Condition thresholds in Australia’s threatened ecological community listings hinder conservation of dynamic ecosystems
Manu E. Saunders A B , Deborah S. Bower A , Sarah Mika A and John T. Hunter
A B , Deborah S. Bower A , Sarah Mika A and John T. Hunter  A
A
A Ecosystem Management, School of Environmental and Rural Sciences, University of New England, Armidale, NSW 2351, Australia.
B Corresponding author. Email: manu.saunders@une.edu.au
Pacific Conservation Biology - https://doi.org/10.1071/PC20040
Submitted: 25 April 2020 Accepted: 26 October 2020 Published online: 13 November 2020
Journal Compilation © CSIRO 2021 Open Access CC BY-NC-ND
Abstract
Environmental degradation is threatening biodiversity and ecosystem function globally. Mandating ecosystem-level protection in policy and legislative frameworks is essential to prevent biodiversity loss. Australia’s Environment Protection and Biodiversity Conservation Act 1999 is the key legislative mechanism for supporting biodiversity at the national level, but has so far been ineffective at protecting habitat and ecological communities. Here we identify a major flaw in the current approach to listing threatened ecological communities (TECs): restrictive condition thresholds that threaten ecosystem function in dynamic ecosystems. Using two wetland TECs as a case study (Upland Wetlands and Coolibah-Black Box Woodlands), we argue that Australia’s environmental legislation should adopt a landscape-scale approach to TEC protection that acknowledges ecosystem function, accounts for different states in temporally dynamic systems, and sustains landscape connectivity of TEC distribution. We present a state-and-transition model for each TEC to show how human activities affect the reference-state continuum of wet and dry phases. We also show that the current listed condition thresholds do not acknowledge alternative ecosystem states and exclude areas that may be important for restoration and conservation of the TEC at the landscape-scale. Description of alternative and transitional states for dynamic systems, including how, when and why ecological communities shift between different states, should be formally integrated into the TEC listing process to protect Australia’s vulnerable ecosystems from further degradation and loss.
Keywords: biodiversity conservation, ecosystem communities, ecosystem function, environmental law, EPBC Act, ephemeral wetlands, floodplains, IUCN Red List, spatial diversity, state and transition, temporal diversity, threatened species.
Introduction
Mandating biodiversity conservation in political and legislative frameworks is a global imperative to address environmental degradation (IPBES 2019). Threatened species and community lists are used to inform conservation priorities in many countries, but many have limitations that can hinder effective action, often because of lack of knowledge, outdated legislation and taxonomic or jurisdictional mismatches (Taylor et al. 2011; Wallace and Fluker 2015; Zhou et al. 2016; Dorey and Walker 2018). A focus on species affects a core goal of conservation, i.e. the ability to understand environmental change and prevent the loss of ecosystem function (Possingham et al. 2002). Ecosystem-level efforts are therefore essential to biodiversity conservation and are becoming increasingly relevant worldwide (Bland et al. 2019). However, a major obstacle to ecosystem-level conservation is driven by semantics. For most conservation goals, the boundaries of a species can be defined through genetics, taxonomy and systematics. Identifying the physical boundaries of an ecosystem or ecological community is more difficult (Willis 1997; Post et al. 2007; Morin 2011) and this complexity and ambiguity translates directly into legislative instruments focused on ecosystem-level conservation, with different jurisdictions using different terminology and definitions (Nicholson et al. 2015). These discrepancies can lead to difficulties in formal description and definition of an ecosystem or ecological community, which may cause major problems for regulatory and conservation frameworks. In addition, current legislative methods focus on a static understanding of diversity. However, diversity is dynamic and has spatial and temporal components. Diversity in time is particularly important in highly dynamic systems, and is exemplified by hidden or dark diversity, i.e. species that occur in the region and can potentially inhabit the system, even if absent during surveys (Pärtel et al. 2011; Lewis et al. 2017).
In Australia, the Environment Protection and Biodiversity Conservation Act 1999 (Cth) (the EPBC Act) is an important mechanism for protecting biodiversity at the national level. The EPBC Act’s principal objects (s 3) are to protect the environment and promote biodiversity conservation, and the legislation mandates higher levels of rigour and integrity for environmental impact assessment than were previously supported under State and Territory legislation (McGrath 2005). Under the EPBC Act, the main unit of assessment for ecosystem-level protection is an ‘ecological community’, which is defined as “an assemblage of native species that inhabits a particular area in nature” (s 528). The guidelines for defining and describing an ecological community for listing purposes are intentionally vague (White et al. 2017), to discourage prescriptive approaches to protecting biological diversity (Preston and Adam 2004; Keith 2009). Uncertainty is therefore inherent in threatened ecological community (TEC) listings and is hard to overcome because of limited availability of information (Preston and Adam 2004; Adam 2009). However, this affects practical applications of the legislation, because regulation, recovery planning and conservation actions are ultimately informed by the description provided in the listing advice (Beeton and McGrath 2009; Keith 2009). Australian Commonwealth and State and Territory governments recently signed a Memorandum of Understanding to move to a new Common Assessment Method for listing species and ecological communities, based on IUCN Red List criteria.1 Using the IUCN criteria will likely improve cross-jurisdictional issues for listing; however, there is no clear timeline or objectives for updating currently listed species and no available information on practical applications of the new listing approach. Furthermore, the IUCN approach will assist only in determining whether communities meet the requirements for listing as threatened. It does not describe how communities are to be defined, which differs across Australian jurisdictions (Gellie et al. 2018). In this paper, we focus on the current EPBC Act framework for defining the existing list of threatened ecological communities.
Ecosystem dynamics in threatened ecological communities
Ecological communities are naturally dynamic and have no explicit physical or biological boundaries. Yet, from a regulatory perspective, the description of a TEC should be clear and able to be understood and applied by users of the relevant legislation (Preston and Adam 2004; White 2013). Under the EPBC Act, the onus is on landholders to interpret listing descriptions and seek required approvals for new activities on their property that may affect TECs. Yet, a recent review of interactions between the EPBC Act and agriculture noted that landholders are often not equipped with the knowledge and awareness to make accurate judgements about whether they require approval for particular actions, especially in relation to recognising examples of a TEC (Craik 2018). This could be one reason why the EPBC Act has been ineffective at protecting critical habitat and threatened ecological communities, as the majority of clearing activities have not been referred to the Federal Government for assessment (Ward et al. 2019).
In direct contrast to the vagueness and uncertainty inherent in defining and describing TECs, most listings contain explicit ‘condition thresholds’, which were introduced in 2005 to prioritise habitat considered higher in function or quality. These thresholds are defined in consultation with experts and often describe minimum patch sizes and habitat attributes. Where areas of a TEC do not meet these specific minimum thresholds, they are considered too degraded to warrant conservation and are not protected under the EPBC Act. This means a landholder does not require approval for actions such as clearing or developing on parts of the TEC that meet these exclusion criteria, and it can also make it hard to justify conservation funding and action for areas that do not meet the minimum condition thresholds. Although condition classes may be a useful tool to identify the relative condition of different patches in an ecological community, the condition thresholds in the listing process exclude potentially valuable areas of habitat regardless of the conservation context in which they exist.
These specified condition thresholds present a major flaw in the approach to TEC protection, for two reasons. First, excluding areas that do not meet certain thresholds for protection assumes that these areas have no functional value. Yet evidence shows that small, disturbed or degraded remnants have high conservation value in many systems, as a target for restoration, a source of regeneration for nearby areas of the TEC as part of a larger scale corridor, or a habitat for species of conservation importance (Hobbs and Huenneke 1992; Smallbone et al. 2014; Kendal et al. 2017; Morgan et al. 2018). Broader landscape approaches to conservation and management are essential in multifunctional landscapes, where human land uses coincide with biodiversity conservation goals (Sayer et al. 2013). When aiming to conserve or restore ecological communities that already have restricted distributions, ignoring the value of small or degraded remnants can have damaging consequences for the future distribution of the TEC.
Second, restrictive condition thresholds are rarely appropriate for conservation and management of temporally dynamic systems, especially in the context of global environmental change. Regime shifts, i.e. abrupt changes in abiotic conditions, community structure and composition, occur naturally in many ecological communities, especially in ephemeral ecosystems such as wetlands (Davis et al. 2010; Leigh et al. 2016; O’Neill 2016; T. J. Hunter, unpubl. data). These shifts may represent cycles of alternative states (Beisner et al. 2003), or may be permanent responses to environmental drivers, creating novel ecosystems in the landscape (Seastedt et al. 2008; Hobbs et al. 2014). Permanent shifts may occur more frequently in response to anthropogenic change, causing massive, sometimes irreversible, changes to community composition and structure (Brock et al. 1999; Brandt et al. 2013; Dieleman et al. 2015; Rocha et al. 2015). For example, extreme drought can significantly change wetland communities and drive novel community trajectories (Bogan and Lytle 2011; Wassens et al. 2017; T. J. Hunter, unpubl. data). Understanding how dynamic communities transition through multiple states, including temporary novel community types, within an ecologically relevant timescale is key to developing effective conservation strategies (Mushet et al. 2020).
Removing the prioritisation of condition thresholds from the listing process would allow inclusion of all areas of habitat that meet the description based on species assemblages, associated interactions and the physical environment, in line with global best practice for listing of threatened ecosystems (Bland et al. 2019). Any habitats in lower condition would then be afforded protective measures and opportunity for conservation action. Prioritisation could then be tailored around context specific issues, such as capacity and interest of stakeholders. This would enable more conservation opportunities driven in part by regional interests, rather than exclusion by experts who may not be familiar with the range of future opportunities afforded to all condition classes of habitat.
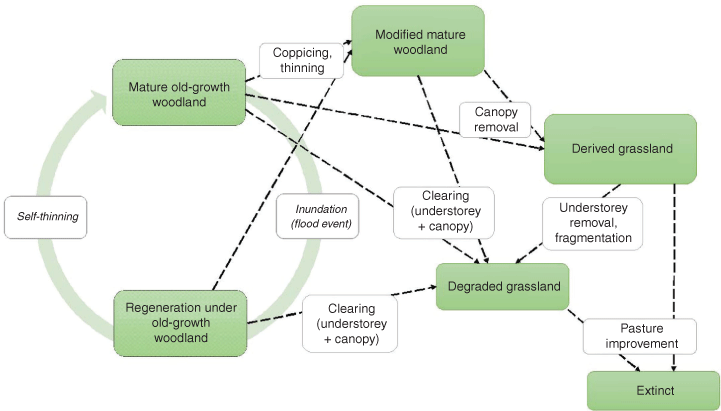
Here, we argue that Australia’s environmental legislation should take a landscape-scale approach to TEC protection that acknowledges ecosystem function, accounts for different states in temporally dynamic systems and sustains landscape connectivity of TEC distribution, regardless of perceived condition of individual sites or patches. As case studies, we focus on two listed TECs that exhibit high variability in regimes and conditions (Fig. 1):
-
Upland Wetlands of the New England Tablelands (New England Tableland Bioregion) and the Monaro Plateau (South Eastern Highlands Bioregion); hereafter, ‘Upland Wetlands’.
-
Coolibah-Black Box Woodlands of the Darling Riverine Plains and the Brigalow Belt South Bioregions; hereafter, ‘Coolibah-Black Box Woodlands’.
We present a state-and-transition model for each TEC to show how human activities affect the reference state cycle through wet and dry phases. We then show that the condition thresholds used in TEC listings under the EPBC Act are not appropriate for conservation of these systems because they do not acknowledge alternative ecosystem states and exclude areas of the TEC that may be important for restoration and conservation of the community at the landscape scale. Finally, we discuss the importance of acknowledging ecological processes in TEC listings using a state-and-transition framework to describe a gradient of conditions exhibited by each TEC.
Case study: Upland Wetlands
Upland Wetlands are generally oval in shape and are distinguished by having a well-defined bank with a sandy lunette on their downward shores formed under previous climatic conditions (Bell et al. 2008). These systems are shallow (<2 m depth) ephemeral water bodies in depression areas of closed or semi-closed drainage on flat landscapes. They receive water from relatively small catchments by a combination of hydrological processes: some mainly stream-fed, some spring-fed and some only by overland flow. The nomenclature used to describe these systems is diverse, but they are best described as semi-permanent or ephemeral marshes (Bell et al. 2008; Hunter and Hunter 2020; T. J. Hunter, unpubl. data). Without human modification, upland wetlands follow a natural cycle of drying and wetting phases (Fig. 2), each of which supports different plant community alliances (Hunter and Hunter 2020). Human modification (i.e. damming, draining, intensive grazing) interrupts this regime and leads to extinction of the TEC when modified conditions are maintained.
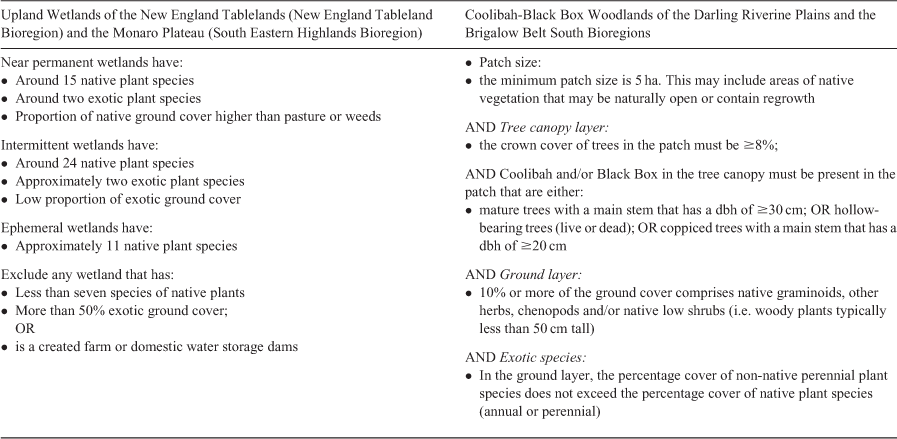
The TEC listing advice2 distinguishes three forms of upland wetlands based upon their hydrological regime: near permanent (rarely dry); intermittent (often seasonally dry); and ephemeral (without free standing water for a majority of the year). These three wetland ‘types’ are arbitrary and undefined. In reality, any wetland can transition across any of these states over time. The listing excludes any ephemeral form of these wetlands that “have a low native species richness (less than seven species, including both wet and dry conditions) and/or have an average cover of introduced species of more than 50% of plant cover present” (Table 1). Farm dams and domestic water storages are also excluded. This is problematic, because most upland wetlands (85%) are on private property and almost all have had some form of alteration in drainage including damming, draining, and changes to the lunette (Bell et al. 2008). This can create confusion about which wetlands justify protection under the TEC listing. For example, a wetland that has been dammed on private property (27% in the Northern Tablelands; Bell et al. 2008) could be excluded from protection under the TEC definition, but it could be an important site for restoration efforts to improve the connectivity of upland wetlands. Very few examples of this TEC remain (58 lagoons in the Northern Tablelands); therefore, even a small number of exclusions based on condition translate to a large overall proportion of the TEC that is excluded from protection, greatly increasing its risk of extinction.
|
The advice also states specific numbers of native plant species that define wetlands in the arbitrary hydrological regimes (Table 1). Near permanent wetlands are expected to support “around 15 species”, intermittent wetlands “around 24 species”, and ephemeral wetlands “approximately 11 species, including both wet and dry conditions”. The listing states the number of exotic species should be low (around two species). These simplistic quantitative thresholds are problematic for identifying whether a particular wetland deserves protection under the listing criteria. First, there is no information on how species richness should be measured and at what scale, or on how to define the wetland boundary. Richness metrics can differ over time or space, both within and between wetlands (Winning 2010; Hunter 2016; Hunter and Hunter 2020; T. J. Hunter, unpubl. data). Second, temporal and within-wetland variation in species richness is high in these wetlands, making specific vegetation community descriptions difficult, as they are in fact not a single community but a mosaic of very different wetland vegetation types (Bell et al. 2008; Hunter 2016). The plant community in the submerged part of an upland wetland can be very different to the fringing vegetation communities, and communities can shift in composition across time and with climatic variability and disturbance (e.g. fire) (T. J. Hunter, unpubl. data). These differences among within-wetland communities may be more important to ecosystem function than differences between wetlands and are an important part of the overall temporal biodiversity of the systems (Bell et al. 2008). Third, a maximum threshold of two exotic plant species is unrealistic for a dynamic wetland system that supports constant movement of animals, including migratory birds (White 1987; Brock et al. 1999, 2003). A threshold of 50% cover (for exotic species) also ignores inherent temporal and spatial variation in upland wetlands, whereby novel communities can exist for short periods of time only to be replaced by native types under different inundation regimes (T. J. Hunter, unpubl. data). Relative proportions of native and introduced plant species can shift dramatically across zones, between wet and dry cycles, or in response to disturbance events (Hunter 2018). For example, within Racecourse Lagoon and Little Llangothlin Lagoon, sites containing no exotic plant taxa during the semi-permanent wet phase shifted to 100% exotic plant cover during a transitional dry phase (T. J. Hunter, unpubl. data). In October 2019, an unplanned fire burned the majority of Dangars Lagoon, which was dry at the time. In February 2020, a vegetation survey of the lagoon found that the post-fire vegetation cover was almost 100% exotic species (27 of 30 plots dominated by exotic species), meaning the lagoon temporarily will not meet the condition threshold for the TEC (T. J. Hunter, unpubl. data).
Case study: Coolibah-Black Box Woodlands
Eucalyptus coolabah (Coolibah) and Eucalyptus largiflorens (Black Box) are extensive woodland trees in the Murray-Darling Basin and depend on regular flooding to maintain growth and regeneration (Roberts and Marston 2011). Woodlands undergo a natural cycle of regeneration and growth, mostly after flooding and self-thinning (Fig. 3). Human modification of woodlands (i.e. coppicing, thinning, clearing) leads to an increasingly degraded state, with extinction following complete removal of canopy and understorey vegetation.
The listing advice3 for this TEC is limited to patches that meet detailed condition thresholds (Table 1). A key problem with this definition is the minimum patch size of at least 5 ha, suggesting that many patches smaller than this can be cleared or developed without approval. This could have damaging effects on landscape connectivity, because small, isolated habitat patches are critical for supporting animal communities and this can have a cumulative effect over time. For example, in modified landscapes, small reserves or habitat remnants provide important refuges for vertebrates (Fischer and Lindenmayer 2002), plants (Arroyo‐Rodríguez et al. 2009; Kendal et al. 2017), and invertebrates (Tscharntke et al. 2002), and play an important role in network connectivity at the landscape scale (Götmark and Thorell 2003; Bodin et al. 2006; Fitzsimons and Michael 2017). Even isolated trees on agricultural land are essential to support biodiversity and ecosystem services (Gibbons and Boak 2002; Wilson 2002; Oliver et al. 2006; Fischer et al. 2010) and are an important seed source for restoration actions (Ottewell et al. 2010).
In addition, the listing excludes any small patches containing mature or coppiced trees with diameter at breast height (dbh) of <30 or 20 cm respectively (Table 1). This exclusion aligns with New South Wales management policy, which lists E. coolabah and E. largiflorens as Invasive Native Species under the Local Land Services Land Management (Native Vegetation) Code 2018,4 allowing landholders to clear any tree of these species with dbh <30 cm. However, in some areas, trees with dbh > 30 cm account for less than 30% of standing trees within mature old growth stands. This condition also excludes dense regeneration stands, which may be critical to restoration and protection of biodiversity in this TEC (Good et al. 2011, 2012). In addition, there is no clarity on identifying variation between wet and dry communities of this floodplain TEC. Similar to upland wetlands, putting specific limits on the number or coverage of native and exotic species overlooks the fact that the relative proportions of exotic and native species can shift rapidly between ecosystem states, or due to variation in topography or flooding history (Hunter 2005).
Traits, states, and transitions
Ecological communities are structured by ecological processes and cross-taxon interactions between plants, animals and microorganisms, yet ecological processes and functions are rarely acknowledged in TEC listings. Instead, TECs are predominantly defined on limited descriptions of plant communities and simplistic condition thresholds. Lack of knowledge (or acknowledgement) of species interactions and processes and the temporal aspects of diversity can be a major hindrance to conservation and recovery efforts for TECs (Auld and Tozer 2004; Nicholson et al. 2015). Despite the traditional focus on plant communities, the TEC listing protocol can also be used to describe animal communities, as Fraser et al. (2019) show by formally describing the Temperate and Subtropical Woodland Bird Community. Similar explicit definitions of other types of animal communities will likely be more difficult, especially in the case of invertebrates, for which there is currently very little knowledge of most species distributions and their overlap with TECs (Braby 2018). However, this approach is an important starting point for acknowledging fauna communities as an important part of sustaining ecosystem function in TECs.
Knowledge of relevant fauna communities is vital to understand how environmental change will impact ecological interactions, as plant and animal communities can respond in contrasting ways to the same community-level processes (Craig et al. 2015). Trait linkage frameworks have been used successfully to understand how associations between plant and animal communities influence ecosystem function and associated services (de Bello et al. 2010; Luck et al. 2012; Lavorel et al. 2013), and integrating this approach with current listing protocols would be useful. For example, identifying key trait combinations relevant to each TEC phase can inform effective restoration activities. A major obstacle will be the lack of data available for most ecological processes and animal populations, especially invertebrates; however, a precautionary approach that acknowledges data deficiencies will be more effective for long-term conservation than simply ignoring these critical components of TECs (Whelan et al. 2004; Nicholson et al. 2015).
State-and-transition models are a useful tool to consider multiple components of an ecological community, which is critical for ecosystem-level conservation. The models originated in rangelands ecology and management as a way to understand non-linear dynamics in ecological systems, and demonstrate the transition process between alternative states (Westoby et al. 1989; Stringham et al. 2003). They are most useful as a flexible tool to understand variability on a site-by-site basis, rather than a rigid management guide (Breshears et al. 2002). In Australia, state-and-transition models have mostly been applied to rangelands and woodlands, often with a focus on restoration (Yates and Hobbs 1997; Prober et al. 2002; Grant 2006; Craig et al. 2015; Wright et al. 2019). They can also be applied to animal communities. For example, Letnic and Dickman (2010) adapted a state-and-transition framework to small mammal assemblages in spinifex grasslands, showing how specific environmental conditions and processes (such as predation pressure or weather events) drive transitions in the composition of the mammal community. Identifying landscape-scale linkages is essential, because community patterns and processes that influence transition operate at multiple spatial scales (Bestelmeyer et al. 2011; Gonzalez et al. 2020). Yet the assumption that animal community patterns follow vegetation community patterns does not hold in every ecosystem (Craig et al. 2015), and animal- and plant-focused models should be assessed together, or integrated into the one model.
Although acknowledgement of alternative ecosystem states and transitions between states is fundamental to application of the EPBC Act’s legal framework (Beeton and McGrath 2009), there have been relatively few attempts to use this approach to explicitly describe TECs (but see Sinclair et al. 2019). Here, we present a state-and-transition model for each of our case study TECs (Figs. 2, 3), showing how communities shift between states and especially how human modification affects the reference-state cycle. Without recognition of such changing states, assessment criteria risk focusing on static condition thresholds that apply to a single state of the community. This can result in exclusion of ecosystems that meet the description of assemblages and physical attributes. Such ecosystems may constitute a large proportion of the TEC (e.g. in the case of dammed upland wetlands), or they could be areas that would otherwise provide a valuable addition to the TEC on a landscape level (e.g. Coolibah-Black Box patches <5 ha), lowering their level of protection and hindering the overall conservation efforts. These models are most relevant to describe major changes at large time scales. At smaller scales, TECs exhibit rapid change in time and space in response to local conditions, which require more detailed approaches, such as trait linkage frameworks. State-and-transition models have many synergies with trait linkage frameworks and integrating trait information within the model can provide better understanding of community-level changes in ecosystem function (Quétier et al. 2007).
Conclusion
In this era of unprecedented human influence on the Earth system, human activities are creating new ecological processes and interactions, redistributing biodiversity, and transforming ecological systems (Ellis 2016; Pecl et al. 2017). Yet governance frameworks have struggled to keep up with these changes (Craig 2010; McDonald et al. 2016) and are often based on the assumption of ecological stationarity, i.e. that natural systems fluctuate to a small degree within an unchanging boundary of variability (Milly et al. 2008; Craig 2010). This poses a major problem for setting objectives and targets for management and conservation of dynamic systems, especially wetlands (Finlayson et al. 2017). Understanding how, when and why ecological communities shift between different states is critical to mitigate threats and manage recovery and conservation actions. Therefore, description of alternative states for dynamic systems should be formally integrated into the listing process for ecological communities, particularly for wetland systems. This is an established legal recommendation (Beeton and McGrath 2009) but, in practice it has not been standardised across TEC listings, leaving Australia’s threatened ecological communities at risk of degradation and loss. Additionally, although condition classes may be useful tools for identifying the relative condition and even comparing threats in different ecosystems, this prioritisation process and the associated use of thresholds needs to be decoupled from the listing process otherwise important remaining habitat will be further lost. When the listing criteria acknowledges the range of states and conditions in which ecological communities exists, more ecosystems that meet TEC descriptions will be protected and conservation of ecological communities will benefit.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
The authors acknowledge support from the Glen Innes Natural Resources Advisory Committee and a NSW Government grant through a partnership between the Saving our Species program and the Environmental Trust. DSB acknowledges support from the Australian Research Council (DE200101424). Joanne Ocock provided helpful comments on manuscript drafts.
References
Adam, P. (2009). Ecological communities - the context for biodiversity conservation or a source of confusion? Australasian Journal of Natural Resources Law and Policy 13, 7–59.Arroyo-Rodríguez, V., Pineda, E., Escobar, F., and Benítez-Malvido, J. (2009). Value of small patches in the conservation of plant-species diversity in highly fragmented rainforest. Conservation Biology 23, 729–739.
| Value of small patches in the conservation of plant-species diversity in highly fragmented rainforest.Crossref | GoogleScholarGoogle Scholar | 19040651PubMed |
Auld, T. D., and Tozer, M. (2004). Endangered ecological communities and landscape conservation in NSW: successes and failures in the Sydney Basin. In ‘Threatened species legislation’. Other RZS NSW Publications. pp. 94–101. (Royal Zoological Society of New South Wales.)
Beeton, R. J. S., and McGrath, C. (2009). Developing an approach to the listing of ecological communities to achieve conservation outcomes. Australasian Journal of Natural Resources Law and Policy 13, 61.
Beisner, B., Haydon, D., and Cuddington, K. (2003). Alternative stable states in ecology. Frontiers in Ecology and the Environment 1, 376–382.
| Alternative stable states in ecology.Crossref | GoogleScholarGoogle Scholar |
Bell, D. M., Hunter, J. T., and Haworth, R. J. (2008). Montane lakes (lagoons) of the New England Tablelands Bioregion. Cunninghamia 10, 475–492.
de Bello, F., Lavorel, S., Díaz, S., Harrington, R., Cornelissen, J. H. C., Bardgett, R. D., Berg, M. P., Cipriotti, P., Feld, C. K., Hering, D., Martins da Silva, P., Potts, S. G., Sandin, L., Sousa, J. P., Storkey, J., Wardle, D. A., and Harrison, P. A. (2010). Towards an assessment of multiple ecosystem processes and services via functional traits. Biodiversity and Conservation 19, 2873–2893.
| Towards an assessment of multiple ecosystem processes and services via functional traits.Crossref | GoogleScholarGoogle Scholar |
Bestelmeyer, B. T., Goolsby, D. P., and Archer, S. R. (2011). Spatial perspectives in state-and-transition models: a missing link to land management? Journal of Applied Ecology 48, 746–757.
| Spatial perspectives in state-and-transition models: a missing link to land management?Crossref | GoogleScholarGoogle Scholar |
Bland, L. M., Nicholson, E., Miller, R. M., Andrade, A., Carré, A., Etter, A., Ferrer‐paris, J. R., Herrera, B., Kontula, T., Lindgaard, A., Pliscoff, P., Skowno, A., Valderrábano, M., Zager, I., and Keith, D. A. (2019). Impacts of the IUCN Red List of Ecosystems on conservation policy and practice. Conservation Letters 12, e12666.
| Impacts of the IUCN Red List of Ecosystems on conservation policy and practice.Crossref | GoogleScholarGoogle Scholar |
Bodin, Ö., Tengö, M., Norman, A., Lundberg, J., and Elmqvist, T. (2006). The value of small size: loss of forest patches and ecological thresholds in southern Madagascar. Ecological Applications 16, 440–451.
| The value of small size: loss of forest patches and ecological thresholds in southern Madagascar.Crossref | GoogleScholarGoogle Scholar | 16711035PubMed |
Bogan, M. T., and Lytle, D. A. (2011). Severe drought drives novel community trajectories in desert stream pools. Freshwater Biology 56, 2070–2081.
| Severe drought drives novel community trajectories in desert stream pools.Crossref | GoogleScholarGoogle Scholar |
Braby, M. F. (2018). Threatened species conservation of invertebrates in Australia: an overview. Austral Entomology 57, 173–181.
| Threatened species conservation of invertebrates in Australia: an overview.Crossref | GoogleScholarGoogle Scholar |
Brandt, J. S., Haynes, M. A., Kuemmerle, T., Waller, D. M., and Radeloff, V. C. (2013). Regime shift on the roof of the world: Alpine meadows converting to shrublands in the southern Himalayas. Biological Conservation 158, 116–127.
| Regime shift on the roof of the world: Alpine meadows converting to shrublands in the southern Himalayas.Crossref | GoogleScholarGoogle Scholar |
Breshears, D. D., Whitlock, C., Jackson, R. D., Bartolome, J. W., and Allen-Diaz, B. (2002). State and transition models: response to an ESA symposium. Bulletin of the Ecological Society of America 83, 194–196.
Brock, M. A., Nielsen, D. L., Shiel, R. J., Green, J. D., and Langley, J. D. (2003). Drought and aquatic community resilience: the role of eggs and seeds in sediments of temporary wetlands. Freshwater Biology 48, 1207–1218.
| Drought and aquatic community resilience: the role of eggs and seeds in sediments of temporary wetlands.Crossref | GoogleScholarGoogle Scholar |
Brock, M. A., Smith, R. G. B., and Jarman, P. J. (1999). Drain it, dam it: alteration of water regime in shallow wetlands on the New England Tableland of New South Wales, Australia. Wetlands Ecology and Management 7, 37–46.
| Drain it, dam it: alteration of water regime in shallow wetlands on the New England Tableland of New South Wales, Australia.Crossref | GoogleScholarGoogle Scholar |
Craig, M. D., Stokes, V. L., Fontaine, J. B., Hardy, G. E. S., Grigg, A. H., and Hobbs, R. J. (2015). Do state-and-transition models derived from vegetation succession also represent avian succession in restored mine pits? Ecological Applications 25, 1790–1806.
| Do state-and-transition models derived from vegetation succession also represent avian succession in restored mine pits?Crossref | GoogleScholarGoogle Scholar | 26591446PubMed |
Craig, R. K. (2010). Stationarity is dead – long live transformation: five principles for climate change adaptation law. Harvard Environmental Law Review , 9–74.
Craik, W. (2018). Review of interactions between the EPBC Act and the agriculture sector. (Commonwealth Department of Environment and Energy, Australia.)
Davis, J., Sim, L., and Chambers, J. (2010). Multiple stressors and regime shifts in shallow aquatic ecosystems in antipodean landscapes. Freshwater Biology 55, 5–18.
| Multiple stressors and regime shifts in shallow aquatic ecosystems in antipodean landscapes.Crossref | GoogleScholarGoogle Scholar |
Dieleman, C. M., Branfireun, B. A., McLaughlin, J. W., and Lindo, Z. (2015). Climate change drives a shift in peatland ecosystem plant community: Implications for ecosystem function and stability. Global Change Biology 21, 388–395.
| Climate change drives a shift in peatland ecosystem plant community: Implications for ecosystem function and stability.Crossref | GoogleScholarGoogle Scholar | 24957384PubMed |
Dorey, K., and Walker, T. R. (2018). Limitations of threatened species lists in Canada: a federal and provincial perspective. Biological Conservation 217, 259–268.
| Limitations of threatened species lists in Canada: a federal and provincial perspective.Crossref | GoogleScholarGoogle Scholar |
Ellis, E. C. (2016). Ecology in an anthropogenic biosphere. Ecological Monographs , 287–331.
| Ecology in an anthropogenic biosphere.Crossref | GoogleScholarGoogle Scholar |
Finlayson, C. M., Capon, S. J., Rissik, D., Pittock, J., Fisk, G., Davidson, N. C., Bodmin, K. A., Papas, P., Robertson, H. A., Schallenberg, M., Saintilan, N., Edyvane, K., and Bino, G. (2017). Policy considerations for managing wetlands under a changing climate. Marine and Freshwater Research 68, 1803–1815.
| Policy considerations for managing wetlands under a changing climate.Crossref | GoogleScholarGoogle Scholar |
Fischer, J., and Lindenmayer, D. B. (2002). Small patches can be valuable for biodiversity conservation: two case studies on birds in southeastern Australia. Biological Conservation 106, 129–136.
| Small patches can be valuable for biodiversity conservation: two case studies on birds in southeastern Australia.Crossref | GoogleScholarGoogle Scholar |
Fischer, J., Stott, J., and Law, B. S. (2010). The disproportionate value of scattered trees. Biological Conservation 143, 1564–1567.
| The disproportionate value of scattered trees.Crossref | GoogleScholarGoogle Scholar |
Fitzsimons, J. A., and Michael, D. R. (2017). Rocky outcrops: a hard road in the conservation of critical habitats. Biological Conservation 211, 36–44.
| Rocky outcrops: a hard road in the conservation of critical habitats.Crossref | GoogleScholarGoogle Scholar |
Fraser, H., Simmonds, J. S., Kutt, A. S., and Maron, M. (2019). Systematic definition of threatened fauna communities is critical to their conservation. Diversity and Distributions 25, 462–477.
| Systematic definition of threatened fauna communities is critical to their conservation.Crossref | GoogleScholarGoogle Scholar |
Gellie, N. J. H., Hunter, J. T., Benson, J. S., Kirkpatrick, J. B., Cheal, D. C., McCreery, K., and Brocklehurst, P. (2018). Overview of plot-based vegetation classification approaches within Australia. Phytocoenologia 48, 251–272.
| Overview of plot-based vegetation classification approaches within Australia.Crossref | GoogleScholarGoogle Scholar |
Gibbons, P., and Boak, M. (2002). The value of paddock trees for regional conservation in an agricultural landscape. Ecological Management & Restoration 3, 205–210.
| The value of paddock trees for regional conservation in an agricultural landscape.Crossref | GoogleScholarGoogle Scholar |
Gonzalez, A., Germain, R. M., Srivastava, D. S., Filotas, E., Dee, L. E., Gravel, D., Thompson, P. L., Isbell, F., Wang, S., Kéfi, S., Montoya, J., Zelnik, Y. R., and Loreau, M. (2020). Scaling-up biodiversity-ecosystem functioning research. Ecology Letters 23, 757–776.
| Scaling-up biodiversity-ecosystem functioning research.Crossref | GoogleScholarGoogle Scholar | 31997566PubMed |
Good, M. K., Price, J. N., Clarke, P. J., and Reid, N. (2012). Dense regeneration of floodplain Eucalyptus coolabah: invasive scrub or passive restoration of an endangered woodland community? The Rangeland Journal 34, 219–230.
| Dense regeneration of floodplain Eucalyptus coolabah: invasive scrub or passive restoration of an endangered woodland community?Crossref | GoogleScholarGoogle Scholar |
Good, M. K., Price, J. N., Clarke, P., and Reid, N. (2011). Densely regenerating coolibah (Eucalyptus coolabah) woodlands are more species-rich than surrounding derived grasslands in floodplains of eastern Australia. Australian Journal of Botany 59, 468–479.
| Densely regenerating coolibah (Eucalyptus coolabah) woodlands are more species-rich than surrounding derived grasslands in floodplains of eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Götmark, F., and Thorell, M. (2003). Size of nature reserves: densities of large trees and dead wood indicate high value of small conservation forests in southern Sweden. Biodiversity & Conservation 12, 1271–1285.
| Size of nature reserves: densities of large trees and dead wood indicate high value of small conservation forests in southern Sweden.Crossref | GoogleScholarGoogle Scholar |
Grant, C. D. (2006). State-and-transition successional model for bauxite mining rehabilitation in the jarrah forest of Western Australia. Restoration Ecology 14, 28–37.
| State-and-transition successional model for bauxite mining rehabilitation in the jarrah forest of Western Australia.Crossref | GoogleScholarGoogle Scholar |
Hobbs, R. J., Higgs, E., Hall, C. M., Bridgewater, P., Chapin, F. S., Ellis, E. C., Ewel, J. J., Hallett, L. M., Harris, J., Hulvey, K. B., Jackson, S. T., Kennedy, P. L., Kueffer, C., Lach, L., Lantz, T. C., Lugo, A. E., Mascaro, J., Murphy, S. D., Nelson, C. R., Perring, M. P., Richardson, D. M., Seastedt, T. R., Standish, R. J., Starzomski, B. M., Suding, K. N., Tognetti, P. M., Yakob, L., and Yung, L. (2014). Managing the whole landscape: historical, hybrid, and novel ecosystems. Frontiers in Ecology and the Environment 12, 557–564.
| Managing the whole landscape: historical, hybrid, and novel ecosystems.Crossref | GoogleScholarGoogle Scholar |
Hobbs, R. J., and Huenneke, L. F. (1992). Disturbance, diversity, and invasion: implications for conservation. Conservation Biology 6, 324–337.
| Disturbance, diversity, and invasion: implications for conservation.Crossref | GoogleScholarGoogle Scholar |
Hunter, J. T. (2016). Differences in disturbance type and nutrient availability favour different functional traits across three co-occurring montane wetland systems in eastern Australia. Australian Journal of Botany 64, 526–529.
| Differences in disturbance type and nutrient availability favour different functional traits across three co-occurring montane wetland systems in eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Hunter, J. T. (2018). Survey and monitoring of Upland Lagoons on the Northern Tablelands. Technical Report. Northern Tablelands Local Land Services
Hunter, J. T. (2005). Vegetation of Culgoa National Park, central northern New South Wales. Cunninghamia 9, 275–284.
Hunter, J. T., and Hunter, V. H. (2020). Montane mire vegetation of the New England Tablelands bioregion of eastern Australia. Vegetation Classification and Survey 1, 37–51.
| Montane mire vegetation of the New England Tablelands bioregion of eastern Australia.Crossref | GoogleScholarGoogle Scholar |
IPBES (2019). Global assessment report on biodiversity and ecosystem services of the Intergovernmental Science – Policy Platform on Biodiversity and Ecosystem Services. IPBES Secretariat, Bonn, Germany.
Keith, D. A. (2009). The interpretation, assessment and conservation of ecological communities. Ecological Management & Restoration 10, S3–S15.
| The interpretation, assessment and conservation of ecological communities.Crossref | GoogleScholarGoogle Scholar |
Kendal, D., Zeeman, B. J., Ikin, K., Lunt, I. D., McDonnell, M. J., Farrar, A., Pearce, L. M., and Morgan, J. W. (2017). The importance of small urban reserves for plant conservation. Biological Conservation 213, 146–153.
| The importance of small urban reserves for plant conservation.Crossref | GoogleScholarGoogle Scholar |
Lavorel, S., Storkey, J., Bardgett, R. D., de Bello, F., Berg, M. P., Le Roux, X., Moretti, M., Mulder, C., Pakeman, R. J., Díaz, S., and Harrington, R. (2013). A novel framework for linking functional diversity of plants with other trophic levels for the quantification of ecosystem services. Journal of Vegetation Science 24, 942–948.
| A novel framework for linking functional diversity of plants with other trophic levels for the quantification of ecosystem services.Crossref | GoogleScholarGoogle Scholar |
Leigh, C., Boulton, A. J., Courtwright, J. L., Fritz, K., May, C. L., Walker, R. H., and Datry, T. (2016). Ecological research and management of intermittent rivers: an historical review and future directions. Freshwater Biology 61, 1181–1199.
| Ecological research and management of intermittent rivers: an historical review and future directions.Crossref | GoogleScholarGoogle Scholar |
Letnic, M., and Dickman, C. R. (2010). Resource pulses and mammalian dynamics: conceptual models for hummock grasslands and other Australian desert habitats. Biological Reviews 85, 501–521.
| Resource pulses and mammalian dynamics: conceptual models for hummock grasslands and other Australian desert habitats.Crossref | GoogleScholarGoogle Scholar | 20015313PubMed |
Lewis, R. J., De bello, F., Bennett, J. A., Fibich, P., Finerty, G. E., Götzenberger, L., Hiiesalu, I., Kasari, L., Lepš, J., Májeková, M., Mudrák, O., Riibak, K., Ronk, A., Rychtecká, T., Vitová, A., and Pärtel, M. (2017). Applying the dark diversity concept to nature conservation. Conservation Biology 31, 40–47.
| Applying the dark diversity concept to nature conservation.Crossref | GoogleScholarGoogle Scholar | 27027266PubMed |
Luck, G. W., Lavorel, S., McIntyre, S., and Lumb, K. (2012). Improving the application of vertebrate trait-based frameworks to the study of ecosystem services. The Journal of Animal Ecology 81, 1065–1076.
| Improving the application of vertebrate trait-based frameworks to the study of ecosystem services.Crossref | GoogleScholarGoogle Scholar | 22435774PubMed |
McDonald, J., McCormack, P. C., Fleming, A. J., Harris, R. M. B., and Lockwood, M. (2016). Rethinking legal objectives for climate-adaptive conservation. Ecology and Society 21, 25.
| Rethinking legal objectives for climate-adaptive conservation.Crossref | GoogleScholarGoogle Scholar |
McGrath, C. (2005). Key concepts of the Environment Protection and Biodiversity Conservation Act 1999 (Cth). Environmental and Planning Law Journal 22, 20–39.
Milly, P. C. D., Betancourt, J., Falkenmark, M., Hirsch, R. M., Kundzewicz, Z. W., Lettenmaier, D. P., and Stouffer, R. J. (2008). Stationarity is dead: whither water management? Science 319, 573–574.
| Stationarity is dead: whither water management?Crossref | GoogleScholarGoogle Scholar |
Morgan, J., Wright, J., Whelan, J., Clarke, M., Coulson, G., Lunt, I., Stoner, J., Varcoe, T., and Shannon, J. (2018). What does it take to do successful adaptive management? A case study highlighting Coastal Grassy Woodland restoration at Yanakie Isthmus. Ecological Management & Restoration 19, 111–123.
| What does it take to do successful adaptive management? A case study highlighting Coastal Grassy Woodland restoration at Yanakie Isthmus.Crossref | GoogleScholarGoogle Scholar |
Morin, P. J. (2011). ‘Community ecology’, 2nd edn. (Blackwell Science Inc.: Chichester, West Sussex.)
Mushet, D. M., McKenna, O. P., and McLean, K. I. (2020). Alternative stable states in inherently unstable systems. Ecology and Evolution 10, 843–850.
| Alternative stable states in inherently unstable systems.Crossref | GoogleScholarGoogle Scholar | 32015848PubMed |
Nicholson, E., Regan, T. J., Auld, T. D., Burns, E. L., Chisholm, L. A., English, V., Harris, S., Harrison, P., Kingsford, R. T., Leishman, M. R., Metcalfe, D. J., Pisanu, P., Watson, C. J., White, M., White, M. D., Williams, R. J., Wilson, B., and Keith, D. A. (2015). Towards consistency, rigour and compatibility of risk assessments for ecosystems and ecological communities. Austral Ecology 40, 347–363.
| Towards consistency, rigour and compatibility of risk assessments for ecosystems and ecological communities.Crossref | GoogleScholarGoogle Scholar |
Oliver, I., Pearce, S., Greenslade, P. J. M., and Britton, D. R. (2006). Contribution of paddock trees to the conservation of terrestrial invertebrate biodiversity within grazed native pastures. Austral Ecology 31, 1–12.
| Contribution of paddock trees to the conservation of terrestrial invertebrate biodiversity within grazed native pastures.Crossref | GoogleScholarGoogle Scholar |
O'neill, B. J. (2016). Community disassembly in ephemeral ecosystems. Ecology 97, 3285–3292.
| Community disassembly in ephemeral ecosystems.Crossref | GoogleScholarGoogle Scholar | 27861768PubMed |
Ottewell, K. M., Donnellan, S. C., and Paton, D. C. (2010). Evaluating the demographic, reproductive, and genetic value of eucalypt paddock trees for woodland restoration in agricultural landscapes. Restoration Ecology 18, 263–272.
| Evaluating the demographic, reproductive, and genetic value of eucalypt paddock trees for woodland restoration in agricultural landscapes.Crossref | GoogleScholarGoogle Scholar |
Pärtel, M., Szava-Kovats, R., and Zobel, M. (2011). Dark diversity: shedding light on absent species. Trends in Ecology & Evolution 26, 124–128.
| Dark diversity: shedding light on absent species.Crossref | GoogleScholarGoogle Scholar |
Pecl, G. T., Araújo, M. B., Bell, J. D., Blanchard, J., Bonebrake, T. C., Chen, I.-C., Clark, T. D., Colwell, R. K., Danielsen, F., Evengård, B., Falconi, L., Ferrier, S., Frusher, S., Garcia, R. A., Griffis, R. B., Hobday, A. J., Janion-Scheepers, C., Jarzyna, M. A., Jennings, S., Lenoir, J., Linnetved, H. I., Martin, V. Y., McCormack, P. C., McDonald, J., Mitchell, N. J., Mustonen, T., Pandolfi, J. M., Pettorelli, N., Popova, E., Robinson, S. A., Scheffers, B. R., Shaw, J. D., Sorte, C. J. B., Strugnell, J. M., Sunday, J. M., Tuanmu, M.-N., Vergés, A., Villanueva, C., Wernberg, T., Wapstra, E., and Williams, S. E. (2017). Biodiversity redistribution under climate change: impacts on ecosystems and human well-being. Science 355, eaai9214.
| Biodiversity redistribution under climate change: impacts on ecosystems and human well-being.Crossref | GoogleScholarGoogle Scholar | 28360268PubMed |
Possingham, H. P., Andelman, S. J., Burgman, M. A., Medellı´n, R. A., Master, L. L., and Keith, D. A. (2002). Limits to the use of threatened species lists. Trends in Ecology & Evolution 17, 503–507.
| Limits to the use of threatened species lists.Crossref | GoogleScholarGoogle Scholar |
Post, D. M., Doyle, M. W., Sabo, J. L., and Finlay, J. C. (2007). The problem of boundaries in defining ecosystems: a potential landmine for uniting geomorphology and ecology. Geomorphology 89, 111–126.
| The problem of boundaries in defining ecosystems: a potential landmine for uniting geomorphology and ecology.Crossref | GoogleScholarGoogle Scholar |
Preston, B. J., and Adam, P. (2004). Describing and listing threatened ecological communities under the Threatened Species Conservation Act 1995 (NSW): Part 1 – The assemblage of species and the particular area. Environmental and Planning Law Journal 21, 250–263.
Prober, S. M., Thiele, K. R., and Lunt, I. D. (2002). Identifying ecological barriers to restoration in temperate grassy woodlands: soil changes associated with different degradation states. Australian Journal of Botany 50, 699–712.
| Identifying ecological barriers to restoration in temperate grassy woodlands: soil changes associated with different degradation states.Crossref | GoogleScholarGoogle Scholar |
Quétier, F., Thébault, A., and Lavorel, S. (2007). Plant traits in a state and transition framework as markers of ecosystem response to land-use change. Ecological Monographs 77, 33–52.
| Plant traits in a state and transition framework as markers of ecosystem response to land-use change.Crossref | GoogleScholarGoogle Scholar |
Roberts, J., and Marston, F. (2011). ‘Water regime for wetland and floodplain plants. A source book for the Murray–Darling Basin’. (National Water Commission, Commonwealth of Australia: Canberra, ACT.)
Rocha, J. C., Peterson, G. D., and Biggs, R. (2015). Regime shifts in the Anthropocene: drivers, risks, and resilience. PLOS ONE 10, e0134639.
| Regime shifts in the Anthropocene: drivers, risks, and resilience.Crossref | GoogleScholarGoogle Scholar | 26267896PubMed |
Sayer, J., Sunderland, T., Ghazoul, J., Pfund, J.-L., Sheil, D., Meijaard, E., Venter, M., Boedhihartono, A. K., Day, M., Garcia, C., Van oosten, C., and Buck, L. E. (2013). Ten principles for a landscape approach to reconciling agriculture, conservation, and other competing land uses. Proceedings of the National Academy of Sciences 110, 8349–8356.
| Ten principles for a landscape approach to reconciling agriculture, conservation, and other competing land uses.Crossref | GoogleScholarGoogle Scholar |
Seastedt, T. R., Hobbs, R. J., and Suding, K. N. (2008). Management of novel ecosystems: are novel approaches required? Frontiers in Ecology and the Environment 6, 547–553.
| Management of novel ecosystems: are novel approaches required?Crossref | GoogleScholarGoogle Scholar |
Sinclair, S. J., Zamin, T., Gibson-Roy, P., Dorrough, J., Wong, N., Craigie, V., Garrard, G. E., and Moore, J. L. (2019). A state-and-transition model to guide grassland management. Australian Journal of Botany 67, 437–453.
| A state-and-transition model to guide grassland management.Crossref | GoogleScholarGoogle Scholar |
Smallbone, L. T., Matthews, A., and Lunt, I. D. (2014). Regrowth provides complementary habitat for woodland birds of conservation concern in a regenerating agricultural landscape. Landscape and Urban Planning 124, 43–52.
| Regrowth provides complementary habitat for woodland birds of conservation concern in a regenerating agricultural landscape.Crossref | GoogleScholarGoogle Scholar |
Stringham, T. K., Krueger, W. C., and Shaver, P. L. (2003). State and transition modeling: An ecological process. Journal of Range Management 56, 106–113.
| State and transition modeling: An ecological process.Crossref | GoogleScholarGoogle Scholar |
Taylor, M. F. J., Sattler, P. S., Evans, M., Fuller, R. A., Watson, J. E. M., and Possingham, H. P. (2011). What works for threatened species recovery? An empirical evaluation for Australia. Biodiversity and Conservation 20, 767–777.
| What works for threatened species recovery? An empirical evaluation for Australia.Crossref | GoogleScholarGoogle Scholar |
Tscharntke, T., Steffan-Dewenter, I., Kruess, A., and Thies, C. (2002). Contribution of small habitat fragments to conservation of insect communities of grassland–cropland landscapes. Ecological Applications 12, 354–363.
| Contribution of small habitat fragments to conservation of insect communities of grassland–cropland landscapes.Crossref | GoogleScholarGoogle Scholar |
Wallace, P., and Fluker, S. (2015). Protection of threatened species in New Zealand. New Zealand Journal of Environmental Law 19, 179–206.
Ward, M. S., Simmonds, J. S., Reside, A. E., Watson, J. E. M., Rhodes, J. R., Possingham, H. P., Trezise, J., Fletcher, R., File, L., and Taylor, M. (2019). Lots of loss with little scrutiny: the attrition of habitat critical for threatened species in Australia. Conservation Science and Practice 1, e117.
| Lots of loss with little scrutiny: the attrition of habitat critical for threatened species in Australia.Crossref | GoogleScholarGoogle Scholar |
Wassens, S., Ning, N., Hardwick, L., Bino, G., and Maguire, J. (2017). Long-term changes in freshwater aquatic plant communities following extreme drought. Hydrobiologia 799, 233–247.
| Long-term changes in freshwater aquatic plant communities following extreme drought.Crossref | GoogleScholarGoogle Scholar |
Westoby, M., Walker, B., and Noy-Meir, I. (1989). Opportunistic management for rangelands not at equilibrium. Journal of Range Management 42, 266–274.
| Opportunistic management for rangelands not at equilibrium.Crossref | GoogleScholarGoogle Scholar |
Whelan, R. J., Brown, C. L., and Farrier, D. (2004). The precautionary principle: what is it and how might it be applied in threatened species conservation? In ‘Threatened species legislation’. Other RZS NSW Publications. pp. 49–58. (Royal Zoological Society of New South Wales.)
White, J. M. (1987). The New England lagoons as drought refuges for waterbirds. Emu - Austral Ornithology 87, 253–255.
| The New England lagoons as drought refuges for waterbirds.Crossref | GoogleScholarGoogle Scholar |
White, M. (2013). Doing what’s best for the ecological community – protection under national environment law. Australasian Plant Conservation: Journal of the Australian Network for Plant Conservation 21, 13.
White, M., Ward, N., and Barraclough, P. (2017). Guidelines for nominating and assessing the eligibility for listing of ecological communities as threatened according to the Environment Protection and Biodiversity Conservation Act 1999 and the EPBC Regulations 2000. Commonwealth of Australia. Available at https://www.environment.gov.au/biodiversity/threatened/nominations/forms-and-guidelines [accessed 8 June 2019].
Willis, A. J. (1997). The ecosystem: an evolving concept viewed historically. Functional Ecology 11, 268–271.
| The ecosystem: an evolving concept viewed historically.Crossref | GoogleScholarGoogle Scholar |
Wilson, B. (2002). Influence of scattered paddock trees on surface soil properties: a study of the Northern Tablelands of NSW. Ecological Management & Restoration 3, 211–219.
| Influence of scattered paddock trees on surface soil properties: a study of the Northern Tablelands of NSW.Crossref | GoogleScholarGoogle Scholar |
Winning, G. (2010). Some problems in determining the boundaries of SEPP 14 Wetlands. Wetlands Australia 11, 10–20.
| Some problems in determining the boundaries of SEPP 14 Wetlands.Crossref | GoogleScholarGoogle Scholar |
Wright, B. R., Albrecht, D. E., Silcock, J. L., Hunter, J., and Fensham, R. J. (2019). Mechanisms behind persistence of a fire-sensitive alternative stable state system in the Gibson Desert, Western Australia. Oecologia 191, 165–175.
| Mechanisms behind persistence of a fire-sensitive alternative stable state system in the Gibson Desert, Western Australia.Crossref | GoogleScholarGoogle Scholar | 31372894PubMed |
Yates, C. J., and Hobbs, R. J. (1997). Woodland restoration in the Western Australian wheatbelt: a conceptual framework using a state and transition model. Restoration Ecology 5, 28–35.
| Woodland restoration in the Western Australian wheatbelt: a conceptual framework using a state and transition model.Crossref | GoogleScholarGoogle Scholar |
Zhou, Z.-M., Newman, C., Buesching, C. D., Meng, X., Macdonald, D. W., and Zhou, Y. (2016). Revised taxonomic binomials jeopardize protective wildlife legislation. Conservation Letters 9, 313–315.
| Revised taxonomic binomials jeopardize protective wildlife legislation.Crossref | GoogleScholarGoogle Scholar |
1 Details on Common Assessment Method available at https://www.environment.gov.au/biodiversity/threatened/cam
2 Threatened Species Scientific Committee (2005). Commonwealth Listing Advice on Upland Wetlands of the New England Tablelands and the Monaro Plateau. Available at http://www.environment.gov.au/biodiversity/threatened/conservation-advices/upland-wetlands-new-england-tablelands-monaro-plateau
3 Threatened Species Scientific Committee (TSSC) (2011). Commonwealth Listing Advice on Coolibah-Black Box Woodlands of the Darling Riverine Plains and the Brigalow Belt South Bioregions. Available at http://www.environment.gov.au/cgi-bin/sprat/public/publicshowcommunity.pl?id=66
4 Available at https://www.lls.nsw.gov.au/help-and-advice/land-management-in-nsw/archive/land-management-code-on-invasive-native-species