Arginine increases development of in vitro-produced porcine embryos and affects the protein arginine methyltransferase–dimethylarginine dimethylaminohydrolase–nitric oxide axis
Bethany K. Redel A , Kimberly J. Tessanne A , Lee D. Spate A , Clifton N. Murphy A and Randall S. Prather A BA Division of Animal Science, Animal Science Research Center, 920 East Campus Drive, Columbia, MO 65211, USA.
B Corresponding author. Email: pratherr@missouri.edu
Reproduction, Fertility and Development 27(4) 655-666 https://doi.org/10.1071/RD14293
Submitted: 9 August 2014 Accepted: 14 February 2015 Published: 13 March 2015
Journal Compilation © CSIRO Publishing 2015 Open Access CC BY-NC-ND
Abstract
Culture systems promote development at rates lower than the in vivo environment. Here, we evaluated the embryo’s transcriptome to determine what the embryo needs during development. A previous mRNA sequencing endeavour found upregulation of solute carrier family 7 (cationic amino acid transporter, y+ system), member 1 (SLC7A1), an arginine transporter, in in vitro- compared with in vivo-cultured embryos. In the present study, we added different concentrations of arginine to our culture medium to meet the needs of the porcine embryo. Increasing arginine from 0.12 to 1.69 mM improved the number of embryos that developed to the blastocyst stage. These blastocysts also had more total nuclei compared with controls and, specifically, more trophectoderm nuclei. Embryos cultured in 1.69 mM arginine had lower SLC7A1 levels and a higher abundance of messages involved with glycolysis (hexokinase 1, hexokinase 2 and glutamic pyruvate transaminase (alanine aminotransferase) 2) and decreased expression of genes involved with blocking the tricarboxylic acid cycle (pyruvate dehydrogenase kinase, isozyme 1) and the pentose phosphate pathway (transaldolase 1). Expression of the protein arginine methyltransferase (PRMT) genes PRMT1, PRMT3 and PRMT5 throughout development was not affected by arginine. However, the dimethylarginine dimethylaminohydrolase 1 (DDAH1) and DDAH2 message was found to be differentially regulated through development, and the DDAH2 protein was localised to the nuclei of blastocysts. Arginine has a positive effect on preimplantation development and may be affecting the nitric oxide–DDAH–PRMT axis.
Additional keywords: amino acid, arginine transporter, culture, gene expression, metabolism, Warburg effect.
Introduction
Culture in vitro is at the heart of many assisted reproductive technologies. However, current systems still do not adequately mimic an in vivo environment, resulting in reduced blastocyst and pregnancy rates (Kikuchi et al. 1999). In addition, genetic and epigenetic effects due to culture are well documented (for a review, see Fleming et al. 2004). Therefore, to produce an ideal culture system, there is a need to understand what the embryo needs in vivo. In an effort to identify ways to improve culture conditions, a next-generation sequencing analysis was completed using in vivo-produced embryos that were cultured to the blastocyst stage in vitro (IVC) or in vivo (IVV; Bauer et al. 2010). The arginine transporter solute carrier family 7 (cationic amino acid transporter, y+ system), member 1 (SLC7A1) was found to be upregulated by at least 63-fold in IVC compared with IVV blastocyst stage embryos. Arginine is a vital amino acid for many metabolic processes in the cell, such as protein synthesis, creatine production, polyamine synthesis and nitric oxide (NO) generation (Wheatley and Campbell 2003). Removal of arginine from the culture medium by either medium formulation or arginase treatment quickly leads to death in 80% of tumour cell lines (Scott et al. 2000). Because early rapidly dividing embryos appear to be metabolically similar to cancer cells (Krisher and Prather 2012; Redel et al. 2012), we hypothesised that in vitro-produced embryos also require significant amounts of arginine.
Arginine is used to produce NO by NO synthases (NOS). There are three NOS isoforms, namely neuronal (NOS1), inducible (NOS2) and endothelial (NOS3). Expression of all three NOS isoforms has been detected in murine and bovine preimplantation embryos from the zygote to blastocyst stage (Tranguch et al. 2003; Tesfaye et al. 2006). l-Arginine is depleted from porcine embryo culture medium in vitro, suggesting that it is metabolised during embryonic development (Humpherson et al. 2005). Manser and Houghton (2006) used the NO-sensitive probe 4-amino-5- methylamino-2′,7′-difluorofluorescien diacetate (DAF-FM-DA) and found that NO was present at all stages of murine preimplantation development. Supplementation of culture media with additional arginine also improves porcine embryo development, suggesting that this amino acid plays a critical role in preimplantation development (Bauer et al. 2010).
NO production is regulated, in part, by the production of endogenous NOS inhibitors through the protein arginine methyltransferase (PRMT)–dimethylarginine dimethylaminohydrolase (DDAH)–NO axis. Proteins that contain arginine methylation via PRMTs release methylated arginine residues upon proteolysis. These residues, specifically monomethylarginine (MMA) and asymmetric dimethylarginine (ADMA), then act on NOS within the cell to reduce NO production. DDAH1 and DDAH2 degrade excess MMA and ADMA within the cell. The DDAH1 null mutation is embryonic lethal, whereas DDAH2-null mice reproduce normally (Breckenridge et al. 2010). This reveals an important role for DDAH and proper regulation of NO in the early embryo.
In the present study we investigated these pathways in embryos that were produced in vitro and show that arginine can enhance the development of these embryos and that these embryos are developmentally competent. We also present evidence supporting a functional PRMT–DDAH–NO axis in early porcine embryonic development.
Materials and methods
Chemical components
Unless indicated otherwise, all chemical components were purchased from Sigma Chemical (St Louis, MO, USA).
In vitro embryo production
Prepubertal porcine oocytes were obtained from ovaries collected from a local slaughterhouse and were subjected to in vitro maturation as described previously (Zhang et al. 2010). Cumulus–oocyte complexes (COCs) were aspirated from follicles of ovaries collected from the local slaughterhouse. COCS were selected on the basis of multiple layers of cumulus cells and evenly distributed cytoplasm; they were a washed in Tyrode’s lactate (TL) HEPES medium supplemented with 0.1% polyvinyl alcohol (PVA). Between 200 and 250 COCs were cultured in 2 mL maturation medium (TCM-199 with 0.1% PVA, 3.05 mM glucose, 0.91 mM sodium pyruvate, 10 µg mL–1 gentamicin, 0.57 mM cysteine, 10 ng mL–1 epidermal growth factor, 0.5 µg mL–1 LH and 0.5 µg mL–1 FSH) for 42–44 h in a humidified atmosphere with 5% CO2 in air at 38.5°C. Forty four hours after culture in maturation medium, mature oocytes were identified by extrusion of a polar body and washed in modified Tris-buffered medium (mTBM) containing 2 mg mL–1 bovine serum albumin (BSA) and 2 mM caffeine (IVF medium). Thirty oocytes were placed into 50-µL droplets of IVF medium covered with mineral oil and incubated at 38.5°C until spermatozoa were added. The spermatozoa used for fertilisation were obtained from a single boar and were used throughout the entire experiment. For IVF, a 0.1-mL frozen semen pellet was thawed in 3 mL sperm washing medium (Dulbecco’s phosphate-buffered saline (dPBS; Gibco, Grand Island, NY, USA) supplemented with 0.1% BSA). Spermatozoa were washed twice by centrifugation. The sperm pellet was resuspended with fertilisation medium to 0.5 × 106 cells mL–1. Finally, 50 µL sperm suspension was added to the oocytes in IVF medium, giving a final concentration of 0.25 × 106 cells mL–1. Spermatozoa and oocytes were incubated together for 5 h.
Embryo culture
After fertilisation, oocytes were removed from the droplets and washed in porcine zygote medium 3 (PZM3; Yoshioka et al. 2002). Fifty presumptive zygotes were then cultured in each well of a four-well dish in PZM3 in a humidified atmosphere with 5% CO2 in air for 28–30 h at 38.5°C. After the 28–30 h culture, embryos that cleaved and were at the 2- to 4-cell stage were selected and 15 cleaved embryos were moved to 25-µL droplets in one of five treatment groups: (1) PZM3 (0 mM arginine); (2) PZM3 control (0.12 mM arginine); (3) PZM3 (0.36 mM arginine); (4) PZM3 (0.72 mM arginine); or (5) PZM3 (1.69 mM arginine), hereafter referred to as MU1. Embryos were cultured in a humidified atmosphere of 5% CO2, 90% N2 and 5% O2 at 38.5°C until Day 6. The concentration of 1.69 mM arginine is the highest physiological concentration found in Day 3 oviductal fluid in gilts (Li et al. 2007) and thus was used as our high arginine concentration in vitro. On Day 6 after fertilisation, blastocysts from each treatment group were collected and the percentage of embryos that had developed to the blastocyst stage was recorded. To determine the effect of arginine on development, the percentage of blastocysts in each treatment group was analysed using PROC GENMOD in SAS (SAS Institute, Cary, NC, USA). A least significant difference (LSD) post-test comparison was performed to determine whether significant differences existed between treatment groups, with significance set at two-tailed P < 0.05. For subsequent experiments, embryos were cultured in one of three treatment groups: (1) PZM3 (0 mM arginine); (2) PZM3 control (0.12 mM arginine); or (3) MU1. The blastocysts were then collected and used for RNA isolation or stained for determination of nuclear number.
Differential nuclear staining
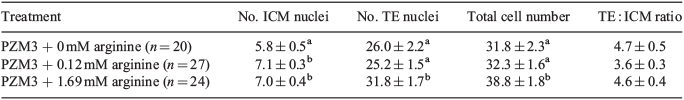
A comparison of the number of trophectoderm (TE) and inner cell mass (ICM) nuclei for embryos cultured in each of the three treatment groups was conducted after differential nuclear staining using procedures described previously (Macháty et al. 1998). Briefly, the zona pellucida was removed using physiological saline lowered to pH 1.79. Zona-free embryos were exposed to a 1 : 7 dilution of rabbit anti-pig whole serum for 60 min (Bauer et al. 2010). The embryos were then washed three times for 5 min each time in TL HEPES medium. Finally, embryos were incubated in a 1 : 10 dilution of the guinea-pig complement containing 10 µg mL–1 propidium iodide and 10 µg mL–1 bisbenzimide for 35 min. The embryos were then observed under ultraviolet (UV) light at ×40 magnification using a Nikon Eclipse E600 inverted microscope (Nikon, Tokyo, Japan). ICM nuclei stained blue, whereas TE nuclei stained pink. Mean ICM, TE, total cell number and the ratio of TE/ICM were first analysed for normality using the UNIVARIATE procedure in SAS. The data were then log2 transformed and analysed by analysis of variance using the MIXED procedure in SAS. An LSD post-test comparison was then performed for each variable to determine whether significant differences (P < 0.05) existed between groups.
Extraction and amplification of RNA for real-time polymerase chain reaction of SLC7A1
Three replicates were obtained for each treatment group, namely PZM3 (0 mM arginine), PZM3 control (0.12 mM arginine) and MU1. Total RNA was extracted from pools of 10 embryos in each replicate using the AllPrep Genomic DNA/RNA Micro Isolation Kit (Qiagen, Germantown, MD, USA). Total RNA was suspended in 12 µL of RNase free water, and 5 μL was amplified using the WT-Ovation Pico RNA Amplification System (NuGEN Technologies, San Carlos, CA, USA). After amplification, samples were purified using Micro Bio-Spin P-30 Columns (Bio-Rad Laboratories, Hercules, CA, USA). Real-time polymerase chain reaction (PCR) was then performed using these amplified embryo pools to determine message abundance of SLC7A1 and genes that regulate the Warburg effect (WE; Bauer et al. 2010).
Relative real-time PCR
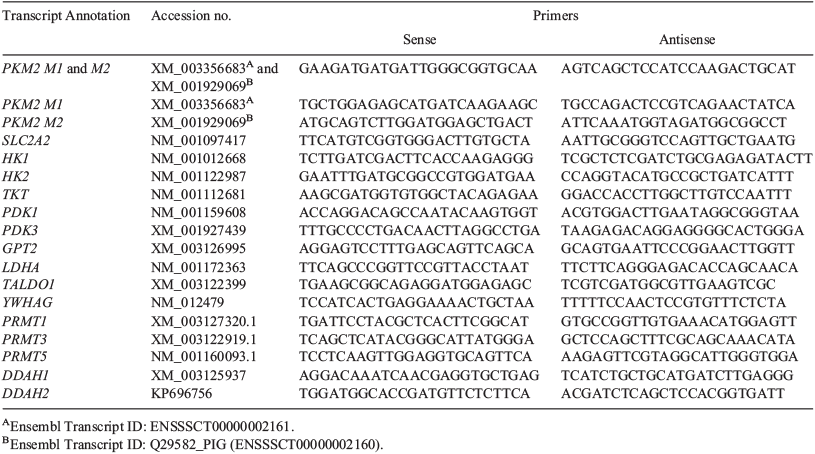
Relative real-time PCR was conducted on each of the amplified samples for the genes involved with the WE and embryo metabolism using IQ SYBR Green Supermix (Bio-Rad Laboratories) and the amplified cDNA from each biological replicate (diluted to 5 ng µL–1) as template. Primers were designed using Integrated DNA Technology (Coralville, IA, USA) software and real-time PCR was run in triplicate for every biological replicate on the MyiQ Single-Colour Real-Time PCR Detection System (Bio-Rad Laboratories) to verify the differential abundance of the chosen transcripts. Primer efficiency tests were completed for each primer set by generating a standard curve using 1 : 10 dilutions of the 5 ng µL–1 reference cDNA pool. Real-time PCR was run in triplicate for each concentration (5, 0.5 and 0.05 ng µL–1) to validate each primer set. Abundance was calculated for each mRNA transcript as described by Bauer et al. (2010) relative to the reference sample and the housekeeping gene tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein, gamma (YWHAG; Whitworth et al. 2005; Bauer et al. 2010). YWHAG has been used as a stable reference message in many of our pig oocyte and embryo studies. Whitworth et al. (2005) found, by both microarray and real-time PCR, that YWHAG expression did not differ among samples, and Bauer et al. (2010) found that YWHAG expression did not differ between treatments in mRNA deep sequencing. For these reasons, YWHAG was used as our reference gene in the present study. The reference sample contained four biological replicates of in vivo-fertilised and then in vivo-cultured blastocysts and in vivo-fertilised and then in vitro-cultured blastocysts pooled together (Bauer et al. 2010). Expression levels between treatments were determined using the comparative threshold cycle (CT) method for each gene. The 2–ΔΔCT values were analysed for normality before being log transformed if not normally distributed. The resulting values were then analysed using the general linear model (PROC GLM) in SAS. The significance of differences in expression was evaluated using the least-squares means (LSMeans) generated by PROC GLM; significance was set at P < 0.05.
Quantitative real-time PCR of PRMT and DDAH transcripts
To examine the expression of PRMT1, PRMT3 and PRMT5, as well as DDAH1 and DDAH2, quantitative real-time PCR analysis was performed on three pools each of 18–22 MII oocytes, 4-cell embryos and blastocyst stage embryos cultured in either control (0.12 mM arginine) or MU1 (1.69 mM arginine). Briefly, RNA was isolated using the Dynabeads mRNA Direct micro kit (Life Technologies, Carlsbad, CA, USA) and cDNA was synthesised using Superscript III (Life Technologies). Real-time PCR was performed using iQ SYBR Green Supermix (BioRad) and run on a BioRad platform using a two-step protocol with melting curve analysis. Reactions were run in triplicate with the following program: 95°C for 2 min, followed by 40 cycles of 95°C for 10 s and 60°C for 30 s. A melting curve analysis followed, with temperature increments of 0.3°C from 55 to 95°C. Quantitative real-time PCR data were analysed using the ΔΔCt method with comparison against YWHAG expression as an endogenous control (Whitworth et al. 2005). Relative mean expression was compared by analysis of variance (ANOVA) after log transformation.
Immunocytochemistry
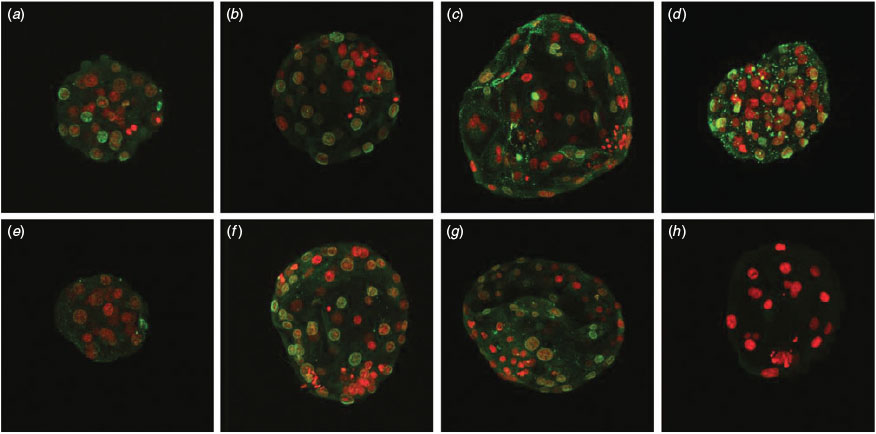
To examine DDAH2 protein localisation, Day 6 blastocysts were collected and the zona pellucida removed using low-pH (pH 1.79) PBS. Embryos were then fixed for 20 min in 4% paraformaldehyde, washed twice in TL HEPES medium and held at 4°C until processing. After permeabilisation in 0.1% Triton in PBS for 3 h at 37°C, embryos were washed through PBS with 0.01% Tween and 3% BSA and then incubated in 2 M HCl for 30 min at room temperature. This was followed by incubation in 100 mM TRIS-HCl, pH 8.5, for 10 min at room temperature. Embryos were then blocked for 1 h at room temperature (blocking buffer 0.01% Tween and 3% BSA) before being incubated overnight in primary antibody (1 : 100; goat polyclonal DDAH2; Santa Cruz Biotechnology, Santa Cruz, CA, USA) diluted in blocking buffer. After three 10-min washes with PBS containing 0.01% Tween and 3% BSA (with rocking), embryos were incubated in secondary antibody (1 : 250; donkey anti-goat Alexa fluor 488; Life Technologies). After three 10-min washes with rocking, embryos were counterstained with propidium iodide and mounted using Prolong Antifade (Life Technologies). Images were taken at 12-µm cross-sections using a Zeiss (Zeiss United States, Thornwood, NY, USA) LSM 510 META NLO two-photon point scanning confocal microscope.
DAF-FM imaging and fluorescence analysis
For DAF imaging, cleaved embryos were cultured in one of four treatments: (1) PZM3 control (0.12 mM arginine); (2) MU1; (3) 50 μM Arginine N-Methyltransferase Inhibitor-1 (AMI-1) (PRMT1 inhibitor) in 0.12 mM arginine; or (4) 100 μM ADMA (NOS inhibitor) in 0.12 mM arginine. Embryos were moved 30 h after insemination to 25-μL culture drops and cultured to either Day 2 (4-cell) or Day 6 (blastocyst). On Day 2 or 6, embryos were washed through TL HEPES medium and into 500-μL wells of PZM3 with 0.1% PVA (no BSA) plus 10 μM DAF-FM-DA (Life Technologies). Embryos were incubated in DAF-FM-DA for 40 min in 5% O2 at 38.5°C and then washed into PZM3 without DAF-FM-DA and incubated for an additional 15 min in 5% O2 at 38.5°C. For imaging, embryos were placed individually in 5-μL drops of TL HEPES medium under mineral oil, held in place using a microinjection holding pipette and imaged with a Nikon inverted fluorescence microscope. All treatments were present throughout the incubation and imaging steps. Images were taken using an exposure time that was the average for embryos treated with 5 mM NG-nitro-l-arginine methyl ester (l-NAME), a non-specific NOS inhibitor, to control for background fluorescence. The resulting images were analysed using ImageJ (National Institutes of Health, Bethesda, MD, USA), and corrected fluorescence intensity values were calculated according to Burgess et al. (2010) as integrated density – (area of embryo measurement × background). Values were then log transformed and compared using PROC GLM ANOVA with fixed effects of treatment and IVF group.
Embryo transfer
Day 6 after fertilisation, blastocysts cultured in MU1 were selected and placed in 3 mL manipulation medium (9.50 g TCM-199, 0.05 g NaHCO3, 0.75 g Hepes, 1.76 g NaCl, 3.00 g BSA, 1 mL gentamicin and 1000 mL Milli Q H2O) in polystyrene tubes (BD Falcon 352054, San Jose, CA, USA). Embryos were transported at 37°C to the University of Missouri Swine Research Complex, where they were loaded with a minimal volume of medium into a tomcat catheter and surgically transferred to the ampullary–isthmic junction of the cycling gilt on Day 5 or 6 of her oestrous cycle (Spate et al. 2010; Lee et al. 2013). Pregnancy was monitored by ultrasound after Day 25 and checked weekly until the gilt returned to oestrus or farrowed. Sex and birthweights were recorded.
Results
Supplementing our current culture (PZM3 with 0.12 mM arginine) with 1.69 mM arginine increased the development of porcine embryos to the blastocyst stage above no arginine (Table 1), and increased the number of TE nuclei and total nuclei above the control PZM3 without significantly changing the ratio of ICM/TE (Table 2). The higher arginine concentration (1.69 mM) in culture produced embryos that were developmentally competent because they produced live piglets (Table 3). A comparison of term development from these two culture systems was beyond the scope of the present study.

|

|
Message for SLC7A1 was decreased in embryos cultured in 1.69 mM arginine compared with 0 or 0.12 mM arginine (Fig. 1). The abundance of two WE-related genes, namely transaldolase 1 (TALDO1) and pyruvate dehydrogenase kinase, isozyme 1 (PDK1), were decreased by culture in the presence of 1.69 mM arginine, whereas expression of hexokinase 1 (HK1), hexokinase 2 (HK2) and glutamic pyruvate transaminase (alanine aminotransferase) 2 (GPT2) was increased (Table 4).

|
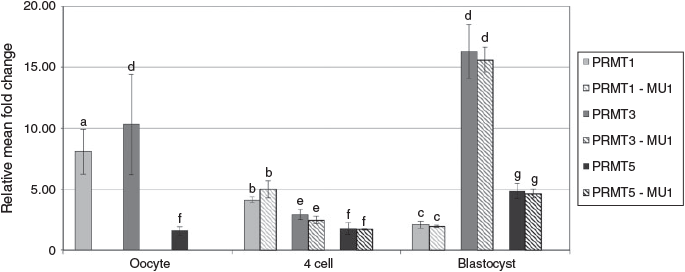
The abundance of message for PMRT1 and PMRT3 decreased from the MII to 4-cell stage, whereas expression of PRMT5 increased from the MII to 4-cell stage (Fig. 2). The abundance of PRMT1 then increased from the 4-cell to blastocyst stage. There was no effect on PRMT1, PRMT3 or PRMT5 message abundance if embryos were cultured in the presence of 0.12 or 1.69 mM arginine. There was no difference in the expression of DDAH1 between the MII oocytes and 4-cell embryos, but both were higher than expression at the blastocyst stage (Fig. 3; P < 0.01). In contrast, DDAH2 expression remained low in MII oocytes and 4-cell embryos, but was then fivefold higher by the blastocyst stage (P < 0.01). DDAH2 protein localisation was analysed using confocal imaging of Day 6 in vivo-derived, PZM3 (0.12 mM arginine) and MU1 cultured IVF embryos. The DDAH2 protein was localised to the nucleus in both in vivo- and in vitro-produced blastocysts, and this localisation appeared to be less abundant in Day 5 in vitro-produced blastocysts cultured in MU1 compared with Day 5 blastocysts cultured in 0.12 mM arginine (Fig. 4). In both treatments, DDAH2 nuclear localisation appeared to be lower in ICM cells compared with TE. A similar pattern of protein expression was seen in Day 6 in vivo-derived blastocysts.

|
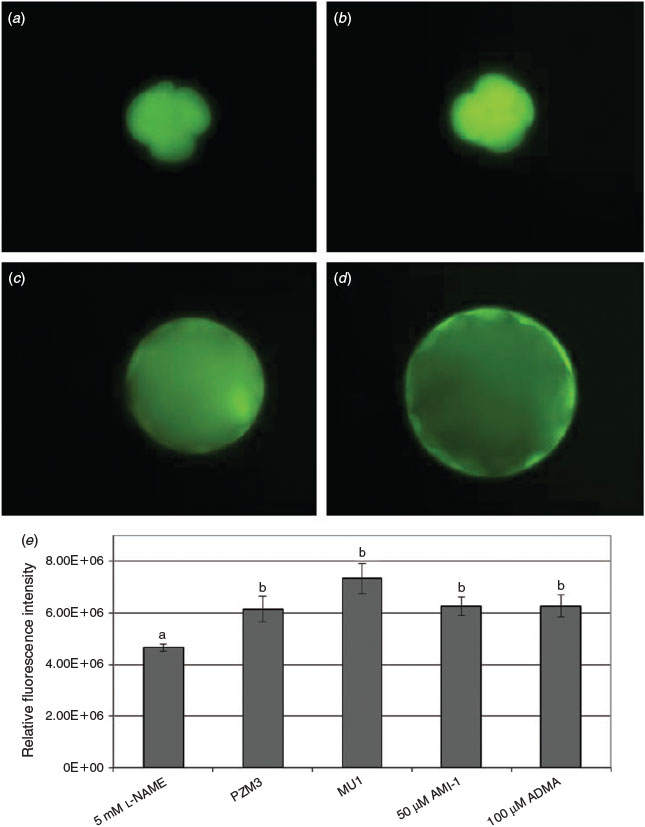
To explore the role of NO production during development, 2-cell embryos were cultured in 2, 5 and 10 mM l-NAME until the blastocyst stage. At 5 and 10 mM, l-NAME inhibited development to the blastocyst stage (P < 0.05; Fig. 5). NO production in 4-cell in vitro-produced embryos was estimated using DAF-FM fluorescence compared with embryos cultured with 5 mM l-NAME (Fig. 6). Quantification of fluorescence on Day 2 of culture (4–6 cells) revealed an increase in NO production in embryos cultured in either 0.12 mM arginine (PZM3) or 1.69 mM arginine (MU1) compared with embryos cultured in 0.12 mM arginine plus 5 mM l-NAME. There was also a tendency for greater NO production in 1.69 mM arginine-treated embryos compared with those treated with 0.12 mM arginine treatment, however the difference did not reach statistical significance (P = 0.11). AMI-1, an inhibitor of class 1 PRMTs, and ADMA dihydrochloride did not reduce DAF-FM fluorescence in embryos cultured in 0.12 mM arginine. Culture of embryos treated with DAF-FM-DA to Day 6 resulted in a 70% blastocyst rate (data not shown), not only confirming that developmentally competent embryos were chosen for imaging, but also that the DAF did not have a negative effect on development.

|
Discussion
Our overall goal is to better understand the metabolism of the embryo so that embryo culture conditions can be improved to increase the efficiency of pig production. One way that this can be done is by assessing the transcriptional profile of IVC and IVV embryos. A previous transcriptional profiling study found a transcript involved with arginine transport was increased in IVC compared with IVV embryos (Bauer et al. 2010). Using those data, we assessed the effect additional arginine would have on embryo development in vitro. Here, we provide evidence illustrating the positive effect that the addition of arginine to porcine embryo culture can have on development.
Arginine is a nutritionally essential amino acid for conceptus growth and development (Wu et al. 2010). Arginine is a precursor not only for protein synthesis, but also for NO, urea, proline, glutamate, creatine and agmatine (Wu and Morris 1998). Most of the l-arginine is transported by the Na+-independent system y+ for cationic amino acids into cells. This cationic amino acid transporter (CAT) system contains three different members encoded by the SLC7A1, SLC7A2, and SLC7A3 genes. Message for SLC7A1 and SLC7A2 has been previously identified in mouse preimplantation embryos (Van Winkle 2001). The SLC7A1 message has also been detected in ovine conceptuses between Days 13 and 18 of pregnancy (Gao et al. 2009), and in porcine conceptuses on Days 12 and 15 of pregnancy (Bazer et al. 2013). More recently, SLC7A1 was found to be the key transporter of arginine by ovine TE (Wang et al. 2014a). Using in vivo morpholino antisense oligonucleotide-mediated knockdown of SLC7A1, Wang et al. (2014a) found that the conceptuses were retarded with abnormal function compared with controls. They concluded that arginine is essential for conceptus survival and development (Wang et al. 2014a).
In a previous study, a threefold greater concentration of arginine than used in PZM3 was added to evaluate the effect on SLC7A1 message levels (Bauer et al. 2010). Increasing arginine from 0.12 to 0.36 mM and culturing in vivo-produced 2-cell embryos to the blastocyst stage decreased the SLC7A1 message level to that seen in in vivo embryos; however, SLC7A1 expression did not differ from what was seen following in vitro culture with 0.12 mM arginine (Bauer et al. 2010). Therefore, a higher concentration of arginine was used to evaluate the effects on in vitro-produced embryo development. Five different treatments were used in the present study: PZM3 with 0, 0.12 (control), 0.36, 0.72 or 1.69 mM arginine. Embryos were also cultured in the presence of 2.5 and 5 mM arginine to see whether there was even more of an improvement in embryo development; however, there was no difference compared with embryos cultured in 1.69 mM arginine (data not shown), so 1.69 mM was used for the remainder of the experiments. Li et al. (2007) characterised the concentrations of amino acids in porcine oviductal and uterine fluid on Day 3 and Day 5 after insemination and found that the concentration of arginine ranged from 0.22 mM in Day 5 uterine fluid to 1.69 mM in Day 3 oviductal fluid. For this reason, 1.69 mM arginine, which is 14-fold higher than the control concentration, was used as the high physiological level of arginine. Embryos cultured with 1.69 mM arginine had decreased levels of SLC7A1 message compared with embryos cultured in 0 or 0.12 mM arginine. This validates our hypothesis that by adding arginine to our current culture medium, we will decrease the expression of this arginine transporter. It appears as though the embryo is trying to overcompensate for the lack of arginine in the 0.12 mM arginine-containing medium and upregulating SLC7A1 message compared with in vivo embryos.
Adding additional arginine to culture improved the percentage of embryos that developed to the blastocyst stage. All three treatments cultured with additional arginine had higher blastocyst percentages than the control embryos. Specifically, embryos cultured in 1.69 mM arginine had the highest percentage of blastocysts compared with each of the treatments, with 70% of cleaved embryos developing into blastocysts (vs ~51% for controls). A few studies have completed amino acid profiling of porcine embryo culture media and found that arginine was depleted from the media (Booth et al. 2005, 2007; Humpherson et al. 2005). Booth et al. (2005) found that arginine was consistently depleted from the medium at each preimplantation stage of development and morulas producing ≥25% blastocysts had more arginine depleted than morulas producing ≤14% blastocysts. Depletion of arginine from the medium is consistent with what is illustrated here, in that providing the embryos additional arginine in culture can improve the blastocyst percentage.
An indicator of embryo quality is total cell number of the resulting blastocysts. Adding a higher concentration of arginine (1.69 mM) increased the average total cell number compared with control blastocysts. After differential staining, the two different cell types in the blastocyst were analysed and there was no effect on ICM number, but there was an increase in the TE number. A porcine TE-derived cell line was cultured in the presence or absence of arginine in a customised medium and when 2 mM arginine was added, there was increased cellular proliferation by approximately eightfold compared with cells cultured in the presence of 0 mM arginine (Kim et al. 2013). This, again, is consistent with what was demonstrated in the present study, because the TE cells appeared to be the cells with increased proliferation compared with controls. There was no difference in the number of ICM between control embryos or embryos cultured in the presence of high arginine.
Arginine has been shown to stimulate cell signalling via the AKT1/mammalian target of rapamycin complex (mTORC) 1/mTORC2 pathway to affect survival and development of the conceptus (Bazer et al. 2013). Arginine induces the phosphorylation of proteins in the mammalian target of rapamycin (mTOR) cell signalling pathway, including ribosomal protein S6 kinase (RPS6K) and ribosomal protein S6 (RPS6) in ovine TE cells (Kim et al. 2011), as well as in porcine TE cells (Kong et al. 2012; Kim et al. 2013). mTOR is a highly conserved serine/threonine protein kinase and is a regulator of mRNA translation. mRNA translation is a key event in the regulation of protein synthesis (Wu 2010). Porcine embryos undergo rapid proliferation, elongation and cellular remodelling, and we propose that the mTOR pathway stimulates TE proliferation, mRNA translation, protein synthesis and cytoskeletal remodelling (Kong et al. 2012; Bazer et al. 2013; Kim et al. 2013).
Like embryos, cancer cells have stimulated mTOR signalling to drive cellular proliferation (Guertin and Sabatini 2007). These cancer cells have an altered metabolism that illustrates the unique characteristics of the WE (Vander Heiden et al. 2009), that is, they have increased glucose uptake and decreased metabolism through the tricarboxylic acid (TCA) cycle. mTORC1 responds to the nutritional status of the cell and is often deregulated in cancer cells (Zoncu et al. 2011). Arginine has been shown to activate mTORC1 via the lysosome amino acid transporter solute carrier family 38, member 2 (SLC38A9) (Wang et al. 2015). Once mTORC1 is stimulated, AKT is activated, promoting glucose uptake (Howell and Manning 2011). Thus, arginine may be directly affecting the WE pathway. To determine whether arginine was affecting transcripts associated with the WE, real-time PCR was conducted on WE-defining genes (Krisher and Prather 2012; Redel et al. 2012; Table 5). There was an upregulation of HK1 and HK2; these are hexokinases involved with the first step of glucose metabolism. HK2 is the main hexokinase expressed in cancer cells and, in these Day 6 blastocysts, it is the more highly abundant compared with HK1. This is consistent with the WE because HK2 is required for tumour initiation and maintenance in cancer cells (Patra et al. 2013). Cancer cells also have increased lactic acid and alanine production. Here, additional arginine caused an increase in the GPT2 message in embryos, which is consistent with cancer cells metabolising pyruvate to alanine and away from the TCA cycle (Beuster et al. 2011). Additional arginine decreased PDK1 expression. This enzyme is important in blocking pyruvate from entering the TCA cycle and shunting it towards alanine or lactate production. The TALDO1 message was also decreased in embryos cultured in 1.69 mM arginine. This enzyme is part of the pentose phosphate pathway that, in rapidly dividing cells, is important for producing NADPH and ribose to assist with the increased cellular proliferation (Krisher et al. 2014). Although only gene expression was measured, and changes seen in transcript abundance do not always translate to changes in protein levels, the results do give a predictor of what could be occurring at the protein and/or enzyme level.

|
Overexpression of PRMTs has been associated with many different cancers. Recently, there has been research linking the deregulation of PRMTs in cellular processes such as proliferation, transformation and anti-apoptotic processes that promote tumourigenesis (Yang and Bedford 2013). In the present study, PRMT1 expression was highest at the MII stage and then decreased, suggesting that less arginine methylation (and therefore less ADMA) is being produced as the embryo progresses through development (Fig. 2). Conditional knockout of PRMT1 in mice led to embryonic lethality, emphasising a need for this protein in the early stages of development (Breckenridge et al. 2010). Further investigation in mouse PRMT1-null embryonic fibroblasts revealed chromosomal aberrations and hypersensitivity to DNA damage (Yu et al. 2009), both of which would be detrimental to the early embryo. The expression of PRMT3, which adds ADMA to arginine residues, also decreased from the oocyte to 4-cell stage, but then markedly increased by the blastocyst stage. This suggests a particular role for this PRMT later in development. PRMT5 is the main symmetric arginine methyltransferase, and PRMT5-knockout mice die very early during development, probably when the maternal RNA pool is depleted (Yang and Bedford 2013). Arginine concentration had no effect on the abundance of the PRMT5 message.
DDAH1 did not appear to be present in porcine blastocysts, but was expressed in oocytes and 4-cell embryos; which could be in response to higher NO production in early embryos, because DAF staining of porcine embryos appeared to have a higher intensity at the 4-cell stage that at the blastocyst stage (Figs 3, 6). In contrast, DDAH2 appears to be expressed throughout development, with a marked increase between the oocyte and 4-cell stages, as well as an increase from the 4-cell stage to blastocyst. This is consistent with earlier work using microarray analysis to determine differences in gene expression between oocytes, 4-cell embryos and blastocysts, where a significant upregulation of DDAH2 was found between the oocyte and 4-cell stages (Whitworth et al. 2005). DDAH2 has been found to be expressed primarily in fetal rather than adult tissues, and has also been found to be upregulated in rapidly dividing cells, such as melanoma cells (Tran et al. 2003). DDAH2 overexpression also enhanced proliferation and migration of endothelial cells (Hasegawa et al. 2006), which suggests that this upregulation could be involved in the dividing embryo.
DDAH2 protein localisation was analysed using confocal imaging of Day 6 in vivo-derived IVF embryos cultured in the presence of PZM3 (0.12 mM arginine) and MU1. In vitro-derived embryos were also analysed on Days 5 and 7 of development. The DDAH2 protein was localised to the nucleus in both in vivo-derived and in vitro-produced blastocysts, suggesting a possible role for this protein in gene regulation. This expression pattern is in contrast with that seen in endothelial cells, where DDAH2 protein was found only in the cytosol (Chen et al. 2005). However, localisation of DDAH2 in the nucleus has been reported in endothelial cells of mesenteric vessels as well as vascular smooth muscle, and translocation of DDAH2 protein localisation to the mitochondria has been reported with interleukin-1β treatment in chondrocytes (Palm et al. 2007; Cillero-Pastor et al. 2012). More importantly, DDAH2 has been shown to be localised in the nucleus of oral squamous cell carcinoma cells, and this localisation relates early embryos to tumour cells (Khor et al. 2013). In the present study, immunocytochemical imaging of porcine blastocysts also showed localisation of DDAH2 primarily in the TE rather than ICM cells. Analysis of a differentially methylated region (DMR) in the promoter of DDAH2 in mice revealed epigenetic regulation of this gene in trophoblast cells (Tomikawa et al. 2006). Further analysis will be needed to determine whether this expression pattern is due to epigenetic differences between these two embryonic cell types.
To examine the role of NO production in the early porcine embryo, the non-specific NOS inhibitor l-NAME was added during in vitro culture. Addition of varying concentrations of l-NAME to porcine embryo culture starting on Day 1 of culture revealed a dose-dependent decrease in blastocyst percentages. Although a low concentration (2 mM) of l-NAME did not affect development rates, the addition of 5 mM l-NAME significantly (P < 0.05) reduced the ability of cleaved embryos to progress to the blastocyst stage. Furthermore, increasing this concentration to 10 mM l-NAME completely abolished blastocyst development (Fig. 5). Similar levels of l-NAME inhibited mouse and bovine embryonic development when added to in vitro culture, supporting a role for NOS in embryonic development in vitro (Amiri et al. 2003; Manser et al. 2004; Schwarz et al. 2010). NOS3 has been shown to be a key enzyme for NO production in ovine TE. Knockdown using an in vivo morpholino antisense oligonucleotide for NOS3 resulted in thin, small and under-developed conceptuses (Wang et al. 2014b). These conceptuses also had decreased SLC7A1 message in the TE. In our transcriptional profiling database, we found that IVC blastocysts had 12 reads that aligned to the NOS3 transcript compared with three reads aligned for IVV blastocysts (P = 0.2; Bauer et al. 2010). This may illustrate a role for NOS3 in porcine embryos.
Examination of embryos on Day 2 of culture (4- to 6-cell stage) using DAF-DM-DA staining revealed a significant increase in DAF fluorescence in embryos cultured in either 0.12 or 1.69 mM arginine-supplemented medium compared with 0.12 mM arginine-supplemented medium containing 5 mM l-NAME (P < 0.05). DAF fluorescence is an effective means of measuring NO production in live porcine embryos, and the findings suggest dynamic production of NO during early embryonic development. DAF fluorescence also demonstrates a marked response of embryos to NOS inhibitor treatment because l-NAME was only present during DAF incubation. Embryos exposed to 1.69 mM arginine during DAF incubation tended to have a higher amount of DAF fluorescence than control 0.12 mM embryos, but this increase was not significant (P = 0.11). This could reflect a need for embryos to have a longer exposure to higher arginine concentrations before NO measurement. Culture in 0.12 mM arginine with inhibitors of the PRMT–DDAH–NOS axis (i.e. AMI-1 and ADMA) did not affect NO production, as assessed by DAF staining, and these embryos still exhibited significantly higher DAF fluorescence than embryos treated with 5 mM l-NAME. This may be due to differences in the effects or activity of exogenous and endogenous forms of these inhibitors in aqueous culture.
Optimising the in vitro environment is crucial to promote embryo development. By evaluating the embryo’s transcriptome, we were able to modify our culture conditions to improve embryo development to the blastocyst stage by adding arginine to our culture medium. Future studies need to determine the exact mechanism by which arginine stimulates increased embryo development to the blastocyst stage, but here we provide the framework to show a dynamic role for the NO–PRMT–DDAH axis in preimplantation porcine embryos.
Acknowledgements
The authors thank Jiude Mao, Jennifer Hamm and Alyssa Davis for their assistance in this project. This work was funded, in part, by Food for the 21st Century at the University of Missouri and by the National Institutes of Health (U42 OD011140).
References
Amiri, I., Sobhani, A., Abolhassani, F., Omidinia, E., Akbari, M., and Farimani, M. (2003). The effect of nitric oxide on mouse pre-implantation embryo development and apoptosis. Iran. Biomed. J. 7, 107–111.| 1:CAS:528:DC%2BD3sXnslSjtbY%3D&md5=b56cb8a9c0e4070f7cff808031dd58c3CAS |
Bauer, B. K., Isom, S. C., Spate, L. D., Whitworth, K. M., Spollen, W. G., Blake, S. M., Springer, G. K., Murphy, C. N., and Prather, R. S. (2010). Transcriptional profiling by deep sequencing identifies differences in mRNA transcript abundance in in vivo-derived versus in vitro-cultured porcine blastocyst stage embryos. Biol. Reprod. 83, 791–798.
| Transcriptional profiling by deep sequencing identifies differences in mRNA transcript abundance in in vivo-derived versus in vitro-cultured porcine blastocyst stage embryos.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtlGls77F&md5=ad9de95e3893d28e4c956ec3b2b7c8e7CAS | 20668257PubMed |
Bazer, F. W., Kim, J., Song, G., Ka, H., Wu, G., Johnson, G. A., and Vallet, J. L. (2013). Roles of selected nutrients in development of the porcine conceptus during pregnancy. In: ‘Control of Pig Reproduction IX, Volume 68’. (Eds H. Rodriquez-Martinez, N. M. Soede, W. L. Flowers.) pp. 159–174. (Context Products Ltd: Leicestershire, UK.)
Beuster, G., Zarse, K., Kaleta, C., Thierbach, R., Kiehntopf, M., Steinberg, P., Schuster, S., and Ristow, M. (2011). Inhibition of alanine aminotransferase in silico and in vivo promotes mitochondrial metabolism to impair malignant growth. J. Biol. Chem. 286, 22 323–22 330.
| Inhibition of alanine aminotransferase in silico and in vivo promotes mitochondrial metabolism to impair malignant growth.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXns1Kksb8%3D&md5=230b2d38f05fdde1a9b39a14cea056d5CAS |
Booth, P. J., Humpherson, P. G., Watson, T. J., and Leese, H. J. (2005). Amino acid depletion and appearance during porcine preimplantation embryo development in vitro. Reproduction 130, 655–668.
| Amino acid depletion and appearance during porcine preimplantation embryo development in vitro.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXht1OnsLjP&md5=204e201fdd3ad49395e949e6677737a3CAS | 16264095PubMed |
Booth, P. J., Watson, T. J., and Leese, H. J. (2007). Prediction of porcine blastocyst formation using morphological, kinetic, and amino acid depletion and appearance criteria determined during the early cleavage of in vitro-produced embryos. Biol. Reprod. 77, 765–779.
| Prediction of porcine blastocyst formation using morphological, kinetic, and amino acid depletion and appearance criteria determined during the early cleavage of in vitro-produced embryos.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXht1CnurfP&md5=b84d7960121baa7f3f8afdd9f09c169dCAS | 17652665PubMed |
Breckenridge, R. A., Kelly, P., Nandi, M., Vallance, P. J., Ohun, T. J., and Leiper, J. (2010). A role for Dimethylarginine Dimethylaminohydrolase 1 (DDAH1) in mammalian development. Int. J. Dev. Biol. 54, 215–220.
| A role for Dimethylarginine Dimethylaminohydrolase 1 (DDAH1) in mammalian development.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXjvFWis7c%3D&md5=a76b682e37d941f388cf8e0053bdf87bCAS | 19757398PubMed |
Burgess, A., Vigneron, S., Brioudes, E., Labbe, J. C., Lorca, T., and Castro, A. (2010). Loss of human Greatwall results in G2 arrest and multiple mitotic defects due to deregulation of the cyclin B-Cdc2/PP2A balance. Proc. Natl Acad. Sci. USA 107, 12 564–12 569.
| Loss of human Greatwall results in G2 arrest and multiple mitotic defects due to deregulation of the cyclin B-Cdc2/PP2A balance.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXpt1eiu7w%3D&md5=585e7b8c5a52d0e9f3a946c935bf39f1CAS |
Chen, Y., Li, Y., Zhang, P., Traverse, J. H., Hou, M., Xu, X., Kimoto, M., and Bache, R. J. (2005). Dimethylarginine dimethylaminohydrolase and endothelial dysfunction in failing hearts. Heart Circ. Physiol. 289, H2212–H2219.
| Dimethylarginine dimethylaminohydrolase and endothelial dysfunction in failing hearts.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXht1CntL%2FN&md5=5b1a4becdb0a9b66ebcdfa861087485eCAS |
Cillero-Pastor, B., Mateos, J., Fernandez-Lopez, C., Oreiro, N., Ruiz-Romero, C., and Blanco, F. J. (2012). Dimethylarginine dimethylaminohydrolase 2, a newly identified mitochondrial protein modulating nitric oxide synthesis in normal human chondrocytes. Arthritis Rheum. 64, 204–212.
| Dimethylarginine dimethylaminohydrolase 2, a newly identified mitochondrial protein modulating nitric oxide synthesis in normal human chondrocytes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhs12mtbrL&md5=32732f3f34465263629e4f8eae670bafCAS | 21898353PubMed |
Fleming, T. P., Kwong, W. Y., Porter, R., Ursell, E., Fesenko, I., Wilkins, A., Miller, D. J., Watkins, A. J., and Eckert, J. J. (2004). The embryo and its future. Biol. Reprod. 71, 1046–1054.
| The embryo and its future.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXnvVGqtrg%3D&md5=a7b10ebbc94aeed769f1e48615b3860aCAS | 15215194PubMed |
Gao, H., Wu, G., Spencer, T. E., Johnson, G. A., and Bazer, F. W. (2009). Select nutrients in the ovine uterine lumen. III. Cationic amino acid transporters in the ovine uterus and peri-implantation conceptuses. Biol. Reprod. 80, 602–609.
| Select nutrients in the ovine uterine lumen. III. Cationic amino acid transporters in the ovine uterus and peri-implantation conceptuses.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXis1amtbc%3D&md5=68c1a4846431d60b4c33c9e1c23cff78CAS | 19038856PubMed |
Guertin, D. A., and Sabatini, D. M. (2007). Defining the role of mTOR in cancer. Cancer Cell 12, 9–22.
| Defining the role of mTOR in cancer.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXotVyqsr0%3D&md5=25b6af20a591a2c29aaf3ea5989919c0CAS | 17613433PubMed |
Hasegawa, K., Wakino, S., Tanaka, T., Kimoto, M., Tatematsu, S., Kanda, T., Yoshioka, K., Homma, K., Sugano, N., Kurabayashi, M., Saruta, T., and Hayashi, K. (2006). Dimethylarginine dimethylaminohydrolase 2 increases vascular endothelial growth factor expression through Sp1 transcription factor in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 26, 1488–1494.
| Dimethylarginine dimethylaminohydrolase 2 increases vascular endothelial growth factor expression through Sp1 transcription factor in endothelial cells.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XmvFyjtLY%3D&md5=59e83e1824a95388ebb91705e26e33d6CAS | 16574895PubMed |
Howell, J. J., and Manning, B. D. (2011). mTOR couples cellular nutrient sensing to organismal metabolic homeostasis. Trends Endocrinol. Metab. 22, 94–102.
| mTOR couples cellular nutrient sensing to organismal metabolic homeostasis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXjsFCqsrc%3D&md5=5b9f862287cffe53672d63212087be8cCAS | 21269838PubMed |
Humpherson, P. G., Leese, H. J., and Sturmey, R. G. (2005). Amino acid metabolism of the porcine blastocyst. Theriogenology 64, 1852–1866.
| Amino acid metabolism of the porcine blastocyst.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtFWhsLfE&md5=8291eb24cb72c74483622d31272bcbbcCAS | 15923030PubMed |
Khor, G. H., Froemming, G. R., Zain, R. B., Abraham, M. T., Omar, E., Tan, S. K., Tan, A. C., Vincent-Chong, V. K., and Thong, K. L. (2013). DNA methylation profiling revealed promoter hypermethylation-induced silencing of p16, DDAH2 and DUSP1 in primary oral squamous cell carcinoma. Int. J. Med. Sci. 10, 1727–1739.
| DNA methylation profiling revealed promoter hypermethylation-induced silencing of p16, DDAH2 and DUSP1 in primary oral squamous cell carcinoma.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXltlGktr8%3D&md5=84d12e649dafef94263c998287b070c5CAS | 24155659PubMed |
Kikuchi, K., Kashiwazaki, N., Noguchi, J., Shimada, A., Takahashi, R., Hirabayashi, M., Shino, M., Ueda, M., and Kaneko, H. (1999). Developmental competence, after transfer to recipients, of porcine oocytes matured, fertilized, and cultured in vitro. Biol. Reprod. 60, 336–340.
| Developmental competence, after transfer to recipients, of porcine oocytes matured, fertilized, and cultured in vitro.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXotlyhtQ%3D%3D&md5=9ab97c8ddf02d6cc2d5686e6cbcb6657CAS | 9915999PubMed |
Kim, J. Y., Burghardt, R. C., Wu, G., Johnson, G. A., Spencer, T. E., and Bazer, F. W. (2011). Select nutrients in the ovine uterine lumen. VIII. Arginine stimulates proliferation of ovine trophectoderm cells through mTOR–RPS6K–RPS6 signaling cascade and synthesis of nitric oxide and polyamines. Biol. Reprod. 84, 70–78.
| Select nutrients in the ovine uterine lumen. VIII. Arginine stimulates proliferation of ovine trophectoderm cells through mTOR–RPS6K–RPS6 signaling cascade and synthesis of nitric oxide and polyamines.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXlvVegtbc%3D&md5=e1d4de181cd76733dc22d4e54d746c3fCAS | 20844281PubMed |
Kim, J., Song, G., Wu, G., Gao, H., Johnson, G. A., and Bazer, F. W. (2013). Arginine, leucine, and glutamine stimulate proliferation of porcine trophectoderm cells through the mTOR–RPS6K–RPS6–EIF4EBP1 signal transduction pathway. Biol. Reprod. 88, 113.
| Arginine, leucine, and glutamine stimulate proliferation of porcine trophectoderm cells through the mTOR–RPS6K–RPS6–EIF4EBP1 signal transduction pathway.Crossref | GoogleScholarGoogle Scholar | 23486913PubMed |
Kong, X., Tan, B., Yin, Y., Gao, H., Li, X., Jaeger, L. A., Bazer, F. W., and Wu, G. (2012). l-Arginine stimulates the mTOR signaling pathway and protein synthesis in porcine trophectoderm cells. J. Nutr. Biochem. 23, 1178–1183.
| l-Arginine stimulates the mTOR signaling pathway and protein synthesis in porcine trophectoderm cells.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhtF2rtbvJ&md5=c6dd16f201854fbc124b0fe2d28cda99CAS | 22137265PubMed |
Krisher, R. L., and Prather, R. S. (2012). A role for the Warburg effect in preimplantation embryo development: metabolic modification to support rapid cell proliferation. Mol. Reprod. Dev. 79, 311–320.
| A role for the Warburg effect in preimplantation embryo development: metabolic modification to support rapid cell proliferation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XlsFygs7Y%3D&md5=c0eff42a155facc9c59aef5762dc984fCAS | 22431437PubMed |
Krisher, R. L., Spate, L. D., Redel, B. K., Lee, K., Park, K.-W., Heuberger, A. L., Herrick, J. R., Schoolcraft, W. B., and Prather, R. S. (2014). Metabolomic profile of porcine blastocysts: Role of intracellular lipids and glutamine metabolism. Biol. Reprod. Suppl. 1, Abstract 420.
Lee, K., Redel, B. K., Spate, L., Teson, J., Brown, A. N., Park, K. W., Walters, E., Samuel, M., Murphy, C. N., and Prather, R. S. (2013). Piglets produced from cloned blastocysts cultured in vitro with GM-CSF. Mol. Reprod. Dev. 80, 145–154.
| Piglets produced from cloned blastocysts cultured in vitro with GM-CSF.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhtFOis7w%3D&md5=5e7b8c50e202f0fb406a58d11c848a8cCAS | 23239239PubMed |
Li, R., Whitworth, K., Lai, L., Wax, D., Spate, L., Murphy, C. N., Rieke, A., Isom, C., Hao, Y., Zhong, Z., Katayama, M., Schatten, H., and Prather, R. S. (2007). Concentration and composition of free amino acids and osmolalities of porcine oviductal and uterine fluid and their effects on development of porcine IVF embryos. Mol. Reprod. Dev. 74, 1228–1235.
| Concentration and composition of free amino acids and osmolalities of porcine oviductal and uterine fluid and their effects on development of porcine IVF embryos.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXpt1altb4%3D&md5=811b0f102e352fdccc26dbd639893085CAS | 17342727PubMed |
Macháty, Z., Day, B. N., and Prather, R. S. (1998). Development of early porcine embryos in vitro and in vivo. Biol. Reprod. 59, 451–455.
| Development of early porcine embryos in vitro and in vivo.Crossref | GoogleScholarGoogle Scholar | 9687321PubMed |
Manser, R. C., and Houghton, F. D. (2006). Ca2+-linked upregulation and mitochondrial production of nitric oxide in the mouse preimplantation embryo. J. Cell Sci. 119, 2048–2055.
| Ca2+-linked upregulation and mitochondrial production of nitric oxide in the mouse preimplantation embryo.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XmtFSmur8%3D&md5=143c135c6bd500d9a5bd5427f98c0eadCAS | 16638811PubMed |
Manser, R. C., Leese, H. J., and Houghton, F. D. (2004). Effect of inhibiting nitric oxide production on mouse preimplantation embryo development and metabolism. Biol. Reprod. 71, 528–533.
| Effect of inhibiting nitric oxide production on mouse preimplantation embryo development and metabolism.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXmtFWgtbY%3D&md5=09d64c1d68b9f4f9672617a80c79ed41CAS | 15070826PubMed |
Palm, F., Onozato, M. L., Luo, Z., and Wilcox, C. S. (2007). Dimethylarginine dimethylaminohydrolase (DDAH): expression, regulation, and function in the cardiovascular and renal systems. Am. J. Physiol. Heart Circ. Physiol. 293, H3227–H3245.
| Dimethylarginine dimethylaminohydrolase (DDAH): expression, regulation, and function in the cardiovascular and renal systems.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhsVOgtLfK&md5=4107a5cdf05854be22607a854f3cb0daCAS | 17933965PubMed |
Patra, K. C., Wang, Q., Bhaskar, P. T., Miller, L., Wang, Z., Wheaton, W., Chandel, N., Laakso, M., Muller, W. J., Allen, E. L., Jha, A. K., Smolen, G. A., Clasquin, M. F., Robey, R. B., and Hay, N. (2013). Hexokinase 2 is required for tumor initiation and maintenance and its systemic deletion is therapeutic in mouse models of cancer. Cancer Cell 24, 213–228.
| Hexokinase 2 is required for tumor initiation and maintenance and its systemic deletion is therapeutic in mouse models of cancer.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXht1Wrur%2FL&md5=bbbcc645a09b29391f9f896cb284254bCAS | 23911236PubMed |
Redel, B. K., Brown, A. N., Spate, L. D., Whitworth, K. M., Green, J. A., and Prather, R. S. (2012). Glycolysis in preimplantation development is partially controlled by the Warburg effect. Mol. Reprod. Dev. 79, 262–271.
| Glycolysis in preimplantation development is partially controlled by the Warburg effect.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhs12mtbfE&md5=4dec75f68c5037e3bf58f44f296fcc66CAS | 22213464PubMed |
Schwarz, K. R., Pires, P. R., de Bem, T. H., Adona, P. R., and Leal, C. L. (2010). Consequences of nitric oxide synthase inhibition during bovine oocyte maturation on meiosis and embryo development. Reprod. Domest. Anim. 45, 75–80.
| Consequences of nitric oxide synthase inhibition during bovine oocyte maturation on meiosis and embryo development.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXislagurg%3D&md5=edb34ca046ba8f6a44740fcb04fbee67CAS | 20137060PubMed |
Scott, L., Lamb, J., Smith, S., and Wheatley, D. N. (2000). Single amino acid (arginine) deprivation: rapid and selective death of cultured transformed and malignant cells. Br. J. Cancer 83, 800–810.
| Single amino acid (arginine) deprivation: rapid and selective death of cultured transformed and malignant cells.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXntFOhsLw%3D&md5=2921403ce7c015f658c66d0825b04581CAS | 10952786PubMed |
Spate, L. D., Whitworth, K. M., Walker, K. A., Bauer, B. K., Murphy, C. N., and Prather, R. S. (2010). Low-density lipoprotein (LDL) receptor mRNA and protein may enable LDL to replace bovine serum albumin during the in vitro swine embryo development. Mol. Reprod. Dev. 77, 298.
| 1:CAS:528:DC%2BC3cXisFemtrg%3D&md5=82d4f11ecb27ec7483876d85a6818033CAS | 20017142PubMed |
Tesfaye, D., Kadanga, A., Rings, F., Bauch, K., Jennen, D., Nganvongpanit, K., Holker, M., Tholen, E., Ponsuksili, S., Wimmers, K., Montag, M., Gilles, M., Kirfel, G., Herzog, V., and Schellander, K. (2006). The effect of nitric oxide inhibition and temporal expression patterns of the mRNA and protein products of nitric oxide synthase genes during in vitro development of bovine pre-implantation embryos. Reprod. Domest. Anim. 41, 501–509.
| The effect of nitric oxide inhibition and temporal expression patterns of the mRNA and protein products of nitric oxide synthase genes during in vitro development of bovine pre-implantation embryos.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXmslKqug%3D%3D&md5=0f752aae85d1aea5cc21d9a9d6ccaed0CAS | 17107508PubMed |
Tomikawa, J., Fukatsu, K., Tanaka, S., and Shiota, K. (2006). DNA methylation-dependent epigenetic regulation of dimethylarginine dimethylaminohydrolase 2 gene in trophoblast cell lineage. J. Biol. Chem. 281, 12 163–12 169.
| DNA methylation-dependent epigenetic regulation of dimethylarginine dimethylaminohydrolase 2 gene in trophoblast cell lineage.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xjslentr4%3D&md5=6ec17890f97cf59bb8e594716015afccCAS |
Tran, C. T., Leiper, J. M., and Vallance, P. (2003). The DDAH/ADMA/NOS pathway. Atheroscler. Suppl. 4, 33–40.
| The DDAH/ADMA/NOS pathway.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXps1Gmtbk%3D&md5=14d0d22700f7bae57270019df2c0023dCAS | 14664901PubMed |
Tranguch, S., Steuerwald, N., and Huet-Hudson, Y. M. (2003). Nitric oxide synthase production and nitric oxide regulation of preimplantation embryo development. Biol. Reprod. 68, 1538–1544.
| Nitric oxide synthase production and nitric oxide regulation of preimplantation embryo development.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXjt12lsb8%3D&md5=b469b4cb326f2ffc94e60af294c512efCAS | 12606428PubMed |
Van Winkle, L. J. (2001). Amino acid transport regulation and early embryo development. Biol. Reprod. 64, 1–12.
| Amino acid transport regulation and early embryo development.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXhtFCntw%3D%3D&md5=6e1e60cefe3aaba9ef7c362b782241d0CAS | 11133652PubMed |
Vander Heiden, M. G., Cantley, L. C., and Thompson, C. B. (2009). Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324, 1029–1033.
| Understanding the Warburg effect: the metabolic requirements of cell proliferation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXmtVKlsbg%3D&md5=e7d3918336fa1c261eea67a2a87ee7a6CAS | 19460998PubMed |
Wang, X., Frank, J. W., Little, D. R., Dunlap, K. A., Satterfield, M. C., Burghardt, R. C., Hansen, T. R., Wu, G., and Bazer, F. W. (2014a). Functional role of arginine during the peri-implantation period of pregnancy. I. Consequences of loss of function of arginine transporter SLC7A1 mRNA in ovine conceptus trophectoderm. FASEB J. 28, 2852–2863.
| Functional role of arginine during the peri-implantation period of pregnancy. I. Consequences of loss of function of arginine transporter SLC7A1 mRNA in ovine conceptus trophectoderm.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhtFaltb%2FL&md5=96e047b4906e88155d9ec23fdc6e7688CAS | 24627544PubMed |
Wang, X., Frank, J. W., Xu, J., Dunlap, K. A., Satterfield, M. C., Burghardt, R. C., Romero, J. J., Hansen, T. R., Wu, G., and Bazer, F. W. (2014b). Functional role of arginine during the peri-implantation period of pregnancy. II. Consequences of loss of function of nitric oxide synthase NOS3 mRNA in ovine conceptus trophectoderm. Biol. Reprod. 91, 59.
| Functional role of arginine during the peri-implantation period of pregnancy. II. Consequences of loss of function of nitric oxide synthase NOS3 mRNA in ovine conceptus trophectoderm.Crossref | GoogleScholarGoogle Scholar | 25061098PubMed |
Wang, S., Tsun, Z. Y., Wolfson, R. L., Shen, K., Wyant, G. A., Plovanich, M. E., Yuan, E. D., Jones, T. D., Chantranupong, L., Comb, W., Wang, T., Bar-Peled, L., Zoncu, R., Straub, C., Kim, C., Park, J., Sabatini, B. L., and Sabatini, D. M. (2015). Metabolism. Lysosomal amino acid transporter SLC38A9 signals arginine sufficiency to mTORC1. Science 347, 188–194.
| Metabolism. Lysosomal amino acid transporter SLC38A9 signals arginine sufficiency to mTORC1.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXitFelsA%3D%3D&md5=4acaaade92e115ac349826b9177e80d6CAS | 25567906PubMed |
Wheatley, D. N., and Campbell, E. (2003). Arginine deprivation, growth inhibition and tumour cell death: 3. Deficient utilisation of citrulline by malignant cells. Br. J. Cancer 89, 573–576.
| Arginine deprivation, growth inhibition and tumour cell death: 3. Deficient utilisation of citrulline by malignant cells.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXlsl2rsb0%3D&md5=98ddfdce106f45573f11b53bda42fe49CAS | 12888832PubMed |
Whitworth, K. M., Agca, C., Kim, J. G., Patel, R. V., Springer, G. K., Bivens, N. J., Forrester, L. J., Mathialagan, N., Green, J. A., and Prather, R. S. (2005). Transcriptional profiling of pig embryogenesis by using a 15-K member unigene set specific for pig reproductive tissues and embryos. Biol. Reprod. 72, 1437–1451.
| Transcriptional profiling of pig embryogenesis by using a 15-K member unigene set specific for pig reproductive tissues and embryos.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXksFGqsbg%3D&md5=235a3033584deb78449d8e37a2543005CAS | 15703372PubMed |
Wu, G. (2010). Functional amino acids in growth, reproduction, and health. Adv. Nutr. 1, 31–37.
| Functional amino acids in growth, reproduction, and health.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXpsF2lur8%3D&md5=392055ffbd1e1bd1efe6c7425701e3ceCAS | 22043449PubMed |
Wu, G., and Morris, S. M. (1998). Arginine metabolism: nitric oxide and beyond. Biochem. J. 336, 1–17.
| 1:CAS:528:DyaK1cXotVGltrs%3D&md5=bad9277b9b6f57e7f29b87c3822bec20CAS | 9806879PubMed |
Wu, G., Bazer, F. W., Burghardt, R. C., Johnson, G. A., Kim, S. W., Li, X. L., Satterfield, M. C., and Spencer, T. E. (2010). Impacts of amino acid nutrition on pregnancy outcome in pigs: mechanisms and implications for swine production. J. Anim. Sci. 88, E195–E204.
| Impacts of amino acid nutrition on pregnancy outcome in pigs: mechanisms and implications for swine production.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3c3pslOrsA%3D%3D&md5=1afd7cc51bb241bc8e49e165f76f3b17CAS | 19854987PubMed |
Yang, Y., and Bedford, M. T. (2013). Protein arginine methyltransferases and cancer. Nat. Rev. Cancer 13, 37–50.
| Protein arginine methyltransferases and cancer.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhvVajur7I&md5=2ae806cadc66e541471eb5cfede2ac2eCAS | 23235912PubMed |
Yoshioka, K., Suzuki, C., Tanaka, A., Anas, I. M., and Iwamura, S. (2002). Birth of piglets derived from porcine zygotes cultured in a chemically defined medium. Biol. Reprod. 66, 112–119.
| Birth of piglets derived from porcine zygotes cultured in a chemically defined medium.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38Xht1yksQ%3D%3D&md5=561964c8beff5173933d7dff62eac0c9CAS | 11751272PubMed |
Yu, Z., Chen, T., Hebert, J., Li, E., and Richard, S. (2009). A mouse PRMT1 null allele defines an essential role for arginine methylation in genome maintenance and cell proliferation. Mol. Cell. Biol. 29, 2982–2996.
| A mouse PRMT1 null allele defines an essential role for arginine methylation in genome maintenance and cell proliferation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXmvVCqsLs%3D&md5=53fb85e03a6231bce1643222cc1f0bcfCAS | 19289494PubMed |
Zhang, X., Miao, Y., Zhao, J. G., Spate, L., Bennett, M. W., Murphy, C. N., Schatten, H., and Prather, R. S. (2010). Porcine oocytes denuded before maturation can develop to the blastocyst stage if provided a cumulous cell-derived coculture system. J. Anim. Sci. 88, 2604–2610.
| Porcine oocytes denuded before maturation can develop to the blastocyst stage if provided a cumulous cell-derived coculture system.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXpvVOrsbY%3D&md5=6dd9ab36f3e1a5d43b732b5c751f2440CAS | 20382876PubMed |
Zoncu, R., Efeyan, A., and Sabatini, D. M. (2011). mTOR: from growth signal integration to cancer, diabetes and ageing. Nat. Rev. Mol. Cell Biol. 12, 21–35.
| mTOR: from growth signal integration to cancer, diabetes and ageing.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhsFGqsbnK&md5=ab06aee63af527cd79d929f4c4dc986aCAS | 21157483PubMed |
*These authors contributed equally to this work.