High prevalence and incidence of HIV, sexually transmissible infections and penile foreskin cutting among sexual health clinic attendees in Papua New Guinea
Andrew Vallely A B I , Claire E. Ryan B C , Joyce Allen B D , Joyce C. Sauk E , Cassey S. Simbiken B , Johanna Wapling B C , Petronia Kaima D , Zure Kombati D , Greg Law F , Glenda Fehler G , John M. Murray H , Peter Siba B and John M. Kaldor on behalf of the Male Circumcision Acceptability and Impact Study (MCAIS) team AA The Kirby Institute, University of New South Wales, Darlinghurst, NSW 2010, Australia.
B Papua New Guinea Institute of Medical Research, Goroka, Papua New Guinea.
C The Burnet Institute, 85 Commercial Road, Melbourne, Vic. 3004, Australia.
D Mount Hagen General Hospital, Mount Hagen, Papua New Guinea.
E National Capital District Department of Health, Port Moresby, Papua New Guinea.
F National Department of Health, Port Moresby, Papua New Guinea.
G Melbourne Sexual Health Centre, 580 Swanston Street, Carlton, Vic. 3053, Australia.
H School of Mathematics and Statistics, University of New South Wales, Sydney, NSW 2052, Australia.
I Corresponding author. Email: avallely@kirby.unsw.edu.au
Submitted: 18 December 2013 Accepted: 2 February 2014 Published: 12 March 2014
Abstract
Background: Papua New Guinea (PNG) has one of the highest prevalences of HIV and sexually transmissible infections (STIs) in the Asia-Pacific region, and one of the highest burdens of maternal syphilis and cervical cancer globally. Despite this disease burden, only limited clinical research in sexual and reproductive health has been conducted in PNG. Methods: A longitudinal clinical cohort study was conducted at two sexual health clinics. Participants completed a behavioural interview, clinical assessment and genital examination at baseline, and at 12, 24 and 50 weeks, including specimen collection for STI diagnostics. Results: In total, 154 people attended a screening visit. Reattendance at 12, 24 and 50-weeks was 87%, 78% and 80% respectively. At baseline, HIV prevalence was 3.3%; chlamydia (Chlamydia trachomatis), 29.2%; gonorrhoea (Neisseria gonorrhoeae), 22.1%; Trichomonas vaginalis 15.6%; herpes simplex type-2 (HSV-2), 46.1%; active syphilis, 11.7%. Multiple infections were common particularly among women. The incidence of chlamydia was 27 per 100 person-years (PY); gonorrhoea, 15 out of 100 PY; T. vaginalis, 29 out of 100 PY; HSV-2, 12 out of 100 PY; syphilis, 8 out of 100 PY. No incident HIV cases were recorded. At baseline, 39% of men in Mt Hagen and 65% in Port Moresby had a penile foreskin cut, with a dorsal slit being the most common. Two men underwent penile cutting during the follow-up period. Conclusions: The prevalence and incidence of STIs, HIV and penile cutting were high among sexual health clinic attendees. High retention figures suggest that this population may be suitable for future interventions research and clinical trials.
Background
Papua New Guinea (PNG) has one of the highest prevalences of HIV, syphilis and other sexually transmissible infections (STIs) in the Asia-Pacific region.1–8 The HIV epidemic in PNG is primarily linked to heterosexual transmission, with over half of reported HIV diagnoses coming from three of its 22 provinces.5,9 Adult HIV prevalence is currently estimated at 0.9%;5 however, a prevalence of 12–17% has been reported among women and men who sell or exchange sex10,11 and an antenatal HIV prevalence of ~2.0% has been reported from several Highland provinces.9 PNG is among 12 high-burden countries selected by the World Health Organisation (WHO) for intensified support for the elimination of mother-to-child transmission of syphilis.2 PNG also has one of the highest estimated burdens of cervical cancer globally, with an age-standardised incidence of 23.7 per 100 000, compared with 5.0 per 100 000 in Australia.12 Cervical cancer is the commonest cancer in women in PNG, resulting in an estimated 1500 premature deaths per year.1 The high proportion of cervical cancers attributable to human papillomavirus (HPV) Types 16 and 18 suggest that polyvalent HPV vaccines could have a significant public health impact in this setting.4,8
Despite this high burden of disease, no longitudinal clinical cohort studies, clinical trial feasibility studies or intervention research in sexual and reproductive health have been conducted to date in PNG. Recognising the need to evaluate the acceptability, operational feasibility and potential public health impact of new biomedical technologies and other interventions for the control of HIV, STIs and HPV infection among women and men at different levels of sexual risk in PNG, we undertook a longitudinal clinical cohort study at two sites in order to investigate the prevalence and incidence of HIV and STIs and determinants of risk in this population, and the feasibility of conducting interventions research and clinical trials in this setting in future. This research was conducted as part of a 4-year multi-disciplinary research program (the Male Circumcision Acceptability and Impact Study) that integrated mathematical modelling, and qualitative, health system and longitudinal clinical research.11,13–21
Methods
Study design and procedures
A longitudinal clinical cohort study was conducted at two urban sexual health clinics in PNG from 2011 to 2012: Tininga Clinic, Mount Hagen, Western Highlands Province and Nine Mile Clinic, Port Moresby, National Capital District. Both clinics are located close to informal urban settlement areas and provide services designed to reach those at different levels of HIV or STI acquisition risk. For example, a volunteer peer outreach program at Nine Mile Clinic has promoted service uptake by men who have sex with men (MSM), and male and female sex workers.
Local communities in each clinic’s catchment area were informed of the study through community-based networks established by provincial AIDS committees, nongovernmental and community-based organisations (e.g. Wansa in Western Highlands, a community-based organisation providing care and support to people living with HIV and their families). Men and women who expressed interest in participating in the study were given a referral slip for participating clinics. Participatory community meetings, and announcements at market places, after church services and on local radio were also used to encourage participation and to raise awareness of key study objectives.
Women and men attending for sexual health services were provided with information about the study on arrival at participating clinics and invited to take part. Following the completion of written informed consent procedures, participants took part in a face-to-face behavioural interview and clinical review, which included a genital examination and the collection of laboratory specimens for STI diagnostics, at baseline, and at 12, 24 and 52 weeks. Those diagnosed with an STI based on their clinical presentation were treated according to PNG national syndromic management guidelines, with additional treatment initiated as indicated by the results of subsequent laboratory investigations.
For women, a cervical swab was collected for chlamydia (Chlamydia trachomatis) and gonorrhoea (Neisseria gonorrhoeae, a posterior fornix swab was collected for Trichomonas vaginalis and a swab from the lateral vaginal wall was collected for Gram stain microscopy. In men with urethral discharge, a urethral swab was collected for chlamydia, gonorrhoea and T. vaginalis. In men without urethral discharge, a first-void urine specimen (or a sample no less than 3 h after previous micturition)22 was collected for the detection of these STIs. A 10-mL venepuncture blood specimen was collected at baseline and scheduled clinical follow-up visits for syphilis and herpes simplex virus Type 2 (HSV-2). Participants were offered voluntary HIV counselling and testing (VCT) at baseline and during follow-up, and were advised to return at any time for additional unscheduled visits should they experience any ongoing or new sexual health problem.
Participants who failed to reattend for scheduled clinical follow-up within 4 weeks of their appointment date were visited in the community by a member of the community outreach team at each site and advised to reattend. Clinical services were provided free of charge to both study participants and nonparticipants.
Laboratory methods
Screening tests for HIV (Determine HIV-1/2, Alere, Jena, Germany) and syphilis (SD Bioline anti-TP 3.0, Alere) were performed at the point of clinical care according to national guidelines. HIV-reactive samples were sent to clinic laboratories, where the serum was separated and tested with the laboratory-based Serodia HIV 1/2 particle agglutination assay (Fujirebio, Japan) and the Immunocomb enzyme immunoassay (Alere). Syphilis infections were confirmed with a rapid plasma reagin test; positive samples (any titre) were considered to be active infections. Genital swabs, urine and serum specimens were stored at −20°C on site until transfer to the PNG Institute of Medical Research in Goroka.
All other laboratory investigations were conducted at the PNG Institute of Medical Research HIV/STI Research Laboratory, with the exception of Nugent score assessment, which was performed at the Melbourne Sexual Health Centre in Australia. Testing for HSV-2 was performed on serum samples using a standard IgG specific ELISA (Kalon Biologicals, Aldershot, UK) according to the manufacturer’s instructions and using the recommended optical density ratios for the interpretation of results.
For extraction of DNA from urine samples, a 3-mL aliquot was centrifuged at 13 000g for 5 min. The supernatant was discarded and the pellet was resuspended in 1 mL of phosphate buffered saline (PBS). Material from genital swabs was suspended in 1 mL of PBS by vortexing for 30 s. For both urine and swab samples, 200 μL of the PBS suspension was used for DNA extraction using the NucleoSpin Tissue kit (Macherey-Nagal, Duren, Germany) according to the manufacturer’s standard protocol for cultured cells.
Real-time polymerase chain reaction (PCR) and analysis was performed using a CFX 96 machine (Bio-Rad, Hercules, CA, USA) and CFX Manager Software ver. 2.1 (Bio-Rad). Amplification of the human β-globin gene was used as a control to confirm the integrity of DNA extracts. A previously published primer pair (GH20–PC04) was used to amplify a 268-bp region of the β-globin gene.23 Amplification reactions were performed in a total of 10 μL with 2 μL of extracted DNA, 0.3 μM of each primer and 1× SsoFast EvaGreen Supermix (Bio-Rad). The cycling protocol consisted of 98°C for 120 s, followed by 45 cycles of 98°C for 5 s and 58°C for 20 s, followed by a standard melt protocol from 65°C to 95°C at 0.5°C each 5 s. Adequate amplifiable DNA in the sample was indicated by the presence of a peak fluorescence change at 85°C. For T. vaginalis, amplification reactions were performed in a total of 15 uL using 5 μL of DNA. A 102-bp fragment of genomic sequence was amplified using 4 μL of previously published primers (TVA5 and TVA6)24 at a final concentration of 0.4 μM and 1× SSoFast Eva Green Supermix (Bio-Rad). The cycling protocol consisted 92°C for 2 min, followed by 40 cycles of 98°C for 5 s and 55°C for 5 s, followed by a standard melt protocol from 65°C to 95°C at 0.5°C each 5 s. Samples were positive if they demonstrated a quantification cycle value less than 40 and a peak fluorescence change at 73°C.
Previously published primer and probe sets were used for the detection of C. trachomatis targeting the multicopy cryptic plasmid25 and N. gonorrhoeae targeting the multicopy opa gene.26 These protocols were adapted for the CFX 96 platform (Bio-Rad) and the QuantiFast Probe PCR Kit (Qiagen, Dusseldorf, Germany). Amplification reactions for C. trachomatis were performed in a total of 15 μL with 5 μL of extracted DNA, 0.25 μM of each primer, 0.01 μM of the probe, 1× QuantiFast Probe PCR mastermix (Qiagen) with an additional 1.5 mM of MgCl2. The cycling protocol consisted of 95°C for 180 s, followed by 45 cycles of 95°C for 5 s, and 55°C for 60 s. Amplification reactions for N. gonorrhoeae were performed in a total of 15 μL with 5 μL of extracted DNA, 0.4 μM each primer, 0.16 μM of the probe, 1× QuantiFast Probe PCR mastermix (Qiagen) with an additional 3 mM of MgCl2. The cycling protocol consisted of 95°C for 180 s, followed by 45 cycles of 95°C for 5 s and 60°C for 60 s.
Positive and negative controls were included on each assay run for all analytes, including standards for N. gonorrhoeae and C. trachomatis, which demonstrated a limit of detection of 10 copies.
The laboratory was enrolled in an external quality assurance program through the Royal College of Pathologists of Australia for syphilis and HSV-2 serology, and for gonorrhoea and chlamydia PCR. There was no available program for T. vaginalis PCR. Gram-stained slides were prepared on site and transferred to Melbourne Sexual Health Centre for microscopic examination. A diagnosis of bacterial vaginosis was inferred on the basis of the Nugent score.27
Data management and statistical methods
Participant study folders (containing completed case record forms and laboratory results slips) were subject to 100% quarterly clinical audits by the study lead investigator (AV) throughout. Data were entered at each clinical site into a study-specific MS Access database (Microsoft Corporation, Redmond, WA, USA). Database entries were validated against participant study folders for accuracy during quarterly clinical audits. Laboratory test results entered into the clinical database were checked for accuracy against source documents (laboratory result slips) for all participants at all visits at the end of the study by the lead investigator.
Fisher’s Exact Test was used to compare statistical differences in outcomes of interest between groups (e.g. between women and men at individual sites; between women or men at one site compared with another). There were no modifications for multiple comparisons. STI incidence rates and confidence intervals were calculated using the Poisson distribution. All statistical analyses were performed with Matlab R2012b (MathWorks Inc., MA, USA).
Participants who had attended at least one clinical follow-up visit and had provided laboratory STI data were included in the STI incidence calculations. Incident cases were defined as laboratory-confirmed infections at the current visit among participants who had a negative laboratory result for the same STI at their previous visit. Person-years (PY) were measured by the time since the previous clinic visit, assuming that the infection occurred at the midpoint between visits. Reinfections were calculated as the proportion of those with a laboratory-confirmed STI at baseline who tested negative for the same STI at their next clinic visit but who had a positive laboratory test during subsequent clinical follow-up.
Ethical approval
The study was approved by the Medical Research Advisory Committee of the National Department of Health in PNG (No. 08.21); and by the Human Research Ethics Committee of the University of New South Wales in Australia (No. 10 266). Written informed consent (signature or witnessed thumbprint) was obtained from all participants before enrolment.
Results
General characteristics of the study population
A total of 154 men and women participated in a baseline screening visit. Statistically significant differences in median age, marital status, educational attainment and occupation were observed between sites (Table 1). For example, subsistence farming was a more common occupation among those attending Tininga Clinic, reflecting the peri-urban and rural local catchment population; sex work was reported as an occupation only at Nine Mile Clinic, where there is an established community outreach program designed to reach those at increased risk of acquiring HIV and STIs. The majority of respondents (149 out of 185; 81%) self-identified as Christian, among whom a majority (93 out of 149; 62%) were of the Catholic, Lutheran or Seventh Day Adventist denominations. No significant differences in religious faith were observed by study site (data not shown).
Baseline behavioural, clinical and laboratory findings
Significant differences were observed both within and between sites in self-reported sexual behaviour, clinical symptoms and signs, syndromic STI diagnoses and laboratory findings (Table 1).
Men in Mt Hagen were significantly more likely to report multiple (≥2) sexual partners in the previous week compared with men in Port Moresby. Among women, multiple sexual partners were reported in Port Moresby only. Men in Port Moresby were significantly more likely to report ever having had anal sex compared with men in Mt Hagen (P = 0.04). Reported condom use at last vaginal sex, at last anal sex and during vaginal sex in the previous month were all extremely low among both men and women in this population (e.g. 81% of women in Mt Hagen and 67% of women in Port Moresby reported never using a condom during vaginal sex in the previous month). A linear model to investigate determinants of never having used a condom in the previous month (based on age, gender, study site, having had multiple vaginal sex partners in the last month, having had anal sex in the last month and the number of STIs at screening) found that gender was the only significant factor associated with nonuse (P = 0.02), with women (59 out of 81; 73%) being more likely to report never using a condom than men (36 out of 73; 49%).
At both clinical sites, the most common presenting symptoms among men were dysuria and genital discharge; those among women were genital discharge and abdominal pain. The most common syndromic diagnosis made was urethral discharge syndrome in men and vaginal discharge syndrome in women at both clinical sites. Abdominal pain was uncommon at baseline among men in Port Moresby but was reported by 30% of men in Mt Hagen (P = 0.03).
Among 154 participants at baseline, the prevalence of HIV was 3.3%, that of chlamydia was 29.2%, that of gonorrhoea was 22.1%, that of T. vaginalis was 15.6%, that of HSV-2 was 46.1%, that of active syphilis was 11.7%, and that of bacterial vaginosis was 58%. Multiple infections were common, particularly among women (e.g. 61% of women in Mt Hagen and 58% of women in Port Moresby had two or more STIs at baseline compared with 15% and 20% of men at each site respectively). Significantly higher prevalences of HSV-2 were reported among women (70% and 71%) compared with men (21% and 36%) in Port Moresby and Mt Hagen respectively. The prevalence of chlamydia was significantly higher among men in Port Moresby compared with men in Mt Hagen (29% v. 6%; P = 0.02). The prevalence of gonorrhoea was significantly higher among women in Port Moresby compared with women in Mt Hagen (49% v. 17%; P = 0.006).
There was a poor association between syndromic clinical diagnosis and laboratory findings, both for individual STIs and when STIs were grouped: chlamydia, gonorrhoea or T. vaginalis were not associated with either vaginal discharge syndrome in women (P = 0.110) or urethral discharge syndrome in men (P = 0.065), with similar findings observed for associations between HSV-2 or syphilis and genital ulcer syndrome in either men or women.
Cohort retention and STI prevalence
Of the 154 participants who attended a baseline clinical screening visit, 141 (92%) attended an enrolment visit 2–3 weeks later, after which reattendance for scheduled clinical follow-up was 87% (122 out of 141), 78% (110 out of 141) and 80% (113 out of 141) respectively at 12, 24 and 50 weeks, and was similar across sites (Table 2).
Reported condom use at last vaginal sex increased at scheduled follow-up visits compared with baseline in this cohort but remained around 20% at final visit (Table 2). Reported multiple sexual partnerships (≥2) in the previous week declined from 23% at baseline to 4% at 50 weeks.
The prevalences of syphilis, chlamydia, gonorrhoea and bacterial vaginosis were lower at 12, 24 and 50 weeks compared with baseline but remained elevated at 50 weeks (4%, 10%, 3% and 50% in Mt Hagen; 1%, 11%, 9% and 38% in Port Moresby, respectively). The prevalence of T. vaginalis declined in the first 24 weeks of follow-up at both sites but in Port Moresby, it was higher at 50 weeks compared with baseline (22% v. 13%).
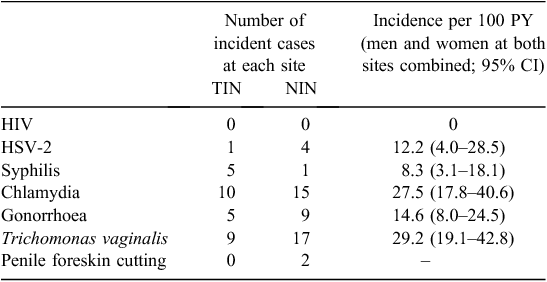
Incidence of HIV and STIs
The incidence of HSV-2 and other STIs was high in this cohort (Table 3). There were no statistically significant associations observed between baseline sociodemographic, behavioural or clinical characteristics with incident infection for any STI, or for all STIs combined (e.g. condom use at last vaginal sex and any incident infection, P = 0.17; self-reported sex worker and any incident infection, P = 0.33). Reinfections were also common among men and women in this cohort, with 18%, 12% and 13% of those diagnosed with chlamydia, gonorrhoea and T. vaginalis, respectively, at baseline subsequently being diagnosed with the same STI at some point during the clinical follow-up period (data not shown). No incident HIV infections were observed in the cohort.
|
Penile foreskin cuts
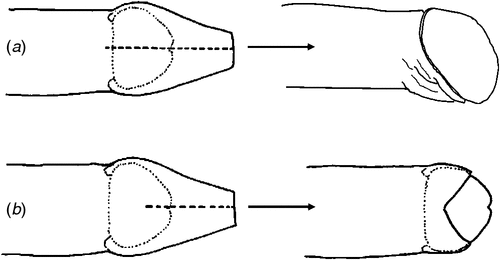
At baseline examination, 39% of male participants in Mt Hagen and 65% in Port Moresby had a penile foreskin cut (Table 1). The predominant type of foreskin cut at each site was the dorsal longitudinal slit or ‘straight cut’ (Fig. 1). Men with a foreskin cut in Mt Hagen were significantly younger than men without a cut (mean age = 28.0 years v. 35.0 years; P = 0.001). No difference in mean age was observed between cut and uncut men in Port Moresby (26.0 years v. 25.5 years respectively). There was no difference in sexual behavioural characteristics at baseline between men with and those without a cut (e.g. condom use at last vaginal sex, P = 0.9; multiple sexual partners, P = 0.9) and no significant differences in STI laboratory diagnoses at baseline between these groups (HIV, P = 1.0; HSV-2, P = 1.0; syphilis, P = 0.7; chlamydia, P = 0.4; gonorrhoea, P = 0.3; T. vaginalis, P = 1.0). Men with a cut were less likely to become infected with syphilis (relative risk: 0.1: 95% confidence interval: 0.01–1.84; P = 0.032) but were more likely to acquire chlamydia infection (relative risk: 3.4; 95% confidence interval: 1.03–11.1; P = 0.027) during the follow-up period. The risk of acquisition of other STIs was not associated with penile cutting. Two men reported undergoing penile foreskin cutting (both had a dorsal slit) during the follow-up period (Table 3).
|
Discussion
High prevalence and incidence rates of HIV, STIs and penile cutting were observed in this study, PNG’s first longitudinal clinical cohort study in sexual health. The prevalence of chlamydia, gonorrhoea and T. vaginalis observed at baseline is comparable to earlier clinic-based cross-sectional studies in PNG, summarised in a recent systematic review and meta-analysis conducted by our group.11 Our study is the first to estimate the incidence of HIV, HSV-2 and other STIs in any population in PNG, and provides estimates that are among the first of any Asia-Pacific country.
The high STI prevalences and incidence rates observed in our cohort are comparable to those reported among subpopulations at risk of HIV or STI infection in other Asia-Pacific countries. For example, among 10 198 VCT clinic attendees in Taiwan, the prevalence of HIV and syphilis was 3.5% and 2.2% respectively, and the incidence of HIV was 3.4 per 100 PY and that of syphilis was 1.6 per 100 PY among reattendees.28 In a study among 4762 MSM attending a community-based STI clinic in Thailand, the prevalence of HIV and syphilis was 28.3% and 9.8% respectively at first visit, and the incidence of HIV was 6.3 per 100 PY and that of syphilis 3.6 per 100 PY among reattendees.29 Male clients, MSM and those reporting one-night stands and casual sexual partnerships were more likely to reattend for VCT within 12 months, and those who used noninjection recreational drugs and who reported unprotected anal sex were at significantly higher risk of acquiring HIV infection and syphilis. These findings suggest that men and women attending sexual and reproductive health clinics in PNG and in other Asia-Pacific countries remain at increased risk of STI acquisition following initial presentation and clinical management, and that more should be done to mitigate risk in these subpopulations.
Low reported condom use and high STI cross-sectional prevalence, incidence rates and reinfection risk were observed in our cohort despite HIV and sexual risk reduction counselling, syndromic STI management with presumptive antibiotic treatment and sexual partner contact tracing at each scheduled clinical follow-up visit. Although we did not track partner contact treatment in this study, anecdotal evidence from discussion with clinic staff and study participants indicates that only very low success rates of partner contact tracing were achieved, suggesting this was a major contributing factor to the high reinfection risks observed in our cohort. Similar findings have been reported in other settings,28–30 underscoring the need for innovative, locally appropriate and evidence-based public health strategies for the effective control of HIV and STIs in all populations at increased risk of infection. Strategies to enable more effective partner contact tracing and treatment; to define the role and potential public health impact of periodic presumptive STI treatment and mass drug administration; and the acceptability, cost-effectiveness and impact of clinical management based on point-of-care STI diagnostics rather than syndromic STI management are urgently needed in PNG and other high-burden, resource-constrained settings.
The prevalence of penile foreskin cutting was high among men in this cohort, reflecting earlier research by our group and others in PNG.13,16,31 We have previously postulated13 that the dorsal longitudinal slit may confer some degree of protection against HIV acquisition in men in a similar way to full removal of the foreskin, because the lateral retraction and eversion of the foreskin that follows a full dorsal slit results in complete exposure of the glans and inner foreskin, and a final appearance that closely resembles medical circumcision (Fig. 1a). Our finding that men with a dorsal slit had a significantly lower incidence of syphilis compared with men without a foreskin cut is consistent with earlier research suggesting that male circumcision may confer protection against syphilis (as well as HSV-2 and chancroid) in men,32–35 but these associations are contentious.36 The present study was underpowered to detect a difference in prevalent or incident HIV infection among men with and without a foreskin cut. Ongoing clinical epidemiological and immunohistological research by our group is investigating the association and mechanisms by which penile foreskin cutting may protect men against HIV infection. This research will clarify the potential role that alternative forms of penile cutting may have in future culturally sensitive HIV prevention programs in PNG and other settings where such practices are common.
A limitation of our study is that it was not designed to provide data representative of the wider adult population in PNG and hence we have not attempted to extrapolate our findings more widely. The study was undertaken to establish the feasibility of conducting longitudinal clinical research in this challenging environment, and to investigate sexual risk among men and women in this study population, in order to inform future public health interventions and biomedical research in this setting.
High cohort retention was achieved at all follow-up visits in our study, despite this research being conducted during a period of intermittent ethnic tension, particularly at the Port Moresby site, where periodic clinic closures were necessary to ensure staff and client security.37 High retention in a population with high STI prevalence and incidence rates suggests that this may be suitable setting for future interventions research and clinical trials. Large-scale feasibility studies are warranted in order to confirm our findings and to inform the design of future field trials.
Conflicts of interest
None declared.
Acknowledgements
The authors thank the men and women who participated in this study for their valuable contribution to public health research in PNG. The authors also acknowledge the support of the PNG National Department of Health and the PNG National AIDS Council, without whom this study would not have been possible. This research was conducted as part of the Male Circumcision Acceptability and Impact Study PNG, which was funded by an Australian Development Research Award from the Australian Agency for International Development.
References
[1] Vallely A, Mola G, Kaldor JM. Human papillomavirus and cervical cancer in Papua New Guinea. P N G Med J 2013; In press.[2] World Health Organization (WHO). Investment case for eliminating mother-to-child transmission of syphilis: promoting better maternal and child health and stronger health systems. Geneva: WHO; 2012.
[3] Vallely A, Ryan C, Mola GDL, Kaldor JM. Human papillomavirus and cervical cancer in Papua New Guinea. Invited oral presentation No. 1150. International Union against Sexually Transmitted Infections World Congress, 15–17 October, 2012;Melbourne, Australia.
[4] Tabone T, Garland SM, Mola G, O’Connor M, Danielewski J, Tabrizi SN. Prevalence of human papillomavirus genotypes in women with cervical cancer in Papua New Guinea. Int J Gynaecol Obstet 2012; 117 30–2.
| Prevalence of human papillomavirus genotypes in women with cervical cancer in Papua New Guinea.Crossref | GoogleScholarGoogle Scholar | 22325971PubMed |
[5] National AIDS Council (NAC). Papua New Guinea HIV prevalence: 2010 estimates. Port Moresby: NAC; 2011.
[6] Joint United Nations Programme on HIV/AIDS (UNAIDS) and World Health Organization (WHO). Oceania: AIDS epidemic update. Regional summary. Geneva: UNAIDS/WHO;. 2008.
[7] National AIDS Council (NAC). The 2007 estimation report on the HIV epidemic in Papua New Guinea. Port Moresby: NAC; 2008.
[8] Garland SM, Brotherton JM, Skinner SR, Pitts M, Saville M, Mola G, et al Human papillomavirus and cervical cancer in Australasia and Oceania: risk-factors, epidemiology and prevention. Vaccine 2008; 26 M80–8.
| Human papillomavirus and cervical cancer in Australasia and Oceania: risk-factors, epidemiology and prevention.Crossref | GoogleScholarGoogle Scholar | 18945417PubMed |
[9] National AIDS Council (NAC). 2013 HIV/AIDS estimations and projections. Port Moresby: NAC; 2013.
[10] Kelly A, Kupul M, Man WYN, et al. Askim na save (ask and understand): People who sell and/or exchange sex in Port Moresby. Key quantitative findings. Sydney: Papua New Guinea Institute of Medical Research and University of New South Wales; 2011.
[11] Vallely A, Page A, Dias S, Siba P, Lupiwa T, Law G, et al The prevalence of sexually transmitted infections in Papua New Guinea: a systematic review and meta-analysis. PLoS ONE 2010; 5 e15586
| The prevalence of sexually transmitted infections in Papua New Guinea: a systematic review and meta-analysis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXjvVSksQ%3D%3D&md5=6a5e2f4d6b4a8a9e8323dc6d1f9d61feCAS | 21203468PubMed |
[12] Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011; 61 69–90.
| Global cancer statistics.Crossref | GoogleScholarGoogle Scholar | 21296855PubMed |
[13] Hill PS, Tynan A, Law G, Millan J, Browne K, Sauk J, et al A typology of penile cutting in Papua New Guinea: results of a modified Delphi study among sexual health specialists. AIDS Care 2012; 24 77–86.
| A typology of penile cutting in Papua New Guinea: results of a modified Delphi study among sexual health specialists.Crossref | GoogleScholarGoogle Scholar | 21756071PubMed |
[14] Kelly A, Kupul M, Aeno H, Shih P, Naketrumb R, Neo J, et al Why women object to male circumcision to prevent HIV in a moderate-prevalence setting. Qual Health Res 2013; 23 180–93.
| Why women object to male circumcision to prevent HIV in a moderate-prevalence setting.Crossref | GoogleScholarGoogle Scholar | 23188385PubMed |
[15] Kelly A, Kupul M, Fitzgerald L, Aeno H, Neo J, Naketrumb R, et al “Now we are in a different time; various bad diseases have come.” Understanding men’s acceptability of male circumcision for HIV prevention in a moderate prevalence setting. BMC Public Health 2012; 12 67
| “Now we are in a different time; various bad diseases have come.” Understanding men’s acceptability of male circumcision for HIV prevention in a moderate prevalence setting.Crossref | GoogleScholarGoogle Scholar | 22264256PubMed |
[16] Kelly A, Kupul M, Nake Trumb R, Aeno H, Neo J, Fitzgerald L, et al More than just a cut: a qualitative study of penile practices and their relationship to masculinity, sexuality and contagion and their implications for HIV prevention in Papua New Guinea. BMC Int Health Hum Rights 2012; 12 10
| More than just a cut: a qualitative study of penile practices and their relationship to masculinity, sexuality and contagion and their implications for HIV prevention in Papua New Guinea.Crossref | GoogleScholarGoogle Scholar | 22818494PubMed |
[17] Tynan A, Vallely A, Kelly A, Kupul M, Neo J, Naketrumb R, et al Socio-cultural and individual determinants for motivation of sexual health workers in Papua New Guinea and their implications for male circumcision as an HIV prevention strategy. Hum Resour Health 2013; 11 7
| Socio-cultural and individual determinants for motivation of sexual health workers in Papua New Guinea and their implications for male circumcision as an HIV prevention strategy.Crossref | GoogleScholarGoogle Scholar | 23418879PubMed |
[18] Tynan A, Vallely A, Kelly A, Law G, Millan J, Siba P, et al Vasectomy as a proxy: extrapolating health system lessons to male circumcision as an HIV prevention strategy in Papua New Guinea. BMC Health Serv Res 2012; 12 299
| Vasectomy as a proxy: extrapolating health system lessons to male circumcision as an HIV prevention strategy in Papua New Guinea.Crossref | GoogleScholarGoogle Scholar | 22943659PubMed |
[19] Vallely A, Fitzgerald L, Fiya V, Aeno H, Kelly A, Sauk J, et al Intravaginal practices and microbicide acceptability in Papua New Guinea: implications for HIV prevention in a moderate-prevalence setting. BMC Res Notes 2012; 5 613
| Intravaginal practices and microbicide acceptability in Papua New Guinea: implications for HIV prevention in a moderate-prevalence setting.Crossref | GoogleScholarGoogle Scholar | 23116431PubMed |
[20] Vallely A, MacLaren D, Kaleva W, et al Male circumcision for HIV prevention in Papua New Guinea: summary of research evidence and recommendations for public health policy following a national policy forum. P N G Med J 2013; In press
[21] Gray RT, Murray JM, Wilson DP, Vallely A, Kaldor J. The PNG HIV model – summary and results: explaining the past, describing the present, and forecasting the future of the HIV epidemic in PNG. Sydney: The Kirby Institute, The University of New South Wales; 2011.
[22] Wisniewski CA, White JA, Michel C-EC, Mahilum-Tapay L, Magbanua JPV, Nadala ECB, et al Optimal method of collection of first-void urine for diagnosis of Chlamydia trachomatis infection in men. J Clin Microbiol 2008; 46 1466–9.
| Optimal method of collection of first-void urine for diagnosis of Chlamydia trachomatis infection in men.Crossref | GoogleScholarGoogle Scholar | 18234860PubMed |
[23] Saiki RK, Gelfand DH, Stoffel S, Scharf SJ, Higuchi R, Horn GT, et al Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science 1988; 239 487–91.
| Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL1cXht1Ogt7k%3D&md5=30cb6b8a4628e9fd34597ed4adde4fefCAS | 2448875PubMed |
[24] Riley DE, Roberts MC, Takayama T, Krieger JN. Development of a polymerase chain reaction-based diagnosis of Trichomonas vaginalis. J Clin Microbiol 1992; 30 465–72.
| 1:CAS:528:DyaK38Xhs1Ogs78%3D&md5=1c0079f7885ddf87b570db13125ebe1aCAS | 1537918PubMed |
[25] Jalal H, Stephen H, Curran MD, Burton J, Bradley M, Carne C. Development and validation of a rotor-gene real-time PCR assay for detection, identification, and quantification of Chlamydia trachomatis in a single reaction. J Clin Microbiol 2006; 44 206–13.
| Development and validation of a rotor-gene real-time PCR assay for detection, identification, and quantification of Chlamydia trachomatis in a single reaction.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhtVymtL4%3D&md5=9af612a6b2fc3f1a34d9206687f8171eCAS | 16390971PubMed |
[26] Tabrizi SN, Chen S, Tapsall J, Garland SM. Evaluation of opa-based real-time PCR for detection of Neisseria gonorrhoeae. Sex Transm Dis 2005; 32 199–202.
| Evaluation of opa-based real-time PCR for detection of Neisseria gonorrhoeae.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhsF2ltrg%3D&md5=4d6dd55eef231ea7a42cf7dcfe8e77e0CAS | 15729160PubMed |
[27] Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol 1991; 29 297–301.
| 1:STN:280:DyaK3M7nvVSmsQ%3D%3D&md5=c16db37e5f75105fde388a4da8137ec2CAS | 1706728PubMed |
[28] Wu H, Wu PY, Li SY, et al Maximising the potential of voluntary counselling and testing for HIV: sexually transmitted infections and HIV epidemiology in a population testing for HIV and its implications for practice. Sex Transm Infect 2012; 88 612–6.
| Maximising the potential of voluntary counselling and testing for HIV: sexually transmitted infections and HIV epidemiology in a population testing for HIV and its implications for practice.Crossref | GoogleScholarGoogle Scholar | 22717470PubMed |
[29] Morbidity and Mortality Weekly Report. HIV and syphilis infection among men who have sex with men – Bangkok, Thailand, 2005–2011. MMWR Morb Mortal Wkly Rep 2013; 62 518–20.
| 23803960PubMed |
[30] Das A, Pathni AK, Narayanan P, George B, Morineau G, Saidel T, et al High rates of reinfection and incidence of bacterial sexually transmitted infections in a cohort of female sex workers from two Indian cities: need for different STI control strategies? Sex Transm Infect 2013; 89 5–10.
| High rates of reinfection and incidence of bacterial sexually transmitted infections in a cohort of female sex workers from two Indian cities: need for different STI control strategies?Crossref | GoogleScholarGoogle Scholar | 23196329PubMed |
[31] MacLaren D, Tommbe R, Mafile OT, Manineng C, Fregonese R, Redman-MacLaren M, et al Foreskin cutting beliefs and practices and the acceptability of male circumcision for HIV prevention in Papua New Guinea. BMC Public Health 2013; 13 818
| Foreskin cutting beliefs and practices and the acceptability of male circumcision for HIV prevention in Papua New Guinea.Crossref | GoogleScholarGoogle Scholar | 24015786PubMed |
[32] Mugo N, Dadabhai SS, Bunnell R, Williamson J, Bennett E, Baya I, et al Prevalence of herpes simplex virus type 2 infection, human immunodeficiency virus/herpes simplex virus type 2 coinfection, and associated risk factors in a national, population-based survey in Kenya. Sex Transm Dis 2011; 38 1059–66.
| Prevalence of herpes simplex virus type 2 infection, human immunodeficiency virus/herpes simplex virus type 2 coinfection, and associated risk factors in a national, population-based survey in Kenya.Crossref | GoogleScholarGoogle Scholar | 21992985PubMed |
[33] Tobian AA, Charvat B, Ssempijja V, Kigozi G, Serwadda D, Makumbi F, et al Factors associated with the prevalence and incidence of herpes simplex virus type 2 infection among men in Rakai, Uganda. J Infect Dis 2009; 199 945–9.
| Factors associated with the prevalence and incidence of herpes simplex virus type 2 infection among men in Rakai, Uganda.Crossref | GoogleScholarGoogle Scholar | 19220138PubMed |
[34] Tobian AA, Kigozi G, Gravitt PE, Xiao C, Serwadda D, Eaton K, et al Human papillomavirus incidence and clearance among HIV-positive and HIV-negative men in sub-Saharan Africa. AIDS 2012; 26 1555–65.
| Human papillomavirus incidence and clearance among HIV-positive and HIV-negative men in sub-Saharan Africa.Crossref | GoogleScholarGoogle Scholar | 22441255PubMed |
[35] Weiss HA. Male circumcision as a preventive measure against HIV and other sexually transmitted diseases. Curr Opin Infect Dis 2007; 20 66–72.
| Male circumcision as a preventive measure against HIV and other sexually transmitted diseases.Crossref | GoogleScholarGoogle Scholar | 17197884PubMed |
[36] Van Howe RS. Sexually transmitted infections and male circumcision: a systematic review and meta-analysis. ISRN Urol 2013; 2013 109846
| 23710368PubMed |
[37] Fox L. Three dead after violent PNG riot. Sydney: ABC News; 2011. Available online at: http://www.abc.net.au/news/2011-08-21/three-dead-in-png-riot/2849024 [verified February 2014].




