Involvement of peroxiredoxin 2 in cumulus expansion and oocyte maturation in mice
You-Jee Jang A , Jin-Seon Kim B , Pu-Reum Yun B , Young-Woo Seo A , Tae-Hoon Lee C , Jae-Il Park A D and Sang-Young Chun B D
B D
A Animal Facility of Aging Science, Korea Basic Science Institute, Gwangju 61186, Republic of Korea.
B School of Biological Sciences and Biotechnology, Faculty of Life Science, Chonnam National University, Gwangju 61186, Republic of Korea.
C Department of Oral Biochemistry, College of Dentistry, Chonnam National University, Gwangju 61186, Republic of Korea.
D Corresponding authors. Email: jaeil74@kbsi.re.kr; sychun@jnu.ac.kr
Reproduction, Fertility and Development 32(8) 783-791 https://doi.org/10.1071/RD19310
Submitted: 3 April 2019 Accepted: 30 November 2019 Published: 7 May 2020
Journal Compilation © CSIRO 2020 Open Access CC BY-NC-ND
Abstract
Peroxiredoxin 2 (Prdx2), an antioxidant enzyme, is expressed in the ovary during the ovulatory process. The aim of the present study was to examine the physiological role of Prdx2 during ovulation using Prdx2-knockout mice and mouse cumulus–oocyte complex (COC) from WT mice. Two days of treatment of immature mice (21–23 days old) with equine chorionic gonadotrophin and followed by treatment with human chorionic gonadotrophin greatly impaired cumulus expansion and oocyte maturation in Prdx2-knockout but not wild-type mice. Treatment of COCs in culture with conoidin A (50 µM), a 2-cys Prdx inhibitor, abolished epiregulin (EPI)-induced cumulus expansion. Conoidin A treatment also inhibited EPI-stimulated signal molecules, including signal transducer and activator of transcription-3, AKT and mitogen-activated protein kinase 1/2. Conoidin A treatment also reduced the gene expression of EPI-stimulated expansion-inducing factors (hyaluronan synthase 2 (Has2), pentraxin 3 (Ptx3), TNF-α induced protein 6 (Tnfaip6) and prostaglandin-endoperoxide synthase 2 (Ptgs2)) and oocyte-derived factors (growth differentiation factor 9 (Gdf9) and bone morphogenetic protein 15 (Bmp15)). Furthermore, conoidin A inhibited EPI-induced oocyte maturation and the activity of connexins 43 and 37. Together, these results demonstrate that Prdx2 plays a role in regulating cumulus expansion and oocyte maturation during the ovulatory process in mice, probably by modulating epidermal growth factor receptor signalling.

Additional keywords: cumulus cell, Graafian follicle, ovary, ovulation.
Introduction
Reactive oxygen species (ROS) play an important role in ovulation. Administration of antioxidant agents to rodents reduces the ovulation rate and cumulus expansion (Shkolnik et al. 2011; Park et al. 2012). A moderate increase in ROS in preovulatory follicles triggers oocyte maturation in rats. However, the excess generation of ROS causes oxidative stress, affecting ovarian physiology (Sugino 2005; Tsai-Turton and Luderer 2006).
Excess ROS are eliminated by antioxidant enzymes, such as superoxide dismutase (SOD), catalase and peroxiredoxins (PRDXs). The six isoforms of mammalian PRDXs are classified into three subtypes with four typical two-cysteine (2-Cys) residues (PRDX1–4), one atypical 2-Cys residue (PRDX5) and one 1-Cys residue (PRDX6; Wood et al. 2003). PRDX2 has been implicated in several cellular functions, such as cell proliferation, apoptosis and intracellular signalling (Latimer and Veal 2016; Rhee 2016). PRDX2 is also associated with cancer, cardiovascular dysfunction and neurodegeneration (Rhee 2016; Nicolussi et al. 2017). However, Prdx2-deficient mice appear normal and fertile because of a compensation mechanism (Lee et al. 2003).
To prevent the detrimental effects of excess ROS, the ovary possesses antioxidant defences. Ascorbic acid, a water-soluble antioxidant vitamin, accumulates during ovulation (Guarnaccia et al. 2000). The antioxidant tripeptide glutathione prevents the apoptosis of preovulatory rat follicles (Tsai-Turton and Luderer 2006; Tsai-Turton et al. 2007). The major ROS scavenging enzymes SOD1 and SOD2 are expressed in the ovaries of several species, including humans and rodents (Sasaki et al. 1994; Tilly and Tilly 1995; Nomura et al. 1996). SOD2-deficient female mice have reduced fertility (Ho et al. 1998; Matzuk et al. 1998) and impaired progesterone secretion (Noda et al. 2012). PRDX1 and PRDX2 are the major PRDXs expressed in the ovary during ovulation (Park et al. 2012). PRDX2 is expressed in cumulus cells and oocytes, as well as in the granulosa cells of preovulatory follicles. Prdx2 plays a vital role in inhibiting ovarian apoptosis through the clearance of H2O2 in mice (Yang et al. 2011). In addition, Prdx2 is required for spindle assembly and chromosome organisation during meiotic maturation in mice (Jeon et al. 2017).
Although the expression of PRDX2 has been demonstrated in the ovary, its physiological role during ovulation remains to be determined. In the present study, the function of Prdx2 was examined in vivo using Prdx2-null mice and in vitro using culture of cumulus–oocyte complexes (COCs). We assumed that the expression of Prdx2 in preovulatory follicles of Prdx2-null mice may be gradually replaced by other Prdxs during follicular growth from prepubertal to adult age. To shorten this compensation period, the growth of preovulatory follicles was artificially induced by administration of gonadotrophins to prepubertal Prdx2-null mice. In addition to the in vivo study in mice, the function of Prdx1/2 was examined in an in vitro culture of COCs using conoidin A, an inhibitor of typical 2-Cys PRDX1–4. Conoidin A has been shown to inhibit PRDX1 and PRDX2 by blocking their hyperoxidation activity (Haraldsen et al. 2009). The results of the present study demonstrate the regulation of cumulus expansion and oocyte maturation by Prdx2 in mice.
Materials and methods
Animals
Immature female C57BL/6 mice were purchased from Samtako. Prdx2–/– (knockout) and Prdx2+/– (heterozygous) mice with the C57BL6/J background were kindly provided by DY Yu (Korea Research Institute of Bioscience and Biotechnology; Lee et al. 2003). Animals were housed in groups under a 14-h light : 10-h dark regimen and provided food and water ad libitum. All animals were maintained and treated in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (National Research Council 2011), as approved by the Institution Animal Care and Use Committee at Chonnam National University.
Examination of ovulation in Prdx2–/– mice in vivo
To induce superovulation, wild-type, Prdx2+/– and Prdx2–/– mice (21–23 days old) were injected subcutaneously with 5 IU equine chorionic gonadotrophin (eCG, also referred to as pregnant mare’s serum gonadotrophin (PMSG); Sigma) to stimulate the growth of multiple follicles. Two days later, some eCG-primed mice were injected intraperitoneally with 5 IU human chorionic gonadotrophin (hCG; Sigma).
To examine ovulation rate, mice were killed, the oviducts were excised and flushed and the oocytes were counted under a dissecting microscope 24 h after hCG administration.
To examine cumulus expansion, ovaries were isolated 6 h after hCG injection and placed in α-minimum essential medium (MEM; Invitrogen) supplemented with 25 mM HEPES, 0.25 mM sodium pyruvate, 3 mM l-glutamine, 100 U mL–1 penicillin and 100 µg mL–1 streptomycin. Preovulatory follicles were then punctured using 26-gauge needles to release COCs. Cumulus expansion was assessed by microscopic examination using a previously described scoring system (Vanderhyden et al. 1990). Scores of 0–1 indicate no expansion or minimum expansion; a score of 2 indicates that cells in the outer two layers have expanded; a score of 3 indicates expansion of all layers of the cumulus except corona radiata cells; and a score of 4 indicates expansion of the whole cumulus, including corona radiata cells. Using these scores, a mean cumulus expansion index (CEI; range 0.0–4.0) was calculated for each group.
To examine oocyte maturation, ovaries were isolated 6 h after hCG injection and placed in α-MEM. COCs released from preovulatory follicles were denuded of cumulus cells by gentle pipetting. Denuded oocytes were examined under a stereomicroscope for evidence of the dissolution of the oocyte nuclear membrane (a process called germinal vesicle breakdown (GVBD)), a hallmark of oocyte meiotic resumption. The experimental design is outlined in Fig. S1, available as Supplementary Material to this paper.
Cumulus expansion and oocyte maturation in the culture of COCs
Unexpanded COCs were collected in complete α-MEM from the ovaries of immature wild-type mice primed with eCG for 48 h. For in vitro COC expansion, 30–35 COCs were plated in separate wells of Nunclon four-well dishes (Nunc) in 150 µL α-MEM with 3 mg mL–1 bovine serum albumin and 5% fetal bovine serum under a cover of mineral oil. The COCs treated with 100 nM epiregulin (EPI) and/or 1–100 µM conoidin A (Cayman), an inhibitor of typical 2-Cys PRDX1–4, and were incubated at 37°C in a modular incubation chamber (Billups Rothenberg) infused with 5% O2, 5% CO2 and 90% N2. COCs were pretreated with conoidin A for 1 h prior to EPI treatment and then cultured in the presence of conoidin A for the duration of the culture period (14 h). The degree of cumulus expansion was then assessed by microscopic examination using the previously described scoring system (Vanderhyden et al. 1990).
To assess oocyte maturation, 30 COCs were cultured in 150 µL α-MEM containing 10 µM 3-isobutyl-1-methylxanthine (IBMX) to block spontaneous GVBD in Nunclon four-well dishes in the presence of 100nM EPI with or without 1–100 µM conoidin A at 37°C for 4 h in a humidified atmosphere of 5% CO2 in air. After removal of cumulus cells by pipetting, oocyte maturation was assessed by scoring GVBD. Experiments were repeated three times with three different mice.
RNA isolation and real-time polymerase chain reaction analysis
Total RNA was isolated using a Total RNA mini kit (Favourgen Biotech) according to the manufacturer’s instructions. Total RNA was reverse transcribed, and real-time polymerase chain reaction (PCR) was performed on a Rotor-Gene Q 5plex (QIAGEN), located at the Korea Basic Science Institute, using a QuantiTect SYBR Green PCR Kit (QIAGEN). Primers were designed using Primer3 software (http://primer3.ut.ee/, accessed 6 March 2020) and are listed in Table S1. The mean Ct value of three determinations for each gene was divided by the linear Ct of the β-actin (Actb) gene to obtain the relative abundance of the transcript. Mean values were obtained from three or four separate experiments. Actb was used as an internal control for all measurements.
Western blot analysis
To evaluate the effects of conoidin A on the downstream signalling pathway for EPI activation, 100 COCs were cultured in 500 µL α-MEM without serum in four-well dishes in the presence of EPI (100 nM) with or without conoidin A (50 µM) for 15 min at 37°C. To assess the expression of connexin (Cx) 43 and Cx37, 200 COCs were cultured in a four-well dish for up to 2 h, in 500 µL α-MEM at 37°C in a humidified atmosphere of 5% CO2 in air. Lysates of COCs were resolved by 4–20% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (Invitrogen) and transferred to nitrocellulose membranes (Amersham Bioscience), per the procedure and incubation conditions as described by Park et al. 2012 and Jeon et al. 2017. Membranes were blocked with 3% skim milk before immunoblotting using a primary antibody (1 : 500 final dilution) and a horseradish peroxidase-conjugated secondary IgG (1 : 1000 final dilution). The anti-Cx37 (ab185820) and anti-Cx43 (ab11370) antibodies were purchased from Abcam. The antibody against phosphorylated (at Ser368) Cx43 (3511S) and all the antibodies used for the signalling study were purchased from Cell Signaling. After washing in 1× Tris-buffered saline Tween-20 (TBST), signals were visualised with enhanced chemiluminescence. Band intensities were quantified using UNSCAN-IT Gel 6.1 software (Silk Scientific) after subtraction of background signal and normalised against glyceraldehyde-3-phosphate dehydrogenase (Gapdh; Santa Cruz Biotechnology).
Statistical analysis
Data are presented as the mean ± s.e.m. One-way analysis of variance (ANOVA) followed by the Dunnett test was used for comparisons among multiple groups. Comparisons between any two points were evaluated using Student’s two-tailed t-test. P < 0.05 was considered significant.
Results
Cumulus expansion and oocyte meiotic resumption in immature Prdx2-deficient mice
Mice deficient in the Prdx2 gene exhibit normal postnatal development and fertility, perhaps due to a compensatory mechanism from other Prdxs (Lee et al. 2003). To circumvent this compensation even partially, prepubertal mice were treated with gonadotrophins in the present study to induce superovulation. The ovulation rate in hCG-treated Prdx2-null mice (38 ± 17 oocytes) was not significantly different to that in wild-type or heterozygous mice (50 ± 17 and 42.3 ± 7.3 oocytes respectively; Fig. 1a).
Cumulus expansion and oocyte maturation were examined in COCs collected from ovarian preovulatory follicles of mice 6 h after hCG administration. Only 14.3 ± 24.8% of COCs in Prdx2-null mice were expanded, compared with 85% and 87% of COCs in the wild-type and heterozygous mice respectively, in which cumulus cells reached almost full expansion (i.e. CEI 3–4; Fig. 1b). Interestingly, the rate of oocyte meiotic resumption, scored as percentage GVBD, was markedly reduced in Prdx2-null mice (2.2 ± 3.8%), whereas more than 80% of oocytes exhibited GVBD in wild-type and heterozygous mice (Fig. 1c).
Regulation of cumulus expansion by conoidin A in mouse COC culture
Complementary to the in vivo analysis, we examined in vitro cumulus expansion using conoidin A, a 2-Cys Prdx inhibitor. Unexpanded COCs isolated from eCG-primed mice were pretreated with conoidin A for 1 h. When COCs were cultured with 100 nM EPI for 14 h in the absence of conoidin A, a maximum degree of expansion was observed (Fig. 2a). The addition of higher concentrations of conoidin A (50–100 μM) to the EPI-containing medium reduced expansion up to 66%, whereas lower concentrations (1–30 μM) had no effect (Fig. 2b). Exposure to and culture of unexpanded COCs in the presence of conoidin A alone resulted in a similar degree of cumulus expansion to that seen in the control group.
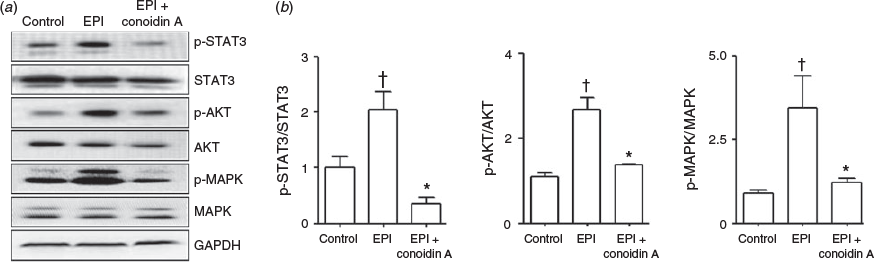
To examine the effect of Prdx2 on epidermal growth factor (EGF) receptor signalling, unexpanded COCs were treated with EPI and/or conoidin A for 15 min in serum-free medium. EPI increased the phosphorylation of signal transducer and activator of transcription (STAT) 3, AKT and mitogen-activated protein kinase (MAPK) 1/2 (Fig. 3a), whereas the addition of conoidin A suppressed EPI-stimulated STAT3, AKT and MAPK 1/2 phosphorylation by 85%, 48% and 65% respectively (Fig. 3b).
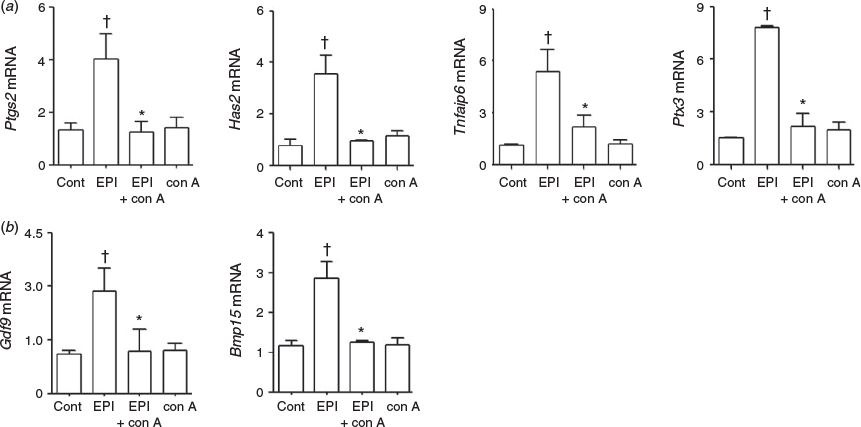
We next examined the effect of conoidin A on the regulation of genes known to affect expansion during COC expansion. The addition of conoidin A suppressed EPI-stimulated expression of hyaluronan synthase 2 (Has2), pentraxin 3 (Ptx3), TNF-α induced protein 6 (Tnfaip6) and prostaglandin-endoperoxide synthase 2 (Ptgs2) by 60–72% (Fig. 4a). Interestingly, the expression of the oocyte-derived factors growth differentiation factor 9 (Gdf9) and bone morphogenetic protein 15 (Bmp15) was stimulated by EPI, and the addition of conoidin A abolished this effect (Fig. 4b).
Regulation of oocyte maturation by conoidin A in COC culture
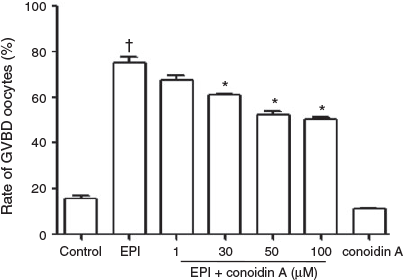
To demonstrate the in vitro function of 2-Cys Prdxs in oocyte maturation, unexpanded COCs were cultured for 4 h in the presence of 10 µM IBMX (to block spontaneous maturation) with 100 nM EPI and/or 1–100 µM conoidin A. In EPI-treated COCs, release from meiotic arrest was significantly higher (75% GVBD) than in COCs cultured with IBMX alone (control; Fig. 5). The addition of conoidin A inhibited EPI-induced GVBD in a dose-dependent manner, with maximum inhibition at 50 μM conoidin A (52% GVBD). Treatment with conoidin A alone resulted in a similar level of GVBD as that in the control.
|
Cx43 and Cx37 play an important role in oocyte maturation (Richard and Baltz 2014). Treatment of COCs with EPI for 1 h resulted in a twofold increase in Cx43 phosphorylation (Fig. 6a). The addition of conoidin A for 1 h inhibited this EPI-induced increase in Cx43 phosphorylation by 46.3%. Treatment with EPI and/or conoidin A for 2 h had no significant effect on Cx43 phosphorylation (Fig. 6b). Expression of Cx37 was markedly increased by EPI treatment for 1 and 2 h (approximately twofold), and the addition of conoidin A completely abolished the effects of EPI.
Discussion
Although Prdx2 is expressed in preovulatory follicles (Park et al. 2012), its physiological function has not been reported. To the best of our knowledge, the present study demonstrated, for the first time, Prdx2 regulation of cumulus expansion and oocyte maturation during ovulation in mice. Prdx2-deficient mice showed less cumulus expansion and oocyte maturation, but no difference in ovulation rate, than Prdx2 wild-type mice after administration of eCG and hCG to prepubertal mice. The role of Prdx1/2 in cumulus expansion and oocyte maturation was demonstrated in the in vitro culture of COCs using conoidin A, an inhibitor of 2-Cys Prdxs. Conoidin A inhibited EPI-induced cumulus expansion by suppressing the expansion-inducing transcripts Has2, Ptx3, Tnfaip6 and Ptgs2 and the oocyte-derived factors Gdf9 and Bmp15. Conoidin A also inhibited oocyte maturation by suppressing the expression of gap junction proteins Cx37 and Cx43. Prdx2 plays an important role in the meiotic maturation of mouse oocytes (Jeon et al. 2017).
The fact that Prdx2-deficient mice exhibit normal fertility (Lee et al. 2003) indicates the existence of a redundant pathway in the ovary. The structure and function of Prdx1 and Prdx2 are similar (Kang et al. 2005), and both are expressed in the ovary (Leyens et al. 2003; Park et al. 2012). In preovulatory follicles, the expression of Prdx2 is higher than that of Prdx1 (Park et al. 2012), indicating that Prdx2 is a major form of Prdxs expressed during ovulation. Therefore, when Prdx2 expression is absent in Prdx2-deficient mice, Prdx1 may be highly expressed up to the levels of Prdx2 for functional compensation, which may explain the normal fertility reported in Prdx2-deficient mice. The present results regarding reduced cumulus expansion and oocyte maturation may indicate that injection of Prdx2-deficent mice with gonadotrophin at an early age (prepubertal) partially eliminated this redundant pathway. The ovary of adult mice contains many preovulatory follicles, whereas the ovary of prepubertal mice primarily contains immature preantral follicles (Komatsu and Masubuchi 2018). In Prdx2-deficent mice, the expression of Prdx2 in preovulatory follicles may be compensated for by other Prdxs, especially by Prdx1, during growth from preantral follicles in prepubertal mice to preovulatory follicles in adult mice. Therefore, the induction of growth from preantral follicles to preovulatory follicles by gonadotrophins within 2 days in prepubertal Prdx2-deficient mice may shorten the time for compensation by other Prdxs, and thus Prdx2 may be temporally expressed in preovulatory follicles. Unfortunately, however, the detailed mechanism by which Prdx2 regulates cumulus expansion and oocyte maturation could not be examined using Prdx2-deficient mice because the breeding of these mice was stopped due to a viral infection. The ovulation rate was not altered in Prdx2-deficient mice, suggesting that Prdx2 may not be involved in regulating factors important for follicle rupture, such as the progesterone receptor and its downstream molecules (Richards et al. 2002a).
The present data obtained from superovulated Prdx2-deficient mice suggest that Prdx2 may be a major antioxidant enzyme regulating the generation of ROS during the ovulatory process. Because large amounts of ROS are generated during the inflammatory process (Johnson et al. 1986), the analogy of ovulation to acute inflammation (Richards et al. 2002b) indicates a role for ROS in this process (Shkolnik et al. 2011). ROS have both deleterious and beneficial effects depending on their concentration. Such dual effects of ROS can be modulated by PRDX2 antioxidant enzyme during ovulation, which acts as a floodgate to maintain low resting levels of ROS and permit higher levels during signal transduction (Laloraya et al. 1988).
Although conoidin A inhibits 2-Cys PRDX1–4 (Haraldsen et al. 2009), the effect of conoidin A on cumulus expansion and oocyte maturation observed in the present in vitro study may be mediated through the inhibition of Prdx2 and/or Prdx1, because Prdx1/2 are the major Prdxs expressed in granulosa cells, cumulus cells and oocytes of preovulatory follicles. In rats, Prdx2, but not Prdx1, is expressed in the oocytes of preovulatory follicles (Park et al. 2012). Granulosa cells and corpora lutea express both PRDX1 and PRDX2 in mice. However, Prdx1 and Prdx2 protein is expressed in mouse oocytes (Jeon et al. 2017). Nevertheless, there is limited ability to come to any conclusion regarding the effects of Prdx2 on cumulus expansion and oocyte maturation in the present study because of the use of the non-specific PRDX inhibitor conoidin A.
The inhibitory effect of conoidin A in COC culture suggests that Prdx1/2 may regulate the EGF receptor (EGFR) signalling pathway to stimulate cumulus expansion-inducing transcripts, resulting in the expansion of cumulus cells. The most prominent EGFR signalling pathway is the MAPK cascade for cumulus expansion, which is activated by EGF-like factors such as EPI (Richards and Ascoli 2018). Although PRDXs inhibit activation of H2O2-activated signalling pathways, PRDXs have also been found to be important for the activation of EGFR signalling, including MAPK (Latimer and Veal 2016). PRDX2 facilitates protein tyrosine phosphatases 1B (PTPIB) inactivation (Dagnell et al. 2017; Kim et al. 2018), a key protein tyrosine phosphatase in the regulation of EGFR-dependent singling (DeYulia and Carcamo 2005). Therefore, PRDX2 may potentiate EGFR signalling by inactivating PTPIB, and thus contribute to cumulus expansion.
Gonadotrophin treatment of prepubertal Prdx2-deficient mice resulted in a defect in cumulus expansion without affecting the ovulation rate. Defects in cumulus expansion often reduce ovulation efficiency (Zhuo and Kimata 2001). Cumulus expansion is severely impaired in mice deficient in the prostanoid Ep2 receptor, one of the receptors for prostaglandin E2, with ovulation efficiency reduced by 28.9% (Kennedy et al. 1999). The ovulated COCs in Ep2-null mice show an irregularly aggregated cumulus oophorus leading to severely impaired fertilisation. Mice deficient in bikunin, which is necessary for the formation of the cumulus hyaluronan-rich matrix, show reduced ovulation efficiency (by 57%) with rarely fertilised oocytes (Zhuo et al. 2001). Mice deficient in natriuretic peptide receptor 2 (Npr2) show a defect in cumulus expansion leading to severe female infertility but not affecting ovulation efficiency (Kiyosu et al. 2012). Therefore, it seems that in knockout mice the magnitude of the effect of the defect in cumulus expansion on ovulation efficiency may depend on the gene deleted. In the present study, deletion of Prdx2 impaired cumulus expansion without affecting ovulation rate. Another possibility is that the defect in cumulus expansion observed in Prdx2-deficient mice was due simply to delayed cumulus expansion, rather than to a complete block. It is possible that, in early time of LH/hCG injection to induce ovulation, Prdx1 was weakly expressed but near the time of follicle rupture Prdx1 was highly expressed to compensate for the lack of Prdx2 function on cumulus expansion and GVBD in preovulatory follicles of superovulated Prdx2-deficient mice. In fact, based on dissecting microscope observations, the cumulus cells seemed to be intact without denuded oocytes in ovulated COCs (data not shown). Further elaboration of the state of cumulus cells in ovulated COCs and examination of fertilisation efficiency after superovulation of Prdx2-null mice is needed. In addition, the generation of ovulation-specific Prdx2-deficient mice, and mice deficient in both Prdx1 and Prdx2 is necessary.
The inhibitory effect of conoidin A on cumulus expansion may be mediated by regulation of phosphatase and tensin homologue (PTEN) activity to modulate the phosphatidylinositol 3-kinase (PI3K)/AKT pathway for cumulus expansion. Targeted depletion of the Pten gene in mouse granulosa cells increases ovulation rates by enhancing proliferation (Fan et al. 2012). EGFR and FSH activate the PI3K/AKT signalling pathway to regulate granulosa cell differentiation (Wayne et al. 2007; Richards and Ascoli 2018). The binding of PRDX1/2 to PTEN alters its downstream signalling to inhibit tumourigenesis (Cao et al. 2009; Verrastro et al. 2016). However, activation of the PI3K/AKT pathway of EGFR signalling in cumulus cells has not been clearly demonstrated. It is also likely that PRDX2 regulates the PTEN/AKT signalling pathway of cumulus expansion-inducing cytokines, such as interleukin (IL)-6 and interferon (IFN)-α which were generated by EGF-like factors. IL-6 (Liu et al. 2009) and IFN-α (Jang et al. 2015) induce cumulus expansion in mice by activating the MAPK and AKT signalling pathways. It is also possible that PRDX2 may contribute to the activation of STAT3 of IL-6 or IFN-α singling. PRDX2 participates directly in the activation of STAT3 (Sobotta et al. 2015).
Another way that PRDX may contribute to cumulus expansion is by regulating the oocyte-derived factors GDF9 and BMP15. The expression and activation of EGFR in cumulus cells are dependent on GDF9 and BMP15 (Fan et al. 2012). GDF9 promotes cumulus expansion by stimulating Ptgs2 and Has2 expression (Elvin et al. 1999) and BMP15 regulates cumulus expansion via a mechanism requiring EGFR signalling (Yoshino et al. 2006).
PRDX regulates oocyte maturation by modulating the disruption of cumulus cell–oocyte gap junctions mediated via EGF-like factors. One of the pathways for the maintenance of oocyte meiotic arrest is via the supply of cGMP generated by the NPR2–C-type natriuretic peptide (CNP) system in granulosa and cumulus cells (Zhang et al. 2010; Conti et al. 2012) through gap junction Cx43 (granulosa–cumulus cell junction) and Cx37 (cumulus–oocyte junction) to the oocyte, which increases the activity of phosphodiesterase (PDE) 3A, the predominant cAMP PDE in the oocyte (Masciarelli et al. 2004). In addition to downregulating Cx43 and Cx37 expression, LH and hCG stimulate the phosphorylation of Cx43 protein (Gershon et al. 2007). EGFR activation causes gap junction closure by MAPK-dependent Cx phosphorylation, which blocks cGMP supply to the oocyte to induce meiotic resumption (Norris et al. 2010; Conti et al. 2012). Thus, it is likely that PRDX regulates the MAPK activity of EGFR signalling and thus stimulates the phosphorylation of Cxs.
In summary, the present study demonstrated that Prdx2 plays an important role in cumulus expansion and oocyte maturation during the ovulatory process, probably by modulating the EGFR signalling pathway. Further studies are needed to determine the mechanism underlying this role of Prdx2 by inducing the tissue-specific knockdown of Prdx2 during ovulation. Because ROS have been suggested to be associated with female infertility (Fujii et al. 2005), it would be of interest to investigate the clinical relevance of PRDX2 in women undergoing IVF.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgement
This work was supported by grants from the Basic Science Research Program through the National Research Program of Korea (NRF) funded by the Ministry of Science and Education (NRF-2015R1A2A2A01006519 and NRF-2018R1D1A1B07040623).
References
Cao, J., Schulte, J., Knight, A., Leslie, N. R., Zagozdzon, A., Bronson, R., Manevich, Y., Beeson, C., and Neumann, C. A. (2009). Prdx1 inhibits tumorigenesis via regulating PTEN/AKT activity. EMBO J. 28, 1505–1517.| Prdx1 inhibits tumorigenesis via regulating PTEN/AKT activity.Crossref | GoogleScholarGoogle Scholar | 19369943PubMed |
Conti, M., Hsieh, M., Zamah, A. M., and Oh, J. S. (2012). Novel signaling mechanisms in the ovary during oocyte maturation and ovulation. Mol. Cell. Endocrinol. 356, 65–73.
| Novel signaling mechanisms in the ovary during oocyte maturation and ovulation.Crossref | GoogleScholarGoogle Scholar | 22101318PubMed |
Dagnell, M., Pace, P. E., Cheng, Q., Frijhoff, J., Ostman, A., Arner, E. S. J., Hampton, M. B., and Winterbourn, C. C. (2017). Thioredoxin reductase 1 and NADPH directly protect protein tyrosine phosphatase 1B from inactivation during H2O2 exposure. J. Biol. Chem. 292, 14371–14380.
| Thioredoxin reductase 1 and NADPH directly protect protein tyrosine phosphatase 1B from inactivation during H2O2 exposure.Crossref | GoogleScholarGoogle Scholar | 28684416PubMed |
DeYulia, G. J., and Carcamo, J. M. (2005). EGF receptor–ligand interaction generates extracellular hydrogen peroxide that inhibits EGFR-associated protein tyrosine phosphatases. Biochem. Biophys. Res. Commun. 334, 38–42.
| EGF receptor–ligand interaction generates extracellular hydrogen peroxide that inhibits EGFR-associated protein tyrosine phosphatases.Crossref | GoogleScholarGoogle Scholar | 15982634PubMed |
Elvin, J. A., Clark, A. T., Wang, P., Wolfman, N. M., and Matzuk, M. M. (1999). Paracrine actions of growth differentiation factor-9 in the mammalian ovary. Mol. Endocrinol. 13, 1035–1048.
| Paracrine actions of growth differentiation factor-9 in the mammalian ovary.Crossref | GoogleScholarGoogle Scholar | 10379900PubMed |
Fan, H. Y., Liu, Z., Mullany, L. K., and Richards, J. S. (2012). Consequences of RAS and MAPK activation in the ovary: the good, the bad and the ugly. Mol. Cell. Endocrinol. 356, 74–79.
| Consequences of RAS and MAPK activation in the ovary: the good, the bad and the ugly.Crossref | GoogleScholarGoogle Scholar | 22197887PubMed |
Fujii, J., Iuchi, Y., and Okada, F. (2005). Fundamental roles of reactive oxygen species and protective mechanisms in the female reproductive system. Reprod. Biol. Endocrinol. 3, 43–52.
| Fundamental roles of reactive oxygen species and protective mechanisms in the female reproductive system.Crossref | GoogleScholarGoogle Scholar | 16137335PubMed |
Gershon, E., Plaks, V., and Dekel, N. (2007). Gap junctions in the ovary: expression, localization and function. Mol. Cell. Endocrinol. 282, 18–25.
| Gap junctions in the ovary: expression, localization and function.Crossref | GoogleScholarGoogle Scholar | 18162286PubMed |
Guarnaccia, M. M., Takami, M., Jones, E. E., Preston, S. L., and Behrman, H. R. (2000). Luteinizing hormone depletes ascorbic acid in preovulatory follicles. Fertil. Steril. 74, 959–963.
| Luteinizing hormone depletes ascorbic acid in preovulatory follicles.Crossref | GoogleScholarGoogle Scholar | 11056240PubMed |
Haraldsen, J. D., Liu, G., Botting, C. H., Walton, J. G., Storm, J., Phalen, T. J., Kwok, L. Y., Soldati-Favre, D., Heintz, N. H., Müller, S., Westwood, N. J., and Ward, G. E. (2009). Identification of conoidin A as a covalent inhibitor of peroxiredoxin II. Org. Biomol. Chem. 7, 3040–3048.
| Identification of conoidin A as a covalent inhibitor of peroxiredoxin II.Crossref | GoogleScholarGoogle Scholar | 21359112PubMed |
Ho, Y. S., Gargano, M., Cao, J., Bronson, R. T., Heimler, I., and Hutz, R. J. (1998). Reduced fertility in female mice lacking copper–zinc superoxide dismutase. J. Biol. Chem. 273, 7765–7769.
| Reduced fertility in female mice lacking copper–zinc superoxide dismutase.Crossref | GoogleScholarGoogle Scholar | 9516486PubMed |
Jang, Y. J., Park, J. I., Moon, W. J., Dam, P. T., Cho, M. K., and Chun, S. Y. (2015). Cumulus cell-expressed type I interferons induce cumulus expansion in mice. Biol. Reprod. 92, 20–27.
| Cumulus cell-expressed type I interferons induce cumulus expansion in mice.Crossref | GoogleScholarGoogle Scholar | 25429090PubMed |
Jeon, H. J., Park, Y. S., Cho, D. H., Kim, J. S., Kim, E., Chae, H. Z., Chun, S. Y., and Oh, J. S. (2017). Peroxiredoxins are required for spindle assembly, chromosome organization, and polarization in mouse oocytes. Biochem. Biophys. Res. Commun. 489, 193–199.
| Peroxiredoxins are required for spindle assembly, chromosome organization, and polarization in mouse oocytes.Crossref | GoogleScholarGoogle Scholar | 28552528PubMed |
Johnson, K. J., Ward, P. A., Kunkel, R. G., and Wilson, B. S. (1986). Mediation of IgA induced lung injury in the rat. Role of macrophages and reactive oxygen products. Lab. Invest. 54, 499–506.
| 3009967PubMed |
Kang, S. W., Rhee, S. G., Chang, T. S., Jeong, W., and Choi, M. H. (2005). 2-Cys peroxiredoxin function in intracellular signal transduction: therapeutic implications. Trends Mol. Med. 11, 571–578.
| 2-Cys peroxiredoxin function in intracellular signal transduction: therapeutic implications.Crossref | GoogleScholarGoogle Scholar | 16290020PubMed |
Kennedy, C. R., Zhang, Y., Brandon, S., Guan, Y., Coffee, K., Funk, C. D., Magnuson, M. A., Oates, J. A., Breyer, M. D., and Breyer, R. M. (1999). Salt-sensitive hypertension and reduced fertility in mice lacking the prostaglandin EP2 receptor. Nat. Med. 5, 217–220.
| Salt-sensitive hypertension and reduced fertility in mice lacking the prostaglandin EP2 receptor.Crossref | GoogleScholarGoogle Scholar | 9930871PubMed |
Kim, J. H., Park, S. J., Chae, U., Seong, J., Lee, H. S., Lee, S. R., Lee, S., and Lee, D. S. (2018). Peroxiredoxin 2 mediates insulin sensitivity of skeletal muscles through regulation of protein tyrosine phosphatase oxidation. Int. J. Biochem. Cell Biol. 99, 80–90.
| Peroxiredoxin 2 mediates insulin sensitivity of skeletal muscles through regulation of protein tyrosine phosphatase oxidation.Crossref | GoogleScholarGoogle Scholar | 29605633PubMed |
Kiyosu, C., Tsuji, T., Yamada, K., Kajita, S., and Kunieda, T. (2012). NPPC/NPR2 signaling is essential for oocyte meiotic arrest and cumulus oophorus formation during follicular development in the mouse ovary. Reproduction 144, 187–193.
| NPPC/NPR2 signaling is essential for oocyte meiotic arrest and cumulus oophorus formation during follicular development in the mouse ovary.Crossref | GoogleScholarGoogle Scholar | 22696190PubMed |
Komatsu, K., and Masubuchi, S. (2018). Mouse oocytes connect with granulosa cells by fusing with cell membranes and form a large complex during follicle development. Biol. Reprod. 99, 527–535.
| Mouse oocytes connect with granulosa cells by fusing with cell membranes and form a large complex during follicle development.Crossref | GoogleScholarGoogle Scholar | 29590310PubMed |
Laloraya, M., Pradeep, K. G., and Laloraya, M. M. (1988). Changes in the levels of superoxide anion radical and superoxide dismutase during the estrous cycle of Rattus norvegicus and induction of superoxide dismutase in rat ovary by lutropin. Biochem. Biophys. Res. Commun. 157, 146–153.
| Changes in the levels of superoxide anion radical and superoxide dismutase during the estrous cycle of Rattus norvegicus and induction of superoxide dismutase in rat ovary by lutropin.Crossref | GoogleScholarGoogle Scholar | 2848516PubMed |
Latimer, H. R., and Veal, E. A. (2016). Peroxiredoxins in regulation of MAPK signalling pathways; sensors and barriers to signal transduction. Mol. Cells 39, 40–45.
| Peroxiredoxins in regulation of MAPK signalling pathways; sensors and barriers to signal transduction.Crossref | GoogleScholarGoogle Scholar | 26813660PubMed |
Lee, T. H., Kim, S. U., Yu, S. L., Kim, S. H., Park, D. S., Moon, H. B., Dho, S. H., Kwon, K. S., Kwon, H. J., Han, Y. H., Jeong, S., Kang, S. W., Shin, H. S., Lee, K. K., Rhee, S. G., and Yu, D. Y. (2003). Peroxiredoxin II is essential for sustaining life span of erythrocytes in mice. Blood 101, 5033–5038.
| Peroxiredoxin II is essential for sustaining life span of erythrocytes in mice.Crossref | GoogleScholarGoogle Scholar | 12586629PubMed |
Leyens, G., Donnay, I., and Knoops, B. (2003). Cloning of bovine peroxiredoxins – gene expression in bovine tissues and amino acid sequence comparison with rat, mouse and primate peroxiredoxins. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 136, 943–955.
| Cloning of bovine peroxiredoxins – gene expression in bovine tissues and amino acid sequence comparison with rat, mouse and primate peroxiredoxins.Crossref | GoogleScholarGoogle Scholar | 14662316PubMed |
Liu, Z., de Matos, D. G., Fan, H. Y., Shimada, M., Palmer, S., and Richards, J. S. (2009). Interleukin-6: an autocrine regulator of the mouse cumulus cell–oocyte complex expansion process. Endocrinology 150, 3360–3368.
| Interleukin-6: an autocrine regulator of the mouse cumulus cell–oocyte complex expansion process.Crossref | GoogleScholarGoogle Scholar | 19299453PubMed |
Masciarelli, S., Horner, K., Liu, C., Park, S. H., Hinckley, M., Hockman, S., Nedachi, T., Jin, C., Conti, M., and Manganiello, V. (2004). Cyclic nucleotide phosphodiesterase 3A-deficient mice as a model of female infertility. J. Clin. Invest. 114, 196–205.
| Cyclic nucleotide phosphodiesterase 3A-deficient mice as a model of female infertility.Crossref | GoogleScholarGoogle Scholar | 15254586PubMed |
Matzuk, M. M., Dionne, L., Guo, Q., Kumar, T. R., and Lebovitz, R. M. (1998). Ovarian function in superoxide dismutase 1 and 2 knockout mice. Endocrinology 139, 4008–4011.
| Ovarian function in superoxide dismutase 1 and 2 knockout mice.Crossref | GoogleScholarGoogle Scholar | 9724058PubMed |
National Research Council (2011). ‘National Institutes of Health Guide for the Care and Use of Laboratory Animals.’ (National Academy Press: Washington DC, USA.)
Nicolussi, A., D’Inzeo, S., Capalbo, C., Giannini, G., and Coppa, A. (2017). The role of peroxiredoxins in cancer. Mol. Clin. Oncol. 6, 139–153.
| The role of peroxiredoxins in cancer.Crossref | GoogleScholarGoogle Scholar | 28357082PubMed |
Noda, Y., Ota, K., Shirasawa, T., and Shimizu, T. (2012). Copper/zinc superoxide dismutase insufficiency impairs progesterone secretion and fertility in female mice. Biol. Reprod. 86, 1–8.
| Copper/zinc superoxide dismutase insufficiency impairs progesterone secretion and fertility in female mice.Crossref | GoogleScholarGoogle Scholar | 21900685PubMed |
Nomura, T., Sasaki, J., Mori, H., Sato, E. F., Watanabe, S., Kanda, S., Matsuura, J., Watanabe, H., and Inoue, M. (1996). Expression of manganese superoxide dismutase mRNA in reproductive organs during the ovulatory process and the estrous cycle of the rat. Histochem. Cell Biol. 105, 1–6.
| Expression of manganese superoxide dismutase mRNA in reproductive organs during the ovulatory process and the estrous cycle of the rat.Crossref | GoogleScholarGoogle Scholar | 8824900PubMed |
Norris, R. P., Freudzon, M., Nikolaev, V. O., and Jaffe, L. A. (2010). Epidermal growth factor receptor kinase activity is required for gap junction closure and for part of the decrease in ovarian follicle cGMP in response to LH. Reproduction 140, 655–662.
| Epidermal growth factor receptor kinase activity is required for gap junction closure and for part of the decrease in ovarian follicle cGMP in response to LH.Crossref | GoogleScholarGoogle Scholar | 20826538PubMed |
Park, J. I., Jeon, H. J., Jung, N. K., Jang, Y. J., Kim, J. S., Seo, Y. W., Jeong, M., Chae, H. Z., and Chun, S. Y. (2012). Periovulatory expression of hydrogen peroxide-induced sulfiredoxin and peroxiredoxin 2 in the rat ovary: gonadotropin regulation and potential modification. Endocrinology 153, 5512–5521.
| Periovulatory expression of hydrogen peroxide-induced sulfiredoxin and peroxiredoxin 2 in the rat ovary: gonadotropin regulation and potential modification.Crossref | GoogleScholarGoogle Scholar | 22989627PubMed |
Rhee, S. G. (2016). Overview on peroxiredoxin. Mol. Cells 39, 1–5.
| Overview on peroxiredoxin.Crossref | GoogleScholarGoogle Scholar | 26831451PubMed |
Richard, S., and Baltz, J. M. (2014). Prophase I arrest of mouse oocytes mediated by natriuretic peptide precursor C requires GJA1 (connexin-43) and GJA4 (connexin-37) gap junctions in the antral follicle and cumulus–oocyte complex. Biol. Reprod. 90, 137–146.
| Prophase I arrest of mouse oocytes mediated by natriuretic peptide precursor C requires GJA1 (connexin-43) and GJA4 (connexin-37) gap junctions in the antral follicle and cumulus–oocyte complex.Crossref | GoogleScholarGoogle Scholar | 24804968PubMed |
Richards, J. S., and Ascoli, M. (2018). Endocrine, paracrine, and autocrine signaling pathways that regulate ovulation. Trends Endocrinol. Metab. 29, 313–325.
| Endocrine, paracrine, and autocrine signaling pathways that regulate ovulation.Crossref | GoogleScholarGoogle Scholar | 29602523PubMed |
Richards, J. S., Russell, D. L., Ochsner, S., Hsieh, M., Doyle, K. H., Falender, A. E., Lo, Y. K., and Sharma, S. C. (2002a). Novel signaling pathways that control ovarian follicular development, ovulation, and luteinization. Recent Prog. Horm. Res. 57, 195–220.
| Novel signaling pathways that control ovarian follicular development, ovulation, and luteinization.Crossref | GoogleScholarGoogle Scholar | 12017544PubMed |
Richards, J. S., Russell, D. L., Ochsner, S., and Espey, L. L. (2002b). Ovulation: new dimensions and new regulators of the inflammatory-like response. Annu. Rev. Physiol. 64, 69–92.
| Ovulation: new dimensions and new regulators of the inflammatory-like response.Crossref | GoogleScholarGoogle Scholar | 11826264PubMed |
Sasaki, J., Sato, E. F., Nomura, T., Mori, H., Watanabe, S., Kanda, S., Watanabe, H., Utsumi, K., and Inoue, M. (1994). Detection of manganese superoxide dismutase mRNA in the theca interna cells of rat ovary during the ovulatory process by in situ hybridization. Histochemistry 102, 173–176.
| Detection of manganese superoxide dismutase mRNA in the theca interna cells of rat ovary during the ovulatory process by in situ hybridization.Crossref | GoogleScholarGoogle Scholar | 7868359PubMed |
Shkolnik, K., Tadmor, A., Ben-Dor, S., Nevo, N., Galiani, D., and Dekel, N. (2011). Reactive oxygen species are indispensable in ovulation. Proc. Natl Acad. Sci. USA 108, 1462–1467.
| Reactive oxygen species are indispensable in ovulation.Crossref | GoogleScholarGoogle Scholar | 21220312PubMed |
Sobotta, M. C., Liou, W., Stocker, S., Talwar, D., Oehler, M., Ruppert, T., Scharf, A. N., and Dick, T. P. (2015). Peroxiredoxin-2 and STAT3 form a redox relay for H2O2 signaling. Nat. Chem. Biol. 11, 64–70.
| Peroxiredoxin-2 and STAT3 form a redox relay for H2O2 signaling.Crossref | GoogleScholarGoogle Scholar | 25402766PubMed |
Sugino, N. (2005). Reactive oxygen species in ovarian physiology. Reprod. Med. Biol. 4, 31–44.
| 29699208PubMed |
Tilly, J. L., and Tilly, K. I. (1995). Inhibitors of oxidative stress mimic the ability of follicle-stimulating hormone to suppress apoptosis in cultured rat ovarian follicles. Endocrinology 136, 242–252.
| Inhibitors of oxidative stress mimic the ability of follicle-stimulating hormone to suppress apoptosis in cultured rat ovarian follicles.Crossref | GoogleScholarGoogle Scholar | 7828537PubMed |
Tsai-Turton, M., and Luderer, U. (2006). Opposing effects of glutathione depletion and follicle-stimulating hormone on reactive oxygen species and apoptosis in cultured preovulatory rat follicles. Endocrinology 147, 1224–1236.
| Opposing effects of glutathione depletion and follicle-stimulating hormone on reactive oxygen species and apoptosis in cultured preovulatory rat follicles.Crossref | GoogleScholarGoogle Scholar | 16339198PubMed |
Tsai-Turton, M., Nakamura, B. N., and Luderer, U. (2007). Induction of apoptosis by 9,10-dimethyl-1,2-benzanthracene in cultured preovulatory rat follicles is preceded by a rise in reactive oxygen species and is prevented by glutathione. Biol. Reprod. 77, 442–451.
| Induction of apoptosis by 9,10-dimethyl-1,2-benzanthracene in cultured preovulatory rat follicles is preceded by a rise in reactive oxygen species and is prevented by glutathione.Crossref | GoogleScholarGoogle Scholar | 17554082PubMed |
Vanderhyden, B. C., Caron, P. J., Buccione, R., and Eppig, J. J. (1990). Developmental pattern of the secretion of cumulus expansion-enabling factor by mouse oocytes and the role of oocytes in promoting granulosa cell differentiation. Dev. Biol. 140, 307–317.
| Developmental pattern of the secretion of cumulus expansion-enabling factor by mouse oocytes and the role of oocytes in promoting granulosa cell differentiation.Crossref | GoogleScholarGoogle Scholar | 2115479PubMed |
Verrastro, I., Tveen-Jensen, K., Woscholski, R., Spickett, C. M., and Pitt, A. R. (2016). Reversible oxidation of phosphatase and tensin homolog (PTEN) alters its interactions with signaling and regulatory proteins. Free Radic. Biol. Med. 90, 24–34.
| Reversible oxidation of phosphatase and tensin homolog (PTEN) alters its interactions with signaling and regulatory proteins.Crossref | GoogleScholarGoogle Scholar | 26561776PubMed |
Wayne, C. M., Fan, H. Y., Cheng, X., and Richards, J. S. (2007). Follicle-stimulating hormone induces multiple signaling cascades: evidence that activation of Rous sarcoma oncogene, RAS, and the epidermal growth factor receptor are critical for granulosa cell differentiation. Mol. Endocrinol. 21, 1940–1957.
| Follicle-stimulating hormone induces multiple signaling cascades: evidence that activation of Rous sarcoma oncogene, RAS, and the epidermal growth factor receptor are critical for granulosa cell differentiation.Crossref | GoogleScholarGoogle Scholar | 17536007PubMed |
Wood, Z. A., Schroder, E., Robin Harris, J., and Poole, L. B. (2003). Structure, mechanism and regulation of peroxiredoxins. Trends Biochem. Sci. 28, 32–40.
| Structure, mechanism and regulation of peroxiredoxins.Crossref | GoogleScholarGoogle Scholar | 12517450PubMed |
Yang, S., Luo, A., Hao, X., Lai, Z., Ding, T., Ma, X., Mayinuer, M., Shen, W., Wang, X., Lu, Y., Ma, D., and Wang, S. (2011). Peroxiredoxin 2 inhibits granulosa cell apoptosis during follicle atresia through the NFKB pathway in mice. Biol. Reprod. 84, 1182–1189.
| Peroxiredoxin 2 inhibits granulosa cell apoptosis during follicle atresia through the NFKB pathway in mice.Crossref | GoogleScholarGoogle Scholar | 21248284PubMed |
Yoshino, O., McMahon, H. E., Sharma, S., and Shimasaki, S. (2006). A unique preovulatory expression pattern plays a key role in the physiological functions of BMP-15 in the mouse. Proc. Natl Acad. Sci. USA 103, 10678–10683.
| A unique preovulatory expression pattern plays a key role in the physiological functions of BMP-15 in the mouse.Crossref | GoogleScholarGoogle Scholar | 16818886PubMed |
Zhang, M., Su, Y. Q., Sugiura, K., Xia, G., and Eppig, J. J. (2010). Granulosa cell ligand NPPC and its receptor NPR2 maintain meiotic arrest in mouse oocytes. Science 330, 366–369.
| Granulosa cell ligand NPPC and its receptor NPR2 maintain meiotic arrest in mouse oocytes.Crossref | GoogleScholarGoogle Scholar | 20947764PubMed |
Zhuo, L., and Kimata, K. (2001). Cumulus oophorus extracellular matrix: its construction and regulation. Cell Struct. Funct. 26, 189–196.
| Cumulus oophorus extracellular matrix: its construction and regulation.Crossref | GoogleScholarGoogle Scholar | 11699635PubMed |
Zhuo, L., Yoneda, M., Zhao, M., Yingsung, W., Yoshida, N., Kitagawa, Y., Kawamura, K., Suzuki, T., and Kimata, K. (2001). Defect in SHAP-hyaluronan complex causes severe female infertility. A study by inactivation of the bikunin gene in mice. J. Biol. Chem. 276, 7693–7696.
| Defect in SHAP-hyaluronan complex causes severe female infertility. A study by inactivation of the bikunin gene in mice.Crossref | GoogleScholarGoogle Scholar | 11145954PubMed |