Phytophthora cinnamomi: extent and impact in Two Peoples Bay Nature Reserve, Western Australia (1983–2024)
Ray P. Hart A , G. Freebury B and S. Barrett B *
B *
A
B
Abstract
Phytophthora cinnamomi has been present in Two Peoples Bay Nature Reserve in Western Australia for decades, with impacts observed in susceptible plant communities.
This study aimed to examine the past and present impact of P. cinnamomi on ecosystems.
The distribution of P. cinnamomi was mapped 1983–1995 and reassessed in 2024. The long-term spread of the pathogen was measured. Plant densities were assessed, dry-weight biomass measured, and visits by avifauna quantified in infested and healthy vegetation. The persistence of chlamydospores was assessed.
Mapping confirmed the widespread distribution of P. cinnamomi in the Reserve, with some areas mapped as disease-free up to 1995 now infested. Phytophthora dieback has spread in Banksia shrubland at an average of 1.5 m/year over a 33-year period. The density of species from the Proteaceae, Fabaceae, Ericaceae, and Hibbertia was significantly reduced; and the biomass of non-sedge species and visits by avifauna lower in infested compared with healthy Banksia shrubland. However, pockets of healthy habitat persist in infested areas and two highly susceptible Ericaceae (Leucopogon glabellus, Styphelia flavescens) were observed in high numbers in infested vegetation. Recovery of chlamydospores confirmed the persistence of the pathogen behind the dieback front.
The impact of P. cinnamomi is long term and irreversible, however, some susceptible species may have mechanisms to ensure their persistence through prolific seed production.
Protection of areas of healthy susceptible habitat from the introduction and spread of P. cinnamomi, the implementation of existing control measures and research into alternative measures continue to be a priority.
Keywords: Banksia, plant disease, Phytophthora cinnamomi, Phytophthora cinnamomi impact, Phytophthora cinnamomi spread, Phytophthora cinnamomi survey, Proteaceae, Two Peoples Bay.
Introduction
Examination of plant decline at Two Peoples Bay Nature Reserve on the Western Australian south coast in 1981 led to the discovery of the soil-borne oomycete Phytophthora cinnamomi Rands (Hart 1983). At that time, the root pathogen P. cinnamomi was regarded as primarily a disease of jarrah (Eucalyptus marginata) forest, although Newhook and Podger (1972) had warned of the threats to a broad range of plant communities. P. cinnamomi is now considered to be one of the world’s top 100 worst invasive alien species and in ecosystems with Mediterranean-type climates the impact has been greatest (Burgess et al. 2017). Disease caused by P. cinnamomi is listed as a key threatening process under the Environment Protection and Biodiversity Conservation Act 1999 (Cth) (EPBC Act) and is regarded as a threat or potential threat to 120 plant species, 32 ecological communities, and 14 fauna species listed under the EPBC Act (Commonwealth of Australia 2018).
Since its initial detection, dieback surveys (Hart 1983; Brittain 1989; Grant, unpublished) revealed that P. cinnamomi was widespread at Two Peoples Bay Nature Reserve. Symptoms and aetiology are similar to those reported elsewhere in south-western Australia, e.g. the jarrah forest and Banksia woodland and heath (Shearer and Tippett 1989; Shearer et al. 2004, 2007). The pattern of infestation and impacts on the vegetation at Two Peoples Bay are typical of areas along the south coast from Augusta to Cape Arid, where soils, rainfall, and topography provide ideal conditions for the persistence of the pathogen (Brandis and Batini 1985; Grant and Barrett 2003; Bishop et al. 2010; Barrett and Yates 2015; Barrett and Rathbone 2018). Human activity has likely caused the most significant rapid and large-scale spread of P. cinnamomi, primarily through the transport of infected soil and roots (Podger 1972; Shearer et al. 2007; Cahill et al. 2008). Once established, the pathogen can spread further by autonomous spread through root-to-root contact, which is relatively slow; and more rapidly by surface and subsurface water flow (Cahill et al. 2008). Dissemination of spores in moving water is rapid and can extend for hundreds of metres in a single episode.
Hart (1983) documented the change in structure and species composition in susceptible plant communities as a result of P. cinnamomi infestation at Two Peoples Bay. The long history of infestation in the Reserve provides an opportunity to review its past and present impact on the flora and vegetation, to examine whether any recovery of the flora and vegetation has occurred, and to re-evaluate the long-term outcomes for conservation values. Specifically, the aims of this paper were to document the extent of P. cinnamomi in Two Peoples Bay Nature Reserve, the rate of spread of P. cinnamomi and its the long-term persistence of pathogen, and the impact of the pathogen on flora, plant biomass, and avifauna.
It is hypothesised that:
Methods
Distribution of Phytophthora dieback in Two Peoples Bay Nature Reserve
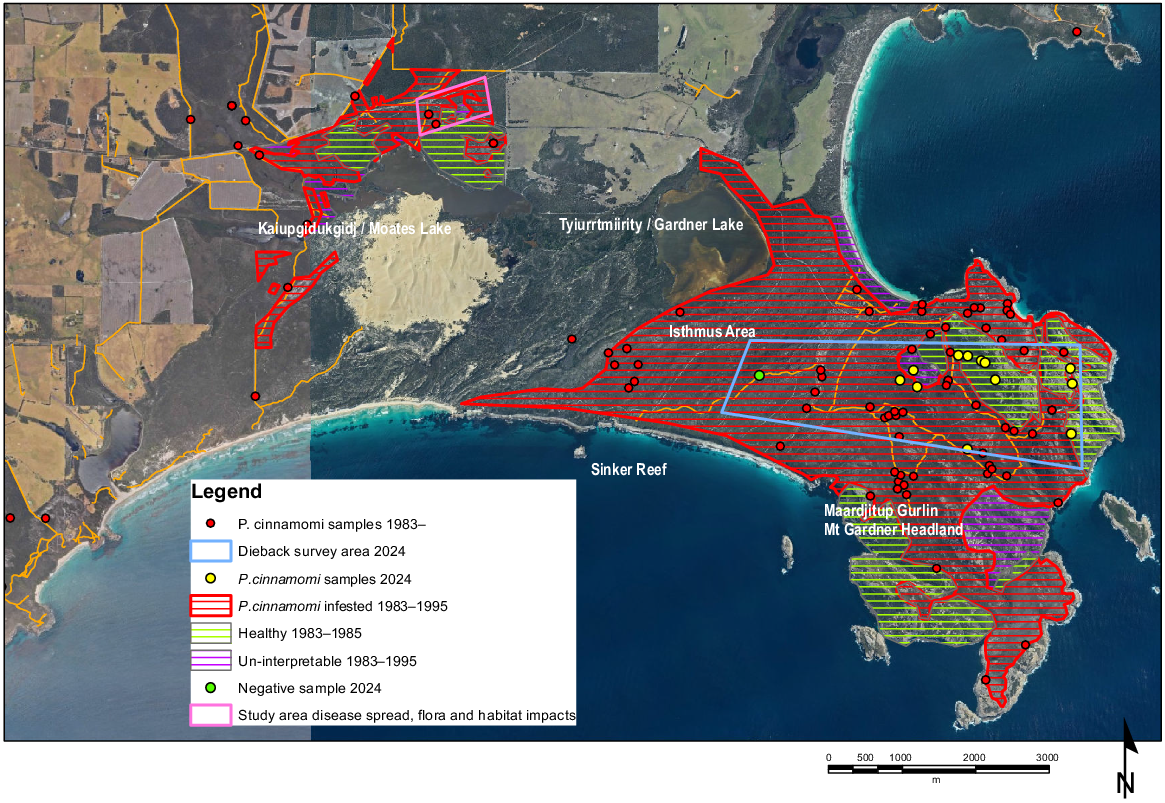
Phytophthora dieback infestations in Two Peoples Bay Nature Reserve were mapped by Hart (1983), 1987–1989 (Brittain 1989) and in 1994–1995 (M. Grant, unpubl. data) by the interpretation of patterns of plant death and by the isolation of the pathogen from roots of dead and dying susceptible species (Department of Parks and Wildlife 2015). A large area south and west of Kaiupgidukgidj (Nooongar name for Moates Lake Knapp et al. 2024)/Moates Lake (Fig. 1) was not assessed as much of this area is a sand dune. In January 2024, areas north of Maardjitup Gurlin/Mt Gardner mapped as dieback-free or non-interpretable and areas mapped as infested in the Isthmus were re-assessed (Fig. 1).
Rate of spread of Phytophthora cinnamomi
The autonomous spread of disease through the soil at Two Peoples Bay Nature Reserve was measured (R. Hart, unpubl. data) by: examining aerial photos and measuring the expansion of infested areas over time; measuring the width of the band of dead vegetation behind an advancing front of the disease, and comparing this with time since the last fire; estimating the time required to produce the successive levels of degradation seen in Banksia trees after infection; and measuring the actual rate of spread of the edges of infested areas over 3 years between January 1983 and January 1986. In an area north of Kaiupgidukgidj/Moates Lake where dieback fronts in a 50-ha area had been delineated (Hart 1983), five isolated occurrences were used to estimate the date of the original infestations, with the origin of the infestation assumed to be in the centre (Fig. 1).
In January 2024, a dieback front north of Kaiupgidukgidj/Moates Lake and within Hart’s study area, which had been delineated by star pickets in December 1990, was reassessed to determine the total spread of Phytophthora dieback over this 33-year period by measuring the distance from the pickets to the edge of the active front.
Persistence of chlamydospores
The persistence of chlamydospores was examined by sampling the soil of infested areas and roots of dying susceptible plants well behind the active front. Soil samples were taken up to 6 m behind the dieback front.
Impact of Phytophthora cinnamomi on flora
In the same 1983 study, an apparently homogeneous area to the north of Kaiupgidukgidj/Moates Lake was selected for a detailed study of disease impact in Banksia shrubland and heath communities. Individual plants were counted in two blocks of 50, adjacent 1 m2 quadrats on either side of an infection front. These blocks were only 12 m apart and, before infection with P. cinnamomi, were assumed to have had similar vegetation (cf., 1946 aerial photography).
Impact of Phytophthora cinnamomi on biomass
Some species that propagate vegetatively, such as in the Cyperaceae and Restionaceae, could not be counted as individual plants and so were measured by total biomass. All live plants were harvested at ground level and divided into rushes and sedges (Restionaceae, Cyperaceae) and all other species. The significance of the differences in plant densities in healthy and infested quadrats was estimated by a chi-squared test. The same study area was re-surveyed in 2024 and observations made on the abundance of the target species assessed in 1983 (Fig. 1).
Impact of Phytophthora cinnamomi on avifauna
The impact of Phytophthora dieback infestation on the avifauna was assessed by observing the use of adjacent (infected and healthy) areas by small bird species that feed in Banksia shrubland vegetation. Four 1-h observations were made in each category, starting just after sunrise in each case.
Results
Distribution of Phytophthora dieback in Two Peoples Bay Nature Reserve
Phytophthora dieback incidence from 1983 to 1995 shows that infestations were already widespread throughout the Reserve in this period (Fig. 1). All major drainage lines were infested and infestations had spread out into the adjacent vegetation. Infestations were also associated with road building, firebreak formation, or other activities involving the movement of soil. Brittain (1989) investigated dieback in 13 different landforms within the Reserve and his survey results indicated that P. cinnamomi had generally reached its peak of activity many years prior in the dunes, interdunal flats, and the granite hills of the Maardjitup Gurlin/Mt Gardner Headland. In areas of old infestation, where susceptible species had been long eradicated by the disease, the changed vegetation appeared healthy with no recent dead or dying plants. Some areas of the Maardjitup Gurlin/Mt Gardner Headland were deemed uninterpretable because of the diffuse impact of infection and the paucity of susceptible indicator species. However, the pathogen was thought to be well established and distributed all over the Headland. The most active infections were in the Dempster Sand units (sand over laterite) (Churchward et al. 1988) north of Kaiupgidukgidj/Moates Lake where the vegetation was undergoing substantial change. In these Banksia communities, the edge of the infestation could be identified as a line of dead and dying plants. There was variable impact and expression in low-lying wetland vegetation, with some communities having few susceptible species e.g. Taxandria juniperina low forest, whereas others had a prominent susceptible component e.g. Banksia quercifolia and B. littoralis. The dune communities on the coastal fringe west of Sinker Reef were not mapped as there were few susceptible species present and these communities were not considered vulnerable. Large areas of Agonis flexuosa heath in the Isthmus area between Tyiurrtmiirity/Gardner Lake and Maardjitup Gurlin/Mt Gardner were mapped as infested.
Sampling of roots of dying plants confirmed the association between the presence of P. cinnamomi and changes to the vegetation (Western Australian Department of Biodiversity, Conservation and Attractions Vegetation Health Services Phytophthora database accessed January 2024) (Fig. 1). Where more intensive sampling was undertaken north of Kaiupgidukgidj/Moates Lake (Hart 1983), P. cinnamomi was not isolated from any of 30 samples with no disease impact; but was isolated from 34 (85%) of 40 root stock samples taken in an area with extensive plant death.
In 2024, the presence of P. cinnamomi was confirmed by sampling in several areas north of Maardjitup Gurlin/Mt Gardner, mapped as disease-free or uninterpretable in surveys to 1995 (Fig. 1). However, pockets of healthy Banksia shrubland and heath persist in these areas as well as on the northeast slope of Maardjitup Gurlin/Mt Gardner (previously mapped as infested) (Fig. 2).
Rate of spread of Phytophthora cinnamomi
Estimates of the rate of spread of P. cinnamomi ranged from 1 to 1.5 m/year: 1 m/year (aerial photography); 0.6 m/year (width of band of dead vegetation behind disease front); 1.5 m/year (time to degradation of Banksia trees after infection), and 1.3 m/year (measured spread of the edges of infested areas). The estimates of rate of spread in five discrete infestations produced disease initiation dates of between 1939 and 1959. The dieback front north of Kaiupgidukgidj/Moates Lake, pegged in 1990 and re-assessed in 2024, was determined to have spread 50.6 m up a gentle incline over the 33-year period, an average of 1.53 m/year.
Persistence of chlamydospores
The overall rate of recovery of positive samples from soil testing was much lower than from roots (12–73%). Chlamydospores were recovered up to 4 m behind the dieback front.
Impact of Phytophthora cinnamomi on flora
Many species were severely reduced in abundance in the infested area, in particular those from the families Proteaceae, Fabaceae, and Ericaceae, and the genus Hibbertia (Table 1). With the exception of B. nutans, differences in Banksia abundance between quadrats were not statistically significant. All Banksia individuals recorded in the infested quadrats were seedlings that had germinated after the initial wave of infection. There were consistently adverse impacts on the Fabaceae and Ericaeae, but the impact on the family Myrtaceae was mixed.
| Species | Healthy | Infested | Chi-squared | Significance | |
|---|---|---|---|---|---|
| Asparagaceae | |||||
| Thysanotus glaucifolius | 0 | 2 | |||
| Dasypogonaceae | |||||
| Dasypogon bromeliifolius | 20 | 12 | 2.0 | n.s. | |
| Hemerocallidaceae | |||||
| Johnsonia lupulina | 9 | 17 | 2.4 | n.s. | |
| Apiaceae | |||||
| Actinotus glomeratus | 31 | 78 | 20.3 | *** | |
| Platysace compressa | 6 | 18 | 6.0 | * | |
| Xanthosia rotundifolia | 13 | 22 | 2.3 | n.s. | |
| All Apiaceae | 50 | 118 | 27.5 | *** | |
| Casuarinaceae | |||||
| Allocasuarina fraseriana | 4 | 5 | |||
| Dilleniaceae | |||||
| Hibbertia pulchra var acutibractea | 226 | 1 | 223 | *** | |
| Ericaceae | |||||
| Andersonia caerulea | 302 | 25 | 235 | *** | |
| Andersonia simplex | 254 | 6 | 237 | *** | |
| Leucopogon elegans | 3239 | 16 | 3191 | *** | |
| Styphelia flavescens | 560 | 19 | 505 | *** | |
| Leucopogon glabellus | 456 | 30 | 373 | *** | |
| Lysinema ciliatum | 601 | 30 | 517 | *** | |
| All Ericaceae | 5412 | 126 | 5045 | *** | |
| Fabaceae | |||||
| Daviesia incrassata | 6 | 0 | |||
| Daviesia flexuosa | 2 | 0 | |||
| Jacksonia spinosa | 3 | 0 | |||
| Phyllota barbata | 109 | 0 | 109 | *** | |
| Latrobea brunonis | 117 | 1 | 114 | *** | |
| All Fabaceae | 237 | 1 | 234 | *** | |
| Goodeniaceae | |||||
| Dampiera pedunculata | 1 | 15 | 12.2 | *** | |
| Myrtaceae | |||||
| Agonis theiformis | 0 | 7 | |||
| Taxandria linearifolia | 12 | 3 | 5.4 | * | |
| Beaufortia anisandra | 111 | 31 | 45.1 | *** | |
| Calytrix asperula | 9 | 29 | 10.5 | ** | |
| Darwinia vestita | 5 | 7 | 0.3 | n.s. | |
| Melaleuca thymoides | 50 | 35 | 265 | n.s. | |
| Proteaceae | |||||
| Adenanthos cuneatus | 24 | 7 | 9.3 | ** | |
| Adenanthos obovatus | 10 | 3 | 3.7 | n.s. | |
| Banksia attenuata | 6 | 1 | |||
| Banksia coccinea | 8 | 1 | |||
| Banksia grandis | 1 | 0 | |||
| Banksia nutans | 31 | 1 | 28.1 | *** | |
| Petrophile acicularis | 13 | 0 | 13.0 | *** | |
| Petrophile rigida | 12 | 7 | 1.32 | n.s. | |
| All Proteaceae | 105 | 20 | 57.8 | *** | |
| Stylidiaceae | |||||
| Stylidium scandens | 68 | 219 | 79.4 | *** | |
| Stylidium spathulatum | 78 | 44 | 9.5 | *** | |
| Restionaceae sp. | 17 | 0 | 17.0 | *** | |
| Schoenus sp. | 37 | 0 | 37.0 | *** | |
| Total of all species | 6451 | 692 (10.7%) | |||
All values of chi-squared have one degree of freedom.
*P < 0.05; **P < 0.01; ***P < 0.001.
For the species counted within the 50 m2 study area, healthy quadrats recorded 6451 plants compared with 692 in infested quadrats (Table 1). This represents a loss of almost 90% of individual plants of the species assessed. Conversely, some species were unaffected or even increased in abundance (e.g. Calytrix asperula, Actinotus glomeratus).
Observations in 2024 within the long-infested vegetation originally studied in 1983, confirmed the absence and significant and ongoing decline of prominent susceptible genera including Banksia, Conospermum, Petrophile, Leucopogon, Daviesia, Phyllota, Latrobea, and Jacksonia (Fig. 3). Bushfires in 2012 and 2015 have now removed all evidence of dead Banksia cones and mature stags in many areas. However, two Ericaceous species, Leucopogon glabellus and Styphelia flavescens, were relatively abundant in long-infested areas despite continuing to demonstrate high mortality along active dieback fronts. Similarly, Adenanthos cuneatus and A. obovatus persisted as scattered individuals in infested areas.
Recent deaths of Phytophthora-susceptible species in study area north of Kaiupgidukgidj/Moates Lake, January 2024 (Photo S. Barrett).

The impact of infestation by P. cinnamomi on other plant communities at Two Peoples Bay Nature Reserve was not measured in detail. However, the results of 1983 and 1987–1989 surveys indicated that the impact of infestation was low on coastal dunes; variable on wetland and drainage line vegetation; severe on Banksia woodland communities; moderate on jarrah forests; and high on the scrubs and heaths of the Maardjitup Gurlin/Mt Gardner Headland.
Impact of Phytophthora cinnamomi on biomass
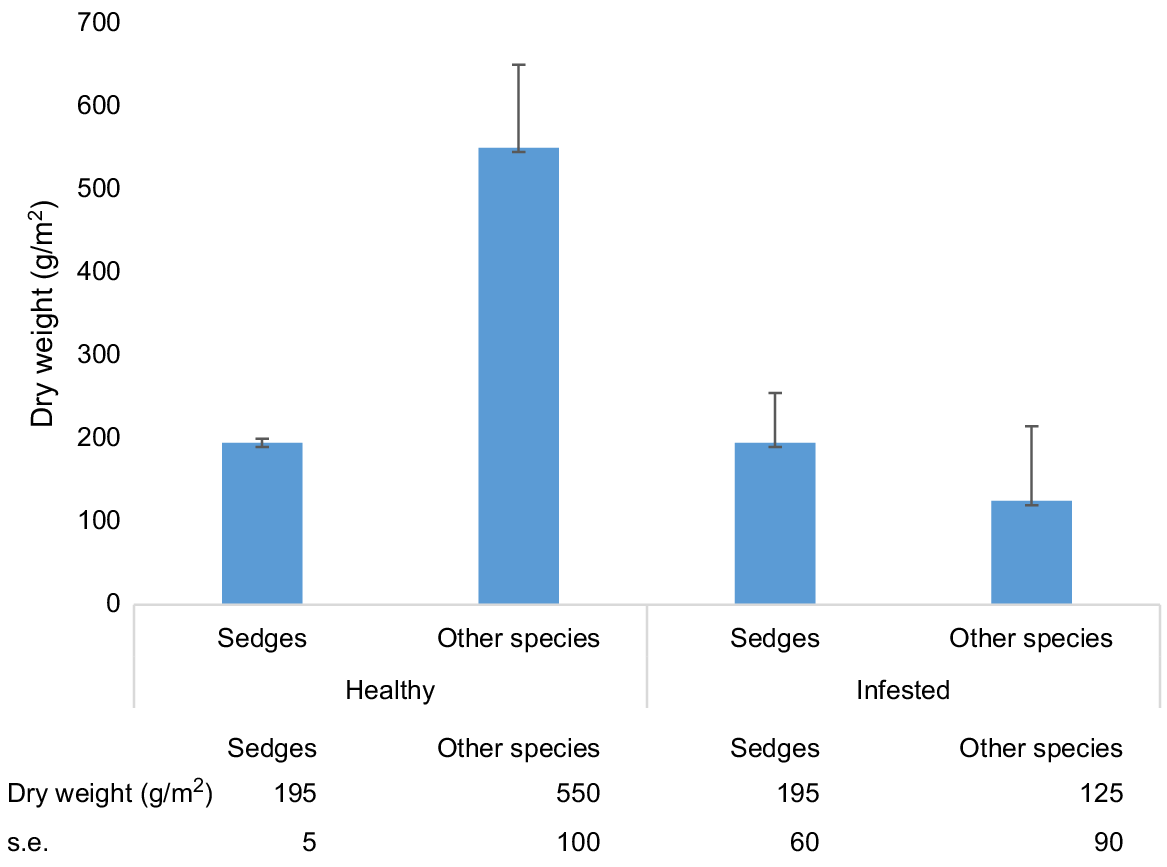
The results for standing biomass show that, with the exception of the rushes and sedges, there was a dramatic decline in biomass in the infested quadrats (Fig. 4). Biomass of species other than sedges and rushes were reduced from 556 g/m2 dry weight to 119 g/m2 by infestation, whereas the former were unchanged at approximately 200 g/m2 in healthy and non-infested quadrats. All sedge and rush species studied appeared to be resistant to P. cinnamomi. The species present included Anarthria gracilis, A. prolifera, A. scabra, Hypolaena exsulca, Lyginia barbata, and unidentified species of Restionaceae and Schoenus.
Discussion
Distribution of Phytophthora dieback
Clearly, Phytophthora dieback caused by P. cinnamomi has been present in Two Peoples Bay Nature Reserve since at least 1959, but the original infestation was probably initiated well before this date. Although the original source of the infection was not known, it was evident that as well as autonomous spread, surface or subsurface water movement had facilitated dispersal, and that the pathogen may have been introduced on more than one occasion (Hart 1983).
The earliest aerial photographs available (1946) showed no clear dieback infections, but the photographs were of poor quality and dieback is likely to have been present. The establishment of early settlements for grazing, training of military personnel, war-time surveillance of coastal waters, hiking, 4WD activity, and the construction of firebreaks have all contributed to the introduction and spread of P. cinnamomi in the Albany hinterland (Grant and Barrett 2003). Oral history suggests that the 10th Australian Light Horse Regiment trained in the area early in World War I, and the United States army visited high points on the coast using jeeps during World War II (M. Grant, pers. comm.).
Observations in 2024 support our first hypothesis and P. cinnamomi has continued to spread into non-infested areas based on sampling on the Maardjitup Gurlin/Mt Gardner Headland and assessment of the spread of a dieback front north of Kaiupgidukgidj/Moates Lake. P. cinnamomi was also sampled in areas previously deemed non-interpretable. In the 1980s, the scrubs and heaths of the Maardjitup Gurlin/Mt Gardner headland contained relatively few susceptible species, but it was not clear whether these susceptible species had been largely eliminated from these areas or had never occurred. The long unburnt nature of the habitat in the 1980s would have been associated with limited disease expression in contrast to the more obvious symptoms observed in regenerating vegetation in 2024, 8 years after fire. It is well established that recent fire is associated with increased disease activity (Moore et al. 2014). It is now suspected that many of the susceptible genera such as Banksia were already in significant decline by this time.
However, despite the extensive areas mapped as infested, significant pockets of healthy vegetation persist that are less than 4 ha in area. Four ha is the lowest threshold for areas deemed ‘protectable’ under dieback interpretation guidelines (Department of Parks and Wildlife 2015). Significant areas of subcoastal dune communities (Agonis flexuosa heath) were originally mapped as infested and Banksia sessilis, known to be susceptible, is common in this landform. Observations from this landform in 2024 suggest that much of this plant community is still intact and notably a sample from the Sinker Reef Track in 2024 returned negative. Coastal dune systems on calcareous soils have been shown to be less conducive to the survival of P. cinnamomi (Shearer and Crane 2014).
Rate of spread of Phytophthora cinnamomi
The autonomous spread of 1.3 m/year measured over a 3-year period in the 1980s is lower than the average of 1.53 m/year recorded over the 33-year period to January 2024 and likely relates to climatic variability over shorter time periods. The estimate of 1.53 m/year, however, corresponds well with an average of 1.5 m/year recorded over a 10-year period across Banksia shrublands with similar soils and topography in south coastal WA from 1991 to 2001 (Grant and Barrett 2003). Clearly, P. cinnamomi continues to spread autonomously and relentlessly in this susceptible habitat, and steadily increases the area of infestation annually. Although discounted by Hart (1983), observations by Greg Freebury and Sarah Barrett in south coast reserves as well as notes on dieback sample sheets point to the association of kangaroo pads and P. cinnamomi infestations. Animal vectors may have also played a role in disease spread in the Reserve.
Persistence of chlamydospores
In 1983, it was considered that with sufficient testing, chlamydospores would have been found more than 4 m from the dieback front, as recently dead Banksia plants were found up to 30 m behind the edge of the infested area. However, combined with field inspection and aerial photography, it was clear that there was survival of spores behind the dieback front and that the infestation persisted for many years.
McDougall et al. (2002) found that P. cinnamomi persisted in the jarrah forest on sites infested 50 years or more previously, although it was spatially rare. It is now known that P. cinnamomi can persist in asymptomatic hosts as oospores, thick walled chlamydospores, and stromata; providing a mechanism for long-term survival (Crone et al. 2013) and enabling the pathogen to infect any susceptible plant that germinates in an infected area. The result of this pattern of infection, survival, and re-infection is that the impact of the infection is permanent. Although there has been limited evidence for recolonisation of infested sites by susceptible species in eastern Australia (McDougall et al. 2002; Weste et al. 2002), most studies suggest that this does not occur (Barrett and Yates 2015; Barrett and Rathbone 2018; Wilson et al. 2020).
Impact of Phytophthora cinnamomi on flora
The range of impacts encountered at a family and generic level agree with what is now known of the host range of P. cinnamomi and variability in susceptibility (Zentmyer 1980; Hill 1990; Shearer et al. 2004, 2007, 2013; O’Gara et al. 2005). Notably, Allocasuarina fraseriana, considered to be a host species by Zentmyer (1980), displayed low susceptibility. This is likely explained by variability due to site characteristics (Shearer and Crane 2011), with A. fraseriana being more susceptible on heavier lateritic soils compared with the free-draining sandy soils encountered on Two Peoples Bay Nature Reserve. Although differences in the abundance of several Banksia species in healthy versus infested quadrats were not significant, this may be attributed to the low representation of large Banksia trees in healthy quadrats within a 1 m2 area. Banksia seedlings were observed in the infested area in the original 1983 study, but it was considered that they were unlikely to reach maturity. Very scattered juvenile or mature Banksia were observed in the same study area in 2024. These individuals are likely to have germinated from seed dispersed from Banksia stands outside the infestation after the 2012 fire. However, this low level of recolonisation is insufficient to have any remedial impact on the modified structure of the upper tall shrub/tree stratum. Similarly, substantial decline of key members of the Fabaceae has significantly reduced the cover of the middle shrub stratum. Contrary to expectations and our second hypothesis, there was relatively high abundance of two susceptible members of the Ericaceae, Leucopogon glabellus, and Styphelia flavescens, but not of Leucopogon elegans. These are likely to have germinated from a soil-stored seed bank after the 2012 fire, although interfire recruitment of S. flavescens was also noted in 2024. In jarrah forest, Banksia sessilis, despite being susceptible, can colonise infested sites due to its prolific seed production and its ability to fruit 3 years after germination (McDougall et al. 2002). Further research is needed to determine whether the soil seed bank of susceptible species in families such as the Ericaceae and Myrtaceae can ensure their long-term persistence and the importance of fire interval. The persistence of scattered individuals of the moderately susceptible Adenanthos cuneatus and A. obovatus in infested vegetation has been observed by Greg Freebury and Sarah Barrett in similar habitat along the south coast.
Several species which had increased in abundance in infested areas in the original study, continued to be prominent in this habitat in 2024. These presumed resistant species appear to have benefited from the reduced competition at infested sites. Herbaceous species such as Stylidium scandens were also abundant and were observed to flower more profusely in infested areas despite predictions of indirect adverse impacts on this species due to changes in structure (Wills 1993).
Impact of Phytophthora cinnamomi on biomass
The methodology used to assess the impact of P. cinnamomi on plant biomass in this study has not been replicated in other studies. However, the significant loss of biomass of species other than sedges reported here is supported by previous studies that have shown significant reductions in cover in the shrub layer, and reductions in basal area and canopy closure (e.g. Bishop et al. 2010; Barrett and Rathbone 2018). Although there was no apparent increase in sedge biomass in the 1983 study, other studies have found an increase in sedge cover after infestation by P. cinnamomi in both dry sclerophyll woodland in Victoria (Weste 1981; Wilson et al. 2020) and in long-infested shrubland in WA (Barrett and Rathbone 2018); further assessment of sedge and bare ground cover in long-infested areas is needed to confirm the results of the 1983 study or otherwise.
In 1983, Hart predicted that all the major Banksia shrubland communities would be infested and lost in the Reserve. He also predicted that many susceptible plant species could become locally extinct including Banksia attenuata, B. coccinea, B. ilicifolia, B. nutans, and B. quercifolia. As discussed above, the 2024 survey shows that small pockets of healthy Banksia shrubland and heath persist and that the above-mentioned species, although severely depleted, continue to survive in these small pockets or persist as scattered individuals in infested vegetation. However, in the long term, loss of genetic diversity in these fragmented populations is likely to be substantial and species may become, or are already, functionally extinct. One such species, Banksia verticillata, is now likely to be extinct within the Reserve with the last plant sighted in 1993. What is uncertain is whether this was due to P. cinnamomi, aerial canker causing fungi (Yates et al. 2021), senescence, or a combination of these factors.
Impact of Phytophthora cinnamomi on avifauna
The 88% reduction in the number of individuals of bird species in infested compared with healthy habitat was similar to the percentage loss of individual plants and biomass, and presumably relates directly to the loss of habitat and feeding opportunities. Davis et al. (2014) found that although total bird abundance in Banksia woodlands did not differ significantly, bird community composition did. The abundance of brown honeyeaters Lichmera indistincta, western spinebills Acanthorhynchus superciliosus, and silvereyes Zosterops lateralis was lower in diseased sites, suggesting that P. cinnamomi was a serious threat for nectivores.
The impact of Phytophthora dieback on elements of the terrestrial fauna other than avifauna was not measured, but it was predicted to be significant because of the loss of habitat including shelter and food resources. Nectar provided by the Proteaceae may be critical for mammals e.g. honey possum Tarsipes rostratus, birds, and insects. The deleterious impacts of the disease on fauna and faunal habitats have been reviewed by Cahill et al. (2008) and investigated in more recent studies in south-west WA (Whelan 2003; Dundas et al. 2013, 2016; Davis et al. 2014).
Conclusion
Newhook and Podger (1972), in a review of P. cinnamomi in Australia and New Zealand, and reiterated in subsequent reviews and plans (e.g. Cahill et al. 2008; Commonwealth of Australia 2018), stated: ‘The epidemic [of Phytophthora cinnamomi] poses a serious problem for conservation in dry sclerophyll heath, woodland, and forest communities, not only for the flora including the wildflowers for which these communities are famous, but also for the many dependent species in the fauna. Many of Australia’s national parks and reserves are in jeopardy; it is a matter of the utmost urgency that they be protected from P. cinnamomi infestation for as long as possible. Without protection all of the highly susceptible elements of vulnerable communities will be eliminated.’ Unfortunately, this process of disease spread had already begun in the Two Peoples Bay area long before 1972, and even before the Nature Reserve was gazetted in its present extent in 1967.
Further dissemination of the disease by human activities can be limited by standard hygiene procedures that are now being widely used throughout the south-west of Western Australia (Department of Biodiversity, Conservation and Attractions 2020). These have evolved in response to the problem of dieback due to P. cinnamomi and other species of this genus. In the Two Peoples Bay Nature Reserve Management Plan (Department of Conservation and Land Management 1995) strategies were proposed to limit disease spread, including control of access, assessment of operations for potential disease impact and required hygiene measures, monitoring of disease spread, mapping, and education. Although the spread of the disease by the transport of soil on footwear and machinery can be controlled by hygiene procedures, the autonomous spread and fauna vectoring cannot be prevented and control and treatment methods remain limited.
In 1983, Hart considered that management may be too late to save much of Two Peoples Bay Nature Reserve from damage and the information gathered then highlighted the importance of protecting other areas in the region that were largely Phytophthora-free, such as the Fitzgerald River and Cape Arid National Parks. The protection of conservation areas in the region that are not already infected continues to be an important priority for management. Research to find alternative practical control methods and implementation of current methods continues to be a priority, with the increasing recognition of the scale and impact of Phytophthora on a range of ecosystems (Burgess et al. 2017).
Date availability
Phytophthora cinnamomi sampling data is available from the Western Australia Department of Biodiversity, Conservation and Attractions Phytophthora database.
Conflicts of interest
The authors declare no conflicts of interest. To the best of our knowledge, the authors have determined that there was no conflict of interest for deceased author Ray Hart.
Acknowledgements
Malcom Grant, Shire of Ravensthorpe for providing an historical context and details on past Phytophthora dieback mapping at Two Peoples Bay Nature Reserve; Todd Brittain and Kelly Gillen for their contribution to dieback interpretation and management in the Reserve. We thank Rod Fensham and Grant Wardell-Johnson for reviewing the paper and providing valuable comments. We also thank Denis Saunders for his editorial assistance. Ray Hart wrote the original version of this paper for a special bulletin of CALMScience on the natural history of Two Peoples Bay Nature Reserve. The paper was subject to peer review, revised and accepted for publication in 1991. The special bulletin was never published. Over 30 years later the paper has been resurrected for publication in a special issue of Pacific Conservation Biology. As Ray Hart was deceased, SB and GF updated the paper and edited it for publication. Ray Hart would have met the criteria for authorship if alive so he has been included as an author on this version.
References
Barrett S, Rathbone D (2018) Long-term phosphite application maintains species assemblages, richness and structure of plant communities invaded by Phytophthora cinnamomi. Austral Ecology 43, 360-374.
| Crossref | Google Scholar |
Barrett S, Yates CJ (2015) Risks to a mountain summit ecosystem with endemic biota in southwestern Australia. Austral Ecology 40, 423-432.
| Crossref | Google Scholar |
Bishop CL, Wardell-Johnson GW, Williams MR (2010) Community-level changes in Banksia woodland following plant pathogen invasion in the Southwest Australian Floristic Region. Journal of Vegetation Science 21, 888-898.
| Crossref | Google Scholar |
Brandis AJ, Batini F (1985) Dieback on the South Coast. Landscope 1, 6-11.
| Google Scholar |
Burgess TI, Scott JK, Mcdougall KL, Stukely MJC, Crane C, Dunstan WA, Brigg F, Andjic V, White D, Rudman T, Arentz F, Ota N, Hardy GESJ (2017) Current and projected global distribution of Phytophthora cinnamomi, one of the world’s worst plant pathogens. Global Change Biology 23, 1661-1674.
| Crossref | Google Scholar | PubMed |
Cahill DM, Rookes JE, Wilson BA, Gibson L, McDougall KL (2008) Phytophthora cinnamomi and Australia’s biodiversity: impacts, predictions and progress towards control. Australian Journal of Botany 56, 279-310.
| Crossref | Google Scholar |
Crone M, McComb JA, O’Brien PA, Hardy GESJ (2013) Survival of Phytophthora cinnamomi as oospores, stromata, and thick-walled chlamydospores in roots of symptomatic and asymptomatic annual and herbaceous perennial plant species. Fungal Biology 117, 112-123.
| Crossref | Google Scholar | PubMed |
Davis RA, Valentine LE, Craig MD, Wilson B, Bancroft WJ, Mallie M (2014) Impact of Phytophthora-dieback on birds in Banksia woodlands in south west Western Australia. Biological Conservation 171, 136-144.
| Crossref | Google Scholar |
Dundas SJ, Fleming PA, Hardy GESJ (2013) Flower visitation by honey possums (Tarsipes rostratus) in a coastal banksia heathland infested with the plant pathogen Phytophthora cinnamomi. Australian Mammalogy 35, 166-174.
| Crossref | Google Scholar |
Dundas SJ, Hardy GESJ, Fleming PA (2016) The plant pathogen Phytophthora cinnamomi influences habitat use by the obligate nectarivore honey possum (Tarsipes rostratus). Australian Journal of Zoology 64, 122-131.
| Crossref | Google Scholar |
Grant M, Barrett S (2003) The distribution and impact of Phytophthora cinnamomi Rands in the south coast region of Western Australia. In ‘Phytophthora in Forests and Natural Ecosystems, Proceedings of the 2nd International IUFRO Working Party Meeting, Albany 30th Sept – 5th Oct 2001’. (Eds JA McComb, GESJ Hardy, IC Tommerup) pp. 115–119. (Murdoch University Print: Perth)
Knapp L, Cummings D, Cummings S, Fielder PL, Hopper SD (2024) A Merningar Bardok family’s Noongar oral history of Two Peoples Bay Nature Reserve and surrounds. Pacific Conservation Biology PC24018.
| Crossref | Google Scholar |
McDougall KL, Hobbs RJ, Hardy GESJ (2002) Vegetation of Phytophthora cinnamomi-infested and adjoining uninfested sites in the northern jarrah (Eucalyptus marginata) forest of Western Australia. Australian Journal of Botany 50, 277-288.
| Crossref | Google Scholar |
Moore N, Barrett S, Howard K, Craig MD, Bowen B, Shearer B, Hardy G (2014) Time since fire and average fire interval are the best predictors of Phytophthora cinnamomi activity in heathlands of south-western Australia. Australian Journal of Botany 62, 587-593.
| Crossref | Google Scholar |
Newhook FJ, Podger FD (1972) The role of Phytophthora cinnamomi in Australian and New Zealand forests. Annual Review of Phytopathology 10, 299-326.
| Crossref | Google Scholar |
O’Gara E, Howard K, Wilson B, Hardy GESJ (2005) The responses of native Australian plant species to Phytophthora cinnamomi. Appendix 4. In ‘Management of Phytophthora cinnamomi for biodiversity conservation in Australia: Part 2. National Best Practice’. (Eds E O’Gara, K Howard, B Wilson, GESJ Hardy). pp 1–52. (Department of the Environment and Heritage: Canberra)
Podger FD (1972) Phytophthora cinnamomi, a cause of lethal disease in indigenous plant communities in Western Australia. Phytopathology 62, 972-981.
| Crossref | Google Scholar |
Shearer BL, Crane CE (2011) Habitat suitability of soils from a topographic gradient across the Fitzgerald River National Park for invasion by Phytophthora cinnamomi. Australasian Plant Pathology 40, 168-179.
| Crossref | Google Scholar |
Shearer BL, Crane CE (2014) Phytophthora cinnamomi disease expression and habitat suitability of soils on a topographic gradient across a coastal plain from dunes to forested peneplain. Australasian Plant Pathology 43, 131-142.
| Crossref | Google Scholar |
Shearer BL, Crane CE, Cochrane A (2004) Quantification of the susceptibility of the native flora of the South-West Botanical Province, Western Australia, to Phytophthora cinnamomi. Australian Journal of Botany 52, 435-443.
| Crossref | Google Scholar |
Shearer BL, Crane CE, Barrett S, Cochrane A (2007) Phytophthora cinnamomi invasion, a major threatening process to conservation of flora diversity in the South-west Botanical Province of Western Australia. Australian Journal of Botany 55, 225-238.
| Crossref | Google Scholar |
Shearer BL, Crane CE, Cochrane JA (2013) Variation in susceptibility of Banksia (including Dryandra) to Phytophthora cinnamomi. Australasian Plant Pathology 42, 351-361.
| Crossref | Google Scholar |
Weste G (1981) Changes in the vegetation of sclerophyll shrubby woodland associated with invasion by Phytophthora cinnamomi. Australian Journal of Botany 29, 261-276.
| Crossref | Google Scholar |
Weste G, Brown K, Kennedy J, Walshe T (2002) Phytophthora cinnamomi infestation—a 24-year study of vegetation change in forests and woodlands of the Grampians, Western Victoria. Australian Journal of Botany 50, 247-274.
| Crossref | Google Scholar |
Wills RT (1993) The ecological impact of Phytophthora cinnamomi in the Stirling range National Park, Western Australia. Australian Journal of Ecology 18, 145-159.
| Crossref | Google Scholar |
Wilson BA, Annett K, Laidlaw WS, Cahill DM, Garkaklis MJ, Zhuang-Griffin L (2020) Long term impacts of Phytophthora cinnamomi infestation on heathy woodland in the Great Otway National Park in south-eastern Australia. Australian Journal of Botany 68, 542-556.
| Crossref | Google Scholar |
Yates CJ, Barrett S, Dilly M, Hopper SD, Stewart B, Williams MR (2021) Modelling the impact of canker disease and fire regimes on the population dynamics and extinction risk of the Critically Endangered and granite endemic shrub Banksia verticillata R. Br. Australian Journal of Botany 69, 274-284.
| Crossref | Google Scholar |