Extrafloral nectaries in Leguminosae: phylogenetic distribution, morphological diversity and evolution
Brigitte Marazzi A F , Ana Maria Gonzalez B , Alfonso Delgado-Salinas C , Melissa A. Luckow D , Jens J. Ringelberg E and Colin E. Hughes E
A F , Ana Maria Gonzalez B , Alfonso Delgado-Salinas C , Melissa A. Luckow D , Jens J. Ringelberg E and Colin E. Hughes E
A Natural History Museum of Canton Ticino, Viale C. Cattaneo 4, 6900 Lugano, Switzerland.
B Instituto de Botánica del Nordeste (UNNE-CONICET), Sargento Cabral 2131, 3400 Corrientes, Argentina.
C Universidad Nacional Autónoma de México, Instituto de Biología, Departamento de Botánica, Apartado Postal 70-233, 04510 Ciudad de México, Mexico.
D Department of Plant Biology, Cornell University, Ithaca, NY 14853, USA.
E Department of Systematic and Evolutionary Botany, University of Zurich, Zollikerstrasse 107, CH-8008 Zurich, Switzerland.
F Corresponding author. Email: marazzibrigitte@gmail.com
Australian Systematic Botany 32(6) 409-458 https://doi.org/10.1071/SB19012
Submitted: 1 February 2019 Accepted: 28 May 2019 Published: 30 September 2019
Journal Compilation © CSIRO 2019 Open Access CC BY-NC-ND
Abstract
Extrafloral nectaries (EFNs) mediating ecologically important ant–plant protection mutualisms are especially common and unusually diverse in the Leguminosae. We present the first comprehensively curated list of legume genera with EFNs, detailing and illustrating their systematic and phylogenetic distributions, locations on the plant, morphology and anatomy, on the basis of a unified classification of EFN categories and a time-calibrated phylogeny, incorporating 710 of the 768 genera. This new synthesis, the first since Mckey (1989)’s seminal paper, increases the number of genera with EFNs to 153 (20% of legumes), distributed across subfamilies Cercidoideae (1), Detarioideae (19), Caesalpinioideae (87) and Papilionoideae (46). EFNs occur at nine locations, and are most prevalent on vegetative plant parts, especially leaves (74%) and inflorescence axes (26%). Four main categories (with eight subcategories) are recognised and include the following: formless, trichomatic (exposed, hollow), parenchymatic (embedded, pit, flat, elevated) and abscission zone EFNs (non-differentiated, swollen scars). Phylogenetic reconstruction of EFNs suggests independent evolutionary trajectories of different EFN types, with elevated EFNs restricted almost exclusively to Caesalpinioideae (where they underwent spectacular morphological disparification), flat EFNs in Detarioideae, swollen scar EFNs in Papilionoideae, and Cercidoideae is the only subfamily bearing intrastipular EFNs. We discuss the complex evolutionary history of EFNs and highlight future research directions.
Additional keywords: ant–plant interactions, extranuptial nectaries, Fabaceae, mutualism, nectar, plant defense, legume phylogeny.
Introduction
Extrafloral nectaries (EFNs) mediate the most widespread and ecologically important indirect plant mutualistic defence mechanism against herbivores (Heil 2015). EFNs secrete a carbohydrate-rich nectar reward to attract especially ants, but also other aggressive arthropods, and exploit and rely on them as ‘bodyguards’ (Bentley 1977; Kessler and Heil 2011). These ecologically important ant–plant mutualisms involving EFNs have evolved many times independently in over 100 angiosperm families and some ferns (Weber and Keeler 2013) and are hypothesised to have spurred plant diversification (Weber and Agrawal 2014). EFNs are particularly common in the legume family, Leguminosae (=Fabaceae), the third largest angiosperm family, which is well known for its rich diversity of interactions with ants (Mckey 1989), including textbook examples such as ant ‘acacias’ and their obligate aggressive ant mutualists, which are critical to enhance plant competitive ability and survival (Janzen 1966).
Extrafloral nectaries in legumes have been known since even before the term ‘extrafloral’ nectary had been coined, with reports of nectar-secreting glandulae (lit. glands) on leaves in the genera, Bauhinia, Cassia s.l., and Mimosa (Hall 17621). Yet, it took nearly 200 years to gradually assemble a more complete understanding of EFNs in this family, culminating in Mckey’s (1989) seminal paper, which provided the most recent comprehensive review of EFNs and their associated interactions in legumes. Since then, there has been much renewed interest in exploring aspects of EFN diversity, ecology and evolution in and beyond legumes (see Marazzi et al. 2013a) and, equally importantly, the taxonomy of the family has undergone substantial realignments in terms of generic delimitation, and the tribal and subfamily classification, largely as a result of insights from molecular phylogenies (see Lewis et al. 2005; Legume Phylogeny Working Group 2013, 2017). Recent collaborative work by the Legume Phylogeny Working Group (LPWG) resulted in a community-endorsed revised subfamily classification of the family, recognising the following six robustly supported monophyletic subfamilies (Legume Phylogeny Working Group 2017): (1) the re-circumscribed Caesalpinioideae DC. (including the former subfamily Mimosoideae now referred to informally as the mimosoid clade), (2) Cercidoideae LPWG (revised circumscription), (3) Detarioideae Burmeist., (4) Dialioideae LPWG (revised circumscription), (5) Duparquetioideae LPWG (revised circumscription) and (6) Papilionoideae DC.
Here, we provide a comprehensively updated synthesis of EFNs in Leguminosae, investigating their systematic distribution and the diversity of their locations, morphology and anatomy. We document the occurrence and diversity of EFNs at the generic level, including numerous new generic records of EFN occurrence, and present a comprehensive phylogenetic overview of EFNs across the family, placed within the context of the new subfamily classification and updated generic delimitation based on Legume Phylogeny Working Group (2017).
The term EFN is here used in the broad sense of Elias and Gelband (1976), i.e. including nectaries on floral parts that do not participate in pollination. In a study drawing on all available literature on EFNs, it is important to define the scope of the structure encompassed by the term ‘nectary’. Schmid (1988, p. 187) defined the nectary as ‘a more or less localized, multicellular glandular structure that occurs on vegetative or reproductive organs and that regularly secretes nectar, a sweet solution containing mainly sugars and generally serving as a reward for pollinators or for protectors (e.g., ants) against herbivores, or, in carnivorous plants, as a lure for animal prey’. Mckey (1989) proposed that, for a secretory structure to be considered an EFN, it must meet one or more of the following three criteria: (i) nectar secretion documented, (ii) ant visitation and (iii) homology apparent with nectary glands in related genera. We suggest adding a fourth criterion, as defined by Schmid (1988), namely, that EFNs are localised structures that never extend over the entire leaf or foliar organs (as can be the case for glandular trichomes).
Materials and methods
We compiled a list of legume genera possessing EFNs (Table 1) on the basis of literature reports, herbarium specimens and observations of cultivated and wild plants by the authors or, in some cases, other researchers, during the past 10 years. We have attempted to confirm all published records of EFNs by using also published images, scientific illustrations, and reliably identified and high-quality photographs supplied by colleagues, in addition to herbarium specimens and living plants. Whenever possible, original field observations were documented with colour photographs and verified by presence of a nectar droplet, foraging ants or both. For each genus, we note the location(s) of EFNs in terms of the main plant organ bearing the secretory structure (e.g. stipules, leaves, inflorescences) and provide a description, with further details, on the position and morphology. We do not review reports of secretion of floral nectaries that continue to secrete post-anthesis and, thus, attract ants during fruit development, despite the similar ecological role of such post-floral nectar secretion to that of EFNs. Separately, we list taxa in which EFNs are explicitly reported to be absent, and taxa in which their presence is considered doubtful, where descriptions are inconclusive or reports are contradictory. We compared our total number of EFN-bearing genera with totals listed in previous accounts.

|
We follow the subfamily classification of Legume Phylogeny Working Group (2017), and lists of accepted genera of Lewis et al. (2005) and updates in Lewis et al. (2013) and Legume Phylogeny Working Group (2017), which already encompass the revised generic systems of Vigna by Delgado-Salinas et al. (2011), Paloue by Redden et al. (2018), and the Caesalpinia group by Gagnon et al. (2015, 2016). New genera published after Legume Phylogeny Working Group (2017), such as Parasenegalia and Pseudosenegalia (Seigler et al. 2017) and Lachesiodendron (Ribeiro et al. 2018), were also included. In several cases, known issues of non-monophyly of genera were taken into account when assessing occurrence of EFNs (e.g. Prosopis; see Table 1). For the Bauhinia alliance, we discuss the occurrence of EFNs in relation to the forthcoming phylogenetic analysis (C. Sinou and A. Bruneau, unpubl. data). We follow the new tribal classification of Detarioideae (de la Estrella et al. 2018), whereas we omitted tribes in Caesalpinioideae because of the limitations and rampant non-monophyly of the current tribes (Legume Phylogeny Working Group 2013), but instead indicated robustly supported clades where necessary (e.g. the mimosoid clade; Legume Phylogeny Working Group 2017). Tribes of Papilionoideae follow Lewis et al. (2005).
To assemble an account of the main morphological and anatomical categories of EFNs, 32 species from 18 genera (including new reports) were studied by scanning electron microscopy (SEM) and light microscopy of histological sections. A complete list of the species studied is found in ‘Specimens studied and voucher information’ section in the Supplementary material to this paper. Plant material for SEM and light microscopy analyses was fixed in formaldehyde–acetic acid–70% ethanol (FAA, 5 : 5 : 90), dehydrated and embedded in paraffin (Johansen 1940). Transverse and longitudinal serial sections between 5–7 and 10 μm thick were cut with a rotary microtome (Microm, Walldorf, Germany). Histological sections were stained with two different combinations, namely, safranin–astra blue (Luque et al. 1996) or ruthenium red–toluidine blue (Weber and Igersheim 1994). For SEM, samples were prepared following standard procedures of dehydration through a graded acetone series, following critical-point drying using liquid CO2 and sputter-coating with gold or palladium. Gold-sputtered samples were examined and photographed with a Jeol LV 5800 SEM (20 kV), at the Electron Microscopy Service of the Universidad Nacional del Nordeste (Corrientes, Argentina), and with a Hitachi SU510 SEM (15 kV) at the Laboratorio de Microscopía y Fotografía de la Diversidad, Instituto de Biología, Universidad Nacional Autónoma de México (Mexico); palladium-sputtered samples were examined and photographed with a Hitachi S-3400 N Type II VPSEM (15 kV) at the University Spectroscopy and Imaging Facilities of the University of Arizona (Arizona, USA).
To illustrate the phylogenetic distribution of EFNs, we mapped the presence of the main categories onto a time-calibrated ultrametric consensus tree derived from the Legume Phylogeny Working Group (2017) matK phylogeny, which includes 710 of the 768 genera, coding genera by category of EFN (and subcategories of parenchymatic EFNs), and, in cases where genera are known to include more than one EFN state, then coding species where known. All optimisations and visualisations were performed in R (ver. 3.5.1, R Foundation for Statistical Computing, Vienna, Austria, see http://www.R-project.org/), using a preliminary time-calibrated version of the Legume Phylogeny Working Group (2017) phylogeny that was inferred with treePL (Smith and O’Meara 2012), using nine fossil calibrations to calibrate internal nodes and 15 secondary calibrations that were derived from a chloroplast exome analysis (E. J. Koenen, unpubl. data) to calibrate the deeper nodes in the family. This chronogram is a preliminary version from a study of macro-evolutionary dynamics in legumes (E. J. Koenen, unpubl. data). Ancestral EFN states were obtained by stochastic character mapping using the make.simmap function of the phytools package (Revell 2012), with an equal-rates model and 200 simulations. Figures were made using various functions of the phytools, ape (Paradis and Schliep 2019) and plotrix (Lemon 2006) packages.
Results and discussion
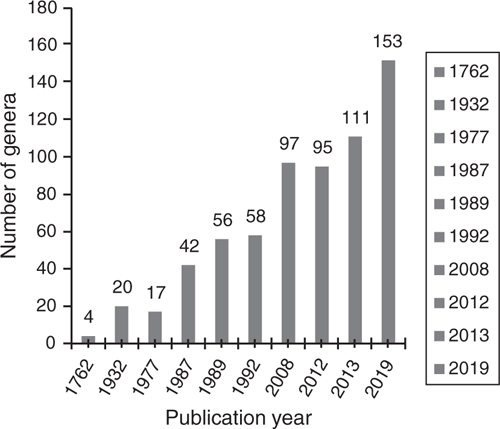
Ever since the first reports of nectar-producing ‘glandulae’ (EFNs) in legumes by Hall (1762), the number of legume genera documented as possessing EFNs has steadily increased (Fig. 1). Here, we present the first comprehensively curated list of legume genera with EFNs, detailing their systematic and phylogenetic distribution (Tables 1, 2, Fig. 2), locations on the plant (Table 3, Fig. 3), morphology and anatomy (including proposing a revised and unified classification of EFN categories; Table 4, Fig. 4–7), all of this amply illustrated with 111 images covering taxa in all four legume subfamilies with EFNs (Fig. 8–15). We confirm the presence of EFNs in 153 genera, i.e. 20% of total legume genera, distributed across the following four subfamilies (Tables 1, 2), in a systematic order: Cercidoideae (1 genus), Detarioideae (19 genera), Caesalpinioideae (87) and Papilionoideae (46). Of the six subfamilies, Caesalpinioideae has the highest proportion of genera with EFNs, with ~60%, followed by Detarioideae and Papilionoideae, with 23 and 9% respectively (Table 2). We could find no reports or evidence for EFNs in subfamilies Dialioideae and Duparquetioideae. Reports from 43 genera remain unclear or lack sufficient reliable detail to confirm the mentioned structures as EFNs (Table S1, available as Supplementary material to this paper), in line with doubts expressed by previous authors (e.g. Mckey 1989; Koptur 1992a). Although, we found explicit reports of absence of EFNs for 36 genera (Table S2 in the Supplementary material), it was beyond the scope of this study to verify these. In the following sections, we present an overview of the diversity of locations, morphology and anatomy of EFNs, discuss this diversity and relevant ecological information for each subfamily and, finally, outline preliminary ideas on the evolutionary history of EFNs within Leguminosae and future research priorities.
|

|

|

|

|

|
Diversity in location, morphology and anatomy
Leguminosae is one of the most, if not the most, diverse plant family in terms of the location of EFNs on a plant, i.e. the organs that bear EFNs, and in terms of EFN morphology and anatomy. Although here presented separately, EFN location, morphology and anatomy are closely connected, because the development, ecology and evolution of EFNs ultimately depend, at least to some degree, on that of its bearing organ (Marazzi et al. 2013a; see also below, section ‘Legume EFNs: a phylogenetic and evolutionary perspective’).
Location of EFNs
The locations of nectaries on plants have long been used to distinguish extrafloral from floral nectaries (Caspary 1848), well before their functional significance and very different ecological roles were recognised (Delpino 1868, 1869, 1870, 1873, 1874)2. EFN locations can be divided into vegetative and reproductive (i.e. extra-reproductive v. reproductive sensu Schmid 1988), given their different protective functions for developing shoots and leaves v. developing flowers (buds) and fruits respectively. In Leguminosae, we identify the following nine different EFN locations (Table 3, Fig. 3): (1) shoots and stems, (2) stipules, (3) between stipules (intrastipular), (4) leaves, (5) inflorescence axes, (6) pedicels, (7) bracts, (8) bracteoles and (9) sepals. Furthermore, the exact position can vary on single organs; for example, stipule EFNs can occur on the lobe or on the abaxial lamina side, or in bracts, they can be on the bract petiole or on the dorsal lamina side. Variation is particularly outstanding in leaves of legumes, because EFNs can occur on many different parts of the compound leaves (pinnate or bipinnate), including (1) the petiole (Fig. 10D–E, P), (2) along the primary and secondary (for bipinnate leaves) rachises between the pairs of leaflets or pinnae (Fig. 10F), including at the apex of the rachis (Fig. 10D), and (3) on the mucro (apparently only in Senna scabriuscula), together with a petiole EFN (Fig. 10N), (4) on stipels (usually the abaxial side), (5) on the leaflet lamina (usually the abaxial side, Fig. 9A–D) and (6) on leaflet margins (Fig. 9E, J, K). Schmid (1988)’s topographical classification of nectaries lacks some of these leaf locations, probably because he only considered simple leaves. EFNs located on the abscission zones of abortive buds or caducous bracts are here referred to inflorescence axes because they are more accurate than locating them on the abscised organs themselves. These assignments of EFNs to specific plant locations allow us to identify patterns in the frequencies of EFNs across different plant parts.
Extrafloral nectaries are not equally distributed across locations. In the majority of genera (nearly 80%), EFNs apparently are found at a single location, and, more generally, on vegetative parts (64%) only, whereas fewer bear them on reproductive parts only or on both parts (20.9 and 14.4% respectively; Table 3). However, it is possible that EFNs in a few genera (especially Caesalpinioideae) also develop on leaves that subtend inflorescences but these have been reported simply as leaves, instead of inflorescence bracts. The 27 genera with EFNs at multiple locations are scattered across legumes, but the caesalpinioid genus Senna is outstanding, with EFNs at up to five different locations across the genus as a whole, although individual plants bear EFNs in up to two to three locations at most (depending on the species and clade; Marazzi et al. 2013b). Beyond these trends, specific locations of EFNs are, in general, associated with particular clades; intrastipular EFNs are characteristic of subfamily Cercidoideae (Bauhinia), leaflet-lamina and leaflet-margin EFNs occur exclusively in subfamily Detarioideae, EFNs on inflorescence axes and stipels are, with a few exceptions, restricted to subclades of Papilionoideae, whereas EFNs on the petiole and the leaf rachis are prevalent in Caesalpinioideae. However, patterns in the evolution of EFN diversity can be correctly interpreted only when considering location alongside morphology and anatomy.
Morphological and anatomical categories and terminology of EFNs
Review of the legume anatomical literature shows a broad range of terms used to describe and categorise EFNs in different studies and different taxonomic groups. Given this terminological disparity, we propose a revised classification of EFN types and a unified and standardised terminology for EFNs that builds on the two most widely used existing classifications of Zimmermann (1932) and Elias (1983). One previous study attempting a classification was by Ono (1907).
Extrafloral nectaries can be fundamentally divided into two main groups, namely, non-structural and structural. The first represents the gestaltlose Nektarien of Zimmermann (1932), equivalent to the ‘formless’ EFNs of Elias (1983). These lack any specifically differentiated nectariferous structure, meaning that nectar is secreted from tissues without any structural specialisation at the tissue or organ level (Wettstein 1889; Zimmermann 1932; Elias 1983; Bernardello 2007).
Formless EFNs are one of the most overlooked types of EFN in angiosperms (Bernardello 2007), including legumes, because they can be detected and located only on the basis of observations of the presence of droplets of nectar or presence of ants. For instance, in this study, we confirmed the occurrence of this EFN category only in the caesalpinioid genus Senna (Table 1, Fig. 4A–C), on the basis of studies by Marazzi et al. (2013b) who observed nectar secretion on the dorsal surfaces of bracts and sepals in species of Senna Clade II; however, detailed anatomical studies did not find any nectariferous structure or tissue associated with the nectar secretion (data not shown).
In structural EFNs, the nectary is recognised by the presence of specialised nectar-producing and -secreting tissues. In general, nectaries (both floral and extrafloral) conform to the structure proposed by Nepi (2007), comprising a nectary epidermis (that mediates nectar release to the outside), nectary parenchyma (directly involved in nectar production and secretion) and subnectary parenchyma (tissue related to nectar production). Structural EFNs may or may not be vascularised, and the vascular supply can be specific to the nectary itself or derived from that of the nearest vascular system. Several distinct types of structural EFNs can be recognised.
First, nectar producing multicellular glandular trichomes are here designated as trichomatic EFNs. In most cases, such trichomes are aggregated, forming a secretory unit that is visible as an EFN. Zimmermann (1932) and Elias (1983) did not recognise this category, whereas Vogel (1977) did (calling it’trichomatous’ EFNs). Trichomatic EFNs are found in at least six legume genera (Table 4). These trichomatic EFNs can be divided into two subcategories, namely, exposed (simply lined on the organ surface) and hollow (sunken into a depression or cavity), the latter corresponding to the ‘Hohlnektarien’ of Zimmermann (1932), and equivalent to the ‘hollow’ nectaries of Elias (1983). For the few legume genera with trichomatic EFNs, most have exposed trichomatic EFNs (Lablab, Mucuna, Phaseolus, Vicia and Vigna; Table 1, Fig. 4E–H, 15M, N). These comprise nectariferous patches of densely packed clavate trichomes on the abaxial side of auriculate stipules, stipels and bracteoles, forming an irregular or triangular to circular surface (1–3 mm in diameter in the species studied) lacking stomata. However, in at least one species of Mucuna, the trichomes are sparsely distributed on the filiform stipels (Lersten and Brubaker 1987). According to Ono (1907), Vicia was the first genus to be studied anatomically (Fuckel 1846). Hollow trichomatic EFNs have been found within legumes, only in the genus Erythrina, on stipels and calyx lobes (Table 1, Fig. 4I–O). In this case, the trichomes themselves are barely visible externally, the stipels and calyx lobes appear swollen, and are structurally modified by the EFN (Fig. 15A, B).
The majority of structural EFNs are parenchymatic, meaning that they are characterised by the presence of secretory tissue formed by small, densely packed thin-walled cells, with a dense and glandular cytoplasm. In these EFNs, the epidermis is the tissue through which nectar exudes; therefore, it is of glandular nature and lacks stomata or secretory trichomes. Parenchymatic EFNs encompass the remaining five types of Elias (1983, based on Zimmermann 1932), namely, ‘embedded’, ‘pit’, ‘flattened’, ‘elevated’ and ‘scale-like’. We follow this classification, modifying it only slightly, and found all of these types of parenchymatic EFNs in legumes (Table 4), except for scale-like EFNs, which are not known to occur.
In embedded EFNs, the secretory cells are completely embedded in the tissue of the organ bearing the EFN, with a minimal area reaching the surface to release nectar. Embedded EFNs occur in only three legume genera (Table 4), namely, Bauhinia (Cercidoideae) and two Detarioideae, Leonardoxa and Plagiosiphon. In Bauhinia, they are intrastipular secretory structures or secretory prickles (Fig. 5A–D). Anatomically, the secretory tissue is inside the prickle, between the parenchyma and the cortex, and extends to the prickle epidermis, and, in this way, the EFNs appear like one-sided spindles (Gonzalez and Marazzi 2018). In the two detarioids, the EFNs are on the abaxial side of the leaflet lamina, embedded in the mesophyll (Fig. 5E, F; Elias 1980; Mckey 1989).
Pit EFNs are sunken in the tissues of other organs, and the ‘depressions in which they lie are usually steep-sided and have a diameter that equals or exceeds that of the nectaries’ (Elias 1983, p. 177). In Leguminosae, pit EFNs occur apparently in only one genus, the mimosoid Entada (Table 1; reported as such by Blüthgen and Reifenrath 2003; Fig. 5G). Entada pit EFNs lack glandular trichomes. However, it should be noted that many of the pit nectaries mentioned by Zimmermann (1932) as ‘Grubennektarien’ do have trichomes; for clarity, we suggest restricting the use of the ‘pit’ EFN category to those nectar-secreting cavities lacking trichomes.
Flat EFNs correspond to Elias’ ‘flattened’ EFNs (‘Flachnektarien’ of Zimmermann 1932). Here, it is important to bear in mind that ‘flat’ should not be confused with ‘flattened’, which is widely used to describe EFN shapes of, for example, elevated nectaries. The secretory surface of flat EFNs is at the level of the organ-bearing tissue (Fig. 5J–O). In legumes, flat EFNs occur in 19 genera (Table 4), on stipule lobes where they are visible as a coloured area (Senna, Fig. 10K), on leaves (Acacia s.str.), on twig junctions and bracts (Entada) or bracts only (Alexa), scattered over the stem, rachis and leaflets (Castanospermum; Table 1), and on the abaxial surfaces of leaflets (14 genera of detarioids), where they are visible as tiny circular structures with a flattened, slightly concave or convex surface, generally no larger than 1 mm in diameter (Fig. 9). Hawthorne and Jongkind (2006) referred to these detarioid flat EFNs as ‘knotted vein glands’, probably because the leaflet secondary veins are often radially arrayed around the EFN (but separated from the nectary parenchyma), a pattern described as stellate by de la Estrella et al. (2012). These flat detarioid EFNs are within the foliar mesophyll, with the nectary parenchyma being located between the vascular tissues and the epidermis, and the secretory surface of the EFN is formed by an epidermis and nectar accumulates under the cuticle (Paiva and Machado 2006; Melo et al. 2010b; Chanam et al. 2015). They can also be noticed as a bulge on the adaxial leaflet surface.
Elevated EFNs (or ‘Hochnektarien’, Zimmermann 1932) are more or less protruding from the organ that bears them (Fig. 6, 11–13). They are the most common category of EFN in legumes, present in 85 genera (55% of all EFN-bearing genera), of which only four are outside the Caesalpinioideae (Table 4). These EFNs are most often associated with leaves, where they occur at many different positions on the petiole, leaf rachis and secondary rachises of bipinnate leaves (see also Table 3). They are larger in size than all other categories of EFN and usually easily visible on herbarium material; hence, they have often been used as taxonomic characters. They are also extremely diverse in shape, being sessile, cupuliform or stalked, elongated or rounded, their surfaces concave or convex (Fig. 6A–J). Their anatomical organisation (shared with flat EFNs) is more constant, comprising a secretory epidermis, nectary parenchyma and subnectary parenchyma that is often served by vascular bundles derived from the nearest vascular system (Fig. 6K–O). In the elevated EFNs of Senna, there is an additional layer of meristematic cells between the nectary and the subnectary parenchyma (Marazzi et al. 2013b; Gonzalez and Marazzi 2018). EFNs in the detarioid genera Gilbertiodendron and Copaifera are slightly elevated, located on leaflet margins, and are reminiscent of (and possibly a transitional form to) the flat EFNs typical of Detarioideae, but they are protruding from the leaflet margins (as opposed to the lamina where most detarioid EFNs are found), and are hence best categorised as elevated EFNs.
The last category, here designated as abscission-zone EFNs, are modifications of the insertion region of organs, mainly in the inflorescence, such as pedicels and bracts, but also of stipules. Two subcategories can be recognised, namely, non-differentiated abscission zones (found only in 5 caesalpinioid genera) and differentiated swollen scars (in 36 papilionoid genera, restricted to tribe Phaseoleae; Tables 1, 3, Fig. 7). In most non-differentiated abscission-zone EFNs, the flowers develop normally but the subtending bract may fall early and nectar is released through the groove that marks the separation zone, delimiting the abscission zone between the pedicel and the inflorescence axis (in Caesalpinia s.str. (Fig. 10B, C), Gelrebia, Libidibia, and Senna (Fig. 10J)). Such a nectary can also occur in the abscission zone of stipules (in Caesalpinia s.str. and Pseudoprosopis). These EFNs lack differentiated nectariferous tissue, but because nectar release is associated with an abscission zone, we do not consider them to be formless EFNs. Like formless EFNs, they are also probably largely overlooked, because only the presence of nectar or ants allows their detection. The second subcategory of abscission-zone EFNs, swollen-scar EFNs, are always associated with abortion of flower buds (Fig. 7, 14). After flower abortion, instead of simply forming a typical abscission-scar zone, a complex, volcano-shaped structure develops consisting of a central depressed area, with a mix of secretory cells and the pre-existing vascular supply, and a marginal swollen area, lacking obvious secretory features (Fig. 7). For a detailed discussion of this type of nectary, see Gonzalez and Marazzi (2018, and references therein).
Finally, reviewing the legume anatomical literature also shows a miscellany of other secretory structures that have been reported as EFNs, possibly because they were called ‘glands’ (see Tables S1, S2, available as Supplementary material to this paper), but which do not fulfill the required criteria for an EFN. ‘Gland’ is a very general term, used to indicate any secretory structure, and has been used to refer to both true EFNs, but also to other secretory structures, notably glandular trichomes (non-nectar secreting or of unknown secretion) and colleters (i.e. secretory emergences producing sticky mixtures of mucilage and terpenes). For example, the presence of glandular trichomes distributed throughout the plant and erroneously interpreted as EFNs were described in Rhynchosia minima, Papilionoideae (Bhattacharyya and Maheshwari 1970; Khan et al. 2017), and Poincianella bracteosa (now Cenostigma bracteosum, see Gagnon et al. 2016), Caesalpinioideae (Melo et al. 2010a, 2010b). The capitate trichomes in the caesalpinioid genus Hoffmannseggia and in Erythrostemon gillesii were described as ‘glands’ and considered as EFNs by authorities like Delpino (1886, 1887, 1889) and Zimmermann (1932), but we consider these reports to be doubtfully true EFNs in line with Mckey (1989). More examples of this sort of confusion with other secretory structures are elaborated in the following sections on each subfamily.
Cercidoideae
Of the 12 genera in subfamily Cercidoideae (formerly placed in the more broadly circumscribed Caesalpinioideae, Legume Phylogeny Working Group 2017), only Bauhinia appears to include species with EFNs (Table 1, Fig. 8). This suggests an independent origin of EFNs within this subfamily that is phylogenetically isolated with respect to the other EFN-bearing lineages (Fig. 2). Known for its taxonomic complexity, Bauhinia is currently undergoing a comprehensive systematic revision, with papers on Phanera Lour. and Schnella Raddi providing necessary combinations in those segregate genera (e.g. Wunderlin 2010; Bandyopadhyay et al. 2012; MacKinder and Clark 2014; Trethowan et al. 2015). On the basis of the forthcoming new generic classification of this group (C. Sinou, W. Cardinal-McTeague and A. Bruneau, unpubl. data), all species with EFNs in subfamily Cercidoideae belong to Bauhinia s.str. The presence of EFNs in Gigasiphon, reported to occur on the sepals as an ‘apical nectary’ (Wunderlin 2010), remains to be confirmed (Table S1).
In Bauhinia, all EFNs fall into the embedded subcategory of parenchymatic EFNs and are primarily located around vegetative nodes (i.e. around the insertion of leaves, prickles and other intrastipular structures and stipules on the shoot axes). Only in one species (B. variegata, Fig. 8G, H), EFNs occur on the inflorescences, where a nectar droplet accumulates laterally to the bracteoles, but the secretory unit remains unknown. An isolated report of EFNs ‘on rachis of compound leaf’ in B. pauletia (Baker et al. 1978, table 1) is unusual and remains to be confirmed.
Intrastipular embedded EFNs have long proven to be useful taxonomically in the American members of Bauhinia to differentiate taxa with EFNs from those taxa lacking EFNs, but bearing prickles instead (Bentham 1870). However, this distinction has turned out to be misplaced. Gonzalez and Marazzi (2018), who studied B. forficata subsp. pruinosa, found that the EFN tissue is completely embedded in the young prickle, which itself is secretory (Fig. 5A, C, 8C, D). In this case, the surface of the prickle is smooth and lacks pores or stomata for nectar release; instead, the cuticle is significantly thinner in the secretory area (Gonzalez and Marazzi 2018). Prickles of B. forficata are not modified stipules and represent the first documented case of secretory prickles in legumes. All other intrastipular nectaries described so far in Bauhinia appear to be homologous with these secretory prickles, including those that characterise species of the traditionally recognised section Pauletia (da Fonseca Vaz and Tozzi 2005). They have been described as ‘intrastipular secretory trichomes’ (Oliveira and Freitas 2004, fig. 1B), or ‘calyciform and elevated’ EFNs (Rezende et al. 1994; Melo et al. 2010a, 2010b).
The EFNs in subfamily Cercidoideae remain the least well understood in the Leguminosae. Although EFNs appear to be limited to the Bauhinia s.str. clade, they may have been overlooked in the genera segregated from Bauhinia s.l., and might, therefore, be more widespread in Cercidoideae than is currently documented. As noted also by Gonzalez and Marazzi (2018), thorough field-based phenological and anatomical investigations are needed to show other cryptic EFNs that are visible only during particular plant ontogenetic or phenological stages. For instance, because they are rather cryptic, the apparently vestigial EFNs reported in some species of Bauhinia, such as B. cheilantha (Melo et al. 2010b), might, in fact, secrete nectar at other stages or under different conditions from those observed by these authors. In addition, so as to verify the occurrence of EFNs in unstudied taxa, comparative anatomical studies are necessary to assess organ identity and, hence, the homology of the intrastipular embedded EFNs, as well as to show the structure of the unknown inflorescence EFNs. Furthermore, ant–EFN interactions remain unstudied in Bauhinia and, to our knowledge, no ecological studies involving EFNs have been published so far for this genus. Filling all these gaps will be crucial, so as to infer an accurate picture of the occurrence of EFNs in this subfamily and to understand their evolutionary history and significance in relation to other types of EFNs found elsewhere in the family.
Detarioideae
Of the 84 genera in subfamily Detarioideae (like Cercidoideae, this subfamily was also formerly placed in the more broadly circumscribed Caesalpinioideae; Legume Phylogeny Working Group 2017), up to 19 genera apparently include species with EFNs (Table 1, Fig. 9; and eight unclear reports of EFNs need further investigations as to whether the mentioned structures are EFNs; Table S1). This is a significant increase in the number of genera documented to possess EFNs in this subfamily compared with previous studies, and, notably, approximately three times as many as listed by Koptur (1992a). Previously Mckey (1989) had concluded that EFNs were confirmed only in one genus, Leonardoxa, although he suspected that EFNs were more widespread in this clade. The new data presented here suggest that EFNs are found in genera scattered across the subfamily, except for tribes Schotieae and Barnebydendreae (sensu de la Estrella et al. 2018); however, even for these tribes, absence needs to be confirmed. EFNs in Detarioideae have been overlooked probably because most species form large trees not easy to study in the field, and field work has focused more on flower morphology and phenology, and not leaf development. Furthermore, in most cases, detarioid EFNs are small, cryptic and not easily visible on herbarium specimens without a hand-lens. Detarioid EFNs are all parenchymatic (Tables 3, 4, Fig. 9), occur exclusively on the lamina of leaflets, and may be flat or embedded (characterising most genera), or occasionally elevated on leaflet margins, which are more conspicuous (Fig. 9J, K). Another reason why EFNs have been overlooked in Detarioideae is perhaps that this clade is well known for another kind of secretory structure, namely, resin-producing glands.
Researchers have long been intrigued by resin-producing detarioid genera. The resin is composed of various sesquiterpenes and diterpenes secreted by epithelial cells that line small pockets, i.e. intercellular spaces produced schizogenously (Langenheim 1981, 2003). These pockets are visible on the surfaces of leaflets (and elsewhere on the plant) as translucent dots (also called ‘punctae’, Dwyer 1951, or simply punctate glands). Their anatomy and ecological roles in plant defence have been investigated in detail, especially in the genus Hymenaea (e.g. Langenheim 1967; Langenheim et al. 1982, and references therein). In contrast to this interest in resin-production, EFNs in Hymenaea (e.g. Paiva and Machado 2006), and several other detarioid genera (e.g. Copaifera, Oliveira and Isaias 2010; Cynometra, A. Radosavljevic and I. Coutinho, unpubl. data), have been studied anatomically only recently. Resin-producing detarioids are now known to be restricted to a subclade within tribe Detarieae (Fougère-Danezan et al. 2007; de la Estrella et al. 2018), suggesting no evident correlation between the presence of EFNs and resin glands, although this needs further study, and their relative ecological roles in plant defence strategies remain poorly understood.
Although most detarioid EFNs are rather cryptic to the untrained eye, Detarioideae, nevertheless, include two well-studied myrmecophyte genera, Humboldtia and Leonardoxa, belonging to unrelated clades within tribe Amherstieae (de la Estrella et al. 2018). These genera possess domatia in addition to their flat and embedded EFNs respectively. Species of the small Indian and Sri Lankan genus Humboldtia display a range of specialised myrmecophytic interactions, including true myrmecophytes in which domatia are consistently formed (e.g. H. laurifolia; Krombein et al. 1999), hemi-myrmecophytes in which only some individuals of a population form domatia (e.g. H. brunonis; Gaume et al. 2005; Shenoy et al. 2012; Chanam et al. 2014), and non-myrmecophytes that lack domatia or any resident ant colony and simply attract ants to leaf EFNs (e.g. H. unijuga; Krombein et al. 1999). Domatia of Humboldtia are particularly interesting because of the highly diverse invertebrate fauna they harbour in addition to ants (e.g. Krombein et al. 1999; Rickson et al. 2003), which include the plant’s pollinators (Shenoy and Borges 2008).
The monospecific African genus Leonardoxa, comprising four subspecies that make up the Leonardoxa africana complex, has been investigated in detail since the early 1980s (e.g. Elias 1980; Mckey 1984). Domatia are consistently present in mature individuals of this species complex, but there is infraspecific variability in the timing of development of the first domatia during plant ontogeny, i.e. the seedling stage, and in the amount of extrafloral nectar produced (Brouat and Mckey 2000). Leonardoxa has emerged as a model system for investigating the evolutionary ecology of ant–plant symbioses (e.g. Heil and Mckey 2003, and references therein; Brouat et al. 2004; Léotard et al. 2008; Blatrix et al. 2012). These studies have shown that co-evolutionary interactions with ants can be an important factor driving infraspecific differentiation (Mckey 2000) and have shown novel tripartite co-evolutionary interactions involving the myrmecophytic plant, its associated ants and fungi (e.g. Defossez et al. 2009).
Detarioideae is the legume subfamily in which further research on EFNs is most likely to produce exciting new discoveries, not only in terms of their phylogenetic distribution, morphological and anatomical diversity and evolution within the subfamily, but also their ecological and evolutionary roles. In this respect, studies in Humboldtia and Leonardoxa serve as exemplars to explore ant–plant interactions in the other detarioid genera with EFNs, where such interactions have yet to be investigated in detail.
Caesalpinioideae
Under the new subfamily classification of legumes (Legume Phylogeny Working Group 2017), subfamily Caesalpinioideae was re-circumscribed as a clade that excludes Cercidoideae, Detarioideae, Dialioideae and Duparquetioideae, but which now includes the nested mimosoid clade (former subfamily Mimosoideae; Fig. 2), and now comprises ~150 genera and ~4400 species (Legume Phylogeny Working Group 2017). Within the mimosoid clade, 87 genera are currently recognised, on the basis of the generic list in Legume Phylogeny Working Group (2017) plus three new mimosoid genera, i.e. Lachesiodendron, Parasenegalia and Pseudosenegalia, described since then. It is within this new-sense subfamily Caesalpinioideae, and especially the mimosoid clade, where the greatest concentration, diversity and abundance of EFN-possessing taxa within the legumes occur. Indeed, 87 genera, i.e. well over half of the caesalpinioid genera, possess EFNs, and, within the mimosoid clade, 78 of the 87 genera currently recognised, or 90%, possess EFNs (Tables 1, 2). This represents a major update on previous surveys of caesalpinioid EFNs (Lewis and Elias 1981; Mckey 1989; Pascal et al. 2000), plugging many generic gaps and updating generic delimitation (Table 1). Five of the mimosoid genera lacking EFNs (Amblygonocarpus, Aubrevillea, Elephanthorriza, Fillaeopsis and Tetrapleura) are early branching lineages in the mimosoid phylogeny. Across most of the core mimosoid clade, EFNs are universal except for three apparently independent evolutionary losses of EFNs involving just four genera (see below). In all but a handful of EFN-possessing Caesalpinioideae genera (Chamaecrista, Entada, Mimosa, Pentaclethra, Senna, Zapoteca), occurrence of EFNs within genera appears to be constant, being either present or absent. Taken together, these numbers suggest that more than 3000 of the 4400 species of Caesalpinioideae are likely to possess EFNs. Nowhere else within the legumes are EFNs so prevalent, abundant and conspicuous as within Caesalpinioideae.
It is also within subfamily Caesalpinioideae that three of the legume myrmecophyte lineages, i.e. true ant plants with domatia, are found, including the emblematic swollen-thorn ant ‘acacias’ in the genus Vachellia in Africa and the Neotropics (Janzen 1974; Mckey 1989; Mayer et al. 2014, Chomicki et al. 2015) and in the genus Tachigali, albeit, in this case, the mutualism lacks EFNs (Mckey 1989; Chomicki et al. 2015). The Neotropical Vachellia myrmecophyte lineage with 12–15 species is often cited as one of the best-studied examples of co-evolution involving an obligate symbiotic mutualism between the Pseudomyrmex ferrugineus group of ~10 species of ants that all nest exclusively in the swollen stipular spine domatia of Vachellia species (Janzen 1966, 1974; Gómez-Acevedo et al. 2010). In return, the nectar from the multiple conspicuous EFNs on the petiole and leaf rachis (see Fig. 13O), as well as specialised beltian food bodies on the tips of the leaflets, are specific for the resident ants (Heil 2004; Heil et al. 2009). Studies of these spectacular protective ant–acacia mutualisms, alongside those on Chamaecrista (especially C. fasciculata; Barton 1986; Kelly 1986; Rutter and Rausher 2004) and Inga (e.g. Koptur 1984, 1985, 1994), were among the first in-depth studies of the protective function of EFNs in obligate and facultative ant–plant mutualisms respectively, and have made important contributions to understanding of the ecology of myrmecophytes more generally (e.g. Heil and Mckey 2003). It is also notable that another legume, the Australian Acacia terminalis, is the only example, to our knowledge, of EFNs as an adaptation to bird pollination (Knox et al. 1985).
Many of the non-mimosoid Caesalpinioideae genera and almost all mimosoid genera have bipinnate leaves (with the notable exceptions in the mimosoid clade of the genus Inga and Cojoba rufescens, which have once-pinnate leaves), and EFNs in Caesalpinioideae are largely restricted to genera with bipinnate leaves, with only a few exceptions, notably Senna, Chamaecrista and Inga. In the large majority of caesalpinioid genera, EFNs are conspicuous raised structures classified as elevated parenchymatic leaf nectaries that are visible on dried herbarium specimens. Although most species possess a single petiolar EFN per leaf, in many species, multiple EFNs are present on the petiole, along the primary leaf rachis at the point of insertion of some or all of the leaflets or pinnae (also called jugal EFNs) and very frequently between the terminal pair of leaflets and pinnae. For some genera with bipinnate leaves, EFNs are also found along the secondary rachises at the point of insertion of the terminal pair(s) of leaflets. In a few species, EFNs have proliferated, with up to 75 EFNs being observed on a single leaf (e.g. Leucaena trichandra, Hughes 1998). It is clear that the compound leaf, and especially the evolutionarily labile and highly variable bipinnate leaf formula prevalent across Caesalpinioideae, provides a flexible and powerful template for legumes to fine-tune exactly where and when during development EFNs are activated and presented to ants in return for protection.
These elevated Caesalpinioid EFNs are extremely diverse in shape and size, and include sessile or stalked nectaries, rounded, oval, elliptic, crateriform, patelliform, verruciform, slit-like and cupular (Fig. 10–13). This morphological diversity is attributable to the protruding morphologies that allow elevated EFNs to occupy morphological space independent of that of their bearing organs, an idea embodied by the concept of EFN individualisation (Marazzi et al. 2013b). Increasing individualisation (i.e. the more an EFN protrudes from the surface) is associated with increasing disparification of EFNs, which are decoupled from the constraints of their bearing organs, culminating in the more elaborate stalked or sessile convex morphologies of elevated EFNs (see examples in Fig. 10, 11; see in the following paragraphs).
This diversity in form and size of elevated EFNs is apparently evolutionarily highly labile, with repeated occurrences of similar suites of morphologically diverse EFNs within many mimosoid genera (e.g. Archidendron, Nielsen et al. 1984; Leucaena, Hughes 1998; Desmanthus, Luckow 1993; Inga, Pennington 1997; Senna, Marazzi et al. 2013b). In a few cases, more conspicuous larger or unusually shaped nectaries have been observed, such as the following: double heart-shaped nectaries in Parkia, sometimes two or three EFNs at each point of insertion of leaflet pairs on the once-pinnate leaves of Inga species; enlarged conspicuous coloured funnel-shaped nectaries up to 12 mm in length (Abarema adenophora) or campanulate and becoming subligneous up to 11 mm in length (Abarema macradenia); nest-shaped nectaries with the orifice pointing upward or the upper part bent over the cavity as a lid (Archidendron merrillii and A. crateradenum respectively, Nielsen et al. 1984, fig. 18); stalked nectaries with a clavate head (Inga allenii); enlarged cup-shaped nectaries up to 7.4 mm in diameter (Pithecellobium macradenium; references in Table 1); in Acacia terminalis red-coloured EFNs attract birds that consume nectar and can act as pollinators (Knox et al. 1985).
In many cases, fully developed, enlarged and functional EFNs are present even on the reduced leaves subtending inflorescences that sometimes show partial and incomplete development, in line with the idea that EFNs are most active on young developing leaves (Mckey 1989). In extreme cases, highly reduced leaves are produced that appear to be completely truncated after formation of a fully functional EFN, especially for species that have extensive compound panicles of capitula, such as Leucaena esculenta (Fig. 13E, F) and Parasenegalia santosii (fig. 42 in Rico-Arce 2007), such that just the petiole and EFN are present.
Aside from this general pattern of occurrence of leaf nectaries, EFNs in Caesalpinioideae have also been reported in a few scattered taxa from other plant organs, including (1) in the genus Senna on inflorescence axes at the base of bracts, and also with non-elevated EFNs on stipules, bracts and sepals (Marazzi et al. 2013a; Table 1, Fig. 10F–N), (2) modified stipules (Archidendron, e.g. A. brachycarpum, A. molle; Nielsen et al. 1984, fig. 56; Nielsen 1992, fig. 16) or at the base of the stipules or stipule scars (Piptadeniastrum), (3) at the base of the floral bracts (Archidendron series Stipulatae in Asia; Nielsen et al. 1984, fig. 59, 65; Nielsen 1992, fig. 17), and Macrosamanea in the Neotropics; Fig. 12I, J), (4) on the bracts subtending inflorescences (Calpocalyx) and (5) on the stems (Entada phaseoloides, Pentaclethra macroloba (Fig. 13D, E) and Pseudoprosopis sericeus).
Thus, in both the genus Senna and across much of the mimosoid clade and within many mimosoid genera, EFNs show their greatest proliferation and morphological disparification, this involving multiple locations on the same plant (e.g. on leaves and inflorescence bracts), multiple locations on the same EFN-bearing organ (e.g. petiole and between leaflets and pinnae), and highly individualised morphologies (e.g. shapes, colours and sessile v. stalked).
Ancestral reconstruction of EFNs across Caesalpinioideae (Fig. 2) suggests a complex pattern of multiple independent gains (and potentially some losses) across non-mimosoid Caesalpinioideae and the first-branching lineages within the mimosoid clade. However, within core mimosoids, EFNs are almost universal apart from three clear cases of the evolutionary loss of EFNs. First, EFNs are absent for species of the genera Acaciella, Calliandra, and its recently segregated sister genus Afrocalliandra (the latter despite suggestions in the original description of this genus (de Souza et al. 2013) that one of the two species possesses EFNs, it is now clear that this is not the case; see Tables 1, S2). In forthcoming mimosoid phylogenies, these three genera form a clade (E. J. Koenen, unpubl. data), suggesting a single loss in this part of the phylogeny. A second loss is postulated within the genus Mimosa where EFNs have been reported to be restricted to section Mimadenia, which is sister to the rest of the genus (Barneby 1991; Simon et al. 2011). However, more recently, Gonzalez and Marazzi (2018) showed that Mimosa bifurca, which is placed in series Stipellares of section Batocaulon and is nested deep within Mimosa (Clade K in Simon et al. 2011) possesses EFNs, suggesting that with more careful observations, additional species of Mimosa with EFNs may well be discovered in the future. It is perhaps notable that the EFN-possessing Mimadenia clade of Mimosa comprises mainly lianas in Amazonian rainforest, in line with the idea that EFNs are especially common on leaves of lianas, which have a continuous production of young leaves (Mckey 1989). Finally, in the genus Zapoteca, only 3 of the ~20 species possess EFNs (Hernández 1989). Given that Zapoteca is deeply nested within the large EFN clade of mimosoids and that the three EFN-possessing species comprise the first branching lineages within the genus (Ferm 2019), this implies one other loss of EFNs within the mimosoid clade. In a few species in other genera (e.g. Albizia), EFNs have apparently become highly reduced or even obsolete.
The idea that there could be an association or correlation between the presence of EFNs and nodulation was suggested by Mckey (1989), perhaps reflecting underlying ecological differences related to competitive strategies and the phenology of leaf production. Our survey provides some tantalising hints supporting this idea within Caesalpinioideae, in that all the genera of early branching mimosoids that lack typical elevated caesalpinioid EFNs (Adenanthera, Amblygonocarpus, Aubrevillea, Fillaeopsis and Tetrapleura) are either known to be non-nodulating or of unknown nodulation status (Sprent 2009). Similarly, it is striking that in the genus Pentaclethra, the African species P. macrophylla is non-nodulating and lacks EFNs, whereas the American species P. macroloba is nodulating and possesses EFNs on the stems. However, beyond these striking examples, there are many nodulating taxa that lack EFNs (e.g. Calliandra, Acaciella, Campsiandra, Melanoxylon and Moldenhawera and, of course, the majority of Papilionoideae), suggesting that this association, if it is significant, is a rather loose one.
Papilionoideae
Of the six subfamilies, Papilionoideae is the largest, comprising over 500 genera, but it includes only 46 genera with EFNs (Tables 1, 2). Therefore, in Papilionoideae absence of EFNs is clearly prevalent, especially compared with subfamilies Caesalpinioideae and Detarioideae where presence of EFNs predominates (Fig. 2, Table 2). This proportionately low number of Papilionoideae genera with EFNs is even more stark, considering that our account lists almost three times more papilionoid genera with EFNs than did previous summaries by Mckey (1989) and Koptur (1992a). Although this increase is, in part, because of generic splitting, it does not take into account that some of the genera previously listed are not confirmed here (Table S1). Despite the relatively sparse occurrence of EFNs in Papilionoideae, their phylogenetic distribution indicates several independent evolutionary origins scattered disparately across the subfamily (Fig. 2A), involving various types of EFNs, and with the majority being concentrated in tribe Phaseoleae.
Four EFN genera of the early diverging lineages of the ADA clade (consisting of the Angylocalyx, Dipterygeae and Amburana clades; Cardoso et al. 2012, 2013), Alexa and Castanospermum (both tribe Angylocalyceae), Monopteryx and Pterodon (both Dipterygeae), are clearly isolated within the subfamily. The rest of the papilionoid EFN genera occur in the large 50-kb inversion clade (cf. Fig. 2 in the present study with fig. 1 in Cardoso et al. 2013). The occurrence of EFN genera in these early branching papilionoid lineages contrasts with earlier hypotheses of EFN evolution in Papilionoideae, which suggested that papilionoid EFNs had evolved in more derived groups and were absent from early branching papilionoid lineages (Lersten and Brubaker 1987; Mckey 1989). Moreover, these isolated reports of ADA clade EFNs are the only ones of parenchymatic EFNs in Papilionoideae, being flat in Alexa and its sister genus Castanospermum and elevated in Monopteryx and Pterodon. The latter are strongly reminiscent of caesalpinioid elevated EFNs (see references in Table 1, Fig. 15C), and are apparently homoplasious across these different groups, but the primary homology of these distantly related elevated parenchymatic EFNs remains speculative, because the ADA clade EFNs remain poorly studied and understood. It is also possible that EFNs have been overlooked or confused with other secretory structures in the ADA clade. For instance, Pterodon also possesses oil glands (Rodrigues et al. 2011), although anatomical analyses suggest that these are distinct from the elevated EFNs in this genus. Thorough investigation of the occurrence of EFNs in these early papilionoid lineages is needed to obtain a more complete picture of the evolution of elevated EFNs in Leguminosae and to address the question of why the Papilionoideae did not develop elevated EFNs in more taxa.
Another phylogenetically isolated occurrence of EFNs within Papilionoideae are the scattered occurrences of EFNs within the genus Crotalaria (tribe Crotalarieae, genistoid clade; sensu Cardoso et al. 2013). EFNs have been reliably reported from just 4 of the ~700 species of Crotalaria, namely, C. incana (e.g. Baker et al. 1978), C. intermedia (mentioned in Mckey 1989, p. 693), C. micans (Noack 1903) and C. pallida (Guimarães et al. 2006; Pereira and Trigo 2013), but Noack’s findings remain unconfirmed (simply cited by Mckey 1989 and Vogel 1998). A fifth EFN-possessing taxon, C. aff. striata (Noack 1903), is probably best treated as C. pallida (G. Lewis, Royal Botanic Gardens Kew, pers. comm.). In Crotalaria, EFNs occur on both reproductive and vegetative parts in the form of abscission-zone EFNs on the scars of fallen stipules, prophylls, bracts and flower pedicels (Noack 1903; Mckey 1989; Díaz-Castelazo et al. 2005). Detailed anatomical studies are needed to ascertain whether these abscission-zone EFNs are non-differentiated or swollen scars. Recent ecological studies of C. pallida (Pereira and Trigo 2013) have shown that these EFNs form part of a complex multispecies interaction involving ants, a specialised seed predator, and predatory wasps.
Among the other 41 papilionoid genera with EFNs (all within the non-protein–amino-acid-accumulating clade, NPAAA; Cardoso et al. 2013), Robinia represents another poorly known and apparently isolated occurrence of EFNs in the robinioid clade. The morphology and anatomy of the EFNs reported on the stipules and stipels of a single species, R. pseudoacacia (Pemberton 1990), are still unknown (see Table 1).
The large majority of papilionoid EFN genera is concentrated within tribe Phaseoleae, except for Vicia (Fabeae; see Table 1), and involves two categories of EFNs, namely, trichomatic (exposed or sunken) and abscission-zone EFNs, and notably swollen-scar EFNs (Table 2). Most taxa apparently have either trichomatic EFNs or swollen-scar EFNs, but in three genera, Dolichos, Lablab and Vigna, species bearing both these EFN categories occur simultaneously (Fig. 4D–G, 7H, I, 15M–P respectively). It is possible that more taxa for which only swollen-scar EFNs are currently reported, also bear trichomatic EFNs on the stipules, stipels or both, but this remains to be verified. By contrast, on taxa that have only trichomatic EFNs, the EFNs may be found also in the inflorescences, such as in Erythrina on the calyx lobes (Fig. 4K, L) and in Vicia on the bracts (Fig. 15L). Erythrina is outstanding among Phaseoleae not only for its calyx-lobe EFNs, but also as the only genus of legumes with pericarpial nectaries, i.e. EFNs on the surface of developing fruits (in E. speciosa), which are visited by ants (Paiva 2009).
These trichomatic inflorescence EFNs are functionally equivalent to swollen-scar EFNs, in that both attract mutualistic ants to the reproductive parts of the plant (Sherbrooke and Scheerens 1979; Priest and Loveless 2009). In contrast, Phaseolus bears trichomatic EFNs on stipels, but lacks inflorescence EFNs (Delgado-Salinas et al. 2011). One species of Phaseolus, P. lunatus L. (Fig. 4H, 15I) has emerged as a model system for investigating the ecological and evolutionary roles of volatile organic compounds in the context of plant defence strategies (e.g. Heil 2004; Choh et al. 2006; Godschalx et al. 2015). The ecology of EFNs in Vicia has also been thoroughly studied (e.g. Koptur 1979; Koptur and Lawton 1988; Mondor and Addicott 2003; Mondor et al. 2006; Gish et al. 2015).
Swollen-scar EFNs are a distinctive feature of papilionoid legumes (Table 1; Delgado-Salinas et al. 2011; Marazzi et al. 2012; Gonzalez and Marazzi 2018), displaying diversity in numbers and positions on the inflorescences, depending on the proportions and total numbers of flowers either developing or aborting. For example, these EFNs can be aligned between anthetic flowers (e.g. Macroptilium prostratum, Fig. 7E; Vigna unguiculata, Fig. 7H) or arranged in a more or less helicoidal order (e.g. Ancistrotropis peduncularis, Fig. 7A; Condylostylis candida, Fig. 7B; Macroptilium gibbosifolium, Fig. 7G). The inflorescence axes themselves can appear short and inflated, bearing multiple swollen-scar EFNs (e.g. Cleobulia multiflora, Fig. 15F; Cochliasanthus caracalla, Fig. 15 G) and these lateral inflorescences with highly compressed axes (Ojeda et al. 2014) are commonly referred to as pseudoraceme nodes (e.g. Delgado-Salinas et al. 2011). Swollen-scar EFNs are surely more widespread across Phaseoleae than is currently documented, and detailed mapping of their diversity in terms of numbers, arrangements and the complexity of their inflorescence axes is likely to show interesting evolutionary patterns for this specialised kind of EFN, which is unique within, and most likely beyond, the legume family. The ecological role of swollen-scar EFNs has long remained hypothetical, following the general reasoning that EFNs are usually located on leaves, flowers and fruits, where they develop and start secreting nectar to attract ants at a time when attacks by herbivores, florivores and seed predators would result in the greatest damage (Mckey 1989; Rico-Gray and Oliveira 2007). To our knowledge, this hypothesis has been investigated in only one species, i.e. Vigna luteola, by Aguirre et al. (2018), who suggested that ants attracted by the swollen-scar EFNs could have a dual function for the plants, namely, protecting them against potential herbivores as well as protecting flowers against nectar thieves. In this case, the presence of ants did not appear to interfere significantly with pollination, because the main pollinator was not deterred by ants.
Several hypotheses and explanations for the absence of EFNs in most of the Papilionoideae have been suggested. Polhill (1994, p. 35) pointed out that, in papilionoids, ‘the function of ant attraction [via EFNs] sometimes was replaced by glandular hairs and pearl bodies’. Indeed, other structures, notably glandular trichomes (unicellular and multicellular), for instance, in tribes Indigofereae and Dalbergieae (Polhill 1994; tribes sensu Cardoso et al. 2013), and some types of hydathodes, pearl bodies, and colleters, have often been misinterpreted as EFNs, probably because of the persistent lack of knowledge concerning their occurrence, contents and functions. The genus Indigofera is a good example; ants collect trichome heads found to secrete lipophilic substances (Marquiafável et al. 2009) and some hydathodes in this genus have been called ‘hydathode extrafloral nectaries’ because of their morphological similarity with nectaries (Schrire 1995; Schrire et al. 2009).
Another hypothesis could be that Papilionoideae are perhaps more versatile in defending themselves from herbivores than are Caesalpinioideae (including mimosoid legumes), Cercidoideae and Detarioideae by increased toxicity of highly diverse nitrogen-based compounds and other toxic compounds found in this subfamily (Harborne 1994; Zarucchi 1994). Chemical constituents have been named and described, including toxic non-protein aminoacids and peptides, aliphatic nitro-compounds, alkaloids and flavonoid constituents such as flavonol glycosides, isoflavonoids, furano coumarins, and xanthones for the Papilionoideae (see also Wink 2013).
Legume EFNs: a phylogenetic and evolutionary perspective
Accurately documenting and understanding the diversity, phylogenetic distribution and temporal and geographical evolutionary trajectories of EFNs, in terms of evolutionary gains and losses across legumes, is fundamental if we are going to be able to properly assess the significance, origins, maintenance and breakdown of these important evolutionary morphological and functional traits and their mutualisms (e.g. Heil et al. 2009; Chomicki and Renner 2015; Chomicki et al. 2015). For example, it has been suggested that EFNs could represent a possible key evolutionary innovation, contributing to diversification in the genus Senna (Marazzi and Sanderson 2010; but see also Marazzi et al. 2013b), whereas in another evolutionarily successful genus, Mimosa, an apparent evolutionary loss of EFNs coincides with a clade comprising the large majority of the ~550 species in the genus (Simon et al. 2011). Losses of mutualisms are also of considerable evolutionary interest (e.g. Gutiérrez-Valencia et al. 2017). For example, the multiple losses of EFNs hypothesised in three independent mimosoid clades documented here remain poorly understood, with no obvious environmental or other correlates for the large cohort of EFN-lacking Calliandra, Acaciella, Mimosa and Zapoteca species, which span a wide range of neotropical biomes.
In this paper, we map and illustrate the global occurrence of legume EFNs (of all types) on a time-calibrated legume phylogeny (Fig. 2), building on the only previous phylogenetic reconstruction by Marazzi et al. (2012), but here using a phylogeny that includes almost all genera of legumes and the much-updated list of generic occurrences (and species occurrences for polymorphic genera) of EFNs across the family (Table 1). Taken at face value, these phylogenetic reconstructions suggest that the ancestral legume lacked EFNs and that EFNs first evolved in subfamily Caesalpinioideae in the early to mid-Eocene and have been prevalent in certain legume clades throughout most of the Cenozoic. However, the ancestral condition in legumes should be further evaluated using data spanning outgroups at least across Fabales where EFNs are known to occur in several genera of Polygalaceae (Eriksen and Persson 2007). Our reconstruction also shows that the evolution of EFNs in legumes substantially predates the evolution of the various legume myrmecophyte lineages, which arose much later in the mid- to late Miocene (Chomicki and Renner 2015), and also significantly predates the multiple origins of EFNs in subfamily Papilionoideae, which all date from the Miocene (Fig. 2). The phylogeny also suggests multiple independent origins of EFNs across legumes as a whole, depicting a pattern of clustered homoplasy for this trait within the legume family, a pattern that is further accentuated by multiple evolutionary losses in certain clades, such as at least four times within the mimosoid clade (see Caesalpinioideae above).
One explanation for such a pattern of clustered homoplasy is the evolution of cryptic precursor traits (genetic or developmental), as suggested by Marazzi et al. (2012). In the analysis of Marazzi et al. (2012), the precursor model explained the phylogenetic diversity of elevated parenchymatic EFNs significantly better than did conventional models of character evolution, and performed equally well in the case of the other, less specialised EFNs. Therefore, although it is entirely possible that at least some legumes are in some way predisposed to evolve EFNs, as suggested by Marazzi et al. (2012), it is also possible that the relative ease of evolving an EFN may mean that no cryptic precursor is required to prompt many independent origins, as depicted in Fig. 2. Indeed, growing knowledge of the diversity of legume EFNs (in terms of topographic locations on the plant, morphology, anatomy and inferred functional significance; Tables 3, 4) casts doubt on the homology of the full gamut of EFNs that are not topographically correspondent, nor structurally, anatomically or morphologically similar structures, but rather represent a set of non-homologous structures that are functionally broadly convergent, as observed at higher taxonomic scales (Weber and Keeler 2013). Clearly, homology assessment of legume EFNs is far from straightforward, but given the non-homology of, for example, parenchymatic EFNs on leaves of Caesalpinioideae and Detarioideae and trichomatic and abscission-zone EFNs in Papilionoideae, a precursor model to explain the full diversity of legume EFNs seems perhaps less compelling than it is for, for example, parenchymatic EFNs alone, where the occurrences across many lineages of Detarioideae, most Caesalpinioideae and a few early diverging lineages of Papilionoideae are more suggestive of a precursor, or even a scenario of a single gain of EFNs deeper in the phylogeny and massive losses within legumes, as has recently been proposed for the evolution of nodulation (van Velzen et al. 2019).
Such scenarios demand further testing but suggest that the complex evolutionary history of EFNs in Leguminosae may better be interpreted in terms of a set of distinct EFN evolutionary trajectories, some potentially with and some without cryptic precursors, some undergoing significant rapid and repeated disparification, and others disappearing in the form of evolutionary losses. In this regard, the possible role that EFN-bearing organs themselves may play in shaping the evolutionary trajectories of legume EFNs, including the morphological disparification of EFNs and evolutionary losses, should not be underestimated. Disparification can be interpreted in terms of the idea of individualisation (Marazzi et al. 2013b), whereas losses could have been precipitated as a ‘by-product’ of sudden or gradual evolutionary changes in the Bauplan of the EFN-bearing organ, which may occur independently from positive selection experienced by the EFNs. Such ‘accidental’ losses are perhaps more likely than are losses resulting from a negative selection on EFNs, because EFNs appear cheap to produce (O’Dowd 1979; see also Rosenzweig 2002) and are likely to persist by genetic or phylogenetic inertia even in the absence of mutualistic interactions (Pemberton 1998; Nogueira et al. 2012).
On another level, phylogenetic patterns with respect to where on a plant EFNs develop are also apparent in that the location of EFNs itself is far from random. The idea that EFNs on non-reproductive structures (e.g. leaves and stems) are associated with long-lived woody perennials and EFNs on reproductive structures (e.g. inflorescences, bracts and bracteoles) are more prevalent on short-lived herbaceous plants (Mckey 1989) is strongly borne out by our results (Table 3, Fig. 2). EFNs are prevalent on leaves and other non-reproductive organs across Detarioideae and the mimosoid clade of Caesalpinioideae (almost all genera bear EFNs on vegetative parts only), which are almost all long-lived woody perennials. In contrast, in papilionoids, EFNs are prevalent on inflorescences (30–46 genera bear EFNs on reproductive structures only) and, especially, almost exclusively in tribe Phaseoleae, which comprises mainly shorter-lived climbing herbs, lianas or scandent shrubs (Lewis et al. 2005), and in the genus Vicia, all of which are short-lived herbaceous species. Interestingly, the isolated elevated parenchymatic EFNs on leaves of early diverging Papilionoideae lineages also occur in genera that comprise long-lived perennial trees up to 25 m tall, adding further support to Mckey’s (1989) hypothesis. Comparative ecological studies are necessary to test this hypothesis and understand whether these fundamentally different protective strategies associated with these distinct life-history strategies (i.e. vegetative v. reproductive plant parts) are matched by differences in the kind of herbivory damage suffered (e.g. leaf herbivory v. florivory or seed predation).
Conclusions and future directions
Several legume EFN questions and issues requiring further work have been highlighted throughout this paper. First, it is clear that EFNs in several legume clades remain poorly known and very likely incompletely documented, most notably in subfamilies Cercidoideae and Detarioideae, where EFNs are inconspicuous and not easily detected without careful and sustained observation in the field. In many cases, EFNs are barely visible on herbarium specimens. Furthermore, because herbarium specimens usually consist of fertile material with mature foliage, younger foliage and their associated caduceus stipules where EFNs are most active are often not included. Second, more detailed reconstructions of the evolutionary history of EFNs across legumes could prove productive at several levels, including within Caesalpinioideae using species-level data and a more resolved phylogeny, but also across legumes as a whole, including a range of legume outgroups and considering early diverging papilionoid lineages where elevated EFNs are found. Such analyses could shed light on whether absence of elevated EFNs in Papilionoideae reflects a loss early in the evolutionary history of the subfamily and, hence, why Papilionoideae did not develop elevated EFNs in more taxa. Third, as our knowledge of EFNs in legumes increases, there is scope to examine the broad-scale geographic distribution and environmental (e.g. bioclimatic, fire) correlates of EFN occurrence and abundance for the first time. It is clear that EFNs in legumes are strongly concentrated in tropical lineages, with there being few examples of temperate legumes with EFNs (in line with patterns for myrmecophytes across angiosperms more generally; see Chomicki and Renner 2015); however, within the tropics, it is unclear whether EFN-bearing legumes are equally abundant across savannas, rain forests and seasonally dry tropical forests, or even deserts. These questions set the stage for exciting future research on legume EFNs in the coming years.
Conflicts of interest
C. E. Hughes is also an Associate Editor of the ‘Advances in Legume Systematics 13’ special issue. Despite this relationship, he did not at any stage have Associate Editor-level access to this manuscript while in peer review, as is the standard practice when handling manuscripts submitted by an editor to this journal. Australian Systematic Botany encourages its editors to publish in the journal and they are kept totally separate from the decision-making process for their manuscripts. The authors have no further conflicts of interest to declare.
Declaration of funding
This study was partly funded by the National Geographic Research Program (Grant 8775-10 to B. Marazzi) and the Universidad Nacional del Nordeste (Grant SGCyT-16A003 to A. M. Gonzalez). Jens J. Ringelberg is funded by Swiss National Science Foundation (Grant 31003A_182453/1 to C. E. Hughes).
Acknowledgements
We thank Berenit Mendoza Garfias and Al Agellon (for assistance with SEM); Andrea Grantham (for assistance with microtome sections of Senna martiana and S. pleurocarpa); João Paulo Basso-Alves, Rumsaïs Blatrix, Domingos Cardoso, Matheus Cota, Thaïs Cury de Barros, Oscar Dorado, Erik Koenen, William Hawthorne, Martin Heil, Karime López, Luciano Paganucci de Queiroz, Nicola Patocchi, Aleksandar Radosavljevic, Leticia Torres-Colín (for beautiful photographs credited in the figures); Erik Koenen (for the preliminary time-calibrated version of the LPWG, 2017, matK phylogeny used to generate Fig. 2); Domingos Cardoso, Gwilym Lewis, William Hawthorne, Luciano Paganucci de Queiroz and Patrick Herendeen (for information about the status of EFNs in particular taxa); Peter Endress, Gwilym Lewis and Daniel Murphy (for extremely valuable and meticulous comments that greatly improved the maunscript). B. Marazzi and A. M. Gonzalez thank Gina (for her happy wagging tail, moral support, and inspiring walks).
References
Aguirre A, Dáttilo W, Rodríguez-Morales D, Canchola-Orozco S, Cocoletzi E, Coates R, Ángeles G (2018) Foraging ants on the extrafloral nectaries repel nectar thieves but not the effective pollinator of Vigna luteola (Fabaceae) in a Mexican coastal sand dune. Sociobiology 65, 621–629.| Foraging ants on the extrafloral nectaries repel nectar thieves but not the effective pollinator of Vigna luteola (Fabaceae) in a Mexican coastal sand dune.Crossref | GoogleScholarGoogle Scholar |
Anjos DVS, Caserio BM, Rezende FT, Ribeiro SP, Claro KD, Fagundes R (2017) Extrafloral-nectaries and interspecific aggressiveness regulate day/night turnover of ant species foraging for nectar on Bionia coriacea. Austral Ecology 42, 317–328.
| Extrafloral-nectaries and interspecific aggressiveness regulate day/night turnover of ant species foraging for nectar on Bionia coriacea.Crossref | GoogleScholarGoogle Scholar |
Bajpai O, Srivastava AK, Kushwaha AK, Chaudhary LB (2014) Taxonomy of a monotypic genus Indopiptadenia (Leguminosae–Mimosoideae). Phytotaxa 164, 61–78.
| Taxonomy of a monotypic genus Indopiptadenia (Leguminosae–Mimosoideae).Crossref | GoogleScholarGoogle Scholar |
Baker HG, Opler PA, Baker I (1978) A comparison of the amino acid complements of floral and extrafloral nectars. Botanical Gazette 139, 322–332.
| A comparison of the amino acid complements of floral and extrafloral nectars.Crossref | GoogleScholarGoogle Scholar |
Bandyopadhyay S, Ghoshal PP, Pathak MK (2012) Fifty new combinations in Phanera Lour. (Leguminosae: Caesalpinioideae) from Paleotropical region. Bangladesh Journal of Plant Taxonomy 19, 55–61.
| Fifty new combinations in Phanera Lour. (Leguminosae: Caesalpinioideae) from Paleotropical region.Crossref | GoogleScholarGoogle Scholar |
Barneby RC (1991) Sensitivae censitae, a description of the genus Mimosa Linnaeus (Mimosaceae) in the New World. Memoirs of the New York Botanical Garden 65, 1–835.
Barneby RC, Grimes JW (1984) Two leguminous forest trees new to the flora of French Guiana Brittonia 36, 45–48.
| Two leguminous forest trees new to the flora of French GuianaCrossref | GoogleScholarGoogle Scholar |
Barneby RC, Grimes JW (1996) Silk tree, guanacaste, monkey’s earring. A generic system for the synandrous Mimosaceae of the Americas, Part 1. Abarema, Albizia and allies. Memoirs of the New York Botanical Garden 74, 1–292.
Barneby RC, Grimes JW (1997) Silk tree, guanacaste, monkey’s earring. A generic system for the synandrous Mimosaceae of the Americas, Part 2. Pithecellobium, Cojoba and Zygia. Memoirs of the New York Botanical Garden 74, 1–149.
Barton AM (1986) Spatial variation in the effect of ants on an extrafloral nectary plant. Ecology 67, 495–504.
| Spatial variation in the effect of ants on an extrafloral nectary plant.Crossref | GoogleScholarGoogle Scholar |
Bennett B, Breed MD (1985) On the association between Pentaclethra macroloba (Mimosaceae) and Paraponera clavata (Hymenoptera, Formicidae) colonies. Biotropica 17, 253–255.
| On the association between Pentaclethra macroloba (Mimosaceae) and Paraponera clavata (Hymenoptera, Formicidae) colonies.Crossref | GoogleScholarGoogle Scholar |
Bentham G (1870) Leguminosae II: Swartzieae et Caesalpinieae. In ‘Flora Brasiliensis. Vol. 15’. (Ed. CFP de Martius) pp.1–254 (Frid. Fleischer: Leipzig, Germany)
Bentley BL (1977) Extrafloral nectaries and protection by pugnacious bodyguards. Annual Review of Ecology and Systematics 8, 407–427.
| Extrafloral nectaries and protection by pugnacious bodyguards.Crossref | GoogleScholarGoogle Scholar |
Bernardello G (2007) A systematic survey of floral nectaries. In ‘Nectaries and Nectar’. (Eds SW Nicolson, M Nepi, E Pacini) pp. 19–128. (Springer: Dordrecht, Netherlands)
Bhattacharyya B, Maheshwari JK (1970) Studies on extrafloral nectaries of the Leguminales. I. Papilionaceae: with a discussion of the systematics of Leguminales. Proceedings of the Indiana Academy of Sciences 37B, 1–30.
Bixenmann RJ, Coley PD, Kursar TA (2011) Is extrafloral nectar production induced by herbivores or ants in a tropical facultative ant–plant mutualism? Oecologia 165, 417–425.
| Is extrafloral nectar production induced by herbivores or ants in a tropical facultative ant–plant mutualism?Crossref | GoogleScholarGoogle Scholar | 20872232PubMed |
Bixenmann RJ, Coley PD, Kursar TA (2013) Developmental changes in direct and indirect defenses in the young leaves of the neotropical tree genus Inga (Fabaceae). Biotropica 45, 175–184.
| Developmental changes in direct and indirect defenses in the young leaves of the neotropical tree genus Inga (Fabaceae).Crossref | GoogleScholarGoogle Scholar |
Blatrix R, Renard D, Djieto-Lordon C, Mckey D (2012) The cost of myrmecophytism: insights from allometry of stem secondary growth. Annals of Botany 110, 943–951.
| The cost of myrmecophytism: insights from allometry of stem secondary growth.Crossref | GoogleScholarGoogle Scholar | 22875811PubMed |
Blüthgen N, Reifenrath K (2003) Extrafloral nectaries in an Australian rainforest: structure and distribution. Australian Journal of Botany 51, 515–527.
| Extrafloral nectaries in an Australian rainforest: structure and distribution.Crossref | GoogleScholarGoogle Scholar |
Blüthgen N, Verhaagh M, Goitía W, Jaffé K, Morawetz W, Barthlott W (2000) How plants shape the ant community in the Amazonian rainforest canopy, the key role of extrafloral nectaries and homopteran honeydew. Oecologia 125, 229–240.
| How plants shape the ant community in the Amazonian rainforest canopy, the key role of extrafloral nectaries and homopteran honeydew.Crossref | GoogleScholarGoogle Scholar | 24595834PubMed |
Blüthgen N, Gottsberger G, Fiedler K (2004) Sugar and amino acid composition of ant-attended nectar and honeydew sources from an Australian rainforest. Austral Ecology 29, 418–429.
| Sugar and amino acid composition of ant-attended nectar and honeydew sources from an Australian rainforest.Crossref | GoogleScholarGoogle Scholar |
Boughton VH (1981) Extrafloral nectaries of some Australian phyllodineous acacias. Australian Journal of Botany 29, 653–664.
| Extrafloral nectaries of some Australian phyllodineous acacias.Crossref | GoogleScholarGoogle Scholar |
Boughton VH (1985) Extrafloral nectaries of some Australian bipinnate acacias. Australian Journal of Botany 33, 175–184.
| Extrafloral nectaries of some Australian bipinnate acacias.Crossref | GoogleScholarGoogle Scholar |
Bower FO (1887) On Humboldtia launfolia, Vahl, as a myrmekophilous plant. Proceedings of the Philosophical Society of Glasgow 18, 320–326.
Brenan JPM (1959) ‘Flora of Tropical East Africa Leguminosae, Subfamily Mimosoideae.’ (Crown Agents for Oversea Governments and Administrations: London, UK)
Brenes-Arguedas T, Coley PD, Kursar TA (2008) Divergence and diversity in the defensive ecology of Inga at two Neotropical sites. Journal of Ecology 96, 127–135.
Brouat C, Mckey D (2000) Origin of caulinary ant domatia and timing of their onset in plant ontogeny: evolution of a key trait in horizontally transmitted ant–plant symbioses. Biological Journal of the Linnean Society. Linnean Society of London 71, 801–819.
Brouat C, Mckey D, Douzery EJP (2004) Differentiation in a geographical mosaic of plants coevolving with ants: phylogeny of the Leonardoxa africana complex (Fabaceae: Caesalpinioideae) using amplified fragment length polymorphism markers. Molecular Ecology 13, 1157–1171.
| Differentiation in a geographical mosaic of plants coevolving with ants: phylogeny of the Leonardoxa africana complex (Fabaceae: Caesalpinioideae) using amplified fragment length polymorphism markers.Crossref | GoogleScholarGoogle Scholar | 15078453PubMed |
Burkart A (1944) Tres nuevas leguminosas del Paraguay, coleccionadas por el Señor Teodoro Rojas. Darwiniana 6, 477–493.
Büsgen M (1903) Einige Wachstumsbeobachtungen aus den Tropen. Berichte der Deutschen Botanischen Gesellschaft 21, 435–440.
Cardoso D, De Queiroz LP, Pennington RT, De Lima HC, Fonty É, Wojciechowski MF, Lavin M (2012) Revisiting the phylogeny of papilionoid legumes: new insights from comprehensively sampled early‐branching lineages. American Journal of Botany 99, 1991–2013.
| Revisiting the phylogeny of papilionoid legumes: new insights from comprehensively sampled early‐branching lineages.Crossref | GoogleScholarGoogle Scholar | 23221500PubMed |
Cardoso D, Pennington RT, De Queiroz LP, Boatwright JS, Van Wyk BE, Wojciechowski MF, Lavin M (2013) Reconstructing the deep-branching relationships of the papilionoid legumes. South African Journal of Botany 89, 58–75.
| Reconstructing the deep-branching relationships of the papilionoid legumes.Crossref | GoogleScholarGoogle Scholar |
Carmona-Galindo VD, Morales K, Maser R, Doyle J, Gobrial M (2014) Characterization of sugar diversity in floral and extra-floral nectar from the coastal coral tree (Erythrina caffra Thunb.) in southern California. Open Journal of Ecology 04, 23–27.
| Characterization of sugar diversity in floral and extra-floral nectar from the coastal coral tree (Erythrina caffra Thunb.) in southern California.Crossref | GoogleScholarGoogle Scholar |
Caspary R (1848) ‘De Nectariis.’ (Adolphus Marcus: Bonn, Germany)
Chanam J, Kasinathan S, Pramanik GK, Jagdeesh A, Joshi KA, Borges RM (2014) Context dependency of rewards and services in an Indian ant–plant interaction, southern sites favour the mutualism between plants and ants. Journal of Tropical Ecology 30, 219–229.
| Context dependency of rewards and services in an Indian ant–plant interaction, southern sites favour the mutualism between plants and ants.Crossref | GoogleScholarGoogle Scholar |
Chanam J, Kasinathan S, Pramanik GK, Jagdeesh A, Joshi KA, Borges RM (2015) Foliar extrafloral nectar of Humboldtia brunonis (Fabaceae), a paleotropic ant–plant, is richer than phloem sap and more attractive than honeydew. Biotropica 47, 1–5.
| Foliar extrafloral nectar of Humboldtia brunonis (Fabaceae), a paleotropic ant–plant, is richer than phloem sap and more attractive than honeydew.Crossref | GoogleScholarGoogle Scholar |
Choh Y, Kugimiya S, Takabayashi J (2006) Induced production of extrafloral nectar in intact lima bean plants in response to volatiles from spider mite-infested conspecific plants as a possible indirect defense against spider mites. Oecologia 147, 455–460.
| Induced production of extrafloral nectar in intact lima bean plants in response to volatiles from spider mite-infested conspecific plants as a possible indirect defense against spider mites.Crossref | GoogleScholarGoogle Scholar | 16341892PubMed |
Chomicki G, Renner S (2015) Phylogenetics and molecular clocks reveal the repeated evolution of ant-plants after the late Miocene in Africa and the early Miocene in Australasia and the Neotropics. New Phytologist 207, 411–424.
| Phylogenetics and molecular clocks reveal the repeated evolution of ant-plants after the late Miocene in Africa and the early Miocene in Australasia and the Neotropics.Crossref | GoogleScholarGoogle Scholar | 25616013PubMed |
Chomicki G, Ward PS, Renner S (2015) Macroevolutionary assembly of ant/plant symbioses, Pseudomyrmex ants and their ant-housing plants in the Neotropics. Proceedings of the Royal Society of London – B. Biological Sciences 282, 20152200
| Macroevolutionary assembly of ant/plant symbioses, Pseudomyrmex ants and their ant-housing plants in the Neotropics.Crossref | GoogleScholarGoogle Scholar |
Coutinho ÍAC, Meira RMSA (2015) Structural diversity of extrafloral nectaries in Chamaecrista sect. Apoucouita. Botany 93, 379–388.
| Structural diversity of extrafloral nectaries in Chamaecrista sect. Apoucouita.Crossref | GoogleScholarGoogle Scholar |
Coutinho ÍAC, Francino DMT, Azevedo AA, Meira RMSA (2012) Anatomy of the extrafloral nectaries in species of Chamaecrista section Absus subsection Baseophyllum (Leguminosae, Caesalpinioideae). Flora 207, 427–435.
| Anatomy of the extrafloral nectaries in species of Chamaecrista section Absus subsection Baseophyllum (Leguminosae, Caesalpinioideae).Crossref | GoogleScholarGoogle Scholar |
Croat TB (1978) ‘Flora of Barro Colorado Island’ (Stanford University Press: Stanford, CA, USA)
da Fonseca Vaz AMSF, Tozzi AMGA (2003) Bauhinia ser. Cansenia (Leguminosae, Caesalpinioideae) no Brasil. Rodriguésia 54, 55–143.
| Bauhinia ser. Cansenia (Leguminosae, Caesalpinioideae) no Brasil.Crossref | GoogleScholarGoogle Scholar |
da Fonseca Vaz AMSF, Tozzi AMGA (2005) Sinopse de Bauhinia sect. Pauletia (Cav.) DC. (Leguminosae, Caesalpinioideae, Cercideae) no Brasil. Revista Brasileira de Botanica 28, 477–491.
de la Estrella M, Aedo C, Mackinder B, Velayos M (2010) Taxonomic revision of Daniellia (Leguminosae, Caesalpinioideae). Systematic Botany 35, 296–324.
| Taxonomic revision of Daniellia (Leguminosae, Caesalpinioideae).Crossref | GoogleScholarGoogle Scholar |
de la Estrella M, Devesa JA, Wieringa JJ (2012) A morphological re-evaluation of the taxonomic status of the genus Pellegriniodendron (Harms) J.Léonard (Leguminosae–Caesalpinioideae–Detarieae) and its inclusion in Gilbertiodendron J.Léonard. South African Journal of Botany 78, 257–265.
| A morphological re-evaluation of the taxonomic status of the genus Pellegriniodendron (Harms) J.Léonard (Leguminosae–Caesalpinioideae–Detarieae) and its inclusion in Gilbertiodendron J.Léonard.Crossref | GoogleScholarGoogle Scholar |
de la Estrella M, Forest F, Klitgård B, Lewis GP, Mackinder BA, de Queiroz LP, Wieringa JJ, Bruneau A (2018) A new phylogeny-based tribal classification of subfamily Detarioideae, an early branching clade of florally diverse tropical arborescent legumes. Scientific Reports 8, 6884
| A new phylogeny-based tribal classification of subfamily Detarioideae, an early branching clade of florally diverse tropical arborescent legumes.Crossref | GoogleScholarGoogle Scholar | 29720687PubMed |
de Souza ER, Lewis GP, Forest F, Schnadelbach AS, van den Berg C, de Queiroz LP (2013) Phylogeny of Calliandra (Leguminosae, Mimosoideae) based on nuclear and plastid molecular markers. Taxon 62, 1200–1219.
| Phylogeny of Calliandra (Leguminosae, Mimosoideae) based on nuclear and plastid molecular markers.Crossref | GoogleScholarGoogle Scholar |
de Souza ER, Krishnaraj MV, de Queiroz LP (2016) Sanjappa, a new genus in the tribe Ingeae (Leguminosae, Mimosoideae) from India. Rheedea 26, 1–12.
de Souza Conceição A, de Queiroz LP, Lewis GP, de Andrade G, de Almeida JM, Ricardo P, Schnadelbach AS (2009) Phylogeny of Chamaecrista Moench (Leguminosae–Caesalpinioideae) based on nuclear and chloroplast DNA regions. Taxon 58, 1168–1180.
| Phylogeny of Chamaecrista Moench (Leguminosae–Caesalpinioideae) based on nuclear and chloroplast DNA regions.Crossref | GoogleScholarGoogle Scholar |
Defossez E, Selosse MA, Dubois MP, Mondolot L, Faccio LA, Djieto‐Lordon C, Mckey D, Blatrix R (2009) Ant‐plants and fungi: a new threeway symbiosis. New Phytologist 182, 942–949.
| Ant‐plants and fungi: a new threeway symbiosis.Crossref | GoogleScholarGoogle Scholar | 19383109PubMed |
Delgado-Salinas A, Thulin M, Pasquet R, Weeden N, Lavin M (2011) Vigna (Leguminosae) sensu lato, the names and identities of the American segregate genera. American Journal of Botany 98, 1694–1715.
| Vigna (Leguminosae) sensu lato, the names and identities of the American segregate genera.Crossref | GoogleScholarGoogle Scholar | 21980163PubMed |
Delpino F (1868) Ulteriori osservazioni e considerazioni sulla Dicogamia nel regno vegetale. Atti della Società Italiana di Scienze Naturali 11, 265–332.
Delpino F (1869) Ulteriori osservazioni e considerazioni sulla Dicogamia nel regno vegetale. Atti della Società Italiana di Scienze Naturali 12, 21–233.
Delpino F (1870) Ulteriori osservazioni e considerazioni sulla Dicogamia nel regno vegetale. Atti della Società Italiana di Scienze Naturali 13, 167–205.
Delpino F (1873) Ulteriori osservazioni e considerazioni sulla Dicogamia nel regno vegetale. Atti della Società Italiana di Scienze Naturali 16, 151–349.
Delpino F (1874) Ulteriori osservazioni e considerazioni sulla Dicogamia nel regno vegetale. Atti della Società Italiana di Scienze Naturali 17, 266–407.
Delpino F (1886) Funzione mirmecofila nel regno vegetale Memorie della Reale Accademia delle Scienze dell’Istituto di Bologna Ser. 4 7, 215–323.
Delpino F (1887) Funzione mirmecofila nel regno vegetale Memorie della Reale Accademia delle Scienze dell’Istituto di Bologna Ser. 4 8, 601–650.
Delpino F (1889) Funzione mirmecofila nel regno vegetale Memorie della Reale Accademia delle Scienze dell’Istituto di Bologna Ser. 4 10, 115–147.
Díaz-Castelazo C, Rico-Gray V, Oliveira PS, Cuautle M (2004) Extrafloral nectary-mediated ant–plant interactions in the coastal vegetation of Veracruz, Mexico, richness, occurrence, seasonality, and ant foraging patterns. Ecoscience 11, 472–481.
| Extrafloral nectary-mediated ant–plant interactions in the coastal vegetation of Veracruz, Mexico, richness, occurrence, seasonality, and ant foraging patterns.Crossref | GoogleScholarGoogle Scholar |
Díaz-Castelazo C, Rico-Gray V, Ortega F, Ángeles G (2005) Morphological and secretory characterization of extrafloral nectaries in plants of coastal Veracruz, Mexico. Annals of Botany 96, 1175–1189.
| Morphological and secretory characterization of extrafloral nectaries in plants of coastal Veracruz, Mexico.Crossref | GoogleScholarGoogle Scholar | 16227307PubMed |
Drewes S (1998) Nectarios en Macroptilium erythroloma (Fabaceae). Anales del Instituto de Biología, Serie Botánica 69, 23–25.
Dwyer JD (1951) The Central American, West Indian and South American species of Copaifera (Caesalpiniaceae). Brittonia 7, 143–172.
| The Central American, West Indian and South American species of Copaifera (Caesalpiniaceae).Crossref | GoogleScholarGoogle Scholar |
Egan AN, Pan B (2015) Resolution of polyphyly in Pueraria (Leguminosae, Papilionoideae): the creation of two new genera, Haymondia and Toxicopueraria, the resurrection of Neustanthus, and a new combination in Teyleria. Phytotaxa 218, 201–226.
| Resolution of polyphyly in Pueraria (Leguminosae, Papilionoideae): the creation of two new genera, Haymondia and Toxicopueraria, the resurrection of Neustanthus, and a new combination in Teyleria.Crossref | GoogleScholarGoogle Scholar |
Egan AN, Puttock CF (2016a) The genus Haymondia AN Egan & B Pan bis (Fabaceae) in Thailand. Thai Forest Bulletin (Botany) 44, 26–31.
| The genus Haymondia AN Egan & B Pan bis (Fabaceae) in Thailand.Crossref | GoogleScholarGoogle Scholar |
Egan AN, Puttock CF (2016b) Toxicopueraria peduncularis, a new genus and species native to Thailand. Thai Forest Bulletin (Botany) 44, 15–21.
| Toxicopueraria peduncularis, a new genus and species native to Thailand.Crossref | GoogleScholarGoogle Scholar |
Elias TS (1972) Morphology and anatomy of foliar nectaries of Pithecellobium macradenium (Leguminosae). Botanical Gazette 133, 38–42.
| Morphology and anatomy of foliar nectaries of Pithecellobium macradenium (Leguminosae).Crossref | GoogleScholarGoogle Scholar |
Elias TS (1980) Foliar nectaries of unusual structure in Leonardoxa africana (Leguminosae) an African obligate myrmecophyte. American Journal of Botany 67, 423–425.
| Foliar nectaries of unusual structure in Leonardoxa africana (Leguminosae) an African obligate myrmecophyte.Crossref | GoogleScholarGoogle Scholar |
Elias TS (1983) Extrafloral nectaries, their structure and distribution. In ‘The Biology of Nectaries’. (Eds B Bentley, T Elias) pp. 174–203. (Columbia University Press: New York, NY, USA)
Elias TS, Gelband H (1976) Morphology and anatomy of floral and extrafloral nectaries in Campsis (Bignoniaceae). American Journal of Botany 63, 1349–1353.
| Morphology and anatomy of floral and extrafloral nectaries in Campsis (Bignoniaceae).Crossref | GoogleScholarGoogle Scholar |
Endo Y, Ohashi H (1998) Morphological and anatomy features of nectary on calyx-tooth of the genus Vicia (Leguminosae) and their systematic utility. Shokubutsu Kenkyu Zasshi 73, 92–101.
Eriksen B, Persson C (2007) Polygalaceae. In ‘The Families and Genera of Vascular Plants. Vol. 9’. (Ed. K Kubitzki) pp. 345–363. (Springer-Verlag: Berlin, Germany)
Feinsinger P, Linhart YB, Swarm LA, Wolfe JA (1979) Aspects of the pollination biology of three Erythrina species on Trinidad and Tobago. Annals of the Missouri Botanical Garden 66, 451–471.
| Aspects of the pollination biology of three Erythrina species on Trinidad and Tobago.Crossref | GoogleScholarGoogle Scholar |
Ferm J (2019) A preliminary phylogeny of Zapoteca (Fabaceae: Caesalpinioideae: Mimosoid clade). Plant Systematics and Evolution.
| A preliminary phylogeny of Zapoteca (Fabaceae: Caesalpinioideae: Mimosoid clade).Crossref | GoogleScholarGoogle Scholar |
Fiala B, Linsenmair KE (1995) Distribution and abundance of plants with extrafloral nectaries in the woody flora of a lowland primary rainforest in Malaysia. Biodiversity and Conservation 4, 165–182.
| Distribution and abundance of plants with extrafloral nectaries in the woody flora of a lowland primary rainforest in Malaysia.Crossref | GoogleScholarGoogle Scholar |
Fougère-Danezan M, Maumont S, Bruneau A (2007) Relationships among resin-producing Detarieae s.l. (Leguminosae) as inferred by molecular data. Systematic Botany 32, 748–761.
| Relationships among resin-producing Detarieae s.l. (Leguminosae) as inferred by molecular data.Crossref | GoogleScholarGoogle Scholar |
Fuckel L (1846) Ueber die Honigabsonderung der Benebblärrchen (Stipulae) bei Vicia sativa L. Flora 27, 417–418.
Gadd ME, Young TP, Palmer TM (2001) Effects of simulated shoot and leaf herbivory on vegetative growth and plant defense in Acacia drepanolobium. Oikos 92, 515–521.
| Effects of simulated shoot and leaf herbivory on vegetative growth and plant defense in Acacia drepanolobium.Crossref | GoogleScholarGoogle Scholar |
Gagnon E, Hughes CE, Lewis GP, Bruneau A (2015) A new cryptic species in a new cryptic genus in the Caesalpinia group (Leguminosae) from the seasonally dry inter-Andean valleys of South America. Taxon 64, 468–490.
| A new cryptic species in a new cryptic genus in the Caesalpinia group (Leguminosae) from the seasonally dry inter-Andean valleys of South America.Crossref | GoogleScholarGoogle Scholar |
Gagnon E, Bruneau A, Hughes CE, de Queiroz LP, Lewis GP (2016) A new generic system for the pantropical Caesalpinia group (Leguminosae). PhytoKeys 71, 1–160.
| A new generic system for the pantropical Caesalpinia group (Leguminosae).Crossref | GoogleScholarGoogle Scholar |
Gale SW, Pennington TD (2004) Lysiloma (Leguminosae, Mimosoideae) in Mesoamerica. Kew Bulletin 59, 453–467.
| Lysiloma (Leguminosae, Mimosoideae) in Mesoamerica.Crossref | GoogleScholarGoogle Scholar |
Gaume L, Zacharia M, Borges RM (2005) Ant–plant conflicts and a novel case of castration parasitism in a myrmecophyte. Evolutionary Ecology Research 7, 435–452.
Gish M, Mescher MC, De Moraes CM (2015) Targeted predation of extrafloral nectaries by insects despite localized chemical defences. Proceedings of the Royal Society of London – B. Biological Sciences 282, 20151835
| Targeted predation of extrafloral nectaries by insects despite localized chemical defences.Crossref | GoogleScholarGoogle Scholar |
Godschalx AL, Schädler M, Trisel JA, Balkan MA, Ballhorn DJ (2015) Ants are less attracted to the extrafloral nectar of plants with symbiotic, nitrogen‐fixing rhizobia. Ecology 96, 348–354.
| Ants are less attracted to the extrafloral nectar of plants with symbiotic, nitrogen‐fixing rhizobia.Crossref | GoogleScholarGoogle Scholar | 26240856PubMed |
Gómez-Acevedo S, Rico-Arce L, Delgado-Salinas A, Magallón S, Eguiarte LE (2010) Neotropical mutualism between Acacia and Pseudomyrmex: phylogeny and divergence times. Molecular Phylogenetics and Evolution 56, 393–408.
| Neotropical mutualism between Acacia and Pseudomyrmex: phylogeny and divergence times.Crossref | GoogleScholarGoogle Scholar | 20307674PubMed |
Gonzalez AM, Marazzi B (2018) Extrafloral nectaries in Fabaceae, filling gaps in structural and anatomical diversity in the family. Botanical Journal of the Linnean Society 187, 26–45.
| Extrafloral nectaries in Fabaceae, filling gaps in structural and anatomical diversity in the family.Crossref | GoogleScholarGoogle Scholar |
Guimarães PR, Raimundo RL, Bottcher C, Silva RR, Trigo JR (2006) Extrafloral nectaries as a deterrent mechanism against seed predators in the chemically protected weed Crotalaria pallida (Leguminosae). Austral Ecology 31, 776–782.
| Extrafloral nectaries as a deterrent mechanism against seed predators in the chemically protected weed Crotalaria pallida (Leguminosae).Crossref | GoogleScholarGoogle Scholar |
Gutiérrez-Valencia J, Chomicki G, Renner S (2017) Recurrent breakdowns of mutualisms with ants in the neotropical ant–plant genus Cecropia. Molecular Phylogenetics and Evolution 111, 196–205.
| Recurrent breakdowns of mutualisms with ants in the neotropical ant–plant genus Cecropia.Crossref | GoogleScholarGoogle Scholar | 28408324PubMed |
Hall BM (1762) Nectaria florum. In ‘Dissertatio Botanica Nectaria Florum’. (Ed. C Linnaeus) pp. 1–16. (Uppsala, Sweden)
Harborne JB (1994) Phytochemistry of the Leguminosae. In ‘Phytochemical Dictionary of the Leguminosae ILDIS & CHCD’. (Eds FA Bisby, J Buckingham, JB Harborne) pp. 20–23. (Chapman & Hall: London, UK)
Hartshorn GS (1983) Pentaclethra macroloba. In ‘Costa Rican Natural History’. (Ed. DH Janzen) pp. 301–303 (University of Chicago Press: Chicago, IL, USA)
Harvey AW (2009) Extrafloral nectaries in Kudzu, Pueraria montana (Lour.) Merr., and Groundnut, Apios americana Medicus (Fabaceae). Castanea 74, 360–371.
| Extrafloral nectaries in Kudzu, Pueraria montana (Lour.) Merr., and Groundnut, Apios americana Medicus (Fabaceae).Crossref | GoogleScholarGoogle Scholar |
Hawthorne W, Jongkind C (2006) ‘Woody Plants of Western African forests. A Guide to the Forest Trees, Shrubs and Lianas from Senegal to Ghana.’ (Royal Botanic Gardens, Kew: London, UK)
Heil M (2004) Induction of two indirect defences benefits lima bean (Phaseolus lunatus, Fabaceae) in nature. Journal of Ecology 92, 527–536.
| Induction of two indirect defences benefits lima bean (Phaseolus lunatus, Fabaceae) in nature.Crossref | GoogleScholarGoogle Scholar |
Heil M (2015) Extrafloral nectar at the plant–insect interface, a spotlight on chemical ecology, phenotypic plasticity, and food webs. Annual Review of Entomology 60, 213–232.
| Extrafloral nectar at the plant–insect interface, a spotlight on chemical ecology, phenotypic plasticity, and food webs.Crossref | GoogleScholarGoogle Scholar | 25564741PubMed |
Heil M, Mckey D (2003) Protective ant–plant interactions as a model system in ecological and evolutionary research. Annual Review of Ecology Evolution and Systematics 34, 425–453.
| Protective ant–plant interactions as a model system in ecological and evolutionary research.Crossref | GoogleScholarGoogle Scholar |
Heil M, González-Teuber M, Clement LW, Kautz S, Verhaagh M, Silva Bueno JC (2009) Divergent investment strategies of Acacia myrmecophytes and the coexistence of mutualists and exploiters. Proceedings of the National Academy of Sciences of the United States of America 106, 18091–18096.
| Divergent investment strategies of Acacia myrmecophytes and the coexistence of mutualists and exploiters.Crossref | GoogleScholarGoogle Scholar | 19717429PubMed |
Heneidak S, Hassan EA (2007) Morphological and anatomical studies of floral and extrafloral nectaries in some Vicia taxa (Fabaceae). International Journal of Botany 3, 329–341.
| Morphological and anatomical studies of floral and extrafloral nectaries in some Vicia taxa (Fabaceae).Crossref | GoogleScholarGoogle Scholar |
Herendeen PS, Lewis GP, Bruneau A (2003) Floral morphology in caesalpinioid legumes, testing the monophyly of the ‘Umtiza clade’. International Journal of Plant Sciences 164, S393–S407.
| Floral morphology in caesalpinioid legumes, testing the monophyly of the ‘Umtiza clade’.Crossref | GoogleScholarGoogle Scholar |
Hernández HM (1989) Systematics of Zapoteca (Leguminosae). Annals of the Missouri Botanical Garden 76, 781–862.
| Systematics of Zapoteca (Leguminosae).Crossref | GoogleScholarGoogle Scholar |
Hernández HM, Guinet Ph (1990) Calliandropsis, a new genus of Leguminosae, Mimosoideae from Mexico. Kew Bulletin 45, 609–620.
| Calliandropsis, a new genus of Leguminosae, Mimosoideae from Mexico.Crossref | GoogleScholarGoogle Scholar |
Hernandez LM, Otero JT, Manzano MR (2013) Biological control of the greenhouse whitefly by Amitus fuscipennis, understanding the role of extrafloral nectaries from crop and non-crop vegetation. Biological Control 67, 227–234.
| Biological control of the greenhouse whitefly by Amitus fuscipennis, understanding the role of extrafloral nectaries from crop and non-crop vegetation.Crossref | GoogleScholarGoogle Scholar |
Hughes C (1998) Monograph of Leucaena (Leguminosae–Mimosoideae). Systematic Botany Monographs 55, 1–244.
| Monograph of Leucaena (Leguminosae–Mimosoideae).Crossref | GoogleScholarGoogle Scholar |
Iganci JR, Soares MV, Guerra E, Morim MP (2016) A preliminary molecular phylogeny of the Abarema alliance (Leguminosae) and implications for taxonomic rearrangement. International Journal of Plant Sciences 177, 34–43.
| A preliminary molecular phylogeny of the Abarema alliance (Leguminosae) and implications for taxonomic rearrangement.Crossref | GoogleScholarGoogle Scholar |
Irwin HS, Barneby RC (1982) The American Cassiinae. Memoirs of the New York Botanical Garden 35, 1–918.
Janzen DH (1966) Coevolution of mutualism between ants and acacias in Central America. Evolution 20, 249–275.
| Coevolution of mutualism between ants and acacias in Central America.Crossref | GoogleScholarGoogle Scholar | 28562970PubMed |
Janzen DH (1974) ‘Swollen-thorn Acacias of Central America.’ (Smithsonian Institution Press: Washington, DC, USA)
Janzen DH, Carroll CR (1983) Paraponera clavata (Bala, giant tropical ant). In ‘Costa Rican Natural History’ (Ed. DH Janzen) pp. 752–753. (University of Chicago Press: Chicago, IL, USA)
Johansen DA (1940) ‘Plant Microtechnique.’ (McGraw-Hill Book Company, Inc.: New York, NY, USA)
Keeler KH (2009) World list of angiosperm species with extrafloral nectaries. Available at https://biosci.unl.edu/emeriti/keeler/extrafloral/worldlistfamilies.htm [Verified 25 October 2008].
Kelly CA (1986) Extrafloral nectaries: ants, herbivores and fecundity in Cassia fasciculata. Oecologia 69, 600–605.
| Extrafloral nectaries: ants, herbivores and fecundity in Cassia fasciculata.Crossref | GoogleScholarGoogle Scholar | 28311622PubMed |
Kessler A, Heil M (2011) The multiple faces of indirect defences and their agents of natural selection. Functional Ecology 25, 348–357.
| The multiple faces of indirect defences and their agents of natural selection.Crossref | GoogleScholarGoogle Scholar |
Khan D, Javed Zaki M, Khan Y (2017) Micromorphological structure of seedlings of Rhynchosia minima (L.) DC. (Papilionaceae) growing in a dry ruderalized site in Karachi. International Journal of Biology and Biotechnology 14, 539–559.
Knox RB, Kenrick J, Bernhardt P, Marginson R, Beresford G, Baker I, Baker HG (1985) Extrafloral nectaries as adaptations for bird pollination in Acacia terminalis. American Journal of Botany 72, 1185–1196.
| Extrafloral nectaries as adaptations for bird pollination in Acacia terminalis.Crossref | GoogleScholarGoogle Scholar |
Koptur S (1979) Facultative mutualism between weedy vetches bearing extrafloral nectaries and weedy ants in California. American Journal of Botany 66, 1016–1020.
| Facultative mutualism between weedy vetches bearing extrafloral nectaries and weedy ants in California.Crossref | GoogleScholarGoogle Scholar |
Koptur S (1984) Experimental evidence for defense of Inga (Mimosoideae) saplings by ants. Ecology 65, 1787–1793.
| Experimental evidence for defense of Inga (Mimosoideae) saplings by ants.Crossref | GoogleScholarGoogle Scholar |
Koptur S (1985) Alternative defenses against herbivores in Inga (Fabaceae, Mimosoideae) over an elevational gradient Ecology 66, 1639–1650.
| Alternative defenses against herbivores in Inga (Fabaceae, Mimosoideae) over an elevational gradientCrossref | GoogleScholarGoogle Scholar |
Koptur S (1992a) Extrafloral nectary-mediated interactions between insects and plants. In ‘Insect–Plant Interactions, Vol. IV’. (Ed. E Bernays) pp. 81–129. (CRC Press: London, UK)
Koptur S (1992b) Plants with extrafloral nectaries and ants in Everglades habitats. The Florida Entomologist 75, 38–50.
| Plants with extrafloral nectaries and ants in Everglades habitats.Crossref | GoogleScholarGoogle Scholar |
Koptur S (1994) Floral and extrafloral nectars of Costa Rican Inga trees, a comparison of their constituents and composition. Biotropica 26, 276–284.
| Floral and extrafloral nectars of Costa Rican Inga trees, a comparison of their constituents and composition.Crossref | GoogleScholarGoogle Scholar |
Koptur S, Lawton JH (1988) Interactions among vetches bearing extrafloral nectaries, their biotic protective agents, and herbivores. Ecology 69, 278–283.
| Interactions among vetches bearing extrafloral nectaries, their biotic protective agents, and herbivores.Crossref | GoogleScholarGoogle Scholar |
Krombein KV, Norden BB, Rickson MM, Rickson FR (1999) Biodiversity of the domatia occupants (ants, wasps, bees, and others) of the Sri Lankan myrmecophyte Humboldtia laurifolia Vahl (Fabaceae). Smithsonian Contributions to Zoology 603, 1–34.
| Biodiversity of the domatia occupants (ants, wasps, bees, and others) of the Sri Lankan myrmecophyte Humboldtia laurifolia Vahl (Fabaceae).Crossref | GoogleScholarGoogle Scholar |
Kuo JS, Pate JS (1985) The extrafloral nectaries of cowpea (Vigna unguiculata (L.) Walp.). I. Morphology, anatomy and fine structure. Planta 166, 15–27.
| The extrafloral nectaries of cowpea (Vigna unguiculata (L.) Walp.). I. Morphology, anatomy and fine structure.Crossref | GoogleScholarGoogle Scholar |
Lamont B (1979) Extrafloral nectaries in Australian plants, with special reference to Acacia. Mulga Research Centre Annual Report 2, 15–18.
Langenheim JH (1967) Preliminary investigations of Hymenaea courbaril as a resin producer. Journal of the Arnold Arboretum Harvard University 48, 203–229.
Langenheim JH (1981) Terpenoids in the Leguminosae. In ‘Advances in Legume Systematics, Part 2’. (Eds RM Polhill, PH Raven) pp. 627–655. (Royal Botanic Gardens, Kew: London, UK)
Langenheim JH (2003) ‘Plant Resins: Chemistry, Evolution, Ecology, and Ethnobotany.’ (Timber Press: Portland, OR, USA)
Langenheim JH, Lincoln DE, Stubblebine WH, Gabrielli AC (1982) Evolutionary implications of leaf resin pocket patterns in the tropical tree Hymenaea (Caesalpinioideae: Leguminosae). American Journal of Botany 69, 595–607.
| Evolutionary implications of leaf resin pocket patterns in the tropical tree Hymenaea (Caesalpinioideae: Leguminosae).Crossref | GoogleScholarGoogle Scholar |
Legume Phylogeny Working Group (2013) Legume phylogeny and classification in the 21st century: progress, prospects and lessons for other species-rich clades. Taxon 62, 217–248.
| Legume phylogeny and classification in the 21st century: progress, prospects and lessons for other species-rich clades.Crossref | GoogleScholarGoogle Scholar |
Legume Phylogeny Working Group (2017) A new subfamily classification of the Leguminosae based on a taxonomically comprehensive phylogeny. Taxon 66, 44–77.
| A new subfamily classification of the Leguminosae based on a taxonomically comprehensive phylogeny.Crossref | GoogleScholarGoogle Scholar |
Lemon J (2006) Plotrix, a package in the red light district of R. R News 6, 8–12.
Léonard J (1957) Généra des Cynometreae et des Amherstieae africaines. Memoires de l’Académie Royale de Belgique, Classe des Sciences 8, 3–312.
Léotard G, Saltmarsh A, Kjellberg F, Mckey D (2008) Mutualism, hybrid inviability and speciation in a tropical ant–plant. Journal of Evolutionary Biology 21, 1133–1143.
| Mutualism, hybrid inviability and speciation in a tropical ant–plant.Crossref | GoogleScholarGoogle Scholar | 18422532PubMed |
Lersten NR, Brubaker CL (1987) Extrafloral nectaries in Leguminosae, review and original observations in Erythrina and Mucuna (Papilionoideae; Phaseoleae). Bulletin of the Torrey Botanical Club 114, 437–447.
| Extrafloral nectaries in Leguminosae, review and original observations in Erythrina and Mucuna (Papilionoideae; Phaseoleae).Crossref | GoogleScholarGoogle Scholar |
Lersten NR, Curtis JD (1996) Survey of leaf anatomy, especially secretory structures, of tribe Caesalpinieae (Leguminosae, Caesalpinioideae). Plant Systematics and Evolution 200, 21–39.
| Survey of leaf anatomy, especially secretory structures, of tribe Caesalpinieae (Leguminosae, Caesalpinioideae).Crossref | GoogleScholarGoogle Scholar |
Lewis GP, Elias TS (1981) Tribe 3 Mimosae. In ‘Advances in Legume Systematics, Part 1’. (Eds RM Polhill, PH Raven) pp. 155–168. (Royal Botanic Gardens, Kew: London, UK)
Lewis GP, Guinet P (1986) Notes on Gagnebina (Leguminosae, Mimosoideae) in Madagascar and neighbouring islands. Kew Bulletin 41, 463–470.
| Notes on Gagnebina (Leguminosae, Mimosoideae) in Madagascar and neighbouring islands.Crossref | GoogleScholarGoogle Scholar |
Lewis GP, Owen PE (1989) ‘Legumes of the Ilha de Maracá.’ (Royal Botanic Gardens, Kew: London, UK)
Lewis G, Schrire B, Mackinder B, Lock M (2005) ‘Legumes of the World.’ (Royal Botanic Gardens, Kew: London, UK)
Lewis GP, Schrire BD, Mackinder BA, Rico L, Clark R (2013) A 2013 linear sequence of legume genera set in a phylogenetic context: a tool for collections management and taxon sampling. South African Journal of Botany 89, 76–84.
| A 2013 linear sequence of legume genera set in a phylogenetic context: a tool for collections management and taxon sampling.Crossref | GoogleScholarGoogle Scholar |
Lorence DH, Wood KR (1994) Kanaloa, a new genus of Fabaceae (Mimosoideae) from Hawaii. Novon 4, 137–145.
| Kanaloa, a new genus of Fabaceae (Mimosoideae) from Hawaii.Crossref | GoogleScholarGoogle Scholar |
Luckow M (1993) Monograph of Desmanthus (Leguminosae–Mimosoideae). Systematic Botany Monographs 38, 1–166.
| Monograph of Desmanthus (Leguminosae–Mimosoideae).Crossref | GoogleScholarGoogle Scholar |
Luque RH, Sousa C, Kraus JE (1996) Métodos de coloração de Roeser (1972) – modificado – e Kropp (1972) visando a subtituição do azul de astra por azul de alcião 8 GS ou 8 GX. Acta Botanica Brasílica 10, 199–212.
| Métodos de coloração de Roeser (1972) – modificado – e Kropp (1972) visando a subtituição do azul de astra por azul de alcião 8 GS ou 8 GX.Crossref | GoogleScholarGoogle Scholar |
Machado SR, Morellato LPC, Sajo MG, Oliveira PS (2008) Morphological patterns of extrafloral nectaries in woody plant species of the Brazilian cerrado. Plant Biology 10, 660–673.
| Morphological patterns of extrafloral nectaries in woody plant species of the Brazilian cerrado.Crossref | GoogleScholarGoogle Scholar | 18761504PubMed |
MacKinder BA, Clark R (2014) A synopsis of the Asian and Australasian genus Phanera Lour. (Cercideae: Caesalpinioideae: Leguminosae) including 19 new combinations. Phytotaxa 166, 49–68.
| A synopsis of the Asian and Australasian genus Phanera Lour. (Cercideae: Caesalpinioideae: Leguminosae) including 19 new combinations.Crossref | GoogleScholarGoogle Scholar |
MacKinder BA, Pennington RT (2011) Monograph of Berlinia (Leguminosae). Systematic Botany Monographs 91, 1–117.
Marazzi B, Sanderson MJ (2010) Large-scale patterns of diversification in the widespread legume genus Senna and the evolutionary role of extrafloral nectaries. Evolution 64, 3570–3592.
| Large-scale patterns of diversification in the widespread legume genus Senna and the evolutionary role of extrafloral nectaries.Crossref | GoogleScholarGoogle Scholar | 21133898PubMed |
Marazzi B, Endress PK, Paganucci de Queiroz L, Conti E (2006) Phylogenetic relationships within Senna (Leguminosae, Cassiinae) based on three chloroplast regions, patterns in the evolution of floral symmetry and extrafloral nectaries. American Journal of Botany 93, 288–303.
| Phylogenetic relationships within Senna (Leguminosae, Cassiinae) based on three chloroplast regions, patterns in the evolution of floral symmetry and extrafloral nectaries.Crossref | GoogleScholarGoogle Scholar | 21646190PubMed |
Marazzi B, Ané C, Simon M, Luckow M, Delgado-Salinas A, Sanderson MJ (2012) Locating evolutionary precursors on a phylogenetic tree. Evolution 66, 3918–3930.
| Locating evolutionary precursors on a phylogenetic tree.Crossref | GoogleScholarGoogle Scholar | 23206146PubMed |
Marazzi B, Bronstein JL, Koptur S (2013a) The diversity, ecology and evolution of extrafloral nectaries, current perspectives and future challenges. Annals of Botany 111, 1243–1250.
| The diversity, ecology and evolution of extrafloral nectaries, current perspectives and future challenges.Crossref | GoogleScholarGoogle Scholar | 23704115PubMed |
Marazzi B, Conti E, Sanderson MJ, McMahon MM, Bronstein JL (2013b) Diversity and evolution of a trait mediating ant-plant interactions, insights from extrafloral nectaries in Senna (Leguminosae). Annals of Botany 111, 1263–1275.
| Diversity and evolution of a trait mediating ant-plant interactions, insights from extrafloral nectaries in Senna (Leguminosae).Crossref | GoogleScholarGoogle Scholar | 23104672PubMed |
Marazzi B, Rossi-Pedruzzi A, Giacalone-Forini I, Maspoli G (2014) Ant–plant interactions between native ants and non-native plants with extrafloral nectaries: new insights from the Brissago Islands (Canton Ticino, Switzerland). Bollettino della Società Ticinese di Scienze Naturali 102, 47–56.
Marquiafável FS, Seabra Ferreira MD, Teixeira SP (2009) Novel reports of glands in Neotropical species of Indigofera L. (Leguminosae, Papilionoideae). Flora 204, 189–197.
| Novel reports of glands in Neotropical species of Indigofera L. (Leguminosae, Papilionoideae).Crossref | GoogleScholarGoogle Scholar |
Maslin BR, George AS, Kodela PG, Ross JH, Wilson AJG (2001) Generic description, key to species. In ‘Flora of Australia 11A’. pp. 41–195. (CSIRO Publishing: Melbourne, Vic., Australia)
Mayer VE, Fredrickson ME, Mckey D, Blatrix R (2014) Current issues in the evolutionary ecology of ant–plant symbioses. New Phytologist 202, 749–764.
| Current issues in the evolutionary ecology of ant–plant symbioses.Crossref | GoogleScholarGoogle Scholar | 24444030PubMed |
Mckey D (1984) Interaction of the ant–plant Leonardoxa africana (Caesalpiniaceae) with its obligate inhabitants in a rainforest in Cameroon. Biotropica 16, 81–99.
| Interaction of the ant–plant Leonardoxa africana (Caesalpiniaceae) with its obligate inhabitants in a rainforest in Cameroon.Crossref | GoogleScholarGoogle Scholar |
Mckey D (1989) Interactions between ants and leguminous plants. Monographs in Systematic Botany from the Missouri Botanical Garden 29, 673–718.
Mckey D (2000) Leonardoxa africana (Leguminosae: Caesalpinioideae): a complex of mostly allopatric subspecies. Adansonia 22, 71–109.
Melo Y, Córdula E, Machado SR, Alves M (2010a) Morfologia de nectarios em Leguminosae sensu lato em areas de Caatinga no Brasil. Acta Botanica Brasílica 24, 1034–1045.
| Morfologia de nectarios em Leguminosae sensu lato em areas de Caatinga no Brasil.Crossref | GoogleScholarGoogle Scholar |
Melo Y, Machado SR, Alves M (2010b) Anatomy of extrafloral nectaries in Fabaceae from dry-seasonal forest in Brazil. Botanical Journal of the Linnean Society 163, 87–98.
| Anatomy of extrafloral nectaries in Fabaceae from dry-seasonal forest in Brazil.Crossref | GoogleScholarGoogle Scholar |
Mondor EB, Addicott JF (2003) Conspicuous extrafloral nectaries are inducible in Vicia faba. Ecology Letters 6, 495–497.
| Conspicuous extrafloral nectaries are inducible in Vicia faba.Crossref | GoogleScholarGoogle Scholar |
Mondor EB, Tremblay MN, Messing RH (2006) Extrafloral nectary phenotypic plasticity is damage- and resource-dependent in Vicia faba. Biological Letters 2, 583–585.
| Extrafloral nectary phenotypic plasticity is damage- and resource-dependent in Vicia faba.Crossref | GoogleScholarGoogle Scholar |
Moog J (2009) The associations of the plant–ant Cladomyrma with plants in Southeast Asia. Dissertation zur Erlangung des Doktorgrades der Naturwissenschaften vorgelegt beim Fachbereich Biowissenschaften der Johann Wolfgang Goethe Universität, Frankfurt am Main, Germany.
Morellato LPC, Oliveira PS (1991) Distribution of extrafloral nectaries in different vegetation types of Amazonian Brazil. Flora 185, 33–38.
| Distribution of extrafloral nectaries in different vegetation types of Amazonian Brazil.Crossref | GoogleScholarGoogle Scholar |
Morini F (1886) Contributo all’anatomia ed alla fisiologia dei nettarii estranuziali. Memorie della Reale Accademia delle Scienze dell’Istituto di Bologna 7, 325–391.
Moya-Raygoza G, Larsen KJ (2001) Temporal resource switching by ants between honeydew produced by the five spotted gama grass leafhopper (Dalbulus quinquenotatus) and nectar produced by plants with extrafloral nectaries. American Midland Naturalist 146, 311–320.
| Temporal resource switching by ants between honeydew produced by the five spotted gama grass leafhopper (Dalbulus quinquenotatus) and nectar produced by plants with extrafloral nectaries.Crossref | GoogleScholarGoogle Scholar |
Muehleisen A, Queenborough SA, Alvia P, Valencia R, Fiala B (2016) Incidence of extrafloral nectaries and their relationship with growth and survival of lowland tropical rain forest trees. Biotropica 48, 321–331.
| Incidence of extrafloral nectaries and their relationship with growth and survival of lowland tropical rain forest trees.Crossref | GoogleScholarGoogle Scholar |
Nepi M (2007) Nectary structure and ultrastructure. In ‘Nectaries and Nectar’. (Eds SW Nicolson, M Nepi, E Pacini) pp. 129–166. (Springer: Dordrecht, Netherlands)
Nevling LI, Niezgoda CJ (1978) On the genus Schleinitzia (Leguminosae–Mimosoideae). Adansonia 18, 345–363.
Nielsen IC (1981) Légumineuses–Mimosoidées. In ‘Flore du Cambodge du Laos et du Vietnam 19’. (Eds A Aubreville, JF Le Roy) pp. 1–159. (Museum National d’Histoire Naturelle: Paris, France)
Nielsen IC (1985) Leguminosae–Mimosoideae. In ‘Flora of Thailand 4(2)’. (Eds T Smitinand, K Larsen) pp. 131–222. (Forest Herbarium, Royal Forest Department: Bangkok, Thailand)
Nielsen IC (1992) Mimosaceae (Leguminosae–Mimosoideae). In ‘Flora Malesiana. Ser. 1, 11 (part 1)’. pp. 1–226. (Hortus Botanicus: Leiden, Netherlands)
Nielsen I, Guinet Ph, Baretta-Kuipers T (1983) Studies in the Melanesian, Australian, and Pacific Ingeae (Leguminosae–Mimosoideae), the genera Archidendropsis, Wallaceodendron, Paraserianthes and Serianthes (part 1). Adansonia 5, 303–329.
Nielsen I, Baretta-Kuipers T, Guinet Ph (1984) The genus Archidendron (Leguminosae–Mimosoideae). Opera Botanica 76, 1–120.
Noack F (1903) Blütenbiologische Beobachtungen aus Brasilien. Beihefte zum Botanischen Centralblatt 13, 112–114.
Nogueira A, Guimarães E, Machado SR, Lohmann LG (2012) Do extrafloral nectaries present a defensive role against herbivores in two species of the family Bignoniaceae in a Neotropical savanna? Plant Ecology 213, 289–301.
| Do extrafloral nectaries present a defensive role against herbivores in two species of the family Bignoniaceae in a Neotropical savanna?Crossref | GoogleScholarGoogle Scholar |
O’Dowd DJ (1979) Foliar nectar production and ant activity on a neotropical tree, Ochroma pyramidale. Oecologia 43, 233–248.
| Foliar nectar production and ant activity on a neotropical tree, Ochroma pyramidale.Crossref | GoogleScholarGoogle Scholar | 28309715PubMed |
Ochoterena-Booth H, Delgado-Salinas A (1994) Contribuciones a la taxonomía de Ramirezella (Leguminosae, Papilionoideae). Anales del Instituto de Biología de la Universidad Nacional Autónoma de México, Serie Botánica 65, 7–19.
Ojeda FS, Hoc PS, Galati BG, Amela García MT (2014) Ontogeny of the extrafloral nectaries of Vigna adenantha (Leguminosae, Phaseoleae). Botanical Studies 55, 74–81.
| Ontogeny of the extrafloral nectaries of Vigna adenantha (Leguminosae, Phaseoleae).Crossref | GoogleScholarGoogle Scholar | 28510960PubMed |
Oliveira PS, Freitas AVL (2004) Ant–plant-herbivore interactions in the neotropical cerrado savanna. Naturwissenschaften 91, 557–570.
| Ant–plant-herbivore interactions in the neotropical cerrado savanna.Crossref | GoogleScholarGoogle Scholar | 15551026PubMed |
Oliveira DC, Isaias RMS (2010) Redifferentiation of leaflet tissues during midrib gall development in Copaifera langsdorffii (Fabaceae). South African Journal of Botany 76, 239–248.
| Redifferentiation of leaflet tissues during midrib gall development in Copaifera langsdorffii (Fabaceae).Crossref | GoogleScholarGoogle Scholar |
Oliveira PS, Leitão-Filho HF (1987) Extrafloral nectaries, their taxonomic distribution and abundance in the woody flora of cerrado vegetation in southeast Brazil. Biotropica 19, 140–148.
| Extrafloral nectaries, their taxonomic distribution and abundance in the woody flora of cerrado vegetation in southeast Brazil.Crossref | GoogleScholarGoogle Scholar |
Oliveira PS, Oliveira-Filho AT (1991) Distribution of extrafloral nectaries in the woody flora of tropical communities in western Brazil. In ‘Plant–Animal Interactions, Evolutionary Ecology in Tropical and Temperate Regions’. (Eds PW Price, TM Lewinsohn, GW Fernandes, WW Benson) pp. 163–175. (Wiley: New York, NY, USA)
Oliveira PS, Pie MR (1998) Interaction between ants and plants bearing extrafloral nectaries in cerrado vegetation. Anais da Sociedade Entomológica do Brasil 27, 161–176.
| Interaction between ants and plants bearing extrafloral nectaries in cerrado vegetation.Crossref | GoogleScholarGoogle Scholar |
Ono K (1907) Studies on some extranuptial nectaries. Journal of the College of Science, Imperial University of Tokyo 23, 1–28.
Paiva EAS (2009) Ultrastructure and post-floral secretion of the pericarpial nectaries of Erythrina speciosa (Fabaceae). Annals of Botany 104, 937–944.
| Ultrastructure and post-floral secretion of the pericarpial nectaries of Erythrina speciosa (Fabaceae).Crossref | GoogleScholarGoogle Scholar |
Paiva EAS, Machado SR (2006) Ontogênese, anatomia e ultra-estrutura dos nectários extraflorais de Hymenaea stigonocarpa Mart. ex Hayne (Fabaceae–Caesalpinioideae). Acta Botanica Brasílica 20, 471–482.
| Ontogênese, anatomia e ultra-estrutura dos nectários extraflorais de Hymenaea stigonocarpa Mart. ex Hayne (Fabaceae–Caesalpinioideae).Crossref | GoogleScholarGoogle Scholar |
Paiva EAS, Morais HC, Isaias RMS, Rocha DMS, Oliveira PO (2001) Occurrence and structure of extrafloral nectaries in Pterodon pubescens Benth. and P. polygalaeflorus Benth. Pesquisa Agropecuária Brasileira 36, 219–224.
| Occurrence and structure of extrafloral nectaries in Pterodon pubescens Benth. and P. polygalaeflorus Benth.Crossref | GoogleScholarGoogle Scholar |
Palacios RA, Hoc PS (2005) Revisión del género Prosopidastrum (Leguminosae) para la Argentina. Boletín de la Sociedad Argentina de Botánica 40, 113–128.
Paradis E, Schliep K (2019) Ape 5.0, an environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics 35, 526–528.
| Ape 5.0, an environment for modern phylogenetics and evolutionary analyses in R.Crossref | GoogleScholarGoogle Scholar | 30016406PubMed |
Pascal LM, Motte-Florac EF, Mckey DB (2000) Secretory structure on the leaf rachis of Caesalpinieae and Mimosoideae (Leguminosae): implications for the evolution of nectary glands. American Journal of Botany 87, 327–338.
| Secretory structure on the leaf rachis of Caesalpinieae and Mimosoideae (Leguminosae): implications for the evolution of nectary glands.Crossref | GoogleScholarGoogle Scholar | 10718993PubMed |
Pemberton RW (1988) The abundance of plants bearing extrafloral nectaries in Colorado and Mojave desert communities of southern California. Madrono XX, 238–246.
Pemberton RW (1990) The occurrence of extrafloral nectaries in Korean plants. The Korean Journal of Ecology 12, 251–266.
Pemberton RW (1998) The occurrence and abundance of plants with extrafloral nectaries, the basis for antiherbivore defensive mutualisms along a latitudinal gradient in east Asia. Journal of Biogeography 25, 661–668.
| The occurrence and abundance of plants with extrafloral nectaries, the basis for antiherbivore defensive mutualisms along a latitudinal gradient in east Asia.Crossref | GoogleScholarGoogle Scholar |
Pennington TD (1997) ‘The Genus Inga Botany.’ (Royal Botanic Gardens, Kew: London, UK)
Pereira MF, Trigo JR (2013) Ants have a negative rather than a positive effect on extrafloral nectaried Crotalaria pallida performance. Acta Oecologica 51, 49–53.
| Ants have a negative rather than a positive effect on extrafloral nectaried Crotalaria pallida performance.Crossref | GoogleScholarGoogle Scholar |
Pereira LB, Costa-Silva R, Felix LP, Agra ML (2018) Leaf morphoanatomy of ‘mororó’ (Bauhinia and Schnella, Fabaceae). Revista Brasileira de Farmacognosia 28, 383–392.
| Leaf morphoanatomy of ‘mororó’ (Bauhinia and Schnella, Fabaceae).Crossref | GoogleScholarGoogle Scholar |
Polhill RM (1994) Classification of the Leguminosae. In ‘Phytochemical Dictionary of the Leguminosae, ILDIS and CHCD’. (Eds FA Bisby, J Buckingham, JB Harborne) pp. 35–48. (Chapman and Hall: London, UK)
Priest GV, Loveless MD (2009) Ant–plant interactions in Erythrina flabelliformis, investigation of a mutualism. In ‘The 94th ESA Annual Meeting’, 2–7 August 2009, Albuquerque, NM, USA. Paper COS 29-7. (Ecological Society of America: Albuquerque, NM, USA) Available at https://eco.confex.com/eco/2009/techprogram/P19041.HTM [Verified 20 August 2019]
Redden KM, Herendeen PS (2006) Morphology and phylogenetic analysis of Paloue and related genera in the Brownea clade (Detarieae, Caesalpinioideae). International Journal of Plant Sciences 167, 1229–1246.
| Morphology and phylogenetic analysis of Paloue and related genera in the Brownea clade (Detarieae, Caesalpinioideae).Crossref | GoogleScholarGoogle Scholar |
Redden KM, Herendeen PS, Lewis GP (2018) Understanding Paloue (Leguminosae: Detarioideae): revision of a predominantly Guiana Shield endemic. Smithsonian Contributions to Botany 109, 2–44.
| Understanding Paloue (Leguminosae: Detarioideae): revision of a predominantly Guiana Shield endemic.Crossref | GoogleScholarGoogle Scholar |
Revell LJ (2012) Phytools. An R package for phylogenetic comparative biology (and other things). Methods in Ecology and Evolution 3, 217–223.
| Phytools. An R package for phylogenetic comparative biology (and other things).Crossref | GoogleScholarGoogle Scholar |
Rezende MH, Alves Cardoso L, Vannucci AL (1994) Morfologia e anatomia foliar de Bauhinia curvula Benth. (Leguminosae–Caesalpinioideae). Acta Botanica Brasílica 8, 19–34.
| Morfologia e anatomia foliar de Bauhinia curvula Benth. (Leguminosae–Caesalpinioideae).Crossref | GoogleScholarGoogle Scholar |
Ribeiro PG, Luckow M, Lewis GP, Simon MF, Cardoso D, de Souza ÉR, Silva APC, Jesus MC, dos Santos FA, Azevedo V, de Queiroz LP (2018) Lachesiodendron, a new monospecific genus segregated from Piptadenia (Leguminosae, Caesalpinioideae, mimosoid clade), evidence from morphology and molecules. Taxon 67, 37–54.
| Lachesiodendron, a new monospecific genus segregated from Piptadenia (Leguminosae, Caesalpinioideae, mimosoid clade), evidence from morphology and molecules.Crossref | GoogleScholarGoogle Scholar |
Rickson FR, Rickson MM, Ghorpade K, Norden BB, Krombein KV (2003) Invertebrate biodiversity (ants, bees and others) associated with stem domatia of the Indian myrmecophyte Humboldtia brunonis Wallich (Magnoliophyta: Fabaceae). Proceedings of the Entomological Society of Washington 105, 73–79.
Rico-Arce L (2007) ‘A Checklist and Synopsis of American Species of Acacia (Leguminosae, Mimosoideae).’ Comisión Nacional para el Conocimiento y Uso de la Biodiversidad. (CONABIO: Mexico City, Mexico).
Rico-Gray V, Oliveira PS (2007) ‘The Ecology and Evolution of Ant–Plant Interactions.’ (University of Chicago Press: Chicago, IL, USA)
Riley-Hulting ET, Delgado-Salinas A, Lavin M (2004) Phylogenetic systematics of Strophostyles (Fabaceae), a North American temperate genus within a Neotropical diversification. Systematic Botany 29, 627–653.
| Phylogenetic systematics of Strophostyles (Fabaceae), a North American temperate genus within a Neotropical diversification.Crossref | GoogleScholarGoogle Scholar |
Rodrigues TM, Santos DC, Machado SR (2011) The role of the parenchyma sheath and PCD during the development of oil cavities in Pterodon pubescens (Leguminosae–Papilionoideae). Comptes Rendus Biologies 334, 535–543.
| The role of the parenchyma sheath and PCD during the development of oil cavities in Pterodon pubescens (Leguminosae–Papilionoideae).Crossref | GoogleScholarGoogle Scholar | 21784363PubMed |
Rodrigues MDS, Martins-da-Silva RC, Secco RDS (2012) Caesalpinieae (Leguminosae–Caesalpinioideae) from the experimental field of the Embrapa eastern Amazon, Moju, Pará State, Brazil. Hoehnea 39, 489–516.
| Caesalpinieae (Leguminosae–Caesalpinioideae) from the experimental field of the Embrapa eastern Amazon, Moju, Pará State, Brazil.Crossref | GoogleScholarGoogle Scholar |
Rosenzweig ML (2002) The distraction hypothesis depends on relatively cheap extrafloral nectaries. Evolutionary Ecology Research 4, 307–311.
Rutter MT, Rausher MD (2004) Natural selection on extrafloral nectar production in Chamaecrista fasciculata: the costs and benefits of a mutualism trait. Evolution 58, 2657–2668.
| Natural selection on extrafloral nectar production in Chamaecrista fasciculata: the costs and benefits of a mutualism trait.Crossref | GoogleScholarGoogle Scholar | 15696745PubMed |
Schmid R (1988) Reproductive versus extra-reproductive nectaries. Historical perspective and terminological recommendations. Botanical Review 54, 179–232.
| Reproductive versus extra-reproductive nectaries. Historical perspective and terminological recommendations.Crossref | GoogleScholarGoogle Scholar |
Schnell R, Cusset G, Quenum M (1963) Contribution à l’étude des glandes extra-florales chez quelques groupes de plantes tropicales. Revue Générale de Botanique 70, 269–342.
Schrire BD (1995) Evolution of the tribe Indigoferae (Leguminosae–Papilionoideae). Advances in Legume Systematics 7, 161–244.
Schrire BD, Lavin M, Barker NP, Forest F (2009) Phylogeny of the tribe Indigofereae (Leguminosae–Papilionoideae), geographically structured more in succulent-rich and temperate settings than in grass-rich environments. American Journal of Botany 96, 816–852.
| Phylogeny of the tribe Indigofereae (Leguminosae–Papilionoideae), geographically structured more in succulent-rich and temperate settings than in grass-rich environments.Crossref | GoogleScholarGoogle Scholar | 21628237PubMed |
Seigler DS, Ebinger JE, Riggin CW, Terra V, Miller JT (2017) Parasenegalia and Pseudosenegalia (Fabaceae), new genera of the Mimosoideae. Novon 25, 180–205.
| Parasenegalia and Pseudosenegalia (Fabaceae), new genera of the Mimosoideae.Crossref | GoogleScholarGoogle Scholar |
Shenoy M, Borges RM (2008) A novel mutualism between an ant-plant and its resident pollinator. Naturwissenschaften 95, 61–65.
| A novel mutualism between an ant-plant and its resident pollinator.Crossref | GoogleScholarGoogle Scholar | 17657468PubMed |
Shenoy M, Radhika V, Satish S, Borges RM (2012) Composition of extrafloral nectar influences interactions between the myrmecophyte Humboldtia brunonis and its ant associates. Journal of Chemical Ecology 38, 88–99.
| Composition of extrafloral nectar influences interactions between the myrmecophyte Humboldtia brunonis and its ant associates.Crossref | GoogleScholarGoogle Scholar | 22234428PubMed |
Sherbrooke WC, Scheerens JC (1979) Ant-visited extrafloral (calyx and foliar) nectaries and nectar sugars of Erythrina flabelliformis Kearney in Arizona. Annals of the Missouri Botanical Garden 66, 472–481.
| Ant-visited extrafloral (calyx and foliar) nectaries and nectar sugars of Erythrina flabelliformis Kearney in Arizona.Crossref | GoogleScholarGoogle Scholar |
Silva MDS, Coutinho ÍAC, Araújo MN, Meira RMSA (2017) Morphoanatomy of nectaries of Chamaecrista (L.) Moench sections Chamaecrista, Caliciopsis and Xerocalyx (Leguminosae: Caesalpinioideae). Acta Botanica Brasílica 31, 445–458.
| Morphoanatomy of nectaries of Chamaecrista (L.) Moench sections Chamaecrista, Caliciopsis and Xerocalyx (Leguminosae: Caesalpinioideae).Crossref | GoogleScholarGoogle Scholar |
Simon MF, Grether R, de Queiroz LP, Särkinen TE, Dutra VF, Hughes CE (2011) The evolutionary history of Mimosa (Leguminosae), toward a phylogeny of the sensitive plants. American Journal of Botany 98, 1201–1221.
| The evolutionary history of Mimosa (Leguminosae), toward a phylogeny of the sensitive plants.Crossref | GoogleScholarGoogle Scholar | 21730340PubMed |
Smith SA, O’Meara BC (2012) treePL: divergence time estimation using penalized likelihood for large phylogenies. Bioinformatics 28, 2689–2690.
| treePL: divergence time estimation using penalized likelihood for large phylogenies.Crossref | GoogleScholarGoogle Scholar | 22908216PubMed |
So ML (2004) The occurrence of extrafloral nectaries in Hong Kong plants. Botanical Bulletin of Academia Sinica 45, 237–245.
Sprent JI (2009) ‘Legume Nodulation, a Global Perspective’ (Wiley-Blackwell: Oxford, UK)
Stenglein SA (2004) Micromorphological variability of leaf epidermis in Mesoamerican common bean (Phaseolus vulgaris, Leguminosae). Australian Journal of Botany 52, 73–80.
| Micromorphological variability of leaf epidermis in Mesoamerican common bean (Phaseolus vulgaris, Leguminosae).Crossref | GoogleScholarGoogle Scholar |
Thulin M, Lavin M, Pasquet R, Delgado-Salinas A (2004) Phylogeny and biogeography of Wajira (Leguminosae), a monophyletic segregate of Vigna centered in the Horn of Africa region. Systematic Botany 29, 903–920.
| Phylogeny and biogeography of Wajira (Leguminosae), a monophyletic segregate of Vigna centered in the Horn of Africa region.Crossref | GoogleScholarGoogle Scholar |
Trethowan L, Clark R, Mackinder B (2015) A synopsis of the neotropical genus Schnella (Cercideae: Caesalpinioideae: Leguminosae) including 12 new combinations. Phytotaxa 204, 237–252.
| A synopsis of the neotropical genus Schnella (Cercideae: Caesalpinioideae: Leguminosae) including 12 new combinations.Crossref | GoogleScholarGoogle Scholar |
van Velzen R, Doyle JJ, Geurts R (2019) A resurrected scenario: single gain and massive loss of nitrogen-fixing nodulation. Trends in Plant Science 24, 49–57.
| A resurrected scenario: single gain and massive loss of nitrogen-fixing nodulation.Crossref | GoogleScholarGoogle Scholar | 30409687PubMed |
Villiers JF (1989) ‘Flore du Gabon, 31 Leguminosae–Mimosoideae.’ (Museum National d’Histoire Naturelle: Paris, France)
Villiers JF (2002a) Tribe Ingeae. In ‘The Leguminosae of Madagascar’. (Eds DJ Du Puy, J-N Labat, R Rabevohitra, J-F Villiers, J Bosser, J Moat) pp. 243–288. (Royal Botanic Gardens, Kew: London, UK)
Villiers JF (2002b) Tribe Mimosae. In ‘The Leguminosae of Madagascar’. (Eds Eds DJ Du Puy, J-N Labat, R Rabevohitra, J-F Villiers, J Bosser, J Moat) pp. 159–223 (Royal Botanic Gardens, Kew: London, UK)
Villiers JF, Guinet P (1989) Lemurodendron Villiers et Guinet, genre nouveau de Leguminosae Mimosoideae de Madagascar. Bulletin du Muséum National d’Histoire Naturelle – B. Adansonia 11, 3–10.
Vogel S (1977) Nectaries and their ecological significance. Apidologie 8, 321–335.
| Nectaries and their ecological significance.Crossref | GoogleScholarGoogle Scholar |
Vogel S (1998) Remarkable nectaries, structure, ecology, organophyletic perspectives IV. Miscellaneous cases. Flora 193, 225–248.
| Remarkable nectaries, structure, ecology, organophyletic perspectives IV. Miscellaneous cases.Crossref | GoogleScholarGoogle Scholar |
Warwick MC, Lewis GP (2003) Revision of Plathymenia (Leguminosae–Mimosoideae). Edinburgh Journal of Botany 60, 111–119.
| Revision of Plathymenia (Leguminosae–Mimosoideae).Crossref | GoogleScholarGoogle Scholar |
Weber MG, Agrawal AA (2014) Defense mutualisms enhance plant diversification. Proceedings of the National Academy of Sciences of the United States of America 111, 16 442–16 447.
| Defense mutualisms enhance plant diversification.Crossref | GoogleScholarGoogle Scholar |
Weber M, Igersheim A (1994) Pollen buds in Ophiorrhiza (Rubiaceae) and their role in pollenkit release. Botanica Acta 107, 257–262.
| Pollen buds in Ophiorrhiza (Rubiaceae) and their role in pollenkit release.Crossref | GoogleScholarGoogle Scholar |
Weber MG, Keeler KH (2013) The phylogenetic distribution of extrafloral nectaries in plants. Annals of Botany 111, 1251–1261.
| The phylogenetic distribution of extrafloral nectaries in plants.Crossref | GoogleScholarGoogle Scholar | 23087129PubMed |
Weber MG, Porturas LD, Keeler KH (2015) World list of plants with extrafloral nectaries. Available at www.extrafloralnectaries.org [Verifed 11 January 2019].
Wettstein RR (1889) Über die Composition der österreichisch-ungarischen Flora mit zuckerabscheidenden Hüllschuppen. Österreichische Akademie der Wissenschaften Sitzungsberichte. Abteilung I. Mineralogie, Krystallographie, Botanik 97, 570–589.
Wink M (2013) Evolution of secondary metabolites in legumes (Fabaceae). South African Journal of Botany 89, 164–175.
| Evolution of secondary metabolites in legumes (Fabaceae).Crossref | GoogleScholarGoogle Scholar |
Wunderlin RP (2010) Reorganization of the Cercideae (Fabaceae, Caesalpinioideae). Phytoneuron 48, 1–5.
Yamashiro A, Yamashiro T (2008) Utilization on extrafloral nectaries and fruit domatia of Canavalia lineata and C. cathartica (Leguminosae) by ants. Arthropod-Plant Interactions 2, 1–8.
| Utilization on extrafloral nectaries and fruit domatia of Canavalia lineata and C. cathartica (Leguminosae) by ants.Crossref | GoogleScholarGoogle Scholar |
Zarucchi JL (1994) Summary of phytochemical reports in this dictionary. In ‘Phytochemical Dictionary of the Leguminosae ILDIS & CHCD’. (Eds FA Bisby J Buckingham, JB Harborne) pp. 24–34. (Chapman & Hall: London, UK)
Zimmermann JG (1932) Über die extrafloralen Nektarien der Angiospermen. Beihefte zum Botanischen Centralblatt 49, 99–196.
1 Also published in Carl von Linné’s Amoenitates academica, vol 6. Laurentius salvius (pp. 263–278).
2 According to Schmid (1988), the part on nectaries appears in Delpino (1873, pp. 233–275 and, although dated 1873, apparently was issued 1874. The entire work was issued as a separate publication in 1875, with new pagination, Abstracts in Botanische Zeitung vol. 29, pp. 443–445, 447–459, 463–467, vol. 33, p. 807 and Just’s Botanischer Jahresbericht vol. 2, pp. 881–896, the last by Hermann Müller.














