Using experimental trials to improve translocation protocols for a cryptic, endangered passerine
William F. Mitchell A H , Rebecca L. Boulton B C , Luke Ireland B , Thomas J. Hunt B C , Simon J. Verdon D E , Liberty G. M. Olds F , Chris Hedger G and Rohan H. Clarke A
A H , Rebecca L. Boulton B C , Luke Ireland B , Thomas J. Hunt B C , Simon J. Verdon D E , Liberty G. M. Olds F , Chris Hedger G and Rohan H. Clarke A
A Monash University, School of Biological Sciences, Clayton, Vic., Australia.
B Government of South Australia, Department for Environment and Water, Murray Bridge, SA, Australia.
C The University of Adelaide, School of Biological Sciences, Adelaide, SA, Australia.
D La Trobe University, Department of Ecology, Environment and Evolution, Melbourne, Australia.
E La Trobe University, Research Centre for Future Landscapes, Melbourne, Australia.
F Zoos SA, Adelaide, SA, Australia.
G National Parks and Wildlife Service South Australia, Riverland and Murraylands, Murray Bridge, SA, Australia.
H Corresponding author. Email: William.Mitchell@monash.edu
Pacific Conservation Biology 28(1) 68-79 https://doi.org/10.1071/PC20097
Submitted: 9 December 2020 Accepted: 12 April 2021 Published: 13 May 2021
Journal Compilation © CSIRO 2022 Open Access CC BY-NC-ND
Abstract
When key ecological information is lacking, conservation translocations should be conducted within an adaptive, experimental framework to maximise knowledge gained and to increase the probability of success. Here we investigated whether timing of release or composition of release groups influenced indices of success during a trial reintroduction of the mallee emu-wren, Stipiturus mallee, to Ngarkat Conservation Park, South Australia. We translocated cohorts of 40 and 38 birds in the Austral autumn and spring of 2018 respectively. We released individuals in small groups, comprising either familiar or unfamiliar birds, and intensively monitored all treatments for 2 weeks post-release to quantify short-term survival and dispersal. We used occupancy modelling to assess persistence of the translocated population for 2 years following releases. We also monitored source populations to assess the impact of removals. Mallee emu-wrens released in spring were more likely to remain at the release site and attempt breeding. Familiarity within a release group did not influence short-term survival. Mallee emu-wren occupancy at the release sites declined following releases and by July 2019 (12–15 months after release), we could no longer detect any emu-wrens. Density at source populations was lower 12 months after removal compared with pre-harvest levels, though these differences were not significant. Despite the failure to establish a population, we gained valuable management insights regarding both the focal species, and translocation practice more broadly. Timing of release can influence short-term indices of success. Spring releases should be considered priority actions in future mallee emu-wren translocations.
Keywords: arid ecology, birds, conservation, mallee emu-wren, management, population management, reintroduction, Stipiturus mallee, threatened species.
Introduction
The translocation of threatened species to establish new populations or augment existing populations is frequently employed to conserve species (Fischer and Lindenmayer 2000; Taylor et al. 2017). Despite notable successes, translocations have historically been subject to a high failure rate (Taylor et al. 2017; Berger‐Tal et al. 2019). Funding is often a limiting factor in conservation management, and when dealing with vulnerable species, it is critical to maximise positive conservation outcomes (IUCN/SSC 2013). In this light, factors that may influence the success of conservation translocations including husbandry, social interactions, habitat suitability, number of founders, and genetic diversity, have received considerable research attention (Griffith et al. 1989; Dickens et al. 2010; Jamieson 2011; Mihoub et al. 2011; Parker et al. 2012; Tetzlaff et al. 2019). For social animals, an ability to form new group associations may increase probability of survival following translocation (Franks et al. 2020). In ecosystems susceptible to variable or harsh environmental conditions, timing releases such that they occur when conditions are favourable may increase the likelihood of successful population establishment (Bright and Morris 1994; Hellstedt and Kallio 2005). For efficient resource allocation, managers require a thorough understanding of how these factors will influence their target species (Armstrong et al. 2007). Where uncertainty exists, translocations should be designed within an adaptive experimental framework, as far as practicable, to inform future management (Armstrong et al. 2007; Taylor et al. 2017).
An increase in the use of translocations as a conservation management tool has led to improvements in practices and outcomes for species (Taylor et al. 2017). However, several areas have been highlighted where translocation practice and theory can be further aligned. One such example is that long-term persistence following translocation is rarely included as a component of success in the translocation literature (Taylor et al. 2017). Research should also more often provide explicit comparisons of alternative management strategies to aid on-ground decision-making (Taylor et al. 2017). Managers must also ensure that harvesting for translocation does not negatively impact source populations (Stevens and Goodson 1993; Dimond and Armstrong 2007; Bain and French 2009; Easton et al. 2019). This is of particular concern for management of threatened species where source populations are often small, and harvesting individuals for translocation may exacerbate threatening processes. Publication bias may also distort perceptions of the effectiveness of translocations as success stories are more likely to be published than failures (Møller and Jennions 2001; Miller et al. 2014).
Here we report on the trial reintroduction of the mallee emu-wren Stipiturus mallee to Ngarkat Conservation Park, South Australia. We investigate whether the timing of release and familiarity of release groups influenced the probability of successful population establishment as these are considered important elements of translocation success (Bright and Morris 1994; Franks et al. 2020). As this was the first translocation of the mallee emu-wren, significant logistical challenges due to the ecological characteristics of the species (e.g. crypsis and evasiveness) also needed to be overcome. We framed the reintroduction in two distinct phases. In ‘phase one’ (this study), our aim was to trial and optimise capture, transfer and release protocols for mallee emu-wrens, while seeking to establish the foundations of a new population if possible. The management insights gained during this first phase will inform a larger scale ‘phase two’ translocation, where the over-arching goal is to re-establish a population of mallee emu-wrens in South Australia. In phase one we adopted an experimental approach where mallee emu-wrens were translocated in one of two distinct seasonal cohorts to determine whether timing of release would affect post-release dispersal, survival or the probability of successful reproduction. Release groups comprised either familiar or unfamiliar individuals to determine whether sociality would increase the probability of successful population establishment in future releases. We monitored the translocated population to track population trends and assess the outcomes of the different treatments. In parallel, we monitored the source population trends to assess the impact of harvesting birds for translocation.
Materials and methods
Study species and system
Endemic to mallee habitats south of the Murray River in South Australia and Victoria, the Endangered mallee emu-wren is a diminutive, hummock grass Triodia scariosa specialist, although the species has historically also been found in Xanthorrhoea sp. dominated habitats (Fig. 1; Brown et al. 2009; Paton et al. 2009; Verdon et al. 2019). Mallee emu-wrens are found in small social groups and are secretive, often only detectable by their high pitched call (Menkhorst et al. 2017). Breeding is thought to occur between late August and November (though it likely varies with environmental conditions) and females lay clutches of two to three eggs (Higgins et al. 2001). Nests are invariably obscured from view within a Triodia hummock (Higgins et al. 2001). Breeding ecology of mallee emu-wrens remains poorly known, though it is likely similar to that of other emu-wrens (e.g. Maguire and Mulder 2004). Low to moderate levels of genetic diversity have been recorded across mallee emu-wren populations with some evidence of gene flow across the species’ range (Brown et al. 2013). For management purposes the mallee emu-wren can be considered a single genetic unit (Brown et al. 2013). In recent decades the global population of the species has declined due to habitat loss, drought, and a series of catastrophic wildfires (Brown et al. 2009). By 2014 it was considered extinct in South Australia, and all remaining populations were confined to a network of Victorian reserves comprising Murray-Sunset National Park, Hattah-Kulkyne National Park, Wyperfeld National Park, and Nowingi State Forest (hereafter Murray-Sunset, Hattah, Wyperfeld, and Nowingi; Verdon et al. 2019; Fig. 1). In today’s fragmented landscapes, mallee emu-wrens have no capacity to naturally recolonise most areas of suitable habitat following local extinctions due to reserve-scale wildfire. Additionally, the ever-present threat of catastrophic wildfire in currently occupied habitat jeopardises the long-term persistence of the mallee emu-wren (Department of Environment, Land, Water and Planning 2016). A successful translocation would increase the global population of the species, while providing an insurance population against further wildfires in currently occupied habitat. As such, translocation was highlighted as a potential conservation strategy in the national recovery plan for the mallee emu-wren (Department of Environment, Land, Water and Planning 2016). In 2018 we implemented a trial reintroduction of mallee emu-wrens to Ngarkat Conservation Park (hereafter Ngarkat), South Australia.
Emu Springs Track in Ngarkat was chosen as the release site due to the presence of suitable Triodia heath habitat, and because it was formerly occupied by mallee emu-wrens, prior to their extirpation by wildfires in 2006 (Paton et al. 2009; Fig. 1). To mitigate the threat of wildfire at the release site, fuel reduction burns were undertaken by South Australia’s Department of Environment, Water and Natural Resources north of Emu Springs Track prior to the translocation to establish a protective fire-break. Additionally, the release site was listed as an environmental asset to be prioritised for protection in the event of wildfire in the area. Mallee emu-wren populations in western Murray-Sunset, Hattah, and Nowingi were chosen as sources for translocation. Although mallee emu-wrens occupy Triodia mallee habitats at these sites, which is structurally different to the Triodia heath dominated habitat at the release site, these source sites were selected for their high density of emu-wrens, accessibility by road, relatively high levels of genetic diversity compared with other populations and because areas of Triodia heath habitat with sufficient mallee emu-wrens to sustain harvesting no longer exist (Brown et al. 2013; Boulton and Hedger 2018). The number of mallee emu-wrens in the Victorian reserve network was estimated to be ~16 000 for the period 1999–2006 (Brown et al. 2009; Boulton and Lau 2015), although a more recent study (conducted after the translocation) estimated the population to number 6449 (95% CI: 1923–12 013) individuals in 2019 (Verdon et al. 2021).
Capture and transport
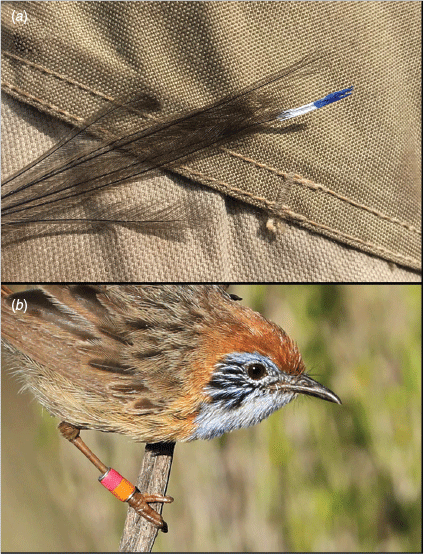
Within their contemporary distribution, mallee emu-wrens have a strong association with Triodia scariosa – a dense, interwoven hummock-forming grass (hereafter Triodia; Howe 1910; Verdon et al. 2020). Mallee emu-wrens have adapted to move ‘rodent-like’ through this complex vegetation and are consequently adept at avoiding capture in mist-nets. For this reason, mallee emu-wrens were captured using a weighted throw-net that was placed over a Triodia hummock in which birds were observed to be sheltering (Brown 2011). Each bird was uniquely marked with a combination of two colours. Given an initial concern that leg-bands or VHF transmitters (Hill and Elphick 2011) may cause birds to become snared by Triodia spines, individuals released in autumn were marked by painting the 5–10 mm terminal tip of the central two tail feathers with unique combinations of nail polish (Fig. 2a). These tail-markings remained for approximately 2 weeks, but were difficult to discern due to degradation of colours and visual obstruction from vegetation. As a result, identification during post-release monitoring was challenging. After a captive trial with rufous-crowned emu-wrens, Stipiturus ruficeps, leg bands were approved for birds released in spring. For this cohort, each bird was marked with a single Australian Bird and Bat Banding Scheme (ABBBS) metal band to which two bands of coloured tape were affixed before a protective epoxy coating was applied (males = right leg, females = left leg; Koronkiewicz et al. 2005, Fig. 2b). Following capture, mallee emu-wrens were held, singly or in pairs, in custom-made transport boxes approximately 300 × 150 × 150 mm. Each box had slide access-doors on both ends and a full length soft fly-wire mesh-covered opening at the side, with a ventilated sliding cover for airflow and to allow birds to be observed if required (e.g. during transfer). They were provisioned with live food (mini meal-worms and crickets), and driven to Ngarkat (190 km by road from Murray-Sunset and 270 km from Hattah/Nowingi; Fig. 1). The distance between catch and release sites meant that birds were held overnight for approximately 24 h before release. At the point of release, birds were held for a 30-min period within transport boxes where the mesh window was positioned to face Triodia vegetation (allowing the birds to gain at least some familiarity with their immediate surroundings), but otherwise the protocol was a ‘hard’ release with no supplementary food or shelter. Each group was released in a patch of dense Triodia habitat that was at least 400 m distant from any other release group. This density approximated that found within the source population. Mallee emu-wrens from the spring cohort were released in the same general area as the autumn cohort but all spring releases were at least 2 km from any autumn-released birds that were known to remain at that time.
|
Timing of release
During autumn, mallee emu-wrens form social groups of up to eight birds and territories are only loosely maintained. As spring approaches these groups divide into pairs (though sometimes supported by one or more helpers) and establish fixed territories (W. Mitchell, unpubl. data). Greater dispersal in autumn may increase the probability that mallee emu-wrens move away from the release site and, in doing so, fail to form a cohesive population (Ward and Schlossberg 2004). Translocation closer to the breeding season may reduce dispersal probability but the stress associated with capture and handling at a time when individuals could already be reproductively active may reduce breeding opportunities immediately after release (Dickens et al. 2010). Translocated individuals may suffer elevated mortality compared with the first generation of offspring born at the release site (Armstrong et al. 2017). Therefore, it is essential that managers maximise reproductive output in the first breeding season following release. To determine the optimum timing for release, this translocation involved both April (hereafter ‘Austral autumn’) and August (hereafter ‘Austral spring’) release cohorts.
Familiarity of release groups
Maintaining intact social groupings through the capture and transport phases of a translocation may reduce dispersal distances and stress experienced by individuals following release (Franks et al. 2020). However, this protocol requires additional time and labour for effective implementation. Unplanned events, such as mortality during holding, may also necessitate the release of group members that do not have prior familiarity. As such, there is value in understanding how separation from a cohesive social unit will influence an individual’s probability of post-release survival. To test this, release groups within each seasonal cohort contained either ‘familiar’ or ‘unfamiliar’ individuals based on association at capture. Mallee emu-wren groups are typically distributed sparsely and birds within a group forage close to one another. When separate groups do intercept, males and females engage in territorial behaviour, often perching in prominent locations and singing loudly (W. Mitchell, pers. obs.). During such displays it is not unusual for group members perceived by the observer to be subordinate or young to remain quiet and hidden until rival birds have moved away. Given these distinctive behaviours, it is unlikely that mallee emu-wrens from separate groups could be confused for familiar individuals during capture for translocation.
Establishment and persistence at the release site
Monitoring of translocated mallee emu-wrens in Ngarkat followed two distinct protocols: short-term intensive monitoring (10–18 days following final release), and longer-term occupancy monitoring (~2 and ~12 months post release). Short-term intensive monitoring commenced immediately following the first release of each cohort. Experienced observers conducted exhaustive area searches on a daily basis in habitat surrounding release points. For the autumn release, searches began on 17 April and finished on 3 May. For the spring release, searches began on 23 August and finished on 8 September. Searching began at ~dawn each day and continued until ~midday or until conditions became unsuitable for searching (e.g. high winds, temperatures exceeding 35°C). Searches were also conducted in the late afternoon when conditions were typically cooler and bird activity increased. Birds meeting the criteria for ‘short-term survival’ were those known to be present at the conclusion of the short-term monitoring period. This assessment did not account for imperfect detectability, however, search effort per unit area was far higher than typical surveys. During these monitoring periods, over 1200 ‘person hours’ were invested in comprehensively searching suitable habitat surrounding release sites. We defined dispersal as the distance between point of release and last known position for all unique individuals that could be positively identified. For the reasons mentioned above, fewer birds from the autumn cohort were able to be identified. We tested for differences in dispersal between autumn and spring cohorts using linear regression with season as sole predictor variable and dispersal as response variable. We also used linear regression to test for differences in dispersal between familiar and unfamiliar groups from the spring cohort with familiarity of release group as predictor and dispersal distance as response. We examined diagnostics plots to ensure that our data did not violate assumptions of linear models. We assessed group cohesion between familiar and unfamiliar groups using Fisher’s exact test (Fisher 1992). We used a binomial generalised linear model with a logit link function and no additional covariates to assess differences in short-term survival between familiar and unfamiliar groups. We checked assumptions using simulated residuals (no. iterations = 1000) in the ‘DHARMa’ package (Hartig 2017). All analyses were performed in the statistical environment R (R Core Team 2020).
During the longer-term monitoring protocol we assessed persistence of the translocated population using occupancy modelling based on repeated call-broadcast surveys at 122 key habitat points surrounding the release area. Surveys of fauna populations can be biased when individuals that are present remain undetected (MacKenzie et al. 2017). Occupancy modelling uses detection histories from repeated visits at multiple survey points to estimate the probability of detection (ρ) and occupancy (ψ) at each point (MacKenzie et al. 2017). Occupancy modelling relies on the assumption of ‘closure’, or that the occupancy state (whether the target species is present or absent) at each survey point remains consistent between repeated visits (MacKenzie et al. 2017). During the July surveys, at least one group of birds was suspected of moving between survey points, so estimated occupancy for this period may represent an upper estimate. The purpose of occupancy surveys was to assess the long-term persistence of the entire translocated population including both seasonal cohorts. To observe colour markings on a mallee emu-wren leg band, one must typically invest a significant amount of time in careful stalking. This was not feasible during occupancy surveys and, consequently, no attempt was made to differentiate between seasonal cohorts during occupancy modelling. Analyses were carried out using the package ‘unmarked’ in R (Fiske and Chandler 2011). Ngarkat is characterised by semiarid heath interspersed with patches of Triodia that form at the base of dunes on the south-eastern face. In this habitat mallee emu-wrens move through heath vegetation but are dependent on Triodia, and territories typically incorporate these Triodia patches. All such patches within a ~2000 ha area surrounding release points were identified using a combination of topographic data, satellite imagery and ground-truthing. At each point, an observer played a 30 s recording of mallee emu-wren contact calls at a volume that approximated free-ranging emu-wren calls, and then listened and watched for 30 s. This survey protocol was repeated once if no birds were detected. Experienced observers visited each point daily for 4 days between 25–28 July and 16–20 October 2018; 9–13 April and 30 July–3 August 2019. The time period between releases and follow-up surveys varied between the two cohorts due to logistical constraints.
Impact of harvesting at source sites
We conducted power analyses following Guillera-Arroita and Lahoz-Monfort (2012) to assess the feasibility of using occupancy modelling to quantify the impact of harvesting on source populations. The statistical power required to detect a change in occupancy between seasons is influenced by ψ, p, the number of occupancy sites being surveyed (S), and the number of visits to each site (k). Increasing S, to obtain a reasonable confidence (0.8) of detecting a change in occupancy across seasons, diluted the size of the effect we were trying to detect. This was because the number of birds being harvested did not change despite the increase in survey area. Given this inverse relationship between sampling area and relative effect size, we found that occupancy modelling would not be informative in this scenario.
Mallee emu-wrens are highly cryptic and detectability can vary between days. However, during spring, territories typically remain fixed. Therefore, the true abundance of mallee emu-wrens in any area is unlikely to change significantly over 1–5 days. Prior to the removal of mallee emu-wrens that made up the spring cohort, the primary harvest sites in Murray-Sunset and Nowingi were surveyed to establish baseline emu-wren density. Birds sourced for the spring translocation were not removed from any sites used as a source during the autumn translocation. We established 50 survey points, encompassing 200 ha and spaced at 200-m intervals, in a grid at each primary harvest site. Experienced observers visited each point daily for 3 days and played a 30 s recording of mallee emu-wren contact calls, followed by a 30 s period during which the observer listened carefully and scanned for any responding individuals. If no birds were detected this protocol was repeated once (this method mirrored that used during occupancy surveys described above). Where a group was detected, observers approached the group and carefully counted the number of individuals present. Following Bain and French (2009), the survey with the largest number of detections was designated as being closest to the ‘true’ abundance. Control sites, which were anticipated to support a similar density of mallee emu-wrens, were surveyed using the same method. Each capture site was paired with a ‘distant control’ at least 2 km from the treatment. A further control site was established adjacent to the treatment site in Nowingi, but this did not occur at the Murray-Sunset site as a patch of suitable habitat of approximately equal size was not available adjacent to the harvest site. These surveys were repeated 12 months after the translocation (August 2019) to assess the impact of removing birds for translocation, and are ongoing. Here, we report results of monitoring that was undertaken 12 months post-removal. Differences in abundance of mallee emu-wrens between years and treatments at each site were assessed with negative binomial generalised linear models with log link functions using the R package ‘MASS’ (equation = emu-wren detections ~ 1 + year × treatment, family = poisson, link-function = log; Venables and Ripley 2002). Interactions between groups were assessed using a post-hoc Tukey test. Assumptions were checked using simulated residuals (no. iterations = 1000) in the ‘DHARMa’ package.
Results
The autumn cohort of mallee emu-wrens were released in Ngarkat between 17 and 22 April 2018. Twenty-four birds (captured from eight groups) were sourced from Murray-Sunset and 16 (captured from four groups) were sourced from Hattah. The autumn cohort comprised seven ‘familiar’ groups and two ‘unfamiliar’ (3–7 birds per group). Spring releases took place between 23 and 28 August 2018. Twenty-two birds (captured from 13 groups) were sourced from Murray-Sunset and 16 birds (captured from eight groups) were sourced from Nowingi. The cohort comprised eight ‘familiar’ groups and nine ‘unfamiliar’ groups (2–3 birds per group). All birds within a group were captured at the same reserve. During autumn two individuals died in transit, and a third was injured during the catching process, resulting in mortality. Five mallee emu-wren mortalities occurred during transit in spring. Aside from the individual injured during capture, histopathology and gross necropsy examinations revealed no obvious cause of death for the other seven individuals. However, it was likely that stress relating to the capture and translocation process contributed to the deaths of at least some individuals. In total, 85 mallee emu-wrens were harvested from source populations.
Measures of success
Of the 40 birds released in autumn, 14–17 individuals distributed across 4–5 groups were known to be present at the conclusion of short-term intensive area searches on 3 May 2018. Painted-tail markings had been difficult to read throughout this monitoring period due to cryptic behaviour and frequent, rapid tail movements. Difficulties were exacerbated by tail-moult and further deterioration of markings, leading to uncertainty of the number of individuals remaining. Of the 38 birds released in spring, at least 27 individuals distributed across 15 groups persisted until the conclusion of intensive short-term monitoring on 8 September 2018. This included an additional individual from this cohort that was subsequently identified at this site in October 2018.
Birds released in spring were more likely to remain close to their release site when compared with autumn releases, and many exhibited behaviour indicative of breeding within 2 weeks of release (Fig. 3). By contrast, known dispersal was significantly higher in autumn when compared with spring (F1,59 = 44.2, P < 0.001, Fig. 3). Values presented here relating to short-term survival, dispersal and reproductive output relate only to birds of known fate. Several birds were never resighted following release and this may have resulted from any combination of cryptic behaviour, mortality or dispersal. However, the highest concentration of suitable mallee emu-wren habitat in Ngarkat occurs in the immediate area surrounding release sites. This area was comprehensively surveyed throughout the post-release monitoring period and birds dispersing beyond this area can reasonably be considered lost from the population, as any chance of renewed contact was unlikely, and a decline in suitable Triodia habitat beyond this area further reduced the probability of long-term persistence.
The challenge of identifying birds from the autumn cohort meant that it was not possible to assess how familiarity between release group members influenced group cohesion, dispersal, and persistence at the release site. By contrast, with colour banded individuals in the spring release, individual identifications were more readily obtained. Of those spring cohort birds present at the release site at the conclusion of monitoring, 82% of birds released in familiar groups maintained group fidelity compared with 38% released with unfamiliar birds (P = 0.047; Table 1). There was no significant difference in known survival (z1,37 = 1.19, P = 0.232) between birds released with either familiar (probability of survival = 0.65, 95% CI = 0.40–0.83) or unfamiliar conspecifics (probability of survival = 0.76, 95% CI = 0.54–0.90). Similarly, dispersal did not differ significantly between familiar and unfamiliar groups (Fig. 3; F1,19 = 0.06, P = 0.817).
For longer-term success mallee emu-wren occupancy in Ngarkat declined steadily following releases until August 2019, after which point we no longer detected any birds (Fig. 4).
Reproduction at the release site
During occupancy surveys at Ngarkat in October 2018 and additional unstructured searches in December 2018, breeding behaviour was observed on multiple occasions. Ultimately, 15 birds from seven distinct groups were confirmed to have contributed to a nesting attempt (based on discoveries of nests containing eggs/chicks or the presence of fledglings with adults). Of the 12 birds that could be identified, 9 were from the spring release cohort (Table 1). Of the three unidentified birds, in two cases a parent had disappeared prior to discovery of the nest and was therefore not identified, while for one female the presence or absence of a band could not be confirmed due to cryptic behaviour. We observed some indication of attempted breeding in an additional three pairs (e.g. courtship displays, distraction displays indicative of active nests), but breeding could not be confirmed.
Impact of harvest on the source population
At Nowingi, inter-annual variation (i.e. the factor ‘year’) was an important predictor of mallee emu-wren abundance with greater numbers estimated pre-harvest compared with post-harvest (z1,299 = 2.291, P = 0.022; Fig. 5a). Treatment was not an important predictor of emu-wren abundance, indicating that the effect of year was not due to the impact of harvest. At Murray-Sunset, abundance of mallee emu-wrens did not significantly differ by year or treatment (z1,199 = 0.480, P = 0.631; Fig. 5b). At both Murray-Sunset and Nowingi the difference in population size between years was greater at harvest and adjacent control sites when compared with distant controls, though these differences were non-significant (all z < 2.515; all P-values > 0.05). We detected no significant interaction between year and treatment at either site.
Discussion
Here we document the first conservation translocations of the Endangered mallee emu-wren. These actions serve as a key step in a recovery process that seeks to implement larger-scale translocations as a conservation tool. We gained valuable insights regarding both the mallee emu-wren specifically and translocation in a broader context. Familiarity of release groups and timing of release can influence post-release behaviour and persistence at release sites. Despite cryptic behaviour, we were able to capture an adequate number of mallee emu-wrens in this first phase for future large-scale translocations to be considered feasible. Individuals persisted at the release site for at least 12 months and a number of translocated individuals formed or maintained pairs and were able to successfully fledge young. Ultimately, the reintroduced population did not persist and we explore possible reasons for this below.
Release in autumn or spring?
The season in which release occurred had a measurable impact on post-release behaviour, persistence at the release site and likelihood of producing offspring. Birds released in autumn dispersed further and were less likely to survive in the short-term than those released in spring, although reduced dispersal and higher incidences of territorial behaviour associated with breeding may have positively biased estimates of persistence for the spring cohort. Additionally, smaller group size during autumn may have been a source of bias to estimates of abundance. Small group size meant there were more groups available to be detected during this period, but the smaller group size may also have decreased detectability when compared with the autumn cohort. However, given exhaustive survey coverage during short-term intensive area searches, we believe that we were able to detect the majority of birds that remained within the release area. Few of the mallee emu-wrens released in autumn were known to have persisted at the release site by spring, and consequently few birds from this cohort were available to integrate with the spring cohort or to breed. Maximising reproductive output of translocated populations is critical for ongoing persistence (Sigg et al. 2005; Batson et al. 2015). Selecting the most beneficial season of release is one way that translocation managers might influence post-release behaviour, increase survival and increase reproductive opportunities. In light of our findings, any future mallee emu-wren translocations should prioritise spring releases.
Changes in animal behaviour between time periods is a near-universal phenomenon (Sutherland 1998). In semiarid systems, behavioural variation occurs both seasonally (e.g. breeding versus non-breeding seasons), and over decadal timescales (e.g. breeding events triggered by periods of above-average rainfall; Verdon-Kidd and Kiem 2009). In this translocation, season of release influenced post-release dispersal, survival, and reproduction. Aligning translocations with favourable longer-term climatic events (e.g. during periods of above-average rainfall as forecast by La Niña climate cycles in southern Australia), may also increase the probability of long-term persistence of translocated populations (Letnic et al. 2005; Chen et al. 2020).
Familiar or unfamiliar?
Translocating cohesive social groups increased the likelihood of post-release group fidelity but had no detectable effect on known dispersal or survival in spring. Release protocols have been found to influence post-release group cohesion in other translocated species (Armstrong 1995; Armstrong and Craig 1995; Anstee and Armstrong 2001; Clarke et al. 2002; Bennett et al. 2012; Moseby et al. 2018; Franks et al. 2020). Whether or not this has led to improved survival rates appears to have been largely species-dependent. In translocated juvenile hihi, Notiomystis cincta, a positive association was found between the number of associates gained during a period of experimental social mixing and post-release survival (Franks et al. 2020). A similar mechanism may have been operating among mallee emu-wren groups released in Ngarkat. More mallee emu-wrens survived when they were released with unfamiliar individuals, although this difference was not significant. For translocation managers, increased post-release group cohesion represents a potential trade-off against increased capture effort and potential for stress in focal individuals. For cryptic species it may not always be possible to capture an intact group. Similarly, mortality during transit may leave managers with an isolated individual. In such a scenario it is advantageous to know the probability of survival of a release group comprising unfamiliar birds compared with that of an intact social group. The precautionary principle still applies, and wherever possible, mallee emu-wren social groups should be kept intact. However, in future translocations, managers may be able to further limit capture stress in birds during harvest, by avoiding extended pursuits of cryptic individuals if all members of a social unit are not required for translocation.
Impact of harvest on the source population
There was a trend towards larger declines at harvest sites, indicative of an impact of harvesting, although this was not significant. However, the recorded decrease in population exceeded the number of mallee emu-wrens removed for translocation, and detections were also lower at distant control sites 12 months after pre-removal surveys, indicating that the removal of birds was not the sole cause of population decrease. Managers must account for abiotic factors that might influence sustainable harvest rates of source populations where possible. Little is known about mallee emu-wren recruitment, but it is possible that below average rainfall in the 12 months following harvesting contributed to the significantly lower abundance across both treatment and control sites at Nowingi. Ongoing monitoring at these sites will improve our understanding of mallee emu-wren recruitment and the long-term sustainability of current populations as a source for future translocations.
Despite repeated calls to routinely investigate impacts of harvesting on source populations the reporting of outcomes on this aspect of translocation practice remains rare (e.g. Stevens and Goodson 1993; Dimond and Armstrong 2007; IUCN/SSC 2013; Furlan et al. 2020). In contrast to our results, no significant reduction in abundance was detected following harvest of 44 eastern bristlebirds, Dasyornis brachypterus, another small cryptic passerine (Bain and French 2009). It is essential that source populations are managed conservatively in settings where the impact of harvest is uncertain (Dimond and Armstrong 2007).
Population decline
A key measure of success in any translocation program is long-term persistence of the translocated population (Taylor et al. 2017). Our explicit focus lay in developing protocols for effective transport and establishment. However, understanding population decline during this early stage is crucial for planning future releases. Several factors may have contributed to the decline in mallee emu-wren occupancy following release. Allee effects – the suppression of population vital rates at low density – have been detected in translocated populations (Courchamp et al. 2008; Armstrong and Wittmer 2011). Little is known about mallee emu-wren vital rates; however, naturally high mortality has been reported in the closely related southern emu-wren S. malachurus with few birds surviving beyond two breeding seasons (Maguire and Mulder 2004). High mortality may have been exacerbated at low density through several mechanisms including reduced social cohesion or greater susceptibility to harsh environmental conditions, e.g. if mallee emu-wrens rely on huddling for thermal protection during cold nights (Gilbert et al. 2010). Emu-wrens may suffer increased susceptibility to mortality following death of a group member, particularly if opportunities to find new associates are rare. Harvest and release sites suffered below average rainfall and higher than average temperatures in the 12 months following release (Fig. 4, Bureau of Meteorology 2020). Mallee emu-wrens are adapted to semiarid environments and variable conditions, but populations typically contract in dry years, as evidenced by reduced abundance at source sites over this same period (Connell 2019). Adverse conditions culminated in an extended period of low rainfall in early 2019 that may have severely limited available resources for mallee emu-wrens in Ngarkat. Days of extreme heat can also have a significant effect on passerine mortality in semiarid systems. Sharpe et al. (2019) found that mortality of jacky winter Microeca fascinans increased by a factor of three during extreme climatic events. Further inference here is limited by a lack of data on the effect of environmental conditions on mallee emu-wrens.
Post-release dispersal behaviour may also have affected long-term persistence (Berger‐Tal et al. 2019). Though source and release sites both contained abundant Triodia, Ngarkat differs in vegetation structure to source sites (Brown et al. 2009). Previously occupied habitat is not necessarily an indicator of suitable habitat, and it is difficult to identify all requirements for a translocated population (Osborne and Seddon 2012). Mallee emu-wrens may have dispersed from the release area seeking habitat more similar to that in which they were captured. We found that releasing birds immediately prior to the breeding season significantly reduced dispersal compared with those released in autumn, but this may have been related to biological cues driving birds to reproduce. At the conclusion of the breeding season groups may have continued to disperse seeking natal habitat.
Financial accountability
Disclosure of the financial details of conservation actions serves as a valuable guide for other researchers and managers in the planning and funding phases of translocation actions (Fischer and Lindenmayer 2000). In addition to formal funding and in-kind support from partner organisations, dedicated volunteers provided hundreds of person-hours of in-kind labour during this project. In total, the phase one translocation of mallee emu-wrens to Ngarkat Conservation Park cost $538 882 AUD, comprising $287 958 AUD of funding and $250 924 AUD of in-kind support (see Supplementary Material Table S1).
Conclusions
In threatened species research, sample sizes are invariably small, and disentangling the many factors that may influence conservation outcomes is a common hurdle. Despite such limitations, insight can be gained when explicit trials are incorporated into conservation management. Season of release can have a significant impact on dispersal, persistence in the short term and reproductive output of translocated individuals. Translocating socially cohesive units can increase post-release group fidelity but, in this case, did not affect survival or dispersal. The mallee emu-wren remains vulnerable to catastrophic wildfire in currently occupied habitat. Given a demonstrated inability to halt many wildfires (Boer et al. 2020), translocation remains an important tool to mitigate this threat. Any future mallee emu-wren translocations should prioritise release immediately prior to the breeding season. Capture and transport of socially cohesive groups should be pursued but is not here considered essential when scarce resources might be better spent. Harvesting mallee emu-wrens for translocation impacted source populations and additional research should focus on population demographics at source sites to ensure that harvesting for translocation is sustainable. To improve the likelihood of success in future conservation measures for mallee emu-wrens, further studies that investigate factors that contribute to longer-term population decline are warranted. Despite not establishing a self-sustaining population, the outcomes of this study provide insight for future translocation programs and valuable learnings for the ongoing conservation of the mallee emu-wren.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
This project was supported by the South Australian Murray–Darling Basin Natural Resources management Board (permit no. A26732-2) through funding from the Australian Government’s National Landcare Program and South Australian NRM levies. Contributions were also made from all project partners including BirdLife Australia; Zoos South Australia; the Victorian Department of Environment, Land, Water and Planning; Parks Victoria; Rotary International; La Trobe University; Monash University; and Zoos Victoria. Research was carried out under South Australia Department of Environment and Water Animal Ethics Approval (37/2017). BirdLife Australia, Zoos Victoria, and Nature Foundation South Australia are thanked for additional research funding. We would like to acknowledge the contribution of three anonymous reviewers who provided valuable feedback. We are grateful to the following for their contributions to the translocations and subsequent monitoring: Shandiya Balasubramaniam, Joseph Birkhead, Jemma Cripps, Sam Gordon, Claire Hartvigsen-Power, Cassie Hlava, Grace Hodder, Tom Hurley, Sierra Jamieson, Stephanie Johnson, Dona Kireta, Owen Lishmund, Marina Louter, Rachel McIntosh, Clementine Menz, Hayley Merigot, Casey O’Brien, Ned Pisconieri, Steve Sass, Angela Simms, Amy Slender, Diane Smith, Peri Stenhouse, Wendy Stubbs, Ben Viola, Ross Williamson, and Elspeth Young.
References
Anstee, S., and Armstrong, K. (2001). The effect of familiarity and mound condition in translocations of the western pebble-mound mouse, Pseudomys chapmani, in the Pilbara region of Western Australia. Wildlife Research 28, 135–140.| The effect of familiarity and mound condition in translocations of the western pebble-mound mouse, Pseudomys chapmani, in the Pilbara region of Western Australia.Crossref | GoogleScholarGoogle Scholar |
Armstrong, D. P. (1995). Effects of familiarity on the outcome of translocations, II. A test using New Zealand robins. Biological Conservation 71, 281–288.
| Effects of familiarity on the outcome of translocations, II. A test using New Zealand robins.Crossref | GoogleScholarGoogle Scholar |
Armstrong, D. P., and Craig, J. L. (1995). Effects of familiarity on the outcome of translocations, I. A test using saddlebacks Philesturnus carunculatus rufusater. Biological Conservation 71, 133–141.
| Effects of familiarity on the outcome of translocations, I. A test using saddlebacks Philesturnus carunculatus rufusater.Crossref | GoogleScholarGoogle Scholar |
Armstrong, D. P., and Wittmer, H. U. (2011). Incorporating Allee effects into reintroduction strategies. Ecological Research 26, 687–695.
| Incorporating Allee effects into reintroduction strategies.Crossref | GoogleScholarGoogle Scholar |
Armstrong, D. P., Castro, I., and Griffiths, R. (2007). Using adaptive management to determine requirements of re-introduced populations: the case of the New Zealand hihi. Journal of Applied Ecology 44, 953–962.
| Using adaptive management to determine requirements of re-introduced populations: the case of the New Zealand hihi.Crossref | GoogleScholarGoogle Scholar |
Armstrong, D. P., Le Coeur, C., Thorne, J. M., Panfylova, J., Lovegrove, T. G., Frost, P. G. H., and Ewen, J. G. (2017). Using Bayesian mark-recapture modelling to quantify the strength and duration of post-release effects in reintroduced populations. Biological Conservation 215, 39–45.
| Using Bayesian mark-recapture modelling to quantify the strength and duration of post-release effects in reintroduced populations.Crossref | GoogleScholarGoogle Scholar |
Bain, D., and French, K. (2009). Impacts on a threatened bird population of removals for translocation. Wildlife Research 36, 516–521.
| Impacts on a threatened bird population of removals for translocation.Crossref | GoogleScholarGoogle Scholar |
Batson, W., Abbott, R., and Richardson, K. M. (2015). Release strategies for fauna reintroductions: theory and tests. In ‘Advances in reintroduction biology of Australian and New Zealand Fauna’. (Eds D.P. Armstrong, M.W. Hayward, D. Moro, and P.J. Seddon.). pp 7–16. (CSIRO Publishing: Clayton South, Vic., Australia.)
Bennett, V. A., Doerr, V. A. J., Doerr, E. D., Manning, A. D., and Lindenmayer, D. B. (2012). The anatomy of a failed reintroduction: a case study with the Brown Treecreeper. Emu - Austral Ornithology 112, 298–312.
| The anatomy of a failed reintroduction: a case study with the Brown Treecreeper.Crossref | GoogleScholarGoogle Scholar |
Berger‐Tal, O., Blumstein, D. T., and Swaisgood, R. R. (2019). Conservation translocations: a review of common difficulties and promising directions. Animal Conservation 23, 121–131.
| Conservation translocations: a review of common difficulties and promising directions.Crossref | GoogleScholarGoogle Scholar |
Boer, M. M., de Dios, V. R., and Bradstock, R. A. (2020). Unprecedented burn area of Australian mega forest fires. Nature Climate Change 10, 171–172.
| Unprecedented burn area of Australian mega forest fires.Crossref | GoogleScholarGoogle Scholar |
Boulton, R. L., and Lau, J. (2015). Threatened Mallee Birds Conservation Action Plan, Report June 2015. Birdlife Australia Report to the Threatened Mallee Birds Implementation Team.
Boulton, R. L., and Hedger, C. (2018). Reintroduction of the mallee emu-wren Stipiturus mallee to Ngarkat Conservation Park, Phase 1. Report to Fauna Translocation Evaluation Panel, Department of Environment, Land, Water and Planning.
Bright, P. W., and Morris, P. A. (1994). Animal translocation for conservation: Performance of dormice in relation to release methods, origin and season. Journal of Applied Ecology 31, 699–708.
| Animal translocation for conservation: Performance of dormice in relation to release methods, origin and season.Crossref | GoogleScholarGoogle Scholar |
Brown, S. (2011). Mallee emu-wren (Stipiturus mallee): Multi-scale habitat requirements and population structure. PhD thesis, Deakin University, Melbourne.
Brown, S., Clarke, M., and Clarke, R. H. (2009). Fire is a key element in the landscape-scale habitat requirements and global population status of a threatened bird: The mallee emu-wren (Stipiturus mallee). Biological Conservation 142, 432–445.
| Fire is a key element in the landscape-scale habitat requirements and global population status of a threatened bird: The mallee emu-wren (Stipiturus mallee).Crossref | GoogleScholarGoogle Scholar |
Brown, S. M., Harrisson, K. A., Clarke, R. H., Bennett, A. F., and Sunnucks, P. (2013). Limited population structure, genetic drift and bottlenecks characterise an endangered bird species in a dynamic, fire-prone ecosystem. PLoS One 8, e59732.
| Limited population structure, genetic drift and bottlenecks characterise an endangered bird species in a dynamic, fire-prone ecosystem.Crossref | GoogleScholarGoogle Scholar | 23626668PubMed |
Bureau Of Meteorology (2020). Lameroo (Austin Plains) Weather Station: monthly mean temperature. Available at http://www.bom.gov.au/climate/data/(Accessed 1 January 2020).
Chen, H. C., Tseng, Y. H., Hu, Z. Z., and Ding, R. (2020). Enhancing the ENSO Predictability beyond the Spring Barrier. Scientific Reports 10, 984.
| Enhancing the ENSO Predictability beyond the Spring Barrier.Crossref | GoogleScholarGoogle Scholar | 31969614PubMed |
Clarke, R. H., Boulton, R. L., and Clarke, M. F. (2002). Translocation of the socially complex Black-eared Miner Manorina melanotis: a trial using hard and soft release techniques. Pacific. Conservation Biology 8, 223–234.
| Translocation of the socially complex Black-eared Miner Manorina melanotis: a trial using hard and soft release techniques. Pacific.Crossref | GoogleScholarGoogle Scholar |
Connell, J. (2019). Fire and rain: Investigating how major ecological drivers shape a semi-arid bird community over space and time. PhD thesis, Department of Ecology, Environment and Evolution, School of Life Sciences, La Trobe University, Melbourne.
Courchamp, F., Berec, L., and Gascoigne, J. (2008). ‘Allee effects in ecology and conservation.’ (Oxford University Press: Oxford.)
Department of Environment, Land, Water and Planning (2016). National Recovery Plan for the mallee emu-wren Stipiturus mallee, red-lored whistler Pachycephala rufogularis and western whipbird Psophodes nigrogularis leucogaster. (Australian Government: Canberra.)
Dickens, M. J., Delehanty, D. J., and Michael Romero, L. (2010). Stress: An inevitable component of animal translocation. Biological Conservation 143, 1329–1341.
| Stress: An inevitable component of animal translocation.Crossref | GoogleScholarGoogle Scholar |
Dimond, W. J., and Armstrong, D. P. (2007). Adaptive harvesting of source populations for translocation: A case study with New Zealand robins. Conservation Biology 21, 114–124.
| Adaptive harvesting of source populations for translocation: A case study with New Zealand robins.Crossref | GoogleScholarGoogle Scholar | 17298517PubMed |
Easton, L. J., Bishop, P. J., and Whigham, P. A. (2019). Balancing act: modelling sustainable release numbers for translocations. Animal Conservation 23, 434–442.
| Balancing act: modelling sustainable release numbers for translocations.Crossref | GoogleScholarGoogle Scholar |
Fischer, J., and Lindenmayer, D. B. (2000). An assessment of the published results of animal relocations. Biological Conservation 96, 1–11.
| An assessment of the published results of animal relocations.Crossref | GoogleScholarGoogle Scholar |
Fisher, R. A. (1992). Statistical methods for research workers. In ‘Breakthroughs in statistics’. (Eds S. Kotz and N.L. Johnson) pp. 66–70. (Springer. Verlag: New York.)
Fiske, I., and Chandler, R. B. (2011). unmarked: An R Package for Fitting Hierarchical Models of Wildlife Occurrence and Abundance. Journal of Statistical Software 43, 1–23.
| unmarked: An R Package for Fitting Hierarchical Models of Wildlife Occurrence and Abundance.Crossref | GoogleScholarGoogle Scholar |
Franks, V. R., Andrews, C. E., Ewen, J. G., McCready, M., Parker, K. A., and Thorogood, R. (2020). Changes in social groups across reintroductions and effects on post‐release survival. Animal Conservation 23, 443–454.
| Changes in social groups across reintroductions and effects on post‐release survival.Crossref | GoogleScholarGoogle Scholar |
Furlan, E. M., Gruber, B., Attard, C. R. M., Wager, R. N. E., Kerezsy, A., Faulks, L. K., Beheregaray, L. B., and Unmack, P. J. (2020). Assessing the benefits and risks of translocations in depauperate species: A theoretical framework with an empirical validation. Journal of Applied Ecology 57, 831–841.
| Assessing the benefits and risks of translocations in depauperate species: A theoretical framework with an empirical validation.Crossref | GoogleScholarGoogle Scholar |
Gilbert, C., McCafferty, D., Le Maho, Y., Martrette, J. M., Giroud, S., Blanc, S., and Ancel, A. (2010). One for all and all for one: the energetic benefits of huddling in endotherms. Biological Reviews 85, 545–569.
| One for all and all for one: the energetic benefits of huddling in endotherms.Crossref | GoogleScholarGoogle Scholar | 20039866PubMed |
Griffith, B., Scott, J. M., Carpenter, J. W., and Reed, C. (1989). Translocation as a species conservation tool: Status and strategy. Science 245, 477–480.
| Translocation as a species conservation tool: Status and strategy.Crossref | GoogleScholarGoogle Scholar | 17750257PubMed |
Guillera-Arroita, G., and Lahoz-Monfort, J. J. (2012). Designing studies to detect differences in species occupancy: power analysis under imperfect detection. Methods in Ecology and Evolution 3, 860–869.
| Designing studies to detect differences in species occupancy: power analysis under imperfect detection.Crossref | GoogleScholarGoogle Scholar |
Hartig, F. (2017). DHARMa: residual diagnostics for hierarchical (multi-level/mixed) regression models. R package version 0.1, 5. Available at https://CRAN.R-project.org/package=DHARMa
Hellstedt, P., and Kallio, E. R. (2005). Survival and behaviour of captive-born weasels (Mustela nivalis nivalis) released in nature. Journal of Zoology 266, 37–44.
| Survival and behaviour of captive-born weasels (Mustela nivalis nivalis) released in nature.Crossref | GoogleScholarGoogle Scholar |
Higgins, P. J., Peter, J. M., and Steele, W. K. (2001). ‘Handbook of Australian, New Zealand and Antarctic Birds, Volume 5: Tyrant-flycatchers to Chats.’ (Oxford University Press: Melbourne.)
Hill, J. M., and Elphick, C. S. (2011). Are grassland passerines especially susceptible to negative transmitter impacts? Wildlife Society Bulletin 35, 362–367.
| Are grassland passerines especially susceptible to negative transmitter impacts?Crossref | GoogleScholarGoogle Scholar |
Howe, F. (1910). Notes on the mallee emu-wren. Emu – Austral Ornithology 10, 336–337.
| Notes on the mallee emu-wren.Crossref | GoogleScholarGoogle Scholar |
IUCN/SSC (2013). Guidelines for reintroductions and other translocations. Version 1.0. (IUCN Species Survival Commision: Gland, Switzerland.)
Jamieson, I. G. (2011). Founder effects, inbreeding, and loss of genetic diversity in four avian reintroduction programs. Conservation Biology 25, 115–123.
| Founder effects, inbreeding, and loss of genetic diversity in four avian reintroduction programs.Crossref | GoogleScholarGoogle Scholar | 20825445PubMed |
Koronkiewicz, T. J., Paxton, E. H., and Sogge, M. K. (2005). A technique to produce aluminum color bands for avian research. Journal of Field Ornithology 76, 94–97.
| A technique to produce aluminum color bands for avian research.Crossref | GoogleScholarGoogle Scholar |
Letnic, M., Tamayo, B., and Dickman, C. R. (2005). The responses of mammals to La Niña (El Niño Southern Oscillation)-associated rainfall, predation, and wildfire in central Australia. Journal of Mammalogy 86, 689–703.
| The responses of mammals to La Niña (El Niño Southern Oscillation)-associated rainfall, predation, and wildfire in central Australia.Crossref | GoogleScholarGoogle Scholar |
MacKenzie, D. I., Nichols, J. D., Royle, J. A., Pollock, K. H., Bailey, L., and Hines, J. E. (2017). ‘Occupancy estimation and modeling: inferring patterns and dynamics of species occurrence.’ (Elsevier: Amsterdam.)
Maguire, G. S., and Mulder, R. A. (2004). Breeding biology and demography of the southern emu-wren (Stipiturus malachurus). Australian Journal of Zoology 52, 583–604.
| Breeding biology and demography of the southern emu-wren (Stipiturus malachurus).Crossref | GoogleScholarGoogle Scholar |
Menkhorst, P., Rogers, D. I., and Clarke, R. (2017). ‘The Australian bird guide.’ (CSIRO Publishing: Melbourne, Victoria.)
Mihoub, J. B., Robert, A., Le Gouar, P., and Sarrazin, F. (2011). Post-release dispersal in animal translocations: Social attraction and the “vacuum effect”. PLoS One 6, e27453.
| Post-release dispersal in animal translocations: Social attraction and the “vacuum effect”.Crossref | GoogleScholarGoogle Scholar | 22194784PubMed |
Miller, K. A., Bell, T. P., and Germano, J. M. (2014). Understanding publication bias in reintroduction biology by assessing translocations of New Zealand’s herpetofauna. Conservation Biology 28, 1045–1056.
| Understanding publication bias in reintroduction biology by assessing translocations of New Zealand’s herpetofauna.Crossref | GoogleScholarGoogle Scholar | 24606604PubMed |
Møller, A. P., and Jennions, M. D. (2001). Testing and adjusting for publication bias. Trends in Ecology & Evolution 16, 580–586.
| Testing and adjusting for publication bias.Crossref | GoogleScholarGoogle Scholar |
Moseby, K. E., Blumstein, D. T., Letnic, M., and West, R. (2018). Choice or opportunity: Are post-release social groupings influenced by familiarity or reintroduction protocols? Oryx 54, 215–221.
| Choice or opportunity: Are post-release social groupings influenced by familiarity or reintroduction protocols?Crossref | GoogleScholarGoogle Scholar |
Osborne, P. E., and Seddon, P. J. (2012). Selecting suitable habitats for reintroductions: variation, change and the role of species distribution modelling. In ‘Reintroduction biology: integrating science and management’. (Eds J.G. Ewen, D.P. Armstrong, K.A. Parker, and P.J. Seddon.) pp. 73–104. (Wiley-Blackwell: Oxford.)
Parker, K. A., Dickens, M. J., Clarke, R. H., Lovegrove, T. G., Ewen, J., and Armstrong, D. (2012). The theory and practice of catching, holding, moving and releasing animals. In ‘Reintroduction biology: integrating science and management’. (Eds J.G. Ewen, D.P. Armstrong, K.A. Parker, and P.J. Seddon.) pp. 73–104. (Wiley-Blackwell: Oxford.)
Paton, D. C., Rogers, D. J., Cale, P., Willoughby, N., and Gates, J. A. (2009). Birds. In ‘Natural History of the Riverland and Murraylands’. (Ed. J.T. Jennings.) pp. 371–396. (Royal Society of South Australia Inc.: Adelaide)
R Core Team (2020). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available at https://www.R-project.org
Sharpe, L., Cale, B., and Gardner, J. L. (2019). Weighing the cost: the impact of serial heatwaves on body mass in a small Australian passerine. Journal of Avian Biology 50, 1–9.
| Weighing the cost: the impact of serial heatwaves on body mass in a small Australian passerine.Crossref | GoogleScholarGoogle Scholar |
Sigg, D., Goldizen, A., and Pople, A. (2005). The importance of mating system in translocation programs: reproductive success of released male bridled nailtail wallabies. Biological Conservation 123, 289–300.
| The importance of mating system in translocation programs: reproductive success of released male bridled nailtail wallabies.Crossref | GoogleScholarGoogle Scholar |
Stevens, D. R., and Goodson, N. J. (1993). Assessing effects of removals for transplanting on a high-elevation bighorn sheep population. Conservation Biology 7, 908–915.
| Assessing effects of removals for transplanting on a high-elevation bighorn sheep population.Crossref | GoogleScholarGoogle Scholar |
Sutherland, W. J. (1998). The importance of behavioural studies in conservation biology. Animal Behaviour 56, 801–809.
| The importance of behavioural studies in conservation biology.Crossref | GoogleScholarGoogle Scholar | 9790690PubMed |
Taylor, G., Canessa, S., Clarke, R. H., Ingwersen, D., Armstrong, D. P., Seddon, P. J., and Ewen, J. G. (2017). Is reintroduction biology an effective applied science? Trends in Ecology & Evolution 32, 873–880.
| Is reintroduction biology an effective applied science?Crossref | GoogleScholarGoogle Scholar |
Tetzlaff, S. J., Sperry, J. H., and DeGregorio, B. A. (2019). Effects of antipredator training, environmental enrichment, and soft release on wildlife translocations: A review and meta-analysis. Biological Conservation 236, 324–331.
| Effects of antipredator training, environmental enrichment, and soft release on wildlife translocations: A review and meta-analysis.Crossref | GoogleScholarGoogle Scholar |
Venables, W. N., and Ripley, B. D. (2002). ‘Modern Applied Statistics with S’, 4th edn. (Springer, New York.)
Verdon, S. J., Watson, S. J., and Clarke, M. F. (2019). Modeling variability in the fire response of an endangered bird to improve fire-management. Ecological Applications 29, e01980.
| Modeling variability in the fire response of an endangered bird to improve fire-management.Crossref | GoogleScholarGoogle Scholar |
Verdon, S. J., Watson, S. J., Nimmo, D. G., and Clarke, M. F. (2020). Are all fauna associated with the same structural features of the foundation species Triodia scariosa? Austral Ecology 45, 773–787.
| Are all fauna associated with the same structural features of the foundation species Triodia scariosa?Crossref | GoogleScholarGoogle Scholar |
Verdon, S. J., Mitchell, W. F., and Clarke, M. F. (2021). Can flexible timing of harvest for translocation reduce the impact on a fluctuating donor population? Wildlife Research , .
| Can flexible timing of harvest for translocation reduce the impact on a fluctuating donor population?Crossref | GoogleScholarGoogle Scholar |
Verdon‐Kidd, D. C., and Kiem, A. S. (2009). Nature and causes of protracted droughts in southeast Australia: Comparison between the Federation, WWII, and Big Dry droughts. Geophysical Research Letters 36, .
| Nature and causes of protracted droughts in southeast Australia: Comparison between the Federation, WWII, and Big Dry droughts.Crossref | GoogleScholarGoogle Scholar |
Ward, M. P., and Schlossberg, S. (2004). Conspecific attraction and the conservation of territorial songbirds. Conservation Biology 18, 519–525.
| Conspecific attraction and the conservation of territorial songbirds.Crossref | GoogleScholarGoogle Scholar |







