Aseasonal and short life cycles of the protandrous hermaphrodite blue threadfin (Eleutheronema tetradactylum) in a near-equatorial tropical region
Yoshimi Ogino A , Wirot Kongasa B , Keisuke Furumitsu A , Gen Kume C and Atsuko Yamaguchi A *
A *
A Faculty of Fisheries, Nagasaki University, 1-14 Bunkyo, Nagasaki 852-8521, Japan.
B Songkhla Marine Fisheries Research and Development Center, 79/1 Wichianchom Road, Bor-Yang Sub-district, Muang Songkhla District, Songkhla 90000, Thailand.
C Faculty of Fisheries, Kagoshima University, 4-50-20 Shimoarata, Kagoshima 890-0056, Japan.
Marine and Freshwater Research 74(6) 562-572 https://doi.org/10.1071/MF22240
Submitted: 22 October 2022 Accepted: 27 February 2023 Published: 24 March 2023
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution 4.0 International License (CC BY)
Abstract
Context: Eleutheronema tetradactylum is exploited throughout its distribution in the Indo–West Pacific region. However, there is a lack of data on its life cycle in near-equatorial regions and the northern hemisphere.
Aim: To investigate the age, growth, sex change and reproduction of Eleutheronema tetradactylum in southern Thailand.
Methods: Analysis of length, otolith and gonad data of 449 fish.
Key results: Contrary to findings from regions at higher latitudes, otoliths did not show seasonal increment formation. Instead, with the exception of large individuals, daily increments were distinguishable. Within the age range of 69–341 days, growth was linear, with results indicating a total length of 430 mm (69% of maximum length) at 1 year. The youngest mature male was 137 days old. Moreover, the length at which half the individuals changed from male to female was 376 mm, corresponding to 282 days. The gonadosomatic index indicated no specific spawning season, and the hatch-date calculations indicated year-round hatching.
Conclusions: Eleutheronema tetradactylum inhabiting near-equatorial regions is characterised by rapid population turnover compared with populations of higher latitudes.
Implications: Our findings indicated life-history variability, including seasonality loss in near-equatorial habitats, of an important coastal fish. This information is important for species conservation.
Keywords: age, annulus formation, growth, life history, otolith macrostructure, otolith microstructure, reproduction, sex change.
Introduction
The blue threadfin (Eleutheronema tetradactylum) belongs to the family Polynemidae (order Perciformes) and is widely distributed in tropical and subtropical waters of the Indian and western Pacific Oceans (Motomura 2004). This species is fished in many countries because it has excellent meat quality and occurs in shallow coastal and brackish waters (Motomura 2004). However, previous studies on its life history, considered as a basis for fisheries management (e.g. age and growth), were all conducted in Australia (Garrett 1997; Pember et al. 2005; Ballagh et al. 2012), which lies at the southern end of the distribution.
Eleutheronema tetradactylum has been confirmed to be a protandrous hermaphrodite by the presence of males in the smaller size groups and females only in the large size groups, along with observation of degenerating testicular tissue and developing ovarian tissue in transitional gonads (Pember et al. 2005; Shihab et al. 2017). In northern Western Australia, the vast majority of individuals reach maturity at the end of their first year of life and change sex to female by the end of their third year of life (Pember et al. 2005). There are fewer protandrous (male-to-female sex change) than protogynous (female-to-male sex change) species (Kuwamura et al. 2020), and studies on their life history are limited. Protandrous species, having a higher proportion of females in larger individuals, are likely to be vulnerable to size-selective fishing such as gill-net fishing because harvesting of large individuals will result in the selective removal of females, thereby considerably reducing egg production in the population (Moore et al. 2017). Therefore, the management of hermaphroditic fish species must include sex-change schedules, which requires information about not only the age and growth of individuals but also length and age at sex change (Provost and Jensen 2015).
The spatial population subdivision of E. tetradactylum has been extensively studied in Australia (Garrett 1997; Zischke et al. 2009; Horne et al. 2011, 2013; Moore et al. 2011; Newman et al. 2011; Ballagh et al. 2012). These studies have demonstrated a high degree of variation in life-history characteristics (more than two-fold variability in both length at sex change and asymptotic length) among subpopulations (Ballagh et al. 2012). This finding implies that the results of previous studies conducted in Australia cannot be extrapolated to predict the life-history characteristics of populations in other regions. In particular, it is unclear whether the life-history characteristics of this species in near-equatorial regions, where life-history studies on coastal fish are rarely conducted, are similar or different from those studied in higher-latitude regions. Some studies on other fish species have reported differences in reproductive characteristics between low- and high-latitude habitats (e.g. Gray et al. 2012; Wakefield et al. 2015; Coulson and Poad 2021). Hence, there is a need to investigate the life history, including reproductive season, of E. tetradactylum beyond Australia, especially in near-equatorial tropical habitats.
Eleutheronema tetradactylum is listed as Endangered under the International Union for Conservation of Nature (IUCN) Red List, with a population trend towards a rapid decrease in individuals (Motomura et al. 2015). In Thailand, gill-net fisheries have targeted this species (Kongasa and Ruangpatikorn 2020), resulting in growing concerns about overfishing. Thus, there is an urgent need to study the life history of E. tetradactylum in this region. In this context, our study aims to obtain baseline information for the fisheries management of E. tetradactylum populations in near-equatorial environments by analysing age, growth, sex change and reproductive data of the species in Thailand.
Materials and methods
Sample collection and measurement
In southern Thailand, fishers use gill nets with a mesh size of 6.25–7.50 cm in coastal areas at a depth of 2.5–16.0 m to catch E. tetradactylum (Kongasa and Ruangpatikorn 2020). Between June 2015 and May 2016, we randomly collected ~30–40 specimens of this species once a month from some catches of a threadfin gill-net fishery in Songkhla and Nakhon Si Thammarat in the Gulf of Thailand (Fig. 1). Coastal water temperatures at Khanom Beach (9°11′N, 99°52′E), located near the sampling area, were obtained from the sea-temperature database (satellite data available at http://seatemperature.info/khanom-beach-water-temperature.html). During the sampling period (June 2015–May 2016), the annual average sea-surface temperature of Khanom Beach was 29.7°C, with a minimum monthly average of 28.5°C in February and a maximum monthly average of 31.4°C in May (Supplementary Fig. S1). In the laboratory, the total length (TL) of fish samples was recorded to the nearest millimetre, in addition to bodyweight (BW) and gonad weight (GW), both being recorded to the nearest gram. Individuals were classified as female, male, or undifferentiated on the basis of macroscopic examination of the gonads. Length–weight relationships were determined by fitting the allometric model below:
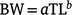
Parameters a and b were estimated using non-linear least-squares (NLS) regression analysis. All model fittings were performed with NLS or linear least-squares regression analyses by using KyPlot (ver. 6.0, Kyenslab, Tokyo, Japan; Yoshioka 2002). To compare the length–weight relationships between females and males, analysis of covariance (ANCOVA) was performed on the log-transformed datasets by using BellCurve (ver. 3.21, Social Survey Research Information, Tokyo, Japan) for MS Excel (ver. 1808, Microsoft, Redmond, WA, USA). To compare the length–weight relationship with results from previous studies based on fork length (FL) rather than TL, we converted FL–BW relationships to TL–BW relationships by using a FL–TL equation (Garrett 1997). Similarly, other FL values from previous studies were also converted to TL values to enable comparisons with our results.
Ethical review and approval was not required for this animal study in accordance with institutional requirements of Institute of Animals for Scientific Purposes Development of Thailand because we bought fish samples that had been taken in commercial catches.
Sex-change size and reproduction
To determine the size at sex change, a logistic function was fitted to the proportion of females in each 25-mm length class, following Ballagh et al. (2012). The logistic function was calculated as follows:
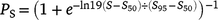
where PS is the proportion of females in each Length class S, and S50 and S95 are the lengths at which 50 and 95% of the population are females respectively. To identify reproductive traits and obtain monthly trends, the gonadosomatic index (GSI) was calculated as follows:
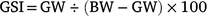
In addition, for individuals collected between January and May 2016 (females: n = 88, males: n = 97), the gonad development stages were determined macroscopically on the basis of Kesteven (1960; Supplementary Table S1).
Otolith macrostructure
Sagittal otoliths were removed from the fish, cleaned and stored dry. Whole sagittal otoliths were immersed in mineral oil and observed under reflected light against a black background by using a stereoscopic microscope (SMZ1000; Nikon, Tokyo, Japan; magnification 8×) and photographed using a digital camera (DS-Fi1; Nikon) mounted on the microscope. To determine the relationship between fish size and otolith size, the otolith radius (OR) was measured from the core to the anterior edge of the otolith (Fig. 2), and the allometric model was fitted to the TL–OR data according to Ballagh et al. (2012). In estimating the age of E. tetradactylum, Pember et al. (2005) and Ballagh et al. (2012) counted the opaque zones observed in the posterior field of the otoliths. Although in the present study, otolith zonation patterns were not evident in the posterior field, translucent zones were detected in the anterior field of the otoliths (Fig. 2) and, hence, were considered in subsequent analyses. For 63 of the sampled specimens, the anterior fields of both the left and right otoliths were broken during storage. Thus, the remaining 386 specimens were used for otolith analysis. The number of translucent zones were counted three times by the same person, without prior knowledge of fish length or previous counts. To investigate the precision of the zone readings, the coefficient of variation (CV) of the three counts was calculated for each specimen and averaged across all specimens (Chang 1982).
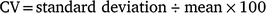
For 210 otoliths for which the counts differed across the three readings, the specimen was deemed illegible and excluded from subsequent analyses. To determine the periodicity of translucent-zone formation, we analysed the edge and marginal increment ratio (MIR). Edge analysis was based on the method described by Yamaguchi et al. (2004) to examine monthly changes in the percentage occurrence of otoliths with translucent margins. In addition, we used MIR analysis, based on the method described by Kume et al. (2010), to examine monthly trends in the marginal increments of otoliths. The MIR was calculated separately for one translucent-zone group and more than one translucent-zone group, as follows.
One translucent zone group:
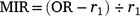
More than one translucent zone group:
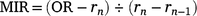
where r1 is the radius of the first translucent zone, rn is the radius of the last translucent zone, and rn−1 is the radius of the second to the last translucent zone. The radius of each translucent zone was measured from the core to the outer edge of the translucent zone along the OR (Fig. 2). Although Garrett (1997) suggested that otoliths of E. tetradactylum be sectioned prior to reading, Pember et al. (2005) found that sectioning did not improve the accuracy of age estimation in this species. In this study, to determine whether otolith sectioning was useful, otoliths of 72 specimens were sectioned transversely following the method described by Ogino et al. (2020) and investigated under a stereoscopic microscope.
Age and growth
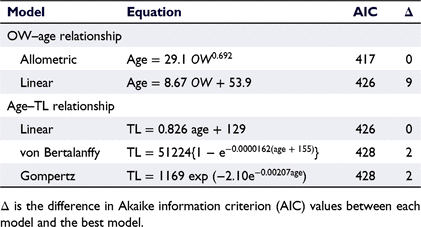
Age estimation by using otolith seasonal increments was not possible (see Discussion section). So, age estimation by using daily increments in otoliths was conducted. Daily increment formation in otoliths of E. tetradactylum has been validated by examining otoliths removed from known-age fish, which were hatched and reared under natural environmental conditions in Taiwan (Su et al. 2019). Therefore, the micro-increments observed in the otolith sections in the present study were presumed to be formed daily. For otoliths that had not been broken during storage, otolith weight (OW) was recorded to nearest 0.1 mg and then transverse sections were cut. The otolith sections were bonded to a microscope slide, ground using a grinding machine (ML-110NT; Maruto, Tokyo, Japan) with 5000-grit waterproof sandpaper until the daily increments were clearly visible, and then polished using a 1-μm alumina suspension. The resulting thin sections were investigated under an upright microscope (ECLIPSE Ci-L; Nikon; magnification 200×) and photographed using a digital camera (DS-Ri1; Nikon) mounted on the microscope. Using digital images, the numbers of daily increments were counted three times by the same person, without prior knowledge of fish length or previous counts. The CV values for the three counts were calculated for each specimen. When the CV was <5%, the mean of the three counts was calculated and recorded as the number of daily increments. When the CV was ≥5%, the specimen was excluded from the analyses (Campana 2001). The relationship between OW and age was established to allow checking the precision of the daily increments (Trip et al. 2014). Following Choat and Axe (1996), the linear and allometric models to the OW–age data were fitted. To analyse growth, three candidate models (linear, von Bertalanffy and Gompertz models) were fitted to the age–length data (Su et al. 2020). The best model for each dataset was selected using the Akaike information criterion (AIC) (Akaike 1974).
Results
Size composition
Of the total 449 specimens collected, 240 were female, 194 were male and 15 were undifferentiated. The size range was 178–624 mm, with bodyweight ranging from 45 to 2460 g (Fig. 3). The mode was observed at 275–300 mm in TL for males and 400–425 mm in TL for females. To investigate the possibility of age estimation using length–frequency analysis, monthly size–frequency distributions were compared. However, no obvious monthly trend in size range or mode was evident (Fig. S2).
|
|
Length–weight relationship
Our results indicated a significant sex difference in the TL–BW relationship (ANCOVA on log-transformed data: slopes, F1, 430 = 0.17, P = 0.7; intercepts, F1, 431 = 10.1, P = 0.002). However, because E. tetradactylum is a protandrous hermaphroditic, we analysed the TL–BW relationship by combining the data of both sexes (Fig. S3). The parameter estimate and standard error (s.e.) of the allometric model were 4.28 × 10−6 and 0.75 × 10−6 for a, and 3.12 and 0.03 for b respectively. The value of coefficient of determination was 0.97.
Sex-change size and reproduction
From our results, the estimated values of S50 and S95 were 376 mm TL (s.e. = 2) and 482 mm TL (s.e. = 7) respectively (Fig. 4). The GSI values showed a large variation among individuals, even when collected in the same month, and no clear seasonal trend was evident for either sex (Fig. 5). Our results did not indicate a clear relationship between TL and GSI for either sex, although an increase in GSI values with TL > 250 mm for males and TL > 350 mm for females was observed (Fig. S4). Of the individuals investigated for maturity stage, the smallest mature male had a TL of 273 mm, and the smallest female that was spawning had a TL of 343 mm. Regarding the monthly proportion of the developmental stages of gonads, mature males were found across all sampled months (January–May), with percentages ranging from 62 to 100% (Fig. 6a). Similarly, females with F4 (spawning stage) and F5 (spent stage) gonads accounted for 30–60% across all sampled months (Fig. 6b).
|
|
|
|
Otolith macrostructure
The relationship between TL and OR was well described by the allometric model (Fig. S5). The parameter estimate of the allometric model was 0.049 (s.e. = 0.004) for a and 0.75 (s.e. = 0.01) for b, with a coefficient of determination of 0.89. The mean CV of the translucent-zone count was 38.8%. Only 176 specimens (45.6%) exhibited a legible otolith zonation pattern. Several examples of otoliths are shown in Fig. S6 of the Supplementary material. Among the legible otoliths, we observed 0–3 translucent zones. The TL ranges of the specimens with 0, 1, 2 and 3 translucent zones were 178–455, 209–624, 281–556 and 422–541 mm respectively (Fig. S7). Both edge and MIR analyses showed no significant seasonal trends, indicating lack of periodicity in translucent-zone formation (Fig. S8). Observation of otolith sections for 72 specimens (178–624 mm TL) showed that the otolith zonation pattern (seasonal increments) could not be clearly observed (Fig. S9).
Age and growth
The daily increments in the sectioned otoliths (Fig. 7) were easier to count for smaller specimens, because daily increments near the otolith margin became more unclear as TL increased. It was impossible to count the daily increments in most specimens with a TL of ≥400 mm. Of the 55 specimens (178–422 mm TL), for which OW could be recorded and otolith daily increments were detectable, 46 (84%) had legible daily increments. Their estimated age range was 69–341 days. Within this age range, the allometric model fitted the OW–age data better than did the linear model (Table 1, Fig. 8a). In addition, the age–TL data were best fitted by the linear model, with the other fitted models (von Bertalanffy and Gompertz) also becoming approximately linear within this age range (Table 1, Fig. 8b). On the basis of the linear growth model, our results indicated a TL of 430 mm at 1 year of age, with the S50 value (376 mm TL) corresponding to 282 days (0.8 years). Among the males whose age was estimated, youngest mature individual (281 mm TL) was 137-day-old (0.4 year). The hatch date of each specimen was calculated from the estimated age and collection date, with results indicating that E. tetradactylum hatched in all months of the sample period (Fig. 9).
|
|
|
Discussion
Body size
A comparison of the length–weight relationship of E. tetradactylum obtained for the southern Gulf of Thailand with results from previous studies (Fig. S10) showed similarities with fish from northern Western Australia (Pember et al. 2005) and the eastern Gulf of Carpentaria, Australia (Garrett 1997). However, individuals in southern Thailand may be heavier on the basis of length than those in northern Australia (Ballagh et al. 2012) and Chilika Lagoon, India (Karna et al. 2012; Panda et al. 2016). In the present study, the highest TL (624 mm) was similar to that of fish in Chilika Lagoon, India (613 mm) (Panda et al. 2016). In contrast, results from three studies conducted in Australia indicated a maximum size range from 463 to 1049 mm (Table 2). Within this context, the maximum TL in the present study is relatively small. Such regional variation in maximum size might represent a sampling bias but is more likely to represent regional differences in growth, including longevity (Ballagh et al. 2012). As such, we suggest that the length–weight relationship of E. tetradactylum may vary depending on the environment or region.
Reproductive seasonality
In Australia, in the southern hemisphere, three studies have reported that E. tetradactylum has a long spawning period of 5–9 months from winter to spring–autumn, specifically, August to January in northern Western Australia (Pember et al. 2005), July to November in the eastern Gulf of Carpentaria (Garrett 1997) and August to April on the eastern coast of Queensland (Russell 1988). However, in the northern hemisphere, no reliable information exists on the spawning season of this species. Nesarul et al. (2014) estimated that the spawning season of E. tetradactylum off the coast of Bangladesh (22°N) is February–March and July–August on the basis of the GSI of 24 females. Similarly, Zamidi et al. (2012) estimated the spawning season of this species in Malaysia (2°N) to be from March to September on the basis of the GSI of 15 females. In Thailand (9°N), on the basis of a sample size of 237 females, we found that the GSI values showed no clear seasonal trend and large inter-individual variation in all the months. This suggests that the timing of spawning in Thailand varies among individuals, and can occur throughout the year. Notably, our results indicate that hatching occurs in all months on the basis of the calculations of hatching dates by using daily age estimates. Although the decrease in the mean GSI of females and increase in the proportion of post-spawning females from January to February (Fig. 5b, 6b) might suggest intense reproduction in January, more samples are needed to estimate the monthly intensity of reproduction, which occurs throughout the year. Further research on the histological analysis of gonads and seasonal changes in sex hormone concentrations of this species in near-equatorial habitats would provide detailed information of the reproductive cycle at the individual level, including information on size and age at maturity. This would improve our understanding of the regional differences in the reproductive cycle at the species level.
Size at sex change
Pember et al. (2005) investigated the sizes at which sex change occurs for E. tetradactylum in northern Western Australia and found that S50 and S95 were 402 mm and 460 mm TL respectively. In addition, Ballagh et al. (2012) conducted an investigation in seven regions of northern Australia and found that the size at which sex change occurred varied considerably by region; namely, S50 ranged from 208 to 465 mm FL (total length equivalent: 254–550 mm) and S95 ranged from 337 to 540 mm FL (total length equivalent: 403–636 mm). When these S50 values were plotted against latitude, there was a decreasing trend at lower latitudes (Fig. 10b). However, the S50 value (376 mm TL) in the present study fell within the range of previous studies, even though the study area was located at a lower latitude (Fig. 10b).
|
|
According to Allsop and West (2003), who demonstrated a life-history invariant for sex change (i.e. that fish change sex at a fixed percentage of their maximum size) using datasets for various fish species, the value of S50 divided by maximum size (S50 ÷ Lmax) was 0.79, regardless of species. Although the study did not refer to any intraspecific variability, the S50 ÷ Lmax value of 0.60 calculated from the results of the present study indicates that E. tetradactylum in southern Thailand changes sex at relatively small sizes relative to maximum size. Moreover, calculations based on data from previous studies on this species showed that the S50 ÷ Lmax value is 0.51 in northern Western Australia (Pember et al. 2005) and ranged between 0.39 and 0.73 for seven populations in northern Australia (Ballagh et al. 2012). Despite being from different environments, the values for the populations of this species were consistently lower than 0.79, contrasting with the finding of Allsop and West (2003). This indicates that for E. tetradactylum, the size at which sex change occurs is small relative to the maximum size. The implications of this are not yet understood. Future investigations may reveal the significance of sex change at small sizes for this species.
Otolith macrostructure
In our study, we initially considered that the estimation of fish age would be possible by counting translucent zones because the otoliths of E. tetradactylum in southern Thailand grew as the body grew and formed zonation patterns with a maximum of three translucent zones. However, the counts of translucent zones were inconsistent for more than half of the individuals because of the difficulty in otolith interpretation related to the low contrast between translucent and opaque zones and large individual differences in zone width (Fig. S6). Moreover, no clear relationship was found between the number of translucent zones and fish TL (Fig. S7). Furthermore, the MIR and edge analyses did not demonstrate the periodicity of translucent-zone formation (Fig. S8). Additionally, no distinct zonation patterns were observed, even when the otoliths were sectioned (Fig. S9). Therefore, it was not possible to estimate fish age using seasonal increments in otoliths for E. tetradactylum from southern Thailand. This contrasts with results from previous studies conducted in Australia that successfully estimated the age of E. tetradactylum by using otolith seasonal increments (Garrett 1997; Pember et al. 2005; Zischke et al. 2009; Ballagh et al. 2012). These fish came from higher latitudes than those considered in present study area.
Several studies that compared the clarity of otolith seasonal increments in the same species among different tropical regions showed that seasonal increments tend to be less clear at lower latitudes (with smaller annual temperature changes) (Fowler and Doherty 1992; Caldow and Wellington 2003; Choat et al. 2003). Considering the inherently large interspecific variation in the clarity of otolith seasonal increments (Fowler 1995; Choat et al. 2009), it is expected that fish from some regions cannot be aged. Although reports of successful ageing for tropical species using otolith seasonal increments have increased in recent years (Longhurst and Pauly 1987; Fowler 2009), few studies have explicitly described the difficulties encountered in interpreting seasonal increments in such species (Morales-Nin and Panfili 2005). One reason for this may be that if the seasonal increment of otoliths is too unclear for age estimation, growth analyses cannot be performed, and studies are unlikely to be reported. Therefore, our findings provide valuable information indicating that a species with distinguishable otolith seasonal increments in regions relatively far from the equator (>14°S) has indistinguishable otolith seasonal increments in a region near the equator (9°N; Table 2).
Age, growth and age at sex change
Although seasonal increments could not be detected in otoliths of E. tetradactylum in the present study, it was possible to estimate age using daily increments. Similar to reports for other species (e.g. Oxenford et al. 1994; Stocks et al. 2019), there is an upper limit to the number of daily increments that can be counted, which in this case was approximately 1 year (341 days). However, it has been reported that OW can be used as an indirect age indicator because age and OW have a linear relationship throughout the life in many fish species (e.g. Anderson et al. 1992; Worthington et al. 1995; Choat and Axe 1996; Newman et al. 1996). By extrapolating the linear relationship between OW and age beyond the first year, we estimated the age of fish with maximum otolith weight (75 mg) to be 1.9 years, or with the allometric model, to be 1.6 years. This suggests that the lifespan of E. tetradactylum in southern Thailand may be shorter than those reported in any of the previously studied regions (lifespan, 4–7 years; Table 2).
On the basis of the linear growth model used in this study, the TL at 1 year of age (L1) of E. tetradactylum in southern Thailand was calculated as 430 mm. For comparison, the L1 of Australian populations, which was calculated using the growth curves reported in previous studies, ranged from 209 to 301 mm (Table 2). This comparison indicated that E. tetradactylum has a faster growth rate in southern Thailand than in Australia (Fig. 10a). In future, smaller individuals should be sampled to clarify early stage growth on the basis of daily age estimation, and growth analysis methods such as mark–recapture should be used for larger fish.
Junnan et al. (2020) reported that 7-month-old (0.6 year) cultured E. tetradactylum (228 specimens) reared at similar water temperatures (28.0–30.7°C) as in the present study area, had an average TL of 264 mm. Moreover, 5.7% of these 7-month-old individuals had already undergone sex changes from male to female (Junnan et al. 2020). In the present study, the TL at 0.6 years of age was estimated as 302 mm, with 11.3% of fish at this length being female (Fig. 4, 8). Considering the variability caused by differences in certain factors, such food and population density, between aquaculture and natural environments, the estimates obtained in the present study are considered reasonable. From macroscopic examination of the gonads, we found that the smallest mature male had a TL of 273 mm, whereas Shihab et al. (2017) reported a TL of 210 mm for the smallest mature male from specimens collected from across India. Moreover, Pember et al. (2005) reported that the length at which 50% of males attain maturity was 201 mm TL in northern Western Australia, on the basis of maturity-stage determination by using a combination of macroscopic and histological examinations of the gonads. These results suggest that the macroscopic criteria for gonadal stages used in the present study may have classified smaller mature males with small flaccid testis (after spawning) as immature, and the maturity size of males in southern Thailand may be larger than that of the same species in other regions. However, this is currently unknown, and future histological examinations of the gonads are required to show the maturity size of near-equatorial populations of E. tetradactylum. The presence of a mature male at 137 days (0.4 years) of age and the estimated S50 of 0.8 years suggested that, in southern Thailand, this species matures as a male within ~6 months of age, and subsequently most individuals change sex to female within the first year. This means that the southern Thailand population matures earlier than the Australian populations, for which the S50 has been reported as 1.4–3.4 years of age (Table 2, Fig. 10c). Hence, the life cycle of E. tetradactylum in regions near the equator is likely to be shorter than that in other regions.
Conclusion
To the best of our knowledge, this is the first study to examine the age and growth of E. tetradactylum in a near-equatorial environment at a latitude of <10°, where sea temperatures remain constant throughout the year. Our study has provided a framework for future research within this field. The population in southern Thailand was characterised by rapid growth (reaching 69% of maximum size at 1 year of age), early maturity and sex change (under 1 year of age), and continuous spawning throughout the year. In other words, this population has a rapid turnover (i.e. short generation time) and the life history variation of E. tetradactylum is wider than previously reported. Populations of species with a short generation time can grow or decline quickly in response to environmental change, and a rapid decline in productivity often requires similarly rapid reductions in fishing effort (Pinsky et al. 2011). Moreover, in the tropical environment, where species diversity is high and many local fisheries catch a variety of large and small fish simultaneously, it is essential to devise management measures that take into account both the life history of the species and the needs of local fisheries.
Supplementary material
Supplementary material is available online.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Declaration of funding
This study was supported in part by JSPS KAKENHI (grant number: 19H02976).
Acknowledgements
The authors are grateful to the staff of the Songkhla Marine Fisheries Research and Development Center for assisting with the field survey. The authors thank M. Horinouchi (Shimane University, Shimane), and K. Hara and M. Watanabe (Nagasaki University, Nagasaki) for discussions regarding the research presented. The authors also thank two anonymous reviewers for constructive comments and valuable suggestions on the manuscript. Finally, the authors thank Editage (www.editage.jp) for English language editing.
References
Akaike, H (1974). A new look at the statistical model identification. IEEE Transactions on Automatic Control 19, 716–723.| A new look at the statistical model identification.Crossref | GoogleScholarGoogle Scholar |
Allsop, DJ, and West, SA (2003). Constant relative age and size at sex change for sequentially hermaphroditic fish. Journal of Evolutionary Biology 16, 921–929.
| Constant relative age and size at sex change for sequentially hermaphroditic fish.Crossref | GoogleScholarGoogle Scholar |
Anderson, JR, Morison, AK, and Ray, DJ (1992). Age and growth of Murray cod, Maccullochella peelii (Perciformes: Percichthyidae), in the Lower Murray–Darling Basin, Australia, from thin-sectioned otoliths. Marine and Freshwater Research 43, 983–1013.
| Age and growth of Murray cod, Maccullochella peelii (Perciformes: Percichthyidae), in the Lower Murray–Darling Basin, Australia, from thin-sectioned otoliths.Crossref | GoogleScholarGoogle Scholar |
Ballagh, AC, Welch, DJ, Newman, SJ, Allsop, Q, and Stapley, JM (2012). Stock structure of the blue threadfin (Eleutheronema tetradactylum) across northern Australia derived from life-history characteristics. Fisheries Research 121–122, 63–72.
| Stock structure of the blue threadfin (Eleutheronema tetradactylum) across northern Australia derived from life-history characteristics.Crossref | GoogleScholarGoogle Scholar |
Caldow, C, and Wellington, GM (2003). Patterns of annual increment formation in otoliths of pomacentrids in the tropical western Atlantic: implications for population age-structure examination. Marine Ecology Progress Series 265, 185–195.
| Patterns of annual increment formation in otoliths of pomacentrids in the tropical western Atlantic: implications for population age-structure examination.Crossref | GoogleScholarGoogle Scholar |
Campana, SE (2001). Accuracy, precision and quality control in age determination, including a review of the use and abuse of age validation methods. Journal of Fish Biology 59, 197–242.
| Accuracy, precision and quality control in age determination, including a review of the use and abuse of age validation methods.Crossref | GoogleScholarGoogle Scholar |
Chang, WYB (1982). A statistical method for evaluating the reproducibility of age determination. Canadian Journal of Fisheries and Aquatic Sciences 39, 1208–1210.
| A statistical method for evaluating the reproducibility of age determination.Crossref | GoogleScholarGoogle Scholar |
Choat, JH, and Axe, LM (1996). Growth and longevity in acanthurid fishes; an analysis of otolith increments. Marine Ecology Progress Series 134, 15–26.
| Growth and longevity in acanthurid fishes; an analysis of otolith increments.Crossref | GoogleScholarGoogle Scholar |
Choat, JH, Robertson, DR, Ackerman, JL, and Posada, JM (2003). An age-based demographic analysis of the Caribbean stoplight parrotfish Sparisoma viride. Marine Ecology Progress Series 246, 265–277.
| An age-based demographic analysis of the Caribbean stoplight parrotfish Sparisoma viride.Crossref | GoogleScholarGoogle Scholar |
Choat JH, Kritzer JP, Ackerman JL (2009) Ageing in coral reef fishes: do we need to validate the periodicity of increment formation for every species of fish for which we collect age-based demographic data? In ‘Tropical fish otoliths: information for assessment, management and ecology’. (Eds BS Green, BD Mapstone, G Carlos, GA Begg) pp. 23–54. (Springer Netherlands: Dordrecht, Netherlands)
Coulson, PG, and Poad, JA (2021). Biological characteristics of the primitive flatfish Indian halibut (Psettodes erumei) from the tropical northeastern Indian Ocean, including implications of the use of incorrect aging methods on mortality estimates. Fishery Bulletin 119, 168–183.
| Biological characteristics of the primitive flatfish Indian halibut (Psettodes erumei) from the tropical northeastern Indian Ocean, including implications of the use of incorrect aging methods on mortality estimates.Crossref | GoogleScholarGoogle Scholar |
Fowler AJ (1995) Annulus formation in otoliths of coral reef fish – a review. In ‘Recent developments in fish otolith research’. (Eds DH Secor, JM Dean, SE Campana) pp. 45–63. (University of South Carolina Press: Columbia, SC, USA)
Fowler AJ (2009) Age in years from otoliths of adult tropical fish. In ‘Tropical fish otoliths: information for assessment, management and ecology’. (Eds BS Green, BD Mapstone, G Carlos, GA Begg) pp. 55–92. (Springer: New York, NY, USA)
Fowler, AJ, and Doherty, PJ (1992). Validation of annual growth increments in the otoliths of two species of Damselfish from the Southern Great Barrier Reef. Marine and Freshwater Research 43, 1057–1068.
| Validation of annual growth increments in the otoliths of two species of Damselfish from the Southern Great Barrier Reef.Crossref | GoogleScholarGoogle Scholar |
Garrett RN (1997) Biology and harvest of tropical fishes in the Queensland Gulf of Carpentaria gillnet fishery. Queensland Fisheries Information Series QI98018, Queensland Department of Primary Industries, Brisbane, Qld, Australia.
Gray, CA, Haddy, JA, Fearman, J, Barnes, LM, Macbeth, WG, and Kendall, BW (2012). Reproduction, growth and connectivity among populations of Girella tricuspidata (Pisces: Girellidae). Aquatic Biology 16, 53–68.
| Reproduction, growth and connectivity among populations of Girella tricuspidata (Pisces: Girellidae).Crossref | GoogleScholarGoogle Scholar |
Horne, JB, Momigliano, P, Welch, DJ, Newman, SJ, and Van Herwerden, L (2011). Limited ecological population connectivity suggests low demands on self-recruitment in a tropical inshore marine fish (Eleutheronema tetradactylum: Polynemidae). Molecular Ecology 20, 2291–2306.
| Limited ecological population connectivity suggests low demands on self-recruitment in a tropical inshore marine fish (Eleutheronema tetradactylum: Polynemidae).Crossref | GoogleScholarGoogle Scholar |
Horne, JB, Momigliano, P, van Herwerden, L, and Newman, SJ (2013). Murky waters: searching for structure in genetically depauperate blue threadfin populations of Western Australia. Fisheries Research 146, 1–6.
| Murky waters: searching for structure in genetically depauperate blue threadfin populations of Western Australia.Crossref | GoogleScholarGoogle Scholar |
Junnan, L, Youjun, O, Jiufu, W, Junwei, L, Yingyue, N, and Jia’er, L (2020). A preliminary study on process of sex reversal in Eleutheronema tetradactylum. South China Fisheries Science 16, 67–74.
| A preliminary study on process of sex reversal in Eleutheronema tetradactylum.Crossref | GoogleScholarGoogle Scholar |
Karna, SK, Sahoo, DK, and Panda, S (2012). Length–weight relationship (LWR), growth estimation and length at maturity of Eleutheronema tetradactylus in the Chilika Lagoon, India. South Asian Journal of Experimental Biology 2, 97–102.
| Length–weight relationship (LWR), growth estimation and length at maturity of Eleutheronema tetradactylus in the Chilika Lagoon, India.Crossref | GoogleScholarGoogle Scholar |
Kesteven GL (Ed.) (1960) ‘Manual of field methods in fisheries biology.’ (Food and Agriculture Organization of the United Nations: Rome, Italy)
Kongasa W, Ruangpatikorn N (2020) การประมงอวนลอยปลากุเราในจังหวัดนครศรีธรรมราชและสงขลา [Threadfin gillnet fishery in Nakhon Si Thammarat and Songkhla Provinces.] Technical Paper Number 4/2020, Department of Fisheries, Ministry of Agriculture and Cooperatives, Thailand. [In Thai]
Kume, G, Kubo, Y, Yoshimura, T, Kiriyama, T, and Yamaguchi, A (2010). Life history characteristics of the protogynous parrotfish Calotomus japonicus from northwest Kyushu, Japan. Ichthyological Research 57, 113–120.
| Life history characteristics of the protogynous parrotfish Calotomus japonicus from northwest Kyushu, Japan.Crossref | GoogleScholarGoogle Scholar |
Kuwamura, T, Sunobe, T, Sakai, Y, Kadota, T, and Sawada, K (2020). Hermaphroditism in fishes: an annotated list of species, phylogeny, and mating system. Ichthyological Research 67, 341–360.
| Hermaphroditism in fishes: an annotated list of species, phylogeny, and mating system.Crossref | GoogleScholarGoogle Scholar |
Longhurst AR, Pauly D (1987) ‘Ecology of tropical oceans.’ (Academic Press: San Diego, CA, USA)
Moore, BR, Stapley, J, Allsop, Q, Newman, SJ, Ballagh, A, Welch, DJ, and Lester, RJG (2011). Stock structure of blue threadfin Eleutheronema tetradactylum across northern Australia, as indicated by parasites. Journal of Fish Biology 78, 923–936.
| Stock structure of blue threadfin Eleutheronema tetradactylum across northern Australia, as indicated by parasites.Crossref | GoogleScholarGoogle Scholar |
Moore, BR, Stapley, JM, Williams, AJ, and Welch, DJ (2017). Overexploitation causes profound demographic changes to the protandrous hermaphrodite king threadfin (Polydactylus macrochir) in Queensland’s Gulf of Carpentaria, Australia. Fisheries Research 187, 199–208.
| Overexploitation causes profound demographic changes to the protandrous hermaphrodite king threadfin (Polydactylus macrochir) in Queensland’s Gulf of Carpentaria, Australia.Crossref | GoogleScholarGoogle Scholar |
Morales-Nin, B, and Panfili, J (2005). Seasonality in the deep sea and tropics revisited: what can otoliths tell us? Marine and Freshwater Research 56, 585–598.
| Seasonality in the deep sea and tropics revisited: what can otoliths tell us?Crossref | GoogleScholarGoogle Scholar |
Motomura H (2004) ‘Threadfins of the world (family Polynemidae): an annotated and illustrated catalogue of polynemid species known to date. FAO Species Catalogue for Fishery Purposes.’ (Food and Agriculture Organization of the United Nations: Rome, Italy)
Motomura H, Matsuura K, Bishop J, Kaymaram F (2015) Eleutheronema tetradactylum (Persian Gulf assessment). In ‘The IUCN red list of threatened species 2015’, e.T46087646A57168342. (International Union for Conservation of Nature and Natural Resources) Available at https://www.iucnredlist.org/species/46087646/57168342
Nesarul, HM, Abu Hena, MK, Saifullah, SM, and Idris, MH (2014). Breeding biology of Eleutheronema tetradactylum (Shaw, 1804) from the Bay of Bengal, Indian Ocean. World Applied Sciences Journal 30, 240–244.
| Breeding biology of Eleutheronema tetradactylum (Shaw, 1804) from the Bay of Bengal, Indian Ocean.Crossref | GoogleScholarGoogle Scholar |
Newman, SJ, Williams, DM, and Russ, GR (1996). Age validation, growth and mortality rates of the tropical snappers (Pisces: Lutjanidae) Lutjanus adetii (Castelnau, 1873) and L. quinquelineatus (Bloch, 1790) from the central Great Barrier Reef, Australia. Marine and Freshwater Research 47, 575–584.
| Age validation, growth and mortality rates of the tropical snappers (Pisces: Lutjanidae) Lutjanus adetii (Castelnau, 1873) and L. quinquelineatus (Bloch, 1790) from the central Great Barrier Reef, Australia.Crossref | GoogleScholarGoogle Scholar |
Newman, SJ, Pember, MB, Rome, BM, Mitsopoulos, GEA, Skepper, CL, Allsop, Q, Saunders, T, Ballagh, AC, Van Herwerden, L, Garrett, RN, Gribble, NA, Stapley, JM, Meeuwig, JJ, Moore, BR, and Welch, DJ (2011). Stock structure of blue threadfin Eleutheronema tetradactylum across northern Australia as inferred from stable isotopes in sagittal otolith carbonate. Fisheries Management and Ecology 18, 246–257.
| Stock structure of blue threadfin Eleutheronema tetradactylum across northern Australia as inferred from stable isotopes in sagittal otolith carbonate.Crossref | GoogleScholarGoogle Scholar |
Ogino, Y, Furumitsu, K, Kiriyama, T, and Yamaguchi, A (2020). Using optimised otolith sectioning to determine the age, growth and age at sexual maturity of the herbivorous fish Kyphosus bigibbus: with a comparison to using scales. Marine and Freshwater Research 71, 855–867.
| Using optimised otolith sectioning to determine the age, growth and age at sexual maturity of the herbivorous fish Kyphosus bigibbus: with a comparison to using scales.Crossref | GoogleScholarGoogle Scholar |
Oxenford, HA, Hunte, W, Deane, R, and Campana, SE (1994). Otolith age validation and growth-rate variation in flyingfish (Hirundichthys affinis) from the eastern Caribbean. Marine Biology 118, 585–592.
| Otolith age validation and growth-rate variation in flyingfish (Hirundichthys affinis) from the eastern Caribbean.Crossref | GoogleScholarGoogle Scholar |
Panda, D, Karna, SK, Mukherjee, M, Manna, RK, Suresh, VR, and Sharma, AP (2016). Length–weight relationships of six tropical fish species from Chilika Lagoon, India. Journal of Applied Ichthyology 32, 1286–1289.
| Length–weight relationships of six tropical fish species from Chilika Lagoon, India.Crossref | GoogleScholarGoogle Scholar |
Pember MB, Newman SJ, Hesp SA, Young GC, Skepper CL, Hall NG, Potter IC (2005) Biological parameters for managing the fisheries for blue and king threadfin salmons, estuary rockcod, Malabar grouper and mangrove jack in north-western Australia. Fisheries Research and Development Corporation, Project Number 2002/003, Centre for Fish and Fisheries Research, Murdoch University, Perth, WA, Australia.
Pinsky, ML, Jensen, OP, Ricard, D, and Palumbi, SR (2011). Unexpected patterns of fisheries collapse in the world’s oceans. Proceedings of the National Academy of Sciences 108, 8317–8322.
| Unexpected patterns of fisheries collapse in the world’s oceans.Crossref | GoogleScholarGoogle Scholar |
Provost, MM, and Jensen, OP (2015). The impacts of fishing on hermaphoditic and treatment of sex change in stock assessments. Fisheries 40, 536–545.
| The impacts of fishing on hermaphoditic and treatment of sex change in stock assessments.Crossref | GoogleScholarGoogle Scholar |
Russell DJ (1988) An assessment of the east Queensland inshore gill net fishery. Information Series QI88024, Number 0727-6273, Queensland Department of Primary Industries, Brisbane, Qld, Australia.
Shihab, I, Gopalakrishnan, A, Vineesh, N, Muktha, M, Akhilesh, KV, and Vijayagopal, P (2017). Histological profiling of gonads depicting protandrous hermaphroditism in Eleutheronema tetradactylum. Journal of Fish Biology 90, 2402–2411.
| Histological profiling of gonads depicting protandrous hermaphroditism in Eleutheronema tetradactylum.Crossref | GoogleScholarGoogle Scholar |
Stocks, JR, Scott, KF, and Gilligan, DM (2019). Daily age determination and growth rates of freshwater fish throughout a regulated lotic system of the Murray–Darling Basin Australia. Journal of Applied Ichthyology 35, 457–464.
| Daily age determination and growth rates of freshwater fish throughout a regulated lotic system of the Murray–Darling Basin Australia.Crossref | GoogleScholarGoogle Scholar |
Su, N-J, Lu, Y-S, Huang, L-C, Cheng, C-Y, and Hsu, Y-W (2019). Validating daily growth increments in otolith for juvenile fourfinger threadfin (Eleutheronema tetradactylum) in waters off western Taiwan. Journal of Fisheries Society of Taiwan 46, 109–116.
Su, N-J, Lu, Y-S, Wang, C-H, Liao, C-H, Chiang, W-C, and Tseng, C-T (2020). Age determination for juvenile fourfinger threadfin (Eleutheronema rhadinum) by using otolith microstructure and length data obtained from commercial fisheries off northwestern Taiwan. Fisheries Research 227, 105560.
| Age determination for juvenile fourfinger threadfin (Eleutheronema rhadinum) by using otolith microstructure and length data obtained from commercial fisheries off northwestern Taiwan.Crossref | GoogleScholarGoogle Scholar |
Trip, EDL, Craig, P, Green, A, and Choat, JH (2014). Recruitment dynamics and first year growth of the coral reef surgeonfish Ctenochaetus striatus, with implications for acanthurid growth models. Coral Reefs 33, 879–889.
| Recruitment dynamics and first year growth of the coral reef surgeonfish Ctenochaetus striatus, with implications for acanthurid growth models.Crossref | GoogleScholarGoogle Scholar |
Wakefield, CB, Potter, IC, Hall, NG, Lenanton, RCJ, and Hesp, SA (2015). Marked variations in reproductive characteristics of snapper (Chrysophrys auratus, Sparidae) and their relationship with temperature over a wide latitudinal range. ICES Journal of Marine Science 72, 2341–2349.
| Marked variations in reproductive characteristics of snapper (Chrysophrys auratus, Sparidae) and their relationship with temperature over a wide latitudinal range.Crossref | GoogleScholarGoogle Scholar |
Worthington, DO, Doherty, PJ, and Fowler, AJ (1995). Variation in the relationship between otolith weight and age: implications for the estimation of age of two tropical damselfish (Pomacentrus moluccensis and P. wardi). Canadian Journal of Fisheries and Aquatic Sciences 52, 233–242.
| Variation in the relationship between otolith weight and age: implications for the estimation of age of two tropical damselfish (Pomacentrus moluccensis and P. wardi).Crossref | GoogleScholarGoogle Scholar |
Yamaguchi, A, Kume, G, and Takita, T (2004). Geographic variation in the growth of white croaker, Pennahia argentata, off the coast of northwest Kyushu, Japan. Environmental Biology of Fishes 71, 179–188.
| Geographic variation in the growth of white croaker, Pennahia argentata, off the coast of northwest Kyushu, Japan.Crossref | GoogleScholarGoogle Scholar |
Yoshioka K (2002) KyPlot as a tool for graphical data analysis. In ‘Compstat’. (Eds W Härdle, B Rönz) pp. 37–46. (Physica-Verlag HD: Heidelberg, Germany)
Zamidi, I, Samat, A, Zaidi, CC, Mazlan, AG, Alam, GM, Al-Amin, AQ, and Simon, KD (2012). Fecundity and temporal reproductive cycle of four finger threadfin (Eleutheronema tetradactylum) in Malaysian coastal water. Asian Journal of Animal and Veterinary Advances 7, 1100–1109.
| Fecundity and temporal reproductive cycle of four finger threadfin (Eleutheronema tetradactylum) in Malaysian coastal water.Crossref | GoogleScholarGoogle Scholar |
Zischke, MT, Cribb, TH, Welch, DJ, Sawynok, W, and Lester, RJG (2009). Stock structure of blue threadfin Eleutheronema tetradactylum on the Queensland east coast, as determined by parasites and conventional tagging. Journal of Fish Biology 75, 156–171.
| Stock structure of blue threadfin Eleutheronema tetradactylum on the Queensland east coast, as determined by parasites and conventional tagging.Crossref | GoogleScholarGoogle Scholar |



