Seasonal variations in kissing and sexual activities among men who have sex with men in Melbourne, Australia: implications for seasonal sexually transmissible infection preventions and interventions
Eric P. F. Chow A B C , Lenka A. Vodstrcil A B and Christopher K. Fairley A B
A B C , Lenka A. Vodstrcil A B and Christopher K. Fairley A B
A Melbourne Sexual Health Centre, Alfred Health, 580 Swanston Street, Carlton, Vic. 3053, Australia.
B Central Clinical School, Monash University, 99 Commercial Road, Melbourne, Vic. 3004, Australia.
C Corresponding author. Email: eric.chow@monash.edu
Sexual Health 17(2) 149-154 https://doi.org/10.1071/SH19046
Submitted: 9 March 2019 Accepted: 15 November 2019 Published: 6 March 2020
Journal compilation © CSIRO 2020 Open Access CC BY-NC-ND
Abstract
Background: Previous studies have shown that there is a peak in sexually transmissible infection (STI) cases and sexual activities around summer, but there has been no study examining whether kissing also follows a similar seasonal pattern. The aim of this study was to examine the seasonal patterns of kissing and sex partners among gay, bisexual and other men who have sex with men (MSM). Methods: A short cross-sectional study was conducted among MSM attending the Melbourne Sexual Health Centre between March 2016 and February 2017. Participants were asked to report the number of kissing-only, sex-only and kissing-with-sex male partners in the last 3 months. The mean number of male partners was calculated and stratified by Australia’s seasons. The seasonal trend in the number of partners was assessed by negative binomial regression models. Results: In total, 4391 MSM were included in the analysis. The number of kissing-only and sex-only partners increased significantly from autumn to summer among MSM in Melbourne (Ptrend <0.001). MSM reported the highest number of male partners for kissing-only (mean: 4.91; 95% confidence intervals (CI): 4.78–5.04) and sex-only (mean: 1.91; 95% CI: 1.83–1.99) around summer compared with other seasons. However, the number of kissing-with-sex partners remained stable across seasons. Conclusions: The study data suggest that there is a peak in kissing-only and sex-only partners among MSM around summer and holiday seasons.
Additional keywords: bisexual, gay, gonorrhoea, holidays, homosexual, kiss, season, sexual behaviours, sexual practice, summer.
Introduction
Rises in gonorrhoea cases have been reported not only in gay, bisexual and other men who have sex with men (MSM), but also in other populations such as female sex workers and heterosexuals worldwide.1–5 The rise of the epidemic is complex and is not well understood. Seasonal variations in human infectious diseases, including some sexually transmissible infections (STIs) such as genital herpes, chlamydia and gonorrhoea, have been observed.6–8 In the northern hemisphere, there is a peak in gonorrhoea cases during summer and autumn8–10 while in the southern hemisphere, similar changes were also observed during summer in Australia.11,12 The peak in STIs has been associated with a greater number of sexual partners during summer.11 Understanding the seasonal patterns of STI and sexual activities is important to implement STI prevention campaigns at the right time, particularly during the peak of STI cases.
Oropharyngeal gonorrhoea has been proposed to contribute significantly to gonorrhoea transmission.13–15 Individuals with oropharyngeal gonorrhoea infection rarely develop symptoms, so individuals are not aware of their infections.16 Hence, undiagnosed and untreated oropharyngeal gonorrhoea infections would lead to ongoing gonorrhoea transmission within the sexual networks. Furthermore, the rise in antimicrobial-resistant Neisseria gonorrhoeae has become an emerging public health concern globally.17–20 Several treatment failure cases, particularly oropharyngeal gonorrhoea infections, have been reported worldwide,21–23 and this is likely due to the oropharynx being considered as an important reservoir of antimicrobial-resistant gonorrhoea infection.16,24 In this context, understanding the transmission of oropharyngeal gonorrhoea, in particular the role of kissing, is critical to its effective control.
Past studies have reported a seasonal variation in gonorrhoea cases, azithromycin resistance in Neisseria gonorrhoeae and number of sexual partners.11,25 Tongue-kissing is considered to be an important risk factor for gonorrhoea transmission, particularly among MSM,26 but there has been no study examining the seasonal patterns in kissing. The aim of this study was to examine whether there are any seasonal variations in kissing and sexual activities among MSM in Melbourne, Australia.
Methods
Study population and setting
We conducted a cross-sectional, computer-based survey, named the ‘Kissing’ study at the Melbourne Sexual Health Centre (MSHC) between March 2016 and February 2017. The primary aim of the ‘Kissing’ study was to examine whether kissing is a risk factor for oropharyngeal gonorrhoea in MSM. The main findings and the methodology have been published elsewhere.26 Results from this study have shown that MSM who had four or more kissing-only partners were 1.5-fold more likely to have oropharyngeal gonorrhoea after adjusting other confounding factors; however, there was no association between the number of sex-only partners and oropharyngeal gonorrhoea positivity. Furthermore, the study also found that younger MSM had more kissing-only partners but fewer sex-only partners than older MSM. However, the seasonal variations in kissing and sexual activities among MSM was not examined in the original study.
In brief, all men who reported having sex with another man in the last 12 months and aged ≥16 years attending MSHC during the study period were invited to participate in a short survey on kissing and sexual activities they had had in the last 3 months. The ‘Kissing’ study asked participants to report the number of male partners in the last 3 months in three different discrete categories: (1) kissing-only partners (the participant who only tongue kissed with their partners but did not have any sexual contacts); (2) sex-only partners (the participant who only had sexual contacts with their partners without kissing); and (3) kissing-with-sex partners (the participant who tongue kissed and had sexual contacts with their partners). A participant information sheet was provided to participants to read before they started the survey and consent was obtained from participants on the first page of the survey by them clicking ‘yes’ to the consent statement. Ethical approval for this study was obtained by the Alfred Hospital Ethics Committee, Melbourne, Australia (project number 69/16).
Statistical analysis
For the purpose of this analysis, we excluded participants who: (1) were aged <16 years; (2) did not have any kissing or sexual partners in the last 3 months; or (3) completed the survey more than once within a 3-month period.
The dates of participation were categorised into four Australia’s seasons: Autumn (March to May), Winter (June to August), Spring (September to November) and Summer (December to February). The mean number of partners by season was calculated and the 95% confidence intervals (CIs) of the mean were calculated. Negative binomial regression models were used to examine whether there was a seasonal trend in the number of partners, and the incidence rate ratio (IRR) and its 95% CIs were calculated from the model. Given the number of partners reported in the last 3 months spans over two seasons and it may lead to bias, we performed a sensitivity analysis among MSM who reported their partners within one season (i.e. men completed the study at the end of each season: May, August, November and February). All statistical analyses were conducted using Stata (version 14; StataCorp, College Station, TX, USA).
Results
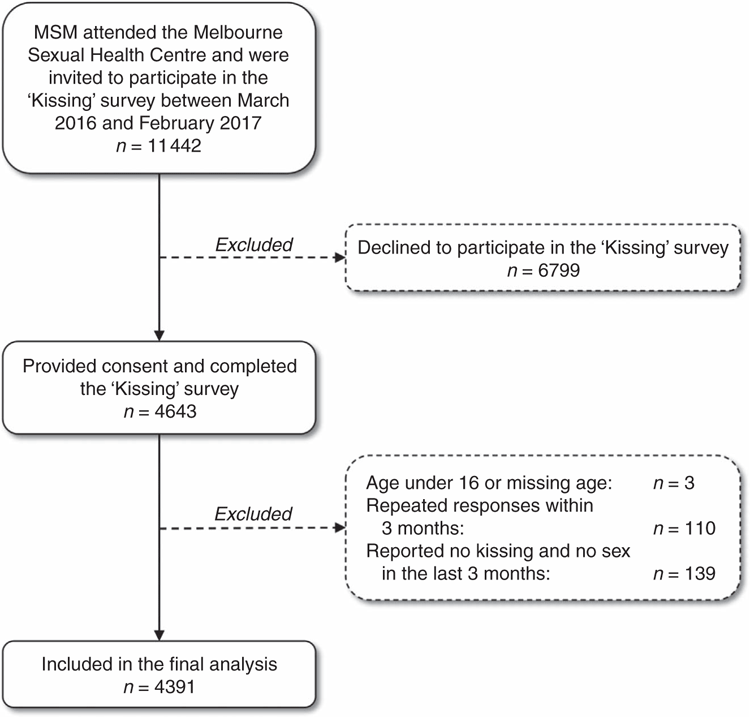
A total of 11 442 MSM were invited to participate in the ‘Kissing’ study and 4643 MSM (40.6%) completed the survey. Of those, 252 (5.4%) MSM did not meet the inclusion criteria and were excluded (Fig. 1). The remaining 4391 MSM were included in the final analysis. Age was not significantly different between participants and non-participants; however, a higher proportion of Australian-born MSM participated in the survey than overseas-born MSM (42.0% (2342/5581) vs 39.3% (2301/5861); P = 0.003). The median age of 4391 MSM was 30 years (IQR: 25–37) and 50.7% (n = 2226) were born in Australia.
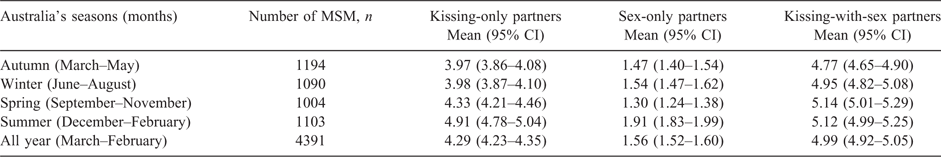
Table 1 shows that the mean number of kissing-only partners was 4.29 (95% CI: 4.23–4.35), sex-only partners was 1.56 (95% CI: 1.52–1.60) and kissing-with-sex partners was 4.99 (95% CI: 4.92–5.05) in the last 3 months.
|
The mean number of kissing-only, sex-only and kissing-with-sex partners fluctuated monthly (Table S1, available as Supplementary Material to this paper). However, the seasonal analysis showed that the number of kissing-only partners (4.91; 95% CI: 4.78–5.04) and sex-only partners (1.91; 95% CI: 1.83–1.99) reported by MSM was the highest around summer compared with other seasons (Table 1). MSM were more likely to report a higher number of kissing-only partners (IRR: 1.24; 95% CI: 1.10–1.39) and sex-only partners (IRR: 1.30; 95% CI: 1.08–1.56) around summer compared with autumn (Table 2). In the sensitivity analyses, by including only those MSM who reported the number of partners within one season, we also found that the number of kissing-only partners peaked around summer (Tables S2 and S3); however, there were no significant seasonal patterns in the number of sex-only partners and kissing-with-sex partners.
Discussion
This study shows that the number of kissing-only and sex-only partners among MSM peaks around the summer period in Australia (between December and February), and this echoes the peak in STI during summer in past studies.11,12 While a few studies have looked at the seasonal variations in the number of partners,11 we were unable to identify other published papers that had investigated the seasonal variations in kissing. In Australia, the summer period starts from December to February, which includes major holidays (e.g. Christmas, New Year and Australia Day) and large gay-related events (e.g. Midsumma Festival (Melbourne) and Mardi Gras (Sydney)). Previous studies have reported that a high number of partners are more likely to occur during the holiday seasons27,28 and gay-related events.29 Large dance parties usually follow on after these events30 and kissing has been associated with men attending these dance parties at clubs or bars.31,32 A case study has identified that a sexual network of kissing and sexual activities occurred in a large annual music festival in Melbourne during summer in 2018.33
Our findings suggest that the number of sex-only partners increases during the summer period compared with other seasons, and this echoes the observation where urethral gonorrhoea peaks in the summer period in Australia.11 We also found that the number of kissing-only partners increases during the summer period but, to our best knowledge, there has been no studies examining the seasonal patterns of oropharyngeal gonorrhoea. Given oropharyngeal gonorrhoea infections are usually asymptomatic, any seasonal patterns of asymptomatic infections may be due to the patterns of screening. Past studies have shown that men are more likely to have kissing-only partners at particular venues such as gay bars or dance parties;31,32 however, sex-only partners may be more likely to be at sex-on-premise venues where men may prefer sex without the intimacy of kissing.34 While, our findings suggest that that there is no seasonal pattern in kissing-with-sex partners, further qualitative research is required to understand the motivation of men having kissing-only, sex-only and kissing-with-sex partners.
Understanding the seasonal variations in kissing and sexual activities may help in the design of STI prevention and intervention programs targeting at-risk populations during particular seasons. For example, a Swedish study found that September and October had the highest number of chlamydia cases, leading to the launch of an annual public health campaign named ‘Chlamydia Monday’, which aimed to increase the knowledge and awareness of chlamydia transmission by providing free testing and condoms on a particular Monday in September.6 Results from the ‘Chlamydia Monday’ campaign successfully showed a subsequent increase in chlamydia testing. Our findings suggest that kissing and sexual activities peak around the summer period, thus STI prevention campaigns on STI testing, knowledge and awareness should be scaled up around the summer period in Australia. More recently, several authors have raised the possibility that gonorrhoea could be transmitted through kissing.13,14,26,35 To date, there has been no intervention to prevent acquiring oropharyngeal gonorrhoea through kissing, although clinical trials and in vitro studies are currently underway in several countries to investigate whether antiseptic mouthwash could be used to prevent gonorrhoea.36,37 If mouthwash is found to be effective in preventing gonorrhoea, it can be translated into a public health campaign advocating mouthwash use, given the concept of using mouthwash to prevent gonorrhoea is highly accepted in MSM.38–41 In addition, such campaigns should be scaled up during the summer period when most men kiss, as shown in this study.
There are some limitations in this study regarding data interpretation. First, this study was conducted at a single urban sexual health centre in Melbourne, which may not be generalisable to other settings or the larger MSM population. A substantial proportion of men declined to participate in the survey, and it is possible that this may have biased our results.42 Second, the number of partners was measured in the last 3 months preceding the survey. Misclassification of the season may have occurred if men answered the survey starting their count at the beginning of the season because the number of partners reported by MSM were in the previous 3 months and therefore correspond to the previous season. This may have affected our estimates and result in an over- or under-estimate of seasonal variations. However, we have performed a sensitivity analysis, which only included MSM who reported the number of partners within one season, and we have similar results and conclusions. Future studies examining weekly or monthly partner numbers may be useful to minimise the bias for seasonal analysis. Third, our cross-sectional design is not ideal to measure changes because it requires a 3-month recall period that may introduce recall bias. Ideally, a cohort design with frequent questionnaires would overcome this limitation, but would be logistically challenging to perform. Finally, this study only included data from a 1-year period, and it is possible that there were some unmeasured factors (e.g. gay-related events) that contributed to the changes in kissing and sexual activities during the study period. Future studies should include data covering more than 1 year to confirm this seasonal variation in kissing and sexual activities.
Our findings conclude that MSM have the highest mean number of kissing-only and sex-only partners around summer in Australia, suggesting that either there are social interactions during these periods or even that some of the increase is driven by biological changes in libido. If STI cases, kissing and sexual activities peak around summer, it is important to scale-up sexual health recourses and public health interventions and promotions during summer and holiday seasons to mitigate the rise in STI. Further research will be required to explore whether these seasonal variations in kissing and sexual activities are also observed among heterosexuals.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgments
E. P. F. Chow was supported by an Australian National Health and Medical Research Council (NHMRC) Early Career Fellowship (GNT1091226) when this study was conducted. We would like to thank Afrizal Afrizal for his assistance with data extraction and Jun Kit Sze for his assistance in implementing the ‘Kissing’ survey on the computer-assisted, self-interviewing system at the Melbourne Sexual Health Centre. This study was funded by an Australian NHMRC Program Grant (GNT568971).
References
[1] Chow EPF, Grulich AE, Fairley CK. Epidemiology and prevention of sexually transmitted infections in men who have sex with men at risk of HIV. Lancet HIV 2019; 6 e396–405.| Epidemiology and prevention of sexually transmitted infections in men who have sex with men at risk of HIV.Crossref | GoogleScholarGoogle Scholar | 31006612PubMed |
[2] Kirkcaldy RD, Weston E, Segurado AC, Hughes G. Epidemiology of gonorrhoea: a global perspective. Sex Health 2019; 16 401–11.
| Epidemiology of gonorrhoea: a global perspective.Crossref | GoogleScholarGoogle Scholar |
[3] Callander D, Guy R, Fairley CK, McManus H, Prestage G, Chow EPF, et al Gonorrhoea gone wild: rising incidence of gonorrhoea and associated risk factors among gay and bisexual men attending Australian sexual health clinics. Sex Health 2019; 16 457–63.
| Gonorrhoea gone wild: rising incidence of gonorrhoea and associated risk factors among gay and bisexual men attending Australian sexual health clinics.Crossref | GoogleScholarGoogle Scholar |
[4] Phillips TR, Fairley CK, Chen MY, Bradshaw CS, Chow EPF. Risk factors for urethral gonorrhoea infection among heterosexual males in Melbourne, Australia: 2007–17. Sex Health 2019; 16 508–13.
| Risk factors for urethral gonorrhoea infection among heterosexual males in Melbourne, Australia: 2007–17.Crossref | GoogleScholarGoogle Scholar |
[5] Chow EP, Williamson DA, Fortune R, Bradshaw CS, Chen MY, Fehler G, et al Prevalence of genital and oropharyngeal chlamydia and gonorrhoea among female sex workers in Melbourne, Australia, 2015–2017: need for oropharyngeal testing. Sex Transm Infect 2019; 95 398–401.
| Prevalence of genital and oropharyngeal chlamydia and gonorrhoea among female sex workers in Melbourne, Australia, 2015–2017: need for oropharyngeal testing.Crossref | GoogleScholarGoogle Scholar | 31113904PubMed |
[6] Hansdotter F, Blaxhult A. ‘Chlamydia Monday’ in Sweden. Euro Surveill 2008; 13 18984
| ‘Chlamydia Monday’ in Sweden.Crossref | GoogleScholarGoogle Scholar | 18801322PubMed |
[7] Martinez ME. The calendar of epidemics: seasonal cycles of infectious diseases. PLoS Pathog 2018; 14 e1007327
| The calendar of epidemics: seasonal cycles of infectious diseases.Crossref | GoogleScholarGoogle Scholar | 30408114PubMed |
[8] Schroeder B, Tetlow P, Sanfilippo JS, Hertweck SP. Is there a seasonal variation in gonorrhea and chlamydia in adolescents? J Pediatr Adolesc Gynecol 2001; 14 25–7.
| Is there a seasonal variation in gonorrhea and chlamydia in adolescents?Crossref | GoogleScholarGoogle Scholar | 11358703PubMed |
[9] Cornelius CE. Seasonality of gonorrhea in the United States. HSMHA Health Rep 1971; 86 157–60.
| Seasonality of gonorrhea in the United States.Crossref | GoogleScholarGoogle Scholar | 5544389PubMed |
[10] Wright RA, Judson FN. Relative and seasonal incidences of the sexually transmitted diseases. A two-year statistical review. Br J Vener Dis 1978; 54 433–40.
| Relative and seasonal incidences of the sexually transmitted diseases. A two-year statistical review.Crossref | GoogleScholarGoogle Scholar | 581655PubMed |
[11] Cornelisse VJ, Chow EP, Chen MY, Bradshaw CS, Fairley CK. Summer heat: a cross-sectional analysis of seasonal differences in sexual behaviour and sexually transmissible diseases in Melbourne, Australia. Sex Transm Infect 2016; 92 286–91.
| Summer heat: a cross-sectional analysis of seasonal differences in sexual behaviour and sexually transmissible diseases in Melbourne, Australia.Crossref | GoogleScholarGoogle Scholar | 26546343PubMed |
[12] Li B, Bi P, Chow EPF, Donovan B, McNulty A, Ward A, et al Seasonal variation in gonorrhoea incidence among men who have sex with men. Sex Health 2016; 13 589–92.
| Seasonal variation in gonorrhoea incidence among men who have sex with men.Crossref | GoogleScholarGoogle Scholar | 27712614PubMed |
[13] Fairley CK, Cornelisse VJ, Hocking JS, Chow EPF. Models of gonorrhoea transmission from the mouth and saliva. Lancet Infect Dis 2019; 19 e360–6.
| Models of gonorrhoea transmission from the mouth and saliva.Crossref | GoogleScholarGoogle Scholar | 31324517PubMed |
[14] Fairley CK, Hocking JS, Zhang L, Chow EP. Frequent transmission of gonorrhea in men who have sex with men. Emerg Infect Dis 2017; 23 102–4.
| Frequent transmission of gonorrhea in men who have sex with men.Crossref | GoogleScholarGoogle Scholar | 27983487PubMed |
[15] Zhang L, Regan DG, Chow EPF, Gambhir M, Cornelisse V, Grulich A, et al Neisseria gonorrhoeae transmission among men who have sex with men: an anatomical site-specific mathematical model evaluating the potential preventive impact of mouthwash. Sex Transm Dis 2017; 44 586–92.
| Neisseria gonorrhoeae transmission among men who have sex with men: an anatomical site-specific mathematical model evaluating the potential preventive impact of mouthwash.Crossref | GoogleScholarGoogle Scholar | 28876289PubMed |
[16] Lewis DA. Will targeting oropharyngeal gonorrhoea delay the further emergence of drug-resistant Neisseria gonorrhoeae strains? Sex Transm Infect 2015; 91 234–7.
| Will targeting oropharyngeal gonorrhoea delay the further emergence of drug-resistant Neisseria gonorrhoeae strains?Crossref | GoogleScholarGoogle Scholar | 25911525PubMed |
[17] Unemo M, Lahra MM, Cole M, Galarza P, Ndowa F, Martin I, et al World Health Organization Global Gonococcal Antimicrobial Surveillance Program (WHO GASP): review of new data and evidence to inform international collaborative actions and research efforts. Sex Health 2019; 16 412–25.
| World Health Organization Global Gonococcal Antimicrobial Surveillance Program (WHO GASP): review of new data and evidence to inform international collaborative actions and research efforts.Crossref | GoogleScholarGoogle Scholar |
[18] Wi T, Lahra MM, Ndowa F, Bala M, Dillon JR, Ramon-Pardo P, et al Antimicrobial resistance in Neisseria gonorrhoeae: global surveillance and a call for international collaborative action. PLoS Med 2017; 14 e1002344
| Antimicrobial resistance in Neisseria gonorrhoeae: global surveillance and a call for international collaborative action.Crossref | GoogleScholarGoogle Scholar | 28746372PubMed |
[19] Whittles LK, White PJ, Paul J, Didelot X. Epidemiological trends of antibiotic resistant gonorrhoea in the United Kingdom. Antibiotics (Basel) 2018; 7 60
| Epidemiological trends of antibiotic resistant gonorrhoea in the United Kingdom.Crossref | GoogleScholarGoogle Scholar |
[20] Williamson DA, Fairley CK, Howden BP, Chen MY, Stevens K, De Petra V, et al Trends and risk factors for antimicrobial-resistant Neisseria gonorrhoeae, Melbourne, Australia, 2007 to 2018. Antimicrob Agents Chemother 2019; 63 e01221-19
| Trends and risk factors for antimicrobial-resistant Neisseria gonorrhoeae, Melbourne, Australia, 2007 to 2018.Crossref | GoogleScholarGoogle Scholar | 31383663PubMed |
[21] Unemo M, Golparian D, Potocnik M, Jeverica S. Treatment failure of pharyngeal gonorrhoea with internationally recommended first-line ceftriaxone verified in Slovenia, September 2011. Euro Surveill 2012; 17 20200
| Treatment failure of pharyngeal gonorrhoea with internationally recommended first-line ceftriaxone verified in Slovenia, September 2011.Crossref | GoogleScholarGoogle Scholar | 22748003PubMed |
[22] Golparian D, Ohlsson A, Janson H, Lidbrink P, Richtner T, Ekelund O, et al Four treatment failures of pharyngeal gonorrhoea with ceftriaxone (500 mg) or cefotaxime (500 mg), Sweden, 2013 and 2014. Euro Surveill 2014; 19 20862
| Four treatment failures of pharyngeal gonorrhoea with ceftriaxone (500 mg) or cefotaxime (500 mg), Sweden, 2013 and 2014.Crossref | GoogleScholarGoogle Scholar | 25108533PubMed |
[23] Read PJ, Limnios EA, McNulty A, Whiley D, Lahra MM. One confirmed and one suspected case of pharyngeal gonorrhoea treatment failure following 500mg ceftriaxone in Sydney, Australia. Sex Health 2013; 10 460–2.
| One confirmed and one suspected case of pharyngeal gonorrhoea treatment failure following 500mg ceftriaxone in Sydney, Australia.Crossref | GoogleScholarGoogle Scholar | 24028864PubMed |
[24] Weinstock H, Workowski KA. Pharyngeal gonorrhea: an important reservoir of infection? Clin Infect Dis 2009; 49 1798–800.
| Pharyngeal gonorrhea: an important reservoir of infection?Crossref | GoogleScholarGoogle Scholar | 19911969PubMed |
[25] Olesen SW, Torrone EA, Papp JR, Kirkcaldy RD, Lipsitch M, Grad YH. Azithromycin susceptibility among Neisseria gonorrhoeae isolates and seasonal macrolide use. J Infect Dis 2019; 219 619–23.
| Azithromycin susceptibility among Neisseria gonorrhoeae isolates and seasonal macrolide use.Crossref | GoogleScholarGoogle Scholar | 30239814PubMed |
[26] Chow EPF, Cornelisse VJ, Williamson DA, Priest D, Hocking JS, Bradshaw CS, et al Kissing may be an important and neglected risk factor for oropharyngeal gonorrhoea: a cross-sectional study in men who have sex with men. Sex Transm Infect 2019; 95 516–21.
| Kissing may be an important and neglected risk factor for oropharyngeal gonorrhoea: a cross-sectional study in men who have sex with men.Crossref | GoogleScholarGoogle Scholar | 31073095PubMed |
[27] Rogstad KE. Sex, sun, sea, and STIs: sexually transmitted infections acquired on holiday. BMJ 2004; 329 214
| Sex, sun, sea, and STIs: sexually transmitted infections acquired on holiday.Crossref | GoogleScholarGoogle Scholar | 15271833PubMed |
[28] Finney H. Contraceptive use by medical students whilst on holiday. Fam Pract 2003; 20 93
| Contraceptive use by medical students whilst on holiday.Crossref | GoogleScholarGoogle Scholar | 12509379PubMed |
[29] Milhausen RR, Reece M, Perera B. A theory-based approach to understanding sexual behavior at Mardi Gras. J Sex Res 2006; 43 97–106.
| A theory-based approach to understanding sexual behavior at Mardi Gras.Crossref | GoogleScholarGoogle Scholar | 16817056PubMed |
[30] Kates SM. Producing and consuming gendered representations: an interpretation of the Sydney Gay and Lesbian Mardi Gras. Consumption Markets & Culture 2003; 6 5–22.
| Producing and consuming gendered representations: an interpretation of the Sydney Gay and Lesbian Mardi Gras.Crossref | GoogleScholarGoogle Scholar |
[31] Cornelisse VJ, Priest D, Fairley CK, Walker S, Bradshaw CS, Phillips T, et al The frequency of kissing as part of sexual activity differs depending on how men meet their male casual sexual partners. Int J STD AIDS 2018; 29 598–602.
| The frequency of kissing as part of sexual activity differs depending on how men meet their male casual sexual partners.Crossref | GoogleScholarGoogle Scholar | 29256822PubMed |
[32] Priest D, Chow EPF. Kissing while high on ecstasy: lessons from a gay dance party attendee. Sex Transm Infect 2018; 94 143
| Kissing while high on ecstasy: lessons from a gay dance party attendee.Crossref | GoogleScholarGoogle Scholar | 29463767PubMed |
[33] Cornelisse VJ, Bradshaw CS, Chow EPF, Williamson DA, Fairley CK. Oropharyngeal gonorrhea in absence of urogenital gonorrhea in sexual network of male and female participants, Australia, 2018. Emerg Infect Dis 2019; 25 1373–6.
| Oropharyngeal gonorrhea in absence of urogenital gonorrhea in sexual network of male and female participants, Australia, 2018.Crossref | GoogleScholarGoogle Scholar | 31211673PubMed |
[34] Grierson J, Smith A, Von Doussa H. An ordinary night out: a report on the research project Pivotal, Peripheral of Positional: understanding SOPVs for intervention. Melbourne: Australian Research Centre in Sex, Health and Society, La Trobe University; 2007.
[35] Chow EP, Fairley CK. The role of saliva in gonorrhoea and chlamydia transmission to extragenital sites among men who have sex with men: new insights into transmission. J Int AIDS Soc 2019; 22 e25354
| The role of saliva in gonorrhoea and chlamydia transmission to extragenital sites among men who have sex with men: new insights into transmission.Crossref | GoogleScholarGoogle Scholar | 31468730PubMed |
[36] Chow EPF, Walker S, Hocking JS, Bradshaw CS, Chen MY, Tabrizi SN, et al A multicentre double-blind randomised controlled trial evaluating the efficacy of daily use of antibacterial mouthwash against oropharyngeal gonorrhoea among men who have sex with men: the OMEGA (Oral Mouthwash use to Eradicate GonorrhoeA) study protocol. BMC Infect Dis 2017; 17 456
| A multicentre double-blind randomised controlled trial evaluating the efficacy of daily use of antibacterial mouthwash against oropharyngeal gonorrhoea among men who have sex with men: the OMEGA (Oral Mouthwash use to Eradicate GonorrhoeA) study protocol.Crossref | GoogleScholarGoogle Scholar | 28659133PubMed |
[37] Chow EP, Howden BP, Walker S, Lee D, Bradshaw CS, Chen MY, et al Antiseptic mouthwash against pharyngeal Neisseria gonorrhoeae: a randomised controlled trial and an in vitro study. Sex Transm Infect 2017; 93 88–93.
| Antiseptic mouthwash against pharyngeal Neisseria gonorrhoeae: a randomised controlled trial and an in vitro study.Crossref | GoogleScholarGoogle Scholar | 27998950PubMed |
[38] Chow EPF, Maddaford K, Trumpour S, Fairley CK. Translating mouthwash use for gonorrhoea prevention into a public health campaign: identifying current knowledge and research gaps. Sex Health 2019; 16 433–41.
| Translating mouthwash use for gonorrhoea prevention into a public health campaign: identifying current knowledge and research gaps.Crossref | GoogleScholarGoogle Scholar |
[39] Cornelisse VJ, Fairley CK, Walker S, Young T, Lee D, Chen MY, et al Adherence to, and acceptability of, Listerine® mouthwash as a potential preventive intervention for pharyngeal gonorrhoea among men who have sex with men in Australia: a longitudinal study. Sex Health 2016; 13 494–6.
| Adherence to, and acceptability of, Listerine® mouthwash as a potential preventive intervention for pharyngeal gonorrhoea among men who have sex with men in Australia: a longitudinal study.Crossref | GoogleScholarGoogle Scholar | 28636870PubMed |
[40] Walker S, Bellhouse C, Fairley CK, Bilardi JE, Chow EP. Pharyngeal gonorrhoea: the willingness of Australian men who have sex with men to change current sexual practices to reduce their risk of transmission – a qualitative study. PLoS One 2016; 11 e0164033
| Pharyngeal gonorrhoea: the willingness of Australian men who have sex with men to change current sexual practices to reduce their risk of transmission – a qualitative study.Crossref | GoogleScholarGoogle Scholar | 27992427PubMed |
[41] Chow EP, Walker S, Phillips T, Fairley CK. Willingness to change behaviours to reduce the risk of pharyngeal gonorrhoea transmission and acquisition in men who have sex with men: a cross-sectional survey. Sex Transm Infect 2017; 93 499–502.
| Willingness to change behaviours to reduce the risk of pharyngeal gonorrhoea transmission and acquisition in men who have sex with men: a cross-sectional survey.Crossref | GoogleScholarGoogle Scholar | 28676558PubMed |
[42] Chow EPF, Carlin JB, Read TRH, Chen MY, Bradshaw CS, Sze JK, et al Factors associated with declining to report the number of sexual partners using computer-assisted self-interviewing: a cross-sectional study among individuals attending a sexual health centre in Melbourne, Australia. Sex Health 2018; 15 350–7.
| Factors associated with declining to report the number of sexual partners using computer-assisted self-interviewing: a cross-sectional study among individuals attending a sexual health centre in Melbourne, Australia.Crossref | GoogleScholarGoogle Scholar | 29966584PubMed |