Fine-scale variability in catch and growth rates of western rock lobsters (Panulirus cygnus) show heterogeneous life-history parameters
A. Miller A B , S. de Lestang A C , J. How A C , B. Gibbons A B , E. Lester A B , M. Navarro A B , J. Fitzhardinge D , M. Brooker A B and T. Langlois
A B and T. Langlois  A B *
A B *
A School of Biological Sciences, University of Western Australia, Crawley, WA 6019, Australia.
B UWA Oceans Institute, University of Western Australia, Crawley, WA 6019, Australia.
C Department of Primary Industries and Regional Development, Hillarys, WA 6025, Australia.
D Southerly Designs, 2 Carol Street, Port Denison, WA 6525, Australia.
Marine and Freshwater Research 74(4) 335-346 https://doi.org/10.1071/MF22084
Submitted: 14 April 2022 Accepted: 9 December 2022 Published: 8 February 2023
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Context: The western rock lobster fishery is recognised to be conservatively managed, with breeding stock levels estimated to be at record levels over the past decade. Despite this, anecdotal reports from commercial fishers identified an area of unexpectedly low catches in the centre of the fishery and lobsters’ biogeographic distribution.
Aim: To confirm the presence of this suspected ‘low-catch’ zone and examine the variability in catch and growth rates of lobsters if identified.
Methods: This study conducted an intensive mark–recapture survey over 8 months to explore catch rate, density, movement and growth rates across this ‘low-catch’ zone and three comparable locations.
Key results: In total, 9318 lobsters were caught and 7565 individuals were tagged during the study. Consistently low catch rates of under-sized lobsters were observed in the ‘low-catch’ zone, with catch rates increasing with distance from the zone. By contrast, similar catch rates of legal-sized lobsters were observed across all locations.
Conclusions: The study confirmed low catch rates, for under-sized lobsters, within an area of perceived low catch rates within the centre of the fishery. The lack of difference found in legal-sized catch rates among locations is likely to be due to the low fishing pressure in the ‘low-catch’ zone, resulting from hyperstability of fishers adapting to the historical perceived low catch rate. Modelled data demonstrated the ‘low-catch’ zone to be associated with faster growth rates and high fine-scale migration, indicating a potential release from density-dependent processes.
Implications: We anticipate that these results will be a useful starting point for future research into the mechanisms responsible for the unexpectedly low catch of sublegal lobsters within the ‘low-catch’ zone and the implications it may have on the wider population, both regionally and across the species distribution.
Keywords: catchability, commercial fishing, fine-scale variability, fisheries ecology, fishery management, mark–recapture, trap fishery, western rock lobster.
Introduction
Understanding the environmental drivers of demographic rates and the catchability of commercially targeted species underpins sustainable fisheries management. The western rock lobster (WRL; Panulirus cygnus George) is found along the lower western coast of Western Australia and is exploited by both commercial and recreational fishers throughout its geographic range. The commercial fishery alone represents Australia’s most valuable wild-caught single-species fisheries, contributing a total revenue of A$424 million in 2017 (Gaughan and Santoro 2018). Owing to its high economic and social value, the western rock lobster fishery has been the focus of over 40 years of extensive research (de Lestang et al. 2016; Bellchambers et al. 2017). Much of this research relies on stock assessments from pot-based surveys to derive size-structured abundance and demographic data. The fishery is currently managed using a strict quota-controlled system with individual transferable quotas (ITQ) and a total allowable commercial (TAC), which is set annually (de Lestang 2014).
Despite the overall high catch rates currently reported within the West Coast Rock Lobster Managed Fishery (WCRLMF), a pre-study workshop with fishers from the Professional Fishermen’s Association (PFA) and perception surveys of fishers on changes in catch rates throughout the fishery identified an area of abnormally low legal-sized catch within the centre of the fishery (Brooker 2022). This ‘low-catch’ zone is centred on the shallow waters at ‘Cliff Head’ (Fig. 1), with lower-than-expected catch rates since 1995 and a continued decline since. Additionally, a gradient of catch rates was identified within the fishery, including a low to moderate catch area (‘boundary’), a moderate to high catch area (‘mid’), and a high-catch area (‘high-catch’) commonly known as ‘Seven Mile Beach’ north of Dongara. Despite this variation in catch rate, all catch areas share broadly comparable environmental conditions and perceived historical (pre-1995) catch rates. The perceived low catch rates within this ‘low-catch’ zone have resulted in the relocation of almost all the commercial fishers that previously fished in this region to fish in adjacent locations. Interestingly, the identified ‘low-catch’ zone allegedly produced some of the highest catch rates in the fishery prior to 1995, supporting a large number of fishing vessels (>60 boats, John Fitzhardinge, pers. comm.). Furthermore, the coastal waters within this region have received historically, and receive currently, some of the highest levels of recruitment within the fishery (Caputi et al. 2014; de Lestang et al. 2016). Moreover, the perceived ‘low-catch’ zone is situated in close proximity (<40 km) to areas that are currently producing some of the highest catch rates in the fishery (de Lestang et al. 2016). Understanding the extent and the likely mechanisms driving these unexpected low-catch rates could be crucial for maintaining the sustainability of the fishery.
Palinurus cygnus has a bi-phasic life cycle composed of pelagic and benthic life stages that cover a large geographical extent, from deep offshore waters to shallow coastal reefs, where the majority of settlement is likely to occur (Chittleborough and Phillips 1975). Post-settlement, P. cygnus is known to undergo a migration from shallow inshore waters (<40 m) between late November and January every year to deeper offshore waters (40−100 m; Caputi et al. 2010). This migration occurs at ~4 years of age before P. cygnus reaches maturity. This process is known as the ‘whites’ migration because of the pale colouration of the lobsters after the moult, prior to the migration event (Caputi et al. 2010). Given the lack of morphological age markers for crustaceans, there is no method to directly age decapods (Kilada et al. 2012), which results in an inability to obtain size-at-age data. Therefore, this ‘whites’ life stage provides an opportunity to compare individuals of likely comparable age.
Low catch rates within the near-shore ‘low-catch’ zone could be caused by a wide range of variables because both environmental and physiological factors have previously been demonstrated to influence the catchability and population density of P. cygnus. Water temperature (Chittleborough 1970), salinity (Morgan 1974), lunar phase (Morgan 1974; Srisurichan et al. 2005) and swell (Srisurichan et al. 2005) can influence the catchability of P. cygnus. Feeding activity, and therefore catch rate, has been shown to be significantly influenced during ecdysis or ‘moulting’, where the hard outer exoskeleton is shed to allow for growth (Needham 1946). For P. cygnus, feeding behaviours have been shown to vary with the stage of the moult cycle, in turn affecting the catchability of the individual (Morgan 1974). In addition, the presence of predators within a trap can affect the catch rate of P. cygnus (Morgan 1974). The main factors thought to influence population density are the levels of recruitment, which are thought to be highly influenced by a range of factors, including environmental processes (de Lestang et al. 2015), post-larval survival, breeding stock abundance (de Lestang et al. 2015), natural mortality (MacArthur et al. 2007), and the degree of immigration or emigration (MacArthur et al. 2008). Disentangling whether the perceived low catch rates at Cliff Head (‘low-catch’ zone) are attributable to certain factors influencing catchability or factors causing a decline in population abundance is a complex, yet vital, task to investigate when exploring the potential implications of the reported low catches in this area and how these mechanisms may relate to the management of the fishery.
The current study conducted an intensive mark–recapture potting survey over 8 successive months to confirm the existence of low-catch rates within the ‘low-catch’ zone reported by fishers, relative to comparable adjacent areas. Specifically, we aimed to test the hypothesis that the ‘low-catch’ zone will be associated with lower standardised catch rates of legal-sized lobster than are nearby locations with comparable environmental and catch rate histories, matching the fishers’ perceptions. Owing to the design of the study, the spatial extent of the ‘low-catch’ zone could be identified if evidence of low catch exists, and variations in growth rates between locations can be analysed. We investigated whether environmental, behavioural, physiological and life-stage variables may be influencing the low catchability coefficient or low abundance within the perceived ‘low-catch’ zone. If the ‘low-catch’ zone is confirmed, a crucial component for future studies will be to explore whether the identified low catch rates are due to a low population density for the area, or whether they are due to a low catchability of the lobster population within the ‘low-catch’ zone.
Materials and methods
Study area
A preliminary workshop was conducted with 18 fishers from the Dongara PFA and a separate perception survey with 47 fishers was conducted as a part of research metholdology of a PhD candidate (M. Brooker, University of Western Australia, UWA). The surveys identified four locations with comparable historical catch rates and environmental variables, yet with contrasting current perceived catch rates (Fig. 1). The ‘low-catch’ location centred on Cliff Head was perceived to currently have the lowest catch and strongest contrast with fishers’ knowledge of historical catch averages, whereas the ‘boundary’ and ‘mid’ locations were perceived to have moderately low catch compared with historical catch averages. The ‘high-catch’ location, commonly known as ‘Seven Mile Beach’, was perceived to currently have the highest and most comparable catch to historical (pre-1995) averages. Each location was chosen to capture this gradient in perceived change in catch-rate across locations representing otherwise comparable lobster habitat.
With the assistance of fishers and historical fishing records, representative and comparable potting sites (between 3 and 5 per location) were chosen. Owing to the mandatory use of sea lion exclusion devices (SLEDs) on all recreational and commercial fishing activities south of 29°35.16′S, it was not possible to find comparable sampling locations further to the south of the southern-most ‘boundary’ location.
Catch rate and mark–recapture experimental design
A catch and mark–recapture survey was conducted over 4–6 successive days every month over an 8-month study period between May and December in 2018. A minimum of 15 baited wooden pots enclosed by mesh were used to capture individuals at each study location, with each pot site being sampled twice on each trip. The pots were adapted from commercial wooden pots, with a 1 cm wire mesh added to cover the interior of the pot. This design of ‘mesh-enclosed’ pot is used as a standard fisheries independent sampling method for sampling both legal size (>76-mm carapace length) and sublegal size (<76-mm carapace length) lobsters across the fishery (Tuffley et al. 2018).
The fishing was conducted over reef habitats pre-identified as suitable fishing grounds and comparable habitats by fishers during the pre-study surveys. Potting was restricted to a maximum depth of 20 m across all locations. All pots were deployed and recovered the following day from a commercial fishing vessel. The bait was standardised across all sampling trips.
Data collection
On capture, lobsters were temporarily placed in a ‘chill tank’ to stun the lobsters, so as to reduce handling damage. Individuals larger than >40-mm carapace length and in suitable condition (no more than four lost appendages and no visible damage) were tagged with a uniquely coded 50-mm plastic T-bar anchor tag (Hallprint, Australia). Tags were inserted, with a tag applicator, into the tail extensor muscle located between the first abdominal segment and the posterior edge of the carapace. During each sampling month a unique pleopod was clipped to allow for estimates of tag loss, where any individual caught with a cut pleopod but no tag was recorded as a tag loss. Biological data on each lobster included carapace length (CL, from the posterior edge of the cephalothorax to the rostral horns), sex, colour, reproductive state (presence or absence of ovigerous setae) and any external damage (lost or newly regenerated legs or antennae). The presence of predators and the type and number of by-catch in each trap was also recorded. All lobsters were placed in a holding tank prior to release to ensure that they were in a suitable condition before being released back into the water. Individuals were returned to the same GPS coordinates as where the pot was pulled from. Sampling sites were left undisturbed for at least 3 weeks before potting was repeated. Water temperatures used in subsequent analyses were derived using the work of Chamberlain et al. (2012).
Additional mark–recapture data were obtained from a simultaneous and comparable on-going research program conducted by the DPIRD (Fisheries Research) at Seven Mile Beach between May 2017 and November 2018. These data extended the spatial coverage and number of recaptures in the study and comprised 534 tagged individuals in total, with 213 total recaptures (39.9%), 141 being unique (26.4%). Historical data from a previous tagging study between November 2017 and December 2017 conducted by the University of Western Australia (UWA) contributed an additional 1682 tagged individuals with 102 total recaptures (6%), 99being unique (5.8%). A further 206 recaptures of individuals marked during these studies were reported by commercial fishers fishing throughout the region between December 2017 and February 2019. Finally, 28 recaptures were obtained during a follow-up sampling trip in June 2019, which re-visited the 2018 study potting locations.
Animal ethics approval
The work conducted for the study was undertaken under animal ethics protocol ‘RA/3/100/1550 Catch and release of western rock lobster (Panulirus cygnus) in an area of unexpectedly low catch rates’ approved by the UWA Animal Ethics Committee on 12 October 2017.
Quality control and validation
Owing to the collaborative nature of the study, an extensive amount of quality control and validation was required. All catch and mark–recapture data collected at the main locations and sites of interest were collected by a single observer; however, historical UWA data and that were provided by commercial fishers and DPRID fisheries researchers were extensively checked prior to incorporation. Inaccurate, incomplete and inconsistent data entries were either corrected or removed prior to statistical analyses.
Statistical analysis
Contribution of location and environmental variables to catch rate and mean length
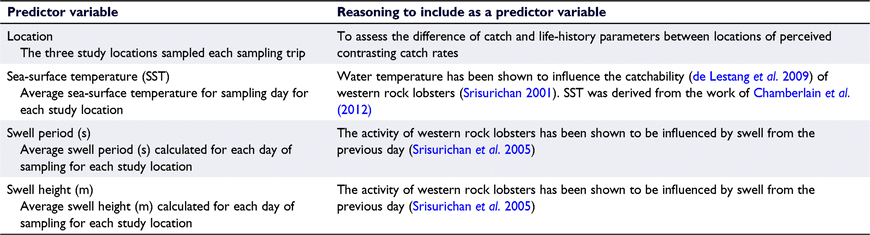
Differences among locations, perceived by fishers to be different, and the influence of environmental variables (Table 1) in catch rate and mean length per pot were assessed using generalised additive mixed models (GAMM) with a full-subset model selection approach (as per Fisher et al. 2018). The full-subset approach tests all possible combinations of a range of predictor variables, to a maximum of three, and then identifies the most parsimonious model (Fisher et al. 2018). Models containing variable combinations with correlations of >0.28 were excluded, as suggested by Graham (2003), to eliminate potential problems with collinearity and overfitting, and model selection was based on Akaike’s Information Criterion optimised for small sample sizes (AICc; Akaike 1973), and parsimony (Graham 2003; Fisher et al. 2018). GAMMs were chosen to standardise catch rates because they utilise smoothing techniques to accommodate for non-linear relationships with predictors and can be modified to suite the error distribution of the data and account for random effects (Hastie and Tibshirani 1986; Lin and Zhang 1999).
|
For catch data, the number of sublegal- and legal-sized lobsters per pot was used as the response variable. The raw data had a high number of zeros (5.7–37%, depending on the location); therefore, a Tweedie error distribution was used (Tweedie 1984). The Tweedie distribution is an extension of a compound Poisson model and has the advantage of handling zero-inflated data in a unified way, in contrast to delta or hurdle models (Tweedie 1984; Candy 2004; Shono 2008). Given that assessing temporal patterns in catch rate was not an objective of this study, and that broad temporal consistency of catch-rate was observed among sampling locations (Fig. 1), the sampling date was considered as a random effect to account for fine-scale temporal variation within the study. In addition, the independent potting sites (3–5) nested within each location were also included as a random effect to account for small-scale spatial variation. All pots that had a predator present, or indication that one had visited, were removed from the catch-rate analysis to reduce the impact of predator presence on variation in catchability and catch rate.
The mean length of the migratory ‘white’ and resident ‘red’ life stages of lobster per pot was analysed in a similar fashion; however, data exploration found the residuals to be normally distributed for both analyses and, therefore, a Gaussian error distribution was used in modelling. The sampling date and the identity of each pot were used as random effects in these models to account for both spatial and temporal small-scale variation and to account for the non-independence of length measures within each pot.
For all models, importance scores of every predictor variable were obtained by summing the AIC weights of each model for which each variable occurred within and these scores were then plotted to identify the relative importance of predictor variables across all possible models (Fisher et al. 2018). All data formatting, plotting and statistical analyses were conducted in the R language for statistical computing (ver. 4.03, R Foundation for Statistical Computing, Vienna, Austria, see https://www.r-project.org/), using the following additional packages: tidyr (ver. 1.12, H. Wickham and M. Girlich, see https://tidyr.tidyverse.org/), dplyr (ver. 1.01, H. Wickham, R. François, L. Henry, K. Müller and RStudio, see https://github.com/tidyverse/dplyr) and ggplot2 (ver. 3.31, H. Wickham, see https://github.com/tidyverse/ggplot2).
Comparison of growth among locations
Growth curves were generated using Fabens (1965) modification of the traditional von Bertalanffy growth equation
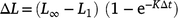
where L1 is the length (mm) at time of marking (tm); Lr is the length (mm) at time of recapture (tr); ΔL is the change in length (mm; Lr– L1); Δt is the time (days) at liberty (tr– tm) and two deterministic growth parameters; K is Brody growth coefficient; and L∞ is the maximum length (mm). The Brody growth coefficient (K) was allowed to vary between sexes and among locations. To avoid potential over-parameterisation of the models, and given that our main objective was to compare growth rates (K) and that the majority of lobsters tagged were immature and well below the maximum length for the species, the maximum size (L∞) was kept constant across the groups being compared (i.e. it was estimated but could not vary among groups). Chi-Square maximum-likelihood ratio tests were conducted to determine whether growth varied significantly with sex or location. Recaptures obtained from commercial fishers and from the follow-up sampling trip conducted in June 2019 were incorporated to increase data abundance and accuracy of the model. Only recapture data from lobsters that were at liberty for more than 1 month were used, to ensure sufficient time for moulting (i.e. growth) to occur between recaptures. The data were standardised by seasons, which resulted in the ‘high-catch’ (Seven Mile) location being excluded owing to the inconsistencies in the temporal coverage of the mark–recapture data from this location. Recaptures that had more than two damaged appendages were excluded because leg loss can affect the growth of lobsters (Goosen and Cockcroft 1995). All analyses were again conducted using the R language for statistical computing (R Foundation for Statistical Computing), using the mixed effects package lme4 (ver. 1.1-27.1, see https://CRAN.R-project.org/package=lme4; Bates et al. 2015).
Results
Catch and release data
In total, 9318 lobsters were captured from 1461 pot lifts across the eight sampling trips (May 2018–December 2018). This resulted in 7565 unique individuals suitable (>40 mm CL and less than five missing appendages) for tagging. The raw catch rates varied temporally across the study but displayed broadly consistent differences among locations (Fig. 2).
The influence of location and environmental variables on catch rate
Legal-sized individuals
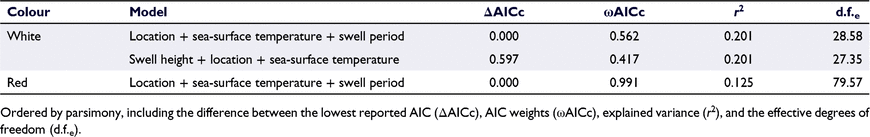
The most parsimonious model for the legal-size class explained 23% of the deviance in the response variables and included the variables swell height, swell period and sea-surface temperature (Table 2). Importance scores indicated that location had low importance for predicting the catch rate of legal-sized individuals, but that all three environmental variables (swell height, period and sea-surface temperature) were more important for predicting the catch rate across all possible models (Fig. 3a). Catch rates were comparable across all four zones. However, the catch rates for the legal-sized class were anticipated to be strongly influenced by fishing pressure within the study locations; as such, the trends across the four zones for this size class were not explored further.

|
Sublegal-sized individuals
Importance scores indicated that swell period had low importance, but that location, swell height and sea-surface temperature were more important for predicting the catch rate of sublegal individuals (Fig. 3). The most parsimonious model for the sublegal-size class catch rate included location, swell height, and sea-surface temperature and explained 31% of the deviance (Table 2). There were lower catch rates in the ‘low-catch’ zone, with a strong trend of increasing catch rate with an increasing distance from the low-catch zone, with the highest catch rate in the ‘high-catch’ location (Fig. 4).
Recapture rates
Of the 7565 unique individuals tagged with a uniquely coded T-bar anchor tag, a total of 1261 individuals (16.7%) was recaptured throughout the study. Of these recaptures, 992 (13.1%) were unique individuals, with more than three-quarters of the recaptured individuals being caught on multiple occasions. A strong pattern was observed with the raw recapture rate of sublegal-size lobsters in the ‘low-catch’ zone being approximately half of that within both the ‘boundary’ and ‘mid’ locations and a quarter of that found in the ‘control’ location. For the legal-size raw recapture rates, a similar pattern was found, except for the ‘mid’ control location, which displayed recapture rates comparable to those in the ‘low-catch’ zone. Unexpectedly, we found considerable along-shore movement of tagged sublegal-sized lobsters among study locations (up to 20 km); therefore, the planned formal mark–recapture analyses were not attempted because of violation of the assumptions of closed, spatially explicit populations.
Mean length frequency
Migratory ‘white’ life stage
The most parsimonious model for the mean length of the ‘white’ life stage explained 20% of the deviance and included location, sea-surface temperature and swell period (Table 3). The mean length of this migratory ‘white’ life stage was greater within the ‘low-catch’, ‘boundary’ and ‘mid’ locations than in the ‘high-catch’ location, with the majority of ‘white’ individuals greater than legal size (>76 mm, Fig. 5). Importance scores indicated that swell height had a low importance for predicting the mean length per pot for the ‘white’ life stage, whereas, location, sea-surface temperature and swell period were relatively more important (Fig. 3b).
|
Resident ‘red’ life stage
The most parsimonious model for the ‘red’ life-stage size class explained 12% of the deviance in the response variable and included the predictor variables location, sea-surface temperature and swell period (Table 3). For this resident ‘red’ life stage, there was a higher mean carapace length found in the ‘low-catch’ and the ‘boundary’ location, with the ‘mid’ and ‘high-catch’ locations demonstrating a comparably lower mean carapace length (Fig. 5). Importance scores indicated that swell height had a very low importance for predicting the mean length per pot for the red life stage, whereas location, sea-surface temperature and swell period were more important (Fig. 3b).
Comparison of growth curves
The Chi-Square maximum-likelihood ratio tests on the growth coefficients obtained from the modified Fabens growth model found that growth rates varied significantly among locations (P < 0.02) but not between lobster sexes (P = 0.4, Fig. 6). Inspection of the residuals for each term in the Fabens found an acceptable distribution of residuals supporting the model. Pairwise comparisons of growth rate among locations found that individuals in the ‘low-catch’ location grew significantly faster than those in the ‘mid’ location (P < 0.01), but no significant differences were found between the ‘low-catch’ and the ‘boundary’, or the ‘boundary’ and ‘mid’ locations.
|
|
Discussion
Our study confirmed the existence of an area of significantly lower standardised catch rates for sublegal-sized lobsters at the centre of western rock lobster fishery. The area was identified to have previously supported high fishing activity and high catch rates, between the 1970s and 1990s (John Fitzhardinge, pers. comm.). By contrast, the catch rates for legal-sized lobsters were comparable across all perceived ‘low’, ‘boundary’, ‘mid’ and ‘high’ catch areas. Additionally, recapture data showed an unexpectedly high movement of individuals among our study locations (up to 20 km), particularly from the ‘low-catch’ area, for both sublegal- and legal-sized lobsters. Movements of these distances have not previously been observed in comparable studies, and thus further research should be conducted to explore this pattern. Furthermore, recaptured lobsters in the ‘low-catch’ zone had the fastest growth rates relative to that in other areas. These results suggest fisher’s perceptions of catch rate could be more reflective of sublegal-size lobster populations or that these populations of sublegal lobster represent the potential and sustained future legal catch rate of the area, which fishers are perceiving through observation of low numbers of sublegal-sized lobsters.
In contrast to the fishers’ perceptions, the current study found the catch rates of the legal-sized lobsters to be comparable among all four study locations. Previous studies have reported that flexibility and adaptation in fishing behaviours result in hyperstability in both fisheries-dependent and -independent data for a target population (Ward et al. 2013). Discussions with fishers at the pre-study workshop and during this current study indicated that hyperstability has occurred, with commercial fishing pressure having been lowest in the ‘low-catch-zone’ and highest in the ‘mid’ and ‘high-catch’ locations for at least the past 20 years. Spatial data on commercial catches collected by DPIRD are unfortunately not of fine-enough spatial resolution to demonstrate such small-scale changes in fishing effort (Gaughan and Santoro 2018). Regardless, given this apparent strong gradient in fishing pressure across the locations sampled, the comparable legal-sized catch rates among the locations support the perception that the ‘low-catch’ zone would have lower sustained catches of legal-sized lobsters if the fishing pressure were higher.
Comparison of raw-recapture rates indicate a strong contrast among the study locations. For both sublegal- and legal-sized lobsters the lowest recapture rate was found within the ‘low-catch’ zone at Cliff Head, with recapture rates generally increasing with an increasing distance from the ‘low-catch’ zone. This trend was particularly evident in the sublegal-size class, with the ‘boundary’ and ‘mid’ locations displaying a two-fold increase, and the ‘high-catch’ location a four-fold increase in recapture rates compared with the ‘low-catch zone’. Interestingly, the recapture rates for the legal-size class displayed a similar trend, except for the ‘mid’ location, which had a recapture rate comparable to that in the ‘low-catch’ location. We suspect that the low recapture rate for legal-sized lobsters within the ‘mid’ location is due to the increased commercial fishing pressure that was observed over the study. Despite approaching and encouraging commercial fishers to submit recapture data by the DPIRD ‘tagging’ app and release any tagged individuals caught, it was expected that not all will be consistently recorded or released, which would contribute to a low rate of recapture.
Similarly, the mean carapace length of the migratory life stage, known colloquially as the ‘whites’, differed among locations. There was a greater mean carapace length in the ‘low-catch’, ‘boundary’ and ‘mid’ locations than in the ‘high-catch’ location. Interestingly, the majority of the size distribution for the ‘high-catch’ location was below the legal-size cut-off (<76 mm), whereas for the other three locations, it was above the legal size (>76 mm). This greater mean length could indicate a faster growth rate of individuals within these areas of interest, allowing them to reach a larger size before they undergo the ‘whites’ migration. However, these results are likely to be confounded by the greater fishing pressure within the ‘high-catch’ location, because commercial fishing would act to remove any individuals greater than 76-mm carapace length, which is evident by the truncated size distribution at legal size in this location. Additionally, swell period was shown to positively influence the mean carapace length of both the ‘white’ and the ‘red’ life stages. Increased swell activity has been shown to increase the activity of lobsters the following day, owing to the stronger water movement disturbing the bottom habitat and increasing food availability (Srisurichan et al. 2005). It is therefore plausible that the greater mean carapace length for the whites is due to (1) the greater likelihood of catching larger individuals at a certain locations because of greater swell increasing the activity of lobsters for the location, (2) greater growth from increasing feeding or moulting events resulting in a higher proportion of large lobsters within certain locations, or (3) fewer larger individuals being located at areas that undergo higher fishing pressure, resulting in a disproportionate population structure.
Comparison of the modelled growth curves derived from the recapture data should provide a comparison that is less confounded by fishing pressure than is any analysis of mean length. Our study indicated that individuals from the ‘low-catch’ zone had a significantly faster rate of growth over the sampling period than did those from the ‘mid’ location. These growth results support the findings from a previous tagging study conducted by Chittleborough (1974), and a SCUBA diver survey conducted by Jernakoff et al. (1994), who both demonstrated faster growth rates of juvenile P. cygnus within the same location as the ‘low-catch’ zone (Cliff Head) than in the more northern ‘high-catch’ location used in the study (Seven Mile Beach). Unfortunately, it was not possible to use the growth data from the ‘high-catch’ location in our study because of seasonal differences in the survey times because temperature has been shown to significantly affect growth of P. cygnus (de Lestang and Melville-Smith 2006). Additionally, the growth rates recorded at Cliff Head in our current study are comparable to those recorded in aquaria-reared P. cygnus that were fed on a highly nutritional diet and reared in optimal conditions (Chittleborough 1974). Chittleborough (1976) suggested that the higher growth rates found at Cliff Head were attributed to the higher invertebrate component found in the Cliff Head population diet than in the diet found at Seven Mile Beach. Regardless of the cause, our analysis found high growth rates that are comparable to those recorded in a study conducted over 40 years ago within the same ‘low-catch’ location (Cliff Head). Importantly, these current growth rates are comparable to a time when, according to fishers, the ‘low-catch’ location produced high catch rates, supported a large number of fishers, and had a much higher fishing pressure.
There are four main competing hypotheses that could explain the observed variations in catch rates across the study locations, namely, the locations vary in (1) catchability, or the relative density of lobster populations at these locations differ due to (2) recruitment limitation, (3) post-recruitment movement patterns, or (4) post-recruitment mortality. These hypotheses are discussed below.
The catchability of lobsters could confound catch-rate estimates across the locations of interest because the ability to capture a lobster has been shown to be influenced by a wide range of environmental, behavioural and physiological factors, with each factor also shown to interact with each other in complex ways (Ziegler et al. 2002, 2003). The additional evidence of faster growth rates within the ‘low-catch’ zone suggests that food availability is relatively higher or has a higher nutritional content, which in turn would decrease the likelihood of a lobster entering a pot (Ziegler et al. 2002). This could be attributed to a lower density of lobsters resulting in abundant food resources that are not found at the other locations. Conversely, research conducted in the 1970s (Chittleborough 1976) found the same high growth rates within the same location now known as the ‘low-catch’ zone, so other factors that influence catchability across the study locations cannot be ruled out. There are obvious limitations associated with utilising commercial fishing techniques as the only methodology to interpret the natural population structure of a species. The most recent study conducted within the area that utilised an alternative sampling technique was that of Jernakoff et al. (1994) over 20 years ago. The study used SCUBA surveys to explore the population densities of juvenile P. cygnus, and reported higher densities of juveniles at the Seven Mile Beach site than at the Cliff Head site. Despite supporting our results, the study also acknowledged the low accuracy of the methodology, and potential for underestimating the population size. This study recommends that future research utilises other techniques, such as diver surveys and underwater camera surveys, to limit deduction of unconfounded data.
Recruitment limitation in the ‘low-catch’ zone may have resulted in the observed differences in catch rates within the fishery. Several environmental factors have historically been shown to strongly effect puerulus recruitment settlement within Western Australia. In particular, the strength of the Leeuwin Current, which brings warm waters southwards along the edge of the West Australian continental shelf, has been shown to be positively correlated with puerulus settlement (Caputi et al. 2014). However, ongoing studies using standard puerulus collection methods suggest that recruitment limitation is unlikely to be a factor in this region. This current ongoing research has recorded comparable recruitment levels between the ‘low-catch’ and ‘high-catch’ locations (Brooker 2022).
Analysis of the recapture data showed unexpectedly high levels of fine-scale movements of individuals among the study locations outside of the migratory time of year. Movements of up to 20 km were recorded for some individuals, with the ‘low-catch’ area being associated with the highest level of movement for both sublegal- and legal-sized lobsters. Previous fine-scale tracking studies have shown that P. cygnus is a typically sedentary species that displays great retention within its home range, only traversing distances of <500 m when foraging (MacArthur et al. 2008). These unusual movement patterns identified within our study are therefore unlikely to be related to normal foraging alone, and instead could be indicating unsuitable habitat or food abundance within the ‘low-catch’ location. These apparent high levels of emigration occurring from the ‘low-catch’ location could also be an attributing factor for the low catch rates and low recapture rates within the location. The contradictory findings of low recapture rates within the ‘low-catch’ zone, usually indicating a high population density, could also be explained by the high levels of movement, because higher emigration from a location would subsequently result in low recapture rates.
High levels of post-recruitment mortality within the ‘low-catch’ location may explain the identified low catch rates of sublegal-sized lobsters within the location. P. cygnus in its first-year post-settlement benthic stage has been shown to experience the highest levels of natural mortality (Phillips et al. 2003). Predation has been recorded as the main cause of natural mortality, with the smaller post-settlement stage being particularly susceptible to predation. Evidence suggests that up to 80% lobsters do not survive the first-year post-settlement stage, with majority of this being due to predation. Various species have been identified to predate on small post-puerulus lobsters from historical studies of the diets of fish species at Seven Mile Beach (Howard 1988). There is a lack of data on fine-scale variations of predator density and activity among our study locations. However, it remains plausible that the ‘low-catch’ area may be associated with a higher level of predation, and further research needs to be conducted.
Marine heat waves have been identified to affect the settlement survival and success of benthic invertebrate species. The extreme marine heatwave that occurred in the austral summer of 2010–2011, affecting ~2000 km of Western Australia’s coastline, was shown to indirectly affect the fishery (Caputi et al. 2019). Previous studies have utilised the abundance of the sublegal-sized lobster population 3–4 years after the heatwave to evaluate the impacts on the puerulus and juvenile phase of the life cycle. Studies on the population abundance in Kalbarri, further north of our study area, have indicated long-term impacts with no sign of recovery evident after 7 years (Caputi et al. 2019). However, locations assessed closer to our study locations, such as the Jurien region, have demonstrated abundance levels prior to marine heatwave conditions. Given that the low-catch zone was identified prior to the marine heat wave, and no significant changes to the population was experienced within the life cycle since, it is unlikely that the marine heatwave has contributed to the existence of the low-catch zone. It is possible that the shift in temperatures has affected other aspects of the zone ecosystem, which indirectly affects the lobster abundance there. However, more research is required to investigate this.
Conclusions
Overall, our study obtained strong, but clearly contrasting, observations on the catch rate, growth, and raw recapture rates of sublegal-size lobsters across the four study locations. The study confirmed that the ‘low-catch’ zone, within the near-shore shallow water habitat at Cliff Head, is characterised by populations with lower-than-expected catch rates of sublegal-sized lobsters, faster growth rates and higher levels of fine-scale movement patterns. This study investigated the main hypotheses on what may be contributing to these fine-scale variations in catch rates within the centre of the fishery, including low catchability, recruitment limitations, high post-recruitment movement patterns, or high post-recruitment mortality. A crucial component is distinguishing whether the lower catch rates of sublegal lobsters are associated with a lower population density, from low recruitment or high post-recruitment movement and mortality, or is simply due to a lower level of catchability. The latter being evidently difficult to disentangle for this study because of the reliance on commercial fishing methodologies. This study aimed to provide an insight into the biological and environmental factors that may be driving the catch and growth rates of P. cygnus. Furthermore, the study was designed to explore a trend that had only previously been anecdotally identified by commercial fishers within the region. Confirmation of the existence of the low catch rate within the ‘low-catch’ zone has emphasised the importance of transparent communication between researchers and relevant stakeholders when it comes to relevant management decisions. This study should be used as a starting point for further research to comprehensively understand the processes that may be driving such patterns and whether these mechanisms pose any threat to the sustainability of the fishery in future.
Data availability
Data used by this study are available here: https://globalarchive.org/geodata/data/project/get/223 (note: users will have to register with an email address before being able to download the data).
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could or be construed to have influenced the work reported in this paper.
Declaration of funding
This research was supported by the Fisheries Research and Development Corporation (Project Number 2016-260).
Author contributions
Ash Miller contributed to the writing of the original draft, formal analysis, data collection, data curation, and visualisation. Emily Lester contributed to the writing of the second draft, reviewing and editing of the draft. Simon de Lestang contributed to conceptualisation, formal analysis, supervision, reviewing and editing of the draft. Jason How contributed to the reviewing and editing of the draft. Brooke Gibbons contributed to data collection, data curation, and formal analysis. Matt Navarro contributed to the formal analysis, reviewing and editing of the draft. Tim Langlois contributed to conceptualisation, formal analysis, supervision, and reviewing and editing of the draft. Michael Brooker contributed to conceptualisation, data collection, data curation, reviewing and editing of the final draft. John Fitzhardinge contributed to conceptualisation, data collection, and reviewing and editing of the draft.
Acknowledgements
This study would not have been possible without the assistance of the following people: the fishers of the western rock lobster fishery, Brian Sloper, Terry Lissiman, Greg Cole, Ashley Cole and the staff at Dongara Marine.
References
Akaike H (1973) Information theory and an extension of the maximum likelihood principle. In ‘Proceedings of the 2nd international symposium on information theory’, 2–8 September 1971, Tsahkadsor, Armenia, USSR. (Ed. BNPA Caskie) pp. 267–281. (Akademiai Kiado: Budapest, Hungary)Bates, D, Mächler, M, Bolker, B, and Walker, S (2015). Fitting linear mixed-effects models using lme4. Journal of Statistical Software 67, 1–48.
| Fitting linear mixed-effects models using lme4.Crossref | GoogleScholarGoogle Scholar |
Bellchambers LM, How J, Evans SN, Pember MB, de Lestang S, Caputi N (2017) Resource assessment report western rock lobster environmental resources of Western Australia. Government of Western Australia, Department of Fisheries.
Brooker MA (2022) Examination of fisheries ecology and fisher perceptions of catch rate in the western rock lobster: with reference to an area of reportedly reduced catch rate. PhD thesis, University of Western Australia, Perth, WA, Australia.
Candy, SG (2004). Modelling catch and effort data using generalised linear models, the Tweedie distribution, random vessel effects and random stratum-by-year effects. CCAMLR Science 11, 59–80.
Caputi, N, Melville-Smith, R, de Lestang, S, Pearce, A, and Feng, M (2010). The effect of climate change on the western rock lobster (Panulirus cygnus) fishery of Western Australia. Canadian Journal of Fisheries and Aquatic Sciences 67, 85–96.
| The effect of climate change on the western rock lobster (Panulirus cygnus) fishery of Western Australia.Crossref | GoogleScholarGoogle Scholar |
Caputi N, Feng M, de Lestang S, Denham A, Penn J, Slawinski D, Pearce A, Weller E, How J (2014) Identifying factors affecting the low western rock lobster puerulus settlement in recent years final FRDC report, project 2009/18. Department of Fisheries, WA, Australia.
Caputi, N, Kangas, M, Chandrapavan, A, Hart, A, Feng, M, Marin, M, and de Lestang, S (2019). Factors affecting the recovery of invertebrate stocks from the 2011 Western Australian extreme marine heatwave. Frontiers in Marine Science 6, 484.
| Factors affecting the recovery of invertebrate stocks from the 2011 Western Australian extreme marine heatwave.Crossref | GoogleScholarGoogle Scholar |
Chamberlain, MA, Sun, C, Matear, RJ, Feng, M, and Phipps, SJ (2012). Downscaling the climate change for oceans around Australia. Geoscientific Model Development 5, 1177–1194.
| Downscaling the climate change for oceans around Australia.Crossref | GoogleScholarGoogle Scholar |
Chittleborough, RG (1970). Studies on recruitment in the Western Australian rock lobster Panulirus longipes cygnus George: density and natural mortality of juveniles. Marine and Freshwater Research 21, 131–148.
| Studies on recruitment in the Western Australian rock lobster Panulirus longipes cygnus George: density and natural mortality of juveniles.Crossref | GoogleScholarGoogle Scholar |
Chittleborough, RG (1974). Western rock lobsters reared to maturity. Marine and Freshwater Research 25, 221–225.
| Western rock lobsters reared to maturity.Crossref | GoogleScholarGoogle Scholar |
Chittleborough, RG (1976). Growth of juvenile Panulirus longipes cygnus George on coastal reefs compared with those reared under optimal environmental conditions. Marine and Freshwater Research 27, 279–295.
| Growth of juvenile Panulirus longipes cygnus George on coastal reefs compared with those reared under optimal environmental conditions.Crossref | GoogleScholarGoogle Scholar |
Chittleborough, RG, and Phillips, BF (1975). Fluctuations of year-class strength and recruitment in the western rock lobster Panulirus longipes (Milne-Edwards). Marine and Freshwater Research 26, 317–328.
| Fluctuations of year-class strength and recruitment in the western rock lobster Panulirus longipes (Milne-Edwards).Crossref | GoogleScholarGoogle Scholar |
de Lestang, S (2014). The orientation and migratory dynamics of the western rock lobster, Panulirus cygnus, in Western Australia. ICES Journal of Marine Science 71, 1052–1063.
| The orientation and migratory dynamics of the western rock lobster, Panulirus cygnus, in Western Australia.Crossref | GoogleScholarGoogle Scholar |
de Lestang, S, and Melville-Smith, R (2006). Interannual variation in the moult cycle and size at double breeding of mature female western rock lobster (Panulirus cygnus). ICES Journal of Marine Science 63, 1631–1639.
| Interannual variation in the moult cycle and size at double breeding of mature female western rock lobster (Panulirus cygnus).Crossref | GoogleScholarGoogle Scholar |
de Lestang, S, Caputi, N, and Melville-Smith, R (2009). Using fine-scale catch predictions to examine spatial variation in growth and catchability of Panulirus cygnus along the west coast of Australia. New Zealand Journal of Marine and Freshwater Research 43, 443–455.
| Using fine-scale catch predictions to examine spatial variation in growth and catchability of Panulirus cygnus along the west coast of Australia.Crossref | GoogleScholarGoogle Scholar |
de Lestang, S, Caputi, N, Feng, M, Denham, A, Penn, J, Slawinski, D, Pearce, A, and How, J (2015). What caused seven consecutive years of low puerulus settlement in the western rock lobster fishery of Western Australia? ICES Journal of Marine Science 72, i49–i58.
| What caused seven consecutive years of low puerulus settlement in the western rock lobster fishery of Western Australia?Crossref | GoogleScholarGoogle Scholar |
de Lestang S, Caputi N, How J (2016) Western Australian Marine Stewardship Council report series no. 9: resource assessment report: western rock lobster resource of Western Australia. Department of Fisheries, WA, Australia.
Fabens, AJ (1965). Properties and fitting of the Von Bertalanffy growth curve. Growth 29, 265–289.
Fisher, R, Wilson, SK, Sin, TM, Lee, AC, and Langlois, TJ (2018). A simple function for full-subsets multiple regression in ecology with R. Ecology and Evolution 8, 6104–6113.
| A simple function for full-subsets multiple regression in ecology with R.Crossref | GoogleScholarGoogle Scholar |
Gaughan DJ, Santoro K (Eds) (2018) Status reports of the fisheries and aquatic resources of Western Australia 2016/17: the state of the fisheries. Department of Primary Industries and Regional Development, WA, Australia.
Goosen, PC, and Cockcroft, AC (1995). Mean annual growth increments for male West Coast rock lobster Jasus lalandii, 1969–1993. South African Journal of Marine Science–Suid-Afrikaanse Tydskrif vir Seewetenskap 16, 377–386.
| Mean annual growth increments for male West Coast rock lobster Jasus lalandii, 1969–1993.Crossref | GoogleScholarGoogle Scholar |
Graham, MH (2003). Confronting multicollinearity in ecological multiple regression. Ecology 84, 2809–2815.
| Confronting multicollinearity in ecological multiple regression.Crossref | GoogleScholarGoogle Scholar |
Hastie, T, and Tibshirani, R (1986). Generalized additive models. Statistical Science 1, 297–310.
| Generalized additive models.Crossref | GoogleScholarGoogle Scholar |
Howard, RK (1988). Fish predators of the western rock lobster (Panulirus cygnus George) in a nearshore nursery habitat. Marine and Freshwater Research 39, 307–316.
Jernakoff, P, Fitzpatrick, J, Phillips, BF, and De Boer, E (1994). Density and growth in populations of juvenile western rock lobsters, Panulirus cygnus (George). Marine and Freshwater Research 45, 69–81.
| Density and growth in populations of juvenile western rock lobsters, Panulirus cygnus (George).Crossref | GoogleScholarGoogle Scholar |
Kilada, R, Sainte-Marie, B, Rochette, R, Davis, N, Vanier, C, and Campana, S (2012). Direct determination of age in shrimps, crabs, and lobsters. Canadian Journal of Fisheries and Aquatic Sciences 69, 1728–1733.
| Direct determination of age in shrimps, crabs, and lobsters.Crossref | GoogleScholarGoogle Scholar |
Lin, X, and Zhang, D (1999). Inference in generalized additive mixed models by using smoothing splines. Journal of the Royal Statistical Society – B. Statistical Methodology 61, 381–400.
| Inference in generalized additive mixed models by using smoothing splines.Crossref | GoogleScholarGoogle Scholar |
MacArthur L, Hyndes G, Babcook R (2007) Western rock lobster in ecosystem processes of south-western Australia. Department of Environment, Water, Heritage and the Arts, Perth, WA, Australia.
MacArthur, LD, Babcock, RC, and Hyndes, GA (2008). Movements of the western rock lobster (Panulirus cygnus) within shallow coastal waters using acoustic telemetry. Marine and Freshwater Research 59, 603–613.
| Movements of the western rock lobster (Panulirus cygnus) within shallow coastal waters using acoustic telemetry.Crossref | GoogleScholarGoogle Scholar |
Morgan, GR (1974). Aspects of the population dynamics of the western rock lobster, Panulirus cygnus George. II. Seasonal changes in the catchability coefficient. Marine and Freshwater Research 25, 249–259.
| Aspects of the population dynamics of the western rock lobster, Panulirus cygnus George. II. Seasonal changes in the catchability coefficient.Crossref | GoogleScholarGoogle Scholar |
Needham, AE (1946). Ecdysis and growth in Crustacea. Nature 158, 667–668.
| Ecdysis and growth in Crustacea.Crossref | GoogleScholarGoogle Scholar |
Phillips BF, Melville-Smith R, Rossbach M, Cheng YW, Caputi N, Thomson AW, Mills D, Crear B (2003) FRDC project 1998/302 – Rock lobster enhancement and aquaculture subprogram: towards establishing techniques for large scale harvesting of pueruli and obtaining a better understanding of mortality rates, fisheries research report. p. 138. Department of Fisheries, WA, Australia.
Shono, H (2008). Application of the Tweedie distribution to zero-catch data in CPUE analysis. Fisheries Research 93, 154–162.
| Application of the Tweedie distribution to zero-catch data in CPUE analysis.Crossref | GoogleScholarGoogle Scholar |
Srisurichan S (2001) Time series modelling of the environmental factors affecting the daily catch rate of western rock lobster. PhD thesis, Edith Cowan University, Perth, WA, Australia. Available at https://ro.ecu.edu.au/theses/1511
Srisurichan, S, Caputi, N, and Cross, J (2005). Impact of lunar cycle and swell on the daily catch rate of western rock lobster (Panulirus cygnus) using time series modelling. New Zealand Journal of Marine and Freshwater Research 39, 749–764.
| Impact of lunar cycle and swell on the daily catch rate of western rock lobster (Panulirus cygnus) using time series modelling.Crossref | GoogleScholarGoogle Scholar |
Tuffley, E-J, de Lestang, S, How, J, and Langlois, T (2018). Methodological comparison for sampling populations of a commercially important rock lobster species. Bulletin of Marine Science 94, 1035–1054.
| Methodological comparison for sampling populations of a commercially important rock lobster species.Crossref | GoogleScholarGoogle Scholar |
Tweedie MCK (1984) An index which distinguishes between some important exponential families. In ‘Statistics: applications and new directions. Proceedings of the Indian Statistical Institute golden jubilee international conference’, 16–19 December 1981, Calcutta, India. (Eds JK Ghosh, J Roy) pp. 579−604. (Indian Statistical Institute: Calcutta, India)
Ward, HGM, Askey, PJ, and Post, JR (2013). A mechanistic understanding of hyperstability in catch per unit effort and density-dependent catchability in a multistock recreational fishery. Canadian Journal of Fisheries and Aquatic Sciences 70, 1542–1550.
| A mechanistic understanding of hyperstability in catch per unit effort and density-dependent catchability in a multistock recreational fishery.Crossref | GoogleScholarGoogle Scholar |
Ziegler, PE, Frusher, SD, Johnson, CR, and Gardner, C (2002). Catchability of the southern rock lobster Jasus edwardsii. I. Effects of sex, season and catch history. Marine and Freshwater Research 53, 1143–1148.
| Catchability of the southern rock lobster Jasus edwardsii. I. Effects of sex, season and catch history.Crossref | GoogleScholarGoogle Scholar |
Ziegler, PE, Frusher, SD, and Johnson, CR (2003). Space–time variation in catchability of southern rock lobster Jasus edwardsii in Tasmania explained by environmental, physiological and density-dependent processes. Fisheries Research 61, 107–123.
| Space–time variation in catchability of southern rock lobster Jasus edwardsii in Tasmania explained by environmental, physiological and density-dependent processes.Crossref | GoogleScholarGoogle Scholar |


