Nurturing the egg: the essential connection between cumulus cells and the oocyte
Claude Robert A B C *A Département des sciences animales, Faculté des sciences de l’agriculture et de l’alimentation, Université Laval, Québec, QC, Canada.
B Centre de Recherche en Reproduction, Développement et Santé Intergénérationnelle (CRDSI), Université Laval, Québec, QC, Canada.
C Réseau Québécois en Reproduction (RQR), Montréal, QC, Canada.
Reproduction, Fertility and Development 34(2) 149-159 https://doi.org/10.1071/RD21282
Published online: 11 October 2021
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing on behalf of the IETS. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
The determinants of oocyte quality remain uncertain. Under suitable conditions, which have yet to be defined, the gamete grows and acquires the competence to resume meiosis, be fertilised and undergo embryonic development at least beyond genome activation, after which the blastomere is autonomous enough to adapt to the specificity of its environment. This review describes the central role played by the oocyte in reproductive success and how communication between cumulus cells and the oocyte are essential to proper oogenesis and the quality of the resulting gamete. While most attempts to improve oocyte quality have been directed at gonadotrophin-based systemic endocrine signalling, it is proposed that parallel control of fertility may act locally within ovarian follicles through intimate cooperation between somatic cells and the oocyte via the network of transzonal projections. This intercellular communication may prove to be more sensitive to environmental conditions than systemic endocrine signalling, which is essential for many non-reproductive tissues.
Keywords: cell signalling, developmental competence, follicle, folliculogenesis, intercellular communications, oocyte, oogenesis, ovary, transzonal projections.
Introduction
Oogenesis and folliculogenesis are two processes that need to be studied to improve scientific understanding of the physiological mechanisms that ensure the production of fully functional eggs. Both processes include stages that are pivotal for female fertility and for the success of livestock herd improvement efforts based on transfer of embryos produced in vitro. The conditions required for the acquisition of developmental competence by oocytes maturing in the follicle or in vitro are still unclear and under investigation.
Intercellular communication
An ovarian follicle is an assembly of communicating interdependent cells that supports the development of the gamete inside. Whereas the development of the antral follicle is controlled remotely to a large degree through the hypothalamic–pituitary–gonadal (HPG) axis, follicular maturation and oogenesis depend more on intra-follicular communication. Folliculogenesis refers to the entire process by which the follicle grows to become fully functional. The natural ovarian cycle involves growth stimulation by follicle stimulating hormone (FSH) with negative feedback via oestradiol secretion by granulosa cells (Liu and Hsueh 1986). Interestingly, no signalling pathway external to the ovary has been found to act directly on the oocyte. In fact, the oocyte has no receptors for FSH or luteinising hormone (Calder et al. 2005) and even secretes repressors of gonadotrophin receptor expression in adjacent cells (Eppig et al. 1997). It therefore must receive its external signals via chains of intermediary cells. For example, the receptor tyrosine kinase KIT is expressed on the oocyte and on theca cells whereas the KIT ligand is secreted by granulosa cells and influences oocyte growth and theca cell differentiation (Reynaud et al. 2001). During the pre-ovulatory period, the LH surge is detected by theca and granulosa cells and transmitted via the epidermal growth factor signalling pathway as epiregulin, amphiregulin, betacellulin, neuregulins and other secretions (Conti et al. 2006) to cumulus cells, which respond by halting the flow of cyclic AMP to the oocyte, thus triggering resumption of meiosis in the gamete (Santiquet et al. 2013; Zeng et al. 2013; Gilchrist et al. 2016). This flow of cAMP is not endocrine but occurs rather through tubular channels that extend from the cumulus cells through the zona pellucida and make direct contact with the oolemma (Santiquet et al. 2013; Gilchrist et al. 2016). These channels are called transzonal projections, and the area of contact between them and the oocyte is not a simple hole but contains active pores called gap junctions.
Meanwhile, the oocyte ensures the maintenance of follicular growth. Its presence is sensed by granulosa and theca cells through secreted factors such as growth-differentiation factor 9 (GDF9) and bone morphogenetic protein 15 (BMP15) (Su et al. 2004; Sugiura et al. 2010). These intercellular communications orchestrate the growth, the differentiation, and the demise of the follicle. The gamete is thus in an essential partnership with its surrounding somatic cells.
Timescale of folliculogenesis and oogenesis
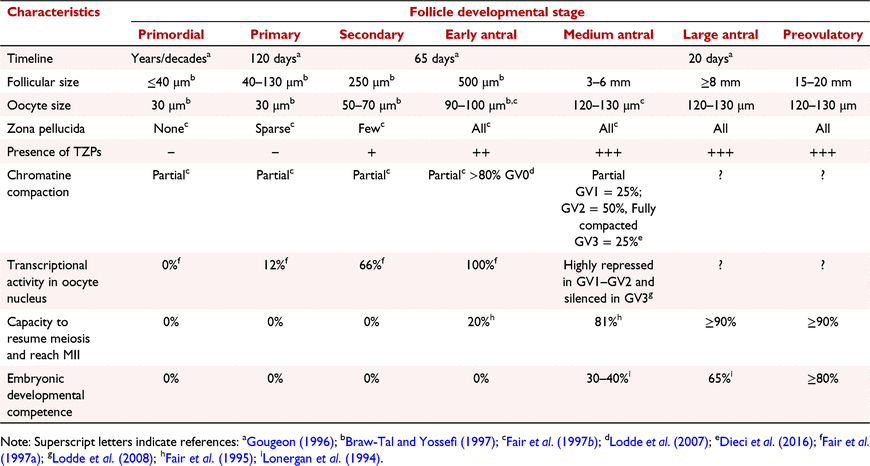
This intimate partnership is established and maintained throughout folliculogenesis. The timescales that apply to ovarian function are unusual. The capacity to remain in a latent state for decades makes the ovarian follicle in large mammals a highly unique structure. Time is similarly discontinuous for the oocyte, which awaits follicular recruitment to initiate intense developmental activity, during which it grows to its full size only to return to a new phase of latency and await fertilisation. Although studies tend to focus on the final phase of follicular growth, namely the 20 days or so during which late antral follicles respond to FSH, the process of recruitment from reserves and pre-antral growth is considerably longer. One of the more comprehensive descriptions of follicular ontogeny in large mammals is that of the human case, in which the initiation and basal growth phases span more than 6 months (Gougeon 1996) (Table 1). It should be kept in mind that in large mammals, follicles reaching the final pre-ovulatory phase have been exposed to the endocrinological cycles of several previous follicular waves.
|
Is FSH needed early?
This exposure to circulating gonadotrophins (primarily FSH) has been the focus of conflicting reports. In many cases, repeated ovarian stimulation does not seem to have any major effect on the number and quality of recovered oocytes or eggs (Caligara et al. 2001; Sood et al. 2017; Paul et al. 2019; Tutt et al. 2021). The number of follicles recruited per wave in a given animal is very repeatable over time (Burns et al. 2005) and is heritable (Bényei et al. 2004). These observations suggest that pre-antral growth is genetically driven and largely unresponsive to external cues including fluctuations in circulating gonadotrophins and steroid hormones. Murine studies confirm this. When cumulus–oocyte complexes from pre-antral follicles in Day 12 animals (in which the ovaries do not contain antral follicles and the oocytes are known to be incapable of resuming meiosis) were cultured for 10 days (in a medium free of FSH but containing fetal calf serum) then fertilised and transferred, live pups were born (Eppig and Schroeder 1989). This suggested that the presence of theca and granulosa cells or FSH was not essential. Using gene inactivation models, it was then shown that in the absence of gonadotrophin receptors, pre-antral folliculogenesis can be sustained but stops at the secondary stage before antral formation (Kumar et al. 1997; Dierich et al. 1998), strengthening the hypothesis that only antral follicles benefit from gonadotrophins.
However, a different picture emerges from in vitro culture of ovarian cortex and follicles. FSH even at low concentrations has been shown to promote pre-antral growth in several species (Demeestere et al. 2005; Kreeger et al. 2005; Wang et al. 2011). Its presence may not be essential, but its effect on the pre-antral follicle is undeniable. In addition, coupling of cumulus cells with the oocyte through transzonal projections fails to occur in the absence of FSH or GDF9 (Albertini et al. 2001). In the experiment with ovarian follicles from day 12 mice, the surface on which the cumulus–oocyte complexes were deposited had to be coated with collagen to prevent cumulus cell migration and to maintain the somatic–gamete interconnectivity, without which oocyte growth and acquisition of competence was compromised (Eppig and Schroeder 1989). These observations suggest that pre-antral development is robust and may progress with minimal support, but that endocrine stimulation and cumulus cell–oocyte physical connectivity are essential for subsequent developmental stages. Since transzonal projections begin to form during the secondary follicle stage (Table 1), pre-antral conditions may have little impact on the endocrine support required at the antral stage but may affect the quality of the transzonal network. In this sense, pre-antral development might not be entirely preprogrammed and could contribute to follicular outcome.
Although mouse oocytes of good quality are obtained in high yield from follicular culture, it is proving to be more difficult to achieve the same success in the case of large mammals (McLaughlin and Telfer 2010). Adequate in vitro support for the early stages of folliculogenesis (e.g. primary or secondary antral) has been designed for cattle, pigs and humans (Pangas et al. 2003; Xiao et al. 2017). The best system so far involves 3D culture of follicles encapsulated sequentially in matrices of different density to mimic the in vivo environment at each developmental stage (Pangas et al. 2003; Kreeger et al. 2005; Xiao et al. 2017). Human follicles thus cultured from the secondary pre-antral stage have brought gametes to full size with the ability to resume meiosis (Xiao et al. 2015). A more sophisticated system involves ectopic culture of up to five tissues (follicle/ovary, Fallopian tube, endometrium, cervix and liver) interconnected through microfluid channels allowing the exchange of metabolites and enabling precise stimulation through exogenous administration (pulse) and wash-off (chase) of hormones, drugs and so on (Xiao et al. 2017). A common observation is that flat culture often leads to poor communication between cumulus cells and the oocyte and ultimately to follicular demise (Eppig and Schroeder 1989; Xiao et al. 2015, 2017). Moreover, even when oocytes reach full-size in such cultures, they often have a limited transzonal network and are meiotically incompetent (Telfer and Andersen 2021). These reports all suggest that physical connection to the cumulus complex via a fully functional network of tubular channels is crucial for the developmental competence of the oocyte.
The transzonal projection network
Oocytes denuded even at late stages of oogenesis are considerably less likely to resume meiosis (Macaulay et al. 2016). Denuded oocytes can sometimes be salvaged by co-culture with fully-enclosed cumulus–oocyte complexes (Luciano et al. 2005; Scantland et al. 2014). It has also been noted that oocytes matured in groups often fare better than those matured individually (O’Doherty et al. 1997). We note that co-cultured cumulus cells are difficult to separate from an oocyte matured in vitro because their expansion tends to surround the previously denuded gamete. Cumulus cells detaching from one oocyte and reattaching to a neighboring denuded oocyte has been observed (Fig. 1). It is not known if such new connections are functional and contribute significantly to supporting the maturation of the oocyte, but the above observations suggest collectively that cumulus cells help with the maturation of oocytes in proximity, by secreting what are likely labile factors.
|
|
The major cytological and physiological developments that occur during folliculogenesis in cattle are summarised in Table 1. Little doubt remains that the intimate physical contact between the oocyte and its surrounding somatic cells lasts throughout the process. As the follicle progresses through its initial growth phase, the number of pre-granulosa cells surrounding the oocyte is limited but definitely increases, especially those in close contact with the gamete (Braw-Tal and Yossefi 1997). Pre-granulosa cell cytoplasmic membranes are initially juxtaposed and attached to the oocyte oolemma via the zonula adherens and desmosome structures (Motta et al. 1994; Fair et al. 1997a, 1997b). Following recruitment, the follicle and its oocyte grow conjointly, the surrounding somatic cells dividing to maintain complete coverage of the gamete surface (Braw-Tal and Yossefi 1997). The initial growth phases are slow, requiring months to complete (Gougeon 1996). Transzonal projections are established as the zona pellucida is secreted, which occurs at the end of the secondary stage of folliculogenesis (Fair et al. 1997b). This marks the onset of a phase during which cells close to the gamete differentiate into cumulus cells while those farther away remain granulosa cells. In mice, the glycoprotein shell is secreted solely by the oocyte (Philpott et al. 1987) whereas in large mammals, cumulus cells also secrete zona pellucida proteins (Sinowatz et al. 2001; Kölle et al. 2007). The ultrastructure of transzonal projections has been investigated thoroughly, beginning with electron microscopic observations in the mid 1960s (Hertig and Adams 1967). Most of our current understanding of these projections comes from the work of Professor David Albertini who pioneered the study of transzonal projection dynamics and interconnectivity between the somatic cells and the oocyte in relation to folliculogenesis and oocyte quality (Anderson and Albertini 1976; Carabatsos et al. 1998; Albertini et al. 2001; Combelles et al. 2004; Plancha et al. 2005; Li and Albertini 2013). It was also shown during this time that the number of transzonal projections in human oocyte complexes evolves during folliculogenesis (Motta et al. 1994). The cellular extensions are not static structures but evolve within the growing follicle. At least two types can be distinguished based on cytoskeleton backbone. Most transzonal projections have an actin filament core while some are made of microtubules (Can and Albertini 1997; Albertini et al. 2001; El-Hayek et al. 2018). Networks of transzonal projections have been described in many mammalian species. Fig. 2 shows those of pig, cattle, and mouse oocytes. Species differences include thinner and less straight networks in mice (Baena and Terasaki 2019). Network development is similar in humans, pigs and cattle (Coticchio et al. 2014).
The network is essential for folliculogenesis, one of its main known roles being to shuttle cyclic nucleotides to the oocyte cytoplasm to maintain meiotic arrest (Gilchrist et al. 2016). Transzonal projections are not open-ended. Molecules are transferred through them via gap junctions, specialised pores that span both membranes (Kidder and Mhawi 2002; Luciano et al. 2011). The LH surge detected by theca and granulosa cells is conveyed to cumulus cells through paracrine signalling, which leads to destabilisation, closure and cessation of molecular transfers via gap junctions (Santiquet et al. 2013; Gilchrist et al. 2016). The cumulus cells thus constitute the final layer of control over the interruption of meiosis. Another essential role played by the same mode of transfer is the supply of energy substrates to the oocyte (Sutton-McDowall et al. 2010). The oocyte is fully dependent on this exogenous delivery of lactate, pyruvate and creatine to produce ATP (Rieger and Loskutoff 1994). It is endowed with a very peculiar contingent of mitochondria estimated to number from 30 000 to 1 000 000 (Van Blerkom 2011) but individually very inefficient at producing ATP by glycolysis or oxidative phosphorylation (Rieger et al. 1992; Rieger and Loskutoff 1994). In many species, including large mammals, oocyte mitochondria undergo conformational transformations during folliculogenesis, passing from small and round to a hooded immature form that progresses to the familiar post-fertilisation oval shape once embryonic genome activation is complete (Van Blerkom 2011). The purpose of this transformation remains unclear. Nevertheless, up to the transzonal projection withdrawal that occurs following meiosis resumption, cumulus cells control the availability of ATP to the oocyte. This is important, since the gamete needs much energy to manage and transcribe its genome to constitute the maternal reserves of stored mRNA and to translate the proteins that will be needed during the upcoming period of transcriptional silence (Fair et al. 1995; Lodde et al. 2008) (Table 1).
The physiological support provided by cumulus cells through transzonal projections was long thought to be limited to small molecules (≤1 kDa) capable of passing through gap junctions. However, larger cargos such as mRNA granules and lipids have been shown to pass from cumulus cells to the oocyte (Macaulay et al. 2014, 2016). These transfers are believed to occur via vesicles secreted at the tip of the projections (Macaulay et al. 2014, 2016). The contact between cumulus cells and the oocyte thus resembles neuronal synapses both structurally and functionally (Allworth and Albertini 1993; Macaulay et al. 2014). And just as neurones do not synthesise each protein in the cell body and transport it over several millimetres or a metre of axon but instead package mRNA into ribonucleoparticles sometimes with ribosomes attached and transport these for local translation (El Fatimy et al. 2016; Muzio and Cascella 2021), so do cumulus cells arrange for remote translation of proteins used in the oocyte. This has been shown by transfecting cumulus cells with a non-endogenous gene (FMRP-GFP) and finding the mRNA in the oocyte cytoplasm (Macaulay et al. 2014, 2016). It is not yet clear if such transcripts are translated in the oocyte cytoplasm or in the transzonal projection bulging ends followed by transfer of the proteins. Both mechanisms are possible and not mutually exclusive.
Other intercellular transfers of large molecules
Direct transfer of large molecules between two cells has been described for many cell types, such as neurones, immune cells and epithelial cells (Domhan et al. 2011; Nawaz and Fatima 2017). It is often mediated through tunnelling nanotubes (TNTs), which are actin-based cytoplasmic projections that can stretch from 50 nm in length to 200 nm (Nawaz and Fatima 2017). Unlike transzonal projections, TNTs are open ended and thus allow bidirectional transfers of material. They have been shown to deliver small molecules such as ions and metabolites but also lipid droplets, lysosomes, viral genomes, entire viruses and even mitochondria (Nawaz and Fatima 2017). Even though cumulus cell transzonal projections are wider than TNTs, it is not known if they are as permissive. Mitochondria do fit inside them and have been found throughout their length (Albertini et al. 2001). However, mitochondrial transfer from cumulus cell to the oocyte has not been documented although proof of such transfer could be evident as mitochondria from both cells do not have the same shape.
The fully developed bovine transzonal network is composed of several thousands of channels. Since the oocyte is the founder cell of the individual animal, transfer of any matter able to destabilise or integrate into the oocyte genome is potentially harmful. Compared to modifying the oocyte genome, modifying a somatic cell genome carries no risk for posterity. On the other hand, this could be a source of random genomic recombination and hence new alleles that provide competitive advantages in specific environments. For example, genomic reorganisation is a major factor in resistance to infectious disease (Rubelt et al. 2017). To put into perspective this risk of transferring potentially genome-destabilising exogenous DNA, the average mutation rate per nucleotide per generation is estimated at <10−7 for a typical large mammalian species (human, cattle) having a genome length of about 3 billion base pairs (Lynch et al. 2016). This low intergenerational mutation rate suggests the presence of a controlled transfer rather than a transfer of random molecules. In addition to supplying the oocyte with essential molecules, cumulus cells likely play a protective role by sequestering harmful compounds absorbed from the environment. They have been shown to internalise many types of molecules including follicular fluid lipids, secreted vesicles and even bacterial particles (Hernandez-Gonzalez et al. 2006; Richards 2007; del Collado et al. 2017). Absorbed material is not transferred spontaneously to the oocyte but seems instead to be the object of intracytoplasmic sorting and possibly repackaging or degradation.
Transzonal projections and oocyte developmental competence
Transzonal projections apparently do not deliver ribonucleoprotein particles constantly, at least not mRNA, before or after the LH surge or during spontaneous meiosis resumption when the cumulus–oocyte complex is removed from the follicle. In cattle ovaries incubated after slaughter, transzonal projections load up on RNA in conjunction with acquisition of oocyte developmental competence (Macaulay et al. 2014, 2016). It has been shown that oocytes aspirated from ovaries quickly after slaughter have limited developmental potential (Blondin et al. 1997). Incubating in saline for 4 h before aspiration more than doubled the percentage of embryos that reached the blastocyst stage after in vitro oocyte maturation, fertilisation and embryo development (Blondin et al. 1997). It is unclear why this difference is so large, since the oocyte remains in meiotic arrest while inside the ovarian follicle. It has been shown that RNA accumulates inside transzonal projections during this time (Macaulay et al. 2014, 2016). Transfer of RNA to the oocyte could be part of the final phase of differentiation leading to the acquisition of developmental competence. This would be consistent with the observation that oocytes in growing follicles are less developmentally competent than those in early atretic follicles (Blondin and Sirard 1995). Oocytes salvaged after slaughter have better developmental potential when surrounded by cumulus complex displaying early signs of expansion than when the complex is very thin or very expanded (Blondin and Sirard 1995). This led to the theory that early atresia is beneficial for the acquisition of developmental potential (Blondin and Sirard 1995; Blondin et al. 1997, 2002; Nivet et al. 2012). The time window of this beneficial effect associated with early demise is rapid and spans only a few hours before an irreversible decline in quality. In vivo uncoupling from the follicular environment occurs after the LH surge, when theca and granulosa cells begin differentiation to become a fully functional corpus luteum while cumulus cells close their gap junctions to cut the flow of cAMP to the oocyte to allow meiosis to resume (Santiquet et al. 2013). Disconnection of the transzonal projections has been shown to involve the EGF signalling pathway (Abbassi et al. 2021). A mild induction of transformation can be induced during follicular growth by withdrawal of FSH. These ‘coasting’ protocols in which oocytes are recovered 48–54 h after FSH injection have been shown to result in very robust developmental competence in Holstein cows (Blondin et al. 2002; Nivet et al. 2012). It is not known if post-FSH timing coincides with RNA loading of transzonal projections. Material transfer during late folliculogenesis is known to occur in Drosophila, in which nurse cells (equivalent to cumulus cells) go through a process called ‘cytoplasmic dumping’ of all their contents into the oocyte, except for the nucleus, which is retained by a mesh of actin filaments (Guild et al. 1997; Spracklen and Tootle 2013). This is an extreme example of cellular devotion to nurturing the egg cell entrusted with the future of the species.
Why so much focus on oocyte quality?
While continuity of physiological support from conception to birth is essential for the delivery of healthy offspring, the key determinants of fertility appear to be developmental competence and a receptive uterine environment. Once gestation is underway, the investment of material resources is already considerable, and halting it would be costly. Based on in vitro production of cattle embryos as a proxy for the pivotal steps, oocyte quality seems to be a crucial component of reproductive success (Fig. 3).
|
|
Most protocols of in vitro production of cattle embryos start with oocytes at the germinal vesicle stage, making a maturation step necessary prior to fertilisation. Most embryo failures occur very early in development, prior to genome activation (Fig. 3). Once this critical step is passed, failure drops to 5–10% during the transition to the blastocyst stage. Of all oocytes fertilised in a typical embryo production run, about 40–50% are viable at transfer (Holm and Callesen 1998; Numabe et al. 2000; van Wagtendonk-de Leeuw et al. 2000; Hansen 2020) and about 18–32% of these fail before or shortly after implantation/attachment, whereas late gestational arrests are far fewer (Romano et al. 2016). These very approximate figures reveal the impact of oocyte quality on the success of embryos produced in vitro. They indicate that most oocytes sent down the standard in vitro pipeline do not generate a live calf, and that 35–65% of all losses occur very early, while the embryo is still under the control of the maternal program. The importance of the last phase of folliculogenesis is revealed by contrasting the developmental competence of oocytes matured in vitro to those matured in vivo (Table 2). Despite confounding factors that could explain some of the observed variance, in vitro maturation clearly does not replicate very adequately this last phase of oocyte preparation. This is true even in mice, despite the relative success of in vitro oogenesis and folliculogenesis with minimal complexity (Eppig and Schroeder 1989).

|
Two aspects determining the effectiveness of transzonal projections to consider are (1) how the network is established and maintained and (2) network functionality in terms of transferring material to the oocyte. The underlying processes could be distinguished based on whether poor follicular support or incomplete follicular and oocyte differentiation has caused developmental incompetence. It has been shown that networks are less developed in women who have long reproductive histories (El-Hayek et al. 2018). In the case of porcine oocytes, the actin filaments are more easily destabilised by heat stress (Yin et al. 2020). Lower competence of bovine oocytes has also been associated with less developed transzonal networks, and oocytes matured in follicular culture under suboptimal conditions frequently display poor connectivity with cumulus cells as well as turning out to be developmentally incompetent (Modina et al. 2007).
Modulating fertility during suboptimal periods
The key steps of folliculogenesis and oogenesis amount to progressive acquisition of maternal reserves that enable the oocyte to resume meiosis. Meiotic capacity is re-acquired before the acquisition of developmental competence, which requires complete shutdown of transcription in conjunction with genome compaction (Table 1). This stepwise progression could allow biological quality control since the follicle and the oocyte must stay synchronous through bidirectional communication. It could also be a modulator of fertility at any point from early folliculogenesis up to the final phase of post-LH preparation.
The need for this multi-step control over oocyte quality is unclear. Many species are known to suffer from reduced fertility periodically and sometimes chronically. Dairy cattle and pigs exhibit lower fertility during periods of heat stress (Graves et al. 2018; Hansen 2020). Although essential for the perpetuation of a species, fertility is expendable for the individual facing a threat to its own survival. Since bearing offspring is such a demanding process for female mammals, it can be a perilous project when conditions are unfavourable. This may explain why reproductive performance is so sensitive to environmental conditions and the metabolic state of the animal. Modulating fertility in accordance with resource availability and body condition is thus believed to be an adaptative trait to improve fitness to maximise the survivability of the progeny (Lawson and Borgerhoff Mulder 2016). This adaptable physiology makes regulation of the reproductive system highly complex. Whereas complete shutdown would hinder secretion of gonadal steroid hormones that have beneficial systemic effects elsewhere, sustaining endocrine secretion while lowering fertility necessarily requires a wide array of metabolic sensing signalling pathways to act in parallel to the canonical endocrine signalling that drives ovarian functions and uterine receptivity.
Transcriptomic surveys and gene expression modelling have shown that follicular cells express in a stage-dependent manner many metabolic pathways including lipid oxidation, which is more active during the transition to the antral follicle (Bunel et al. 2014; Peñalver Bernabé et al. 2019). Such metabolic flexibility throughout follicular development could support the modulation of fertility in response to environmental conditions.
Conclusion
The intimate relationship between the oocyte and the surrounding cumulus cells is essential to the development of a high-quality oocyte capable of resuming meiosis, being fertilised and undergoing embryonic development at least to the blastocyst stage. This connectivity is ensured by the network of transzonal projections. In vitro culture of ovarian follicles under suboptimal conditions has shown that resulting full-size oocytes are incompetent, a consequence of just getting by with an underdeveloped network of transzonal projections. The formation of an extensive and fully functional network is therefore a strong predictor of a good oocyte quality.
Premature completion or partial loss of connectivity with cumulus cells will prevent the oocyte from becoming developmentally competent. Full-sized oocytes acquiring developmental potential through a late maturation step while transzonal projections are still actively shuttling RNA has been reported. The transzonal projection network must therefore be not only extensive but fully functional.
This implies two separate but related aspects of folliculogenesis and oogenesis having a direct impact on oocyte quality, either early through modulation of network development or later through transfers of material via the transzonal projections. This could have biological relevance by adjusting fertility when environmental conditions are suboptimal (food less accessible, heat stress, etc.) without completely shutting down systemic endocrine signalling. This relationship needs to be studied in greater depth.
Data availability
This manuscript does not include any data other than the images presented in the figures.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Declaration of funding
Images were taken during projects supported by the Fond de recherche – Nature et technologies (FQRNT) no. 2015-PR-182922 and by the Natural Sciences and Engineering Research Council of Canada (NSERC) no. RGPIN/04775–2017. Both grants were awarded to the author.
Acknowledgements
The author would like to thank Dr Isabelle Gilbert for critical review of the manuscript along with Dr Angus Macaulay, Alexandre Bastien and Isabelle Laflamme for providing images.
References
Abbassi, L, El-Hayek, S, Carvalho, KF, et al. (2021). Epidermal growth factor receptor signaling uncouples germ cells from the somatic follicular compartment at ovulation. Nature Communications 12, 1438.| Epidermal growth factor receptor signaling uncouples germ cells from the somatic follicular compartment at ovulation.Crossref | GoogleScholarGoogle Scholar | 33664246PubMed |
Albertini, DF, Combelles, CM, Benecchi, E, et al. (2001). Cellular basis for paracrine regulation of ovarian follicle development. Reproduction 121, 647–653.
| Cellular basis for paracrine regulation of ovarian follicle development.Crossref | GoogleScholarGoogle Scholar | 11427152PubMed |
Allworth, AE, and Albertini, DF (1993). Meiotic maturation in cultured bovine oocytes is accompanied by remodeling of the cumulus cell cytoskeleton. Developmental Biology 158, 101–112.
| Meiotic maturation in cultured bovine oocytes is accompanied by remodeling of the cumulus cell cytoskeleton.Crossref | GoogleScholarGoogle Scholar | 8330667PubMed |
Anderson, E, and Albertini, DF (1976). Gap junctions between the oocyte and companion follicle cells in the mammalian ovary. Journal of Cell Biology 71, 680–686.
| Gap junctions between the oocyte and companion follicle cells in the mammalian ovary.Crossref | GoogleScholarGoogle Scholar |
Baena, V, and Terasaki, M (2019). Three-dimensional organization of transzonal projections and other cytoplasmic extensions in the mouse ovarian follicle. Scientific Reports 9, 1262.
| Three-dimensional organization of transzonal projections and other cytoplasmic extensions in the mouse ovarian follicle.Crossref | GoogleScholarGoogle Scholar | 30718581PubMed |
Bényei, B, Gaspardy, A, Komlosi, I, et al. (2004). Repeatability and heritability of ovulation number and embryos in dam-daughters pairs in superovulated Holstein-Friesian cows. Reproduction in Domestic Animals = Zuchthygiene 39, 99–102.
| Repeatability and heritability of ovulation number and embryos in dam-daughters pairs in superovulated Holstein-Friesian cows.Crossref | GoogleScholarGoogle Scholar | 15065991PubMed |
Blondin, P, and Sirard, MA (1995). Oocyte and follicular morphology as determining characteristics for developmental competence in bovine oocytes. Molecular Reproduction and Development 41, 54–62.
| Oocyte and follicular morphology as determining characteristics for developmental competence in bovine oocytes.Crossref | GoogleScholarGoogle Scholar | 7619506PubMed |
Blondin, P, Coenen, K, Guilbault, LA, et al. (1997). In vitro production of bovine embryos: developmental competence is acquired before maturation. Theriogenology 47, 1061–1075.
| In vitro production of bovine embryos: developmental competence is acquired before maturation.Crossref | GoogleScholarGoogle Scholar | 16728056PubMed |
Blondin, P, Bousquet, D, Twagiramungu, H, et al. (2002). Manipulation of follicular development to produce developmentally competent bovine oocytes. Biology of Reproduction 66, 38–43.
| Manipulation of follicular development to produce developmentally competent bovine oocytes.Crossref | GoogleScholarGoogle Scholar | 11751261PubMed |
Braw-Tal, R, and Yossefi, S (1997). Studies in vivo and in vitro on the initiation of follicle growth in the bovine ovary. Journal of Reproduction and Fertility 109, 165–171.
| Studies in vivo and in vitro on the initiation of follicle growth in the bovine ovary.Crossref | GoogleScholarGoogle Scholar | 9068428PubMed |
Bunel, A, Nivet, AL, Blondin, P, et al. (2014). Cumulus cell gene expression associated with pre-ovulatory acquisition of developmental competence in bovine oocytes. Reproduction, Fertility and Development 26, 855–865.
| Cumulus cell gene expression associated with pre-ovulatory acquisition of developmental competence in bovine oocytes.Crossref | GoogleScholarGoogle Scholar |
Burns, DS, Jimenez-Krassel, F, Ireland, JLH, et al. (2005). Numbers of antral follicles during follicular waves in cattle: evidence for high variation among animals, very high repeatability in individuals, and an inverse association with serum follicle-stimulating hormone concentrations. Biology of Reproduction 73, 54–62.
| Numbers of antral follicles during follicular waves in cattle: evidence for high variation among animals, very high repeatability in individuals, and an inverse association with serum follicle-stimulating hormone concentrations.Crossref | GoogleScholarGoogle Scholar | 15744026PubMed |
Calder, MD, Caveney, AN, Sirard, M-A, et al. (2005). Effect of serum and cumulus cell expansion on marker gene transcripts in bovine cumulus-oocyte complexes during maturation in vitro. Fertility and Sterility 83, 1077–1085.
| Effect of serum and cumulus cell expansion on marker gene transcripts in bovine cumulus-oocyte complexes during maturation in vitro.Crossref | GoogleScholarGoogle Scholar | 15831278PubMed |
Caligara, C, Navarro, J, Vargas, G, et al. (2001). The effect of repeated controlled ovarian stimulation in donors. Human Reproduction 16, 2320–2323.
| The effect of repeated controlled ovarian stimulation in donors.Crossref | GoogleScholarGoogle Scholar | 11679512PubMed |
Can, A, and Albertini, DF (1997). Stage specific effects of carbendazim (MBC) on meiotic cell cycle progression in mouse oocytes. Molecular Reproduction and Development 46, 351–362.
| Stage specific effects of carbendazim (MBC) on meiotic cell cycle progression in mouse oocytes.Crossref | GoogleScholarGoogle Scholar | 9041138PubMed |
Carabatsos, MJ, Elvin, J, Matzuk, MM, et al. (1998). Characterization of oocyte and follicle development in growth differentiation factor-9-deficient mice. Developmental Biology 204, 373–384.
| Characterization of oocyte and follicle development in growth differentiation factor-9-deficient mice.Crossref | GoogleScholarGoogle Scholar | 9882477PubMed |
Combelles, CMH, Carabatsos, MJ, Kumar, TR, et al. (2004). Hormonal control of somatic cell oocyte interactions during ovarian follicle development. Molecular Reproduction and Development 69, 347–355.
| Hormonal control of somatic cell oocyte interactions during ovarian follicle development.Crossref | GoogleScholarGoogle Scholar |
Conti, M, Hsieh, M, Park, JY, et al. (2006). Role of the epidermal growth factor network in ovarian follicles. Molecular Endocrinology 20, 715–723.
| Role of the epidermal growth factor network in ovarian follicles.Crossref | GoogleScholarGoogle Scholar | 16051667PubMed |
Coticchio, G, Guglielmo, MC, Albertini, DF, et al. (2014). Contributions of the actin cytoskeleton to the emergence of polarity during maturation in human oocytes. Molecular Human Reproduction 20, 200–207.
| Contributions of the actin cytoskeleton to the emergence of polarity during maturation in human oocytes.Crossref | GoogleScholarGoogle Scholar | 24258450PubMed |
del Collado, M, da Silveira, JC, Sangalli, JR, et al. (2017). Fatty acid binding protein 3 and transzonal projections are involved in lipid accumulation during in vitro maturation of bovine oocytes. Scientific Reports 7, 2645.
| Fatty acid binding protein 3 and transzonal projections are involved in lipid accumulation during in vitro maturation of bovine oocytes.Crossref | GoogleScholarGoogle Scholar | 28572619PubMed |
Demeestere, I, Centner, J, Gervy, C, et al. (2005). Impact of various endocrine and paracrine factors on in vitro culture of preantral follicles in rodents. Reproduction 130, 147–156.
| Impact of various endocrine and paracrine factors on in vitro culture of preantral follicles in rodents.Crossref | GoogleScholarGoogle Scholar | 16049152PubMed |
Dieci, C, Lodde, V, Labreque, R, et al. (2016). Differences in cumulus cell gene expression indicate the benefit of a pre-maturation step to improve in-vitro bovine embryo production. Molecular Human Reproduction 22, 882–897.
| Differences in cumulus cell gene expression indicate the benefit of a pre-maturation step to improve in-vitro bovine embryo production.Crossref | GoogleScholarGoogle Scholar | 27559149PubMed |
Dierich, A, Sairam, MR, Monaco, L, et al. (1998). Impairing follicle-stimulating hormone (FSH) signaling in vivo: targeted disruption of the FSH receptor leads to aberrant gametogenesis and hormonal imbalance. Proceedings of the National Academy of Sciences of the United States of America 95, 13612–13617.
| Impairing follicle-stimulating hormone (FSH) signaling in vivo: targeted disruption of the FSH receptor leads to aberrant gametogenesis and hormonal imbalance.Crossref | GoogleScholarGoogle Scholar | 9811848PubMed |
Domhan, S, Ma, L, Tai, A, et al. (2011). Intercellular communication by exchange of cytoplasmic material via tunneling nano-tube like structures in primary human renal epithelial cells. PLoS One 6, e21283.
| Intercellular communication by exchange of cytoplasmic material via tunneling nano-tube like structures in primary human renal epithelial cells.Crossref | GoogleScholarGoogle Scholar | 21738629PubMed |
El Fatimy, R, Davidovic, L, Tremblay, S, et al. (2016). Tracking the fragile X mental retardation protein in a highly ordered neuronal ribonucleoparticles population: a link between stalled polyribosomes and RNA granules. PLoS Genetics 12, e1006192.
| Tracking the fragile X mental retardation protein in a highly ordered neuronal ribonucleoparticles population: a link between stalled polyribosomes and RNA granules.Crossref | GoogleScholarGoogle Scholar | 27462983PubMed |
El-Hayek, S, Yang, Q, Abbassi, L, et al. (2018). Mammalian oocytes locally remodel follicular architecture to provide the foundation for germline-soma communication. Current Biology 28, 1124–1131.e3.
| Mammalian oocytes locally remodel follicular architecture to provide the foundation for germline-soma communication.Crossref | GoogleScholarGoogle Scholar | 29576478PubMed |
Ellenbogen, A, Shavit, T, and Shalom-Paz, E (2014). IVM results are comparable and may have advantages over standard IVF. Facts, Views & Vision in ObGyn 6, 77–80.
Eppig, JJ, and Schroeder, AC (1989). Capacity of mouse oocytes from preantral follicles to undergo embryogenesis and development to live young after growth, maturation, and fertilization in vitro. Biology of Reproduction 41, 268–276.
| Capacity of mouse oocytes from preantral follicles to undergo embryogenesis and development to live young after growth, maturation, and fertilization in vitro.Crossref | GoogleScholarGoogle Scholar | 2508774PubMed |
Eppig, JJ, Wigglesworth, K, Pendola, F, et al. (1997). Murine oocytes suppress expression of luteinizing hormone receptor messenger ribonucleic acid by granulosa cells. Biology of Reproduction 56, 976–984.
| Murine oocytes suppress expression of luteinizing hormone receptor messenger ribonucleic acid by granulosa cells.Crossref | GoogleScholarGoogle Scholar | 9096881PubMed |
Fair, T, Hyttel, P, and Greve, T (1995). Bovine oocyte diameter in relation to maturational competence and transcriptional activity. Molecular Reproduction and Development 42, 437–442.
| Bovine oocyte diameter in relation to maturational competence and transcriptional activity.Crossref | GoogleScholarGoogle Scholar | 8607973PubMed |
Fair, T, Hulshof, SCJ, Hyttel, P, et al. (1997a). Nucleus ultrastructure and transcriptional activity of bovine oocytes in preantral and early antral follicles. Molecular Reproduction and Development 46, 208–215.
| Nucleus ultrastructure and transcriptional activity of bovine oocytes in preantral and early antral follicles.Crossref | GoogleScholarGoogle Scholar | 9021752PubMed |
Fair, T, Hulshof, SCJ, Hyttel, P, et al. (1997b). Oocyte ultrastructure in bovine primordial to early tertiary follicles. Anatomy and Embryology 195, 327–336.
| Oocyte ultrastructure in bovine primordial to early tertiary follicles.Crossref | GoogleScholarGoogle Scholar | 9108198PubMed |
Gilchrist, RB, Luciano, AM, Richani, D, et al. (2016). Oocyte maturation and quality: role of cyclic nucleotides. Reproduction 152, R143–R157.
| Oocyte maturation and quality: role of cyclic nucleotides.Crossref | GoogleScholarGoogle Scholar | 27422885PubMed |
Gougeon, A (1996). Regulation of ovarian follicular development in primates: facts and hypotheses. Endocrine Reviews 17, 121–155.
| Regulation of ovarian follicular development in primates: facts and hypotheses.Crossref | GoogleScholarGoogle Scholar | 8706629PubMed |
Graves, KL, Seibert, JT, Keating, AF, et al. (2018). Characterizing the acute heat stress response in gilts: II. Assessing repeatability and association with fertility. Journal of Animal Science 96, 2419–2426.
| Characterizing the acute heat stress response in gilts: II. Assessing repeatability and association with fertility.Crossref | GoogleScholarGoogle Scholar | 29788126PubMed |
Guild, GM, Connelly, PS, Shaw, MK, et al. (1997). Actin filament cables in Drosophila nurse cells are composed of modules that slide passively past one another during dumping. Journal of Cell Biology 138, 783–797.
| Actin filament cables in Drosophila nurse cells are composed of modules that slide passively past one another during dumping.Crossref | GoogleScholarGoogle Scholar |
Hansen, PJ (2020). The incompletely fulfilled promise of embryo transfer in cattle-why aren’t pregnancy rates greater and what can we do about it? Journal of Animal Science 98, skaa288.
| The incompletely fulfilled promise of embryo transfer in cattle-why aren’t pregnancy rates greater and what can we do about it?Crossref | GoogleScholarGoogle Scholar | 33141879PubMed |
Hernandez-Gonzalez, I, Gonzalez-Robayna, I, Shimada, M, et al. (2006). Gene expression profiles of cumulus cell oocyte complexes during ovulation reveal cumulus cells express neuronal and immune-related genes: does this expand their role in the ovulation process? Molecular Endocrinology 20, 1300–1321.
| Gene expression profiles of cumulus cell oocyte complexes during ovulation reveal cumulus cells express neuronal and immune-related genes: does this expand their role in the ovulation process?Crossref | GoogleScholarGoogle Scholar | 16455817PubMed |
Hertig, AT, and Adams, EC (1967). Studies on the human oocyte and its follicle. I. Ultrastructural and histochemical observations on the primordial follicle stage. Journal of Cell Biology 34, 647–675.
| Studies on the human oocyte and its follicle. I. Ultrastructural and histochemical observations on the primordial follicle stage.Crossref | GoogleScholarGoogle Scholar |
Holm, P, and Callesen, H (1998). In vivo versus in vitro produced bovine ova: similarities and differences relevant for practical application. Reproduction Nutrition Development 38, 579–594.
| In vivo versus in vitro produced bovine ova: similarities and differences relevant for practical application.Crossref | GoogleScholarGoogle Scholar |
Kidder, GM, and Mhawi, AA (2002). Gap junctions and ovarian folliculogenesis. Reproduction 123, 613–620.
| Gap junctions and ovarian folliculogenesis.Crossref | GoogleScholarGoogle Scholar | 12006089PubMed |
Kölle, S, Dubois, CS, Caillaud, M, et al. (2007). Equine zona protein synthesis and ZP structure during folliculogenesis, oocyte maturation, and embryogenesis. Molecular Reproduction and Development 74, 851–859.
| Equine zona protein synthesis and ZP structure during folliculogenesis, oocyte maturation, and embryogenesis.Crossref | GoogleScholarGoogle Scholar | 17252540PubMed |
Kreeger, PK, Fernandes, NN, Woodruff, TK, et al. (2005). Regulation of mouse follicle development by follicle-stimulating hormone in a three-dimensional in vitro culture system is dependent on follicle stage and dose. Biology of Reproduction 73, 942–950.
| Regulation of mouse follicle development by follicle-stimulating hormone in a three-dimensional in vitro culture system is dependent on follicle stage and dose.Crossref | GoogleScholarGoogle Scholar | 15987824PubMed |
Kumar, TR, Wang, Y, Lu, N, et al. (1997). Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility. Nature Genetics 15, 201–204.
| Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility.Crossref | GoogleScholarGoogle Scholar | 9020850PubMed |
Lawson, DW, and Borgerhoff Mulder, M (2016). The offspring quantity–quality trade-off and human fertility variation. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 371, 20150145.
| The offspring quantity–quality trade-off and human fertility variation.Crossref | GoogleScholarGoogle Scholar | 27022072PubMed |
Li, R, and Albertini, DF (2013). The road to maturation: somatic cell interaction and self-organization of the mammalian oocyte. Nature Reviews Molecular Cell Biology 14, 141–152.
| The road to maturation: somatic cell interaction and self-organization of the mammalian oocyte.Crossref | GoogleScholarGoogle Scholar | 23429793PubMed |
Liu, Y-X, and Hsueh, AJW (1986). Synergism between granulosa and theca-interstitial cells in estrogen biosynthesis by gonadotropin-treated rat ovaries: studies on the two-cell, two-gonadotropin hypothesis using steroid antisera. Biology of Reproduction 35, 27–36.
| Synergism between granulosa and theca-interstitial cells in estrogen biosynthesis by gonadotropin-treated rat ovaries: studies on the two-cell, two-gonadotropin hypothesis using steroid antisera.Crossref | GoogleScholarGoogle Scholar | 3091103PubMed |
Lodde, V, Modina, S, Galbusera, C, et al. (2007). Large-scale chromatin remodeling in germinal vesicle bovine oocytes: interplay with gap junction functionality and developmental competence. Molecular Reproduction and Development 74, 740–749.
| Large-scale chromatin remodeling in germinal vesicle bovine oocytes: interplay with gap junction functionality and developmental competence.Crossref | GoogleScholarGoogle Scholar | 17075796PubMed |
Lodde, V, Modina, S, Maddox-Hyttel, P, et al. (2008). Oocyte morphology and transcriptional silencing in relation to chromatin remodeling during the final phases of bovine oocyte growth. Molecular Reproduction and Development 75, 915–924.
| Oocyte morphology and transcriptional silencing in relation to chromatin remodeling during the final phases of bovine oocyte growth.Crossref | GoogleScholarGoogle Scholar | 17948251PubMed |
Lonergan, P, Monaghan, P, Rizos, D, et al. (1994). Effect of follicle size on bovine oocyte quality and developmental competence following maturation, fertilization, and culture in vitro. Molecular Reproduction and Development 37, 48–53.
| Effect of follicle size on bovine oocyte quality and developmental competence following maturation, fertilization, and culture in vitro.Crossref | GoogleScholarGoogle Scholar | 8129930PubMed |
Luciano, AM, Lodde, V, Beretta, MS, et al. (2005). Developmental capability of denuded bovine oocyte in a co-culture system with intact cumulus–oocyte complexes: role of cumulus cells, cyclic adenosine 3′,5′-monophosphate, and glutathione. Molecular Reproduction and Development 71, 389–397.
| Developmental capability of denuded bovine oocyte in a co-culture system with intact cumulus–oocyte complexes: role of cumulus cells, cyclic adenosine 3′,5′-monophosphate, and glutathione.Crossref | GoogleScholarGoogle Scholar | 15803456PubMed |
Luciano, AM, Franciosi, F, Modina, SC, et al. (2011). Gap junction-mediated communications regulate chromatin remodeling during bovine oocyte growth and differentiation through cAMP-dependent mechanism(s). Biology of Reproduction 85, 1252–1259.
| Gap junction-mediated communications regulate chromatin remodeling during bovine oocyte growth and differentiation through cAMP-dependent mechanism(s).Crossref | GoogleScholarGoogle Scholar | 21816847PubMed |
Lynch, M, Ackerman, MS, Gout, J-F, et al. (2016). Genetic drift, selection and the evolution of the mutation rate. Nature Reviews Genetics 17, 704–714.
| Genetic drift, selection and the evolution of the mutation rate.Crossref | GoogleScholarGoogle Scholar | 27739533PubMed |
Macaulay, AD, Gilbert, I, Caballero, J, et al. (2014). The gametic synapse: RNA transfer to the bovine oocyte. Biology of Reproduction 91, 90.
| The gametic synapse: RNA transfer to the bovine oocyte.Crossref | GoogleScholarGoogle Scholar | 25143353PubMed |
Macaulay, AD, Gilbert, I, Scantland, S, et al. (2016). Cumulus cell transcripts transit to the bovine oocyte in preparation for maturation. Biology of Reproduction 94, 16.
| Cumulus cell transcripts transit to the bovine oocyte in preparation for maturation.Crossref | GoogleScholarGoogle Scholar | 26586844PubMed |
McLaughlin, M, and Telfer, EE (2010). Oocyte development in bovine primordial follicles is promoted by activin and FSH within a two-step serum-free culture system. Reproduction 139, 971–978.
| Oocyte development in bovine primordial follicles is promoted by activin and FSH within a two-step serum-free culture system.Crossref | GoogleScholarGoogle Scholar | 20207724PubMed |
Modina, S, Borromeo, V, Luciano, AM, et al. (2007). Relationship between growth hormone concentrations in bovine oocytes and follicular fluid and oocyte developmental competence. European Journal of Histochemistry 51, 173–180.
| 17921112PubMed |
Motta, PM, Makabe, S, Naguro, T, et al. (1994). Oocyte follicle cells association during development of human ovarian follicle. A study by high resolution scanning and transmission electron microscopy. Archives of Histology and Cytology 57, 369–394.
| Oocyte follicle cells association during development of human ovarian follicle. A study by high resolution scanning and transmission electron microscopy.Crossref | GoogleScholarGoogle Scholar | 7880591PubMed |
Muzio MR, Cascella M (2021) ‘‘Histology, Axon’, in StatPearls’. (StatPearls Publishing: Treasure Island, FL, USA). Available at http://www.ncbi.nlm.nih.gov/books/NBK554388/ [Accessed: 20 July 2021]
Nawaz, M, and Fatima, F (2017). Extracellular vesicles, tunneling nanotubes, and cellular interplay: synergies and missing links. Frontiers in Molecular Biosciences 4, 50.
| Extracellular vesicles, tunneling nanotubes, and cellular interplay: synergies and missing links.Crossref | GoogleScholarGoogle Scholar | 28770210PubMed |
Nivet, A-L, Bunel, A, Labrecque, R, et al. (2012). FSH withdrawal improves developmental competence of oocytes in the bovine model. Reproduction 143, 165–171.
| FSH withdrawal improves developmental competence of oocytes in the bovine model.Crossref | GoogleScholarGoogle Scholar | 22080141PubMed |
Numabe, T, Oikawa, T, Kikuchi, T, et al. (2000). Birth weight and birth rate of heavy calves conceived by transfer of in vitro or in vivo produced bovine embryos. Animal Reproduction Science 64, 13–20.
| Birth weight and birth rate of heavy calves conceived by transfer of in vitro or in vivo produced bovine embryos.Crossref | GoogleScholarGoogle Scholar | 11078963PubMed |
O’Doherty, EM, Wade, MG, Hill, JL, et al. (1997). Effects of culturing bovine oocytes either singly or in groups on development to blastocysts. Theriogenology 48, 161–169.
| Effects of culturing bovine oocytes either singly or in groups on development to blastocysts.Crossref | GoogleScholarGoogle Scholar | 16728116PubMed |
Pangas, SA, Saudye, H, Shea, LD, et al. (2003). Novel approach for the three-dimensional culture of granulosa cell-oocyte complexes. Tissue Engineering 9, 1013–1021.
| Novel approach for the three-dimensional culture of granulosa cell-oocyte complexes.Crossref | GoogleScholarGoogle Scholar | 14633385PubMed |
Paul, LT, Atilan, O, and Tulay, P (2019). The effect of repeated controlled ovarian stimulation cycles on the gamete and embryo development. Zygote 27, 347–349.
| The effect of repeated controlled ovarian stimulation cycles on the gamete and embryo development.Crossref | GoogleScholarGoogle Scholar | 31405397PubMed |
Peñalver Bernabé, B, Thiele, I, Galdones, E, et al. (2019). Dynamic genome-scale cell-specific metabolic models reveal novel inter-cellular and intra-cellular metabolic communications during ovarian follicle development. BMC Bioinformatics 20, 307.
| Dynamic genome-scale cell-specific metabolic models reveal novel inter-cellular and intra-cellular metabolic communications during ovarian follicle development.Crossref | GoogleScholarGoogle Scholar | 31182013PubMed |
Philpott, CC, Ringuette, MJ, and Dean, J (1987). Oocyte-specific expression and developmental regulation of ZP3, the sperm receptor of the mouse zona pellucida. Developmental Biology 121, 568–575.
| Oocyte-specific expression and developmental regulation of ZP3, the sperm receptor of the mouse zona pellucida.Crossref | GoogleScholarGoogle Scholar | 2884155PubMed |
Plancha, CE, Sanfins, A, Rodrigues, P, et al. (2005). Cell polarity during folliculogenesis and oogenesis. Reproductive Biomedicine Online 10, 478–484.
| Cell polarity during folliculogenesis and oogenesis.Crossref | GoogleScholarGoogle Scholar | 15901455PubMed |
Plourde, D, Vigneault, C, Lemay, A, et al. (2012). Contribution of oocyte source and culture conditions to phenotypic and transcriptomic variation in commercially produced bovine blastocysts. Theriogenology 78, 116–131.e3.
| Contribution of oocyte source and culture conditions to phenotypic and transcriptomic variation in commercially produced bovine blastocysts.Crossref | GoogleScholarGoogle Scholar | 22494684PubMed |
Reynaud, K, Cortvrindt, R, Smitz, J, et al. (2001). Alterations in ovarian function of mice with reduced amounts of KIT receptor. Reproduction 121, 229–237.
| Alterations in ovarian function of mice with reduced amounts of KIT receptor.Crossref | GoogleScholarGoogle Scholar | 11226047PubMed |
Richards, JS (2007). Genetics of ovulation. Seminars in Reproductive Medicine 25, 235–242.
| Genetics of ovulation.Crossref | GoogleScholarGoogle Scholar | 17594604PubMed |
Rieger, D, and Loskutoff, NM (1994). Changes in the metabolism of glucose, pyruvate, glutamine and glycine during maturation of cattle oocytes in vitro. Reproduction 100, 257–262.
| Changes in the metabolism of glucose, pyruvate, glutamine and glycine during maturation of cattle oocytes in vitro.Crossref | GoogleScholarGoogle Scholar |
Rieger, D, Loskutoff, NM, and Betteridge, KJ (1992). Developmentally related changes in the metabolism of glucose and glutamine by cattle embryos produced and co-cultured in vitro. Reproduction 95, 585–595.
| Developmentally related changes in the metabolism of glucose and glutamine by cattle embryos produced and co-cultured in vitro.Crossref | GoogleScholarGoogle Scholar |
Rizos, D, Ward, F, Duffy, P, et al. (2002). Consequences of bovine oocyte maturation, fertilization or early embryo development in vitro versus in vivo: implications for blastocyst yield and blastocyst quality. Molecular Reproduction and Development 61, 234–248.
| Consequences of bovine oocyte maturation, fertilization or early embryo development in vitro versus in vivo: implications for blastocyst yield and blastocyst quality.Crossref | GoogleScholarGoogle Scholar | 11803560PubMed |
Romano, JE, Bryan, K, Ramos, RS, et al. (2016). Effect of early pregnancy diagnosis by per rectum amniotic sac palpation on pregnancy loss, calving rates, and abnormalities in newborn dairy calves. Theriogenology 85, 419–427.
| Effect of early pregnancy diagnosis by per rectum amniotic sac palpation on pregnancy loss, calving rates, and abnormalities in newborn dairy calves.Crossref | GoogleScholarGoogle Scholar | 26443235PubMed |
Rubelt, F, Busse, CE, Bukhari, SAC, et al. (2017). Adaptive immune receptor repertoire community recommendations for sharing immune-repertoire sequencing data. Nature Immunology 18, 1274–1278.
| Adaptive immune receptor repertoire community recommendations for sharing immune-repertoire sequencing data.Crossref | GoogleScholarGoogle Scholar | 29144493PubMed |
Santiquet, N, Robert, C, and Richard, FJ (2013). The dynamics of connexin expression, degradation and localisation are regulated by gonadotropins during the early stages of in vitro maturation of swine oocytes. PLoS One 8, e68456.
| The dynamics of connexin expression, degradation and localisation are regulated by gonadotropins during the early stages of in vitro maturation of swine oocytes.Crossref | GoogleScholarGoogle Scholar | 23861906PubMed |
Santiquet, NW, Greene, AF, Becker, J, et al. (2017). A pre-in vitro maturation medium containing cumulus oocyte complex ligand-receptor signaling molecules maintains meiotic arrest, supports the cumulus oocyte complex and improves oocyte developmental competence. Molecular Human Reproduction 23, 594–606.
| A pre-in vitro maturation medium containing cumulus oocyte complex ligand-receptor signaling molecules maintains meiotic arrest, supports the cumulus oocyte complex and improves oocyte developmental competence.Crossref | GoogleScholarGoogle Scholar | 28586460PubMed |
Scantland, S, Tessaro, I, Macabelli, CH, et al. (2014). The adenosine salvage pathway as an alternative to mitochondrial production of ATP in maturing mammalian oocytes. Biology of Reproduction 91, 75.
| The adenosine salvage pathway as an alternative to mitochondrial production of ATP in maturing mammalian oocytes.Crossref | GoogleScholarGoogle Scholar | 25078684PubMed |
Sinowatz, F, Kölle, S, and Töpfer-Petersen, E (2001). Biosynthesis and expression of zona pellucida glycoproteins in mammals. Cells Tissues Organs 168, 24–35.
| Biosynthesis and expression of zona pellucida glycoproteins in mammals.Crossref | GoogleScholarGoogle Scholar | 11114584PubMed |
Sood, P, Zachut, M, Dekel, I, et al. (2017). Preovulatory follicle characteristics and oocyte competence in repeat breeder dairy cows. Journal of Dairy Science 100, 9372–9381.
| Preovulatory follicle characteristics and oocyte competence in repeat breeder dairy cows.Crossref | GoogleScholarGoogle Scholar | 28888606PubMed |
Spracklen, AJ, and Tootle, TL (2013). The utility of stage-specific mid-to-late Drosophila follicle isolation. Journal of Visualized Experiments: JoVE 82, 50493.
| The utility of stage-specific mid-to-late Drosophila follicle isolation.Crossref | GoogleScholarGoogle Scholar |
Stimpfel, M, Jancar, N, Vrtacnik-Bokal, E, et al. (2019). Conventional IVF improves blastocyst rate and quality compared to ICSI when used in patients with mild or moderate teratozoospermia. Systems Biology in Reproductive Medicine 65, 458–464.
| Conventional IVF improves blastocyst rate and quality compared to ICSI when used in patients with mild or moderate teratozoospermia.Crossref | GoogleScholarGoogle Scholar | 31522570PubMed |
Su, Y-Q, Wu, X, O’Brien, MJ, et al. (2004). Synergistic roles of BMP15 and GDF9 in the development and function of the oocyte–cumulus cell complex in mice: genetic evidence for an oocyte–granulosa cell regulatory loop. Developmental Biology 276, 64–73.
| Synergistic roles of BMP15 and GDF9 in the development and function of the oocyte–cumulus cell complex in mice: genetic evidence for an oocyte–granulosa cell regulatory loop.Crossref | GoogleScholarGoogle Scholar | 15531364PubMed |
Sugiura, K, Su, Y-Q, Li, Q, et al. (2010). Estrogen promotes the development of mouse cumulus cells in coordination with oocyte-derived GDF9 and BMP15. Molecular Endocrinology 24, 2303–2314.
| Estrogen promotes the development of mouse cumulus cells in coordination with oocyte-derived GDF9 and BMP15.Crossref | GoogleScholarGoogle Scholar | 21047911PubMed |
Sutton-McDowall, ML, Gilchrist, RB, and Thompson, JG (2010). The pivotal role of glucose metabolism in determining oocyte developmental competence. Reproduction 139, 685–695.
| The pivotal role of glucose metabolism in determining oocyte developmental competence.Crossref | GoogleScholarGoogle Scholar | 20089664PubMed |
Telfer, EE, and Andersen, CY (2021). In vitro growth and maturation of primordial follicles and immature oocytes. Fertility and Sterility 115, 1116–1125.
| In vitro growth and maturation of primordial follicles and immature oocytes.Crossref | GoogleScholarGoogle Scholar | 33823993PubMed |
Truong, T, and Gardner, DK (2017). Antioxidants improve IVF outcome and subsequent embryo development in the mouse. Human Reproduction 32, 2404–2413.
| Antioxidants improve IVF outcome and subsequent embryo development in the mouse.Crossref | GoogleScholarGoogle Scholar | 29136144PubMed |
Tutt, DAR, Silvestri, G, Serrano-Albal, M, et al. (2021). Analysis of bovine blastocysts indicates ovarian stimulation does not induce chromosome errors, nor discordance between inner-cell mass and trophectoderm lineages. Theriogenology 161, 108–119.
| Analysis of bovine blastocysts indicates ovarian stimulation does not induce chromosome errors, nor discordance between inner-cell mass and trophectoderm lineages.Crossref | GoogleScholarGoogle Scholar | 33307428PubMed |
Van Blerkom, J (2011). Mitochondrial function in the human oocyte and embryo and their role in developmental competence. Mitochondrion 11, 797–813.
| Mitochondrial function in the human oocyte and embryo and their role in developmental competence.Crossref | GoogleScholarGoogle Scholar | 20933103PubMed |
van Wagtendonk-de Leeuw, AM, Mullaart, E, de Roos, APW, et al. (2000). Effects of different reproduction techniques: AI MOET or IVP, on health and welfare of bovine offspring. Theriogenology 53, 575–597.
| Effects of different reproduction techniques: AI MOET or IVP, on health and welfare of bovine offspring.Crossref | GoogleScholarGoogle Scholar | 10735051PubMed |
Wang, X, Catt, S, Pangestu, M, et al. (2011). Successful in vitro culture of pre-antral follicles derived from vitrified murine ovarian tissue: oocyte maturation, fertilization, and live births. Reproduction 141, 183–191.
| Successful in vitro culture of pre-antral follicles derived from vitrified murine ovarian tissue: oocyte maturation, fertilization, and live births.Crossref | GoogleScholarGoogle Scholar | 21075829PubMed |
Xiao, S, Zhang, J, Romero, MM, et al. (2015). In vitro follicle growth supports human oocyte meiotic maturation. Scientific Reports 5, 17323.
| In vitro follicle growth supports human oocyte meiotic maturation.Crossref | GoogleScholarGoogle Scholar | 26612176PubMed |
Xiao, S, Coppeta, JR, Rogers, HB, et al. (2017). A microfluidic culture model of the human reproductive tract and 28-day menstrual cycle. Nature Communications 8, 14584.
| A microfluidic culture model of the human reproductive tract and 28-day menstrual cycle.Crossref | GoogleScholarGoogle Scholar | 28350383PubMed |
Yin, C, Liu, J, Chang, Z, et al. (2020). Heat exposure impairs porcine oocyte quality with suppressed actin expression in cumulus cells and disrupted F-actin formation in transzonal projections. Journal of Animal Science and Biotechnology 11, 71.
| Heat exposure impairs porcine oocyte quality with suppressed actin expression in cumulus cells and disrupted F-actin formation in transzonal projections.Crossref | GoogleScholarGoogle Scholar | 32647569PubMed |
Zeng, H-t, Ren, Z, Guzman, L, et al. (2013). Heparin and cAMP modulators interact during pre-in vitro maturation to affect mouse and human oocyte meiosis and developmental competence. Human Reproduction 28, 1536–1545.
| Heparin and cAMP modulators interact during pre-in vitro maturation to affect mouse and human oocyte meiosis and developmental competence.Crossref | GoogleScholarGoogle Scholar | 23559189PubMed |


