Recreational fishery discard practices influence use of tidal estuary by a large marine mesopredator
Joni Pini-Fitzsimmons A * , Nathan A. Knott B and Culum Brown
A * , Nathan A. Knott B and Culum Brown  A
A
A School of Natural Sciences, Macquarie University, North Ryde, NSW, Australia.
B Marine Ecosystems Unit, Fisheries Research, New South Wales Department of Primary Industries, Huskisson, NSW, Australia.
Marine and Freshwater Research 74(4) 320-334 https://doi.org/10.1071/MF22146
Submitted: 23 July 2022 Accepted: 6 January 2023 Published: 30 January 2023
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Context: It is common for recreational anglers to discard waste produced from filleting catches back into the water, which results in a highly spatio-temporally predictable food subsidy for wildlife to scavenge. However, the behavioural responses of these scavengers has received little attention.
Aims: We aimed to assess the visitation of a common mesopredatory scavenger in relation to temporal patterns in waste discarding at a boat ramp in south-eastern Australia.
Methods: Using passive acoustic telemetry, the movements of 13 adult female smooth stingrays (Bathytoshia brevicaudata) were tracked, and patterns in their acoustic detections and duration of time spent in different sections within the study area were compared.
Key results: Use of the study area was strongly focused around the boat ramp, and peaked during periods of increased provisioning activity (i.e. afternoons and weekends). Environmental variables had limited influence on visitation, suggesting that the use of the area was not likely to be linked to natural behaviours.
Conclusions: The observed patterns indicated that the movements of smooth stingrays were linked to waste-discard practices by recreational anglers.
Implications: This study has implications for the management of discard practices for recreational fishing.
Keywords: batoidea, behavioural ecology, elasmobranchs, fishing discards, food provisioning, human–wildlife interactions, movement ecology, recreational fishing.
Introduction
The more predictable resources are in time and space, the faster and more strongly animals can build associations that maximise their access to them (Reebs 1993; Mulder et al. 2013; Heinrich et al. 2020). Due to the stochastic nature of environmental conditions, resources are rarely highly predictable in both time and space, and if conditions do support high predictability, it is typically short-lived. Human activities such as agriculture, hunting and fishing can result in food subsidies being provisioned to wildlife (Oro et al. 2013). Many of these activities have inherent temporal cycles (Margalef 1997; Oro et al. 2013), such as the 9–5 working-day or 7-day week, and typically occur in specific locations, resulting in highly predictable food subsidies. This, in turn, can cause significant changes to animal distribution and behaviour through their consumption of these provisioned resources (Oro et al. 2013). For example, in the Mediterranean, there is a well-studied seabird community that forages on discards (i.e. by-catch) from a trawl fishery that operates during set hours on weekdays and in fishing grounds that are generally consistent in space (Oro et al. 2013). The resulting high spatio-temporal predictability of discards from this fishery has led to a significant reduction in foraging times and foraging areas by seabirds that have synchronised their movement with the operating schedule of the fishery (e.g. Bartumeus et al. 2010; García-Tarrasón et al. 2015; Matos et al. 2018).
In marine systems, fisheries generate large quantities of discards, either as by-catch in the form of undersized or non-target species or waste from processing. For commercial fisheries, it is estimated at ~10% of global catches are discarded annually (~7 × 106 tonnes, Mg; Kelleher 2005; Zeller et al. 2018). Annual catches for recreational fisheries amount to almost 1 × 106 Mg (Freire et al. 2020), and it has been estimated that 30–75% of catches are discarded as by-catch or non-target species (Huddart 2019). For recreational catches that are retained for consumption, it is also widely accepted to discard waste produced from the cleaning and filleting process (i.e. offal, carcasses) back into the water. However, by-catch and waste discards from recreational fisheries are rarely quantified and not currently considered in global fishery discard estimates (Kelleher 2005; Zeller et al. 2018; Freire et al. 2020). In addition, although the direct impacts of fishing are well documented (e.g. impacts to fish stocks, habitat destruction; Dayton et al. 1995; Ortuño Crespo and Dunn 2017; Huddart 2019; Lewin et al. 2019), the potential influence of associated discards has received little attention.
There is a considerable body of research characterising the consumers of commercial fishery discards, such as invertebrates, teleost fishes, elasmobranchs, marine mammals, and seabirds (Oro et al. 2013). Changes to reproduction, spatial distribution, population sizes, and dispersal has been documented in many of these consumers, particularly seabirds, and to a lesser extent marine mammals (reviewed in Oro et al. 2013). However, there is a paucity of research into the impacts on mid-water and benthic species, such as teleost fishes and elasmobranchs, despite a considerable portion of discards sinking below the surface. Likewise, a range of species have been documented foraging on recreational fishing discards, including invertebrates, teleost fishes, marine mammals (Donaldson et al. 2010; Christiansen et al. 2016; Voohris 2016), elasmobranchs (Newsome et al. 2004; Pini-Fitzsimmons et al. 2018; Martin et al. 2019), sea birds, and even terrestrial predators, such as dingoes (Behrendorff et al. 2016; Déaux et al. 2018), but few studies have assessed the potential impacts on these animals. The few existing studies suggest that species alter their behaviour and space use to access these resources. For example, bottlenose dolphins (Tursiops sp.) learned to associate recreational fishing boats with food and began depredating on non-target fishes discarded by recreational anglers (Powell and Wells 2011; Christiansen et al. 2016). Similarly, a repeated-exposure experiment of carcharhinid shark species to fishing boats and food (representing hook and line fishing) resulted in reduced time to arrival and feeding, suggesting that depredation can lead to behavioural modifications (Mitchell et al. 2020).
Furthermore, although fishing activity typically occurs over wide areas, discards tend to be concentrated into smaller areas. This is perhaps truer for recreational fisheries, where anglers return to shore-based fish-cleaning facilities associated with boat ramps to process their catches for consumption and discard waste. This high concentration of food in specific locations in conjunction with repetitive temporal patterns in the activity result in conditions where animals can quickly develop strong associations. Given high participation rates and the widespread prevalence of recreational fishing (Cisneros-Montemayor and Sumaila 2010; Freire et al. 2020; Arlinghaus et al. 2021), and the potentially significant behavioural modifications identified for species that consume fishery discards, the role of these discards as a food subsidy to marine wildlife and their behavioural adaptations for accessing these resources needs to be further examined.
Smooth stingrays (Bathytoshia brevicaudata) are a large demersal ray species found in coastal waters of Australia, New Zealand, southern Africa, Japan, and eastern Russia (Rigby et al. 2021). Throughout their range, they are common scavengers of recreational fishing discards (Australia: J. Pini-Fitzsimmons, pers. obs.; New Zealand: H. Cadwallader, pers. obs.; South Africa: C. Elston, pers. obs.) and are known to take advantage of other anthropogenically provisioned food sources, such as baits used for white shark (Carcharodon carcharias) cage-diving operations (Rizzari et al. 2017; Meyer et al. 2020) and commercial fishery discards (Svane et al. 2008). In Hamelin Bay, Western Australia, smooth stingrays, along with black stingrays (Bathytoshia lata, formerly Dasyatis thetidis) and southern eagle rays (Myliobatis tenuicaudatus) are hand-fed bait and fish carcasses as part of an unmanaged tourist attraction (Newsome et al. 2004). This attraction developed from the rays becoming attracted to commercial anglers cleaning their catches at this location, followed by the installation of fish-cleaning facilities for recreational anglers who discard scraps into the waters (Newsome et al. 2004). Their predilection for utilising provisioned resources makes them a useful study species for determining potential impacts of fishing discards, particularly for large mesopredatory species that play central roles in coastal food webs.
In the Jervis Bay Marine Park, on the southern coast of New South Wales (NSW), Australia, smooth stingrays have scavenged discards from the cleaning of recreational fishing catches at the Woollamia Regional Boat Ramp in Currambene Creek since the 1980s. Previous research has indicated that the stingray’ use of the boat ramp is strongly linked to the timing and intensity of fish-cleaning activity and associated to discarding of fish waste from the shore-based fish-cleaning facilities (i.e. increased presence with increased fish cleaning, particularly in afternoons when fish cleaning is more common; Pini-Fitzsimmons et al. 2018). However, the previous study relied on visual observations and was limited in duration (22 days). How smooth stingrays use the wider creek system within which the fish-cleaning facilities are located in relation to the provisioning of recreational fishing discards remains unknown.
Here we built on our previous study by using passive acoustic telemetry to assess the visitation of smooth stingrays within Currambene Creek, specifically in relation to temporal patterns in discarding at the boat ramp and environmental conditions. If provisioning of fishing discards is the primary driver of smooth stingray use of the creek, we expected (1) smooth stingray visitation to be higher at the Woollamia Regional Boat Ramp than in other areas within the study site where provisioning is not occurring, and (2) for visitation at the boat ramp to match temporal patterns in food provisioning (i.e. higher visitation in afternoons and on weekends) with no such patterns being observed at other areas where provisioning is not occurring. In contrast, if smooth stingray use of the creek system was related to natural behaviours, during which they utilised provisioned resources if and when they were available, we expected visitation to be linked to the physical environment (i.e. water temperature, tides, rainfall, etc.), and for these variables to be of greater influence than patterns in provisioning activity.
Materials and methods
Study site and acoustic-array design
Currambene Creek in the Jervis Bay Marine Park is a mature barrier estuary (Fig. 1a) and the main tributary of Jervis Bay, NSW, Australia (Owers et al. 2016). It is dominated by soft sandy substrata and lined by small areas of Zostera sp. (NSW Department of Primary Industries 2013; Lucieer et al. 2017), and supports an extensive temperate saline wetland of mangroves and saltmarsh (Owers et al. 2016). The creek is ~15 km long, has a waterway area of 1.2 km2, and drains a catchment of 165 km2 (Ricardo et al. 2014). It is influenced by the semi-diurnal tidal range of ~2 m experienced in the neighbouring Jervis Bay (Owers et al. 2016).
The study area for this research was restricted to the lower reaches around the Woollamia Regional Boat Ramp, with the total study area spanning from 250 m inside the mouth to 2.75 km upstream (Fig. 1b). The Woollamia Regional Boat Ramp is a popular public boat ramp with fish-cleaning facilities that, at the time of the study, featured running water and a four-station metal fish-cleaning table with an open pipe in the centre that drained into the creek for disposing of fish discards from cleaning and filleting. The boat ramp and fish-cleaning facilities are used daily, and smooth stingrays have been observed foraging discards here since the 1980s and are seen in the vicinity daily (R. Simpson, Simo’s Afloat Fishing Charts, pers. obs.).
Four acoustic receivers (VR2W 69 kHz; Innovasea Systems, Nova Scotia, Canada) were deployed in the study area, namely, 0.5 km (‘Creek Mouth’), 1.2 km (‘Downstream’), 1.85 km, (‘Woollamia Boat Ramp’) and 2.65 km (‘Upstream’) upstream from the mouth of the creek (Fig. 1). The Woollamia Boat Ramp receiver detection range encompassed the provisioning area at the Woollamia Regional Boat Ramp. Receivers were affixed to moorings ~1–2 m from the benthos, with the hydrophone facing up. The detection range was estimated as 200–300 m and was determined through deployment of transmitters anchored at 50, 100, 200 and 300 m from the Woollamia Boat Ramp receiver. All detections were captured at 200 m and ~50% of detections were captured at 300 m. The widest point of the creek within the study area was ~220 m at the mean high-water mark and therefore the vast majority of detections from tagged animals were expected to be captured by the receivers in the array as they passed. Acoustic coverage of the study area (250 m from mouth to 2.75 km upstream) was estimated to be 55–87% (200–300-m detection range; Fig. 1b). No receiver was placed in the loop in the north-west of the study site, between the Woollamia Boat Ramp and the Upstream receiver (Fig. 1), because this area of the creek is too shallow to deploy a receiver and stingrays are rarely observed (local residents, pers. comm.).
Acoustic tagging
Smooth stingrays were tagged at the Woollamia Regional Boat Ramp. Stingrays were enticed onto the boat ramp by using a bait tube filled with locally sourced fish frames creating a chum trail. When a ray entered the tagging area, a 3- × 2-m sling made from shade cloth with wooden dowels as handles and weights at the base, was walked behind the ray and used to beach it on the boat ramp. The ray was orientated such that most of the body was out of the water, but the mouth, gills and spiracles remained fully submerged, allowing unrestricted respiration. Heavy wet towels were used to disable the tail and barb.
Smooth stingrays were then tagged externally with Vemco V9-2H 69 kHz coded acoustic transmitters (400-mm total length, 2.9 g in water) with a random repeat interval of 30–90 s and estimated battery life of 476 days (Innovasea Systems, Nova Scotia, Canada). Stingrays were tagged using a novel pelvic fin transmitter attachment method that was based on a similar method used by Hunter et al. (2005) on thornback rays (Raja clavata). However, we opted for pelvic fin over pectoral wing attachment because of wing undulation potentially causing necrosis around transmitter attachment wounds (Ward et al. 2019), and the potential for transmitter loss from males biting and scraping the wings of females during mating (Kajiura et al. 2000; Chapman et al. 2003; Le Port et al. 2008). The tagging procedure is described below.
The transmitters were affixed to plastic Petersen discs (250-mm diameter; Fig. 2) by using Shellys Aqua Fix waterproof epoxy adhesive, with a length of 200-lb (~90.7-kg) monofilament fishing line threaded through the disc. A sterile stainless-steel needle (2.5-mm diameter) was used to make a guide hole through the middle of one of the stingrays’ pelvic fins, through which a sterile stainless-steel hypodermic needle (3.5-mm internal diameter) was then passed. The monofilament from the Petersen disc was then threaded through the pelvic fin via the hypodermic needle and the needle was removed through the ventral side. Another Petersen disc was then threaded onto the monofilament on the ventral side of the pelvic fin and crimped in place with a 150-lb (~68-kg) stainless steel fishing crimp (Fig. 2).
|
|
During the tagging procedure, disc width and disc length were measured, sex was determined and any distinguishing features on the ray were noted and photographed for individual identification. Following tagging, the sling was lowered, allowing the ray to swim freely out of the tagging area. The tagging procedure took ~5 min per individual and most stingrays returned to the tagging area within an hour of tagging, indicating limited negative effects from the tagging procedure.
Tagging and tracking were approved by the Macquarie University Animal Ethics Committee under ARA 2014/015 and NSW DPI Scientific Collection Permit Number P08/0010.
Food-provisioning variables
Previous research at this site highlighted that fish-cleaning intensity at the Woollamia Regional Boat Ramp is higher in the afternoons (Pini-Fitzsimmons et al. 2018). This matches patterns in recreational fishing effort documented in the literature; specifically, effort is skewed to daylight hours, with peaks in fishing effort at approximately midday (Askey et al. 2018) and returns to fish-cleaning facilities in the afternoon (Pini-Fitzsimmons et al. 2018; Lynch et al. 2020). Similarly, recreational fishing effort is increased on non-business days (i.e. weekends and holidays; Parnell et al. 2010; van Poorten et al. 2015; Flynn et al. 2018; Kendall et al. 2021). Therefore, hour of the day and day of the week served as temporal variables for investigating the influence of provisioning activity. Receiver location allowed investigation of the spatial influence of provisioning activity (e.g. differences in visitation at the Woollamia Boat Ramp receiver vs other receivers).
Environmental variables
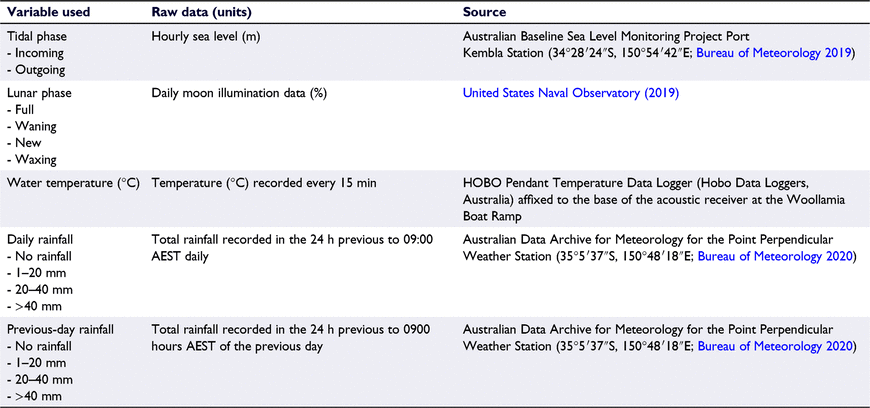
Environmental data were collected to determine the potential influence of the physical environment on smooth stingray use of lower Currambene Creek. Variables used were water temperature, daily rainfall, previous-day rainfall (i.e. to examine delayed effects of rain events), tidal phase, and lunar phase (Table 1). These variables were chosen because they are commonly associated with or examined when assessing drivers of elasmobranch movements in nearshore environments (e.g. Heupel et al. 2003; Smoothey et al. 2019; Niella et al. 2020; Spaet et al. 2020). These data, their treatments, and the sources are summarised in Table 1. It should be noted that water temperature was collected by a single data logger deployed at the provisioning site and used as a proxy for water temperature within the study area generally. Water temperature was included as a continuous variable. Tidal phase, lunar phase, daily rainfall and previous-day rainfall were included as categorical variables to simplify analyses and account for sparseness in some datasets (e.g. rainfall).
|
Data analysis
Pre-processing
Passive acoustic detection data gathered in the 12 h following tagging were excluded to account for potential changes in behaviour from the tagging procedure. Similarly, the first 3 h of water-temperature data were removed to ensure that the data logger was acclimated after deployment.
Periodicity of detections
To identify whether periodicity existed within the acoustic detection data, Rao’s spacing tests were used to determine whether the mean number of detections (for all individuals combined) varied across (1) hour of the day and (2) day of the week for each receiver. Polar plots were then used to visualise these differences. Rao’s spacing tests and polar plots were conducted with the circular package (ver. 0.4-95, C. Agostinelli and U. Lund, see https://CRAN.R-project.org/package=circular) in R (ver. 4.0.0, R Foundation for Statistical Computing, Vienna, Austria, see https://www.r-project.org/). If food provisioning was a major driver of visitation by smooth stingrays, we expected to see detections (1) peak in the afternoon at the Woollamia Boat Ramp receiver but not at other receivers and (2) peak on weekend days at the Woollamia Boat Ramp receiver but not at other receivers.
Visitation patterns
To capture how often tagged stingrays used areas of Currambene Creek, a detection index was estimated for (1) each stingray overall and (2) each stingray at each receiver, on the basis of the number of days detected divided by the total number of days an individual was tracked for the study (Udyawer et al. 2018). Values ranged from 0 (never detected) to 1 (detected every day). The total number of days tracked was calculated as the number of days from 12 h post-release until the last recorded detection. A Kruskal–Wallis test followed by a Dunn’s multiple comparisons post hoc test was used to determine whether detection indices were significantly different across receivers.
The proportion of acoustic detections recorded on each receiver by each individual was calculated to identify site preferences by tagged stingrays. If food provisioning from the Woollamia Regional Boat Ramp was driving the distribution of stingrays within the creek system, it was expected that the highest proportion of detections would be recorded on the receiver closest to the boat ramp.
To assess the time spent by stingrays within each section (defined by receiver location) of Currambene Creek, visitation events were extracted from the acoustic detection data. Visitation events were defined as the continuous periods of time a tagged stingray was within the range of a given receiver. They were initiated when a transmitter was detected by a receiver and terminated either when the transmitter was detected at a different receiver or if no further detections were made within 15 min. The 15-min timeout was selected because this is ample time for a smooth stingray to move through the detection range of a receiver (up to ~600 m) (Campbell et al. 2012). Visitation events less than 5 min in length were not considered to represent meaningful site occupancy and were therefore removed from further analyses.
A generalised additive mixed-effects model (GAMM) was used to assess how food provisioning at the Woollamia Regional Boat Ramp and aspects of the physical environment at the initiation of visitation events influenced the duration of visitation events. In other words, to test what conditions influenced the arrival of smooth stingrays and resulted in longer stays at each acoustic receiver. This model considered hour of day and day of week that visitation events were initiated on in interaction with receiver location, with cyclic cubic regression splines being applied to hour of the day and day of the week to account for their cyclical nature. Categorical (lunar phase, tidal phase, daily rainfall, previous-day rainfall) and continuous (water temperature) environmental variables occurring at the initiation of visitation events were also included additively. No regression spline was applied to water temperature because the relationship in the model was determined to be linear. Transmitter number was included as a random effect to account for the unequal number of visitation events recorded for each individual and lack of independence of individual ray behaviour. The duration of visitation-event data were transformed using Ordered Quantile normalisation (Peterson and Cavanaugh 2020) prior to modelling, and the model used a Gaussian error structure.
The GAMM was run using the mgcv package (ver. 1.8-41, S. Wood, see https://cran.r-project.org/package=mgcv/; Wood 2017) in R (R Foundation for Statistical Computing). Model selection was conducted using the double-penalty approach by setting ‘select = TRUE’ within the bam() function in mgcv. This method penalises variables of limited influence out of the global model, without the need to compare candidate models with all possible combinations of variables. An autocorrelation plot was used to assess whether there was serial correlation among residuals within the model. Temporal autocorrelation was indeed evident and so a first-order auto-regressive structure was included. The final model was validated by inspecting diagnostic plots (Q–Q plots, histograms of residuals, response vs fitted values and linear predictors vs residuals). Predictor variables were visually checked for outliers using Cleveland dot plots and collinearity was checked using variance inflation factors (VIFs) prior to modelling. No outliers or collinearity (VIF < 3; Zuur et al. 2010) were detected.
It should be noted that water-temperature data were available only for 129 days of the 210-day study period. For completeness during data exploration, a GAMM was run using the full visitation event dataset without water temperature as a variable (9001 visitation events) and compared with the GAMM with water temperature included as a variable (8160 visitation events); the former was found to perform substantially worse than the latter model. Therefore, the model with water temperature was retained and the results for this model are presented here.
Results
Acoustic monitoring
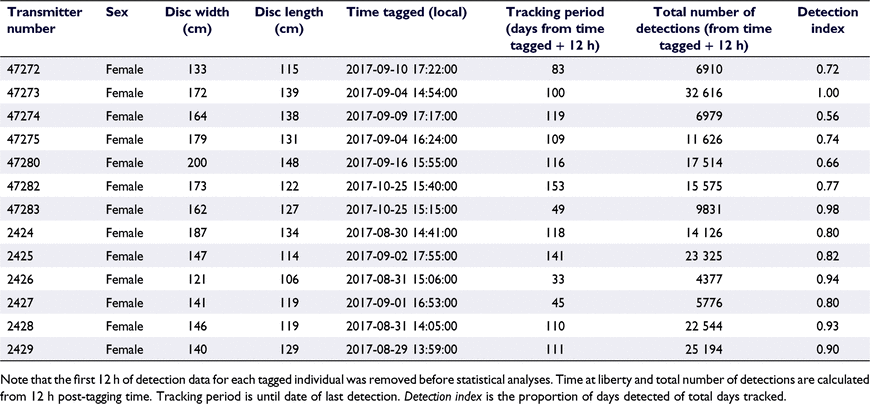
The study spanned August 2017 until April 2018. Acoustic transmitters were deployed on 13 smooth stingrays during August and September 2017 at the Woollamia Regional Boat Ramp (Table 2). Disc widths averaged 158.9 cm (±6.4 cm s.e.) and disc lengths averaged 126.2 cm (±3.3 cm s.e.; Table 2), and all tagged rays were adult females (disc width ≥100 cm; Le Port et al. 2012). All 13 tagged rays were detected within the array post-release. Following the removal of detections recorded within 12 h post-release for each individual, 196 393 detections remained (Fig. 3). Tracking periods for the rays ranged between 33 and 153 days (mean ± s.e. = 99 ± 10 days; Table 2). Owing to the external tagging methodology, tracking periods ended following premature loss of tags rather than by stingrays leaving the system. This was confirmed through resighting of all individuals at the boat ramp in the following months (August–September 2018). Therefore, tracking periods were considerably shorter than expected (tag battery life of ~476 days), but no stingrays appeared to leave the broader study area.
|
The highest proportion of detections (averaged across individuals) were recorded at the Woollamia Boat Ramp receiver (mean ± s.e. = 48 ± 4.569%), followed by the Downstream (mean ± s.e. = 31 ± 2.870%), Creek Mouth (mean ± s.e. = 11 ± 1.438%), and Upstream (mean ± s.e. = 11% ± 3.270%) receivers. Detection indices were high for all stingrays in Currambene Creek (mean ± s.e. = 0.817 ± 0.036; Table 2). By receiver, mean detection indices were high at the Woollamia Boat Ramp, Downstream and Creek Mouth receivers (mean ± s.e. = 0.763 ± 0.039, 0.794 ± 0.035 and 0.763 ± 0.033 respectively), and low at the Upstream receiver (mean ± s.e. = 0.381 ± 0.077). Detection indices varied by receiver (Kruskal–Wallis test: d.f. = 3, χ2 = 16.148, P = 0.001), driven by the significantly lower indices at the Upstream receiver (Dunn’s multiple-comparisons test: Upstream compared with all other receivers, P < 0.01; see Supplementary Table S1).
Periodicity of detections
Mean hourly detections were significantly non-homogeneous across hour of the day at all receivers (Rao’s spacing tests: Upstream: t = 323.578, P < 0.001; Woollamia Boat Ramp: t = 355.211, P < 0.001; Downstream: t = 350.512, P < 0.001; Creek Mouth: t = 333.119, P < 0.001). Diurnal patterns were present in the mean hourly detections at the Woollamia Boat Ramp and the Downstream receivers (Fig. 4b, c, d). At the Woollamia Boat Ramp, mean hourly detections increased from 0900 hours, peaked at 1200–1500 hours, followed by a substantial drop at 1800 hours (Fig. 4b). At the Downstream receiver, a matutinal pattern was seen, with higher detections between 0600 and 0800 hours (Fig. 4c). Mean hourly detections were too low at the Creek Mouth and Upstream receivers (<10 detections at peaks) to discern any clear patterns (Fig. 4a, d).
Detections by day of the week were significantly non-homogeneous at all receivers (Rao’s spacing tests: Upstream: t = 359.823, P < 0.001; Woollamia Boat Ramp: t = 359.977, P < 0.001; Downstream: t = 359.954, P < 0.001; Creek Mouth: t = 359.869, P < 0.001). At the Woollamia Boat Ramp, mean daily detections peaked on Sundays, with generally higher mean daily detections seen Friday to Monday (Fig. 5b). A similar trend was seen at the Creek Mouth receiver, although mean daily detections were substantially lower than at the boat ramp (Fig. 5d). Mean daily detections peaked on Sunday and Monday at the Downstream receiver (Fig. 5c) and the Upstream receiver (Fig. 5a), although mean daily detections were substantially lower at the Upstream receiver.
|
|
Visitation patterns
In total, 9001 visitation events were recorded throughout the study period, lasting between 5 min and 13 h 47 min (mean ± s.e. = 36 min ± 35 s). Visitation events were, on average, longer at the Woollamia Boat Ramp (mean ± s.e. = 53 ± 1.3 min) than at the Downstream (mean ± s.e. = 28 min ± 36 s), Upstream (mean ± s.e. = 24 ± 1.6 min), and Creek Mouth (mean ± s.e. = 23 min ± 41 s) receivers.
The GAMM considered 8160 of the total 9001 visitation events and explained 11.3% of the deviance observed (summarised in Table S2). Overall, visitation events were significantly longer at the Woollamia Boat Ramp than at the Upstream (β = −0.545, t = −13.217, P < 0.001), Downstream (β = −0.299, t = −11.586, P < 0.001), and Creek Mouth (β = −0.524, t = −16.746, P < 0.001) receivers (Fig. 6i), indicating that stingrays spent more time in the vicinity of the Woollamia Regional Boat Ramp than in other parts of the study area. There was a significant effect of day of the week on the duration of visitation events at the Woollamia Boat Ramp (d.f.e = 1.660, F = 0.998, P = 0.039), with visitation events being longest when initiated on Saturday and Sunday and shortest on Wednesday–Thursday (Fig. 6b). No patterns were observed at the other three receivers (Fig. 6a, c, d). There was a significant effect of hour of the day at all receivers except the Creek Mouth (Upstream: d.f.e = 1.932, F = 0.335, P = 0.010; Woollamia Boat Ramp: d.f.e = 5.218, F = 3.997, P < 0.001; Downstream: d.f.e = 3.460, F = 2.602, P < 0.001; Fig. 6e–h). At the Woollamia Boat Ramp, visitation events were longest when initiated between 1000 and 1200 hours (Fig. 6f). That is, stingrays that arrived at the boat ramp between 1000 and 1200 hours stayed the longest, with these events spanning the afternoon. This pattern is also supported by peaking detections during this period shown above (Fig. 4b). A somewhat opposing relationship was observed at the Downstream (i.e. shortest when initiated 1000 to 1700 hours; Fig. 6g) and Upstream (i.e. shortest when initiated 0800 to 1100 hours; Fig. 6e) receivers. Visitation events were also significantly shorter when initiated during an outgoing tide compared with an incoming tide, but the effect was small (β = −0.069, t = −3.101, P = 0.002; Fig. 6j). Similarly, visitation events were significantly shorter with increasing water temperatures, but the effect was also small (β = −0.024, t = −4.048, P < 0.001; Fig. 6k). There was a significant effect of transmitter number as the random effect (d.f.e = 11.382, F = 21.696, P < 0.001), suggesting that individual rays varied in their behaviour. There was no effect of lunar phase, daily rainfall or previous-day rainfall. Therefore, the physical environment had little (tidal phase and water temperature) to no (lunar phase, daily rainfall, and previous-day rainfall) effect on the duration of stingray visits.
Discussion
Previous research has indicated that smooth stingray visitation to the Woollamia Regional Boat Ramp was linked to the intensity of recreational fish cleaning and associated provisioning of discards, with increased presence in the afternoons when fish-cleaning activity peaked (Pini-Fitzsimmons et al. 2018). This study has built on this work to show that smooth stingray use of the broader lower Currambene Creek area is strongly linked to provisioning activity. Specifically, we found that (1) smooth stingrays use Currambene creek frequently (~79% of days tracked) and visited the provisioning site (Woollamia Regional Boat Ramp) with the same frequency (~74% of days tracked), (2) smooth stingray visitation was highest at the provisioning site relative to all other sites, and (3) patterns in their visitation to this site coincided with provisioning activity (i.e. higher mean acoustic detections and longer visitation events during afternoons and weekends). Importantly, these patterns were not observed at the other areas within the creek. In addition, (4) environmental variables had little (tidal phase and water temperature) to no (lunar phase, daily rainfall, and previous-day rainfall) effect on the length of time stingrays spent in areas of the creek, as might have been expected if their use of the creek was predominantly related to natural behaviours based on environmental cues. These spatial and temporal patterns are consistent with our hypothesis that the use of Currambene Creek by smooth stingrays is linked to the discarding of fish-cleaning waste at the Woollamia Regional Boat Ramp.
Daily patterns
Smooth stingrays tracked in this study appeared to show diel movements whereby they enter Currambene Creek in the morning, travel upstream to the Woollamia Regional Boat Ramp in the afternoon where they remain for extended periods and then leave again over night. Natural diel movement patterns for smooth stingrays are currently unknown, but diel patterns in the use of inshore habitats for other batoid species typically involve use of warmer shallow waters during the night and refuging in cooler, deeper waters while they digest during the day (i.e. diel vertical migration; Wearmouth and Sims 2009; Farrugia et al. 2011; Humphries et al. 2017; DeGroot et al. 2020). Indeed, for several dasyatid ray species, presence in shallow habitats is generally higher at night and nocturnal space use is larger as individuals actively forage for food (Cartamil et al. 2003; Farrugia et al. 2011; Corcoran et al. 2013). Smooth stingrays might, therefore, be expected to show similar natural diel patterns if their use of Currambene Creek was related to natural movements. Instead, here we found that their use of the shallow creek area was higher during the day than at night, particularly in the afternoon and was overwhelmingly focussed on the provisioning site. On the basis of comparatively low night-time detections, it is likely that smooth stingrays leave Currambene Creek overnight. However, diel movements related to thermoregulation can also result in higher use of shallow waters during the day (Schlaff et al. 2014). For example, bat rays (Myliobatis californicus) travel from cooler deeper waters to forage intertidal sandflats in the middle of the day, using the warmer waters as a means of behavioural thermoregulation (Matern et al. 2000). Although smooth stingrays in this study exhibited longer visitation events from the middle of the day and peaks in detections in the afternoon, visitation events were actually shorter with increased water temperatures, but the effect was negligible compared with temporal and spatial variables related to food-provisioning activity. In addition, if smooth stingrays were using behavioural thermoregulation, the area around the downstream receiver would be preferable to the boat ramp area, at least during high tide, because of the presence of expansive shallow sandflats that are submerged with the high tide; however, this was not the case. It is unlikely, therefore, that the diel patterns in space use observed are related to behavioural thermoregulation. However, it is important to re-iterate that diurnal behaviours of smooth stingrays are unknown, and other elasmobranchs show mixed diurnal behaviours (Hammerschlag et al. 2017). In addition, thermal and salinity preferences for smooth stingrays are unknown, preventing detailed discussion of such influences on space use. Therefore, further work is needed to understand baseline behaviour and preferences, to identify potential changes caused by provisioning activity.
Food provisioning has been shown to cause the reversal of diel behaviour in southern stingrays (Hypanus americanus, formerly Dasyatis americana) fed as part of ecotourism at Stingray City in the Bahamas (Corcoran et al. 2013). The diel behaviour of non-provisioned stingrays involved resting in cooler deeper waters at night and larger activity spaces over shallow habitats during the day (Corcoran et al. 2013). In comparison, provisioned stingrays were constantly active during the day, particularly during provisioning activity, with high attachment to the provisioning site, and would disperse at night (Corcoran et al. 2013). With the normal diel movements of smooth stingrays being currently unknown, we cannot comment on whether such a behaviour switch has occurred in the studies context and further research is needed.
Weekly patterns
Few, if any, environmental phenomena or combinations of environmental variables function on 7-day cycles. For instance, tides vary in accordance with lunar cycles over short (~11 h) and long (30 day) cycles, and water temperature varies across days and by season. As such, the most likely cue to explain increased detections and longer visitation events on weekends at the Woollamia Regional Boat Ramp is human behaviour, specifically that related to increased recreational fishing effort on weekends (Parnell et al. 2010; van Poorten et al. 2015; Flynn et al. 2018; Kendall et al. 2021). These observed patterns are indicative of a ‘weekend effect’, whereby animals demonstrate differing behavioural patterns on weekends from those on weekdays in areas of increased human recreational activity (Nix et al. 2018). However, this phenomenon typically involves animals retreating from areas with increased human use on weekends (e.g. Stalmaster and Kaiser 1998; Lafferty 2001; Longshore et al. 2013; Nix et al. 2018), rather than increased attraction to such sites as demonstrated here.
However, human behaviour and environmental conditions are not always mutually exclusive. Environmental conditions may determine when and where people go fishing, both in regard to the spatio-temporal distribution of target species (e.g. fish migrations, prey patches) and the level of enjoyment experienced because of weather conditions (e.g. lower angler number during adverse weather; Cabanellas-Reboredo et al. 2014; Lynch et al. 2020; Kendall et al. 2021). Nevertheless, temporal factors related to provisioning (hour of the day and receiver) had a greater effect on smooth stingray visitation than had environmental variables in the present study. However, it is important to note that the modelling used in this study does not consider the periods between visitation events (i.e. stingray absences), and certain environmental variables may be more closely linked to stingray disuse of an area.
Implications of utilising provisioned recreational fishing discards
Even though recreational fishery discards are likely to drive the use of Currambene Creek by smooth stingrays, the broader implications of this shift in behaviour are currently unknown, although, some insights can be gained from wildlife tourism. Feeding wildlife for tourism is based on animals learning associations with provisioned food that is provided predictably in time and space, and there is a growing body of evidence that feeding wildlife in these contexts can lead to long-term changes in abundance, community structure, behaviour, and movement patterns, in both terrestrial and marine species (reviewed in Orams (2002), Newsome and Rodger (2008), Brena et al. (2015), Trave et al. (2017), Patroni et al. (2018)). It is argued that the extent to which food provisioning affects animals is based on the amount and frequency of provisioning (Abrantes et al. 2018; Heinrich et al. 2020). For example, southern stingrays provisioned daily by 1 million tourists annually at Stingray City in the Bahamas (Vaudo et al. 2018) suffer from long-term behavioural impacts (Corcoran et al. 2013), whereas Caribbean reef sharks (Carcharhinus perezi) that have been provisioned daily for over 20 years elsewhere in the Bahamas but are fed a restricted amount of food showed no significant changes in movement patterns (Maljković and Côté 2011). The Woollamia Regional Boat Ramp is one of the most popular ramps in the region and used daily (R. Simpson, pers. comm.). Smooth stingrays are long-lived and have been observed foraging discarded recreational fish-cleaning waste at the boat ramp for over 35 years, during which weekly and daily trends have been reinforced, and may therefore be at high risk of longer-term impacts.
Notably, all smooth stingrays tagged in this study were adult females, and a number were observed in breeding condition (J. Pini-Fitzsimmons, pers. obs.). This female bias has been consistent at the site for a number of years (Pini-Fitzsimmons et al. 2018, 2021), and has been noted at other sites where this species is provisioned (e.g. cage-diving in the Neptune Islands, South Australia, A. Fox, pers. comm.; Hamelin Bay, Western Australia, Newsome et al. 2004). Further, female-bias in provisioned elasmobranch populations is common (e.g. Brunnschweiler and Baensch 2011; Clarke et al. 2011; Maljković and Côté 2011; Clarke et al. 2013; Corcoran et al. 2013; Brunnschweiler et al. 2014; Rizzari et al. 2017; Vaudo et al. 2018). Yet, there has been limited research into the potential implications of this, and the reasons are not yet clear. Adult females in breeding condition may seek to supplement their diets with provisioned foods to assist in meeting the increased energetic demands of reproduction (Wearmouth and Sims 2008). Alternatively, provisioning may simply occur in locations that are already used by these individuals for some purpose (e.g. gestation sites), and the animals make use of the additional resources as they come available (Clarke et al. 2011; Hammerschlag et al. 2012; Sulikowski et al. 2016). Female smooth stingrays are also larger than males and may competitively exclude them from these provisioning sites, as has been suggested for provisioned southern stingrays (H. americanus) at Stingray City in the Caymans Islands (Semeniuk and Rothley 2008; Corcoran et al. 2013; Vaudo et al. 2018). This could induce higher stress or affect energy reserves for females who are exerting extra energy to defend provisioned resources (Pini-Fitzsimmons et al. 2021). Nonetheless, any activity that affects only a subset of a population, such as one sex or reproductive stage, has the potential to disrupt population dynamics (Semeniuk et al. 2009; Clarke et al. 2011). Future research should focus on quantifying the use of provisioning sites by female elasmobranchs, particularly those in breeding condition, compared with non-provisioned populations, to determine how provisioned foods are integrated into and affect their reproductive success and energetic demands (Hammerschlag et al. 2012; Mourier et al. 2021), with foresight to evaluating impacts to population dynamics more broadly. Specifically for smooth stingrays, there is a need for improved understanding of reproductive behaviour, which remains a significant knowledge gap (Rigby et al. 2021).
Given that stingrays in this study were tagged at the provisioning site and no non-provisioned stingrays were tracked for comparison, it is possible that we have sampled only individuals that have a propensity for (1) utilising provisioned resources, (2) using the area around the boat ramp more generally, or (3) being more active in the afternoons. Indeed, elasmobranchs have been shown to form specific individual preferences with provisioning sites. For example, Martin et al. (2019) found a surprising level of individual variation in terms of association with piers where fishing waste is discarded by blacktip sharks (Carcharhinus limbatus). Without tagging individuals from non-provisioning sites in the present study, it is difficult to account for such individualistic differences in behaviour or whether the creek is used by other individuals that do not use the provisioning site. However, our long-term monitoring of the smooth stingray population in this region indicates that all individuals sighted in the creek regularly visit the Woollamia Regional Boat Ramp provisioning site and preliminary tracking data in a concurrent study indicate that there is little overlap in the use of Currambene Creek by smooth stingrays tagged at other provisioning and non-provisioning sites nearby (Pini-Fitzsimmons, unpubl. data). Future research should look to expand this work over a broader spatio-temporal context and include the tracking on non-provisioned smooth stingrays to clarify whether individual preferences play a role in accessing provisioned resources.
Although the present study was limited in temporal and spatial scope, we have shown that the tagged smooth stingrays develop strong associations with the location and timing of the provisioning of recreational fishing discards, and therefore it is reasonable to suggest that these stingrays may be experiencing negative impacts (e.g. changes in behaviour and population dynamics, as detailed above). However, there is evidence that although food provisioning may cause short-term behavioural changes for some species, it may not drive their long-term movements (Laroche et al. 2007; Brunnschweiler and Barnett 2013; Huveneers et al. 2013; see also Trave et al. 2017). Given that smooth stingrays are common scavengers of recreational fishery discards throughout their range (J. Pini-Fitzsimmons, pers. obs.; H. Cadwallader, pers. obs.; C. Elston, pers. obs.), it is important that there is continued research into the role that recreational fishery discards play in the behavioural ecology of smooth stingrays and other animals, to facilitate the effective implementation of management plans.
Supplementary material
Supplementary material is available online.
Data availability
All acoustic telemetry data collected during this study are available through the Integrated Marine Observing System (IMOS) Animal Tracking Facility database (https://animaltracking.aodn.org.au/).
Conflicts of interest
The authors declare that they have no conflicts of interest.
Declaration of funding
Funding for this project was provided by the Department of Biological Sciences, Macquarie University. J. Pini-Fitzsimmons was also supported by an Australian Government Research Training Pathway Scholarship.
Acknowledgements
We are grateful to the staff at NSW Department of Primary Industries, Fisheries, Huskisson Office for their logistical support in maintaining the acoustic arrays used for this study, along with the numerous volunteers who helped in the field.
References
Abrantes, KG, Brunnschweiler, JM, and Barnett, A (2018). You are what you eat: examining the effects of provisioning tourism on shark diets. Biological Conservation 224, 300–308.| You are what you eat: examining the effects of provisioning tourism on shark diets.Crossref | GoogleScholarGoogle Scholar |
Arlinghaus, R, Aas, Ø, Alós, J, Arismendi, I, Bower, S, Carle, S, Czarkowski, T, Freire, KMF, Hu, J, Hunt, LM, Lyach, R, Kapusta, A, Salmi, P, Schwab, A, Tsuboi, J-I, Trella, M, Mcphee, D, Potts, W, Wołos, A, and Yang, Z-J (2021). Global participation in and public attitudes toward recreational fishing: international perspectives and developments. Reviews in Fisheries Science & Aquaculture 29, 58–95.
| Global participation in and public attitudes toward recreational fishing: international perspectives and developments.Crossref | GoogleScholarGoogle Scholar |
Askey, PJ, Ward, H, Godin, T, Boucher, M, and Northrup, S (2018). Angler effort estimates from instantaneous aerial counts: use of high-frequency time-lapse camera data to inform model-based estimators. North American Journal of Fisheries Management 38, 194–209.
| Angler effort estimates from instantaneous aerial counts: use of high-frequency time-lapse camera data to inform model-based estimators.Crossref | GoogleScholarGoogle Scholar |
Bartumeus, F, Giuggioli, L, Louzao, M, Bretagnolle, V, Oro, D, and Levin, SA (2010). Fishery discards impact on seabird movement patterns at regional scales. Current Biology 20, 215–222.
| Fishery discards impact on seabird movement patterns at regional scales.Crossref | GoogleScholarGoogle Scholar |
Behrendorff, L, Leung, LK-P, McKinnon, A, Hanger, J, Belonje, G, Tapply, J, Jones, D, and Allen, BL (2016). Insects for breakfast and whales for dinner: the diet and body condition of dingoes on Fraser Island (K’gari). Scientific Reports 6, 23469.
| Insects for breakfast and whales for dinner: the diet and body condition of dingoes on Fraser Island (K’gari).Crossref | GoogleScholarGoogle Scholar |
Brena, PF, Mourier, J, Planes, S, and Clua, E (2015). Shark and ray provisioning: functional insights into behavioral, ecological and physiological responses across multiple scales. Marine Ecology Progress Series 538, 273–283.
| Shark and ray provisioning: functional insights into behavioral, ecological and physiological responses across multiple scales.Crossref | GoogleScholarGoogle Scholar |
Brunnschweiler, JM, and Baensch, H (2011). Seasonal and long-term changes in relative abundance of bull sharks from a tourist shark feeding site in Fiji. PLoS ONE 6, e16597.
| Seasonal and long-term changes in relative abundance of bull sharks from a tourist shark feeding site in Fiji.Crossref | GoogleScholarGoogle Scholar |
Brunnschweiler, JM, and Barnett, A (2013). Opportunistic visitors: long-term behavioural response of bull sharks to food provisioning in Fiji. PLoS ONE 8, e58522.
| Opportunistic visitors: long-term behavioural response of bull sharks to food provisioning in Fiji.Crossref | GoogleScholarGoogle Scholar |
Brunnschweiler, JM, Abrantes, KG, and Barnett, A (2014). Long-term changes in species composition and relative abundances of sharks at a provisioning site. PLoS ONE 9, e86682.
| Long-term changes in species composition and relative abundances of sharks at a provisioning site.Crossref | GoogleScholarGoogle Scholar |
Bureau of Meteorology (2019) Hourly sea level and meteorological data. In ‘Australian baseline sea level monitoring project’. (BOM, Australian Government) Available at http://www.bom.gov.au/oceanography/projects/abslmp/data/index.shtml
Bureau of Meteorology (2020) Climate data online. In ‘Australian data archive for meteorology’. (BOM, Australian Government) Available at http://www.bom.gov.au/climate/data/index.shtml
Cabanellas-Reboredo, M, Alós, J, March, D, Palmer, M, Jordà, G, and Palmer, M (2014). Where and when will they go fishing? Understanding fishing site and time choice in a recreational squid fishery. ICES Journal of Marine Science 71, 1760–1773.
| Where and when will they go fishing? Understanding fishing site and time choice in a recreational squid fishery.Crossref | GoogleScholarGoogle Scholar |
Campbell, HA, Watts, ME, Dwyer, RG, and Franklin, CE (2012). V-Track: software for analysing and visualising animal movement from acoustic telemetry detections. Marine and Freshwater Research 63, 815–820.
| V-Track: software for analysing and visualising animal movement from acoustic telemetry detections.Crossref | GoogleScholarGoogle Scholar |
Cartamil, DP, Vaudo, JJ, Lowe, CG, Wetherbee, BM, and Holland, KN (2003). Diel movement patterns of the Hawaiian stingray, Dasyatis lata: implications for ecological interactions between sympatric elasmobranch species. Marine Biology 142, 841–847.
| Diel movement patterns of the Hawaiian stingray, Dasyatis lata: implications for ecological interactions between sympatric elasmobranch species.Crossref | GoogleScholarGoogle Scholar |
Chapman, DD, Corcoran, MJ, Harvey, GM, Malan, S, and Shivji, MS (2003). Mating behavior of southern stingrays, Dasyatis americana (Dasyatidae). Environmental Biology of Fishes 68, 241–245.
| Mating behavior of southern stingrays, Dasyatis americana (Dasyatidae).Crossref | GoogleScholarGoogle Scholar |
Christiansen, F, Mchugh, KA, Bejder, L, Siegal, EM, Lusseau, D, Mccabe, EB, Lovewell, G, and Wells, RS (2016). Food provisioning increases the risk of injury in a long-lived marine top predator. Royal Society Open Science 3, 160560.
| Food provisioning increases the risk of injury in a long-lived marine top predator.Crossref | GoogleScholarGoogle Scholar |
Cisneros-Montemayor, AM, and Sumaila, UR (2010). A global estimate of benefits from ecosystem-based marine recreation: potential impacts and implications for management. Journal of Bioeconomics 12, 245–268.
| A global estimate of benefits from ecosystem-based marine recreation: potential impacts and implications for management.Crossref | GoogleScholarGoogle Scholar |
Clarke, C, Lea, JSE, and Ormond, RFG (2011). Reef-use and residency patterns of a baited population of silky sharks, Carcharhinus falciformis, in the Red Sea. Marine and Freshwater Research 62, 668–675.
| Reef-use and residency patterns of a baited population of silky sharks, Carcharhinus falciformis, in the Red Sea.Crossref | GoogleScholarGoogle Scholar |
Clarke, CR, Lea, JSE, and Ormond, RFG (2013). Changing relative abundance and behaviour of silky and grey reef sharks baited over 12 years on a Red Sea reef. Marine and Freshwater Research 64, 909–919.
| Changing relative abundance and behaviour of silky and grey reef sharks baited over 12 years on a Red Sea reef.Crossref | GoogleScholarGoogle Scholar |
Corcoran, MJ, Wetherbee, BM, Shivji, MS, Potenski, MD, Chapman, DD, and Harvey, GM (2013). Supplemental feeding for ecotourism reverses diel activity and alters movement patterns and spatial distribution of the southern stingray, Dasyatis americana. PLoS ONE 8, e59235.
| Supplemental feeding for ecotourism reverses diel activity and alters movement patterns and spatial distribution of the southern stingray, Dasyatis americana.Crossref | GoogleScholarGoogle Scholar |
Dayton, PK, Thrush, SF, Agardy, MT, and Hofman, RJ (1995). Environmental effects of marine fishing. Aquatic Conservation: Marine and Freshwater Ecosystems 5, 205–232.
| Environmental effects of marine fishing.Crossref | GoogleScholarGoogle Scholar |
Déaux, EC, Crowe, T, and Charrier, I (2018). Recreational fishing alters dingo foraging behavior on Fraser Island. The Journal of Wildlife Management 82, 85–92.
| Recreational fishing alters dingo foraging behavior on Fraser Island.Crossref | GoogleScholarGoogle Scholar |
DeGroot, BC, Roskar, G, Brewster, L, and Ajemian, MJ (2020). Fine-scale movement and habitat use of whitespotted eagle rays Aetobatus narinari in the Indian River Lagoon, Florida, USA. Endangered Species Research 42, 109–124.
| Fine-scale movement and habitat use of whitespotted eagle rays Aetobatus narinari in the Indian River Lagoon, Florida, USA.Crossref | GoogleScholarGoogle Scholar |
Donaldson, R, Finn, H, and Calver, M (2010). Illegal feeding increases risk of boat-strike and entanglement in Bottlenose Dolphins in Perth, Western Australia. Pacific Conservation Biology 16, 157–161.
| Illegal feeding increases risk of boat-strike and entanglement in Bottlenose Dolphins in Perth, Western Australia.Crossref | GoogleScholarGoogle Scholar |
Farrugia, TJ, Espinoza, M, and Lowe, CG (2011). Abundance, habitat use and movement patterns of the shovelnose guitarfish (Rhinobatos productus) in a restored southern California estuary. 62, 648–657.
| Abundance, habitat use and movement patterns of the shovelnose guitarfish (Rhinobatos productus) in a restored southern California estuary.Crossref | GoogleScholarGoogle Scholar |
Flynn, DJH, Lynch, TP, Barrett, NS, Wong, LSC, Devine, C, and Hughes, D (2018). Gigapixel big data movies provide cost-effective seascape scale direct measurements of open-access coastal human use such as recreational fisheries. Ecology and Evolution 8, 9372–9383.
| Gigapixel big data movies provide cost-effective seascape scale direct measurements of open-access coastal human use such as recreational fisheries.Crossref | GoogleScholarGoogle Scholar |
Freire, KMF, Belhabib, D, Espedido, JC, Hood, L, Kleisner, KM, Lam, VWL, Machado, ML, Mendonça, JT, Meeuwig, JJ, Moro, PS, Motta, FS, Palomares, M-LD, Smith, N, Teh, L, Zeller, D, Zylich, K, and Pauly, D (2020). Estimating global catches of marine recreational fisheries. Frontiers in Marine Science 7, 12.
| Estimating global catches of marine recreational fisheries.Crossref | GoogleScholarGoogle Scholar |
García-Tarrasón, M, Bécares, J, Bateman, S, Arcos, JM, Jover, L, and Sanpera, C (2015). Sex-specific foraging behavior in response to fishing activities in a threatened seabird. Ecology and Evolution 5, 2348–2358.
| Sex-specific foraging behavior in response to fishing activities in a threatened seabird.Crossref | GoogleScholarGoogle Scholar |
Hammerschlag, N, Gallagher, AJ, Wester, J, Luo, J, and Ault, JS (2012). Don’t bite the hand that feeds: assessing ecological impacts of provisioning ecotourism on an apex marine predator. Functional Ecology 26, 567–576.
| Don’t bite the hand that feeds: assessing ecological impacts of provisioning ecotourism on an apex marine predator.Crossref | GoogleScholarGoogle Scholar |
Hammerschlag, N, Skubel, RA, Calich, H, Nelson, ER, Shiffman, DS, Wester, J, Macdonald, CC, Cain, S, Jennings, L, Enchelmaier, A, and Gallagher, AJ (2017). Nocturnal and crepuscular behavior in elasmobranchs: a review of movement, habitat use, foraging, and reproduction in the dark. Bulletin of Marine Science 93, 355–374.
| Nocturnal and crepuscular behavior in elasmobranchs: a review of movement, habitat use, foraging, and reproduction in the dark.Crossref | GoogleScholarGoogle Scholar |
Heinrich, DDU, Vila Pouca, C, Brown, C, and Huveneers, C (2020). Effects of reward magnitude and training frequency on the learning rates and memory retention of the Port Jackson shark Heterodontus portusjacksoni. Animal Cognition 23, 939–949.
| Effects of reward magnitude and training frequency on the learning rates and memory retention of the Port Jackson shark Heterodontus portusjacksoni.Crossref | GoogleScholarGoogle Scholar |
Heupel, MR, Simpfendorfer, CA, and Hueter, RE (2003). Running before the storm: blacktip sharks respond to falling barometric pressure associated with Tropical Storm Gabrielle. Journal of Fish Biology 63, 1357–1363.
| Running before the storm: blacktip sharks respond to falling barometric pressure associated with Tropical Storm Gabrielle.Crossref | GoogleScholarGoogle Scholar |
Huddart D (2019) Recreational fishing. In ‘Outdoor recreation’. (Eds D Huddart, T Stott) pp. 395–428. (Springer International Publishing)
Humphries, NE, Simpson, SJ, and Sims, DW (2017). Diel vertical migration and central place foraging in benthic predators. Marine Ecology Progress Series 582, 163–180.
| Diel vertical migration and central place foraging in benthic predators.Crossref | GoogleScholarGoogle Scholar |
Hunter, E, Buckley, AA, Stewart, C, and Metcalfe, JD (2005). Migratory behaviour of the thornback ray, Raja clavata, in the southern North Sea. Journal of the Marine Biological Association of the United Kingdom 85, 1095–1105.
| Migratory behaviour of the thornback ray, Raja clavata, in the southern North Sea.Crossref | GoogleScholarGoogle Scholar |
Huveneers, C, Rogers, PJ, Beckmann, C, Semmens, JM, Bruce, BD, and Seuront, L (2013). The effects of cage-diving activities on the fine-scale swimming behaviour and space use of white sharks. Marine Biology 160, 2863–2875.
| The effects of cage-diving activities on the fine-scale swimming behaviour and space use of white sharks.Crossref | GoogleScholarGoogle Scholar |
Kajiura, SM, Sebastian, AP, and Tricas, TC (2000). Dermal bite wounds as indicators of reproductive seasonality and behaviour in the Atlantic stingray, Dasyatis sabina. Environmental Biology of Fishes 58, 23–31.
| Dermal bite wounds as indicators of reproductive seasonality and behaviour in the Atlantic stingray, Dasyatis sabina.Crossref | GoogleScholarGoogle Scholar |
Kelleher K (2005) Discards in the world’s marine fisheries: an update. FAO Fisheries Technical Paper 470, Food and Agriculture Organization of the United Nations, Rome, Italy.
Kendall, MS, Williams, BL, Winship, AJ, Carson, M, Grissom, K, Rowell, TJ, Stanley, J, and Roberson, KW (2021). Winds, waves, warm waters, weekdays, and which ways boats are counted influence predicted visitor use at an offshore fishing destination. Fisheries Research 237, 105879.
| Winds, waves, warm waters, weekdays, and which ways boats are counted influence predicted visitor use at an offshore fishing destination.Crossref | GoogleScholarGoogle Scholar |
Lafferty, KD (2001). Birds at a Southern California beach: seasonality, habitat use and disturbance by human activity. Biodiversity and Conservation 10, 1949–1962.
| Birds at a Southern California beach: seasonality, habitat use and disturbance by human activity.Crossref | GoogleScholarGoogle Scholar |
Laroche, RK, Kock, AA, Dill, LM, and Oosthuizen, WH (2007). Effects of provisioning ecotourism activity on the behaviour of white sharks Carcharodon carcharias. Marine Ecology Progress Series 338, 199–209.
| Effects of provisioning ecotourism activity on the behaviour of white sharks Carcharodon carcharias.Crossref | GoogleScholarGoogle Scholar |
Le Port, A, Sippel, T, and Montgomery, JC (2008). Observations of mesoscale movements in the short-tailed stingray, Dasyatis brevicaudata from new zealand using a novel PSAT tag attachment method. Journal of Experimental Marine Biology and Ecology 359, 110–117.
| Observations of mesoscale movements in the short-tailed stingray, Dasyatis brevicaudata from new zealand using a novel PSAT tag attachment method.Crossref | GoogleScholarGoogle Scholar |
Le Port, A, Lavery, S, and Montgomery, JC (2012). Conservation of coastal stingrays: seasonal abundance and population structure of the short-tailed stingray Dasyatis brevicaudata at a Marine Protected Area. ICES Journal of Marine Science 69, 1427–1435.
| Conservation of coastal stingrays: seasonal abundance and population structure of the short-tailed stingray Dasyatis brevicaudata at a Marine Protected Area.Crossref | GoogleScholarGoogle Scholar |
Lewin, W-C, Weltersbach, MS, Ferter, K, Hyder, K, Mugerza, E, Prellezo, R, Radford, Z, Zarauz, L, and Strehlow, HV (2019). Potential environmental impacts of recreational fishing on marine fish stocks and ecosystems. Reviews in Fisheries Science & Aquaculture 27, 287–330.
| Potential environmental impacts of recreational fishing on marine fish stocks and ecosystems.Crossref | GoogleScholarGoogle Scholar |
Longshore, K, Lowrey, C, and Thompson, DB (2013). Detecting short-term responses to weekend recreation activity: desert bighorn sheep avoidance of hiking trails. Wildlife Society Bulletin 37, 698–706.
| Detecting short-term responses to weekend recreation activity: desert bighorn sheep avoidance of hiking trails.Crossref | GoogleScholarGoogle Scholar |
Lucieer V, Walsh P, Flukes E, Butler C, Proctor R, Johnson C (2017) ‘Seamap Australia – a national seafloor habitat classification scheme.’ (Institute for Marine and Antarctic Studies (IMAS), University of Tasmania (UTAS)) Available at https://metadata.imas.utas.edu.au/geonetwork/srv/eng/catalog.search#/metadata/4739e4b0-4dba-4ec5-b658-02c09f27ab9a
Lynch, TP, Foster, S, Devine, C, Hegarty, A, Mcennulty, F, Burton, M, and Lyle, JM (2020). Trail camera video systems: investigating their utility in interpreting patterns of marine, recreational, trailer-boat fishers’ access to an offshore Marine Park in differing weather conditions. ICES Journal of Marine Science 77, 3110–3126.
| Trail camera video systems: investigating their utility in interpreting patterns of marine, recreational, trailer-boat fishers’ access to an offshore Marine Park in differing weather conditions.Crossref | GoogleScholarGoogle Scholar |
Maljković, A, and Côté, IM (2011). Effects of tourism-related provisioning on the trophic signatures and movement patterns of an apex predator, the Caribbean reef shark. Biological Conservation 144, 859–865.
| Effects of tourism-related provisioning on the trophic signatures and movement patterns of an apex predator, the Caribbean reef shark.Crossref | GoogleScholarGoogle Scholar |
Margalef R (1997) ‘Our biosphere.’ (Ecology Institute: Oldendorf, Germany)
Martin, KL, Abel, DC, Crane, DP, Hammerschlag, N, and Burge, EJ (2019). Blacktip shark Carcharhinus limbatus presence at fishing piers in South Carolina: association and environmental drivers. Journal of Fish Biology 94, 469–480.
| Blacktip shark Carcharhinus limbatus presence at fishing piers in South Carolina: association and environmental drivers.Crossref | GoogleScholarGoogle Scholar |
Matern, SA, Cech, JJ, and Hopkins, TE (2000). Diel movements of bat rays, Myliobatis californica, in Tomales bay, California: evidence for behavioral thermoregulation? Environmental Biology of Fishes 58, 173–182.
| Diel movements of bat rays, Myliobatis californica, in Tomales bay, California: evidence for behavioral thermoregulation?Crossref | GoogleScholarGoogle Scholar |
Matos, DM, Ramos, JA, Calado, JG, Ceia, FR, Hey, J, and Paiva, VH (2018). How fishing intensity affects the spatial and trophic ecology of two gull species breeding in sympatry. ICES Journal of Marine Science 75, 1949–1964.
| How fishing intensity affects the spatial and trophic ecology of two gull species breeding in sympatry.Crossref | GoogleScholarGoogle Scholar |
Meyer, L, Whitmarsh, SK, Nichols, PD, Revill, AT, and Huveneers, C (2020). The effects of wildlife tourism provisioning on non-target species. Biological Conservation 241, 108317.
| The effects of wildlife tourism provisioning on non-target species.Crossref | GoogleScholarGoogle Scholar |
Mitchell, JD, Schifiliti, M, Birt, MJ, Bond, T, Mclean, DL, Barnes, PB, and Langlois, TJ (2020). A novel experimental approach to investigate the potential for behavioural change in sharks in the context of depredation. Journal of Experimental Marine Biology and Ecology 530-531, 151440.
| A novel experimental approach to investigate the potential for behavioural change in sharks in the context of depredation.Crossref | GoogleScholarGoogle Scholar |
Mourier, J, Claudet, J, and Planes, S (2021). Human-induced shifts in habitat use and behaviour of a marine predator: the effects of bait provisioning in the blacktip reef shark. Animal Conservation 24, 230–238.
| Human-induced shifts in habitat use and behaviour of a marine predator: the effects of bait provisioning in the blacktip reef shark.Crossref | GoogleScholarGoogle Scholar |
Mulder, CK, Gerkema, MP, and Van der Zee, EA (2013). Circadian clocks and memory: time-place learning. Frontiers in Molecular Neuroscience 6, 8.
| Circadian clocks and memory: time-place learning.Crossref | GoogleScholarGoogle Scholar |
Newsome D, Rodger K (2008) To feed or not to feed: a contentious issue in wildlife tourism. In ‘Too close for comfort: contentious issues in human–wildlife encounters’. (Eds D Lunney, A Munn, W Meikle) pp. 255–270. (Royal Zoological Society of New South Wales: Sydney, NSW, Australia)
Newsome, D, Lewis, A, and Moncrieff, D (2004). Impacts and risks associated with developing, but unsupervised, stingray tourism at Hamelin Bay, western Australia. International Journal of Tourism Research 6, 305–323.
| Impacts and risks associated with developing, but unsupervised, stingray tourism at Hamelin Bay, western Australia.Crossref | GoogleScholarGoogle Scholar |
Niella, Y, Smoothey, AF, Peddemors, V, and Harcourt, R (2020). Predicting changes in distribution of a large coastal shark in the face of the strengthening East Australian Current. Marine Ecology Progress Series 642, 163–177.
| Predicting changes in distribution of a large coastal shark in the face of the strengthening East Australian Current.Crossref | GoogleScholarGoogle Scholar |
Nix, JH, Howell, RG, Hall, LK, and McMillan, BR (2018). The influence of periodic increases of human activity on crepuscular and nocturnal mammals: testing the weekend effect. Behavioural Processes 146, 16–21.
| The influence of periodic increases of human activity on crepuscular and nocturnal mammals: testing the weekend effect.Crossref | GoogleScholarGoogle Scholar |
NSW Department of Primary Industries (2013) Estuarine macrophytes of NSW. (NSW DPI) Available at http://metadata.imas.utas.edu.au/geonetwork/srv/en/metadata.show?uuid=281FAA64-F6F3-400C-A48F-D342E4ABCA83
Orams, MB (2002). Feeding wildlife as a tourism attraction: a review of issues and impacts. Tourism Management 23, 281–293.
| Feeding wildlife as a tourism attraction: a review of issues and impacts.Crossref | GoogleScholarGoogle Scholar |
Oro, D, Genovart, M, Tavecchia, G, Fowler, MS, and Martínez-Abraín, A (2013). Ecological and evolutionary implications of food subsidies from humans. Ecology Letters 16, 1501–1514.
| Ecological and evolutionary implications of food subsidies from humans.Crossref | GoogleScholarGoogle Scholar |
Ortuño Crespo, G, and Dunn, DC (2017). A review of the impacts of fisheries on open-ocean ecosystems. ICES Journal of Marine Science 74, 2283–2297.
| A review of the impacts of fisheries on open-ocean ecosystems.Crossref | GoogleScholarGoogle Scholar |
Owers, CJ, Rogers, K, Mazumder, D, and Woodroffe, CD (2016). Spatial variation in carbon storage: a case study for Currambene Creek, NSW, Australia. Journal of Coastal Research 75, 1297–1301.
| Spatial variation in carbon storage: a case study for Currambene Creek, NSW, Australia.Crossref | GoogleScholarGoogle Scholar |
Parnell, PE, Dayton, PK, Fisher, RA, Loarie, CC, and Darrow, RD (2010). Spatial patterns of fishing effort off San Diego: implications for zonal management and ecosystem function. Ecological Applications 20, 2203–2222.
| Spatial patterns of fishing effort off San Diego: implications for zonal management and ecosystem function.Crossref | GoogleScholarGoogle Scholar |
Patroni, J, Simpson, G, and Newsome, D (2018). Feeding wild fish for tourism – a systematic quantitative literature review of impacts and management. International Journal of Tourism Research 20, 286–298.
| Feeding wild fish for tourism – a systematic quantitative literature review of impacts and management.Crossref | GoogleScholarGoogle Scholar |
Peterson, RA, and Cavanaugh, JE (2020). Ordered quantile normalization: a semiparametric transformation built for the cross-validation era. Journal of Applied Statistics 47, 2312–2327.
| Ordered quantile normalization: a semiparametric transformation built for the cross-validation era.Crossref | GoogleScholarGoogle Scholar |
Pini-Fitzsimmons, J, Knott, NA, and Brown, C (2018). Effects of food provisioning on site use in the short-tail stingray Bathytoshia brevicaudata. Marine Ecology Progress Series 600, 99–110.
| Effects of food provisioning on site use in the short-tail stingray Bathytoshia brevicaudata.Crossref | GoogleScholarGoogle Scholar |
Pini-Fitzsimmons, J, Knott, NA, and Brown, C (2021). Heterarchy reveals social organisation of a smooth stingray (Bathytoshia brevicaudata) population in a provisioned food context. Frontiers in Marine Science 8, 641761.
| Heterarchy reveals social organisation of a smooth stingray (Bathytoshia brevicaudata) population in a provisioned food context.Crossref | GoogleScholarGoogle Scholar |
Powell, JR, and Wells, RS (2011). Recreational fishing depredation and associated behaviors involving common bottlenose dolphins (Tursiops truncatus) in Sarasota bay, Florida. Marine Mammal Science 27, 111–129.
| Recreational fishing depredation and associated behaviors involving common bottlenose dolphins (Tursiops truncatus) in Sarasota bay, Florida.Crossref | GoogleScholarGoogle Scholar |
Reebs, SG (1993). A test of time-place learning in a cichlid fish. Behavioural Processes 30, 273–281.
| A test of time-place learning in a cichlid fish.Crossref | GoogleScholarGoogle Scholar |
Ricardo, GF, Davis, AR, Knott, NA, and Minchinton, TE (2014). Diel and tidal cycles regulate larval dynamics in salt marshes and mangrove forests. Marine Biology 161, 769–784.
| Diel and tidal cycles regulate larval dynamics in salt marshes and mangrove forests.Crossref | GoogleScholarGoogle Scholar |
Rigby CL, Chin A, Derrick D (2021) Smooth Stingray Bathytoshia brevicaudata. The ‘IUCN Red List of Threatened Species 2021’. e.T104039923A104039985. (International Union for Conservation of Nature and Natural Resources) Available at https://www.iucnredlist.org/species/104039923/104039985
Rizzari, JR, Semmens, JM, Fox, A, and Huveneers, C (2017). Observations of marine wildlife tourism effects on a non-focal species. Journal of Fish Biology 91, 981–988.
| Observations of marine wildlife tourism effects on a non-focal species.Crossref | GoogleScholarGoogle Scholar |
Schlaff, AM, Heupel, MR, and Simpfendorfer, CA (2014). Influence of environmental factors on shark and ray movement, behaviour and habitat use: a review. Reviews in Fish Biology and Fisheries 24, 1089–1103.
| Influence of environmental factors on shark and ray movement, behaviour and habitat use: a review.Crossref | GoogleScholarGoogle Scholar |
Semeniuk, CAD, and Rothley, KD (2008). Costs of group-living for a normally solitary forager: effects of provisioning tourism on southern stingrays Dasyatis americana. Marine Ecology Progress Series 357, 271–282.
| Costs of group-living for a normally solitary forager: effects of provisioning tourism on southern stingrays Dasyatis americana.Crossref | GoogleScholarGoogle Scholar |
Semeniuk, CAD, Bourgeon, S, Smith, SL, and Rothley, KD (2009). Hematological differences between stingrays at tourist and non-visited sites suggest physiological costs of wildlife tourism. Biological Conservation 142, 1818–1829.
| Hematological differences between stingrays at tourist and non-visited sites suggest physiological costs of wildlife tourism.Crossref | GoogleScholarGoogle Scholar |
Smoothey, AF, Lee, KA, and Peddemors, VM (2019). Long-term patterns of abundance, residency and movements of bull sharks (Carcharhinus leucas) in Sydney Harbour, Australia. Scientific Reports 9, 18864.
| Long-term patterns of abundance, residency and movements of bull sharks (Carcharhinus leucas) in Sydney Harbour, Australia.Crossref | GoogleScholarGoogle Scholar |
Spaet, JLY, Manica, A, Brand, CP, Gallen, C, and Butcher, PA (2020). Environmental conditions are poor predictors of immature white shark Carcharodon carcharias occurrences on coastal beaches of eastern Australia. Marine Ecology Progress Series 653, 167–179.
| Environmental conditions are poor predictors of immature white shark Carcharodon carcharias occurrences on coastal beaches of eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Stalmaster, MV, and Kaiser, JL (1998). Effects of recreational activity on wintering bald eagles. Wildlife Monographs 137, 3–46.
Sulikowski, JA, Wheeler, CR, Gallagher, AJ, Prohaska, BK, Langan, JA, and Hammerschlag, N (2016). Seasonal and life-stage variation in the reproductive ecology of a marine apex predator, the tiger shark Galeocerdo cuvier, at a protected female-dominated site. Aquatic Biology 24, 175–184.
| Seasonal and life-stage variation in the reproductive ecology of a marine apex predator, the tiger shark Galeocerdo cuvier, at a protected female-dominated site.Crossref | GoogleScholarGoogle Scholar |
Svane, I, Roberts, S, and Saunders, T (2008). Fate and consumption of discarded by-catch in the Spencer Gulf prawn fishery, South Australia. Fisheries Research 90, 158–169.
| Fate and consumption of discarded by-catch in the Spencer Gulf prawn fishery, South Australia.Crossref | GoogleScholarGoogle Scholar |
Trave, C, Brunnschweiler, J, Sheaves, M, Diedrich, A, and Barnett, A (2017). Are we killing them with kindness? Evaluation of sustainable marine wildlife tourism. Biological Conservation 209, 211–222.
| Are we killing them with kindness? Evaluation of sustainable marine wildlife tourism.Crossref | GoogleScholarGoogle Scholar |
Udyawer, V, Dwyer, RG, Hoenner, X, Babcock, RC, Brodie, S, Campbell, HA, Harcourt, RG, Huveneers, C, Jaine, FRA, Simpfendorfer, CA, Taylor, MD, and Heupel, MR (2018). A standardised framework for analysing animal detections from automated tracking arrays. Animal Biotelemetry 6, 17.
| A standardised framework for analysing animal detections from automated tracking arrays.Crossref | GoogleScholarGoogle Scholar |
United States Naval Observatory (2019) Phases of the moon. (USNO) Available at https://www.usno.navy.mil/USNO/astronomical-applications/data-services/phases-moon
van Poorten, BT, Carruthers, TR, Ward, HGM, and Varkey, DA (2015). Imputing recreational angling effort from time-lapse cameras using an hierarchical Bayesian model. Fisheries Research 172, 265–273.
| Imputing recreational angling effort from time-lapse cameras using an hierarchical Bayesian model.Crossref | GoogleScholarGoogle Scholar |
Vaudo, JJ, Wetherbee, BM, Harvey, GCM, Harvey, JC, Prebble, AJF, Corcoran, MJ, Potenski, MD, Bruni, KA, Leaf, RT, Henningsen, AD, Collie, JS, and Shivji, MS (2018). Characterisation and monitoring of one of the world’s most valuable ecotourism animals, the southern stingray at Stingray city, Grand Cayman. Marine and Freshwater Research 69, 144–154.
| Characterisation and monitoring of one of the world’s most valuable ecotourism animals, the southern stingray at Stingray city, Grand Cayman.Crossref | GoogleScholarGoogle Scholar |
Voohris RR (2016) Impacts of human provisioning from the chatham fish pier on the ecology of grey seals (Halichoerus grypus). BSc(Hons) thesis, University of North Carolina at Chapel Hill.
Ward, CRE, Bouyoucos, IA, Brooks, EJ, and O’Shea, OR (2019). Novel attachment methods for assessing activity patterns using triaxial accelerometers on stingrays in the Bahamas. Marine Biology 166, 53.
| Novel attachment methods for assessing activity patterns using triaxial accelerometers on stingrays in the Bahamas.Crossref | GoogleScholarGoogle Scholar |
Wearmouth, VJ, and Sims, DW (2008). Sexual segregation in marine fish, reptiles, birds and mammals: behaviour patterns, mechanisms and conservation implications. Advances in Marine Biology 54, 107–170.
| Sexual segregation in marine fish, reptiles, birds and mammals: behaviour patterns, mechanisms and conservation implications.Crossref | GoogleScholarGoogle Scholar |
Wearmouth, VJ, and Sims, DW (2009). Movement and behaviour patterns of the critically endangered common skate Dipturus batis revealed by electronic tagging. Journal of Experimental Marine Biology and Ecology 380, 77–87.
| Movement and behaviour patterns of the critically endangered common skate Dipturus batis revealed by electronic tagging.Crossref | GoogleScholarGoogle Scholar |
Wood SN (2017) ‘Generalized additive models: an introduction with R.’ (Chapman & Hall: New York, NY, USA)
Zeller, D, Cashion, T, Palomares, M, and Pauly, D (2018). Global marine fisheries discards: a synthesis of reconstructed data. Fish and Fisheries 19, 30–39.
| Global marine fisheries discards: a synthesis of reconstructed data.Crossref | GoogleScholarGoogle Scholar |
Zuur, AF, Ieno, EN, and Elphick, CS (2010). A protocol for data exploration to avoid common statistical problems. Methods in Ecology and Evolution 1, 3–14.
| A protocol for data exploration to avoid common statistical problems.Crossref | GoogleScholarGoogle Scholar |


