Scholar | PubMed |
35 Li P, Liu Q, Li W, Liu Z, Xing B, Wu S, et al. Characteristics of human papillomavirus infection among women with cervical cytological abnormalities in the Zhoupu District, Shanghai City, China, 2014-2019. Virol J 2021; 18(1): 51.
| Crossref | Google Scholar | PubMed |
36 Tang SY, Liao YQ, Hu Y, Shen HY, Wan YP, Wu YM. HPV prevalence and genotype distribution among women from Hengyang district of Hunan Province, China. Front Public Health 2021; 9: 710209.
| Crossref | Google Scholar | PubMed |
37 Luo Q, Lang L, Han N, Liang L, Shen L, Zhang H. Prevalence and genotype distribution of high-risk human papillomavirus infection among women with cervical cytological abnormalities in Chongqing, China, 2014–2020. Diagn Cytopathol 2021; 49(12): 1237-43.
| Crossref | Google Scholar | PubMed |
38 Wang R, Guo XL, Wisman GBA, Schuuring E, Wang WF, Zeng ZY, et al. Nationwide prevalence of human papillomavirus infection and viral genotype distribution in 37 cities in China. BMC Infect Dis 2015; 15: 257.
| Crossref | Google Scholar | PubMed |
39 Ogilvie GS, van Niekerk D, Krajden M, Smith LW, Cook D, Gondara L, et al. Effect of screening with primary cervical HPV testing vs cytology testing on high-grade cervical intraepithelial neoplasia at 48 months: the HPV FOCAL randomized clinical trial. JAMA 2018; 320(1): 43-52.
| Crossref | Google Scholar | PubMed |
40 Song L, Lyu Y, Ding L, Li X, Gao W, Wang M, et al. Prevalence and genotype distribution of high-risk human papillomavirus infection in women with abnormal cervical cytology: a population-based study in Shanxi Province, China. Cancer Manag Res 2020; 12: 12583-91.
| Crossref | Google Scholar |
41 Lyu Y, Ding L, Gao T, Li Y, Li L, Wang M, et al. Influencing factors of high-risk human papillomavirus infection and DNA load according to the severity of cervical lesions in female coal mine workers of China. J Cancer 2019; 10(23): 5764-69.
| Crossref | Google Scholar | PubMed |
42 Zhang R, Xu W, Yang S, Hu D, Bai L, Xiang R, et al. Prevalence of high-risk human papillomavirus infection, associated risk factors, and relationship with cervical precancerous lesions in perimenopausal and older women in an area with high cervical cancer incidence in China. Cureus 2024; 16(4): e58081.
| Crossref | Google Scholar | PubMed |
43 Mboumba Bouassa RS, Nodjikouambaye ZA, Sadjoli D, Adawaye C, Péré H, Veyer D, et al. High prevalence of cervical high-risk human papillomavirus infection mostly covered by Gardasil-9 prophylactic vaccine in adult women living in N’Djamena, Chad. PLoS ONE 2019; 14(6): e0217486.
| Crossref | Google Scholar | PubMed |
44 Patel SJ, Mugo NR, Cohen CR, Ting J, Nguti R, Kwatampora J, et al. Multiple human papillomavirus infections and HIV seropositivity as risk factors for abnormal cervical cytology among female sex workers in Nairobi. Int J STD AIDS 2013; 24(3): 221-5.
| Crossref | Google Scholar | PubMed |
45 Molina-Pineda A, López-Cardona MG, Limón-Toledo LP, Cantón-Romero JC, Martínez-Silva MG, Ramos-Sánchez HV, et al. High frequency of HPV genotypes 59, 66, 52, 51, 39 and 56 in women from Western Mexico. BMC Infect Dis 2020; 20(1): 889.
| Crossref | Google Scholar | PubMed |
46 de Sanjose S, Quint WG, Alemany L, Geraets DT, Klaustermeier JE, Lloveras B, et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol 2010; 11(11): 1048-56.
| Crossref | Google Scholar | PubMed |
47 Bruni L, Diaz M, Castellsagué X, Ferrer E, Bosch FX, de Sanjosé S. Cervical human papillomavirus prevalence in 5 continents: meta-analysis of 1 million women with normal cytological findings. J Infect Dis 2010; 202(12): 1789-99.
| Crossref | Google Scholar | PubMed |
48 Crow JM. HPV: The global burden. Nature 2012; 488(7413): S2-3.
| Crossref | Google Scholar | PubMed |
49 Li K, Li Q, Song L, Wang D, Yin R. The distribution and prevalence of human papillomavirus in women in mainland China. Cancer 2019; 125(7): 1030-37.
| Crossref | Google Scholar | PubMed |
50 Han C, Huang W, Ye M, Zou R, Lan J, Chen J, et al. HPV prevalence and genotype distribution in 2,306 patients with cervical squamous cell carcinoma in central and eastern China. Front Public Health 2023; 11: 1225652.
| Crossref | Google Scholar | PubMed |
51 Yang X, Li Y, Tang Y, Li Z, Wang S, Luo X, et al. Cervical HPV infection in Guangzhou, China: an epidemiological study of 198,111 women from 2015 to 2021. Emerg Microbes Infect 2023; 12(1): e2176009.
| Crossref | Google Scholar | PubMed |
52 Health Commission of Xinjiang Uygur Autonomous Region. Xinjiang’s 249-valent HPV vaccine basically realizes full coverage of all prefectures (states and cities) and key counties (cities and districts). Available at https://wjw.xinjiang.gov.cn/hfpc/xwxc1/202407/35196c05ebb84263ab5ac0275d223d9f.shtml
53 Ye J, Cao L, Yu W, Song Y, Yin Z. Reported routine immunization coverage with National Immunization Program vaccines in China, 2020–2021. Chinese J Vaccin Immuniz 2022; 28(5): 576-80.
| Google Scholar |