Bryophytes of Two Peoples Bay Nature Reserve, Western Australia
R. Wyatt A , A. Stoneburner A and Stephen D. Hopper B *
B *
A
B
Abstract
Although the vascular flora of Western Australia is renowned for high species diversity and endemism, very little is known about the state’s moss and liverwort flora.
In 1984 we conducted surveys of bryophytes in Two Peoples Bay Nature Reserve.
We searched comprehensively for bryophytes at seven major habitats on the Reserve, collecting voucher specimens subsequently identified with modern literature and with the help of Australian bryophyte experts. We updated the text to reflect modern taxonomy and considering modern literature on phytogeography and hypotheses associated with OCBIL (Old, Climatically Buffered, Infertile Landscapes) theory.
A total of 38 species of mosses representing 25 genera from 13 families were identified along with 10 species of liverworts representing nine genera and six families. Two moss species (Distichium inclinatum and Tortella dakinii) were previously unknown from Western Australia. Another represented a new species (Pleurophascum occidentale) in a heretofore monotypic genus, family, and order. The Pottiaceae, with 11 species, was the most diverse family of mosses. The Lepidoziaceae, with three species, was the most diverse family of liverworts.
Lower levels of endemism in bryophytes versus vascular plants may reflect the bryophytes’ capacity for wide and long-distance dispersal of spores and fragments. Bryophyte diversity and endemism may be less than on extensive OCBILs because much of the Reserve emerged from the ocean as recently as the mid-Pleistocene.
This, the first listing published of bryophytes on a Reserve in WA, indicates the potential rewards for further survey of mosses and liverworts.
Keywords: ancient lineages, Distichium, herbarium studies, Lepidoziaceae, OCBIL theory, phytogeography, Pleurophascum, Pottiaceae, Tortella.
Introduction
Study of the bryophyte flora of Western Australia has been limited. According to Ramsay (1977), the flora is thought to be depauperate, with only about 100 species compared with more than 1200 species for the rest of Australia. In The Mosses of Southern Australia, Scott and Stone (1976) listed a total of 102 species documented for Western Australia. A census published in 1993, however, reported nearly twice as many: 192 species in 78 genera and 31 families (Stoneburner et al. 1993). Ramsay (1977) also predicted low levels of endemism in the bryophyte flora, in contrast to the high degree of endemism known among flowering plants (Beard 1981; Gioia and Hopper 2017). Ramsay suggested that the low incidence of rainforest habitat may explain why the bryophyte flora is not rich and diverse. From our own experience in the Southwest Australian Floristic Region (SWAFR, Fig. 1), it appears that this assumed low diversity may be attributed at least in part to a paucity of bryophyte collecting (Stoneburner et al. 1993).
Maps of Two Peoples Bay Nature Reserve and (inset) the Southwest Australian Floristic Region showing Provinces and Districts identified by Gioia and Hopper (2017). Collecting localities of bryophytes are in green boxes. These include Robinson Gully (RG); forest at Reserve Office (RO); Waterfall Beach (WB); Sinker Reef (SRf); Rock Island (RI); Sheoak Ridge (SRd); and margins of Lake Gardner (LG). See Tables 1 and 2 for species collected. Base map by A. J. M. Hopkins and G. T. Smith.
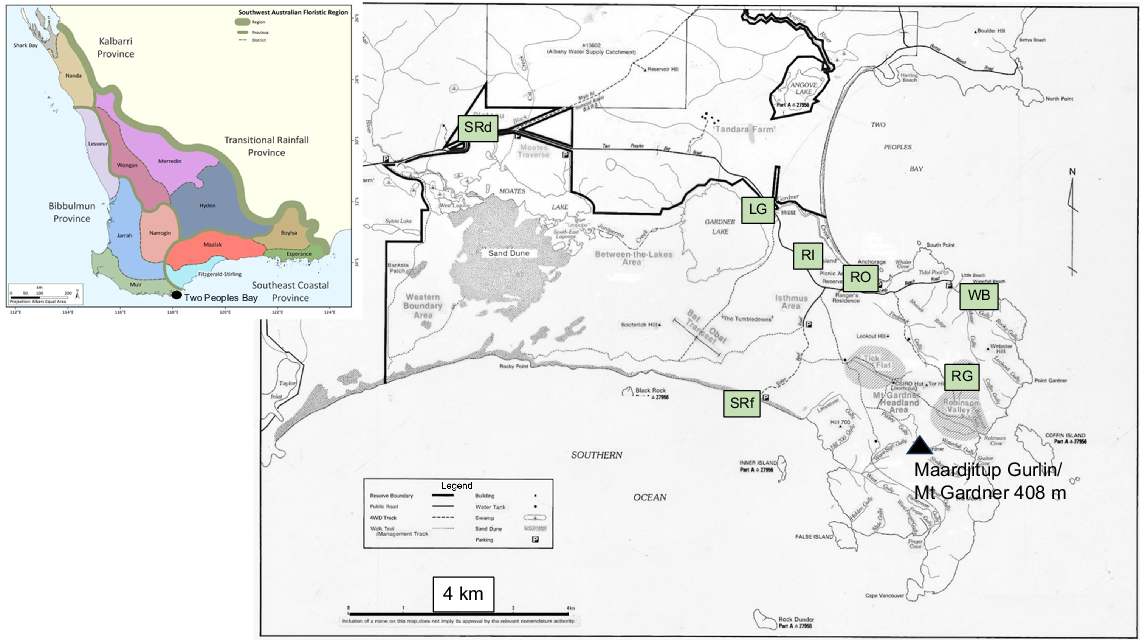
On the other hand, it is probably true that endemism is low, as only four species of mosses and liverworts are known to be restricted to Western Australia. Note, however, that most of our collections were made in the SWAFR, which is famous for high levels of diversity and endemism in the vascular flora (Hopper and Gioia 2004; Gioia and Hopper 2017). Recent development of OCBIL (Old, Climatically Buffered, Infertile Landscapes) theory to explain high diversity and endemism in regions like the SWAFR (Hopper 2009, 2023) may not apply to mosses and liverworts, whose tiny spores are highly dispersible and may be carried over long distances. The first of seven predictions derived from OCBIL theory is that organisms should exhibit reduced dispersibility, leading to high numbers of localised rare endemics and strongly differentiated population systems. Along with many other bryologists, Crum (1972) argued that bryophytes can be considered part of the natural assemblage of whole floras acted upon by the same geological, climatic, and ecological conditions that shape the evolution of the vascular members of that flora. Yet, this seems not to apply to the situation in Western Australia.
At Two Peoples Bay Nature Reserve, the moderate Mediterranean climate with wet, mild winters and cool, dry summers (Hopkins et al. 2024) provides favourable conditions for establishment and growth of bryophyte colonies. The relative youth of much of the landmass of the Reserve, however, may have hindered development of a diverse bryophyte flora. However, the Mt Gardner headland has been above the sea for at least 250 million years (Kohn et al. 2002), likely as a nearshore island during high sea levels (González-Álvarez et al. 2016), thus forming a classic OCBIL (Hopper 2009, 2023), whereas with fluctuating Quaternary sea levels the sands of the isthmus and lakes areas have accumulated only since the mid-Pleistocene (P. E. Playford and I. Tyler, unpubl. data). Crum (1972) argued that, for most species of bryophytes, long-distance dispersal is not common. Nevertheless, wind is an important factor in the environment of the Reserve (Hopkins et al. 2024) and may play a larger role here than expected in long-distance dispersal by spores or plant fragments.
Materials and methods
During the period from April to September 1984, seven sites were visited in the Reserve (Fig. 1) and 105 collections of mosses and 19 of liverworts were made. Voucher specimens of all collections were deposited in the Western Australian Herbarium (PERTH) and in the bryophyte herbarium at Duke University (DUKE). Prior to this survey only one specimen from Two Peoples Bay Nature Reserve (Campylopus bicolor from Mt Gardner) had been collected (PERTH). A limited number of collections made after our survey was completed have been reported to us. These reports are included in Table 1 under the heading ‘Other Collections’.
| Species | RG | RO | WB | SRf | RI | SRd | LG | Other | |
|---|---|---|---|---|---|---|---|---|---|
| Barbula calycina Schwägr. | X | X | X | X | |||||
| Bryum chrysoneuron Müll. Hal. | X | ||||||||
| Campylopus australis Catches. & J.-P. Frahm | X | X | |||||||
| C. bicolor (Hornsch ex Müll. Hal.) Wilson | X | X | X | ||||||
| C. introflexus (Hedw.) Brid. | X | X | X | ||||||
| C. pallidus Hook.f. & Wilson | X | ||||||||
| Codonoblepharon menziesii Schwägr. | X | ||||||||
| Dicranoloma diaphanoneuron (Hampe) Paris | X | ||||||||
| Didymodon australasiae (Hook. & Grev.) R.H. Zander | X | X | |||||||
| D. torquatus (Taylor) Catches. | X | ||||||||
| Distichium inclinatum (Hedw.) Bruch & Schimp. | X | ||||||||
| Fissidens serratus Müll. Hal. | X | X | |||||||
| Funaria hygrometrica Hedw. | X | ||||||||
| F. microstoma Bruch ex Schimp. | X | ||||||||
| F. salcicola C. Müll. | X | ||||||||
| Grimmia laevigata (Brid.) Brid. | X | ||||||||
| Gymnostomum calcareum Nees & Hornsch. | X | X | |||||||
| Hypnum cupressiforme Hedw. | X | ||||||||
| Orthodontium inflatum (Mitt.) Paris | X | ||||||||
| Pleurophascum occidentale R.E. Wyatt & A.H. Stoneb. | X | X | |||||||
| Pseudocrossidium crinitum (Schultz) R.H. Zander | X | ||||||||
| Racopilum cuspidigerum (Schwägr.) Ångstr. | X | X | X | X | X | ||||
| Rhacocarpus purpurascens (Brid.) Paris | X | ||||||||
| Rosulabryum albolimbatum (Hampe ex A. Jaeger) J.R. Spence | X | X | X | ||||||
| R. billarderii (Schwägr.) J.R. Spence | X | X | |||||||
| R. campylothecium (Taylor) J.R. Spence | X | ||||||||
| R. subtomentosum (Hampe ex A. Jaeger) J.R. Spence | X | X | |||||||
| Sematophyllum amoenum (Hedw.) Mitt. | X | X | |||||||
| S. contiguum (Mitt.) Mitt. | X | X | X | ||||||
| S. homomallum (Hampe) Broth. | X | X | X | X | X | X | |||
| Syntrichia princeps (De Not.) Mitt. | X | X | X | ||||||
| Thuidiopsis furfurosa (Hook.f. & Wilson) M. Fleisch. | X | X | |||||||
| Thuidium laeviusculum (Mitt.) A. Jaeger | X | X | |||||||
| Tortella dakinii J.H. Willis | X | ||||||||
| T. rubripes (Mitt.) Broth. | X | ||||||||
| Tortula muralis Hedw. | X | ||||||||
| Trichostomum eckelianum R.H. Zander | X | ||||||||
| Triquetrella papillata (Hook.f. & Wilson) Broth. | X | X | X |
Collection sites are: RG, Robinson Gully; RO, forest at Reserve Office; WB, Waterfall Beach; SRf, Sinker Reef; RI, Rock Island; SRd, Sheoak Ridge; LG, margins of Lake Gardner. ‘Other’ represents reports of specimens collected subsequent to our study.
This survey should be considered a preliminary one. An attempt was made to visit different kinds of habitats in the relatively short time available. More time will be required in the future to study in detail mesic habitats, especially the gullies and sclerophyll woodlands.
Mosses were identified using the manuals of Catcheside (1980) and Scott and Stone (1976) and by comparison with specimens from PERTH and the herbarium of the University of Western Australia (UWA, now incorporated at PERTH). Geographical distributions of the species were noted and compared for provinces within Western Australia, using Beard’s (1980) classification, and within Australia and the rest of the world, as described in the aforementioned manuals and Index Muscorum (van der Wijk et al. 1959–1969). These data were then updated to conform to Gioia and Hopper’s (2017) classification of phytogeographic floristic regions, provinces and districts in the SWAFR. Liverworts were identified using keys made available and subsequently published by Scott (1985). Members of the genus Riccardia were identified using Hewson’s (1970) keys and descriptions. For some problematical specimens we enlisted the help of David G. Catcheside, Ilma G. Stone and George A. M. Scott. Using Tropicos, a plant database maintained by the Missouri Botanical Garden that links more than 1.38 million scientific names with more than 6.94 million specimens, the taxonomy and nomenclature of all the mosses were brought up to date. For liverworts we used the 828-page world checklist of hornworts and liverworts published by Söderström et al. (2016).
Results
Mosses
The 38 species of mosses listed in Table 1 represent 25 genera from 13 families. Two species are reported as new to Western Australia: Distichium inclinatum and Tortella dakinii. Most surprising of our discoveries, however, was a new species of Pleurophascum (Wyatt and Stoneburner 1989), a previously monotypic genus now treated as a separate family and order of mosses (Goffinet et al. 2001; Bechteler et al. 2023). Pleurophascum is estimated to have diverged from its nearest relative Dicranella at about 187 (157–216) million years ago (Bechteler et al. 2023). The Pottiaceae are the largest family represented in the bryophyte flora, with six genera and 11 species.
The habitats occupied by these taxa are not narrowly specific. Most are found on soil at the bases of trees or boulders or on rocks in protected crevices or fissures. In more mesic sites, bryophytes grow on fallen logs or on the trunks and branches of trees and shrubs or over bare rock. Numerous species, although not strictly acidophilic, calciphilic or epiphytic, appear to favour particular habitats over others. Accordingly, granitic sites like Robinson Gully support a different set of bryophytes when compared with Sinker Reef, which is largely limestone. More than one-half of our collections came from just two of the seven sites: Robinson Gully and the forests adjacent to the picnic area at the Reserve Office.
Most of the moss and liverwort species collected at Two Peoples Bay Nature Reserve are found elsewhere in the Southwest Australian Floristic Region (Fig. 1), as defined by Hopper and Gioia (2004) and Gioia and Hopper (2017), notably from the Muir and Jarrah Floristic Districts. Except for Pleurophascum occidentale, all these species have been reported from elsewhere in Australia or New Zealand. The bryophyte flora of South Australia is especially close to that of south-west Western Australia. The greatest number of species (46%) are endemic to Australia and/or New Zealand; 24% demonstrate a Gondwanaland distribution; 27% can be described as nearly cosmopolitan; and only one species (Sematophyllum homomallum) shows an Australasian distribution (i.e. it occurs in Australia, New Zealand and parts of Asia).
Liverworts
Table 2 lists 10 species of liverworts in nine genera and six families, with site notes on their distribution. As with mosses, Robinson Gully supported the most abundant and diverse flora.
| Species | RG | RO | WB | SRf | RI | SRd | LG | |
|---|---|---|---|---|---|---|---|---|
| Riccardia crassa (Schwägr.) C. Massal. | X | X | ||||||
| R. rupicola (Steph.) Hewson | X | |||||||
| Symphyogyna interrupta Carring. & Pearson | X | X | ||||||
| Cephaloziella exiliflora (Taylor) Douin | X | |||||||
| Frullania probosciphora Taylor | X | X | ||||||
| Heteroscyphus planiusculus (Hook.f. & Taylor) J.J. Engel | X | |||||||
| Lophocolea semiteres (Lehm.) Mitt. | X | X | X | |||||
| Paracromastigum longiscyphum (Taylor) R.M. Schust. | X | X | ||||||
| Neolepidozia disparata (J.J. Engel & G.L.Sm.Merr.) E.D. Cooper | X | |||||||
| Tricholepidozia tetradactyla (Hook.f. & Taylor) E.D. Cooper | X | X |
Collection sites were: RG, Robinson Gully; RO, forest at Reserve Office; WB, Waterfall Beach; SRf, Sinker Reef; RI, Rock Island; SRd, Sheoak Ridge; LG, margins of Lake Gardner.
Discussion
Worldwide there are about 14,000 species of mosses and liverworts. Their origin is thought to be more ancient than that of vascular plants (Bechteler et al. 2023). Except for local colonisations of newly available or disturbed sites, their present distributional patterns probably antedate the origin of flowering plants and the breakup of Gondwanaland in the Cretaceous (Crum 1972). A rich and diverse bryophyte flora would not be expected along that portion of the south-west coast that is of geologically recent exposure except where bedrock persists as elevated OCBIL uplands such as Mt Gardner. The genus to species ratio, 1:1.85, is nearly identical to that of mosses of the Hawaiian Islands (1:1.80), another landmass of geologically recent (though pyroclastic) origin (Crum 1972).
The Pottiaceae, the most common family occurring in the Reserve, constitutes a highly polymorphic group. Many of its members are adapted to semi-arid to arid conditions and are commonly thought of as small, tufted mosses of soil and typically basic rocks. There have been many recent changes in the taxonomy of these plants, including recognition of many new genera, based on modern gene sequencing and phylogenetic reconstructions (Bechteler et al. 2023).
Regardless of family relationships, nearly one-half of the species collected in the Reserve have the capacity to occupy open, relatively dry habitats. The morphological adaptations to drought of several of these species have been described in detail by Scott (1982). Those sites with the greatest diversity and cover of bryophytes are the most mesic, especially Robinson Gully and the sclerophyll forests adjacent to the picnic area near the Reserve Office. The driest sites support a distinctly depauperate bryophyte flora, particularly ‘Rock Island’, the shores of Gardner Lake, and Sheoak Ridge.
The bryophyte flora of the Reserve is, as expected, similar to that of adjacent regions. Only three species (Campylopus pallidus, Pseudocrossidium crinitum, and Funaria microstoma) had not been collected previously from the Muir and Jarrah Districts, although they are known from other districts in the SWAFR. Most of the other collections from Two Peoples Bay Nature Reserve represent species that have also been reported from the Muir and Jarrah Districts. The Southeast Coastal Floristic Province appears to have a decidedly lesser overlap with the flora, and the Kalbarri Floristic Province and Boylya Floristic District have the least.
Some distinctive differences in substrate preferences can be identified based on our collections and field experience. Species found primarily on granite rocks, presumed to be acidic, include C. bicolor, Dicranoloma diaphanoneuron, P. occidentale, Rhacocarpus purpurascens, and S. homomallum. P. occidentale has, however, been found on a range of other substrates including quartzite, spongelite and laterite subsequent to our work reported herein. On limestone rocks and soils derived therefrom, presumed to be basic, the following are commonly found: Didymodon australasiae, Gymnostomum calcareum, Funaria salsicola, Tortella rubripes, and Trichostomum eckelianum. An oddity on display near the Reserve Office of Two Peoples Bay is a partial skeleton of a ‘100-year-old right whale’ (Eubalaena australis). The cranium and some of the vertebrae supported growth of several mosses including Barbula calycina, Bryum chrysoneuron, Codonoblepharum menziesii, D. australasiae, Syntrichia princeps, and Thuidium laeviusculum. All of these appear to be species that prefer basic substrates (e.g. Duckett 2017), and recent study of blue (Balaenoptera musculus) and humpback (Megaptera novaeangliae) whale skeletons from Antarctica showed the pH ranges from 7.50 to 8.10 (Putzke et al. 2022).
The flora of south-west Western Australia and South Australia appear very similar, thereby making Mosses of South Australia (Catcheside 1980) an invaluable manual for collectors in Western Australia. Species collected at five or more sites show the following geographical affinities: Racopilum cuspidigerum (Fig. 2) is endemic to Australia and New Zealand; B. calycina and C. bicolor show a Gondwanaland distribution; Campylopus introflexus is cosmopolitan; and S. homomallum (Figs 3 and 4) is found in Australia, New Zealand, and parts of Asia.
Racopilum cuspidigerum, one of the most common mosses from Two Peoples Bay Nature Reserve, was collected from five sites. Photo taken in Porongurup, WA. Except as noted, all photos are courtesy of iNaturalist and used under the Creative Commons Attribution-Non-Commercial (CC BY-NC) license.

Sematophyllum homomallum is very common on acidic rocks, logs, and bases of trees and was collected from six sites in Two Peoples Bay Nature Reserve. Photo taken in Hazelvale, WA.

Sematophyllum homomallum showing its distinctive red and gold colour, though new growth is often green. Photo taken in Denmark, WA.

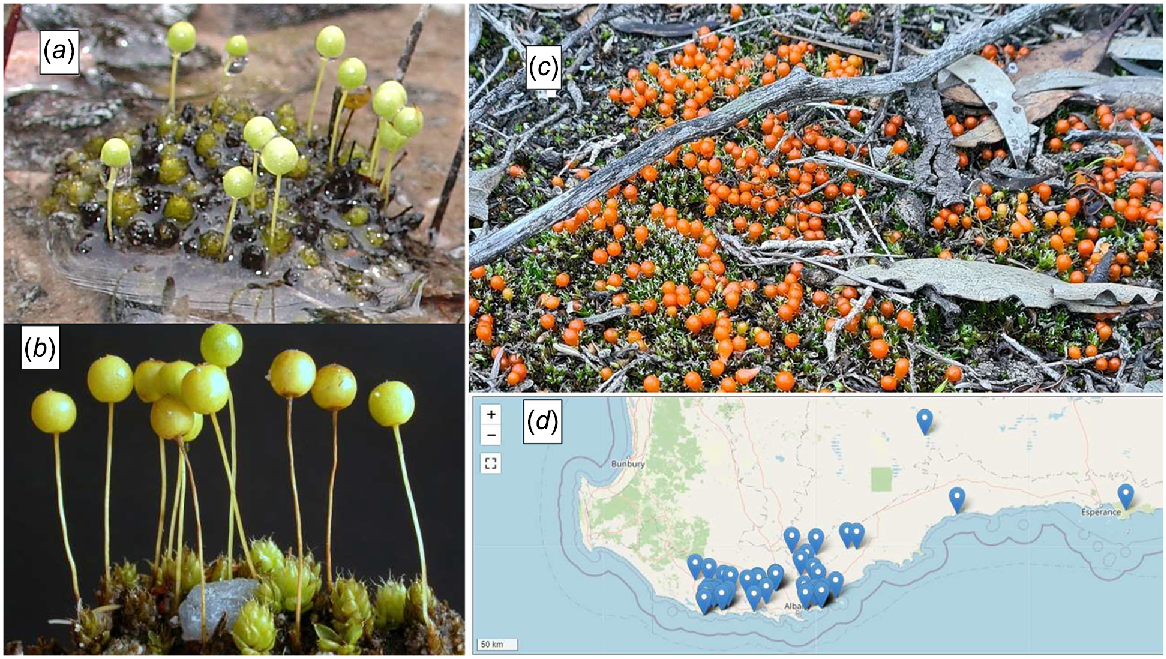
Although the majority of species collected from the Reserve were not unexpected, several collections support the importance of continuing the bryophyte survey: D. inclinatum and T. dakinii had not been reported previously from Western Australia, and initially three species appeared to be new to science. Two of these taxa belonged to genera which are notorious for blurred species boundaries (Bryum and Tortella). With further study these taxa have been assigned to Rosulabryum subtomentosum and T. eckelianum. P. occidentale (Figs 5 and 6), however, differs distinctly from the one described species of the genus, Pleurophascum grandiglobum (Fig. 6), and represents a striking geographical disjunction of the genus from Tasmania and New Zealand, where the latter occurs (Wyatt and Stoneburner 1989). The new species differs in numerous characters from P. grandiglobum, described by Scott and Stone (1976) as a ‘famous bryological rarity’. It is a much larger plant overall with leaves 6 mm × 2.5 mm vs 2–3 mm × 1 mm, a flexuose, hoary, serrate vs rounded to bluntly obtuse leaf tip, and nearly sessile, large, orange, obovoid capsules vs longer-stalked, smaller, and lighter-coloured capsules (Figs 5 and 6). Since our original collection, searching west and east of Two Peoples Bay has significantly extended the range of the species (Fig. 6d). Clearly, it is important to continue floristic investigations of mosses in the SWAFR, a region unique in its geographical diversity and ecological amplitude. More thorough sampling of different habitats and substrates will undoubtedly add many new species of mosses and liverworts to the list of species in the region.
Pleurophascum occidentale with its large, orange, obovoid capsules on very short setae. North-east of Esperance. Photo Michael Warren, iNaturalist.

Pleurophascum grandiglobum immersed in water in situ in Tasmania: (a) photo M. Brown, (b) photo David Tng. Pleurophascum occidentale north-east of Esperance: (c) photo Michael Warren iNaturalist. (d) Pleurophascum occidentale distribution (blue markers) from Florabase February 2024. Pleurophascum occidentale was a new species in a previously monotypic genus and family when first collected and described from Two Peoples Bay Nature Reserve. Its range is now known to extend east to the Esperance district, WA.
The hepatic flora of Australia is even less well-studied than the moss flora. Scott’s (1985) manual has done much to remedy that situation for southern Australia, though he does not consider his manual exhaustive in scope. Distributional information available for Australian liverworts is especially incomplete. The list of species reported here should not be considered definitive, but rather an indication of the orders, families, and genera likely to be most common in the Reserve. Both thalloid (Riccardia crassa: Fig. 7) and leafy (Lophocolea semiteres: Fig. 8) growth forms are represented. For southern Australia, Scott (1985) lists 80 genera in 33 families and five orders.
Riccardia crassa, an impressive thalloid liverwort that grows on streambanks, was collected from two sites in Two Peoples Bay Nature Reserve. Photo taken in Walpole, WA.

Lophocolea semiteres is a leafy liverwort, often growing on bark at the base of Eucalyptus trees, collected from three sites in Two Peoples Bay Nature Reserve. Photo taken in Albany, WA.

Bryophytes play an important role in soil conservation (Moul and Buell 1955; Golding and Stanton 1972), vegetation development (Thieret 1956), and nutrient cycling (Brown 1982). A well-developed colony of a moss or liverwort may be decades in the making. It is well to remember ‘that mosses are vulnerable … and that indiscriminate or wasteful collecting is unethical, immoral and altogether to be deplored’ (Scott and Stone 1976). Of even greater concern in terms of preserving the bryophyte flora is prevention of careless four-wheel driving and use of trail bikes and mountain bikes on granite outcrops and other sensitive habitats that support large colonies of mosses and liverworts (Fig. 9) Research on repair and restoration of such needless destruction of moss mats due to trail damage is only just beginning (e.g. Medeiros et al. 2023). Management policy at Two Peoples Bay Nature Reserve should continue with protection of the flora and fauna as its number one priority.
Granite outcrop moss mats are particularly susceptible to erosion through off road vehicle use such as by mountain bikes, trail bikes, quad bikes and 4WD vehicles. Moss mats often provide habitat for threatened geophytes and annual plants, as well as resurrection plants such as Borya nitida (a). Photos by S.D. Hopper of damage caused to moss mats by: (a) mountain bikes at Mt Clarence Albany; (b) 4WD vehicles at Blue Rock SE of Perth; (c) pedestrian trail Mt Chudulup; (d) trail bikes at Blue Rock; (e) 4WD vehicles and trail bikes at Wandoo National Park east of Perth viewed by participants at a granite rock conservation conference.

Declaration of funding
This research would not have been possible without the invaluable assistance of salaries and operating budgets paid for by the University of Georgia and the Western Australian Government (Departments of Fisheries and Wildlife, Conservation and Land Management, Kings Park and Botanic Garden, Botanic Gardens and Parks Authority), The Royal Botanic Gardens Kew, and The University of Western Australia. Robert Wyatt was in receipt of a Sarah H. Moss Fellowship when the 1984 work was undertaken and also received a John Simon Guggenheim Fellowship.
Acknowledgements
We are grateful for collecting permits issued by the then (1984) Department of Fisheries and Wildlife. Valerie Hobbs and Graeme Smith collected additional specimens after our survey and communicated their identifications to us. Various specimens were referred to David Catcheside, Ilma Stone, and George A. M. Scott, who were most helpful in solving troublesome problems. We thank G. G. Smith and John Green, former curators of the herbaria at UWA and PERTH, respectively, for their assistance with specimens, and Neville Marchant and Marion Blackwell for comments on the manuscript. William R. Buck of the New York Botanical Garden and Sarah Barrett of the Department of Parks and Wildlife in Albany, WA, provided useful comments on the penultimate draft of the manuscript. Mick and Deidre Brown led SDH to a population of Pleurophascum grandiglobum illustrated in Fig. 6a. We are grateful to the Department of Biodiversity, Conservation and Attractions for providing access to Florabase and the map in Fig. 6.
References
Beard JS (1980) A new phytogeographic map of Western Australia. Western Australian Herbarium Research Notes 3, 37-58.
| Google Scholar |
Bechteler J, Peñaloza-Bojacá G, Bell D, et al. (2023) Comprehensive phylogenomic time tree of bryophytes reveals deep relationships and uncovers gene incongruences in the last 500 million years of diversification. American Journal of Botany 110(11), e16249.
| Crossref | Google Scholar |
Brown DH (1982) Mineral nutrition. In ‘Bryophyte ecology’. (Ed. AJE Smith) pp. 383–444. (Cambridge University Press: Cambridge) doi:10.1007/978-94-009-5891-3_11
Crum H (1972) The geographic origins of the mosses of North America’s eastern deciduous forest. The Journal of the Hattori Botanical Laboratory 35, 269-298.
| Google Scholar |
Duckett JG (2017) Whale bones: the world’s most endangered bryophyte habitat. Field Bryology 118, 2-7.
| Google Scholar |
Gioia P, Hopper SD (2017) A new phytogeographic map for the Southwest Australian Floristic Region after an exceptional decade of collection and discovery. Botanical Journal of the Linnean Society 184, 1-15.
| Crossref | Google Scholar |
Goffinet B, Cox CJ, Shaw AJ, Hedderson TAJ (2001) The Bryophyta (Mosses): systematic and evolutionary inferences from an rps4 gene (cpDNA) phylogeny. Annals of Botany 87, 191-208.
| Crossref | Google Scholar |
Golding DL, Stanton CR (1972) Water storage in the forest floor of subalpine forests of Alberta. Canadian Journal of Forest Research 2, 1-6.
| Crossref | Google Scholar |
González-Álvarez I, Salama W, Anand RR (2016) Sea-level changes and buried islands in a complex coastal palaeolandscape in the South of Western Australia: implications for greenfield mineral exploration. Ore Geology Reviews 73, 475-499.
| Crossref | Google Scholar |
Hewson HJ (1970) The family Aneuraceae in Australia and New Guinea. Il. The genus Riccardia. Proceedings of the Linnean Society of New South Wales 95, 60-121.
| Google Scholar |
Hopkins AJM, Smith GT, Saunders DA (2024) Introduction to the special edition of the natural history of Two Peoples Bay Nature Reserve, Western Australia. Pacific Conservation Biology 30, PC24023 https://doi.org/PC24023.
| Google Scholar |
Hopper SD (2009) OCBIL theory: towards an integrated understanding of the evolution, ecology and conservation of biodiversity on old, climatically buffered, infertile landscapes. Plant and Soil 322, 49-86.
| Crossref | Google Scholar |
Hopper SD (2023) Ocbil theory as a potential unifying framework for investigating narrow endemism in Mediterranean climate regions. Plants 12(3), 645.
| Crossref | Google Scholar | PubMed |
Hopper SD, Gioia P (2004) The Southwest Australian floristic region: evolution and conservation of a global hot spot of biodiversity. Annual Review of Ecology, Evolution, and Systematics 35, 623-650.
| Crossref | Google Scholar |
Kohn BP, Gleadow AJW, Brown RW, Gallagher K, O’Sullivan PB, Foster DA (2002) Shaping the Australian crust over the last 300 million years: insights from fission track thermotectonic imaging and denudation studies of key terranes. Australian Journal of Earth Sciences 49, 697-717.
| Crossref | Google Scholar |
Medeiros MB, Cordeiro J, Silva SLL, Salim IH, Reis A, Lacerda TJ, Lobo Seabra EA, Oliveira MF, Moura SP, Santos INR, Bessa L, Fonseca MT, Méndez-Quintero JD, Nero MA, Maciel-Silva AS, Scotti MR (2023) Rehabilitation of eroded trails and gullies on quartzite rock outcrops with native species in a high-altitude grassland. Journal of Environmental Management 326, 116569.
| Crossref | Google Scholar | PubMed |
Moul ET, Buell MF (1955) Moss cover and rainfall interception in frequently burned sites in the New Jersey Pine Barrens. Bulletin of the Torrey Botanical Club 82, 155-162.
| Crossref | Google Scholar |
Putzke J, Schaefer CEGR, Thomazini A, et al. (2022) Changes in plant communities and soil attributes in the “Cousteau’s whale bone skeleton” tourist attraction area in Keller Peninsula after 48 years. Anais da Academia Brasileira de Ciências 94(suppl. 1), e20191467.
| Crossref | Google Scholar |
Ramsay HP (1977) Chromosome numbers of some mosses from Western Australia. Journal of Bryology 9, 343-347.
| Crossref | Google Scholar |
Scott GAM (1982) Desert bryophytes. In ‘Bryophyte ecology’. (Ed. AJE Smith) pp. 105–122. (Cambridge University Press: Cambridge) doi:10.1007/978-94-009-5891-3_4
Söderström L, Hagborg A, von Konrat M, et al. (2016) World checklist of hornworts and liverworts. PhytoKeys 59, 1-828.
| Crossref | Google Scholar |
Stoneburner A, Wyatt R, Catcheside DG, Stone IG (1993) Census of the mosses of Western Australia. The Bryologist 96, 86-101.
| Crossref | Google Scholar |
Thieret JW (1956) Bryophytes as economic plants. Economic Botany 10, 75-91.
| Crossref | Google Scholar |
van der Wijk R, Margadant WD, Florschütz PA (1959–1969) Index Muscorum. Regnum Vegetabile 17, 26, 33, 48, 65. Available at https://search.worldcat.org/title/index-muscorum/oclc/277579807
Wyatt R, Stoneburner A (1989) Pleurophascum occidentale: a new moss from Western Australia. The Bryologist 92, 299-301.
| Crossref | Google Scholar |


