Estimating flying-fox mortality associated with abandonments of pups and extreme heat events during the austral summer of 2019–20
Matthew Mo A Z , Mike Roache A , Janine Davies B , Judith Hopper C , Hugh Pitty D , Natalie Foster E , Sandra Guy F G , Kerryn Parry-Jones
A Z , Mike Roache A , Janine Davies B , Judith Hopper C , Hugh Pitty D , Natalie Foster E , Sandra Guy F G , Kerryn Parry-Jones  H I , Geoff Francis J , Audrey Koosmen C , Leah Colefax K , Chelsea Costello L , Josie Stokes M , Sarah Curran K M , Michael Smith N , Garry Daly O , Carla-Maree Simmons G , Rhonda Hansen K , Desley Prophet K , Sara Judge K , Fiona Major P , Tamsyn Hogarth Q , Carole-Ann McGarry C , Lawrence Pope R , Stephen Brend S , Drew Coxon T , Kimberly Baker T , Kylie Kaye T , Linda Collins U , Michelle Wallis K , Rachel Brown A , Lisa Roberts V , Susan Taylor K , Tim Pearson M W , Tania Bishop X , Pauline Dunne A , Kylie Coutts-McClelland A , Lorraine Oliver A , Chris Dawe A and Justin A. Welbergen Y
H I , Geoff Francis J , Audrey Koosmen C , Leah Colefax K , Chelsea Costello L , Josie Stokes M , Sarah Curran K M , Michael Smith N , Garry Daly O , Carla-Maree Simmons G , Rhonda Hansen K , Desley Prophet K , Sara Judge K , Fiona Major P , Tamsyn Hogarth Q , Carole-Ann McGarry C , Lawrence Pope R , Stephen Brend S , Drew Coxon T , Kimberly Baker T , Kylie Kaye T , Linda Collins U , Michelle Wallis K , Rachel Brown A , Lisa Roberts V , Susan Taylor K , Tim Pearson M W , Tania Bishop X , Pauline Dunne A , Kylie Coutts-McClelland A , Lorraine Oliver A , Chris Dawe A and Justin A. Welbergen Y
A Department of Planning, Industry and Environment, Biodiversity, Conservation and Science; Saving our Species program, 4 Parramatta Square, 12 Darcy Street, Parramatta, NSW 2150, Australia (M. Mo, M. Roache, R. Brown); Hunter Central Coast Branch, 26 Honeysuckle Drive, Newcastle, NSW 2300, Australia (P. Dunne); South East Branch, Level 3, Farrer Place, Queanbeyan, NSW 2620, Australia (L. Oliver); 84 Crown Street, Wollongong, NSW 2500, Australia (K. Coutts-McClelland); North West Branch, 48–52 Wingewarra Street, Dubbo, NSW 2830, Australia (C. Dawe).
B Shoalhaven Bat Clinic and Sanctuary, Wildlife Rescue South Coast, Bomaderry, NSW 2541, Australia.
C Hunter Wildlife Rescue (Native Animal Trust Fund), Shortland, NSW 2307, Australia.
D Friends of Glebe Wetlands, Bega, NSW 2550, Australia.
E Eurobodalla Shire Council, Vulcan Street, Moruya, NSW 2537, Australia.
F Department of Planning, Industry and Environment, Environment Line, Business Information and Services, 4 Parramatta Square, 12 Darcy Street, Parramatta, NSW 2150, Australia.
G Sydney Metropolitan Wildlife Services, Lindfield, Sydney, NSW 2070, Australia.
H Wildlife Animal Rescue and Care Society, Gosford, NSW 2250, Australia.
I School of Life and Environmental Science, Heydon-Laurence Building, University of Sydney, Sydney, NSW 2006, Australia.
J Oatley Flora and Fauna Conservation Society, Oatley, Sydney, NSW 2223, Australia.
K Wildlife Information, Rescue and Education Service, Brookvale, Sydney, NSW 2100, Australia.
L Ku-ring-gai Council, 818 Pacific Highway, Gordon, Sydney, NSW 2072, Australia.
M Ku-ring-gai Bat Conservation Society, Gordon, Sydney, NSW 2072, Australia.
N Shoalhaven City Council, 36 Bridge Road, Nowra, NSW 2541, Australia.
O Gaia Research, North Nowra, NSW 2541, Australia.
P ACT Wildlife, Kambah, ACT 2902, Australia.
Q Fly By Night Bat Clinic, Melbourne, Vic 3788, Australia.
R Friends of Bats and Bushcare, North Carlton, Melbourne, Vic 3054, Australia.
S Parks Victoria, Yarra Bend Park, Yarra Bend Road, Fairfield, Melbourne, Vic 3078, Australia.
T Port Stephens Council, 116 Adelaide Street, Raymond Terrace, NSW 2324, Australia.
U Fauna Rescue of South Australia, Modbury North, Adelaide, SA 5092, Australia.
V Friends of Bats and Habitat Gippsland, Bairnsdale, Vic 3875, Australia.
W Department of Biological Sciences, Macquarie University, Sydney, NSW 2109, Australia.
X University of Queensland, Brisbane, Qld 4072, Australia.
Y Hawkesbury Institute for the Environment, Western Sydney University, Richmond, NSW 2753, Australia.
Z Corresponding author. Email: matthew.mo@environment.nsw.gov.au
Pacific Conservation Biology 28(2) 124-139 https://doi.org/10.1071/PC21003
Submitted: 28 January 2021 Accepted: 12 April 2021 Published: 13 May 2021
Journal Compilation © CSIRO 2022 Open Access CC BY-NC-ND
Abstract
Mass mortalities in flying-foxes occur in summers that reach extremely hot temperatures. In this study, we examine the spatiotemporal distributions of mortality from pup abandonments and extreme heat events in Australian flying-fox camps during the 2019–20 summer. We recorded data on flying-fox mortality in known affected camps and applied a standard method to estimate the number of deaths. Pup mortalities from abandonments were recorded in 10 camps in New South Wales. A minimum estimate of 2612 flying-foxes died in pup abandonments, the majority of which occurred in one camp in Bomaderry. Die-offs from extreme heat events were recorded in 40 camps associated with eight separate heat events in south-eastern Australia. A minimum estimate of 72 175 flying-foxes died during these heat events, which all occurred within the range of the threatened grey-headed flying-fox (Pteropus poliocephalus). Further, 409 and 2251 live flying-foxes were taken into care from pup abandonments and heat events respectively. The minimum mortality estimated represents the highest recorded mortality of Australian flying-foxes within a single summer. This highlights a need to restore vegetation in flying-fox foraging areas and camps, address anthropogenic climate change and gather more empirical data to inform heat stress interventions to minimise flying-fox mortalities.
Keywords: climate change, extreme weather, heat stress, Pteropus poliocephalus, summer, threatened species, wildlife conservation, wildlife rehabilitation.
Introduction
Mass mortality events in animals provide important triggers for wildlife management and conservation as they may remove a substantial proportion of a population within a short period of time, which, in turn, can affect the dynamics and overall viability of wildlife populations (e.g. Frick et al. 2010; Mason-Romo et al. 2018), as well as the structure and function of ecosystems (e.g. McDowell et al. 2017; Godfree et al. 2019). For managers, die-offs highlight the stressors that are potentially important population-level threats for particular species (Kock et al. 2018), such as disease (Isidoro-Ayza et al. 2019; Gizzia et al. 2020), nutritional deficiency (Jones et al. 2019), chemical factors such as poisoning (Richards et al. 2017), and physical factors such as extreme weather events, including those associated with droughts (Foley et al. 2008) and extreme heat (Welbergen et al. 2008; Pruvot et al. 2019; Till et al. 2019). Such extreme weather events are set to escalate under climate change (Easterling et al. 2000; Meehl and Tebaldi 2004), and thus understanding how animal populations are affected is key for addressing future challenges for wildlife management and conservation (Maxwell et al. 2019).
Flying-foxes (genus Pteropus) are phytophagous megachiropteran bats that play an important role in forest ecosystems as long-distance pollinators and seed dispersers (Eby 1991; Fujita and Tuttle 1991; Southerton et al. 2004); however, a large proportion of flying-fox taxa are at some risk of extinction (Vincenot et al. 2017). Their ecological function is likely encumbered by large reductions in their numbers (McConkey and Drake 2006). In Australia, two of the four mainland flying-fox species, the grey-headed flying-fox (P. poliocephalus) and the spectacled flying-fox (P. conspicillatus), are federally listed as threatened with extinction (Westcott et al. 2015). A highly-mobile species (Welbergen et al. 2020), P. poliocephalus occurs in eastern and southern Australia as a single interbreeding population (Webb and Tidemann 1996), sharing substantial parts of its range with the black flying-fox (P. alecto) and little red flying-fox (P. scapulatus) (Roberts et al. 2012). Past surveys indicated a population decline of at least 30% between 1989 (Parry-Jones 2000) and 1998 (Eby et al. 1999). Recent surveys place population estimates at approximately 680 000 individuals (Westcott et al. 2015).
Flying-foxes are seasonal breeders, typically producing one pup per year (Martin et al. 1996). In both P. poliocephalus and P. alecto, males generally establish territories in camps in January prior to nursing females weaning their pups from the previous cycle (Eby 1995; Welbergen 2011). Copulation typically occurs from February to May (Martin and McIlwee 2002; Connell et al. 2006). Pups are born mainly in October and November (Eby 1995), and remain with their mothers for at least 3–4 months (Martin et al. 1996; Markus and Blackshaw 2002; Welbergen 2011). Nursing females initially carry pups during foraging forays but start to leave them in camps after 3 weeks (Eby 1995). At around 12 weeks of age, juveniles begin venturing out of the camps on their own account (Welbergen 2010). As a consequence of this reproductive phenology, the timing of gestation commonly coincides with seasonal food resource bottlenecks in winter and spring (Eby et al. 1999; Parry-Jones and Augee 2001; Law et al. 2002). Furthermore, these bottlenecks are exacerbated by large-scale habitat destruction, particularly the removal of winter foraging habitat (Eby et al. 1999). Mass die-offs in pups are generally caused by dehydration or starvation following the continued absence of nursing females prior to weaning. This may occur for various reasons, including when pups are abandoned by nursing females suffering nutritional stress or when pups are orphaned when nursing females are killed while foraging (Parry-Jones 2000; Divljan et al. 2011). Pups separated from their mothers represent a major component of flying-foxes requiring ex-situ rehabilitation by wildlife carers (Mo et al. 2021a).
Mass die-offs in flying-foxes also occur during extreme heat events (Welbergen et al. 2008). This represents an emerging issue, particularly for P. poliocephalus, P. alecto and P. conspicillatus (Welbergen 2012; McKechnie and Wolf 2019), as extreme heat events become increasingly frequent and severe due to anthropogenic climate change (Meehl and Tebaldi 2004; Steffen et al. 2014). Furthermore, the hottest months of the year coincide with the time when flying-foxes rear their pups (Snoyman et al. 2012). Apart from ambient temperatures, the severity of heat stress in animals is influenced by a range of environmental factors such as humidity, wind speed, solar radiation, roost vegetation condition (affecting availability of shade refuge), and consecutive days of very high temperatures (Bohmanova et al. 2007; Ratnayake et al. 2021). The internal factors influencing heat stress severity include solar reflectance, fur depth, body mass, posture and pre-existing health status (Porter and Gates 1969; Bohmanova et al. 2007; Ratnayake et al. 2021). Both megachiropteran and microchiropteran bats deploy a predictable sequence of thermoregulatory behaviours with increasing ambient temperatures (Welbergen et al. 2008): wing-fanning to induce forced convection (Laburn and Mitchell 1975); shade-seeking to reduce exposure to solar radiation exposure (Licht and Leitner 1975a); and salivation and panting to induce evaporative cooling (Bartholomew et al. 1964; Licht and Leitner 1975b). However, flying-foxes begin dying from hyperthermia when ambient temperatures exceed 42°C (Welbergen et al. 2008), at which stage thermoregulatory behaviours are insufficient to stop body temperatures reaching critical thresholds. Published rescue data for P. poliocephalus suggest that extreme heat events are major causes of mortality, alongside powerline electrocutions and fruit-netting and barbed-wire entanglements (Tidemann and Nelson 2011; Scheelings and Frith 2015; Mo et al. 2021a). Despite frequent representation of this issue in news media, there are few documented estimates of flying-foxes lost in these die-offs in scientific literature (Welbergen et al. 2008; Ratnayake et al. 2019).
During the austral spring and summer of 2019–20, there were die-offs of P. poliocephalus and P. alecto in eastern and south-eastern Australia from a sequence of circumstances associated with prolonged drought. Initially, a starvation event across a broad area of northern New South Wales (NSW) and south-eastern Queensland resulted in a large but unquantified number of adult flying-fox deaths (Cox 2019; Heathcote 2019), possibly in the tens of thousands based on anecdotal reports from wildlife carers. There were then die-offs of flying-fox pups in south-eastern NSW, which we assumed resulted from nursing females abandoning or becoming separated from them. These die-offs were followed by further die-offs of both adults and young from extreme heat events across south-eastern Australia. Finally, there were several concurrent megafires, termed the Black Summer bushfires, which burnt more than 10 million hectares of mostly temperate forests (Boer et al. 2020; Nolan et al. 2020), substantially reducing available foraging habitat for flying-foxes. This was compounded by subsequent heavy rainfall in some areas, which likely induced further starvation from the temporary depletion of pollen and nectar supplies. Although the full extent of the impacts of these events is difficult to establish, the overall impact was likely substantial for flying-foxes, and especially for P. poliocephalus as these events occurred over a large proportion of this species’ range. Die-offs from abandonments of pups and extreme heat events were localised in camps, which made a collaborative data gathering effort possible so that mortality could be quantified.
In this study, we examined the spatiotemporal distributions of flying-fox mortality in camps known to us from abandonments of pups and extreme heat events during the 2019–20 summer in Australia. We provide conservative estimates of the number of flying-foxes killed in these circumstances, and discuss the implications of such events on flying-fox conservation and management, particularly the need for research to inform heat stress management and building capacity for the wildlife rehabilitation sector.
Materials and methods
Data collection
Die-offs of pups were observed in camps prior to any extreme heat events occurring in those regions during the study period. We assumed these mortalities were due to nursing females abandoning their pups, although we did not gather specific evidence to confirm this. We defined abandonments of pups as events that observers found at least 10 dead pups in a camp within a short period of time (e.g. 1–8 days). This definition excludes circumstances in which fewer carcasses are found in camps, which can be considered normal thresholds during pup-rearing season (M. Mo, J. Hopper, pers. obs.). We became aware of these die-offs from site visits, either as part of routine inspections to monitor camp size, searches for injured flying-foxes, or responsive inspections triggered by reports of injured and dead flying-foxes from the public. Abandonments of pups were distinguished from die-offs caused by extreme heat by (1) the lack of high ambient temperatures at these camps within 5 days prior to observations of carcasses (extreme heat events in flying-fox camps typically involve ambient temperatures exceeding 42°C; Welbergen et al. 2008), and (2) carcasses in these camps comprising solely of pups.
We defined die-offs from extreme heat events as a day or series of days during which flying-fox deaths occurred in association with high ambient temperatures, adapted from Ratnayake et al. (2019). Wildlife carers and land managers anticipated extreme heat events in their local areas by observing meteorological forecasts for predictions of ambient temperatures above 42°C. This includes monitoring of forecasts made by the Flying-fox Heat Stress Forecaster (Lab of Animal Ecology 2020a), which presents spatially-explicit forecasts of which flying-fox camps are likely to experience heat stress up to 3 days into the future (Ratnayake et al. 2019). Die-offs from extreme heat events were determined from attending camps during or within 5 days of high ambient temperatures and recording dead flying-foxes. These observations were made by a large number of people including wildlife carers, other volunteers and local and state government staff. Unlike pup abandonments, die-offs from extreme heat events would generally involve deaths in both adult and young flying-foxes (Welbergen et al. 2008). All deaths directly associated with high ambient temperatures were attributed to extreme heat events.
We collected information on die-offs from pup abandonments and extreme heat events from persons attending affected camps. Details of camp locations, dates, indicative temperatures from the nearest weather station (Australian Bureau of Meteorology 2020), flying-fox species present at the camp and numbers of flying-foxes that died or were taken into ex-situ rehabilitation were collated. The information was collected through formal and informal peer-to-peer networks: these included Wildlife Health Australia’s Bat Health Focus Group, which comprises representatives of federal and state government departments of agriculture, public health and environment, research organisations, veterinarians, wildlife carers and other relevant organisations; the NSW Flying-fox Consultative Committee, which comprises representatives of government agencies, not-for-profit organisations and the agricultural sector; and the NSW Flying-fox Land Managers’ Network, an email-based network comprising local councils and other public land managers. Information was also collected through an online data form hosted by Western Sydney University’s Lab of Animal Ecology (2020b). The data captured were therefore dependent on the location of observers available to attend camps and not expected to be evenly distributed throughout the range of P. poliocephalus, P. alecto, and P. conspicillatus.
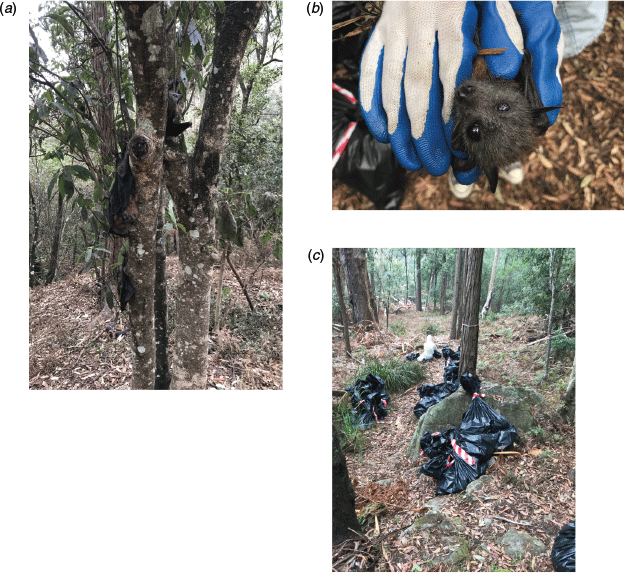
Numbers of dead flying-foxes were obtained through direct counts where possible. However, in some cases where excessive numbers of carcasses were present, numbers were estimated based on different sampling methods. In these cases, estimates were flagged as ’approximate’. The most common estimation method was the grid method, which involved collecting and laying out carcasses in rows of a set number to obtain estimates by multiplication over the number of rows formed (Fig. 1). A weighing method was also used in which samples of up to 20 carcasses were individually weighed to determine the mean mass of a single carcass. Disposal bags filled with all collected carcasses were then weighed to estimate total number based on the mean individual mass. A density method was also used, which involved counting the number of carcasses within 10–15 quadrats of 1 m2 to determine an average density that was then applied to the affected area. For some locations, only an eyeball estimation was done, involving the observer making a judgement of the number of carcasses roughly by sight. In these cases, observers assigned a range estimate (e.g. 100–200 carcasses). For cases when complete counts were not possible (if there were carcasses clumped in trees or the core area of the camp was not accessed to minimise disturbance of live flying-foxes), estimates only included carcasses that were visible to observers and were recorded as ‘greater than’. Injured flying-foxes that were euthanised in-situ were included in the tally of dead animals. Population estimates of affected camps were not recorded at the times mortalities were found, thus it was not possible to scale the numbers of carcasses to population size.

|
The number of flying-foxes taken into care was obtained from records maintained by wildlife rehabilitation organisations. In some cases, the accuracy of these records was limited due to flying-foxes taken into care being distributed to various carers before a proper inventory was undertaken. In these cases, the minimum number of flying-foxes taken into care was recorded and flagged as ‘approximate’ or ‘greater than’ based on the attending wildlife carers’ assessment.
Data analysis
To obtain a conservative estimate of flying-fox deaths from the die-offs recorded from pup abandonment and extreme heat, we standardised records of mortality using the following criteria. For mortalities reported as a range estimate, the lower estimate was used (e.g. ‘100–500 carcasses’ was accounted as 100 deaths). Mortalities reported as ‘hundreds’ or ‘thousands’ were accounted as 150 and 1500 deaths respectively. Mortalities reported as ‘greater than’ were only accounted from the known quantity (e.g. ‘>150 carcasses’ was accounted as 150 deaths). Standardised records of mortalities were combined to generate an overall estimate of flying-fox deaths.
Where the numbers of flying-foxes taken into care were estimates, they were standardised as above. Similarly, standardised records of flying-foxes taken into care were combined to generate an overall estimate of flying-foxes transferred to ex-situ rehabilitation.
The distribution of recorded die-offs from pup abandonments and extreme heat events were plotted by mapping the locations of affected camps. We used meteorological data collected from Australian Bureau of Meteorology (2020) weather stations nearest to each camp to identify the maximum temperatures occurring within 5 days prior to observations of carcasses.
Results
Abandonments of pups
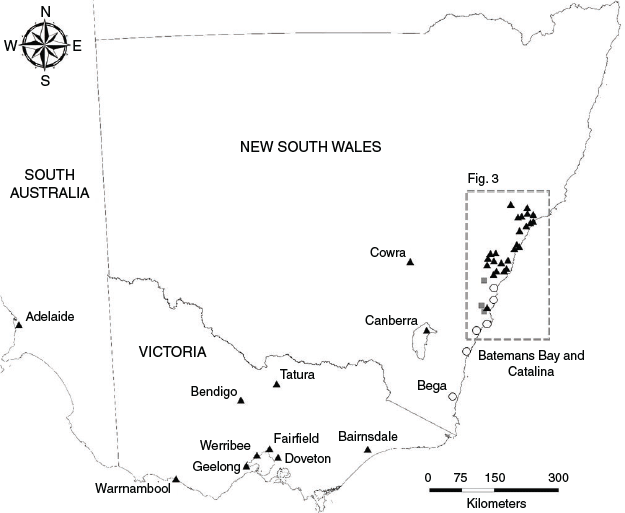
We recorded die-offs from pup abandonments in 10 camps in south-eastern NSW (Figs. 2, 3). These camps were solely occupied by P. poliocephalus, such that we could attribute the total mortality to this species. Meteorological data showed that these events were not associated with extreme heat (Australian Bureau of Meteorology 2020), although extreme heat events were subsequently recorded in three of these camps (Bomaderry, Kangaroo Valley, Picton). Carcasses in one camp (Bega) were first observed on a warmer day (21 November, maximum temperature recorded at the nearest weather station 40.3°C; Supplementary material Table S1); however, carcasses were consistently malnourished and appeared over a 4-week period, mostly between 7 and 14 days after the warmer day. Thus, we deduced that these pups likely died from being separated from their mothers, rather than an extreme heat event. Notably, several P. poliocephalus in these camps, both males and females, were in poor condition indicating nutritional stress.
|
|
In five camps, die-offs were recorded as early as late November (Table 1, and see Table S2 for specific dates). In one of these camps, mortality was only observed on a single day and the camp was not inspected prior or afterward (Corrimal). Other die-offs that commenced in late November occurred over a period of weeks (Bega, Picton) to months (Batemans Bay, Catalina), though there were notable spikes in mortalities that distinguished these die-offs from normal thresholds of pup mortality (e.g. 73 carcasses found in Bega from 3–6 December). In four other camps, die-offs were identified on single days around mid-December (Booderee Botanic Gardens, Bomaderry, Kangaroo Valley, Shellharbour) but three of these camps were not inspected until subsequent extreme heat events. A die-off in a tenth camp, Yatte Yattah was also recorded at the end of December (meteorological data showed there was no prior extreme heat event; Table S1).
Mortalities from pup abandonments ranged from at least 12 deaths (Shellharbour) to greater than 1600 deaths (Bomaderry) recorded on single days. Our conservative estimate is that at least 2612 pups died from abandonments. The die-off in Bomaderry accounted for approximately 62% of this total estimate. There were also live pups taken into care at three camps, totalling at least 409 pups taken into care from pup abandonments.
Extreme heat events
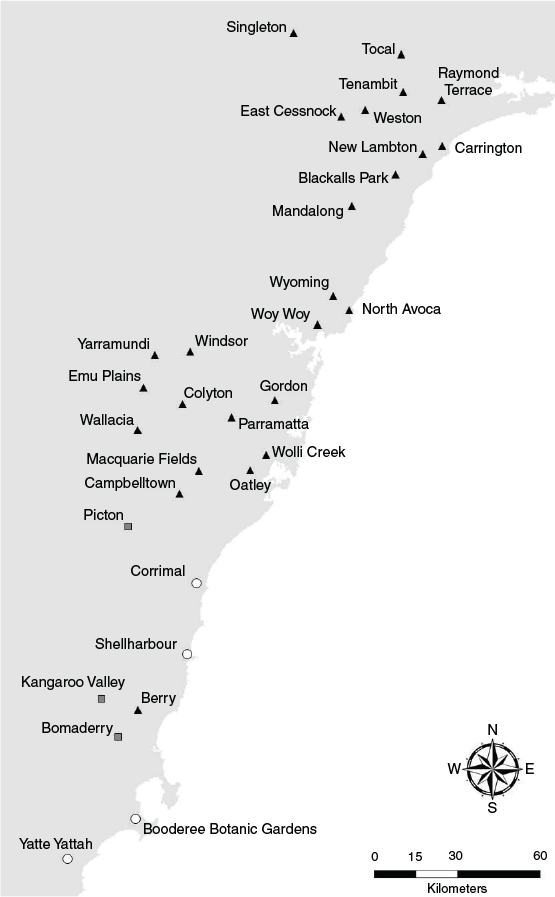
Die-offs from extreme heat events during the 2019–20 summer were observed in 40 camps: 30 in NSW, 1 in the Australian Capital Territory (ACT), 8 in Victoria, and 1 in South Australia (Table 2). Twenty-three camps were solely occupied by P. poliocephalus at the time of mortalities, and the remaining 17 camps were coinhabited by P. poliocephalus and P. alecto (P. scapulatus were also present in some camps but were not recorded as carcasses). All but four affected camps were within 70 km from the coast (Fig. 2). Approximately 65% of affected camps were located within the Sydney metropolitan area and Hunter region (Fig. 3). Of the eight affected camps in Victoria, three were located within the Melbourne metropolitan area. There are few known camps in South Australia and its only permanent camp in Adelaide was the only camp in the state from which die-offs from extreme heat events were recorded. No extreme heat events were recorded within the range of P. conspicillatus.
Meteorological data show that there were eight discrete heat events from November to February (Table S3), one event over 2 days in November, three events over 1–5 days in December, and four events over 1–4 days in January and early February (Table 3). Heat events were associated with masses of hot air moving from South Australia towards the eastern states in six of these heat events (Australian Bureau of Meteorology 2020). For example, in heat event 1, Adelaide experienced a maximum temperature of 42.2°C on 20 November, preceding Werribee experiencing a maximum temperature of 42.4°C the following day (other die-offs may have occurred in Victoria on 21 November, but were not observed). Similarly, in heat events 3 (17–21 December), 4 (27–31 December) and 8 (29 January–2 February), high temperatures in Adelaide preceded die-offs in the Victorian camps, then camps in Commonwealth Park, Canberra and NSW. The highest numbers of heat-affected camps recorded were in heat events 3 (17–21 December), 4 (27–31 December), and 5 (3–5 January).
In camps where the number of flying-foxes killed was recorded, death rates ranged from only a single individual (Adelaide, 30 January) to approximately 10 000 flying-foxes (Tocal, 10 December) on single days of extreme heat (Table 2). In total, we gathered data to conservatively estimate that at least 72 175 adult and young flying-foxes died in extreme heat events (Table 2). The highest mortalities were recorded in eight camps in which the known number of deaths ranged from 4000 to 10 000. Mortalities from these camps accounted for 76% of our estimate of all extreme heat mortalities. In particular, extreme heat mortalities in Adelaide, Kangaroo Valley, Gordon (Fig. 4) and Tocal each comprised more than 9% of our total estimate of extreme heat mortalities. It was generally observed that a large proportion of mortalities were pups, juveniles and lactating females, though demographic resolution was largely absent in the data to provide any meaningful quantification. From photographs of some carcasses, it also appears some mortalities were of pups that had been born prematurely (Fig. 5).
|
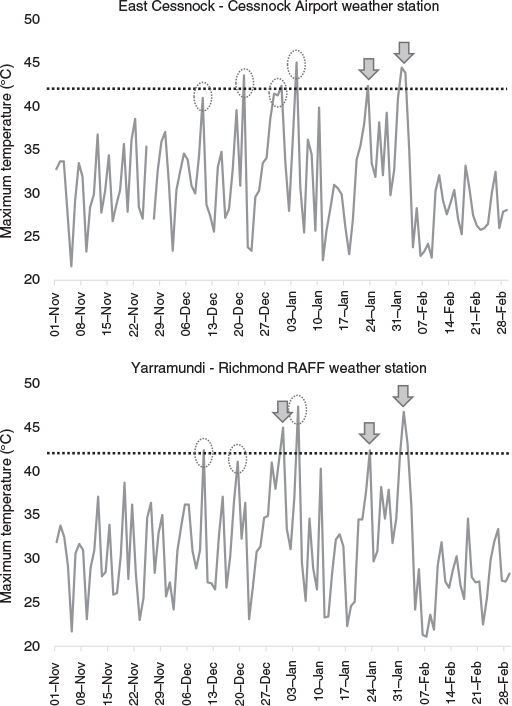
By observing meteorological data from weather stations close to affected camps, we determined there may have been unrecorded extreme heat-related mortality; using examples of East Cessnock and Yarramundi, there were two and three periods of very high temperatures respectively in which the camps were not monitored (Fig. 6). In contrast, we observed camps during ambient temperatures greater than 42°C that did not result in mortality (e.g. Parramatta on 1 February). In particular, observers monitored the Adelaide camp on any days predicted to exceed 40°C, which showed that mortalities did not result from heat events 4, 5, and 6 despite temperatures exceeding 42°C (Table S3).
|
We recorded flying-foxes transferred to ex-situ rehabilitation from extreme heat events in 19 camps (Table 2). Our records show that the minimum number of flying-foxes taken into care in extreme heat events was 2251.
In at least two camps, heat-related mortalities were likely exacerbated by storms prior to heat events. In Gordon, there were tree falls caused by a windstorm on 26 November, which substantially reduced shade refugia within the camp. This windstorm occurred less than 2 months before heat event 5. Similarly, the camp in Canberra was affected by a hailstorm on 20 January, 11 days before heat event 8 (see also Major 2020). Wildlife carers were recovering flying-foxes with hail-related injuries for 6 weeks following the storm, initially daily for the first 13 days. Most of the flying-foxes that died in this camp during heat event 8 had sustained injuries from the hailstorm (Fig. 7). In other camps, prolonged dry conditions had caused wetlands to dry up (e.g. Raymond Terrace, Oatley), which reduced flying-foxes’ access to water during heat events.

|
Interventions by humans most likely influenced whether heat stress was ameliorated or exacerbated. There was great variation in how affected camps were treated: first, whether there were interventions, and second, which intervention methods (see also Mo and Roache 2021) were implemented and the manner in which they were implemented. As an example, in Cowra, flying-foxes that were observed to have descended within 2 m of the ground were lightly sprayed with water, which potentially reduced the number of mortalities. In these interventions, personnel were limited, which likely contributed to minimising additional distress to flying-foxes. In contrast, there were some instances where interventions were either attempted by unlicensed persons or were inconsistent with existing protocols for intervening during extreme heat events (e.g. Department of Environment and Climate Change 2008; Bishop et al. 2019; Collins et al. 2019a). These situations may have increased the numbers of flying-foxes killed that may have otherwise survived. In addition, one of the authors observed members of the public harassing flying-foxes during heat events, which also potentially exacerbated mortalities.
Discussion
As colonial and conspicuous species, flying-foxes represent an important bioindicator of the effects of extreme weather events, raising concern that similar impacts occur in more solitary and cryptic species (Welbergen et al. 2014). Our conservative estimates indicated that during the summer of 2019–20 in Australia, at least 72 175 flying-foxes died in 40 camps during eight extreme heat events, and a further 2612 pups died in 10 camps from pup abandonments. This resulted in a total of more than 74 000 deaths of both adult and young flying-foxes. This represents a high level of mortality even though these estimates did not attempt to capture flying-fox deaths from starvation effects from drought and heavy rainfall and possible mortalities directly from the Black Summer bushfires.
A substantial proportion of the reported deaths involved P. poliocephalus, a vulnerable species with an estimated population size of around 680 000 individuals (Westcott et al. 2015). There were 27 041 mortalities recorded from 23 camps solely occupied by P. poliocephalus (Table 2), which alone represents more than one-third of the total estimate of mortalities. The remaining mortality (45 103) was from 17 camps occupied by both P. poliocephalus and P. alecto (Table 2). Although P. alecto is typically worse affected in extreme heat events than P. poliocephalus (Welbergen et al. 2008), we expect that P. poliocephalus comprised the majority of the total estimate based on wildlife carers and land managers noting that there were visually more P. poliocephalus carcasses than P. alecto carcasses at some shared camps. We also infer this across the affected area from P. poliocephalus being considerably more common within this range (McClelland 2009; Roberts et al. 2012) and at least seven times more abundant than P. alecto at 16 of the shared affected camps from which species composition data are available (Department of Agriculture, Water and the Environment 2020). Although the deaths represent a substantial proportion of the known population, it is unclear whether it represents a true loss to the population or a temporary forward displacement of mortality of already compromised flying-fox individuals that would likely have died from other causes in the short-term anyway (‘harvesting effect’; Huynen et al. 2001). Nevertheless, what is known is that extreme heat events predominantly affect juveniles and lactating adult females, which is of particular concern for flying-fox conservation as this can have disproportionate impacts on recruitment and the size of the effective breeding population respectively (Welbergen et al. 2008). Continued monitoring of population trends is needed to ascertain whether mortality from these series of extreme heat events has had a significant impact on the population of P. poliocephalus.
The recorded deaths likely represent underestimates of the true flying-fox mortality associated with extreme heat events of the 2019–20 summer. We expect there was flying-fox mortality in camps affected by extreme heat events, indicated by meteorological data, but not inspected during or following the events (e.g. Fig. 6). However, it is important to note that microclimatic conditions in camps can differ markedly from those recorded at nearby weather stations (Snoyman and Brown 2010), although Ratnayake et al. (2019) showed that 24‐ and 48‐h weather forecasts can accurately predict 77 and 73% of die‐offs, respectively. Furthermore, in some camps, mortality may have been underestimated due to visually inaccessible carcasses not being counted, carcasses being removed by scavenging animals (N. Foster, pers. obs.) or local residents disposing of them prior to counts. Some of the sampling approaches for estimating the number of carcasses may also have introduced errors. For example, the highest mortality within a camp was recorded in Tocal; here, the number of carcasses within a relatively small representative number of 1 m2 quadrats (10) was used to infer the mortality across the entire ~3 ha camp. In this case, relatively small initial counting errors and/or inadvertent bias in the selection of quadrats could have led to large over- or underestimates of mortality in that camp. However, the need for such sampling is a reflection of the scale of die-offs encountered at the worst affected camps. The same limitations were relevant to eyeball estimations. On balance, we are confident that our mortality estimates are underestimates, despite the serious practical limitations of estimating mortality at such a vast landscape scale.
Extreme heat events represent an important management issue for flying-fox conservation, reflected in at least two studies as one of the leading causes of debilitation in P. poliocephalus and P. alecto requiring rescue and rehabilitation (Tidemann and Nelson 2011; Mo et al. 2021a). Previous researchers have reported die-offs from single summers estimated in the tens of thousands. In the 2013-14 summer, more than 50 000 flying-foxes died in extreme heat events (J. A. Welbergen, unpubl. data), including the 45 500 P. poliocephalus and P. alecto killed across 52 camps reported by Welbergen et al. (2014). More recently, extreme heat events in the 2018-19 summer killed at least 48 000 flying-foxes, including approximately 23 000 P. conspicillatus and 20 000 P. alecto in north-eastern Queensland (Mao 2019; McKechnie and Wolf 2019), more than 4000 P. poliocephalus in Adelaide (Cockburn 2019) and less than 1000 flying-foxes in smaller die-offs (J. A. Welbergen, unpubl. data). In comparison, our conservative estimate of 72 175 deaths from heat events in the 2019-20 summer potentially represents the most severe summer of heat-related mortality in flying-foxes recorded to date. In light of climate change predictions of more frequent, longer and more intense heatwaves in Australia (Steffen et al. 2014), similar or potentially worse summers for flying-fox mortalities can be expected in the future (see also Welbergen et al. 2008).
Flying-fox camps near residential communities are often a source of human-wildlife conflicts (Kung et al. 2015) and there are broad negative public perceptions of bats (Lunney and Moon 2011). Where we observed deliberate disturbance to flying-foxes from humans during heat events, the camp was located on private property and within 20 m of residential dwellings. This suggests that flying-foxes roosting in urban areas may be at greater risk during extreme heat events due to deliberate disturbance if they are perceived by local communities to be a nuisance. During our study, experienced wildlife carers also reported incidents of unlicensed persons spraying flying-foxes in manners contrary to existing protocols (e.g. Department of Environment and Climate Change 2008; Bishop et al. 2019; Collins et al. 2019a). In these situations, there is the risk that these activities could exacerbate flying-fox mortality, rather than ameliorate it.
The severity of heat stress is likely influenced by whether human intervention occurred and the methods used. We were aware that intervention methods aimed at reducing die-offs varied between affected camps, ranging from hand spraying of roost vegetation and ground-based or tree-mounted sprinklers to collecting heat-stressed animals for intensive cooling and rehydration treatments (as described by Bishop et al. 2019; Collins et al. 2019a). Anecdotal observations by experienced wildlife carers suggest that these intervention methods can benefit flying-fox survival during heat events, however carefully controlled studies have not been carried out (Mo and Roache 2021). The absence of empirical evidence for these methods has prompted concerns that some well-meaning actions may cause additional stress for flying-foxes at their thermal limits (Ratnayake et al. 2019); for example, human presence may cause already heat-stressed flying-foxes to take flight, or spraying may decrease vapour pressure deficits within camps and interfere with flying-foxes’ thermoregulatory responses that rely on evaporative cooling (e.g. Bartholomew et al. 1964). These uncertainties highlight the urgent need for controlled experiments to inform improvements in flying-fox heat stress management.
Our observations allude to some preceding factors that potentially influence the extent of die-offs during extreme heat events. First, the amount of exposure to solar radiation contributes to the total heat load of individual flying-foxes (Snoyman et al. 2012; Ratnayake et al. 2021). This highlights the importance of intact vegetation strata in camps during extreme heat events. Notably, during heat event 5, flying-foxes in Gordon were affected in areas of the camp that were previously densely vegetated and would have provided adequate shade refuge if not for the prior storm damage. This possibly influenced the numbers of flying-foxes that died, combined with the large numbers of flying-foxes present at the camp around this time (43 000 individuals counted on 20 December, and 35 000 individuals counted on 14 January; Ku-ring-gai Council, unpubl. data). Maintaining vegetation strata in camps could thus potentially provide an effective long-term measure for mitigating the impacts of extreme heat events on flying-fox camps; however, the defoliation of roost trees by flying-foxes in regularly used camps (Eby 1995; Snoyman and Brown 2010) represents an important potential limitation. Furthermore, a key management strategy for managing flying-foxes in urban areas is to remove roost trees to create buffers between camps and homes (Currey et al. 2018; Mo et al. 2020). These actions may be contrary to vegetation retention objectives, hence the important need for managers to balance efforts to reduce human-wildlife conflict with retaining sufficient roost vegetation. Second, heat-related mortality may possibly be exacerbated when pups are abandoned in the days leading up to extreme heat events. In this study, die-offs were observed from both pup abandonments and subsequent extreme heat events in two camps. It would therefore be possible that pups in other camps were also being abandoned unbeknown to us, especially since some camps were inspected during but not prior to extreme heat events. Knowledge of these preceding influencing factors may assist with anticipating the camps likely to be most affected from future extreme heat events that are forecasted.
At present, the causes of P. poliocephalus pups being abandoned in NSW are not clear. Since there was a starvation event confirmed in northern NSW and the south-eastern Queensland during this time, we surmise that abandonments were possibly also related to nutritional stress. We would expect that lactating females in poor body condition would have had low milk production. Although pup abandonments were mostly recorded in December and January, mortality in some camps was recorded as early as November. Nursing females have the highest nutritional demands in the austral spring (Wade and Schneider 1992; Welbergen 2011), which coincide with time of the year food bottlenecks are most likely to occur (Parry-Jones and Augee 1991; Eby 1995), especially with the effects of large-scale habitat destruction (Eby et al. 1999). Starving lactating flying-foxes have high levels of physiological stress (Parry-Jones et al. 2016). It is therefore possible that nursing females could not maintain lactation as the drought continued (Sadleir 1969) or were unable to reunite with pups at camps if exhaustion occurred during foraging forays. At the time, the Black Summer bushfires had already been burning vast areas of northern NSW (Boer et al. 2020; Nolan et al. 2020) that represent important foraging habitat (Eby et al. 1999). In Batemans Bay and Catalina, there is the additional possibility that bushfire smoke presented obstacles for nursing females trying to return to their pups. Both the starvation event and bushfires are associated with drought and climate change (Sherwin et al. 2012; Collins et al. 2019b), which form broader management issues. However, the exacerbating effect of the destruction of foraging habitat on food shortages likely represents a major factor in nutritional stress in flying-foxes. Thus, a reasonable intervention would be to restore vegetation communities identified as important foraging habitat, especially in winter foraging sites (Eby and Law 2008; Eby et al. 2019).
During seasons of die-offs, wildlife carers may suffer stress from encountering large numbers of deaths and injuries, as well as exhaustion from personal burdens on time and finances when large numbers of animals require rehabilitation (Yeung et al. 2017; Englefield et al. 2018). During this study, large numbers of flying-foxes coming into care created overwhelming workloads for carers, such that many carers did not have capacity to accept further animals. This resulted in some flying-foxes that would normally have been considered viable being euthanised. This issue highlights the need to continue to build capacity in the wildlife rehabilitation sector to withstand such circumstances. Capacity building may involve improved state and national coordination between stakeholders, development of training resources, and partnerships that facilitate the provision or subsidising of resources such as food (e.g. Mo et al. 2021b), equipment and rabies vaccinations. The numbers of flying-foxes taken into care during the 2019–20 summer demonstrate the value of both the collective voluntary efforts of wildlife carers and ongoing research into heat stress management to minimise flying-fox mortality in the first instance (e.g. Ratnayake et al. 2019).
Mass die-offs of flying-foxes also have serious implications for a broader range of stakeholders. For instance, large numbers of decaying carcasses represent both an amenity and public health issue for communities living close to camps (Merone et al. 2020). Heat-stressed flying-foxes on or near the ground also increase opportunities for human-bat contact, which represent an increased risk of transmission of zoonotic diseases such as Australian bat lyssavirus (Francis et al. 2014; Field 2018). Despite public messaging from state government authorities and wildlife rehabilitation organisations advising against directly handling of injured bats, there are numerous cases of members of the public doing so (S. Guy, pers. obs.) and putting themselves at risk. Wildlife carers attending camps during extreme heat events therefore provide barrier measures for the community at these times by facilitating separation between members of the public and flying-foxes. However, the broad social implications associated with mass flying-fox die-offs highlight the importance of collaborative mitigation and assistance from a broader range of stakeholders such as land managers, veterinarians, researchers, health authorities, community groups and corporate partners.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
This project was an extensive and collaborative effort. We recognise the hard work of many volunteers from the ACT Wildlife; Fauna Rescue of South Australia; Fly By Night Bat Clinic; Friends of Bats and Bushcare; Friends of Bats and Habitat Gippsland; Hunter Wildlife Rescue; Ku-ring-gai Bat Conservation Society; Sydney Metropolitan Wildlife Services; Wildlife Aid; Wildlife Animal Rescue and Care Society; Wildlife In Need of Care; Wildlife Information, Rescue and Education Service and Wildlife Rescue South Coast and staff of the NSW Department of Planning, Industry and Environment; Adelaide City Council; Campbelltown City Council; Central Coast Council; Cessnock City Council; Eurobodalla Shire Council; Georges River Council; Greater Shepparton City Council; Ku-ring-gai Council; Lake Macquarie City Council; Newcastle City Council; Parks Victoria; Parramatta Park Trust; Port Stephens Council; Shellharbour City Council; Shoalhaven City Council; Singleton Council; Sutherland Shire Council; Wollondilly Shire Council; South Australian Department for Environment, Water and Natural Resources; Natural Resources Adelaide and Mount Lofty Ranges and Infrastructure NSW. We thank Ann Tierney, Caron Taylor, David Henry, David Kirkland, Deb Little, Denise Kay, Emma McDermott, Freddy Herrera, Gavin Seymour, Hannah McCauley, Heather Caulfield, Jason Van Weenen, Jo Kelly, Jodie Cooper, Katherine Howard, Keren Cox-Witton, Leonie Sweeney, Linda Bell, Marg Peachey, Meg Pethybridge, Nat Blanchford, Nick Godfrey-Smith, Ray Giddins, Ray Morrow, Rebecca Williams, Sarah Burke, Skye Martin, Tracy Ward and Uday Mangalvedhekar. Peggy Eby, Vanessa Wilson, and Sue Ellis provided useful comments on drafts of this paper. This research did not receive any specific funding.
References
Australian Bureau of Meteorology. (2020). Climate data online. Available at http://www.bom.gov.au/climate/data/?ref=ftr [accessed 30 October 2020].Bartholomew, G. A., Leitner, P., and Nelson, J. E. (1964). Body temperature, oxygen consumption and heart rate in three species of Australian flying-foxes. Physiological Zoology 37, 179–198.
| Body temperature, oxygen consumption and heart rate in three species of Australian flying-foxes.Crossref | GoogleScholarGoogle Scholar |
Bishop, T., Pearson, T., Lyons, R., and Brennan, M. (2019). Flying-fox heat event response guidelines. Unpublished report.
Boer, M. M., Resco de Dios, V., and Bradstock, R. A. (2020). Unprecedented burn area of Australian mega forest fires. Nature Climate Change 10, 171–172.
| Unprecedented burn area of Australian mega forest fires.Crossref | GoogleScholarGoogle Scholar |
Bohmanova, J., Misztal, I., and Cole, J. B. (2007). Temperature-humidity indices as indicators of milk production losses due to heat stress. Journal of Dairy Science 90, 1947–1956.
| Temperature-humidity indices as indicators of milk production losses due to heat stress.Crossref | GoogleScholarGoogle Scholar | 17369235PubMed |
Cockburn, H. (2019). Bats dying ‘on biblical scale’ due to record-breaking Australia heatwave. Independent, 16 January 2019. Available at https://www.independent.co.uk/news/world/australasia/australia-bats-death-heatwave-cairns-flying-foxes-new-south-wales-adelaide-a8730626.html
Collins, L., Stanvic, S., and McDonald, V. (2019a). Managing heat stress in flying-foxes colonies. Unpublished report.
Collins, L., Bennett, A. F., Leonard, S. W. J., and Penman, T. D. (2019b). Wildfire refugia in forests: severe fire weather and drought mute the influence of topography and fuel age. Global Change Biology 25, 3829–3843.
| Wildfire refugia in forests: severe fire weather and drought mute the influence of topography and fuel age.Crossref | GoogleScholarGoogle Scholar | 31215102PubMed |
Connell, K. A., Munro, U., and Torpy, F. R. (2006). Daytime behaviour of the grey-headed flying-fox Pteropus poliocephalus Temminck (Pteropodidae: Megachiroptera) at an autumn/winter roost. Australian Mammalogy 28, 7–14.
| Daytime behaviour of the grey-headed flying-fox Pteropus poliocephalus Temminck (Pteropodidae: Megachiroptera) at an autumn/winter roost.Crossref | GoogleScholarGoogle Scholar |
Cox, L. (2019). Flying-foxes found dead and emaciated across eastern Australia as dry weather bites. The Guardian, 17 October 2019. Available at https://www.theguardian.com/environment/2019/oct/17/flying-foxes-found-dead-and-emaciated-across-eastern-australia-as-dry-weather-bites
Currey, K. C., Kendal, D., van der Ree, R., and Lentini, P. E. (2018). Land manager perspectives on conflict mitigation strategies for urban flying-fox camps. Diversity 10, 39.
| Land manager perspectives on conflict mitigation strategies for urban flying-fox camps.Crossref | GoogleScholarGoogle Scholar |
Department of Agriculture, Water and the Environment. (2020). National flying-fox monitoring viewer. Available at http://www.environment.gov.au/webgis-framework/apps/ffc-wide/ffc-wide.jsf [accessed 30 October 2020].
Department of Environment and Climate Change. (2008). ‘Best practice guidelines for the grey-headed flying-fox.’ (Department of Environment and Climate Change: Sydney.)
Divljan, A., Parry-Jones, K., and Eby, P. (2011). Deaths and injuries to grey-headed flying-foxes Pteropus poliocephalus shot at an orchard near Sydney, New South Wales. Australian Zoologist 35, 698–710.
| Deaths and injuries to grey-headed flying-foxes Pteropus poliocephalus shot at an orchard near Sydney, New South Wales.Crossref | GoogleScholarGoogle Scholar |
Easterling, D. R., Meehl, G. A., Parmesan, C., Changnon, S. A., Karl, T. R., and Mearns, L. O. (2000). Climate extremes: observations, modelling, and impacts. Science 289, 2068–2074.
| Climate extremes: observations, modelling, and impacts.Crossref | GoogleScholarGoogle Scholar | 11000103PubMed |
Eby, P. (1991). “Finger-winged night workers”: managing forests to conserve the role of grey-headed flying-foxes as pollinators and seed dispersers. In ‘Conservation of Australia’s Forest Fauna’. (Ed. D. Lunney.) pp. 91–100. (Royal Zoological Society of New South Wales: Sydney.)
Eby, P. (1995). The biology and management of flying-foxes in NSW. Species Management Report Number 18. (NSW National Parks and Wildlife Service: Sydney.)
Eby, P., and Law, B. (2008). Ranking the feeding habitats of grey-headed flying-foxes for conservation management. Report for the NSW Department of Environment and Climate Change and Australian Department of Environment, Water, Heritage and the Arts.
Eby, P., Richards, G., Collins, L., and Parry-Jones, K. (1999). The distribution, abundance and vulnerability to population reduction of a nomadic nectarivore, the grey-headed flying-fox Pteropus poliocephalus in New South Wales, during a period of resource concentration. Australian Zoologist 31, 240–253.
| The distribution, abundance and vulnerability to population reduction of a nomadic nectarivore, the grey-headed flying-fox Pteropus poliocephalus in New South Wales, during a period of resource concentration.Crossref | GoogleScholarGoogle Scholar |
Eby, P., Sims, R., and Bracks, J. (2019). Flying-fox foraging habitat mapping NSW: a seamless map for assessing temporal and spatial patterns of habitat quality for flying-foxes. Report for Local Government NSW. Griffith University and Ecosure Pty Ltd, Brisbane, Qld.
Englefield, B., Starling, M., and McGreevy, P. (2018). A review of roadkill rescue: who cares for the mental, physical and financial welfare of Australian wildlife carers? Wildlife Research 45, 103–118.
| A review of roadkill rescue: who cares for the mental, physical and financial welfare of Australian wildlife carers?Crossref | GoogleScholarGoogle Scholar |
Field, H. E. (2018). Evidence of Australian bat lyssavirus infection in diverse Australian bat taxa. Zoonoses Public Health 65, 742–748.
| Evidence of Australian bat lyssavirus infection in diverse Australian bat taxa.Crossref | GoogleScholarGoogle Scholar | 29785730PubMed |
Foley, C., Pettorelli, N., and Foley, L. (2008). Severe drought and calf survival in elephants. Biology Letters 4, 541–544.
| Severe drought and calf survival in elephants.Crossref | GoogleScholarGoogle Scholar | 18682358PubMed |
Francis, J. R., Nourse, C., Vaska, V. L., Calvert, S., Northill, J. A., McCall, B., and Mattke, A. C. (2014). Australian bat lyssavirus in a child: the first reported case. Pediatrics 133, e1064.
| Australian bat lyssavirus in a child: the first reported case.Crossref | GoogleScholarGoogle Scholar |
Frick, W. F., Pollock, J. F., Hicks, A. C., Langwig, K. E., Reynolds, D. S., Turner, G. G., Butchkoski, C. M., and Kunz, T. H. (2010). An emerging disease causes regional population collapse of a common North American bat species. Science 329, 679–682.
| An emerging disease causes regional population collapse of a common North American bat species.Crossref | GoogleScholarGoogle Scholar | 20689016PubMed |
Fujita, M. S., and Tuttle, M. D. (1991). Flying-foxes (Chiroptera: Pteropodidae): threatened animals of key ecological and economic importance. Conservation Biology 5, 455–463.
| Flying-foxes (Chiroptera: Pteropodidae): threatened animals of key ecological and economic importance.Crossref | GoogleScholarGoogle Scholar |
Gizzia, F., Jiménez, J., Schäfera, S., Castro, N., Costa, S., Lourenço, S., José, R., Canning-Clode, J., and Monteiro, J. (2020). Before and after a disease outbreak: tracking a keystone species recovery from a mass mortality event. Marine Environmental Research 156, 104905.
| Before and after a disease outbreak: tracking a keystone species recovery from a mass mortality event.Crossref | GoogleScholarGoogle Scholar |
Godfree, R. C., Knerr, N., Godfree, D., Busby, J., Robertson, B., and Encinas-Viso, F. (2019). Historical reconstruction unveils the risk of mass mortality and ecosystem collapse during pancontinental megadrought. Proceedings of the National Academy of Sciences of the United States of America 116, 15580–15589.
| Historical reconstruction unveils the risk of mass mortality and ecosystem collapse during pancontinental megadrought.Crossref | GoogleScholarGoogle Scholar | 31308227PubMed |
Heathcote, A. (2019). Community unites to provide ‘apple kebabs’ for starving flying-foxes. Australian Geographic, 26 September 2019. https://www.australiangeographic.com.au/topics/wildlife/2019/09/community-unites-to-provide-apple-kebabs-for-starving-flying-foxes
Huynen, M. M. T. E., Martens, P., Schram, D., Weijenberg, M. P., and Kunst, A. E. (2001). The impact of heat waves and cold spells on mortality rates in the Dutch population. Environmental Health Perspectives 109, 463–470.
| The impact of heat waves and cold spells on mortality rates in the Dutch population.Crossref | GoogleScholarGoogle Scholar |
Isidoro-Ayza, M., Lorch, J. M., Ballmann, A. E., and Businga, N. K. (2019). Mass mortality of green frog (Rana clamitans) tadpoles in Wisconsin, USA, associated with severe infection with the pathogenic Perkinsea clade. Journal of Wildlife Diseases 55, 262–265.
| Mass mortality of green frog (Rana clamitans) tadpoles in Wisconsin, USA, associated with severe infection with the pathogenic Perkinsea clade.Crossref | GoogleScholarGoogle Scholar | 30024771PubMed |
Jones, T., Divine, L. M., Renner, H., Knowles, S., Lefebvre, K. A., Burgess, H. K., Wright, C., and Parrish, J. K. (2019). Unusual mortality of tufted puffins (Fratercula cirrhata) in the eastern Bering Sea. PLoS ONE 14, e0216532.
| Unusual mortality of tufted puffins (Fratercula cirrhata) in the eastern Bering Sea.Crossref | GoogleScholarGoogle Scholar | 31141532PubMed |
Kock, R. A., Orynbayev, M., Robinson, S., Zuther, S., Singh, N. J., Beauvais, W., Morgan, E. R., Kerimbayev, A., Khomenko, S., Martineau, H. M., Rystaeva, R., Omarova, Z., Wolfs, S., Hawotte, F., Radoux, J., and Milner-Gulland, E. J. (2018). Saigas on the brink: multidisciplinary analysis of the factors influencing mass mortality events. Scientific Advances 4, eaao2314.
| Saigas on the brink: multidisciplinary analysis of the factors influencing mass mortality events.Crossref | GoogleScholarGoogle Scholar |
Kung, N. Y., Field, H. E., McLaughlin, A., Edson, D., and Taylor, M. (2015). Flying-foxes in the Australian urban environment – community attitudes and opinions. One Health 1, 24–30.
| Flying-foxes in the Australian urban environment – community attitudes and opinions.Crossref | GoogleScholarGoogle Scholar | 28616461PubMed |
Lab of Animal Ecology. (2020a). The Flying-fox Heat Stress Forecaster. Available at https://www.animalecologylab.org/ff-heat-stress-forecaster.html [accessed 30 October 2020].
Lab of Animal Ecology. (2020b). Flying-fox heat-stress data form. Available at https://www.animalecologylab.org/heat-stress-data-form.html [accessed 30 October 2020].
Laburn, H. P., and Mitchell, D. (1975). Evaporative cooling as a thermoregulatory mechanism in the fruit bat, Rousettus aegyptiacus. Physiological Zoology 21, 195–202.
| Evaporative cooling as a thermoregulatory mechanism in the fruit bat, Rousettus aegyptiacus.Crossref | GoogleScholarGoogle Scholar |
Law, B., Eby, P., and Somerville, D. (2002). Tree-planting to conserve flying-foxes and reduce orchard damage. In ‘Managing the Grey-headed Flying-fox as a Threatened Species in New South Wales’. (Eds P. Eby, and D. Lunney.) pp. 84–90. (Royal Zoological Society of New South Wales: Sydney.)
Licht, P., and Leitner, P. (1975a). Behavioral responses to high temperatures in three species of Californian bats. Journal of Mammalogy 48, 52–61.
| Behavioral responses to high temperatures in three species of Californian bats.Crossref | GoogleScholarGoogle Scholar |
Licht, P., and Leitner, P. (1975b). Physiological responses to high environmental temperatures in three species of microchiropteran bats. Comparative Biochemical Physiology A: Comparative Physiology 22, 371–387.
| Physiological responses to high environmental temperatures in three species of microchiropteran bats.Crossref | GoogleScholarGoogle Scholar |
Lunney, D., and Moon, C. (2011). Blind to bats: traditional prejudices and today’s bad press render bats invisible to public consciousness. In ‘The Biology and Conservation of Australasian Bats’. (Eds B. Law, P. Eby, D. Lunney, and L. Lumsden.) pp. 44–63. (Royal Zoological Society of New South Wales: Sydney.)
Major, F. (2020). Injuries and deaths of grey-headed flying-foxes, Pteropus poliocephalus in Commonwealth Park, ACT, resulting from a hailstorm event occurring on 20th January 2020. (ACT Wildlife: Kambah, ACT.)
Mao, F. (2019). How one heatwave killed ‘a third’ of a bat species in Australia. BBC News, 15 January 2019. Available at https://www.bbc.com/news/world-australia-46859000
Markus, N., and Blackshaw, J. K. (2002). Behaviour of the black flying-fox Pteropus alecto: 1. An ethogram of behaviour, and preliminary characterisation of mother-infant interactions. Acta Chiropterologica 4, 137–152.
| Behaviour of the black flying-fox Pteropus alecto: 1. An ethogram of behaviour, and preliminary characterisation of mother-infant interactions.Crossref | GoogleScholarGoogle Scholar |
Martin, L., and McIlwee, A. P. (2002). The reproductive biology and intrinsic capacity for increase of the grey-headed flying-foxes Pteropus poliocephalus (Megachiroptera), and the implications of culling. In ‘Managing the Grey-headed Flying-fox as a Threatened Species in New South Wales’. (Eds P. Eby, and D. Lunney.) pp. 91–108. (Royal Zoological Society of New South Wales: Sydney.)
Martin, L., Kennedy, J. H., Little, L., Luckhoff, H., O’Brien, G. M., Pow, C. S. T., Towers, P. A., Waldon, A. K., and Wang, D. Y. (1996). The reproductive biology of Australian flying-foxes (genus Pteropus). Symposium Zoological Society of London 67, 167–184.
Mason-Romo, E. D., Ceballos, G., Lima, M., Martínez-Yrízar, A., Jaramillo, V. J., and Maass, M. (2018). Long-term population dynamics of small mammals in tropical dry forests, effects of unusual climate events, and implications for management and conservation. Forest Ecology and Management 426, 123–133.
| Long-term population dynamics of small mammals in tropical dry forests, effects of unusual climate events, and implications for management and conservation.Crossref | GoogleScholarGoogle Scholar |
Maxwell, S. L., Butt, N., Maron, M., McAlpine, C. A., Chapman, S., Ullmann, A., Segan, D. B., and Watson, J. E. M. (2019). Conservation implications of ecological responses to extreme weather and climate events. Diversity and Distributions 25, 613–625.
| Conservation implications of ecological responses to extreme weather and climate events.Crossref | GoogleScholarGoogle Scholar |
McClelland, K. (2009). Challenges and recovery actions for the widespread, threatened grey-headed flying-fox: a review from a New South Wales policy perspective. Ecological Management and Restoration 10, S110–S116.
| Challenges and recovery actions for the widespread, threatened grey-headed flying-fox: a review from a New South Wales policy perspective.Crossref | GoogleScholarGoogle Scholar |
McConkey, K. R., and Drake, D. R. (2006). Flying-foxes cease to function as seed dispersers long before they become rare. Ecology 87, 271–276.
| Flying-foxes cease to function as seed dispersers long before they become rare.Crossref | GoogleScholarGoogle Scholar | 16637350PubMed |
McDowell, W. G., McDowell, W. H., and Byers, J. E. (2017). Mass mortality of a dominant invasive species in response to an extreme climate event: implications for ecosystem function. Limnology and Oceanography 62, 177–188.
| Mass mortality of a dominant invasive species in response to an extreme climate event: implications for ecosystem function.Crossref | GoogleScholarGoogle Scholar |
McKechnie, A. E., and Wolf, B. O. (2019). The physiology of heat tolerance in small endotherms. Physiology 34, 302–313.
| The physiology of heat tolerance in small endotherms.Crossref | GoogleScholarGoogle Scholar | 31389778PubMed |
Meehl, G. A., and Tebaldi, C. (2004). More intense, more frequent, and longer lasting heat waves in the 21st century. Science 305, 994–997.
| More intense, more frequent, and longer lasting heat waves in the 21st century.Crossref | GoogleScholarGoogle Scholar | 15310900PubMed |
Merone, L., Thirlwell, C., Esmonde, J., and Gair, R. (2020). A mass mortality event in bats caused by extreme heat: surprising public health challenges. Public Health Research and Practice 30, e3042032.
| A mass mortality event in bats caused by extreme heat: surprising public health challenges.Crossref | GoogleScholarGoogle Scholar |
Mo, M., and Roache, M. (2021). A review of intervention methods used to reduce flying-fox mortalities in heat stress events. Australian Mammalogy 43, 137–150.
| A review of intervention methods used to reduce flying-fox mortalities in heat stress events.Crossref | GoogleScholarGoogle Scholar |
Mo, M., Roache, M., Williams, R., Drinnan, I. N., and Noël, B. (2020). From cleared buffers to camp dispersal: mitigating impacts of the Kareela flying-fox camp on adjacent residents and schools. Australian Zoologist 41, 19–41.
| From cleared buffers to camp dispersal: mitigating impacts of the Kareela flying-fox camp on adjacent residents and schools.Crossref | GoogleScholarGoogle Scholar |
Mo, M., Roache, M., Haering, R., and Kwok, A. (2021a). Using wildlife carer records to identify patterns in flying-fox rescues: a case study in New South Wales, Australia. Pacific Conservation Biology 27, 61–69.
| Using wildlife carer records to identify patterns in flying-fox rescues: a case study in New South Wales, Australia.Crossref | GoogleScholarGoogle Scholar |
Mo, M., Roache, M., Reid, T., Oliver, D. L., Broome, L., Fawcett, A., Howard, K., Thomas, P., Tracey, S., Andersen, G., and Lowry, R. (2021b). Corporate support for threatened species recovery efforts: three case studies from the 2019–20 Australian bushfire season. Australian Zoologist 41, 186–193.
| Corporate support for threatened species recovery efforts: three case studies from the 2019–20 Australian bushfire season.Crossref | GoogleScholarGoogle Scholar |
Nolan, R. H., Boer, M. M., Collins, L., Resco de Dios, V., Clarke, H., Jenkins, M., Kenny, B., and Bradstock, R. A. (2020). Causes and consequences of eastern Australia’s 2019-20 season of mega‐fires. Global Change Biology 26, 1039–1041.
| Causes and consequences of eastern Australia’s 2019-20 season of mega‐fires.Crossref | GoogleScholarGoogle Scholar | 31916352PubMed |
Parry-Jones, K. (2000). Historical declines since the early 1900’s, and current mortality factors and abundance of the grey-headed flying-fox in NSW. In ‘Proceedings of a Workshop to Assess the Status of the Grey-headed Flying-fox in NSW’. (Eds G. Richards, and L. Hall.) pp. 56–65. (Australasian Bat Society: Canberra.)
Parry-Jones, K. A., and Augee, M. L. (1991). Food selection by grey-headed flying-foxes (Pteropus poliocephalus) occupying a summer colony site near Gosford, New South Wales. Wildlife Research 18, 111–124.
| Food selection by grey-headed flying-foxes (Pteropus poliocephalus) occupying a summer colony site near Gosford, New South Wales.Crossref | GoogleScholarGoogle Scholar |
Parry-Jones, K. A., and Augee, M. L. (2001). Factors affecting the occupation of a colony site in Sydney New South Wales by the grey-headed flying-fox, Pteropus poliocephalus (Pteropodidae). Austral Ecology 26, 47–55.
| Factors affecting the occupation of a colony site in Sydney New South Wales by the grey-headed flying-fox, Pteropus poliocephalus (Pteropodidae).Crossref | GoogleScholarGoogle Scholar |
Parry-Jones, K., Webster, K. N., and Divljan, A. (2016). Baseline levels of faecal glucocorticoid metabolites and indications of chronic stress in the vulnerable grey-headed flying-fox, Pteropus poliocephalus. Australian Mammalogy 38, 195–203.
| Baseline levels of faecal glucocorticoid metabolites and indications of chronic stress in the vulnerable grey-headed flying-fox, Pteropus poliocephalus.Crossref | GoogleScholarGoogle Scholar |
Porter, W. P., and Gates, D. M. (1969). Thermodynamic equilibria of animals with environment. Ecological Monographs 39, 227–244.
| Thermodynamic equilibria of animals with environment.Crossref | GoogleScholarGoogle Scholar |
Pruvot, M., Cappelle, J., Furey, N., Hul, V., Heng, H. S., Duong, V., Dussart, P., and Horwood, P. (2019). Extreme temperature event and mass mortality of insectivorous bats. European Journal of Wildlife Research 65, 41.
| Extreme temperature event and mass mortality of insectivorous bats.Crossref | GoogleScholarGoogle Scholar | 32214949PubMed |
Ratnayake, H., Welbergen, J. A., van der Ree, R., and Kearney, M. (2021). Variation in fur properties may explain differences in heat-related mortality among Australian flying-foxes. Australian Journal of Zoology , .
| Variation in fur properties may explain differences in heat-related mortality among Australian flying-foxes.Crossref | GoogleScholarGoogle Scholar |
Ratnayake, H. U., Kearney, M. R., Govekar, P., Karoly, D., and Welbergen, J. A. (2019). Forecasting wildlife die-offs from extreme heat events. Animal Conservation 22, 386–395.
| Forecasting wildlife die-offs from extreme heat events.Crossref | GoogleScholarGoogle Scholar |
Richards, N., Zorrilla, I., Lalah, J., Otieno, P., Fernandez, I., Calvino, M., and Garcia, J. (2017). Talons and beaks are viable but underutilized samples for detecting organophosphorus and carbamate pesticide poisoning in raptors. Vulture News 72, 3–13.
| Talons and beaks are viable but underutilized samples for detecting organophosphorus and carbamate pesticide poisoning in raptors.Crossref | GoogleScholarGoogle Scholar |
Roberts, B. J., Catterall, C. P., Eby, P., and Kanowski, J. (2012). Latitudinal range shifts in Australian flying-foxes: a re-evaluation. Austral Ecology 37, 12–22.
| Latitudinal range shifts in Australian flying-foxes: a re-evaluation.Crossref | GoogleScholarGoogle Scholar |
Sadleir, R. M. F. S. (1969). ‘The Ecology of Reproduction in Wild and Domestic Mammals.’ (Methven and Co. Ltd: London.)
Scheelings, T. F., and Frith, S. E. (2015). Anthropogenic factors are the major cause of hospital admission of a threatened species, the grey-headed flying-fox (Pteropus poliocephalus), in Victoria, Australia. PLoS ONE 10, e0133638.
| Anthropogenic factors are the major cause of hospital admission of a threatened species, the grey-headed flying-fox (Pteropus poliocephalus), in Victoria, Australia.Crossref | GoogleScholarGoogle Scholar | 26207984PubMed |
Sherwin, H. A., Montgomery, W. I., and Lundy, M. G. (2012). The impact and implications of climate change for bats. Mammal Review 43, 171–182.
| The impact and implications of climate change for bats.Crossref | GoogleScholarGoogle Scholar |
Snoyman, S., and Brown, C. (2010). Microclimate preferences of the grey-headed flying-fox (Pteropus poliocephalus) in the Sydney region. Australian Journal of Zoology 58, 376–383.
| Microclimate preferences of the grey-headed flying-fox (Pteropus poliocephalus) in the Sydney region.Crossref | GoogleScholarGoogle Scholar |
Snoyman, S., Munich, J., and Brown, C. (2012). Nursing females are more prone to heat stress: demography matters when managing flying-foxes for climate change. Applied Animal Behaviour Science 142, 90–97.
| Nursing females are more prone to heat stress: demography matters when managing flying-foxes for climate change.Crossref | GoogleScholarGoogle Scholar |
Southerton, S. G., Birt, P., Porter, J., and Ford, H. A. (2004). Review of gene movement by bats and birds and its potential significance for eucalypt plantation forestry. Australian Forestry 67, 44–53.
| Review of gene movement by bats and birds and its potential significance for eucalypt plantation forestry.Crossref | GoogleScholarGoogle Scholar |
Steffen, W., Hughes, L., and Perkins, S. (2014). ‘Heatwaves: hotter, longer, more often.’ (Climate Council: Potters Point, NSW.)
Tidemann, C. R., and Nelson, J. E. (2011). Life expectancy, causes of death and movements of the grey-headed flying-fox (Pteropus poliocephalus) inferred from banding. Acta Chiropterologica 13, 419–429.
| Life expectancy, causes of death and movements of the grey-headed flying-fox (Pteropus poliocephalus) inferred from banding.Crossref | GoogleScholarGoogle Scholar |
Till, A., Rypel, A. L., Bray, A., and Fey, S. B. (2019). Fish die-offs are concurrent with thermal extremes in north temperate lakes. Nature Climate Change 9, 637–641.
| Fish die-offs are concurrent with thermal extremes in north temperate lakes.Crossref | GoogleScholarGoogle Scholar |
Vincenot, C. E., Florens, F. B. V., and Kingston, T. (2017). Can we protect island flying-foxes. Science 355, 1368–1370.
| Can we protect island flying-foxes.Crossref | GoogleScholarGoogle Scholar | 28360279PubMed |
Wade, G. N., and Schneider, J. E. (1992). Metabolic fuels and reproduction in female mammals. Neuroscience and Biobehavioral Reviews 16, 235–272.
| Metabolic fuels and reproduction in female mammals.Crossref | GoogleScholarGoogle Scholar | 1630733PubMed |
Webb, N. J., and Tidemann, C. R. (1996). Mobility of Australian flying‐foxes, Pteropus spp. (Megachiroptera): evidence from genetic variation. Proceedings of the Royal Society of London Series B 263, 497–502.
| Mobility of Australian flying‐foxes, Pteropus spp. (Megachiroptera): evidence from genetic variation.Crossref | GoogleScholarGoogle Scholar | 8637931PubMed |
Welbergen, J. A. (2010). Growth, bimaturation, and sexual size dimorphism in wild gray-headed flying foxes (Pteropus poliocephalus). Journal of Mammalogy 91, 38–47.
| Growth, bimaturation, and sexual size dimorphism in wild gray-headed flying foxes (Pteropus poliocephalus).Crossref | GoogleScholarGoogle Scholar |
Welbergen, J. A. (2011). Fit females and fat polygynous males: seasonal body mass changes in the grey-headed flying-fox. Oecologia 165, 629–627.
| Fit females and fat polygynous males: seasonal body mass changes in the grey-headed flying-fox.Crossref | GoogleScholarGoogle Scholar | 21153744PubMed |
Welbergen, J. A. (2012). The impacts of extreme events on biodiversity – lessons from die-offs in flying-foxes. In ‘Proceedings of the International Symposium on the Importance of Bats as Bioindicators’. (Eds C. Flaquer, and X. Puig-Montserrat.) pp. 70–75. (Museum of Natural Sciences Edicions: Granollers, Barcelona, Spain.)
Welbergen, J. A., Klose, S. M., Markus, N., and Eby, P. (2008). Climate change and the effects of temperature extremes on Australian flying-foxes. Proceedings of the Royal Society B 275, 419–425.
| Climate change and the effects of temperature extremes on Australian flying-foxes.Crossref | GoogleScholarGoogle Scholar | 18048286PubMed |
Welbergen, J. A., Booth, C., and Martin, J. (2014). Killer climate: tens of thousands of flying-foxes dead in a day. Available at https://theconversation.com/killer-climate-tens-of-thousands-of-flying-foxes-dead-in-a-day-23227 [accessed 30 October 2020].
Welbergen, J. A., Meade, J., Field, H., Edson, D., McMichael, L., Shoo, L., Praszczalek, J., Smith, G., and Martin, J. (2020). Extreme mobility of the world’s largest flying mammals creates key challenges for management and conservation. BMC Biology 18, 101.
| Extreme mobility of the world’s largest flying mammals creates key challenges for management and conservation.Crossref | GoogleScholarGoogle Scholar | 32819385PubMed |
Westcott, D. A., Heersink, D. K., McKeown, A., and Caley, P. (2015). Status and trends of Australia’s EPBC-listed flying-foxes. Report to the Commonwealth of Australia. (Commonwealth Scientific and Industrial Research Organisation: Atherton, Qld.)
Yeung, P., White, B., and Louise Chilvers, B. (2017). Exploring wellness of wildlife carers in New Zealand: a descriptive study. Anthrozoös 30, 549–563.
| Exploring wellness of wildlife carers in New Zealand: a descriptive study.Crossref | GoogleScholarGoogle Scholar |






