Reducing antibiotic prescribing in general practice in Australia: a cluster randomised controlled trial of a multimodal intervention
Minyon L. Avent A B * , Lisa Hall
A B * , Lisa Hall  C , Mieke van Driel D , Annette Dobson C , Laura Deckx D , Mahmoud Galal C , Malene Plejdrup Hansen
C , Mieke van Driel D , Annette Dobson C , Laura Deckx D , Mahmoud Galal C , Malene Plejdrup Hansen  E and Charles Gilks C
E and Charles Gilks C
A
B
C
D
E
Abstract
The health and economic burden of antimicrobial resistance (in Australia is significant. Interventions that help guide and improve appropriate prescribing for acute respiratory tract infections in the community represent an opportunity to slow the spread of resistant bacteria. Clinicians who work in primary care are potentially the most influential health care professionals to address the problem of antimicrobial resistance, because this is where most antibiotics are prescribed.
A cluster randomised trial was conducted comparing two parallel groups of 27 urban general practices in Queensland, Australia: 13 intervention and 14 control practices, with 56 and 54 general practitioners (GPs), respectively. This study evaluated an integrated, multifaceted evidence-based package of interventions implemented over a 6-month period. The evaluation included quantitative and qualitative components, and an economic analysis.
A multimodal package of interventions resulted in a reduction of 3.81 prescriptions per GP per month. This equates to 1280.16 prescriptions for the 56 GPs in the intervention practices over the 6-month period. The cost per prescription avoided was A$148. The qualitative feedback showed that the interventions were well received by the GPs and did not impact on consultation time. Providing GPs with a choice of tools might enhance their uptake and support for antimicrobial stewardship in the community.
A multimodal package of interventions to enhance rational prescribing of antibiotics is effective, feasible and acceptable in general practice. Investment in antimicrobial stewardship strategies in primary care may ultimately provide the important returns for public health into the future.
Keywords: antibiotics, anti-infectives, antimicrobial stewardship, antimicrobials, community, cost effective, general practitioners, prescribing, qualitative feedback.
Introduction
Antimicrobial resistance (AMR) is undeniably an urgent global health threat, with rates of resistance increasing faster than the capacity of industry to develop new drugs. The Global Action Plan on AMR was adopted in 2015 by all countries through decisions endorsed in the World Health Assembly, the Food and Agriculture Organization Governing Conference and the World Organisation for Animal Health. Optimising the use of antimicrobial agents is a key component of this action plan (WHO 2015).
High levels of antibiotic prescribing drive AMR (Bell et al. 2014), and despite a broad range of initiatives within the past 10 years in Australia, reductions have been modest and insufficient to address AMR. Gains have been made in hospital settings, but optimising antibiotic prescribing in the community remains a challenge, with nearly 40% of general practitioners (GPs) admitting to prescribing antibiotics to meet patient expectations, and one in five patients expecting antibiotics for acute respiratory tract infections (ARIs; Hardy-Holbrook et al. 2013). ARIs are predominantly self-limiting and/or caused by viruses, and clinical guidelines generally do not recommend treatment with antibiotics (Therapeutic Guidelines 2019). It is estimated that 40% of the Australian population had at least one antibiotic dispensed in 2019, which is much higher than in most European countries and Canada (Australian Commission on Safety and Quality in Health Care 2021), and the majority of these antibiotics are prescribed by GPs (McKenzie et al. 2013). Inappropriate use of antibiotics is the most important driver of resistance, and primary care has an important part to play in optimising the appropriate use of these agents (van Driel et al. 2022). The Australian National Antimicrobial Resistance Strategy aligns Australia to the World Health Organization’s Global action plan on antimicrobial resistance, and provides the foundation for Australia’s national antimicrobial stewardship (AMS) strategy (van Driel et al. 2022). Improving outpatient antibiotic prescribing at the point of care requires two complementary strategies: (1) changing clinician behaviour to alleviate concerns related to diagnostic uncertainties, and (2) educating patients and families about the role of antibiotics in medical care and their own wellbeing (Tamma and Cosgrove 2016). There are a number of strategies to improve antibiotic prescribing at the point of care. The best evidence supports use of specific educational interventions, point-of-care tests and clinical decision support tools (McDonagh et al. 2018).
Strategies that have employed a combination of these interventions have been shown to be the most effective (Cals et al. 2013; Little et al. 2013; Gulliford et al. 2019). The evaluation of costs of proven effective interventions to reduce antibiotic prescribing are limited (Cals et al. 2011; Coulter et al. 2015; Dekker et al. 2019). Further research is required in real-world settings to determine whether they can be implemented and prove to be effective across a range of contexts (Gerber 2016).
In 2015–2016 we implemented and evaluated an integrated, multimodal package of interventions to reduce antibiotic prescribing for suspected ARIs in general practice through the General Practice Antimicrobial Program for Stewardship (GAPS) trial (Avent et al. 2016). Given the pressures currently experienced by GPs, the continued lack of national action on community-based interventions for antimicrobial stewardship and the changes of the National Prescribing Service in Australia, we have re-examined and reflected on learnings from this work. In this paper, we present key findings on the feasibility, uptake and potential costs of implementing the integrated package of interventions in the GAPS trial.
Methods
Trial design
This was a cluster randomised trial comparing two parallel groups of general practices. The intervention period ran from 1 September 2015 to 29 February 2016. Data on the GPs’ prescribing were available from 1 June 2012, which provided data for a 39-month pre-intervention period, followed by a 6-month intervention period, but no post-intervention observations. The evaluation included both quantitative and qualitative components, as well as an economic analysis.
Participants and settings
Twenty-eight urban general practices in Brisbane and the Gold Coast in Southeast Queensland, Australia, were purposively recruited. All GPs working in these practices were eligible to participate in the trial, provided they consented to the study team obtaining data from Medicare (Australia’s universal health insurance scheme) on their patient encounters and pharmaceutical prescriptions filled. GPs were provided with Continuing Professional Development points for completing the education activities associated with the study. The GP practices were provided with an incentive payment of A$100 per practice.
Interventions
This study evaluated an integrated, multimodal package of interventions shown to be effective at reducing antibiotic use for ARIs in previously published studies (Box 1; Avent et al. 2016).
The package of interventions was implemented in intervention practices following a 1-month engagement period (1−30 June 2015) by research co-ordinators trained in how the interventions should be used. In the study period, the research co-ordinators regularly visited the intervention practices to support the uptake of the interventions and provide any supplementary training. GPs were able to choose from the range of interventions offered (see Box 1) depending on their consultation preferences and the patient’s characteristics – supporting the concept that ‘a one-size does not fit all’. The GPs in the control practices continued their normal clinical practice and were offered the package after completion of the trial.
Available data
GPs provided consent for the release of data from Medicare Benefits Schedule (MBS) and the Pharmaceutical Benefits Scheme (PBS) on the number of patient encounters and prescriptions filled. The MBS data were for all patients seen by the GP when working at the study practice; the PBS data covered all filled prescriptions provided by the GP wherever they were working (i.e. not only at a study practice).
GPs were identified only by identification (ID) numbers. There was no patient identification. Data were available for 39 months before the trial (1 June 2012 to 31 August 2015) and during the 6-month trial period (1 September 2015 to 29 February 2016).
Sample size calculation
The original sample size calculation was based on the antibiotic prescription rate defined as the number of original prescriptions for antibiotic medications coded J01 according to the Anatomical Therapeutic Chemical Classification divided by the number of patient encounters per general practice for the same period. The mean change in antibiotic prescription rates in practices in the intervention group (before – during the intervention) was to be compared with the mean change in practices in the control group over the same period. A difference in the mean change in rates in the range 0.20–0.25 was considered clinically significant and plausible, if the standard deviation in rates was 0.20. With equal numbers of practices in the intervention and control groups, power of 80%, significance level of 5% for a two-tailed test, for a difference of 0.24, 12 practices per group would be needed. As little was known about the numbers of GPs per practice or the extent of clustering within practices, it was planned to recruit 14 practices per group.
Randomisation
A block randomisation list for eight general practices per block was generated using an internet-based system (Sealed Envelope, London, UK). One block used was for practices located on the Gold Coast and other blocks for those in the Brisbane area. The list of recruited practices was prepared by the project manager, MLA. Simple random assignment of practices to the intervention or control groups was performed by the statistician (AJD).
Effectiveness measures – quantitative analysis
The pre-specified primary outcome measure described in the protocol (Avent et al. 2016) was the antibiotic prescription rate, However, there were large discrepancies between the MBS and PBS data, mainly caused by GPs working simultaneously in practices participating in the study and other practices; this occurred both before and during the period of the trial. Thus, the MBS data did not provide a suitable measure of GP activity directly related to this study. Instead, the total number of original prescriptions for any medication by the same GP was used as a measure of activity; this was obtained from the PBS dataset. Therefore, the primary outcome for analysis in this analysis is the number of original prescriptions for J01 medications prescribed by the GP over a specified period, adjusted for the number of original prescriptions by the same GP for any medication for the same period.
For the analysis, only the first or original prescriptions (not repeats) for medications, excluding those supplied as doctor’s bag items, were included.
Statistical methods
Crude mean numbers of antibiotic prescriptions (original J01 prescriptions) per GP per month were compared between GPs in the intervention practices, and the control practices before and during the period of the trial. Adjusted numbers of antibiotic prescriptions per GP per month were estimated using mixed effects negative binomial regression. In this model, the number of original antibiotic prescriptions was the response variable, the total number of original prescriptions was the offset variable, the GPs and practices were treated as random effects (to account for clustering of patients within GPs and GPs within practices), and there were fixed effects defined by indicator variables for the intervention and control groups, the periods before and during the trial, month (to account for seasonal effects), and the interaction between periods and groups. From this model, mean numbers of antibiotic prescriptions per GP per month were estimated, as well as differences between intervention and control groups before and during the intervention period, using marginal predicted means. All analyses were conducted using Stata version 14 (StataCorp LP, College Station, TX, USA).
Uptake and trial feasibility measures – qualitative data collection and analysis
At the end of the intervention period, semi-structured interviews were conducted with the GPs who participated in the intervention arm. A single researcher (LD) conducted the interviews. All interviews were transcribed and analysed using concurrent thematic analysis (Braun and Clarke 2006). The interview guide ensured important dimensions of the interventions were explored, and allowed the participants to discuss and raise further issues or concerns (Kelly 2010). In general, interview questions examined which elements of the intervention were found useful and why, which elements GPs thought had changed practice, and which parts of the intervention could be improved.
Economic methods
Costs were estimated in 2015 Australian dollars (A$) from the perspective of the public health system. Costing worksheets were developed following interviews with study and practice staff. A review of project management and financial records was conducted to collect data on the resources required to implement the integrated package. The time horizon for the valuation was the study period of the trial. Centralised staffing costs were valued based on employee salaries, including superannuation, whereas practice staff time was valued according to professional salary rates, as reported in national surveys (Cheng et al. 2010). Consumable items were valued based on expenditure detailed in project accounting records, or (where items had been provided free of charge) quotes from industry suppliers. The total cost of each antibiotic (government and patient contributions) was obtained from the PBS website on 1 April 2016. The mean cost for all original-prescription antibiotics in the dataset was A$13.55 (standard deviation A$2.61).
We compared the cost of implementation of the trial and the cost savings from reduced prescribing to estimate the total net cost of the intervention. To allow comparison with other prescribing interventions described in the literature, we calculated the cost per practice, the cost per prescription avoided and the total net monetary cost of implementing the integrated package. A number of scenario analyses were used to explore assumptions made in the model: (1) placing no value of practice staff time (akin to considering only study financial expenditure), (2) including cost savings from avoiding repeat prescriptions (based on the observation that 21% of the J01 prescriptions in our dataset were repeats), (3) including cost savings from avoiding antibiotic-related adverse events (comprised of diarrhoea, candidiasis and rash based on a meta-analysis (Gillies et al. 2015), (4) we included the cost savings from avoided cases of antibiotic associated Clostridium difficile (based on epidemiological data (Vardakas et al. 2016). Our final model included the additional cost savings from avoided repeat prescriptions, avoided adverse incidents and avoided cases of C. difficile to give an overall estimate of the efficiency of the study.
Ethics approval and trial registration
Ethical approval was obtained from the University of Queensland (ref: 2015000988). In addition, an administrative review was obtained from Bond University and Queensland University of Technology ethics committees. The Department of Human Services granted approval for consent to be obtained from the GPs to access their MBS and PBS data (ref: MI4140). The GAPS trial is registered under the Australian New Zealand Clinical Trials Register (ANZCTR), reference number: ACTRN12615001128583.
Results
The numbers of participating GPs and practices for each phase of the study, together with observation periods (GP-months) and numbers of original antibiotic prescriptions and total original prescriptions filled are shown in Fig. 1.
Randomisation of GP practices. * Two of the selected practices, both randomised to the intervention group, were subsequently found to be run by the same organisation with the same GPs working at both locations, so they were treated as the same practice.
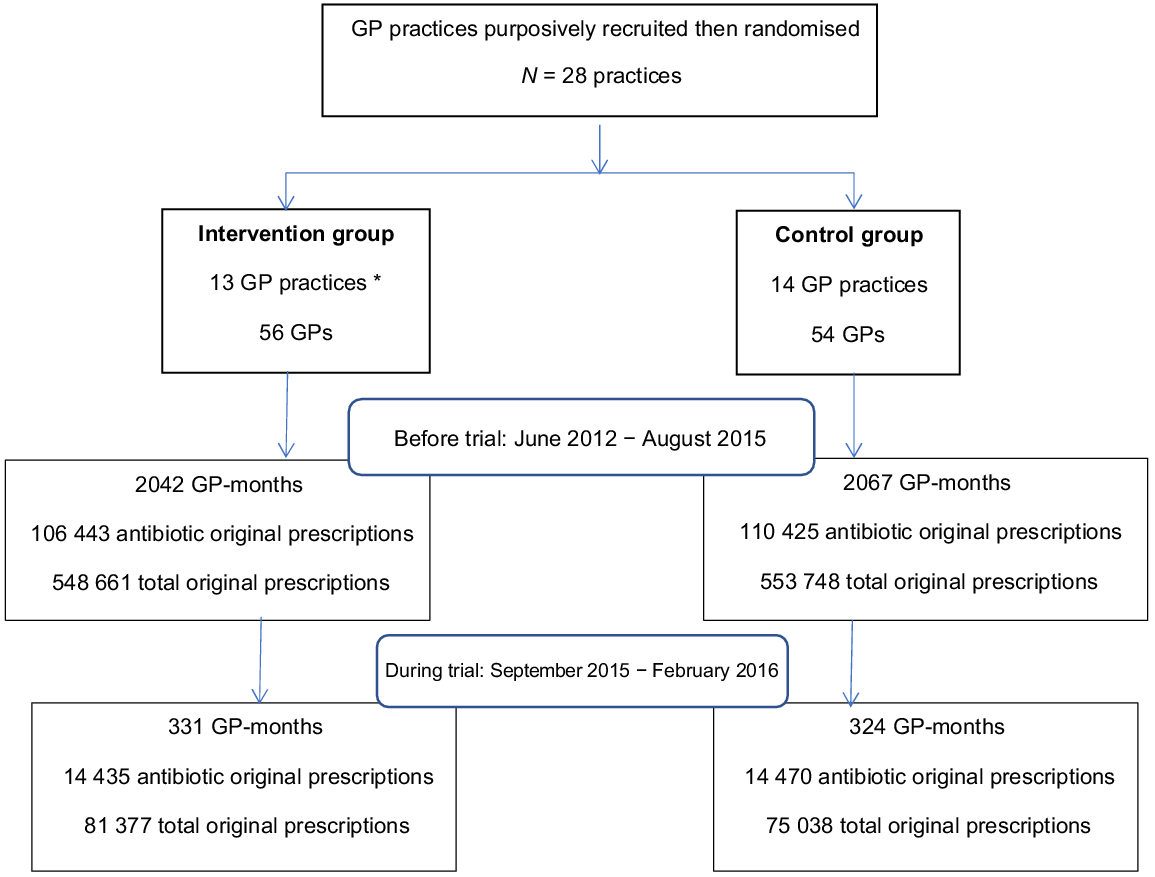
The characteristics of the participating general practices are described in Table 1. In total, 56 (intervention) and 54 (control) GPs took part in the trial, with 46% and 44%, respectively, being women. The GPs in each group had a median of eight sessions per week (either a morning or afternoon session).
| Intervention (n = 13) | Control (n = 14) | ||
|---|---|---|---|
| Practice structure | (%) | (%) | |
| Sole owner | 2 (15) | 8 (57) | |
| Associateship | 2 (15) | 0 (0) | |
| Partnership | 4 (31) | 5 (36) | |
| Corporate owned | 5 (38) | 0 (0) | |
| Other | 0 (0) | 1 (7) | |
| Percentage bulk billingA: median (min, max) | 70 (30, 100) | 78 (26, 100) | |
| Number of participating GPs | 56 | 54 | |
| Practice staff median (min, max) | (FTE)B | (FTE)B | |
| Admin/reception staff | 4.5 (1,8) | 3 (0,8) | |
| Practice manager | 1 (0,2) | 1 (0,1) | |
| Nursing | 1.7 (0, 4) | 1.6 (0, 4.5) | |
| Allied health | 0.65 (0, 8) | 1.25 (0.3,7) | |
| Medical GPs | 5 (2, 10) | 5 (2.5, 9.5) | |
| Patient appointments | |||
| Standard appointment is 10 min | 6 (46) | 4 (31) | |
| Standard appointment is 15 min | 7 (54) | 9 (69) | |
| Patient fee for a standard appointment (AUD) | 70 (62, 75) | 68 (25, 85) |
Quantitative analysis
Table 2 shows the crude and fully adjusted mean numbers of antibiotic prescriptions per GP per month. In the crude analysis, fewer antibiotics were prescribed during the intervention period than before the trial in both groups. This was largely because the intervention period was during the southern hemisphere summer, when the rate of antibiotic prescribing was lower than in winter. When month was included in the model, the season effect was reduced.
| Intervention | Control | Intervention – control | ||
|---|---|---|---|---|
| Crude analysis | ||||
| Antibiotics/GP-months | ||||
| Before | 52.13 (51.81, 52.44) | 53.42 (53.11, 53.74) | −1.30 (−1.74, −0.85) | |
| During | 43.61 (42.90, 44.33) | 44.66 (43.94, 45.39) | −1.05 (−2.07, −0.03) | |
| During – before | −8.52 (−9.29, −7.74) | −8.76 (−9.56, −7.97) | 0.25 (−0.86, 1.36) | |
| Adjusted analysis A | ||||
| Antibiotics/GP-months | ||||
| Before | 55.62 (49.49, 61.76) | 54.79 (48.64, 60.93) | 0.84 (−7.66, 9.34) | |
| During | 52.53 (46.57, 58.49) | 55.49 (49.09, 61.90) | −2.97 (−11.53, 5.59) | |
| During – before | −3.10 (−4.82, −1.37) | 0.71 (−1.05, 2.47) | −3.81 (−6.18, −1.44) | |
In the adjusted model, the mean numbers of antibiotic prescriptions were similar in the intervention and control groups before the intervention (55.62 vs 54.79, difference 0.84, 95% confidence interval (CI) −7.66, 9.34). However, during the intervention period, the mean number of antibiotic prescriptions in the intervention group declined from 55.62 to 52.53 (difference −3.10, 95% CI −4.82, −1.37). In contrast, there was little change in the control group, from 54.79 before the intervention to 55.49 during the intervention period (difference 0.71, 95% CI −1.05, 2.47). This resulted in a small reduction of −3.81 prescriptions per GP per month (95% CI −6.18, −1.44) in the intervention group compared with the control group in the intervention period; compared with the pre-intervention period. This would equate to 1280.16 prescriptions for the 56 GPs in the intervention practices over the 6-month period.
Qualitative analysis
In total, 46 GPs from 11 practices were interviewed. The mean duration of the interview was 11 min (s.d. 3.32). On average, participants had been working as GPs for 14 years (s.d. 11.45), with 44% (20/46) being women. Seventy percent (32/46) of the GPs were trained in Australia, 13% (6/46) in the UK and 17% (8/46) in other countries. The GPs used the interventions that suited their own preferences, their opinions on the specific tools were quite diverse, with each intervention eliciting both positive and negative reactions.
Major findings from the interview are summarised in Table 3 under the following themes:
Themes | Summary statements | Quotes | |
|---|---|---|---|
Perceptions of over-prescribing |
| GP016, male, 20 years of experience: ‘it’s a sort of balance between what the patient is expecting and demanding versus what we think they need, plus we have the issue of running as a business, if the patients are dissatisfied with what they get at the end of a consultation, they are likely to just go to another practice and try again until they get what they want’ GP002, male, 8 years experience: ‘it’s about changing general perceptions and then the rest becomes easier later on.’ GP036, male 32 years experience: ‘certainly amongst the younger population they are more educated about antibiotic usage, whereas the older population they just have expected that over the years if they don’t get one they think there is something wrong, so you know it’s the patient group’ GP023, female, 8 years experience: ‘parents will bring this you know green snotty nose, you know germ-factory in and they’ll say ‘day-cares says they can go back in if they are on antibiotics’ and you look at them and you know it’s clearly like a rhinovirus, it doesn’t need antibiotics it needs to be kept away from the other children’ GP004, female, 22 years of experience: ‘so for the future, we can try and re-educate and re-train the patient’s way of thinking. It takes a long time, it takes more than standard consultation, longer than a standard consultation to educate a patient and try to convince them away from the inappropriate use of antibiotics’ | |
Reception of the interventions |
| GP013, female, 14 years experience: ‘I’ve actually really enjoyed it [being part of the study]. Like I’m so big on the appropriate antibiotic prescribing and it really annoys me how many antibiotics are given out inappropriately’ GP010, female, 7 years experience: ‘I think most patients were pretty positive about it [i.e. the use of the tools] and they found some of the statistics about the number needed to treat and the number needed to harm very helpful, I think it helped them to know that you are not just avoiding antibiotics for you know some global process, but that it actually you know – overall was better for them’ GP013, female, 14 years experience: ‘I didn’t find a big difference between what I am trying to do every day and being part of the study, it was just that I had these little aids to use, which I found useful’ GP025, male, 2 years experience: ‘it seemed that a lot of the focus of the study was on how to not give antibiotics and it seems like not giving antibiotics is like a win but I guess that’s not always the case. The thing I would have appreciated is some discussion about appropriate settings to give antibiotics’ | |
Impact on practice |
| GP030, female, 13 years experience: ‘because I had all this material at my fingers I could persuade them that they didn’t need it [antibiotics]’ GP010, female, 7 years experience: ‘I thought that it was helpful in terms of having a few extra tools to help remind patients that antibiotics aren’t always beneficial and that they don’t always help, that they can do more harm than good, and also just having some of the prompts to help sort of drive the point home was quite helpful’ GP013, female, 14 years experience: ‘I think that patients appreciate the explanation, so I think if you can give them the information and especially if you can show them something visually like the smiley faces and things like that I think it actually does get through, I think they actually appreciate the explanation.’ GP001, female, 8 years experience: ‘I think the strength of the project is that they gave us a suite of tools and you can pick and choose which you liked, so it is not one size fits all … whereas if it would only have been the decision tool then I would have probably zoned out fairly quickly’ |
Economic analysis
Implementation costs per practice are presented in Table 4. The total cost was A$206 508, that is just under A$16 000 ($206 508/13 = $15 885) per practice for the study period. The opportunity cost of practice staff time represented 10% of this total ($19 913/$206 508), with the remainder representing financial expenditure. The largest component of costs was staffing, with the study program manager accounting for nearly 40% of total costs, and the facilitators a further 30%.
| Costs | Total cost (AUD) | Cost/practice (AUD) | |
|---|---|---|---|
| Centralised staff | |||
| Project manager | 81 032 | 6233 | |
| Facilitators | 69 247 | 5327 | |
| Subtotal | 150 279 | 11 560 | |
| Consumables | |||
| Staff travel | 3646 | 280 | |
| Website, laptops and mobile phones | 9850 | 758 | |
| Practice consumables (catering, leaflets, posters etc.) | 1559 | 120 | |
| Software licensing and update | 5000 | 385 | |
| CRP machine (6 month rental includes training and warranty) | 16 259 | 1251 | |
| Subtotal | 36 314 | 2794 | |
| Practice staff time commitment | |||
| Staff time implementation activities | 6778 | 521 | |
| GP time – individual setup | 3417 | 263 | |
| GP time – communication training | 2164 | 166 | |
| Staff time – CRP training and operation | 4963 | 382 | |
| Staff time – ongoing project activities | 2591 | 199 | |
| Subtotal | 19 913 | 1531 | |
| Total costs | 206 506 | 15 831 | |
Total net cost under a variety of scenarios is presented in Table 5. Under baseline assumptions (considering only cost savings from avoided primary prescriptions), the total cost of the study is just under A$210 000, which represents a cost of A$148 per prescription avoided. If only the financial expenditure is included in cost estimates, the net cost drops to A$190 000 (A$132 per prescription avoided).
| Scenario | Cost of study | Prescription cost savings | Other cost savings | Total net cost | Net cost per practice per 6 months | Net cost per prescription avoided | |
|---|---|---|---|---|---|---|---|
| Baseline | 206 508 | 17 346 | – | 189 162 | 14 551 | 148 | |
| Financial expenditure only (no value practice staff time) | 186 595 | 17 346 | – | 169 249 | 13 019 | 132 | |
| Additional benefits | |||||||
| Avoiding repeat prescriptions | 206 508 | 20 989 | – | 185 519 | 14 271 | 145 | |
| Avoided adverse events | 206 508 | 17 346 | 6155 | 183 007 | 14 077 | 143 | |
| Avoided cases Clostridoides difficile | 206 508 | 17 346 | 50 407 | 138 755 | 10 673 | 108 | |
| All avoided events | 206 508 | 20 989 | 56 562 | 128 957 | 9920 | 101 | |
Scenario analyses considered additional benefits that may accrue from reduced prescribing. It was estimated that 269 repeat prescriptions would be averted, and the following antibiotic associated adverse events would be avoided: 127 cases of antibiotic associated diarrhoea, 21 cases of candidiasis, 17 rashes and 16 cases of C. difficile (See Supplementary Appendix 1). These accrue further cost savings of A$77 551, meaning the net cost of implementing the package would drop to approximately A$130 000, representing a cost of approximately A$100 per prescription avoided.
Discussion
The GAPS trial has demonstrated that offering a multimodal package of interventions to GP practices was acceptable and feasible, and reduced antibiotic prescribing by 3.81 prescriptions per GP per month (during the summer season) at a cost of A$148 per prescription avoided. Although the reduction may seem small, it can have important implications for antimicrobial stewardship at a national level and into the future. Interventions that help guide and improve appropriate prescribing for ARIs in the community represent an opportunity to slow the spread of AMR, as well as minimising the associated economic burden (Wozniak et al. 2022). However, implementation and uptake of these strategies has been slow (van Driel et al. 2022) and there is a need for sustained programs (Glasziou et al. 2022).
Our study was conducted prior to the COVID-19 pandemic when a trend of steady decline in antibiotic prescribing in the community was being observed (Australian Commission on Safety and Quality in Health Care 2021). Although promising, the antibiotic prescribing rates in Australia remain high at twice the OECD average and more than twice that of low prescribing countries such as Denmark and The Netherlands. Analysis of data during the first year of the COVID-19 pandemic shows a significant reduction in prescribing for respiratory infections, but this can be attributed to the low incidence of respiratory infections during extended lockdowns (Gillies et al. 2022), and more recent data suggest a rebound when lockdown restrictions were lifted (Imai et al. 2022). Therefore, the learnings from the GAPS trial remain relevant.
A number of studies have evaluated the effectiveness of single interventions and demonstrated a decrease in antibiotic usage (Spurling et al. 2017; McDonagh et al. 2018). The GAPS study was not designed to evaluate the effect of each of the components in this multimodal package of interventions but instead evaluated the feasibility and effectiveness of providing the intervention as a bundle. This is more realistic of a real world setting where practitioners choose to use what best suits them and their patients. In addition, multimodal interventions such as ours have been shown to be most effective in primary care in reducing antibiotic prescribing (Bjerrum et al. 2011; Cals et al. 2013; Gonzales et al. 2013; Little et al. 2013; Dekker et al. 2019; Gulliford et al. 2019).
A strength of our study is that we conducted a comprehensive evaluation, including the experiences of participating GPs and an economic evaluation. Our package proved relatively low-cost to roll out from a public health system perspective. The largest costs were associated with the project staff who facilitated the implementation and education components of the intervention package. In other cost-effectiveness studies of antibiotic interventions targeting GPs (Cals et al. 2013; Dekker et al. 2019) the cost of the project staff was not included in the analysis. It is important to note that uptake of antimicrobial stewardship interventions does not happen spontaneously, rather an active implementation approach over the long term is required and these costs need to be factored into the cost analysis (Grimshaw et al. 2004). The implementation costs maybe important barrier to rolling out such interventions on a large scale. However, the costs of not acting; that is, letting antibiotic prescribing run its course and antimicrobial resistance grow, are far greater (Wozniak et al. 2022).
Overall, the intervention package was well received by the participants. It was considered adaptable to individual practices, and provided GPs with the opportunity to reflect on their management of patients with ARIs. The package was practical and complemented the consultation process and GPs welcomed the interventions, which helped them to reassure and educate the patients about the use of or need for antibiotics. An important advantage of the intervention package was that the ‘one size fits all’ principle was not applied, and that the GP was able to choose from a range of interventions depending on his/her consultation preferences and the patient at hand. Strategies that have utilised multifaceted interventions have shown to be the most effective (Cals et al. 2013; Gonzales et al. 2013; Little et al. 2013; Deckx et al. 2018; Gulliford et al. 2019). Working in the primary care setting is challenging, with many competing financial and time constraints, particularly in a post-COVID-19 world (Kippen et al. 2020). No single intervention strategy is sufficient, and programs should ideally be sustained over the long term. The selection of intervention combinations should be based on known barriers and enablers to changing behaviours (Glasziou et al. 2022). In addition, further research is warranted to determine which interventions would be most effective in reducing antibiotic use and can also be implemented into practice.
Our study had some limitations. Due to contractual arrangements, the study had to be completed in 12 months, starting on 1 July 2015. This meant we were limited to a short intervention period, over summer months when ARIs are less common. This may have limited the uptake and the impact of some of the interventions. Also, GPs are conservative adaptors, and it takes time for them adopt and implement new strategies (Huddy et al. 2016). Nevertheless, we were able to demonstrate a reduction in antibiotic prescribing in the intervention compared with the control practices. Although this study was conducted in urban areas, the interventions are relatively simple and easy to use; therefore, the package would be adaptable on a larger scale in a variety of geographical settings. For this study, the GP practice was not required to contribute to the data collection process, as the analysis was based on the PBS data on and the number of prescriptions filled. This facilitated the recruitment process, as the study was perceived as having minimal impact on the GP practice workflow. It meant, however, that we were not able to obtain individual patient prescribing data or determine the appropriateness of antibiotic therapy.
In conclusion, a multimodal package of interventions to enhance rational prescribing of antibiotics is effective, feasible and acceptable in general practice. Providing GPs with a choice of tools might enhance uptake and support antimicrobial stewardship in the community. The costs of rolling out such a program needs to be offset against the cost of AMR (Wozniak et al. 2022). Investment in primary care antimicrobial stewardship may ultimately provide valuable returns for public health into the future.
Data availability
Restrictions apply to the availability of these data, which were used under ethics approval or the current study, and so data are not publicly available.
Declaration of funding
Funding for the trial has been received from the Department of Health and Aged Care, Australia. This funding source had no role in the design and execution of this study.
Acknowledgements
We thank our co-investigator, the late Professor Chris del Mar, for his valuable contribution to the study. We thank Dr Kate Halton and Dr Chi-Wai Lui for their expertise and assistance with the data analysis. We also express our gratitude to Professor David Paterson for his support. We thank the research facilitators for their enthusiasm and dedication: Mrs Lisa Soo, Mrs Melinda Jesudason, Ms Amanda Murray. Dr Elly Scheermeyer and Ms Nera Komaric.
References
Australian Commission on Safety and Quality in Health Care (2021) AURA: fourth Australian report on antimicrobial use and resistance in human health. Available at www.safetyandquality.gov.au/publications-and-resources/aura-surveillance-system-reports-and-resources [Accessed 17 January 2023]
Avent ML, Hansen MP, Gilks C, Del Mar C, Halton K, Sidjabat H, Hall L, Dobson A, Paterson DL, van Driel ML (2016) General Practitioner Antimicrobial Stewardship Programme Study (GAPS): protocol for a cluster randomised controlled trial. BMC Family Practice 17, 48.
| Crossref | Google Scholar | PubMed |
Bell BG, Schellevis F, Stobberingh E, Goossens H, Pringle M (2014) A systematic review and meta-analysis of the effects of antibiotic consumption on antibiotic resistance. BMC Infectious Diseases 14, 13.
| Crossref | Google Scholar |
Bjerrum L, Munck A, Gahrn-Hansen B, Hansen MP, Jarbol DE, Cordoba G, Llor C, Cots JM, Hernandez S, Lopez-Valcarcel BG, Perez A, Caballero L, von der Heyde W, Radzeviciene R, Jurgutis A, Reutskiy A, Egorova E, Strandberg EL, Ovhed I, Molstad S, Stichele RV, Benko R, Vlahovic-Palcevski V, Lionis C, Ronning M (2011) Health Alliance for prudent antibiotic prescribing in patients with respiratory tract infections (HAPPY AUDIT) -impact of a non-randomised multifaceted intervention programme. BMC Family Practice 12, 52.
| Crossref | Google Scholar | PubMed |
Braun V, Clarke V (2006) Using thematic analysis in psychology. Qualitative Research in Psychology 3, 77-101.
| Crossref | Google Scholar |
Cals JWL, Ament AJHA, Hood K, Butler CC, Hopstaken RM, Wassink GF, Dinant G-J (2011) C-reactive protein point of care testing and physician communication skills training for lower respiratory tract infections in general practice: economic evaluation of a cluster randomized trial. Journal of Evaluation in Clinical Practice 17, 1059-1069.
| Crossref | Google Scholar | PubMed |
Cals JWL, de Bock L, Beckers P-JHW, Francis NA, Hopstaken RM, Hood K, de Bont EGPM, Butler CC, Dinant G-J (2013) Enhanced communication skills and C-reactive protein point-of-care testing for respiratory tract infection: 3.5-year follow-up of a cluster randomized trial. The Annals of Family Medicine 11, 157-164.
| Crossref | Google Scholar | PubMed |
Cheng TC, Scott A, Jeon S-H, Kalb G, Humphreys J, Joyce C (2010) What factors influence the earnings of GPs and medical specialists in Australia? Evidence from the MABEL Survey. Melbourne Institute, Melbourne (AU). 29 p. Cat. No.: 12/10. Available at https://www.melbourneinstitute.com/downloads/working_paper_series/wp2010n12.pdf [Cited 26 March 2016]
Coulter S, Merollini K, Roberts JA, Graves N, Halton K (2015) The need for cost-effectiveness analyses of antimicrobial stewardship programmes: a structured review. International Journal of Antimicrobial Agents 46, 140-149.
| Crossref | Google Scholar | PubMed |
Deckx L, Anthierens S, Magin PJ, Morgan S, McArthur L, Yardley L, Dallas A, Little P, van Driel ML (2018) Focus on early-career GPs: qualitative evaluation of a multi-faceted educational intervention to improve antibiotic prescribing. Family Practices 35, 99-104.
| Crossref | Google Scholar | PubMed |
Dekker ARJ, van der Velden AW, Luijken J, Verheij TJM, van Giessen A (2019) Cost-effectiveness analysis of a GP- and parent-directed intervention to reduce antibiotic prescribing for children with respiratory tract infections in primary care. Journal of Antimicrobial Chemotherapy 74, 1137-1142.
| Crossref | Google Scholar | PubMed |
Gerber JS (2016) Improving outpatient antibiotic prescribing: another nudge in the right direction. JAMA 315, 558-559.
| Crossref | Google Scholar | PubMed |
Gillies M, Ranakusuma A, Hoffmann T, Thorning S, McGuire T, Glasziou P, Del Mar C (2015) Common harms from amoxicillin: a systematic review and meta-analysis of randomized placebo-controlled trials for any indication. Canadian Medical Association Journal 187, E21-E31.
| Crossref | Google Scholar | PubMed |
Gillies MB, Burgner DP, Ivancic L, Nassar N, Miller JE, Sullivan SG, Todd IMF, Pearson S-A, Schaffer AL, Zoega H (2022) Changes in antibiotic prescribing following COVID-19 restrictions: lessons for post-pandemic antibiotic stewardship. British Journal of Clinical Pharmacology 88, 1143-1151.
| Crossref | Google Scholar |
Glasziou P, Dartnell J, Biezen R, Morgan M, Manski-Nankervis J-A (2022) Antibiotic stewardship: a review of successful, evidence-based primary care strategies. Australian Journal of General Practice 51, 15-20.
| Crossref | Google Scholar | PubMed |
Gonzales R, Anderer T, McCulloch CE, Maselli JH, Bloom FJ, Jr, Graf TR, Stahl M, Yefko M, Molecavage J, Metlay JP (2013) A cluster randomized trial of decision support strategies for reducing antibiotic use in acute bronchitis. JAMA Internal Medicine 173, 267-273.
| Crossref | Google Scholar | PubMed |
Grimshaw J, Thomas R, MacLennan G, Fraser C, Ramsay C, Vale L, Whitty P, Eccles M, Matowe L, Shirran L, Wensing M, Dijkstra R, Donaldson C (2004) Effectiveness and efficiency of guideline dissemination and implementation strategies. Health Technology Assessment 8, iii-iv 1–72.
| Crossref | Google Scholar |
Gulliford MC, Prevost AT, Charlton J, Juszczyk D, Soames J, McDermott L, Sultana K, Wright M, Fox R, Hay AD, Little P, Moore MV, Yardley L, Ashworth M (2019) Effectiveness and safety of electronically delivered prescribing feedback and decision support on antibiotic use for respiratory illness in primary care: REDUCE cluster randomised trial. BMJ 364, l236.
| Crossref | Google Scholar | PubMed |
Hardy-Holbrook R, Aristidi S, Chandnani V, DeWindt D, Dinh K (2013) Antibiotic resistance and prescribing in Australia: current attitudes and practice of GPs. Healthcare Infection 18, 147-151.
| Crossref | Google Scholar |
Huddy JR, Ni MZ, Barlow J, Majeed A, Hanna GB (2016) Point-of-care C reactive protein for the diagnosis of lower respiratory tract infection in NHS primary care: a qualitative study of barriers and facilitators to adoption. BMJ Open 6, e009959.
| Crossref | Google Scholar | PubMed |
Imai C, Amin J, Prgomet M, Pearce C, Georgiou A (2022) An increase in antibiotic prescribing for respiratory tract infections through telehealth consultations: retrospective study in Australian general practice. Journal of Medical Internet Research 24, e40876.
| Crossref | Google Scholar | PubMed |
Kippen R, O’Sullivan B, Hickson H, Leach M, Wallace G (2020) A national survey of COVID-19 challenges, responses and effects in Australian general practice. Australian Journal for General Practitioners 49, 745-751.
| Crossref | Google Scholar |
Little P, Stuart B, Francis N, Douglas E, Tonkin-Crine S, Anthierens S, Cals JWL, Melbye H, Santer M, Moore M, Coenen S, Butler C, Hood K, Kelly M, Godycki-Cwirko M, Mierzecki A, Torres A, Llor C, Davies M, Mullee M, O’Reilly G, van der Velden A, Geraghty AWA, Goossens H, Verheij T, Yardley L, Grace consortium (2013) Effects of internet-based training on antibiotic prescribing rates for acute respiratory-tract infections: a multinational, cluster, randomised, factorial, controlled trial. The Lancet 382, 1175-1182.
| Crossref | Google Scholar | PubMed |
McDonagh MS, Peterson K, Winthrop K, Cantor A, Lazur BH, Buckley DI (2018) Interventions to reduce inappropriate prescribing of antibiotics for acute respiratory tract infections: summary and update of a systematic review. Journal of International Medical Research 46, 3337-3357.
| Crossref | Google Scholar | PubMed |
McKenzie D, Rawlins M, Del Mar C (2013) Antimicrobial stewardship: what’s it all about? Australian Prescriber 36, 116-120.
| Crossref | Google Scholar |
Meeker D, Knight TK, Friedberg MW, Linder JA, Goldstein NJ, Fox CR, Rothfeld A, Diaz G, Doctor JN (2014) Nudging guideline-concordant antibiotic prescribing: a randomized clinical trial. JAMA Internal Medicine 174, 425-431.
| Crossref | Google Scholar | PubMed |
NPS MedicineWise (2016) Respiratory tract infections: manage your symptoms action plan. NPS MedicineWise. Available at https://www.nps.org.au/assets/fc2723c4a3fcdec8-31e4936adc6c-NPS-MedicineWise-RTI-Action-Plan-1.pdf [Accessed 17 January 2023]
Spurling GKP, Del Mar CB, Dooley L, Foxlee R, Farley R (2013) Delayed antibiotics for respiratory infections. Cochrane Database of Systematic Reviews 4, CD004417.
| Crossref | Google Scholar |
Spurling GK, Del Mar CB, Dooley L, Foxlee R, Farley R (2017) Delayed antibiotic prescriptions for respiratory infections. Cochrane Database Systematic Reviews 9, CD004417.
| Crossref | Google Scholar |
Tamma PD, Cosgrove SE (2016) Addressing the appropriateness of outpatient antibiotic prescribing in the United States: an important first step. JAMA 315, 1839-1841.
| Crossref | Google Scholar | PubMed |
Therapeutic Guidelines (2019) ‘Therapeutic guidelines: antibiotic.’ (Therapeutic Guidelines) Available at http://online.tg.org.au [Accessed 17 January 2023]
van Driel ML, Morgan S, Tapley A, McArthur L, McElduff P, Yardley L, Dallas A, Deckx L, Mulquiney K, Davis JS, Davey A, Henderson K, Little P, Magin PJ (2016) Changing the Antibiotic Prescribing of general practice registrars: the ChAP study protocol for a prospective controlled study of a multimodal educational intervention. BMC Family Practices 17, 67.
| Crossref | Google Scholar | PubMed |
van Driel ML, Merlo G, Baillie E, Dartnell J, Hall L, Heal C (2022) Preserving antibiotics for the future: where Australian general practice sits on the global spectrum. Australian Journal of General Practice 51, 10-13.
| Crossref | Google Scholar | PubMed |
Vardakas KZ, Trigkidis KK, Boukouvala E, Falagas ME (2016) Clostridium difficile infection following systemic antibiotic administration in randomised controlled trials: a systematic review and meta-analysis. International Journal of Antimicrobial Agents 48, 1-10.
| Crossref | Google Scholar |
WHO (2015) Global action plan on antimicrobial resistance. World Health Organization. Available at https://www.who.int/publications/i/item/9789241509763 [Accessed 17 January 2023]
Wozniak TM, Dyda A, Merlo G, Hall L (2022) Disease burden, associated mortality and economic impact of antimicrobial resistant infections in Australia. The Lancet Regional Health – Western Pacific 27, 100521.
| Crossref | Google Scholar | PubMed |


