Changes in cell cycle and extracellular matrix gene expression during placental development in deer mouse (Peromyscus) hybrids
Amanda R. Duselis A , Craig Obergfell B , Jennifer A. Mack B , Michael J. O’Neill B , Quang K. Nguyen A , Rachel J. O’Neill B and Paul B. Vrana A CA Department of Biological Chemistry, Sprague Hall 350, School of Medicine, University of California Irvine, Irvine, CA 92799-1700, USA.
B Department of Molecular & Cell Biology, University of Connecticut, BH 309A, 345 Mansfield Road, Unit 2131, Storrs, CT 06269-2131, USA.
C Corresponding author. Email: pvrana@uci.edu
Reproduction, Fertility and Development 19(5) 695-708 https://doi.org/10.1071/RD07015
Submitted: 21 January 2007 Accepted: 6 May 2007 Published: 4 July 2007
Abstract
Crosses between two species of the rodent genus Peromyscus produce defects in both growth and development. The defects are pronounced in the hybrid placentas. Peromyscuys maniculatus (strain BW) females mated to P. polionotus (strain PO) males produce placentas half the size of the parental species, as well as growth-retarded embryos. In contrast, PO females mated to BW males result in defective conceptuses that display embryonic and placental overgrowth. These ‘parent-of-origin’-dependent phenotypes are consistent with previous studies that demonstrated altered expression of imprinted genes and genetic linkage of the overgrowth phenotypes to imprinted domains. In the present study, we take a broader approach in assessing perturbations in hybrid placental gene expression through the use of Mus musculus cDNA microarrays. In verifying classes of genes identified in microarray screens differentially regulated during hybrid placental development, we focused on those influencing the cell cycle and extracellular matrix (ECM). Our work suggests that cell cycle regulators at the G1/S phase check-point are downregulated in the large hybrid placenta, whereas the small hybrid placenta is more variable. The ECM genes are typically downstream targets of cell cycle regulation and their misregulation is consistent with many of the dysmorphic phenotypes. Thus, these data suggest imbalances in proliferation and differentiation in hybrid placentation.
Introduction
The placenta is a major endocrine gland that also regulates the maternal–fetal flow of nutrients and waste (Cross 2006). Aberrant placental development may result in fetal defects, as well as gestational trophoblast diseases, such as choriocarcinoma (Cheung 2003). Although the ultimate causes of placental malfunctions are varied, many are accompanied by misexpression of cell cycle genes and corresponding changes in proliferation, differentiation and apoptosis (Arima et al. 1997; Constancia et al. 2002; Frank et al. 2002; Luu et al. 2005; Walter and Schonkypl 2006).
We use rodents of the North American genus Peromyscus as a model system to study placental growth dysplasia. Two species, namely P. polionotus (strain PO) and P. maniculatus (strain BW), produce offspring with marked parent-of-origin growth phenotypes when inter-crossed (Dawson 1965). When BW females are mated with PO males, growth-retarded offspring are produced (bw × po; note the use of lowercase letters, which indicates the result of the cross). The hybrid effect is particularly pronounced in hybrid placentas, which are half the size of those of the parental strain. Conversely, a PO female mated with a BW male (PO × BW; here, uppercase letters denote overgrowth) results in placental and embryonic overgrowth (Fig. 1). This overgrowth is accompanied by developmental abnormalities, as well as frequent fetal and maternal lethality (Rogers and Dawson 1970; Maddock and Chang 1979; Vrana et al. 2000).
These reciprocal phenotypes are associated with changes in genomic imprinting and exhibit linkage to regions known to harbour multiple imprinted loci. Imprinted genes are those that exhibit expression bias of one parental allele over the other (Tilghman 1999). Imprinted genes are typically found in discrete genomic clusters (Lewis and Reik 2006). The two parental alleles in these regions are distinguished by differential distribution of epigenetic marks such as DNA methylation and histone modifications (Monk et al. 1987; Reik et al. 1987; Sanford et al. 1987; Sapienza et al. 1987; Swain et al. 1987). Genomic imprinting is thought to share mechanistic origins with X-chromosome inactivation, which exhibits a bias towards inactivation of the paternal copy (Hajkova and Surani 2004). Further, the X-chromosome has been shown to contain a surfeit of ‘placental genes’ (i.e. genes whose expression is primarily in the placenta and/or whose products have been shown to have critical placental function; Monk 1986; Khil et al. 2004).
Evidence from numerous studies links misregulation of both imprinted and X-linked genes to placental and embryonic growth phenotypes. The best-studied group of these genes is a cluster located on distal mouse chromosome 7/human 11p15.5, which is implicated in sporadic cancer and growth phenotypes (Weksberg et al. 1993; Feinberg 2000; Reik et al. 2003). For example, paternal inheritance of a targeted mutation that reduces placental insulin-like growth factor 2 (Igf2) expression by approximately 10% results in reduced growth of both the placenta and embryo (Constancia et al. 2002). Maternal inheritance of a deletion of the linked Ipl/Phlda2 gene produces an overgrown placenta (Frank et al. 2002). Altered expression of both the Igf2 and Ipl/Phlda2 genes is associated with trophoblast diseases such as choriocarcinoma and biparental complete hydatidiform mole (Arima et al. 1997). The latter condition is characterised by an overgrowth of trophoblast (placental) tissue and lack of a fetus. Other imprinted genes in this region, such as Cdkn1c/p57kip2 (a cyclin-dependent kinase inhibitor), show similar associations (Takahashi et al. 2000; Kim et al. 2005). Such perturbations are typically accompanied by altered patterns of one or more epigenetic marks (e.g. DNA methylation).
Epigenetic defects and subsequent aberrant growth phenotypes are also associated with misexpression of non-imprinted and X-linked genes. For example, misregulation of genes involved in the cell cycle (Rb1, Cdkn1c/p57kip2, p16), mismatch repair (MlH1), Wnt signalling (SFRP1, SFRP2, SFRP4 and SFRP5), ECM (procollagen-encoding loci), metastasis (Timp3 and s100a6/Calcyclin) and many other pathways leads to defects in the control of proliferation, differentiation and apoptosis. All these loci also exhibit altered DNA methylation at the promoter associated with aberrant transcription (Parker et al. 1982; Guenette et al. 1992; Baylin and Ohm 2006).
The goal of the present study was to assess the scope of changes in hybrid placental gene expression. Our hypothesis was that known growth regulators and cell cycle genes would show altered expression in the reciprocal hybrids. In particular, we predicted that imprinted loci and other loci known to be subject to epigenetic regulation would be affected. To our knowledge, this is the first study to take a large-scale approach in examining changes in gene expression that accompany perturbations of imprinted gene expression.
Materials and methods
Animals
We purchased PO and BW animals from the Peromyscus Genetic Stock Center (Columbia, SC, USA). Animals were housed with food and water available ad libitum on a 16 : 8 h light : dark cycle. We bred PO females with male BW and BW females with PO males to obtain reciprocal hybrids. These crosses, along with inter-crossed parental species, were placed in the same cage with a separation cage top to allow the oestrous cycle of the female to be induced by the presence of a male without contact. After 3 days, a normal cage top was used so females and males could mate at will. Vaginal smears were performed morning and night for sperm. Day 0 was defined as the day when spermatozoa were found in vagina of a cycling female. Peromyscus preimplantation development is 4 days longer than that of Mus. However, to facilitate comparisons, we have presented the data without inclusion of these 4 days, such that the developmental stages of the two genera coincide; hence, all age references in the text refer to M. musculus equivalents.
Microarray hybridisation
Sample preparations were conducted using Qiagen (Germantown, MD, USA) RNeasy Mini Kit for isolation of total RNA from animal tissue, with all homogenisation performed using Qiashredder (Qiagen). First- and second-strand cDNA synthesis was conducted using Invitrogen Life Technologies (Carlsbad, CA, USA) SuperScriptTM Choice Systems for cDNA Synthesis with 5 μg starting RNA and HPLC-purified T7-oligodT. Poly-A Spike Controls were added to first strand synthesis as per the Affymetrix (Santa Clara, CA, USA) GeneChip Eukaryotic Poly-A RNA Control Kit. Poly-A control (1 μL) was added to the first-strand reaction to give a final volume of 12 μL. The first-strand incubation was performed at 42°C. Clean up of the double-stranded cDNA was performed using the GeneChip Sample Cleanup Module by Affymetrix. The cRNA was then biotin labelled using the Enzo BioArray High Yield RNA Transcript Labelling Kit (Affymetrix). All samples were labelled for 5 h at 37°C. Clean up, quantification and fragmentation of biotin-labelled cRNA was conducted using the GeneChip Sample Cleanup Module (Affymetrix).
The hybridisation solution was then prepared with 15 μg fragmented cRNA, 3 nm control oligo B2, 20× control cRNA, 10 mg mL–1 herring sperm DNA, 50 mg mL–1 bovine serum albumin (BSA), 2× hybridisation buffer and diethylpyrocarbonate H2O to give a final volume of 300 μL. This solution was incubated at 99°C for 5 min and then 45°C for 5 min. The Affymetrix Mouse Expression Array 430A Gene Chips were equilibrated to room temperature during hybridisation target incubation. Hybridisation to these array chips was performed for 20 h at 45°C according to the manufacturer’s instructions. Analyses of these hybridisations are described in the Results section.
Histological analysis
Placentas were fixed in 4% paraformaldehyde. Fixed samples were then dehydrated through ascending concentrations of ethanol and then sent to Histoserv Inc. (http://www.histoservinc.com) for sectioning and haematoxylin and eosin staining. Sections were used for type I collagen staining using primary antibodies obtained from Abcam (ab21285). The Vectostain ABC elite kit (Vector Laboratories, Burlingame, CA, USA) with horseradish peroxidase and Vector NovaRED substrate kit was used to visualise antibody binding.
Gene expression
Total RNA was obtained from whole placental tissue either through lithium chloride–urea treatment (Auffray and Rougeon 1980) or using the RNeasy Micro Kit or Mini Kit purchased from Qiagen. In all cases, RNA samples were DNased before reverse transcription using Qiagen On-Column DNase digestion. For quantitative real-time polymerase chain reaction (PCR) assays of the Cdkn1c/p57kip2, Rpl-32, Ccnd1, Ccne1, Gas1 and Klf6 genes, Peromyscus-specific Taqman assays were developed using Assays-by-Design technology (Applied Biosystems, Foster City, CA, USA). The Taqman protocol was performed according to the recommendations of Applied Biosystems. Reactions were performed in triplicate in 96-well plates and cycled on an ABI 7900 machine (Applied Biosystems). Quantitative analysis was conducted according to ‘Critical Factors for Successful Real-Time PCR’ (http://www.Qiagen.com). The quantitative real-time reverse transcription (RT)–PCR for Col1a2 and Col3a1 was performed according to the methods described by Raefski and O’Neill (2005). A minimum of n = 3 was used for each sub-grouping (parental strains, PO × BW and bw × po) in the analysis. Error bars denote 95% confidence intervals. Differences in gene expression values between groups were considered significant if the mean values differed by more than one standard deviation.
Semiquantitative PCR assays for the Timp1, Timp2, Timp3 and s100a6/Calcyclin genes were conducted using 32P-labelled deoxy-cytosine. Reverse transcription was performed using Invitrogen SuperScript First Strand Synthesis for RT–PCR with oligo(dT). Polymerase chain reaction experiments were conducted with minimal amounts of radioactivity for incorporation of radioactive cytosine. Primer sets for both experimental and control (Rpl-32) loci were placed in the same reaction and amplified for a maximum of 20 cycles to ensure that measurements were taken in the linear range of amplification. Samples were electrophoresed on 7.5% polyacrylamide gels and then dehydrated. Quantitation and visualisation were performed using an Amersham Bioscience Typhoon 9400 according to the manufacturer’s instructions. Primer sets and annealing temperatures for PCR assays are listed in Table 1.

|
For northern blot analysis, total RNA was isolated as above and then subjected to electrophoresis on a 3-[N-morpholino]propanesulphonic acid (MOPS)–agarose gel. The size-separated RNA molecules were then transferred to a Hybond-N+ nylon filter and probed with radioactive probes made from DecaPrime (Ambion, Austin, TX, USA) in UltraHyb solution (Ambion). Blots were visualised and bands quantified as described above.
Results
Microarray analysis
The Peromyscus genome has not yet been sequenced, nor are there commercially available Peromyscus cDNA microarrays. To circumvent these problems, we used M. musculus microarray chips for genome-wide gene expression profiling in Peromyscus. Although Mus and Peromyscus diverged approximately 25 million years ago (Kass et al. 1992; Lee et al. 1995), our experience is that coding regions typically exhibit high sequence identity between the two genera (i.e. ≥ 70%). However, independent verification of these results is paramount.
We dissected placentas at approximately embryonic Day (E) 12.5 of gestation from eight pregnancies. These litters represented the four genotypic classes (Fig. 1) as follows: BW, two litters; PO, one litter; bw × po, three litters; and PO × BW, two litters.
We isolated total placental RNA from three separate placentas from each class using separate litters where possible. Each of the 12 samples was hybridised to a separate Affymetrix M. musculus microarray, as described in the methods section. Using the chip files, the value of the genes was generated using gcRMA in the software ArrayAssist (version 2.6.1642; Stragene, La Jolla, CA, USA). One-way ANOVA with Bayesian t-test (http://visitor.ics.uci.edu/cgi-bin/genex/cybert/CyberTReg-8.0.form.pl) was performed to construct a list of genes with P values < 0.01. We divided genes into functional groups based on Gene Ontology annotation (http://www.geneontology.org).
In our survey of these loci, those whose products regulate the cell cycle or are involved in regulation of the ECM stood out. We constructed a Venn diagram to depict the relative numbers of these two gene classes suggested to be affected (Fig. 2). A total of 415 ECM/cell cycle genes was found of 5758 transcripts that were deemed significant by ANOVA analysis (P < 0.01). We initially focused on genes that showed significant differences in the F1 hybrids relative to the parental strains (i.e. PO, BW). That is, we initially chose to test genes where the parental strains displayed roughly equal expression.

|
Genes suggested by the array analyses to be downregulated in both F1 hybrids included Col1a2, Col3a1, Timp2, Gas1 and Ccne1. Genes suggested by the array analyses to be downregulated specifically in PO × BW hybrids included s100a6/Calcyclin, Timp1, Ccnd1 and Cdkn1c/p57kip2, whereas downregulation in the bw × po hybrids was predicted for Timp3. Finally, the genes suggested by the array analyses to be upregulated in the PO × BW hybrids included Klf6 and Timp3, whereas genes suggested to be upregulated in bw × po placentas included s100a6/Calcyclin and Cdkn1c/p57kip2.
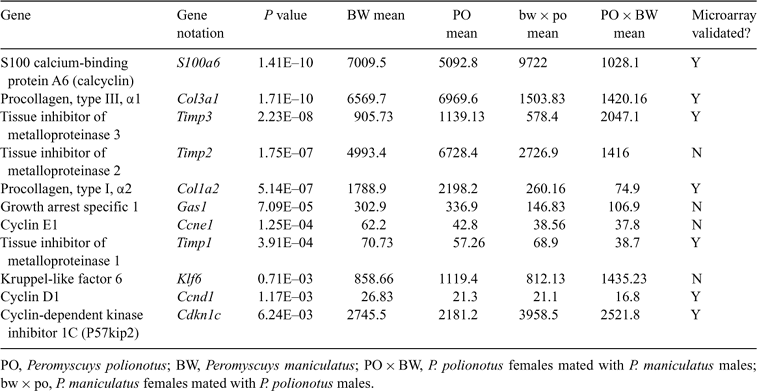
The 50 ECM- and cell cycle-related genes with the lowest P values are listed in Table 2. The P values and average expression values of the loci tested on the basis of the microarray analysis are given in Table 3. Primers sequences used to amplify these loci are given in Table 1. As noted, the products of all these genes have been shown to regulate either the cell cycle or ECM (Prockop 1985; Chen et al. 1989; Tonini et al. 1991;Apte et al. 1994; Lee et al. 1995; Matsuoka et al. 1995). We assessed expression levels of these genes at three different time points, namely E10.5 (early placentation), E13.5 (mid-placentation) and E16 (after placental maturation). These time points were chosen to investigate the temporal expression dynamics of these genes, as well as to test the patterns suggested by the microarray analysis.

|
Perturbation of cell cycle regulatory genes in the F1 hybrids
The s100a6/Calcyclin gene product is thought to have diverse functions, but has been specifically implicated in the G1/G0 cell cycle checkpoint (Tonini et al. 1991). In the placenta, s100a6/Calcycin is expressed in uterine natural killer (uNK) cells and has been implicated in regulation of secretory vesicles (Thordarson et al. 1991; Farnsworth and Talamantes 1998). Uterine NK cells invade the uterine tissue at the zygotic–maternal interface (decidua) and the metrial gland, a pregnancy specific uterine structure. These uNK cells are associated with the formation of the decidua and vascular remodelling (Takeda and Paszkowski 2005).
To ensure that we obtained tissue samples that included all placental s100a6/Calcycin-expressing cell types, we used cross-sections from whole placentas with the entire uterine component for both RNA isolation and histological analyses. A semiquantitative radioactive PCR was used to independently confirm differential gene expression of the s100a6 gene between hybrid and parental placental samples. The microarray gene expression profile suggested the upregulation of s100a6 in the growth-retarded hybrids, with reciprocal underexpression in the hyperplastic PO × BW placentas. The predicted reduction in PO × BW s100a6/Calcyclin microarray gene expression was borne out at E13.5 and E16 (Fig. 3). Although the bw × po data at E13.5 were suggestive of increased expression, the changes were not statistically significant.

|
The cell cycle regulator encoded by the Cdkn1c/p57kip2 gene is a broadly expressed member of the Cip/Kip family. Increased levels of Cdkn1c product result in inhibition of cyclins E/A and are associated with Cdk2 kinase activity (Lee et al. 1995). The Cdkn1c gene is also imprinted, suggesting that hybrid expression perturbations are likely (Hatada and Mukai 1995). We tested Peromyscus placental Cdkn1c expression by both northern analysis (data not shown) and quantitative RT-PCR (Fig. 3). Consistent with the microarray results, both assays indicated reduced Cdkn1c expression levels in the PO × BW samples at E13.5 and E16.
The Cdkn1c gene is expressed at much lower levels at E10.5, a stage when other Cip/Kip family members exhibit higher expression (Kim et al. 2005). An assay to test the predicted increase in bw × po placental Cdkn1c expression was again suggestive of that trend, but the differences were not significant.
The Ccnd1 gene product cyclin D1 is a cell cycle regulator necessary for progression into S phase (Baldin et al. 1993). Microarray analyses predicted reduced Ccnd1 levels in PO × BW relative to the other classes. Our secondary assay verified significantly reduced expression of Ccnd1 in PO × BW at E10.5 and E16. Although the average Ccnd1 levels in PO × BW were also lower at E13.5, the difference was not significant. The bw × po placentas displayed a varied gene expression profile with levels equivalent to the PO/BW strains at E10.5, but lower expression levels at E13.5 and E16.
The Klf6 transcription factor acts to regulate transcription of Ccnd1 (Benzeno et al. 2004). The Klf6 gene was suggested to be overexpressed in the PO × BW class. In contrast with what the array analysis suggested, PO × BW placental Klf6 expression was not significantly different from the parental strains at E13.5. However, the reciprocal bw × po placentas did display significantly increased levels of Klf6 at E13.5.
The Gas1 product is associated with the cell cycle by interacting with p53 and inhibiting G1/S phase transition (Del Sal et al. 1995). During development, the highest levels of expression are found in undifferentiated cells and expression is lost as cells terminally differentiate (Rees et al. 1999). Microarray analysis predicted reduced Gas1 expression in both hybrid classes. Our verification assay confirmed reduced Gas1 expression in PO × BW; however, this difference was observed only at E10.5. Reduced Gas1 expression was never observed in bw × po hybrids.
The final cell cycle gene tested was Ccne1, encoding cyclin E1. Cyclin E1 is a positive regulator of the cell cycle and increases in expression are associated with cancer and excessive growth (Sutherland and Musgrove 2004). Surprisingly, the microarray analyses suggested a decrease in Ccne1 expression in both hybrid classes. Our tests revealed equivalent levels of Ccne1 expression at E10.5 and E13.5, followed by an approximate fourfold increase in PO × BW placentas at E16.
Reduced expression of hybrid ECM genes
Several genes whose products contribute to, or positively regulate, the ECM showed changes specific to the hybrid placentas. We assessed changes in the expression levels of these genes, including Col1a2, Col3a1, Timp1, Timp2 and Timp3, using real-time quantitative RT-PCR assays and semiquantitative radioactive RT-PCR.
The procollagen gene family is involved in numerous aspects of matrix stability and development (Prockop 1985). The specific genes identified in our screen include Col1a2 (type I procollagen) and Col3a1 (type III procollagen). The growth-retarded bw × po placentas had relatively high expression levels of Col1a2 and Col3a1 at E10.5, but reduced levels at E13.5 and E16, compared with the parental strains (Fig. 4). The PO × BW placentas had reduced levels of Col1a2 at E13.5 and E16 and reduced Col3a1 expression at E10.5 and E13.5.

|
The Timp1, Timp2 and Timp3 gene products act positively on the maintenance of the ECM by negatively regulating matrix metalloproteinases, which degrade ECM protein components (Matrisian 1990). In addition, Timp3 inhibits angiogenesis, invasiveness in cancers, increases apoptosis and suppresses growth (Lam et al. 2005). Timp1 and Timp2 are soluble proteins, whereas Timp3 is bound to the ECM. Timp2 is constitutively expressed, whereas Timp1 and Timp3 transcription is regulated by cytokines and growth factors (Kassiri and Khokha 2005).
The gene expression data from the microarray results for Timp1 suggested a reduction in expression for PO × BW placentas. However, this trend was confirmed only at E16 (Fig. 4c). No other significant differences in Timp1 expression were observed. The microarray analysis predicted a reduction of Timp2 expression in both hybrid classes, but our verification assays did not reveal any significant changes in gene expression for either group. However, Timp3 expression was substantially reduced in the bw × po hybrids at all three time points tested. Strikingly, PO × BW hybrids displayed a similar reduction in Timp3 expression at both E10 and E16, but displayed equal or greater amounts of expression than the parental strains at E13.5 (Fig. 4b).
Histology reveals altered collagen expression profile
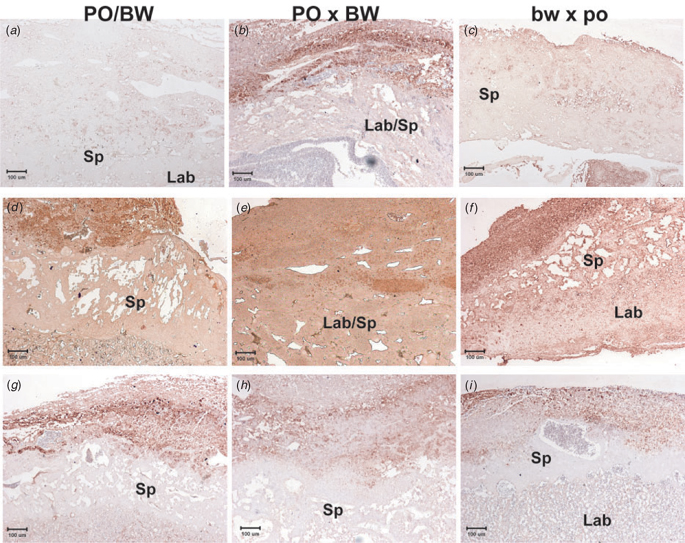
Collagens are typically secreted by differentiated cells and are formed from procollagen polypeptides (Prockop 1985). To assess the correlation between the observed reduction in procollagen gene expression and the extent of the collagen protein content across the placenta, we performed histological staining with the type I collagen antibody.
As suggested, the parental strain (PO and BW) placentas displayed the highest levels of type I collagen staining (Fig. 5a, d, g). A reduction in hybrid type I collagen staining was particularly evident in decidua at E13.5 (Fig. 5d–f) and E16 (Fig. 5g–i). An overall reduction in type I collagen was seen in PO × BW placentas, with areas of staining found limited to the maternal decidua (Fig. 5b, e, h). The dark pockets seen in these hypertrophic placentas are not positive for type I collagen staining, but rather represent pockets of erythrocytes due to haemorrhaging.
Discussion
Misregulation of placental gene expression can have far-ranging effects on both the mother and fetus. Reciprocal crosses between P. polionotus and P. maniculatus offer a unique system for studying the role of natural genetic variation in producing aberrant placental gene expression and its associated phenotypes.
Imprinted genes may be particularly susceptible to such perturbations, given the intricacy of their epigenetic regulation. Further, the placenta is a major site of imprinted gene expression and growth regulation. We have previously established that imprinted loci likely contribute to the hybrid phenotypes in two ways. First, several loci lose their imprinted status in hybrid embryos and placentas, particularly in the PO × BW offspring (Vrana et al. 1998, 2000). This loss-of-imprinting is modulated by a genetic maternal effect (Duselis et al. 2005). Second, we have mapped two loci involved in the overgrowth phenotype to imprinted domains (Vrana et al. 2000).
Although we have confirmed aberrant DNA methylation at several imprinted loci, we do not yet know the genome-wide extent of changes in either DNA methylation or gene expression in the Peromyscus hybrids. In addition to a candidate gene approach, we have used cDNA microarray to finding affected loci. Of the 11 loci assessed initially, our tests validated seven as showing significant changes in hybrid gene expression relative to the parental strains. With the successful validation of approximately 64% genes tested, it seems likely that these analyses of the microarray data will yield insights into additional affected pathways.
The two pathways investigated to date were ECM and cell cycle regulation. No clear linkage has been established between genomic imprinting and ECM-related genes. However, as growth regulators, it seems likely that imprinted loci are typically upstream of genes involved in directly affecting the ECM.
Of the cell cycle genes we found to be perturbed in the PO × BW hybrids, five (Cdkn1c/p57kip2, Ccne1, Gas1, s100a6/Calcyclin and Ccnd1) encode regulators of the G1/S phase checkpoint. Placental misregulation of this checkpoint can cause overgrowth, as well as increases in proliferation and decreases in differentiation (Zhang et al. 1998; Wu et al. 2003). The reduction in PO × BW Cdkn1c/p57kip2 expression particularly suggests an increase in proliferative capacity and a corresponding decrease in differentiation. Similarly, increases in Ccne1 expression are associated with uncontrolled growth and cancer (Yue and Jiang 2005).
Degradation of the ECM is typically necessary for additional growth and angiogenesis (Spurbeck et al. 2002). Both collagens and Timp proteins are required at the maternal–fetal interface of the placenta (Apte et al. 1994; Carbone et al. 2006). Together, these data suggest improper ECM formation both within the fetal placenta and the decidua of the PO × BW cross. Further, numerous cell types have been shown to require specific extra-cellular matrix composition for proper differentiation and/or migration (Pedersen and Swartz 2005). Thus, the lack of proper ECM gene expression may further contribute to an imbalance between proliferation and differentiation, resulting in dysplastic phenotypes. For example, the uNK cell expression of s100a6/Calcyclin requires the migration of this cell type to the decidua from other portions of the uterus (Whitelaw and Croy 1996; Farnsworth and Talamantes 1998). In turn, the secretion of s100a6 by uNK cells is thought to act on invasive trophoblast giant cells to produce placental hormones (Soares et al. 1998). Thus, the diminished levels of collagen and metalloproteinase inhibitor gene expression in the PO × BW hyperplastic placentas may result in ECM that is not competent for uNK cell migration. The resulting reduction in s100a6 signalling is predicted to affect giant cell development and/or placental lactogen (PL) expression from these cells. Altered PL expression may affect both fetal and maternal tissues (Soares 2004). Indeed, previous work has shown temporal misregulation of type I placental lactogen expression in PO × BW placental development (Vrana et al. 2001).
These results bear comparison with Mus placental dysplasias, which have been the subject of broad gene expression analyses. Singh et al. (2004) performed microarray experiments on three groups of hyperplastic placentas: (1) those produced by somatic cell nuclear transfer (i.e. ‘clones’); (2) those produced by targeted mutation (knockout) of the Esx1 homeobox gene; and (3) those produced by crossing Mus spretus females with males of the C57BL/6 strain (B6 or ‘Black 6’). All three classes are potentially relevant to the Peromyscus crosses. First, the cloned placentas display epigenetic aberrations, including perturbations of imprinted gene expression (Humpherys et al. 2002; Suemizu et al. 2003). Esx1 is a candidate gene for one of the loci involved in the PO × BW placental hyperplasia (Loschiavo et al. 2007). Finally, the M. spretus/B6 crosses bear special note because they have also been termed interspecific hybrids (Zechner et al. 2004). However, the B6 strain is a mix of the M. musculus musculus and domesticus subspecies of unknown locales, as well as containing M. spretus sequences (http://www.informatics.jax.org/external/festing/mouse/docs/C57BL.shtml). Thus, the C57BL/6 combination of alleles does not exist naturally, nor is it likely that it could. Surprisingly, the M. spretus/B6 placenta hypertrophy results in few or no discernable effects on the embryo proper (Zechner et al. 1996). The Esx1 knockout results in growth retardation of the associated embryo, whereas the clone phenotypes are variable (Li and Behringer 1998). Despite the similarities of placental hypertrophy, few genes shared common expression trends among these three classes.
Genes identified by Singh et al. (2004) as showing twofold or greater changes relative to controls are listed in Table 4 (from table 2 in Singh et al. 2004). To facilitate comparisons, we have shown the Peromyscus hybrid expression levels relative to an average of PO and BW values (note, these values may be significantly different owing to differential hybridisation of the two alleles to the spotted 25-mer olignonucleotides). For comparative purposes, we counted genes with values of 1.3 or greater as upregulated and those with values 0.7 or less as downregulated. Major caveats to such comparisons are the sequence divergence resulting in lack of hybridisation or paralogous gene hybridisation.
Despite the preliminary nature of these comparisons, several trends are suggested. The PO × BW placentas show the same trend (downregulation) of the one gene common to all three Mus classes, namely Ramp2. The PO × BW placentas displayed three genes with trends in common to those of eight shared by the clone and M. spretus/B6 classes. The PO × BW placentas shared two loci with the same trend (Zfp36l1 and Plac8) with those unique to the M. spretus/B6 hybrids and one gene that displayed the opposite trend (Ada). In contrast, the PO × BW class showed the same trend as eight genes unique in the Mus analysis to the cloned placentae. No loci displayed opposite trends between these two classes. Finally, the Esx1-knockout and PO × BW placentas shared only two genes with the same trend, but seven genes with opposite trends. The latter is intriguing owing to the very dissimilar effects these two hyperplastic placental types have on their associated embryos.
Many of the genes we have identified as misregulated in the Peromyscus hybrids have been shown to be subject to epigenetic regulation, including Timp1, Timp2, Timp3, Procollagen I, S100a6/Calcyclin and Cdkn1c/p57kip2 (Guenette et al. 1992; Wistuba et al. 2001; Feng et al. 2004; Fan et al. 2005; Rehman et al. 2005; Chu et al. 2006). In addition, Cdkn1c/p57kip2 is an imprinted gene and is therefore regulated by epigenetic modifications (Hatada et al. 1996). The finding that multiple classes of genes regulated by DNA methylation are perturbed in these Peromyscus hybrids is striking. Numerous studies have now implicated DNA methylation in gene silencing apart from heterochromatin, X-chromosome inactivation and genomic imprinting (Baylin and Ohm 2006). It is now clear that epigenetic marks play a significant role in regulating tissue- and temporally specific gene expression (Song et al. 2005). This suggests that, in addition to genetic mutations, epigenetic misregulation of genes may be responsible for developmental and growth defects (Humpherys et al. 2001). Indeed, many tumours have now been shown to exhibit multiple epigenetic changes (Baylin and Ohm 2006).
Because the two Peromyscus strains used in the present study represent natural populations, we believe this is the first report to suggest a link between non-deleterious allelic variants and control of multiple classes of epigenetically regulated loci. To our knowledge, this is also the first study to broadly examine changes in gene expression accompanying placental imprinting perturbations at multiple loci.
Acknowledgements
This research was supported by grants from the American Cancer Society (RSG-03–070–01-MGO) and March of Dimes (#5-FY03–17) to PBV, the National Science Foundation (MCB-0093250) to RJO and the University of Connecticut Research Foundation to RJO and MJO. The authors thank the Center for Applied Genetics and Technology for Affymetrix support and the Center for Molecular and Mitochondrial Medicine and Genetics for assistance with microscopy and imaging.
Apte, S. S. , Mattei, M. G. , and Olsen, B. R. (1994). Cloning of the cDNA encoding human tissue inhibitor of metalloproteinases-3 (TIMP-3) and mapping of the TIMP3 gene to chromosome 22. Genomics 19, 86–90.
| Crossref | GoogleScholarGoogle Scholar | PubMed |
Cheung, A. N. (2003). Pathology of gestational trophoblastic diseases. Best Pract. Res. Clin. Obstet. Gynaecol. 17, 849–868.
| Crossref | GoogleScholarGoogle Scholar | PubMed |
Farnsworth, R. L. , and Talamantes, F. (1998). Calcyclin in the mouse decidua: expression and effects on placental lactogen secretion. Biol. Reprod. 59, 546–552.
| Crossref | GoogleScholarGoogle Scholar | PubMed |
Frank, D. , Fortino, W. , Clark, L. , Musalo, R. , Wang, W. , Saxena, A. , Li, C. M. , Reik, W. , Ludwig, T. , and Tycko, B. (2002). Placental overgrowth in mice lacking the imprinted gene Ipl. Proc. Natl Acad. Sci. USA 99, 7490–7495.
| Crossref | GoogleScholarGoogle Scholar |
Luu, H. H. , Zhou, L. , Haydon, R. C. , Deyrup, A. T. , and Montag, A. G. , et al. (2005). Increased expression of s100a6 is associated with decreased metastasis and inhibition of cell migration and anchorage independent growth in human osteosarcoma. Cancer Lett. 229, 135–148.
| Crossref | GoogleScholarGoogle Scholar | PubMed |
Reik, W. , Collick, A. , Norris, M. L. , Barton, S. C. , and Surani, M. A. (1987). Genomic imprinting determines methylation of parental alleles in transgenic mice. Nature 328, 248–251.
| Crossref | GoogleScholarGoogle Scholar | PubMed |
Wu, L. , de Bruin, A. , Saavedra, H. I. , Starovic, M. , and Trimboli, A. , et al. (2003). Extra-embryonic function of Rb is essential for embryonic development and viability. Nature 421, 942–947.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Yue, H. , and Jiang, H. Y. (2005). Expression of cell cycle regulator p57kip2, cyclinE protein and proliferating cell nuclear antigen in human pancreatic cancer: an immunohistochemical study. World J. Gastroenterol. 11, 5057–5060.
| PubMed |

Zechner, U. , Reule, M. , Orth, A. , Bonhomme, F. , Strack, B. , Guenet, J. L. , Hameister, H. , and Fundele, R. (1996). An X-chromosome linked locus contributes to abnormal placental development in mouse interspecific hybrids. Nat. Genet. 12, 398–403.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Zechner, U. , Shi, W. , Hemberger, M. , Himmelbauer, H. , and Otto, S. , et al. (2004). Divergent genetic and epigenetic post-zygotic isolations mechanisms in Mus and Peromyscus. J. Evol. Biol. 17, 453–460.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Zhang, P. , Wong, C. , DePinho, R. A. , Harper, J. W. , and Elledge, S. J. (1998). Cooperation between the Cdk inhibitors p27KIP1 and p57KIP2 in the control of tissue growth and development. Genes Dev. 12, 3162–3167.
| PubMed |