Chlamydia home sampling in the real world: a cross-sectional analysis
Rosalind Foster A B * , Tobias Vickers
A B * , Tobias Vickers  A B , Heng Lu A and Anna McNulty A C
A B , Heng Lu A and Anna McNulty A C
A Sydney Sexual Health Centre, GPO1614, Sydney, NSW, Australia.
B The Kirby Institute, Sexual Health Program, UNSW Medicine, Kensington, NSW, Australia.
C School of Public Health and Community Medicine, UNSW Medicine, Kensington, NSW, Australia.
Sexual Health 19(5) 479-483 https://doi.org/10.1071/SH22054
Submitted: 31 March 2022 Accepted: 21 June 2022 Published: 21 July 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing
Abstract
Background: Retesting rates for chlamydia in Australia are low. Chlamydia home sampling has been shown to increase retesting rates. Sydney Sexual Health Centre introduced chlamydia home sampling in 2019. The aim of this study is to describe home sampling in a real world setting.
Methods: In this retrospective study, the total number of heterosexual males and non-sex-working females who tested positive for chlamydia at a urogenital site (1 November 2019 to 31 October 2020) were identified based on local diagnostic codes. Agreeing participants who were sent a home sampling SMS reminder at 2.5 months were included for further analysis. Descriptive statistics and attrition rates of the home sampling were calculated using frequencies and percentages. Bivariate analyses of the main covariates by each stage, assessing crude associations, were performed using chi-squared tests.
Results: A total of 444 people attending Sydney Sexual Health Centre were eligible for the chlamydia home sampling option, 25.9% agreed to be sent the home sampling SMS invitation, of which 53 (46.1%) replied and were mailed a home sampling kit, with 43.4% returning the kit; of these 3 (13.0%) were positive for chlamydia. The majority (91.3%) of tests were performed within 6 months of original diagnosis. Of those who initially agreed but then did not undertake home sampling, 22.6% subsequently tested in clinic at Sydney Sexual Health Centre. There were no associations between any of the variables measured and undertaking home sampling.
Conclusions: Home sampling process for chlamydia reinfection screening in heterosexual men and non-sex-working women had much lower uptake than seen in a previous trial with high attrition rates at each stage.
Keywords: Australasia, chlamydia, community health, environment, home-obtained specimen, home testing, postal specimen, preventive medicine and public health, re-infection, screening, self-sampling, sexually transmitted infections.
Introduction
Repeat chlamydia testing 3 months post-treatment is recommended by the Australian STI guidelines.1 An Australian prospective cohort study in 2012 identified that 18% of young women had a repeat chlamydia infection 3 months after treatment.2 Analysis of routinely collected data from Australian sexual health clinics during 2004–2008 showed low chlamydia 3-month retesting rates (14.1%), with high positivity (20.1%) in those who retested.3 In 2018, only 12 810 (18%) of all people aged 15–29 treated for chlamydia in Australia had a repeat test within 6 months after diagnosis.4
An Australian randomised trial (known as REACT) conducted between 2011 and 2013 demonstrated that chlamydia home sampling significantly increased retesting rates compared with retesting in clinic.5 Participants diagnosed with chlamydia in sexual health clinics in Melbourne and Sydney were randomised to an intervention arm (postal home collection kit plus reminder short messaging service (SMS) at 3 months post diagnosis) vs standard of care (reminder SMS and retest at clinic). The percentage retesting within 1–4 months of the chlamydia diagnosis was significantly higher in the home vs clinic arm (61% [184/302] vs 39% [117/298], P < 0.001). The NSW STI Strategy 2016–2020 lists chlamydia home-based self-sampling as an option for increasing retesting rates.6
Sydney Sexual Health Centre (SSHC) offered chlamydia home sampling as part of routine care from November 2019. Non-sex-working women and heterosexual men attending SSHC testing positive for chlamydia at a urogenital site could opt in for a home sampling test; vaginal swab or sponge-based urine collection device (UriSWAB, Copan Diagnostics, CA, USA). An automated alert within the electronic medical record (eMR) reminded the clinician to offer the home sampling option. If the client agreed, an automated SMS was sent at 2.5 months post-diagnosis, inviting the client to opt in for home sampling. Those who opted in replied with their address and were sent a home sampling kit by post. The kit could be sent to any Australian state or territory, and included user instructions plus a prepaid envelope to return specimens to the testing laboratory. Laboratory results were communicated to the patient as per the usual, automated SSHC delivery system described in a previous publication.7 Testing was free of charge to the user, regardless of Medicare status. For those who declined home sampling, individual clinicians could set an automated SMS reminder at the time of chlamydia treatment/diagnosis to be sent in 3 months’ time ‘You are due for a repeat test. Please call SSHC to make an appointment.’
The aim of this study is to describe home sampling in a real world setting among clients who would not routinely present to a sexual health service within the subsequent 6 months.
Materials and methods
This study was a retrospective cross sectional analysis of routinely collected data from the SSHC eMR The project was reviewed by the South Eastern Sydney Local Health District HREC on 8 February 2022 and recommended to be a Quality Improvement activity not requiring independent ethics review.
The total number of heterosexual males and non-sex-working females attending SSHC who tested positive for chlamydia at a urogenital site from 1 November 2019 to 31 October 2020 were identified based on local diagnostic codes. Data were extracted from the clinic eMR and entered into a Microsoft Excel spreadsheet. All clients eligible for home sampling were described according to gender, whether sent a reminder SMS, and whether re-tested within 6 months of chlamydia diagnosis. All those who agreed to be sent a home sampling SMS reminder at 2.5 months were included for further analysis.
Descriptive statistics of the home sampling care cascade were calculated using frequencies and percentages to describe each stage: Consented to receive home sampling SMS invitation > Replied to SMS to receive a kit > Returned kit for testing > Result of test. Bivariate analyses of the main covariates by each stage, assessing crude associations, were performed with chi-squared tests using JASP ((Version 0.14.1)).
Results
During the study period, 444 people were eligible for the chlamydia home sampling option; 227 (51.1%) non-sex-working women (226 cisgender and 1 transgender woman) and 217 (48.9%) heterosexual cisgender men.
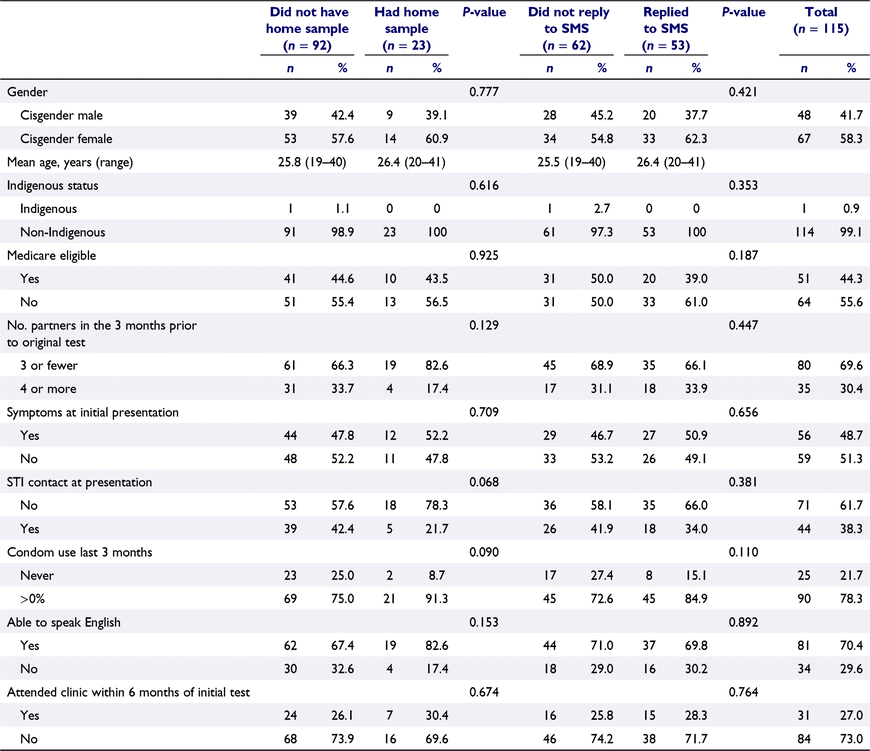
Of the 115 (25.9%) participants who signed up to receive an invitation SMS, 53 (46.1%) replied and were mailed a home sampling kit, 23 (43.4%) of those returned the kit, and of these 3 (13.0%) were positive for chlamydia (Fig. 1). The majority, 21 (91.3%) of tests were performed within 6 months of the initial positive result. All three individuals with positive chlamydia test results were treated at SSHC, times from home sample result to treatment were 0, 1, and 4 days.
Replying to the SMS invitiation, or undertaking home sampling were not associated with any of the variables studied (Table 1).
|
Of those who initially agreed but then did not undertake home sampling, 26/115 (22.6%), subsequently tested at SSHC. The majority, 21 (80.8%), of these tests were performed within 6 months of the original chlamydia diagnosis, and two (7.7%) were positive.
Of the 329 individuals who were eligible but did not opt in for home sampling, 172 (52.3%) cisgender men and 157 (47.7%) women (156 cisgender and 1 transgender woman); 86 (26.1%) were sent SMS reminders for 3-month test of reinfection; 63 (25.9%) of those not sent an SMS, and 23 (26.7%) of those sent an SMS, retested at SSHC within 6 months, and 8 (9.3%) of tests were positive. Being sent an SMS was not associated with retesting within 6 months in this sample.
In summary, of the 115 who signed up to receive a home sampling SMS invitation, a total of 49 (42.6%) had a repeat chlamydia test either sampled at home or in clinic, of which 5 (10.2%) were positive.
Discussion
Overall, uptake of the home sampling option amongst eligible clients at SSHC was lower than in REACT. Of the 115 who signed up for home sampling during the study period, only 43% had a repeat test. This is in comparison with 60% retesting rate in the home sampling arm of REACT.5 One explanation could be due to differences between the REACT protocol and ours; for example, all people in the home arm of REACT were sent the home sampling kit, whereas in our protocol, clients were required to opt in to receive a home sampling kit by replying to an SMS. Additionally, all participants in REACT were invited to undertake a post intervention survey for which they were paid A$40. Another possibility is that staff may be more willing to offer and clients more likely to participate in a novel research study driven locally by a research team than an option that is standard of care.
Cost analyses were not performed as part of this study. A 2016 analysis of the REACT home sampling study found home based sampling to be more cost effective than clinic retesting; with cost per infection estimated as A$1409.20 vs A$3132.60, respectively, despite only a 55.6% kit return rate in the home sampling arm overall.8
SMS reminders alone, rather than the home sampling kit, may have been the catalyst for testing. However among the eligible clients who did not sign up for home sampling in our study, receiving an SMS was not associated with retesting.
There was attrition between responding to the home sampling invitation SMS and returning the test kit, with only 23/53 (43.4%) returning the kit for testing. Interestingly, in REACT, the kit return rate by women and heterosexual men in the home sampling arm and was also quite low; only 93 (45.6%) performed their repeat sample at home, and 30 (14.7%) returned to have their test in clinic. A 2017–2019 study in Queensland, Australia, measured uptake of an online request followed by home postal sampling kit or pathology collection service for urine chlamydia testing, with up to eight phone calls and two SMS reminders.9 In this study, the test return rate (for home and pathology collection service combined) was 61.6%; however, prior to the reminder phone calls and SMS, the return rate was lower at 52.9%. A 2015 uptake and return rate study of an online home-based chlamydia sampling kit in Solihull, UK, showed a return rate of 48.2% in women and heterosexual men who had requested home sampling online, there were no SMS or phone reminders issued to encourage return of kits.10 Both these studies were in asymptomatic individuals wishing to undertake STI testing, for whom perception of risk of infection may be different to people being recommended a 3 month test of reinfection.
Positivity in home samples returned was 13% and is comparable with the 12% positivity in kits returned from women and heterosexual men in REACT.5 We found no variables to be significantly associated with likelihood of home sampling uptake or kit return.
Conclusion
Our study showed that this automated, home sampling process for chlamydia reinfection screening in heterosexual men and non-sex-working women attending SSHC had lower uptake than seen in a previous trial, and attrition rates were high at each stage.
Data availability
The data that support this study cannot be publicly shared due to ethical or privacy reasons and may be shared upon reasonable request to the corresponding author if appropriate.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
This research did not receive any specific funding.
Acknowledgements
The authors acknowledge Rebecca Houghton, Nurse Unit Manager at SSHC and Dr Lara Roeske from The Victorian Cytology Service for their assistance with setting up the home sampling process.
References
[1] Australasian Society for HIV, Viral Hepatitis and Sexual Health Medicine (ASHM). Australian STI Management Guidelines: for use in primary care. 2021. Available at http://www.sti.guidelines.org.au/[2] Walker J, Tabrizi SN, Fairley CK, Chen MY, Bradshaw CS, Twin J, et al. Chlamydia trachomatis incidence and re-infection among young women – behavioural and microbiological characteristics. PLoS One 2012; 7 e37778
| Chlamydia trachomatis incidence and re-infection among young women – behavioural and microbiological characteristics.Crossref | GoogleScholarGoogle Scholar |
[3] Guy R, Wand H, Franklin N, Fairley CK, Chen MY, O’Connor CC, et al. Re-testing for chlamydia at sexual health services in Australia, 2004–08. Sex Health 2011; 8 242–7.
| Re-testing for chlamydia at sexual health services in Australia, 2004–08.Crossref | GoogleScholarGoogle Scholar |
[4] Kirby Institute, UNSW. National update on HIV, viral hepatitis and sexually transmissible infections in Australia: 2009–2018. Sydney: Kirby Institute, UNSW Sydney; 2020.
[5] Smith KS, Hocking JS, Chen MY, Fairley CK, McNulty AM, Read P, et al. Dual intervention to increase chlamydia retesting: a randomized controlled trial in three populations. Am J Prev Med 2015; 49 1–11.
| Dual intervention to increase chlamydia retesting: a randomized controlled trial in three populations.Crossref | GoogleScholarGoogle Scholar |
[6] NSW Ministry of Health. NSW Sexually transmissible infections strategy 2016–2020. 2016. Available at https://www.health.nsw.gov.au/sexualhealth/Publications/nsw-sti-strategy-2016-2020.pdf. [accessed 16 June 2022]
[7] Knight V, Nugent C, Houghton R, O’Reilly K, Scally E, Lu H. An automated, electronic, client-centred results delivery system saves time and improves workflow. Sex Health 2019; 16 88–9.
| An automated, electronic, client-centred results delivery system saves time and improves workflow.Crossref | GoogleScholarGoogle Scholar |
[8] Smith KS, Kaldor JM, Hocking JS, Jamil MS, McNulty AM, Read P, et al. The acceptability and cost of a home based chlamydia retesting strategy: findings from the REACT randomised controlled trial. BMC Public Health 2016; 16 83
| The acceptability and cost of a home based chlamydia retesting strategy: findings from the REACT randomised controlled trial.Crossref | GoogleScholarGoogle Scholar |
[9] Groos A, Peardon-Freeman S, McFarlane K, Braithwaite S, Gajjar D, Murch P. Free online chlamydia and gonorrhoea urine test request in Queensland: sexually transmissible infections testing can be hard for young people even if the process is easy. Sex Health 2020; 17 543–6.
| Free online chlamydia and gonorrhoea urine test request in Queensland: sexually transmissible infections testing can be hard for young people even if the process is easy.Crossref | GoogleScholarGoogle Scholar |
[10] Banerjee P, Thorley N, Radcliffe K. A service evaluation comparing home-based testing to clinic-based testing for Chlamydia and gonorrhoea in Birmingham and Solihull. Int J STD AIDS 2018; 29 974–9.
| A service evaluation comparing home-based testing to clinic-based testing for Chlamydia and gonorrhoea in Birmingham and Solihull.Crossref | GoogleScholarGoogle Scholar |


