Artificial crevice habitats to assess the biodiversity of vagile macro-cryptofauna of subtidal rocky reefs
Mateus de A. Baronio A and Daniel J. Bucher A BA School of Environmental Science and Management, Southern Cross University, Lismore, NSW 2480, Australia.
B Corresponding author. Email: daniel.bucher@scu.edu.au
Marine and Freshwater Research 59(8) 661-670 https://doi.org/10.1071/MF07170
Submitted: 25 September 2007 Accepted: 22 April 2008 Published: 22 August 2008
Abstract
Reef cryptofauna (animals inhabiting cracks and crevices) represent much of a reef’s biodiversity yet are seldom studied owing to their inaccessibility. Subtidal rocky reefs off Brunswick Heads and Byron Bay in northern New South Wales, Australia support benthic communities ranging from coral-dominated offshore reefs to kelp beds of Ecklonia radiata on inshore reefs. It was hypothesised that differential exposure to river discharge and the East Australian Current, as well as proximity to other reef habitats, may produce differences in recruitment and persistence of cryptofauna between superficially similar reefs within a small geographical range. Artificial crevice habitats were deployed at similar depths on three inshore reefs supporting similar Ecklonia densities. Although the species richness of crevice fauna was similar at all reefs, the species composition differed significantly along with the assemblages recruited in different seasons and to different crevice sizes. Neither reef faunas nor that of varying crevice sizes changed consistently with the seasons, yet all crevices appeared equally accessible to colonists. These results demonstrate the potential inadequacy of classifying reef communities for management of regional biodiversity based on the visual dominance of a few species that may not be as sensitive to environmental variables as many of the less obvious taxa.
Additional keywords: crevice fauna, cryptofauna, multi-plate artificial habitats.
Introduction
One of the principal goals of marine protected areas (MPAs) is to conserve biodiversity and provide refuges from human exploitation of the marine environment (Gladstone 2007). The first aim is most efficiently achieved by ensuring that any system of MPAs incorporates representative examples of as wide a range of habitats as possible (Kelleher 1999; Banks and Skilleter 2002). However, planners of MPAs rarely have access to comprehensive assessments of community structure at a wide range of sites within an intended reserve. In place of detailed surveys, broad habitat classifications are generally used as surrogates for finer-scale biodiversity – the assumption is that all areas within each category are similar enough that inclusion of a proportion of such areas in a park is sufficient to ‘capture’ the range of organisms present in areas of the same habitat category (Stevens and Connolly 2004; Newton et al. 2007). This is often done without validation that the chosen categories are relatively homogeneous in the assemblages they support and that the areas chosen for protection are, in fact, representative of the broader habitat type (O’Hara 2001).
Rocky and coral reefs present particular difficulties for comprehensive surveys of biodiversity because of the wealth of organisms that cannot be easily sampled as they occur in deep cracks and crevices of varying size within the reef matrix (Hutchings and Weate 1977; Peyrot-Clausade 1980) and within other living organisms such as sponges, corals and kelp (Uebelacker 1977; Hutchings 1983; Smith 1996). For this reason, various ‘rapid assessment’ techniques have been developed such as video transects and timed searches targeting specific groups of easily-sampled taxa such as fish, large echinoderms and molluscs (e.g. Gladstone 2002; Edwards and Smith 2005; Smith 2005). However, such methods do not sample perhaps the largest component of reef biodiversity – the cryptofauna or crevice fauna (Reaka-Kudla 1997).
Cryptofauna are the macro-invertebrates and some fishes that use cavities in substrata either temporarily or permanently. Some are bioeroders of reefs, creating their own cavities, whereas others are opportunistic colonisers of existing spaces (Hutchings 1983). The cryptofauna are an important part of the reef food web. Cryptofaunal organisms are an important food source for certain reef carnivores, including fishes (Vivien and Peyrot-Clausade 1974), gastropod molluscs (Kohn and Nybakken 1975) and octopuses (Ambrose 1986).
One method for obtaining quantitative comparative data on cryptofauna is to deploy artificial habitats that approximate the essential features of the natural habitat but are accessible to researchers and provide for standardised sampling effort, enabling direct comparisons between different sites and studies (Britton and Greeson 1987). Owing to the difficulty of securing large structures onto exposed marine reefs and the difficulty in recovering heavy structures, most studies of marine cryptofauna that have used artificial substrata have focussed on meiofaunal and smaller macrofaunal sized assemblages. The most commonly used substratum for such studies has been nylon mesh ‘pot scourer’ pads (Myers and Southgate 1980; Pugh 1996; Atilla and Fleeger 2000; Smith and Rule 2002; Rule and Smith 2005, 2007). However, such substrata do not approximate the conditions presented by large cracks and crevices in rock that provide habitat to larger molluscs, crustaceans, polychaetes, echinoderms and small fishes.
Multi-plate samplers (Hester and Dendy 1962) have been used in the study of freshwater and estuarine invertebrates. For this type of work, the crevices between plates range from 0.3 to 1.3 cm and plate areas from 57 to 121 cm2 (Fullner 1971; Slack et al. 1988; Atilla and Fleeger 2000). Many marine reef organisms typically occupy much larger crevices. Larger (38 × 38 cm) multi-plate crevice samplers have been used to routinely monitor settlement of juvenile eastern rock lobsters in New Zealand and south-east Australia (Booth and Tarring 1986; Gardner et al. 2001), but no studies using this method to examine whole assemblages of crevice fauna have been published to our knowledge.
Cape Byron Marine Park (CBMP) includes in no-take management zones several small reefs that are visually dominated by the kelp Ecklonia radiata (C. Agardh) J. Agardh. Do these reefs provide an adequate representation of reef biodiversity of the region? We hypothesised that differential exposure to river discharge and eddies of the East Australian Current, as well as proximity to other reef habitats, will produce differences among cryptic macro-invertebrate assemblages between reefs despite their superficially similar sessile benthic cover and that reefs outside the park may therefore support unique assemblages not found inside the park. Large, multi-plate artificial habitats provide a non-destructive means of quantitatively investigating potential differences in recruitment and persistence of cryptic reef assemblages in no-take marine parks.
Materials and methods
Study location
Covering an area of ~22 700 ha off the coast of northern New South Wales, Cape Byron Marine Park includes Cape Byron, the most easterly point of the Australian mainland (Fig. 1). The Park extends for ~37 km from Brunswick Heads in the north to Lennox Head in the south, and from the mean high water mark to 3 nautical miles (5.6 km) offshore. It includes the estuary of the Brunswick River, and several smaller estuaries. The addition of freshwater, sediments and nutrients from the Brunswick River can affect the coastal water quality (Ferguson et al. 2004) and consequently influence reef communities in the northern part of the park. Furthermore, the water from the river is potentially enriched with larvae derived from mangrove and seagrass habitats. The East Australian Current (EAC) is another important influence on the area. Flowing between 8°S and 27°S, it brings warm waters from tropical Australia in a rapid (up to 10 km h–1) narrow stream down the east Australian coastline (Godfrey et al. 1980). Variations in strength and position of the EAC can produce rapid and large fluctuations in temperature, turbidity and other factors along the east coast of Australia.
The climate in northern NSW is subtropical. Water temperatures range from an average maximum of 18–19°C in September to 25–27°C in February–March (Baronio, unpubl. data). The three reefs used for this study are all within 1 km of the coast and range in depth from 10 to 15 m (Fig. 1). All reefs have been surveyed by side-scan sonar and broadly characterised according to depth, distance offshore and the dominant benthic cover from remote video imagery as ‘inshore Ecklonia reefs’ (Bickers 2004). More detailed surveys by Bucher and Hartley (2004) using diver-held video concluded that the sessile epibenthic assemblages were broadly similar at these three reefs although Wilsons and Bait Reefs were more similar to one another than they were to Brunswick Reef.
Wilsons Reef (28°37′24.92″S, 153°36′37.97″E) and Bait Reef (28°37′41.17″S, 153°36′57.95″E) are both small patch reefs located within the Cape Byron Marine Park, off Byron Bay (Fig. 1). Wilsons Reef is 12 m deep and Bait Reef is the shallowest of the three study sites at 10 m.
The reefs off Brunswick Heads are to the north of Cape Byron Marine Park (28°30′50.39″S, 153°33′39.77″E). Bucher and Hartley (2004) identified the Brunswick Reefs as three complexes, which they labelled as ‘Brunswick Deep’, ‘Brunswick Intermediate’ and ‘Brunswick Shallow’. The area used for this study was part of the ‘Brunswick Intermediate’ reef, colloquially referred to as ‘Locals Reef’ by fishermen. The Brunswick Reef complex stretches for over 5 km parallel to the shore and ~1 km offshore. Depths over the reefs range from 11 to 15 m. The study site was at the southern end of the reef in a depth of 13 m.
Multi-plate artificial habitats
The multi-plate artificial habitats used in this study were designed to target larger animals, be easily deployed and recovered by divers and to withstand the strong wave energy environment of the open coast reef. The plates were constructed of 25 × 25 cm squares of 6-mm thick fibre-cement with a hole drilled through the centre. Ten plates separated by spacers cut from 35-mm polyvinyl chloride (PVC) pressure pipe were used in each habitat, providing nine artificial crevices for recruitment of cryptofauna. The crevices were of three sizes (2.0, 1.0 and 0.5 cm) with three replicates of each size per habitat structure. The sizes were chosen as typical of natural crevices commonly found on these reefs and all three sizes were represented in each habitat to better approximate the intermingled sizes of natural crevices. The three sizes were arranged in three groups (‘top’, ‘middle’ and ‘bottom’) such that no two adjacent crevices were of the same size. Unlike most other multi-plate designs described in the literature, only two opposing sides of each crevice were left open, the two remaining sides were blocked by plastic barriers glued to the plates with silicone. This approximates more closely the sheltered conditions in natural crevices and reduced potential hydrodynamic differences between habitats in different positions on the rack. The structure was placed onto a 30-cm length of PVC conduit (16-mm diameter). To further prevent the plates from flexing, a cable tie was placed around each habitat.
Two triangular steel frames with 1.6-m sides were attached to each reef to act as a supporting rack for the habitats (Fig. 2). The racks were levelled using threaded rods as adjustable supports and attached to the reef using mountaineering pitons and chains tensioned by turn-buckles. Four vertical 12-mm threaded rods were welded to each rack. One multi-plate habitat was fitted over each rod and secured with wing nuts and cable ties. The distance between adjacent habitats was ~20 cm. Black polycarbonate 1-cm square mesh was added to each bar to provide easier access for vagile organisms from the reef to the habitats.
Habitats were retrieved by first clamping a padded plate across both open ends to retain all animals within the crevices. The habitat was then removed from the rack and as soon as possible transferred to a tub of 10% formaldehyde in seawater. After at least 24 h of fixation, the habitats were disassembled and as each crevice was exposed the animals were removed with forceps or a fine brush and stored in 70% ethanol for later sorting, identification and counting. Voucher specimens have been deposited in the museum collection of the School of Environmental Science and Management, Southern Cross University.
Sampling design and analysis
The sampling design is shown in Fig. 3. At each reef there was four 3-month deployment periods approximately corresponding to the four seasons, commencing with summer of 2005/6. The total sampling period was 58 weeks from 24 October 2005 to 28 November 2006. At the end of each season, two multi-plate habitats were removed from each reef and replaced. The remaining habitats were left to examine the effects of longer deployment times, but damage and loss of habitat during storms limited the amount of usable data obtained and the results of multi-season deployments are not reported here. Although the study focussed on vagile or unattached animals, some bivalves that attached by byssal threads and polychaetes that constructed temporary tubes were also included in the counts. Specifically excluded were permanently-cemented sessile organisms such as barnacles, serpulid worms, oysters and clonal or encrusting forms such as sponges, ascideans and bryozoans.
Differences in species composition between treatments were tested by permutational analysis of variance using PERMANOVA (Anderson 2005) on fourth-root transformed abundance data using a Bray–Curtis similarity matrix. The four factors tested in the PERMANOVA were ‘reefs’ (a fixed, orthogonal factor with three levels: Bait, Wilsons and Brunswick Reefs), ‘season’ (a fixed, orthogonal factor with three levels – owing to lack of data from Bait Reef following summer storms we present an analysis for the subsequent three seasons only), ‘crevice size’ (fixed, orthogonal with three levels: 0.5-, 1.0- and 2.0-cm crevices) and ‘position’ (fixed, orthogonal with three levels: lower third, middle third and top third of the habitats). The number of permutations used was 999 and the integer used as a random seed was 5. Where PERMANOVA demonstrated significant differences between levels within a factor, the levels were further contrasted in a pairwise a posteriori comparison. The relationships between significantly different levels were represented in three-dimensional non-metric multi-dimensional scaling (MDS) plots using PRIMER ver. 6 (PRIMER-E, www.primer-e.com). The species that contribute most to dissimilarities between significantly different treatment levels in PERMANOVA were identified using the SIMPER (percentage similarity) routine in PRIMER.
Results
Over 58 weeks, the multi-plate habitats recruited 6000 cryptofaunal specimens from 120 taxa. Owing to storms in early March, 24 habitats were lost including all habitats at Bait Reef. Several further losses occurred in most seasons. This prevented greater replication of quantitative comparisons over longer deployment times.
During the entire study period, the number of species recruited at each reef was remarkably similar. Seventy-four species were recruited at Brunswick Reef at an average of 5.9 ± 2.8 (s.e.) per 0.5-cm crevice, 5.4 ± 3.5 (s.e.) per 1-cm crevice and 5.9 ± 2.9 (s.e.) per 2-cm crevice. Sixty-three species were recorded in only three seasons at Bait Reef at an average of 5.9 ± 2.6 (s.e.) species per 0.5-cm crevice, 5.5 ± 2.3 (s.e.) per 1-cm crevice and 6.0 ± 3.6 (s.e.) per 2-cm crevice and 72 species occurred at Wilsons Reef (5.0 ± 1.7 (s.e.) species per 0.5-cm crevice, 5.0 ± 2.5 (s.e.) per 1-cm crevice and 5.5 ± 2.8 (s.e.) per 2-cm crevice). The most abundant taxa were a species of gammaridean amphipod (especially in spring), the bivalve molluscs Anomia trigonopsis and Hiatella australis, a species of isopod and a snapping shrimp (family Alpheidae). Five taxa were ubiquitous; the bivalves Anomia trigonopsis, Hiatella australis and Marikellia solida, alpheid shrimp and hermit crabs were recorded during all four seasons and at all reefs.
Despite similar species richness there was also a remarkably similar, and high, level of uniqueness in each reef’s assemblage. Only 34 out of 120 taxa occurred at all three reefs. Twenty-five taxa (34% of the total number of taxa for the location) were found only at Brunswick Reef, twenty-three species (32% of all taxa at the site) were found only at Wilsons Reef and sixteen species (25% of all taxa at the site) were found only at Bait Reef. In addition, Bait and Wilsons Reefs shared seven species that were not present at Brunswick Reef. Bait and Brunswick Reefs shared six species that were not present at Wilsons Reef. Brunswick and Wilsons Reefs shared nine species that were not present at Bait Reef.
Although the following analyses include only three seasons, all analyses were also repeated for the two reefs (Brunswick and Wilsons) over all four seasons and the conclusions were similar.
The PERMANOVA analysis (Table 1) showed a significant difference in community structure between the three reefs. Pairwise comparisons (Table 2) showed that each reef was significantly different to the other two. There was also a significant effect of season, with pairwise comparisons (Table 3) showing that all three seasons were different to one another (reanalysis using all four seasons at the two reefs for which there were data showed that summer was also significantly different to the other three seasons: P = 0.0231). A significant reef × season interaction showed that the relationships between the reefs were not always consistent (Table 2). Pairwise comparisons between reefs within each season showed that Bait and Wilsons Reefs were significantly different from each other in all seasons except spring.

|
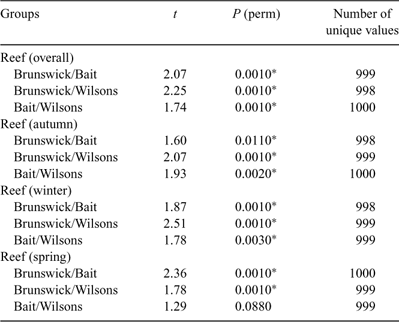
|
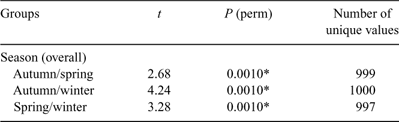
|
There was a significant effect of crevice size, with all three being significantly different to each other overall. However a significant season × crevice size interaction was also evident and pairwise comparisons of crevice sizes within each season (Table 4) showed that the relationships between crevices changed with the seasons. In spring, for example, none of the assemblages in the three crevice sizes were significantly different from each other.
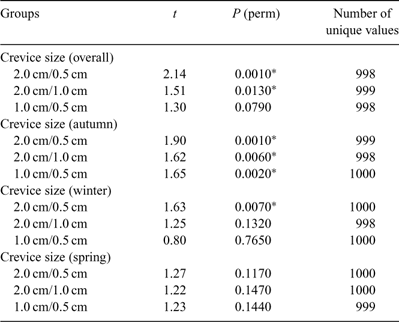
|
The MDS ordination plots comparing assemblages in each crevice size at each reef are shown in Fig. 4. Despite the significant differences in species assemblages, there is considerable overlap of the distribution of samples from all reefs for all crevice sizes. SIMPER analysis (Table 5) shows that the species that contribute most to dissimilarity between all combinations of reefs were the gammaridean amphipod and the bivalve Anomia trigonopsis. The MDS ordination plots that contrast samples from different seasons (Fig. 5) show some segregation of samples from the different seasons, although considerable overlap is also evident for all crevice sizes. SIMPER analysis (Table 5) shows that the bivalve A. trigonopsis contributed substantially to dissimilarity between all pairwise comparisons of seasons except between winter and spring. The gammaridean amphipod was most abundant in spring and dominated dissimilarity measures for all comparisons with that season. The MDS ordination plot that contrasts the three different crevice sizes across all reefs and seasons is shown in Fig. 6. The greatest difference was between the 0.5- and 2.0-cm crevices.
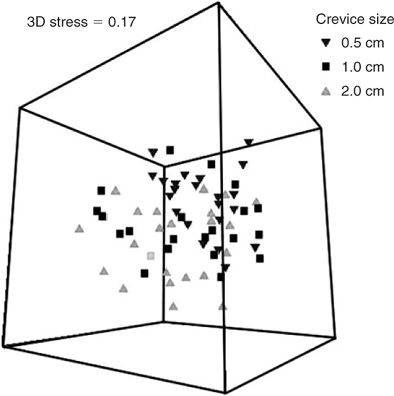
|
SIMPER analysis (Table 5) demonstrated that the gammaridean amphipod contributed most to dissimilarity in all pairwise comparisons of crevice size. A. trigonopsis was also an important contributor to dissimilarity in all pairwise comparisons.
Each multi-plate artificial habitat had three replicates of each crevice size arranged such that there was a crevice of each size in the lower, medium and higher part of each habitat. There was no significant effect of position within each habitat.
Discussion
The usefulness and limitations of artificial crevice habitats in the study of reef cryptofauna
The difficulties of sampling cryptofauna of rocky subtidal reefs have led to this group of animals being relatively unstudied. The results of this study show that provision of multi-plate artificial crevice habitats is an effective way of sampling a wide variety of cryptic macro-invertebrates in such habitats including molluscs (Gastropoda, Polyplacophora and Bivalvia), arthropods (Crustacea and Pycnogonida), polychaetes and echinoderms (Asteroidea, Ophiuroidea and Echinoidea). Despite several studies using multi-plate samplers in fresh water (e.g. Slack et al. 1988), there have been few published studies that have applied this technique to marine environments. Most studies of reef cryptofauna have focussed on meiofauna or small macrofauna that inhabit small spaces within coral rock (Hutchings and Weate 1977) or filamentous algae on hard surfaces in estuarine waters (Atilla and Fleeger 2000) or animals that inhabit the spaces within kelp holdfasts (Smith 1996; Smith et al. 1996; Coleman et al. 2007) or coralline algal turf (Kelaher 2002). The present study is the first to use gaps between large plates to investigate the assemblages of macro-invertebrates that may be expected to inhabit deep cracks and crevices in natural rocky reefs.
Multi-plate samplers can be designed to exclude adult colonisation by immigration, allowing only larval access to the crevices, but if immigration is to be allowed, as in the present study, the samplers must be positioned to maximise accessibility. For the present study, the samplers were placed as close to the reef surface as possible and plastic mesh was secured between the plates and the reef to provide covered access to the structure. Nevertheless, some bias is unavoidable against animals that will not move across open surfaces or climb structures to enter new crevices. However, the lack of a significant difference between the assemblages at different heights suggests that animals were equally able to colonise the higher crevices once they had gained initial access to the habitat structure.
The present study was limited to investigating assemblages that had developed in artificial crevices over approximately 3-month intervals owing to losses of most long-term samples in storm events. The assemblages sampled therefore probably represent mostly opportunistic colonisers of new crevices rather than mature assemblages. Many of the taxa collected were juveniles that may not persist over time or may move to larger crevices as they grow. Some limited data from samples left for 6 months over the winter–spring period suggest that significantly greater diversity was achieved in all three crevice sizes over the longer deployment time and several taxa were only found after longer deployments. Although many natural crevices, especially larger ones, are relatively permanent structures (in relation to invertebrate life spans), others can be more ephemeral. Storms may expose crevices that have previously been buried; large sessile animals may completely fill crevices but the crevice is re-opened when the animal eventually dies. Assemblages such as were recruited to the crevices in this study are therefore likely to be well represented in at least some natural crevices. Further refinement of the attachment system during the latter part of this study has produced a more robust structure that can now be used to further investigate changes in crevice fauna over time.
Effects of crevice size
Natural crevices vary in size, and as many cryptofauna possess shells or inflexible exoskeletons it is clear that reduced crevice size will limit the range of organisms that can inhabit them. However, larger crevices do not necessarily support more diverse assemblages, as they also allow access to a greater range of predators. Large crabs, fish and an octopus were recorded inside the 2-cm crevices during this study. Polychaetes and small gastropods were more common in the smallest crevice size. The mixing of crevice sizes in each habitat in the study was designed to include the modifying effects of biological interactions between adjacent crevices as would occur in natural reefs. If crevice size is to be eliminated as a factor in future studies, we recommend the tapered crevices used for monitoring of lobster recruitment (Booth and Tarring 1986; Gardner et al. 2001). In the present study, we limited our investigation to horizontal crevices, but further studies should examine the effects of crevice orientation on recruitment.
Seasonal differences
Strong seasonal differences were also observed, as might be expected in short-term samples influenced by seasonality of planktonic recruitment (Grantham et al. 2003). Although there is a definite seasonal cycle of water temperatures in this region, temperature loggers (‘Hobo’ pendant style) installed on the samplers and recording temperature every 1 h, regularly recorded fluctuations of up to 5°C in a 6-hourly cycle, especially during periods of spring tides, indicating vertical stratification of the water. Often, similar sudden changes occurred at slightly different times at each reef, indicating exposure to moving large water masses. In one 2-week period in summer, temperatures at all reefs ranged between 18 and 29°C. Short-term temperature variability in this region can therefore be as great as the annual range. Against this variable background, Brunswick Reef was, nevertheless, significantly cooler at a given time on average than the other two reefs by ~0.5°C (Baronio, unpubl. data). Warmer water temperatures were also often correlated with lower turbidity (some of the temperature loggers also had a light sensor), characteristic of water in the East Australian Current. Cooler, turbid waters may have come from shallow coastal areas or from the Brunswick River estuary. Each water body would presumably contain a different planktonic larval assemblage and differences in exposure to these bodies may explain much of the variation in cryptofauna between these reefs.
Differences between reefs
Cryptofauna appear to vary much more between reefs than might be predicted from comparisons of their physical characteristics and the algae and sessile invertebrates inhabiting the open rock surfaces (Bucher and Hartley 2004). All the reefs in this study have similar geology and are less than 2 km from the coast in an average depth of 10–15 m. Another important characteristic is the presence of kelp Ecklonia radiata. However, the cryptofauna at Brunswick Reef was significantly different to that of Bait and Wilsons Reefs in all seasons. A significant difference has also been noted between the fish faunas of these same reefs (Perera 2005; Schultz 2007).
Brunswick Reef is a much larger area of reef than the other two reefs studied. Classic island biogeographical theory (Macarthur and Wilson 1963) would suggest that such a large area would provide a more representative example of the community typical of such habitats. However, in the marine environment, where the majority of organisms recruit from planktonic larvae, small patches of a particular habitat are not necessarily demographically isolated from other patches and may support equally diverse and representative communities as larger patches (Harriott et al. 1999; Banks and Skilleter 2002). The cryptofaunas of Bait and Wilsons Reefs were remarkably similar in species richness to the much larger Brunswick Reef. However, multivariate analyses demonstrated that the species composition of the cryptofauna is significantly different between all reefs, but more so between Brunswick and the other two.
There is a large proportion of the cryptofauna sampled at each reef that is not represented at the other reefs. Some of these taxa are of low abundance and their presence or absence may simply be a matter of chance. Of particular significance are taxa that are abundant at one site but absent or rare at others. These differences are more likely to represent real differences in the cryptofaunal communities of different reefs. For example the gastropod family Costellaridae and grapsid crabs were abundant at Brunswick but rare or absent at the other reefs. The gastropod families Triphoridae and Nassaridae and the bivalve Psammobidae were abundant at Bait and Wilsons Reef but absent or rare at Brunswick. In addition to differences in exposure to estuarine water masses and warm ocean currents, proximity to other reef habitats may also contribute to differences in availability of larvae to each reef. Bucher and Hartley (2004) identified a very different shallow reef community near the mouth of the Brunswick River, and a deeper (23–30 m) reef tract with reduced kelp cover, both within 1 km of the Brunswick Reef site used for this study. Bait and Wilsons Reef are closer to the Ecklonia-free reefs at the eastern end of Byron Bay and at Julian Rocks (Fig. 1).
Conclusion
Despite superficially similar appearances, the Brunswick Reef supports a community that is significantly different to the inshore Ecklonia reefs of Byron Bay and many of the elements of this community are not therefore adequately represented in protected zones of the Cape Byron Marine Park. Any future revision of the boundaries of the Cape Byron Marine Park should give consideration to inclusion of at least a part of the Brunswick Reef complex. Cryptofaunal samplers are a valuable tool for the assessment of rocky or coral reef biodiversity because this diverse assemblage is likely to represent the majority of mobile invertebrates in the reef community and appears to show greater between-reef variation than the epibenthic groups that are more often used to determine representativeness for conservation purposes.
Acknowledgements
This work was made possible by internal research funding from Southern Cross University. Although not specifically funded by the New South Wales Marine Parks Authority, this work was able to make use of sampling opportunities associated with MPA-funded projects. We thank the four anonymous reviewers and the editor for their valuable comments that have greatly improved this manuscript and particularly thank Assoc. Prof. Steve Smith of the University of New England for his assistance with applying PERMANOVA to this study.
Atilla, N. , and Fleeger, J. W. (2000). Meiofaunal colonisation of artificial substrates in an estuarine embayment. Marine Ecology 21, 69–83.
| Crossref | GoogleScholarGoogle Scholar |
Booth, J. D. , and Tarring, S. C. (1986). Settlement of the red rock lobster Jasus edwardsii, near Gisbourne, New Zealand. New Zealand Journal of Marine and Freshwater Research 20, 291–297.
Coleman, M. A. , Vytopil, E. , Goodsell, P. J. , Gillanders, B. M. , and Connell, S. D. (2007). Diversity and depth-related patterns of mobile invertebrates associated with kelp forests. Marine and Freshwater Research 58, 589–595.
| Crossref | GoogleScholarGoogle Scholar |
Hutchings, P. A. , and Weate, P. D. (1977). Distribution and abundance of cryptofauna from Lizard Island, Great Barrier Reef. Marine Research in Indonesia 17, 99–112.
Kohn, A. , and Nybakken, J. (1975). Ecology of Conus on eastern Indian Ocean fringing reefs: diversity of species and resource utilization. Marine Biology (Berlin) 29, 211–234.
| Crossref | GoogleScholarGoogle Scholar |
Peyrot-Clausade, M. (1980). Motile cryptofauna of Tulèar reef flats. Marine Biology 59, 43–47.
| Crossref | GoogleScholarGoogle Scholar |
Rule, M. J. , and Smith, S. D. A. (2005). Spatial variation in the recruitment of benthic assemblages to artificial substrata. Marine Ecology Progress Series 290, 67–78.
| Crossref | GoogleScholarGoogle Scholar |
Slack, K. V. , Ferreira, R. F. , Averett, R. C. , and Kennelly, S. S. (1988). Effects of spatial orientation of multiple plate artificial substrates on invertebrate colonization. Journal of the American Water Resources Association 24, 781–789.
| Crossref | GoogleScholarGoogle Scholar |








