Characterisation of bovine embryos following prolonged culture in embryonic stem cell medium containing leukaemia inhibitory factor
Misa Hosoe A B H , Tadashi Furusawa A , Ken-Go Hayashi B , Toru Takahashi C , Yutaka Hashiyada D E , Keiichiro Kizaki C , Kazuyoshi Hashizume C , Tomoyuki Tokunaga A , Shuichi Matsuyama F G and Ryosuke Sakumoto BA Institute of Agrobiological Sciences, National Agriculture and Food Research Organization, Tsukuba, Ibaraki 305-8602, Japan.
B Institute of Livestock and Grassland Science, National Agriculture and Food Research Organization, Tsukuba, Ibaraki 305-0901, Japan.
C Department of Veterinary Medicine, Faculty of Agriculture, Iwate University, Iwate 020-8550, Japan.
D National Livestock Breeding Center, Nishigo, Fukushima 961-8511, Japan.
E Ishikawa Prefectural University, Nono, Ishikawa, 921-8836, Japan.
F Institute of Livestock and Grassland Science, National Agriculture and Food Reasarch Organization, Nasushiobara, Tochigi 329-2793, Japan.
G Nagoya University, Nagoya, Aichi 464-8601, Japan.
H Corresponding author. Email: hosoe@affrc.go.jp
Reproduction, Fertility and Development 31(6) 1157-1165 https://doi.org/10.1071/RD18343
Submitted: 25 August 2018 Accepted: 2 February 2019 Published: 29 April 2019
Journal Compilation © CSIRO 2019 Open Access CC BY-NC-ND
Abstract
In order to help elucidate the process of epiblast and trophoblast cell differentiation in bovine embryos in vitro, we attempted to develop a suitable culture medium to allow extended embryo culture. Day 7 bovine blastocysts developed in conventional medium were cultured further in embryonic stem cell medium with or without leukaemia inhibitory factor (LIF) until Day 23. At Day 14, the expression of octamer-binding transcription factor 3/4 (OCT3/4) and VIMENTIN was significantly higher in embryos cultured with than without LIF, but embryonic disc formation was not observed. Although expression of SRY (sex determining region Y)-box 17 (SOX17) mRNA was significantly lower in Day 14 embryos cultured with and without LIF than in in vivo embryos, hypoblast cells formed just inside the trophoblast cells of the in vitro-cultured embryos. On Day 23, expression of placental lactogen (PL) and prolactin-related protein 1 (PRP1) was not affected by LIF in in vitro-cultured embryos, levels of both genes were significantly lower in the in vitro than in vivo embryos. Similar to in vivo embryos, binucleate cell clusters seen in Day 23 in vitro-cultured embryos were composed of PL-negative and -positive cells. These results suggest that our culture system partially reproduced the differentiation process of trophoblast cells in vivo.
Additional keywords: binucleate cell, embryo differentiation, epiblast, trophoblast.
Introduction
Bovine embryos generally hatch 8–9 days after fertilisation and begin to transform from a spherical shape into an ovoid, tubular and filamentous shape. The process of cell differentiation continues until implantation, which occurs around Days 20–23 (Maddox-Hyttel et al. 2003; Degrelle et al. 2005; Blomberg et al. 2008). This early period of gestation is crucial for embryo survival, because embryo loss can frequently occur. However, detailed information relating to the developmental processes between hatching and implantation is still lacking.
Several in vitro culture systems for bovine embryos after hatching have been investigated, for example using agarose gel tunnels and/or collagen gel substrates (Brandão et al. 2004; Vajta et al. 2004; Alexopoulos et al. 2005). These systems induce embryo elongation and initial differentiation, but progressive degeneration of the epiblast has been observed in elongated embryos, and the differentiation of binucleate cells from trophoblast cells has not been detected (Brandão et al. 2004; Vajta et al. 2004; Alexopoulos et al. 2005).
In a previous study we established bovine embryonic stem (ES)-like cells from the bovine inner cell mass (ICM; Furusawa et al. 2013) and showed that recombinant human leukaemia inhibitory factor (LIF) was indispensable for the establishment of these cells. LIF maintains pluripotency in mouse ES cells and inhibits the differentiation of these cells (Smith et al. 1988). In addition, endometrial LIF may be crucial for murine implantation; transgenic mice lacking LIF produced normal embryos, but transgenic embryos and transferred wild-type embryos failed to implant (Stewart et al. 1992). In humans, endometrial LIF is an essential cytokine for implantation because it regulates trophoblast differentiation (Nachtigall et al. 1996). In cattle, LIF is present in oviduct epithelial cells (Reinhart et al. 1998) and in the endometrium of the uterus (Oshima et al. 2003). The LIF receptor subunit gp130 and LIF receptor β (LIFR-beta) mRNA are both expressed in bovine blastocysts (Eckert and Niemann 1998; Rizos et al. 2002). Oshima et al. (2003) also reported that LIF affects the development and differentiation of blastocysts. Based on these reports, we speculated that ES cell medium containing LIF would prevent degeneration of the epiblast and thus support cell differentiation in cultured bovine embryos.
In this study we compared gene expression patterns and morphological changes between embryos cultured in ES cell medium with or without LIF, as well as with embryos produced in vivo.
Materials and methods
Animal ethics approvals
The protocols regarding the use of animals were approved by the Animal Care Committee of the National Institute of Agrobiological Sciences (Approval number: H18-36-3(25)) and the Institute of Livestock and Grassland Science, National Agriculture and Food Research Organization (Approval number: 1711C026).
In vitro embryo culture
Ovaries were obtained at a local slaughterhouse. Cumulus–oocyte complexes (COCs) were collected by aspiration from follicles 3–5 mm in diameter. IVM and IVF were performed as described previously (Hosoe and Shioya 1997). Following IVF, putative zygotes were cultured in synthetic oviductal fluid with amino acids (SOFaa) at 38.5°C in 5% O2, 5% CO2 and 90% N2 for 3 days; cleaved embryos were then placed in SOFaa with 27.7 mM glucose for 4 days. On Day 7 (where Day 0 is the day of insemination), when the embryos were at the blastocyst stage, the culture medium was changed to ES cell medium consisting of KnockOut–Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 2 mM GlutaMAX-I, 1% minimum essential medium (MEM) non-essential amino acids, 1% antibiotic–antimycotic liquid, 10% fetal bovine serum (FBS; all from Invitrogen) and 0.5 mM monothioglycerol (instead of β-mercaptoethanol; Wako Pure Chemical), with or without 20 ng mL−1 recombinant human LIF (prepared according to the methods described by Furusawa et al. 2013). Hatched embryos that attached to the bottom of the cell culture dish were detached each day by pipetting. After Day 8, a half volume of medium was changed with fresh medium every other day. Degenerated and shrunken embryos were removed. The number of embryos, and their diameter, was recorded on Days 10, 14, 17, 20 and 23. The embryo survival rate is expressed as the mean ± s.d. Embryo culture experiments were replicated four times. For gene and protein expression analyses, cultured embryos were collected at Days 14, 17, 20 and 23, and the embryo culture experiment was replicated at least three times.
Collection of in vivo-derived embryos
As a control, in vivo-derived embryos were flushed and collected non-surgically from the uteri of superovulated and artificially inseminated Japanese Black cows on Day 14. Superovulation was induced in six cows by administration of a total of 20 mg FSH (Antrin R10; Kyoritsu Seiyaku) twice daily for 3 days in decreasing doses (5, 5, 3, 3, 2 and 2 mg). Embryos were recovered on Days 17, 20 and 23 from artificially inseminated cows after they had been killed. At least three intact embryos were collected on each sampling day. These embryos were used for quantitative reverse transcription–polymerase chain reaction (qRT-PCR) and histochemical analysis.
Quantitative RT-PCR
Total RNA (~1.0 μg) was isolated from embryos using an RNeasy Micro kit (QIAGEN) with DNase treatment and subjected to oligo-dT primed reverse transcription in a total reaction volume of 20 μL using a Primer Script 1st Strand cDNA Synthesis Kit (TaKaRa) according to the manufacturer’s instructions. The PCR was performed using a LightCycler 480 SYBR Green I (Roche) on a LightCycler 480 (Roche). Sequences for the gene-specific primer sets used in this PCR are given in Table 1. The reaction conditions for the PCR were as follows: 95°C for 5 min, followed by 45 cycles of denaturation at 95°C for 10 s, primer annealing at 64°C for 5 s and elongation at 72°C for 10 s. A fluorescent signal was read after each cycle following elongation at 72°C. The relative difference in the amount of each cDNA was determined by comparing Ct values. Standard curves were generated for each gene by serially diluting plasmids containing the sequences of each individual gene to quantify mRNA concentrations. Bovine glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an endogenous control for normalisation. The primer set for interferon-τ (IFNT) was designed to detect most IFNT variants. All values are presented as the mean ± s.d.
Histochemical analysis and immunochemistry
Day 14 embryos produced in vivo and in vitro were fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS) at 4°C and embedded in paraffin using a conventional protocol. Thereafter, 5-μm sections were cut and used for haematoxylin–eosin (HE) staining.
For immunostaining, Day 20 and Day 23 embryos produced in vivo and in vitro were fixed, washed with PBS containing 0.1% Triton X-100 (PBTX) and blocked with PBTX containing 10% FBS for 1 h at room temparature. The tissues were then incubated with primary placental lactogen (PL) antibody (1 : 500 dilution; rabbit polyclonal anti-bovine PL antibody; Nakano et al. 2001) in PBTX containing 10% FBS overnight at 4°C. After three washes with PBTX containing 10% FBS and three washes with PBTX, a fluorescence-labelled secondary antibody (1 : 500 dilution; AlexaFluor 488 goat anti rabbit IgG; Molecular Probe, Thermo Fisher Scientific) in PBTX containing FBS was applied for 1 h at room temperature. After washing with PBTX containing FBS, embryos were incubated with 10 μg mL−1 propidium iodide for 5 min, washed with PBTX and then observed under an epifluorescence microscope (BZ-X710; KEYENCE).
Statistical analysis
The embryo survival rate was compared between groups using a Chi-squared test, whereas diameter was evaluated using Student’s t-test. The embryo culture experiment was replicated four times. A t-test was used to compare the results of qRT-PCR. At least three embryos were used for analysis. GAPDH expression was used for standardisation. Two-sided P < 0.05 was considered significant.
Results
In vitro culture
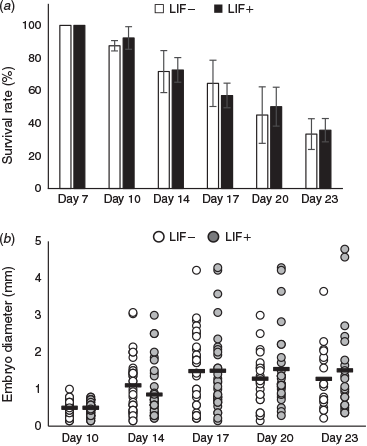
After Day 7, the respective survival rates of hatched embryos in culture medium with and without LIF (n = 56 and 53 embryos respectively; n = 4 replicates) were 92.3 ± 16.9% and 87.5 ± 3.2% at Day 10, 72.7 ± 7.6% and 71.7 ± 12.9% at Day 14, 57.0 ± 7.5% and 64.4 ± 14.1% at Day 17, 50.1 ± 11.9% and 45.0% ± 17.3% at Day 20 and 35.7% ± 7.1% and 33.3 ± 9.4% at Day 23 (Fig. 1a). There were no significant differences between the two groups in survival rate throughout the culture period. The respective mean diameter of embryos developed in culture medium with and without LIF was 0.47 and 0.50 mm at Day 10, 0.86 and 1.11 mm at Day 14, 1.41 and1.49 mm at Day 17, 1.55 and 1.22 mm at Day 20 and 1.51 and 1.30 mm at Day 23 (Fig. 1b). There were no significant differences between the two groups in the mean diameter of embryos throughout the culture period.
|
Quantitative RT-PCR
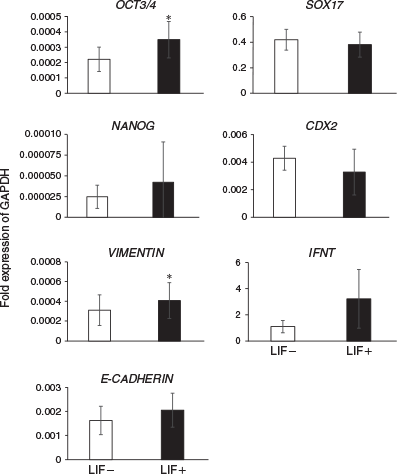
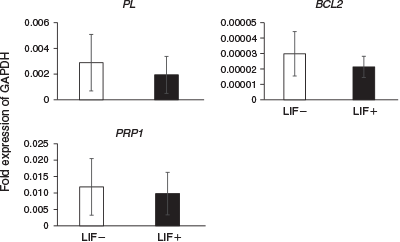
The expression levels of differentiation marker genes were compared between embryos cultured in ES cell medium with or without LIF at Day 14 (Fig. 2) and Day 23 (Fig. 3). On Day 14, the expression of the ICM and epiblast markers octamer-binding transcription factor 3/4 (OCT3/4) and VIMENTIN was significantly higher in embryos cultured with than without LIF. Although there were no significant differences between the two groups, expression of NANOG (an ICM and epiblast marker), E-CADHERIN (an ICM and epiblast marker), SRY (sex determining region Y)-box 17 (SOX17; a hypoblast marker), caudal type homeobox 2 (CDX2; a trophoblast marker) and IFNT (a trophoblast marker) tended to be higher in embryos cultured with LIF. On Day 23, there were no significant differences in the expression of PL (placental cell marker), prolactin-related protein 1 (PRP1; placental cell marker) and BCL2 (an anti-apoptotic protein) in embryos cultured with and without LIF.
|
|
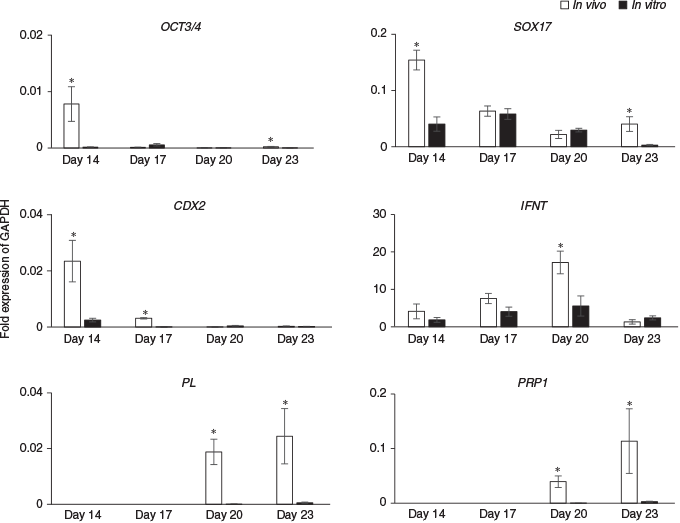
Next, the expression of differentiation marker genes were compared between embryos produced in vivo and those cultured in ES cell medium with LIF on Days 14, 17, 20 and 23 (Fig. 4). On Days 14 and 23, levels of OCT3/4 and SOX17 were significantly lower in in vitro than in vivo embryos, but there were no significant differences in the expression of these genes on Days 17 or 20. CDX2 expression on Days 14 and 17 was significantly lower in in vitro than in in vivo embryos, but there were no significant differences between the two groups on Days 20 or 23. Although there were no significant differences in IFNT expression on Days 14, 17 and 23 between the in vitro and in vivo embryos, on Day 20 IFNT levels were one-third lower in in vitro than in in vivo embryos. The placental cell marker genes PL and PRP1 were detected on Days 20 and 23 in both in vitro and in vivo embryos, but the levels of both mRNAs were significantly lower in in vitro than in vivo embryos.
Histochemical analysis and immunochemistry
Fig. 5 shows stereomicroscopic images (Fig. 5a, b, e, f) and HE-stained images (Fig. 5c, d, g, h) of Day 14 in vivo (Fig. 5a–d) and in vitro (Fig. 5e–h) embryos. The morphology of in vivo embryos changed from spherical to ovoid, tubular or filamentous (Fig. 5a, c), whereas the shape of in vitro embryos remained spherical (Fig. 5e, g) even though these embryos were growing in size. Embryonic disc formation was observed in Day 14 in vivo embryos (Fig. 5b, d); however, in Day 14 in vitro embryos, cells derived from the ICM appeared to be degenerating (Fig. 5h). Hypoblast cells developed just inside the trophoblast cells in both in vivo and in vitro embryos (Fig. 5c, g).
Immunostaining revealed that PL-positive cells were present in Day 20 embryos produced both in vivo (Fig. 6a, c) and in vitro (Fig. 6d, f), and these cells were almost all mononucleate (Fig. 6c, f). PL-positive mononucleate cells were distributed both individually and in groups. A few binucleate cells were observed in Day 20 in vivo and in vitro embryos (data not shown). Immunostaining also revealed that PL-positive cells were present in Day 23 in vivo (Fig. 7a–f) and in vitro (Fig. 7g–l) embryos, and these cells were almost all binucleate. Binucleate cells formed clusters (Fig. 7e, k) that contained both PL-positive and -negative cells in in vivo and in vitro embryos (Fig. 7f, l). Binucleate cell clusters were abundant in in vivo embryos (Fig. 7a–c), but much less frequent in in vitro embryos (Fig. 7g–i).
Discussion
In this study we showed that the expression of ICM and epiblast marker genes was higher in Day 14 embryos cultured with than without LIF, and the embryo culture system using ES cell medium enabled bovine trophoblast cells to differentiate into binucleate cells. In addition, we confirmed the expression of PL in Day 20 and 23 embryos produced in vitro. The timing of the appearance of PL-positive binucleate cells in this study was consistent with previous reports (Morgan et al. 1989). Thus, binucleate cell differentiation can occur punctually without support from the endometrium or chemical induction for differentiation in vitro.
In a previous study, Vajta et al. (2004) reported that on Day 16, 3% (2/67) of embryos survived in SOFaa supplemented sodium citrate and myo-inositol and 5% calf serum on an agarose gel tunnel. Alexopoulos et al. (2005) reported that all embryos degenerated in protein-free SOFaa supplemented sodium citrate and myo-inositol, or with 5% bovine serum albumin (BSA), but that 12% of embryos survived in SOFaaci with 5% FCS on collagen gels at Day 21. In the present study, the survival rate on Day 23 of embryos cultured with or without LIF was 35.7% and 33.3% respectively. This suggests that some components in the ES cell medium, except LIF, support embryo survival. It has been reported that β-mercaptoethanol increases the number of blastocyst cells and assists embryo survival after vitrification and thawing (Nedambale et al. 2006). Therefore, providing β-mercaptoethanol or monothioglycerol in the ES cell medium may be beneficial to embryo survival.
In previous studies, the addition of recombinant human LIF to the culture medium improved the rate of development to the morula and blastocyst stages (Fukui and Matsuyama 1994; Neira et al. 2010), increased the hatching rate after bovine embryo freezing (Han et al. 1995) and increased pregnancy rates after ovine embryo transfer (Fry et al. 1992). Moreover, bovine embryos cultured in SOF with BSA remained viable until Day 9, whereas those cultured in human ES cell medium containing five inhibitors and LIF remained viable until Day 24, even though disc formation failed (Brinkhof et al. 2017). In the present study we showed that LIF can contribute to ICM and epiblast cell survival to Day 14, although it could not contribute to disc formation. LIF did not support the differentiation of hypoblast cells (SOX17 expression) and binucleate cells (PL and PRP1 expression), or prevent apoptosis (BCL2 expression). These data suggest that LIF supports the maintenance of undifferentiated cells (ICM and epiblasts), but has no effect on the induction of differentiation in hatched bovine embryos. Because the embryonic disc had not been formed, cultured embryos may become trophoblast vesicles with hypoblasts cells after Day 14 in culture. In future studies, it will be necessary to screen other candidate supplements, for example compounds derived from the endometrium of a pregnant uterus.
In our culture system, hypoblast cell differentiation was observed. Expression of the hypoblast cell marker SOX17 was also detected, although expression levels were relatively low. Because hypoblast cell differentiation was also observed in previous studies (Brandão et al. 2004; Vajta et al. 2004; Alexopoulos et al. 2005; Vejlsted et al. 2006; Brinkhof et al. 2017), the differentiation process may occur spontaneously, regardless of the culture conditions. Expression of CDX2 is known to be restricted in bovine trophoblast cells (Degrelle et al. 2005) and regulates multiple trophoblast genes, including IFNT (Sakurai et al. 2009, 2010; Schiffmacher and Keefer 2013). However, it has also been reported that CDX2 did not affect IFNT expression in a CDX2-knockdown Day 14 conceptus (Berg et al. 2011). In the present study, expression of CDX2 was lower in in vitro than in vivo embryos on Day 14, whereas IFNT expression did not differ significantly between the two groups on Days 14, 17 and 23. From Day 14 to Day 20, the IFNT mRNA increased gradually and then declined, both in vivo and in vitro. Such changes in IFNT expression are consistent with a previous report (Ealy and Yang 2009). Our results showed that IFNT expression may not be regulated by CDX2 but, rather, by some hitherto unknown system.
Expression of PL and PRP1 genes was detected on Day 20, and expression levels increased on Day 23 in both in vivo and in vitro embryos. Binucleate cell clusters were not found on Day 20, but were present on Day 23. In these clusters, both PL-positive and -negative cells were present. These results led to the hypothesis illustrated in Fig. 8. We speculate that the trophoblast cells predestined to become binucleate cells appeared before Day 20, then proliferated, forming clusters; then, a portion of the cells began to express PL on Day 20 and PL-positive and -negative cells subsequently differentiated into binucleate cells simultaneously. In future studies we shall attempt to clarify the relationship between binucleate cell clusters in the embryo and during formation of the placentome.
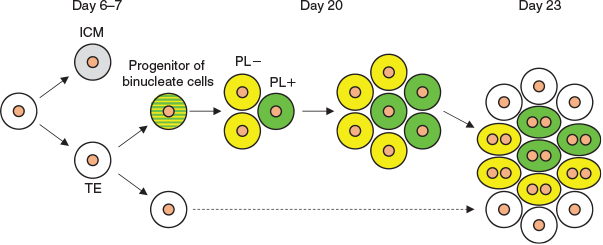
|
Various cell lines have been established from bovine blastocysts in order to investigate the differentiation and function of trophoblast cell linages (Talbot et al. 2000, 2008; Shimada et al. 2001; Hambruch et al. 2010; Suzuki et al. 2011). One of these cell lines, BT-1, can differentiate into bovine placental-specific binucleate cells (Shimada et al. 2001; Nakano et al. 2002; Suzuki et al. 2011), and a previous cDNA microarray analysis showed that the gene expression profile of BT-1 cells was similar to that of trophoblast cells during the peri-implantation period (Ushizawa et al. 2005). Although these trophoblast cell lines are a useful research tool to clarify the mechanism of implantation and the maintenance of pregnancy, it is difficult to analyse changes in the embryo along a developmental time axis and/or the interaction of endoderm- and mesoderm-derived cells. More detailed analyses will become possible through the use of both BT-1 cells and the culture system developed in this study. Furthermore, the extended embryo culture allows us to biopsy a sufficient number of cells for genome-wide analyses related to economic traits before embryo transfer (Fujii et al. 2017). Therefore, the establishment of a post-hatching culture system could be a valuable application in cattle breeding in future research.
In conclusion, our culture system partially reproduced the differentiation process of trophoblast cells from blastocysts to elongated embryos in vivo.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
The authors thank the staff of Livestock Research Support Center of the National Agriculture and Food Research Organization for animal management and their technical assistance with sample collection. This research did not receive any specific funding.
References
Alexopoulos, N. I., Vajta, G., Maddox-Hyttel, P., French, A. J., and Trounson, A. O. (2005). Stereomicroscopic and histological examination of bovine embryos following extended in vitro culture. Reprod. Fertil. Dev. 17, 799–808.| Stereomicroscopic and histological examination of bovine embryos following extended in vitro culture.Crossref | GoogleScholarGoogle Scholar | 16476207PubMed |
Berg, D. K., Smith, C. S., Pearton, D. J., Wells, D. N., Broadhurst, R., Donnison, M., and Pfeffer, P. L. (2011). Trophectoderm lineage determination in cattle. Dev. Cell 20, 244–255.
| Trophectoderm lineage determination in cattle.Crossref | GoogleScholarGoogle Scholar | 21316591PubMed |
Blomberg, L., Hashizume, K., and Viebahn, C. (2008). Blastocyst elongation, trophoblastic differentiation, and embryonic pattern formation. Reproduction 135, 181–195.
| Blastocyst elongation, trophoblastic differentiation, and embryonic pattern formation.Crossref | GoogleScholarGoogle Scholar | 18239048PubMed |
Brandão, D. O., Maddox-Hyttel, P., Løvendahl, P., Rumpf, R., Stringfellow, D., and Callesen, H. (2004). Post hatching development: a novel system for extended in vitro culture of bovine embryos. Biol. Reprod. 71, 2048–2055.
| Post hatching development: a novel system for extended in vitro culture of bovine embryos.Crossref | GoogleScholarGoogle Scholar | 15329327PubMed |
Brinkhof, B., van Tol, H. T. A., Groot Koerkamp, M. J. A., Wubbolts, R. W., Haagsman, H. P., and Roelen, B. A. J. (2017). Characterization of bovine embryos cultured under conditions appropriate for sustaining human naïve pluripotency. PLoS One 12, e0172920.
| Characterization of bovine embryos cultured under conditions appropriate for sustaining human naïve pluripotency.Crossref | GoogleScholarGoogle Scholar | 28241084PubMed |
Degrelle, S. A., Campion, E., Cabau, C., Piumi, F., Reinaud, P., Richard, C., Renard, J.-P., and Hue, I. (2005). Molecular evidence for a critical period in mural trophoblast development in bovine blastocysts. Dev. Biol. 288, 448–460.
| Molecular evidence for a critical period in mural trophoblast development in bovine blastocysts.Crossref | GoogleScholarGoogle Scholar | 16289134PubMed |
Ealy, A. D., and Yang, Q. E. (2009). Control of interferon-tau expression during early pregnancy in ruminants. Am. J. Reprod. Immunol. 61, 95–106.
| Control of interferon-tau expression during early pregnancy in ruminants.Crossref | GoogleScholarGoogle Scholar | 19143673PubMed |
Eckert, J., and Niemann, H. (1998). mRNA expression of leukaemia inhibitory factor (LIF) and its receptor subunits glycoprotein 130 and LIF-receptor-beta in bovine embryos derived in vitro or in vivo. Mol. Hum. Reprod. 4, 957–965.
| mRNA expression of leukaemia inhibitory factor (LIF) and its receptor subunits glycoprotein 130 and LIF-receptor-beta in bovine embryos derived in vitro or in vivo.Crossref | GoogleScholarGoogle Scholar | 9809677PubMed |
Fry, R. C., Batt, P. A., Fairclough, R. J., and Parr, R. A. (1992). Human leukemia inhibitory factor improves the viability of cultured ovine embryos. Biol. Reprod. 46, 470–474.
| Human leukemia inhibitory factor improves the viability of cultured ovine embryos.Crossref | GoogleScholarGoogle Scholar | 1617019PubMed |
Fujii, T., Hirayama, H., Naito, A., Kashima, M., Sakai, H., Fukuda, S., Yoshino, H., Moriyasu, S., Kageyama, S., Sugimoto, Y., Matsuyama, S., Hayakawa, H., and Kimura, K. (2017). Production of calves by the transfer of cryopreserved bovine elongating conceptuses and possible application for preimplantation genomic selection. J. Reprod. Dev. 63, 497–504.
| Production of calves by the transfer of cryopreserved bovine elongating conceptuses and possible application for preimplantation genomic selection.Crossref | GoogleScholarGoogle Scholar | 28781338PubMed |
Fukui, Y., and Matsuyama, K. (1994). Development of in vitro matured and fertilized bovine embryos cultured in media containing human leukemia inhibitory factor. Theriogenology 42, 663–673.
| Development of in vitro matured and fertilized bovine embryos cultured in media containing human leukemia inhibitory factor.Crossref | GoogleScholarGoogle Scholar | 16727572PubMed |
Furusawa, T., Ohkoshi, K., Kimura, K., Matsuyama, S., Akagi, S., Kaneda, M., Ikeda, M., Hosoe, M., Kizaki, K., and Tokunaga, T. (2013). Characteristics of bovine inner cell mass-derived cell lines and their fate in chimeric conceptuses. Biol. Reprod. 89, 28.
| Characteristics of bovine inner cell mass-derived cell lines and their fate in chimeric conceptuses.Crossref | GoogleScholarGoogle Scholar | 23782837PubMed |
Hambruch, N., Haeger, J.-D., Dilly, M., and Pfarrer, C. (2010). EGF stimulates proliferation in the bovine placental trophoblast cell line F3 via Ras and MAPK. Placenta 31, 67–74.
| EGF stimulates proliferation in the bovine placental trophoblast cell line F3 via Ras and MAPK.Crossref | GoogleScholarGoogle Scholar | 19914712PubMed |
Han, Y. M., Lee, E. S., Mogoe, T., Lee, K. K., and Fukui, Y. (1995). Effect of human leukemia inhibitory factor on in vitro development of IVF-derived bovine morulae and blastocysts. Theriogenology 44, 507–516.
| Effect of human leukemia inhibitory factor on in vitro development of IVF-derived bovine morulae and blastocysts.Crossref | GoogleScholarGoogle Scholar | 16727749PubMed |
Hosoe, M., and Shioya, Y. (1997). Distribution of cortical granules in bovine oocytes classified by cumulus complex. Zygote 5, 371–376.
| Distribution of cortical granules in bovine oocytes classified by cumulus complex.Crossref | GoogleScholarGoogle Scholar | 9563685PubMed |
Maddox-Hyttel, P., Alexopoulos, N. I., Vajta, G., Lewis, I., Rogers, P., Cann, L., Callesen, H., Tveden-Nyborg, P., and Trounson, A. (2003). Immunohistochemical and ultrastructural characterization of the initial post-hatching development of bovine embryos. Reproduction 125, 607–623.
| Immunohistochemical and ultrastructural characterization of the initial post-hatching development of bovine embryos.Crossref | GoogleScholarGoogle Scholar | 12683931PubMed |
Morgan, G., Wooding, F. B. P., Beckers, J. F., and Friesen, H. G. (1989). An immunological cryo-ultrastructural study of a sequential appearance of proteins in placental binucleate cells in early pregnancy in the cow. J. Reprod. Fertil. 86, 745–752.
| An immunological cryo-ultrastructural study of a sequential appearance of proteins in placental binucleate cells in early pregnancy in the cow.Crossref | GoogleScholarGoogle Scholar | 2760899PubMed |
Nachtigall, M. J., Kliman, H. J., Feinberg, R. F., Olive, D. L., Engin, O., and Arici, A. (1996). The effect of leukemia inhibitory factor (LIF) on trophoblast differentiation: a potential role in human implantation. J. Clin. Endocrinol. Metab. 81, 801–806.
| The effect of leukemia inhibitory factor (LIF) on trophoblast differentiation: a potential role in human implantation.Crossref | GoogleScholarGoogle Scholar | 8636307PubMed |
Nakano, H., Takahashi, T., Imai, K., and Hashizume, K. (2001). Expression of placental lactogen and cytokeratin in bovine placental binucleate cells in culture. Cell Tissue Res. 303, 263–270.
| Expression of placental lactogen and cytokeratin in bovine placental binucleate cells in culture.Crossref | GoogleScholarGoogle Scholar | 11291772PubMed |
Nakano, H., Shimada, A., Imai, K., Takezawa, T., Takahashi, T., and Hashizume, K. (2002). Bovine trophoblastic cell differentiation on collagen substrata: formation of binucleate cells expressing placental lactogen. Cell Tissue Res. 307, 225–235.
| Bovine trophoblastic cell differentiation on collagen substrata: formation of binucleate cells expressing placental lactogen.Crossref | GoogleScholarGoogle Scholar | 11845329PubMed |
Nedambale, T. L., Du, F., Yang, X., and Tian, X. C. (2006). Higher survival rate of vitrified and thawed in vitro produced bovine blastocysts following culture in defined medium supplemented with β-mercaptoethanol. Anim. Reprod. Sci. 93, 61–75.
| Higher survival rate of vitrified and thawed in vitro produced bovine blastocysts following culture in defined medium supplemented with β-mercaptoethanol.Crossref | GoogleScholarGoogle Scholar | 16099115PubMed |
Neira, J. A., Tainturier, D., Peña, M. A., and Martal, J. (2010). Effect of the association of IGF-I, IGF-II, bFGF, TGF-β1, GM-CSF, and LIF on the development of bovine embryos produced in vitro. Theriogenology 73, 595–604.
| Effect of the association of IGF-I, IGF-II, bFGF, TGF-β1, GM-CSF, and LIF on the development of bovine embryos produced in vitro.Crossref | GoogleScholarGoogle Scholar | 20035987PubMed |
Oshima, K., Watanabe, H., Yoshihara, K., Kojima, T., Dochi, O., Takenouchi, N., Fukushima, M., and Komatsu, M. (2003). Gene expression of leukemia inhibitory factor (LIF) and macrophage colony stimulating factor (M-CSF) in bovine endometrium during early pregnancy. Theriogenology 60, 1217–1226.
| Gene expression of leukemia inhibitory factor (LIF) and macrophage colony stimulating factor (M-CSF) in bovine endometrium during early pregnancy.Crossref | GoogleScholarGoogle Scholar | 14511776PubMed |
Reinhart, K. C., Dubey, R. K., Mummery, C. L., van Rooijen, M., Keller, P. J., and Marinella, R. (1998). Synthesis and regulation of leukaemia inhibitory factor in cultured bovine oviduct cells by hormones. Mol. Hum. Reprod. 4, 301–308.
| Synthesis and regulation of leukaemia inhibitory factor in cultured bovine oviduct cells by hormones.Crossref | GoogleScholarGoogle Scholar | 9570277PubMed |
Rizos, D., Lonergan, P., Boland, M. P., Arroyo-García, R., Pintado, B., de la Fuente, J., and Gutiérrez-Adán, A. (2002). Analysis of differential messenger RNA expression between bovine blastocysts produced in different culture systems: implications for blastocyst quality. Biol. Reprod. 66, 589–595.
| Analysis of differential messenger RNA expression between bovine blastocysts produced in different culture systems: implications for blastocyst quality.Crossref | GoogleScholarGoogle Scholar | 11870062PubMed |
Sakurai, T., Sakamoto, A., Muroi, Y., Bai, H., Nagaoka, K., Tamura, K., Takahashi, T., Hashizume, K., Sakatani, M., Takahashi, M., Godkin, J. D., and Imakawa, K. (2009). Induction of endogenous interferon tau gene transcription by CDX2 and high acetylation in bovine nontrophoblast cells. Biol. Reprod. 80, 1223–1231.
| Induction of endogenous interferon tau gene transcription by CDX2 and high acetylation in bovine nontrophoblast cells.Crossref | GoogleScholarGoogle Scholar | 19211809PubMed |
Sakurai, T., Bai, H., Konno, T., Ideta, A., Aoyagi, Y., Godkin, J. D., and Imakawa, K. (2010). Function of a transcription factor CDX2 beyond its trophectoderm lineage specification. Endocrinology 151, 5873–5881.
| Function of a transcription factor CDX2 beyond its trophectoderm lineage specification.Crossref | GoogleScholarGoogle Scholar | 20962045PubMed |
Schiffmacher, A. T., and Keefer, C. L. (2013). CDX2 regulates multiple trophoblast genes in bovine trophectoderm CT-1 cells. Mol. Reprod. Dev. 80, 826–839.
| CDX2 regulates multiple trophoblast genes in bovine trophectoderm CT-1 cells.Crossref | GoogleScholarGoogle Scholar | 23836438PubMed |
Shimada, A., Nakano, H., Takahashi, T., Imai, K., and Hashizume, K. (2001). Isolation and characterization of a bovine blastocyst-derived trophoblastic cell line, BT-1: development of a culture system in the absence of feeder cell. Placenta 22, 652–662.
| Isolation and characterization of a bovine blastocyst-derived trophoblastic cell line, BT-1: development of a culture system in the absence of feeder cell.Crossref | GoogleScholarGoogle Scholar | 11504534PubMed |
Smith, A. G., Heath, J. K., Donaldson, D. D., Wong, G. G., Moreau, J., Stahl, M., and Rogers, D. (1988). Inhibition of pluripotential embryonic stem cell differentiation by purified polypeptides. Nature 336, 688–690.
| Inhibition of pluripotential embryonic stem cell differentiation by purified polypeptides.Crossref | GoogleScholarGoogle Scholar | 3143917PubMed |
Stewart, C. L., Kaspar, P., Brunet, L. J., Bhatt, H., Gadi, I., Köntgen, F., and Abbondanzo, S. J. (1992). Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature 359, 76–79.
| Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor.Crossref | GoogleScholarGoogle Scholar | 1522892PubMed |
Suzuki, Y., Koshi, K., Imai, K., Takahashi, T., Kizaki, K., and Hashizume, K. (2011). Bone morphogenetic protein 4 accelerates the establishment of bovine trophoblastic cell lines. Reproduction 142, 733–743.
| Bone morphogenetic protein 4 accelerates the establishment of bovine trophoblastic cell lines.Crossref | GoogleScholarGoogle Scholar | 21862694PubMed |
Talbot, N. C., Caperna, T. J., Edwards, J. L., Garrett, W., Wells, K. D., and Ealy, A. D. (2000). Bovine blastocyst-derived trophectoderm and endoderm cell cultures: interferon tau and transferrin expression as respective in vitro markers. Biol. Reprod. 62, 235–247.
| Bovine blastocyst-derived trophectoderm and endoderm cell cultures: interferon tau and transferrin expression as respective in vitro markers.Crossref | GoogleScholarGoogle Scholar | 10642558PubMed |
Talbot, N. C., Powell, A. M., Ocón, O. M., Caperna, T. J., Camp, M., Garrett, W. M., and Ealy, A. D. (2008). Comparison of the interferon-tau expression from primary trophectoderm outgrowths derived from IVP, NT, and parthenogenote bovine blastocysts. Mol. Reprod. Dev. 75, 299–308.
| Comparison of the interferon-tau expression from primary trophectoderm outgrowths derived from IVP, NT, and parthenogenote bovine blastocysts.Crossref | GoogleScholarGoogle Scholar | 17721989PubMed |
Ushizawa, K., Takahashi, T., Kaneyama, K., Tokunaga, T., Tsunoda, Y., and Hashizume, K. (2005). Gene expression profiles of bovine trophoblastic cell line (BT-1) analyzed by a custom cDNA microarray. J. Reprod. Dev. 51, 211–220.
| Gene expression profiles of bovine trophoblastic cell line (BT-1) analyzed by a custom cDNA microarray.Crossref | GoogleScholarGoogle Scholar | 15613779PubMed |
Vajta, G., Alexopoulos, N. I., and Callesen, H. (2004). Rapid growth and elongation of bovine blastocysts in vitro in a three-dimensional gel system. Theriogenology 62, 1253–1263.
| Rapid growth and elongation of bovine blastocysts in vitro in a three-dimensional gel system.Crossref | GoogleScholarGoogle Scholar | 15325552PubMed |
Vejlsted, M., Du, Y., Vajta, G., and Maddox-Hyttel, P. (2006). Post-hatching development of the porcine and bovine embryo – defining criteria for expected development in vivo and in vitro. Theriogenology 65, 153–165.
| Post-hatching development of the porcine and bovine embryo – defining criteria for expected development in vivo and in vitro.Crossref | GoogleScholarGoogle Scholar | 16257443PubMed |