Morphokinetic prediction of embryo viability in cattle
Satoshi Sugimura A * , Tatsuma Yao B C , Satoko Matoba D , Kazuo Yamagata B and Kei Imai EA
B
C
D
E
Abstract
Conventionally, bovine in vitro-produced (IVP) embryos for transfer are morphologically evaluated at day 7–8 of embryo culture, as recommended by the International Embryo Technology Society (IETS). However, this method is subjective, relying on the percentage of degenerated cells and developmental stage, leading to variability depending on the operator. In contrast, we have implemented a novel selection system for bovine IVP blastocysts using time-lapse monitoring in specially developed microwell culture dishes (LinKID micro25). This approach allows for continuous tracking of individual embryo development. Additionally, we have advanced live-cell imaging technology to observe nuclear and chromosomal dynamics during early embryogenesis, identifying prognostic factors indicative of viability post-transfer. Integrating these factors has significantly improved conception rates compared to conventional morphological evaluation. This review discusses morphokinetics relevant to viability and our innovative selection system, which enable accurate prediction of embryo viability after transfer in cattle.
Keywords: bovine, embryo, first cleavage, in vitro production, live-cell imaging, morphokinetics, time-lapse monitoring, viability prediction.
Introduction
Embryo transfer practitioners have long sought a way to identify bovine in vitro-produced (IVP) embryos that are most likely to establish a pregnancy and develop to term. The conventional selection criteria are based on morphological quality and developmental stage at the time of transfer (Stringfellow and Givens 2010; Bo and Mapletoft 2013). However, this approach is considered subjective and inadequate due to significant variation in classification between practitioners (Farin et al. 1995). Additionally, pregnancy outcomes for bovine IVP embryos deemed morphologically good to excellent (code 1) remain low (Sugimura et al. 2017). Therefore, novel criteria that allow for objective and reliable selection of embryos for transfer are needed to advance bovine IVP technology.
Beyond morphological quality, factors such as the number of embryonic cells in the inner cell mass (ICM) and trophectoderm (TE) (Koo et al. 2002), apoptosis incidence (Gjørret et al. 2003), hatching competence (Massip et al. 1982), chromosomal abnormalities (Viuff et al. 1999), and expression of specific genes (Mohan et al. 2004; Rabaglino et al. 2023) have been widely accepted for determining embryo quality. However, histological, cytogenetic, and epigenetic analyses complicate embryo transfer and practical selection. Non-invasive criteria that predict both blastocyst quality and viability may lead to novel methods for selecting bovine embryos for transfer.
In transvaginal ovum pickup (OPU), a limited number of oocytes are collected and then placed into culture systems for in vitro oocyte maturation (IVM), in vitro fertilization (IVF), and embryo culture (IVC) (Matoba et al. 2010). We developed a microwell culture dish (LinKID micro25) based on the well of the well system (WOW) of Vajta et al. (2000), enabling tracking of individual embryos with time-lapse monitoring (Sugimura et al. 2010). Time-lapse monitoring allows continuous imaging of each embryo’s development in vitro, enabling analysis of morphokinetics such as the number of pronuclei (PN) or nuclei, timing of cleavage, and number of blastomeres, which are used to select the best embryos for transfer in human assisted reproduction technology (Shoukir et al. 1997; Ziebe et al. 1997; Wong et al. 2010). In cattle, these criteria predict developmental competence to the blastocyst stage (Lonergan et al. 1999; Holm et al. 2002), but are rarely used for selecting embryos for transfer.
Biological information from time-lapse monitoring is limited, and lipid-rich dark cytoplasm such as that found in bovines prevents pronuclei (PN) and nuclear observation in IVP embryos. We applied live-cell imaging, developed for mice, to bovine embryos (Yao et al. 2018). This method enabled the evaluation of nuclear/chromosomal integrity despite the dark cytoplasm (Fig. 1).
3D live-cell imaging of bovine embryos injected with mRNA encoding histone H2B-mCherry and EGFP-α-tubulin was performed from the one-cell to blastocyst stage for 8 days. Red and green represent H2B-mCherry (nuclei/chromosome) and EGFP-α-tubulin (microtubule), respectively (Yao et al. 2018).
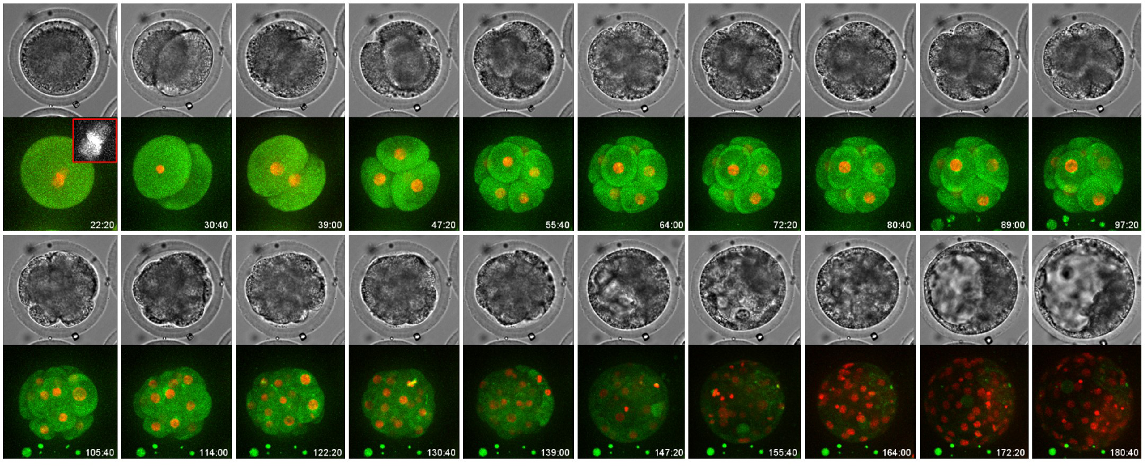
We identified various prognostic factors for predicting viability after embryo transfer using time-lapse monitoring with LinKID micro25 and live-cell imaging. This review describes morphokinetics related to embryo viability and the selection system for bovine IVP embryos using these morphokinetics.
Microwell culture dish suitable for time-lapse monitoring of bovine IVP embryos
Embryos cultured in LinKID micro25 fixed in alternative microwells have excellent visibility during time-lapse monitoring, with no negative side effects in terms of in vitro development or cell numbers at the blastocyst stage. Moreover, blastocysts derived from LinKID micro25 show a lower incidence of apoptosis and a higher conception rate than those cultured with a group in conventional droplets, apparently due to the accumulation of suitable autocrine and/or paracrine factors in the microwells (Sugimura et al. 2010).
In conventional group culture, the effects of positive-acting autocrine/paracrine factors and negative-acting toxic by-products of embryo metabolism depend on droplet size (Ferry et al. 1994), surface-to-volume ratio (Ferry et al. 1994), distance between cultured embryos (Gopichandran and Leese 2006), quality of neighboring embryos (Stokes et al. 2005), and embryo density (the number of embryos relative to medium volume) (Khurana and Niemann 2000; Fujita et al. 2006; Hoelker et al. 2009). Embryo density has been considered a particularly critical factor because it appears to affect embryo development, the number of cells in the ICM and TE (Vutyavanich et al. 2011), IFN-tau secretion (Larson and Kubisch 1999), and gene expression (Hoelker et al. 2009). We tested whether the density of bovine embryos (the number of embryos per volume of medium) affected the rate of in vitro development and gene expression during culture in conventional droplets or LinKID micro25. We did not observe the same effects of embryo density on development rate or gene expression in microwell cultures that we had seen in conventional droplet cultures. These outcomes suggest that blastocyst formation and transcription are not affected by embryo density in LinKID micro25 in the same way as they are in conventional droplet culture (Sugimura et al. 2013).
Timing of first cleavage
Previous studies with humans have indicated that slowly cleaving embryos have a lower likelihood of resulting in pregnancy compared to rapidly cleaving embryos (Fenwick et al. 2002). The reasons for the inferior viability and lower pregnancy and implantation rates of slowly cleaving embryos are not fully understood; however, chromosomal aberrations may play a role and likely reflect the quality of the spermatozoa and oocytes (Magli et al. 2007). We confirmed that slowly cleaving embryos had a higher incidence of abnormal chromosomes and were more often identified as mixoploid compared to rapidly cleaving embryos (Sugimura et al. 2012). While mixoploids are relatively common in IVP embryos and do not seem to critically influence subsequent development, there may be serious consequences of a high frequency (>25%) of abnormal chromosomes per blastocyst (Hare et al. 1980). Furthermore, live cell imaging revealed that embryos with chromosome abnormalities exhibited a delayed first cleavage, suggesting that the delay in first cleavage may reflect cytogenetic abnormalities such as chromosome segregation errors (Yao et al. 2018).
Direct cleavage
Sugimura et al. (2017) reported a lower conception rate following the transfer of blastocysts that had undergone direct cleavage from one cell to 3–4 cells (3–4 blastomeres) than for blastocysts with normal cleavage morphologies (two blastomeres). A recent study on humans indicated that directly cleaved embryos were correlated with impaired implantation and clinical outcomes (Zhan et al. 2016). Moreover, the study revealed a strong correlation between direct cleavage and multiple pronuclei. Similarly, in cattle, we demonstrated a high correlation between direct cleavage and multiple pronuclei or mis-migration of pronuclei (Suzuki et al. 2023). In other words, direct cleavage in cattle reflects abnormalities in fertilization. Indeed, ~80% of blastocysts that had undergone direct cleavage exhibited abnormalities in the number of pronuclei or errors in migration, whereas these abnormalities were present in only 10% of blastocysts that had cleaved normally into two cells.
Interestingly, in humans, it has been reported that even if direct cleavage occurs, if the embryo develops into a blastocyst, the pregnancy success rate is the same as with normal cleavage (Zhan et al. 2016). In cattle, live births have also been achieved after direct cleavage (Sugimura et al. 2010). Although the mechanism behind this is not clear, live-cell imaging analysis has revealed that only two neighboring pronuclei underwent syngamy, resulting in bipolar division and the formation of two blastomeres (Suzuki et al. 2023). Recently, single-cell genome analysis of bovine embryos revealed that embryos that underwent multiple divisions during the first mitosis contained biparental diploid blastomeres (De Coster et al. 2022). Biparental diploid blastomeres were formed if the two neighboring pronuclei that underwent syngamy had maternal and paternal genomes, and such embryos may have both diploid and haploid karyotypes (Han et al. 1999; Sugimura et al. 2012; Destouni et al. 2016; De Coster et al. 2022). The results obtained in this study strongly support previous reports in pigs and suggest why multi-pronuclei zygotes can develop into offspring (Han et al. 1999).
Reverse cleavage
Liu et al. (2014) defined reverse cleavage (RC) as either two daughter blastomeres recombining after complete separation following cleavage division, or incomplete separation of blastomeres that then fully recombine. In humans, it has been reported that the implantation rate is clearly reduced compared to normal cleavage (Bamford et al. 2022). Studies in cattle have shown that embryos that have undergone RC not only have a reduced ability to develop into blastocysts but also, even if they reach the blastocyst stage, have a reduced ability to hatch from the zona pellucida (Magata 2023). It has been suggested that this may be related to cytogenetic abnormalities.
Unevenly sized blastomeres
Human embryos with unevenly sized blastomeres have a lower likelihood of successful pregnancy and implantation (Hardarson et al. 2001). It has been noted that such embryos may have aneuploidy or multinucleation. In cattle, the relationship between uneven cleavage and viability was not well understood, but our research has revealed that an increase in unequal blastomere size at the 2-cell stage leads to a decrease in pregnancy outcomes (data not shown). Furthermore, as in humans, the relationship between uneven blastomeres and multinucleation has been suggested in cattle (Angel-Velez et al. 2023). The mechanism by which unequal cleavage occurs is unclear, but abnormalities in the cytoskeleton have been suggested (Yang et al. 2023).
Number of blastomeres at lag-phase
A better prognostic factor than the cell cycle observed at the lag-phase is the number of blastomeres at the lag-phase. With time-lapse monitoring, it was evident that a low number of blastomeres at the onset of the lag-phase was related to a low ICM percentage and elevated apoptosis in blastocysts (Sugimura et al. 2012). The lag-phase during the fourth cell cycle (5- to 8-cells) or fifth cell cycle (9- to 16-cells), is a key step in embryonic gene activation (EGA) following the maternal-to-zygotic transition (Lequarre et al. 2003). Moreover, the fourth cell cycle may be a conserved step in cell differentiation into ICM or TE cells. Indeed, the first signs of cell polarity in bovine embryos can already be detected at this stage (Koyama et al. 1994), so lag-phase parameters may reflect differentiation and/or EGA and be related to the viability of embryos after transfer.
Chromosome segregation error during early cleavage
In assisted reproductive technology (ART) in humans and cattle, chromosome aneuploidy is common and may be the primary cause of embryo-related pregnancy loss (Vanneste et al. 2009; McCoy et al. 2015; Tšuiko et al. 2017). It is well known that most aneuploidy arises during the mitotic division of preimplantation embryos (Vanneste et al. 2009). Mitotic aneuploidy is thought to result from chromosomal segregation errors caused by chromosomal instability during early embryonic development. In a mouse model, Mashiko et al. (2020) showed that the severity of chromosomal segregation errors in early embryonic development affects blastocyst formation but not viability after blastocyst transfer. However, this has not been clarified in cattle. To investigate this, we performed live-cell imaging to examine the relationship between abnormal chromosomal segregation (ACS) and embryonic development in cattle (Yao et al. 2022).
Errors in chromosome segregation in early cleavage affect blastocyst formation – specifically, embryos that underwent ACS with multiple or large micronucleus formation rarely developed into blastocysts. Embryos with severe ACS had prolonged cell cycles. After transferring blastocysts with live-cell imaging of chromosome segregation to 10 cows, six became pregnant, and four of them gave birth to full-term offspring. Interestingly, two of these offspring were derived from blastocysts with ACS. Hence, chromosomal segregation errors during early cleavage can be a critical hallmark of preimplantation embryogenesis but may not impact viability after blastocyst transfer in cattle (Yao et al. 2022). ‘Self-correction’, such as the elimination of cells with chromosomal abnormalities, may be involved (Bolton et al. 2016; Nagai et al. 2021; Yang et al. 2021).
Improving viable embryo prediction based on morphokinetics
We have established novel criteria for selecting bovine embryos for transfer using time-lapse monitoring with LinKID micro25 (Sugimura et al. 2017). This system successfully identified prognostic factors: (i) timing of the first cleavage (≤27 h: yes/no), (ii) blastomere number at the end of the first cleavage (direct cleavage: yes/no), and (iii) blastomere number at the onset of the lag-phase (≥6: yes/no). These factors reflected viability after transfer. Selecting blastocysts with multiple predictors based on morphokinetics improved the prediction of viable embryos compared to the conventional criteria based on blastocyst morphology, with conception rates at D30 of 31.8% and 59.3%, respectively.
Conclusion
Currently, the quality of bovine IVP embryos is assessed at the time of transfer based on the IETS code of the blastocyst. Our studies have shown that evaluating early cleavage, especially the pattern of the first cleavage, is more critical than assessing blastocyst morphology. To predict the viability of bovine IVP embryos after transfer, practitioners and embryologists are strongly encouraged to focus on the morphokinetics of early cleavage. In human ART, research into embryo selection technology using artificial intelligence is advancing (Hengstschläger 2023). If automatic recognition of early cleavage, particularly the mode of the first cleavage, could be achieved in bovine IVP embryos, it would enable a more objective and efficient prediction of embryo viability. Combining morphokinetics with artificial intelligence will accelerate its acceptance in practical bovine IVP technology.
Data availability
Data sharing is not applicable as no new data were generated or analyzed during this study.
Declaration of funding
This work was supported by JSPS KAKENHI Grant Numbers JP18K05937, JP21K19184, JP22H02495 to S.S., and the JRA Livestock Industry Promotion Project to S.S.
Acknowledgements
Satoshi Sugimura would like to thank IETS for the invitation to write this review article for the lecture at the 51st IETS Annual Conference, and thanks all those involved in the studies that contributed to this review.
References
Angel-Velez D, De Coster T, Azari-Dolatabad N, Fernández-Montoro A, Benedetti C, Pavani K, Van Soom A, Bogado Pascottini O, Smits K (2023) Embryo morphokinetics derived from fresh and vitrified bovine oocytes predict blastocyst development and nuclear abnormalities. Scientific Reports 13(1), 4765.
| Crossref | Google Scholar |
Bamford T, Barrie A, Montgomery S, Dhillon-Smith R, Campbell A, Easter C, Coomarasamy A (2022) Morphological and morphokinetic associations with aneuploidy: a systematic review and meta-analysis. Human Reproduction Update 28(5), 656-686.
| Crossref | Google Scholar | PubMed |
Bo GA, Mapletoft RJ (2013) Evaluation and classification of bovine embryos. Animal Reproduction 10, 344-348.
| Google Scholar |
Bolton H, Graham SJL, Van der Aa N, Kumar P, Theunis K, Fernandez Gallardo E, Voet T, Zernicka-Goetz M (2016) Mouse model of chromosome mosaicism reveals lineage-specific depletion of aneuploid cells and normal developmental potential. Nature Communications 7, 11165.
| Crossref | Google Scholar | PubMed |
De Coster T, Masset H, Tšuiko O, Catteeuw M, Zhao Y, Dierckxsens N, Aparicio AL, Dimitriadou E, Debrock S, Peeraer K, de Ruijter-Villani M, Smits K, Van Soom A, Vermeesch JR (2022) Parental genomes segregate into distinct blastomeres during multipolar zygotic divisions leading to mixoploid and chimeric blastocysts. Genome Biology 23(1), 201.
| Crossref | Google Scholar | PubMed |
Destouni A, Zamani Esteki M, Catteeuw M, Tšuiko O, Dimitriadou E, Smits K, Kurg A, Salumets A, Van Soom A, Voet T, Vermeesch JR (2016) Zygotes segregate entire parental genomes in distinct blastomere lineages causing cleavage-stage chimerism and mixoploidy. Genome Research 26(5), 567-578.
| Crossref | Google Scholar | PubMed |
Farin PW, Britt JH, Shaw DW, Slenning BD (1995) Agreement among evaluators of bovine embryos produced in vivo or in vitro. Theriogenology 44(3), 339-349.
| Crossref | Google Scholar | PubMed |
Fenwick J, Platteau P, Murdoch AP, Herbert M (2002) Time from insemination to first cleavage predicts developmental competence of human preimplantation embryos in vitro. Human Reproduction 17(2), 407-412.
| Crossref | Google Scholar |
Ferry L, Mermillod P, Massip A, Dessy F (1994) Bovine embryos cultured in serum-poor oviduct-conditioned medium need cooperation to reach the blastocyst stage. Theriogenology 42(3), 445-453.
| Crossref | Google Scholar | PubMed |
Fujita T, Umeki H, Shimura H, Kugumiya K, Shiga K (2006) Effect of group culture and embryo-culture conditioned medium on development of bovine embryos. Journal of Reproduction and Development 52(1), 137-142.
| Crossref | Google Scholar | PubMed |
Gjørret JO, Knijn HM, Dieleman SJ, Avery B, Larsson LI, Maddox-Hyttel P (2003) Chronology of apoptosis in bovine embryos produced in vivo and in vitro. Biology of Reproduction 69(4), 1193-1200.
| Crossref | Google Scholar | PubMed |
Gopichandran N, Leese HJ (2006) The effect of paracrine/autocrine interactions on the in vitro culture of bovine preimplantation embryos. Reproduction 131(2), 269-277.
| Crossref | Google Scholar | PubMed |
Han YM, Wang WH, Abeydeera LR, Petersen AL, Kim JH, Murphy C, Day BN, Prather RS (1999) Pronuclear location before the first cell division determines ploidy of polyspermic pig embryos. Biology of Reproduction 61(5), 1340-1346.
| Crossref | Google Scholar | PubMed |
Hardarson T, Hanson C, Sjögren A, Lundin K (2001) Human embryos with unevenly sized blastomeres have lower pregnancy and implantation rates: indications for aneuploidy and multinucleation. Human Reproduction 16(2), 313-318.
| Crossref | Google Scholar | PubMed |
Hare WC, Singh EL, Betteridge KJ, Eaglesome MD, Randall GC, Mitchell D, Bilton RJ, Trounson AO (1980) Chromosomal analysis of 159 bovine embryos collected 12 to 18 days after estrus. Canadian Journal of Genetics and Cytology 22(4), 615-626.
| Crossref | Google Scholar | PubMed |
Hengstschläger M (2023) Artificial intelligence as a door opener for a new era of human reproduction. Human Reproduction Open 2023(4), hoad043.
| Crossref | Google Scholar |
Hoelker M, Rings F, Lund Q, Ghanem N, Phatsara C, Griese J, Schellander K, Tesfaye D (2009) Effect of the microenvironment and embryo density on developmental characteristics and gene expression profile of bovine preimplantative embryos cultured in vitro. Reproduction 137(3), 415-425.
| Crossref | Google Scholar | PubMed |
Holm P, Booth PJ, Callesen H (2002) Kinetics of early in vitro development of bovine in vivo- and in vitro-derived zygotes produced and/or cultured in chemically defined or serum-containing media. Reproduction 123(4), 553-565.
| Crossref | Google Scholar | PubMed |
Khurana NK, Niemann H (2000) Effects of oocyte quality, oxygen tension, embryo density, cumulus cells and energy substrates on cleavage and morula/blastocyst formation of bovine embryos. Theriogenology 54(5), 741-756.
| Crossref | Google Scholar | PubMed |
Koo DB, Kang YK, Choi YH, Park JS, Kim HN, Oh KB, Son DS, Park H, Lee KK, Han YM (2002) Aberrant allocations of inner cell mass and trophectoderm cells in bovine nuclear transfer blastocysts. Biology of Reproduction 67(2), 487-492.
| Crossref | Google Scholar | PubMed |
Koyama H, Suzuki H, Yang X, Jiang S, Foote RH (1994) Analysis of polarity of bovine and rabbit embryos by scanning electron microscopy. Biology of Reproduction 50(1), 163-170.
| Crossref | Google Scholar | PubMed |
Larson MA, Kubisch HM (1999) The effects of group size on development and interferon-tau secretion by in-vitro fertilized and cultured bovine blastocysts. Human Reproduction 14(8), 2075-2079.
| Crossref | Google Scholar | PubMed |
Lequarre AS, Marchandise J, Moreau B, Massip A, Donnay I (2003) Cell cycle duration at the time of maternal zygotic transition for in vitro produced bovine embryos: effect of oxygen tension and transcription inhibition. Biology of Reproduction 69(5), 1707-1713.
| Crossref | Google Scholar | PubMed |
Liu Y, Chapple V, Roberts P, Matson P (2014) Prevalence, consequence, and significance of reverse cleavage by human embryos viewed with the use of the Embryoscope time-lapse video system. Fertility and Sterility 102(5), 1295-1300.e1292.
| Crossref | Google Scholar |
Lonergan P, Khatir H, Piumi F, Rieger D, Humblot P, Boland MP (1999) Effect of time interval from insemination to first cleavage on the developmental characteristics, sex ratio and pregnancy rate after transfer of bovine embryos. Journal of Reproduction and Fertility 117(1), 159-167.
| Crossref | Google Scholar | PubMed |
Magata F (2023) Time-lapse monitoring technologies for the selection of bovine in vitro fertilized embryos with high implantation potential. Journal of Reproduction and Development 69(2), 57-64.
| Crossref | Google Scholar | PubMed |
Magli MC, Gianaroli L, Ferraretti AP, Lappi M, Ruberti A, Farfalli V (2007) Embryo morphology and development are dependent on the chromosomal complement. Fertility and Sterility 87(3), 534-541.
| Crossref | Google Scholar | PubMed |
Mashiko D, Ikeda Z, Yao T, Tokoro M, Fukunaga N, Asada Y, Yamagata K (2020) Chromosome segregation error during early cleavage in mouse pre-implantation embryo does not necessarily cause developmental failure after blastocyst stage. Scientific Reports 10(1), 854.
| Crossref | Google Scholar | PubMed |
Massip A, Mulnard J, Vanderzwalmen P, Hanzen C, Ectors F (1982) The behaviour of cow blastocyst in vitro: cinematographic and morphometric analysis. Journal of Anatomy 134(Pt 2), 399-405.
| Google Scholar | PubMed |
Matoba S, Fair T, Lonergan P (2010) Maturation, fertilisation and culture of bovine oocytes and embryos in an individually identifiable manner: a tool for studying oocyte developmental competence. Reproduction, Fertility and Development 22(5), 839-851.
| Crossref | Google Scholar | PubMed |
McCoy RC, Demko ZP, Ryan A, Banjevic M, Hill M, Sigurjonsson S, Rabinowitz M, Petrov DA (2015) Evidence of selection against complex mitotic-origin aneuploidy during preimplantation development. PLoS Genetics 11(10), e1005601.
| Crossref | Google Scholar | PubMed |
Mohan M, Hurst AG, Malayer JR (2004) Global gene expression analysis comparing bovine blastocysts flushed on day 7 or produced in vitro. Molecular Reproduction and Development 68(3), 288-298.
| Crossref | Google Scholar | PubMed |
Nagai H, Okada M, Nagai Y, Sakuraba Y, Okae H, Suzuki R, Sugimura S (2021) Abnormal cleavage is involved in the self-correction of bovine preimplantation embryos. Biochemical and Biophysical Research Communications 562, 76-82.
| Crossref | Google Scholar | PubMed |
Rabaglino MB, Salilew-Wondim D, Zolini A, Tesfaye D, Hoelker M, Lonergan P, Hansen PJ (2023) Machine-learning methods applied to integrated transcriptomic data from bovine blastocysts and elongating conceptuses to identify genes predictive of embryonic competence. FASEB Journal 37(3), e22809.
| Crossref | Google Scholar | PubMed |
Shoukir Y, Campana A, Farley T, Sakkas D (1997) Early cleavage of in-vitro fertilized human embryos to the 2-cell stage: a novel indicator of embryo quality and viability. Human Reproduction 12(7), 1531-1536.
| Crossref | Google Scholar | PubMed |
Stokes PJ, Abeydeera LR, Leese HJ (2005) Development of porcine embryos in vivo and in vitro; evidence for embryo ‘cross talk’ in vitro. Developmental Biology 284(1), 62-71.
| Crossref | Google Scholar | PubMed |
Sugimura S, Akai T, Somfai T, Hirayama M, Aikawa Y, Ohtake M, Hattori H, Kobayashi S, Hashiyada Y, Konishi K, Imai K (2010) Time-lapse cinematography-compatible polystyrene-based microwell culture system: a novel tool for tracking the development of individual bovine embryos. Biology of Reproduction 83(6), 970-978.
| Crossref | Google Scholar | PubMed |
Sugimura S, Akai T, Hashiyada Y, Somfai T, Inaba Y, Hirayama M, Yamanouchi T, Matsuda H, Kobayashi S, Aikawa Y, Ohtake M, Kobayashi E, Konishi K, Imai K (2012) Promising system for selecting healthy in vitro-fertilized embryos in cattle. PLoS ONE 7(5), e36627.
| Crossref | Google Scholar | PubMed |
Sugimura S, Akai T, Hashiyada Y, Aikawa Y, Ohtake M, Matsuda H, Kobayashi S, Kobayashi E, Konishi K, Imai K (2013) Effect of embryo density on in vitro development and gene expression in bovine in vitro-fertilized embryos cultured in a microwell system. Journal of Reproduction and Development 59(2), 115-122.
| Crossref | Google Scholar | PubMed |
Sugimura S, Akai T, Imai K (2017) Selection of viable in vitro-fertilized bovine embryos using time-lapse monitoring in microwell culture dishes. Journal of Reproduction and Development 63(4), 353-357.
| Crossref | Google Scholar | PubMed |
Suzuki R, Yao T, Okada M, Nagai H, Khurchabilig A, Kobayashi J, Yamagata K, Sugimura S (2023) Direct cleavage during the first mitosis is a sign of abnormal fertilization in cattle. Theriogenology 200, 96-105.
| Crossref | Google Scholar | PubMed |
Tšuiko O, Catteeuw M, Zamani Esteki M, Destouni A, Bogado Pascottini O, Besenfelder U, Havlicek V, Smits K, Kurg A, Salumets A, D’Hooghe T, Voet T, Van Soom A, Robert Vermeesch J (2017) Genome stability of bovine in vivo-conceived cleavage-stage embryos is higher compared to in vitro-produced embryos. Hum Reprod 32(11), 2348-2357.
| Crossref | Google Scholar | PubMed |
Vajta G, Peura TT, Holm P, Páldi A, Greve T, Trounson AO, Callesen H (2000) New method for culture of zona-included or zona-free embryos: the Well of the Well (WOW) system. Molecular Reproduction and Development 55(3), 256-264.
| Crossref | Google Scholar | PubMed |
Vanneste E, Voet T, Melotte C, Debrock S, Sermon K, Staessen C, Liebaers I, Fryns JP, D’Hooghe T, Vermeesch JR (2009) What next for preimplantation genetic screening? High mitotic chromosome instability rate provides the biological basis for the low success rate. Human Reproduction 24(11), 2679-2682.
| Crossref | Google Scholar | PubMed |
Viuff D, Rickords L, Offenberg H, Hyttel P, Avery B, Greve T, Olsaker I, Williams JL, Callesen H, Thomsen PD (1999) A high proportion of bovine blastocysts produced in vitro are mixoploid. Biology of Reproduction 60(6), 1273-1278.
| Crossref | Google Scholar | PubMed |
Vutyavanich T, Saeng-Anan U, Sirisukkasem S, Piromlertamorn W (2011) Effect of embryo density and microdrop volume on the blastocyst development of mouse two-cell embryos. Fertility and Sterility 95(4), 1435-1439.
| Crossref | Google Scholar | PubMed |
Wong CC, Loewke KE, Bossert NL, Behr B, De Jonge CJ, Baer TM, Reijo Pera RA (2010) Non-invasive imaging of human embryos before embryonic genome activation predicts development to the blastocyst stage. Nature Biotechnology 28(10), 1115-1121.
| Crossref | Google Scholar | PubMed |
Yang M, Rito T, Metzger J, Naftaly J, Soman R, Hu J, Albertini DF, Barad DH, Brivanlou AH, Gleicher N (2021) Depletion of aneuploid cells in human embryos and gastruloids. Nature Cell Biology 23(4), 314-321.
| Crossref | Google Scholar | PubMed |
Yang T, Yuan X, Xue Q, Sun L, Xu T, Chen Y, Shi D, Li X (2023) Comparison of symmetrical and asymmetrical cleavage 2-cell embryos of porcine by Smart-seq2. Theriogenology 210, 221-226.
| Crossref | Google Scholar | PubMed |
Yao T, Suzuki R, Furuta N, Suzuki Y, Kabe K, Tokoro M, Sugawara A, Yajima A, Nagasawa T, Matoba S, Yamagata K, Sugimura S (2018) Live-cell imaging of nuclear-chromosomal dynamics in bovine in vitro fertilised embryos. Scientific Reports 8(1), 7460.
| Crossref | Google Scholar | PubMed |
Yao T, Ueda A, Khurchabilig A, Mashiko D, Tokoro M, Nagai H, Sho T, Matoba S, Yamagata K, Sugimura S (2022) Micronucleus formation during early cleavage division is a potential hallmark of preimplantation embryonic loss in cattle. Biochemical and Biophysical Research Communications 617(Pt 2), 25-32.
| Crossref | Google Scholar | PubMed |
Zhan Q, Ye Z, Clarke R, Rosenwaks Z, Zaninovic N (2016) Direct unequal cleavages: embryo developmental competence, genetic constitution and clinical outcome. PLoS ONE 11(12), e0166398.
| Crossref | Google Scholar | PubMed |
Ziebe S, Petersen K, Lindenberg S, Andersen AG, Gabrielsen A, Andersen AN (1997) Embryo morphology or cleavage stage: how to select the best embryos for transfer after in-vitro fertilization. Human Reproduction 12(7), 1545-1549.
| Crossref | Google Scholar | PubMed |


