An integrated approach for assessing the survival of discarded sandbar sharks, Carcharhinus plumbeus, captured in scientific longlines
Taylor Grosse A B * , Cynthia Awruch
A B * , Cynthia Awruch  C D , Euan Harvey
C D , Euan Harvey  B , Benjamin Saunders
B , Benjamin Saunders  B , Chris Dowling A , Daniela Waltrick
B , Chris Dowling A , Daniela Waltrick  A and Matias Braccini
A and Matias Braccini  A
A
A
B
C
D
Abstract
The sandbar shark (Carcharhinus plumbeus) has a global distribution and is caught by commercial fishers and recreational anglers.
To assess the stress physiology, release condition, and post-release survival of sandbar sharks caught in longline surveys conducted in Western Australia.
Post-release survival of sandbar sharks caught in longlining surveys was assessed using an integrated approach that combined the use of hook-timers, qualitative release conditions, satellite-tagging, and blood physiology.
Of 57 individuals examined, there was 100% post-capture survival after a maximum of 4 h on the hook. Most of these animals (88%) displayed a strong release condition, exhibiting minimal behavioural impairment. All 13 satellite-tagged individuals survived 30 days post-capture. Sharks dived up to 307 m deep and showed cyclical depth movement patterns, with some individuals moving through the water column both day and night, whereas others moved almost exclusively at night. The concentration of blood metabolites did not significantly change with time-on-hook.
Post-capture and post-release survival of 100% after up to 4 h on hooks suggested that the use of longlines for surveying sandbar shark abundance had no deleterious effects on captured sharks.
This will support future stock assessments of sharks by quantifying the survival rates in the methods used for long-term monitoring of sandbar shark populations.
Keywords: Chondrichthyes, fisheries, post-capture survival, post-release survival, PSAT tags, release condition, stress physiology, survivorship.
Introduction
Elasmobranchs are one of the most vulnerable vertebrate groups living in our oceans. An increasing number of elasmobranchs is being classified as either Endangered or Threatened by the International Union for Conservation of Nature Red List because of the life-history characteristics (slow growth rates, late age at maturity, low fecundity, and long life span) that make the group vulnerable to overfishing (Clementi et al. 2021; Dulvy et al. 2021; Pacoureau et al. 2021). The economic value of fins and other elasmobranch by-products has resulted in an increased fishing pressure worldwide (Clarke et al. 2006, 2007; Cardeñosa et al. 2020). Sharks are directly targeted for the fin trade and ‘flake’ consumption, but are caught and discarded by fishers targeting other species (Clarke et al. 2007; Oliver et al. 2015; Dulvy et al. 2021).
Empirical estimates of discard mortality are often unavailable when estimating commercial or recreational fishing mortality, and the fate of discarded sharks is largely unknown (Clarke et al. 2006; Davidson et al. 2016; Braccini et al. 2021). This information is key for the accurate assessment of stocks and the development of effective fisheries management and conservation policies (Braccini et al. 2021). Different approaches have been used to assess the fate of discarded sharks on release, either by quantifying post-capture survival (PCS; survival observed at the vessel) or post-release survival (PRS; survival after discarding). For example, changes in blood chemistry (e.g. lactate, glucose, pH, urea) can be used to estimate the likelihood of discard survival (Dapp et al. 2016), whereas methodologies such as cages to monitor survival (Rulifson 2007), tag-recapture (Hueter et al. 2006), and electronic tracking (Kneebone et al. 2013) are also used (Braccini et al. 2012; Ellis et al. 2017). However, estimates of both PCS and PRS are needed to quantify the overall mortality of the captured sharks (Braccini et al. 2012; French et al. 2015; Hutchinson et al. 2015; Hutchinson and Bigelow 2019).
Several blood-physiology indicators have been used to quantify how sharks respond to the catch-and-release. In recent years, adrenocorticotropic hormone (ACTH) concentrations have been used to evaluate the role of ACTH, a pituitary hormone responsible for stimulating the release of glucocorticoids (GCs) associated with physical stress events. However, these studies are very limited in sharks and the roles of ACTH are not entirely understood concerning capture stress (Fuller et al. 2020). Although 1α-hydroxycorticosterone (1α-OH-B) is the primary internal corticosteroid produced by elasmobranchs (Anderson 2012) to help the body respond to physical stress (Armour et al. 1993; Anderson 2012; Ruiz-Jarabo et al. 2019; Schoen et al. 2021), corticosterone (CORT) has also been linked to initial stress-coping mechanisms in elasmobranchs (Iki et al. 2020) and has been previously used to assess the stress response in conjunction with other metabolites (Manire et al. 2007; Brinn et al. 2012). However, CORT can be separately expressed with maturity and reproductive state, which should be considered when evaluating concentrations (Rasmussen and Crow 1993; Snelson et al. 1997; Manire et al. 2007). These initial endocrine coping mechanisms to stress trigger a range of secondary stress responses such as lactate, glucose, and hydromineral balance (Karsten 2000; Heberer et al. 2010; Hammerschlag et al. 2017).
Satellite tagging, such as with pop-up satellite archival transmitting (PSAT) tags, is an effective method for assessing PRS (Mohan et al. 2020, Schaefer et al. 2021). However, the cost of satellite tags limits the number of individuals tagged (Kohler and Turner 2001; French et al. 2015). The PSAT tag transmits light, temperature, and depth data in a range of temporal frequencies that can be altered to suit the species of shark studied (Lynch et al. 2017; Sulikowski et al. 2020). Many charismatic species have been monitored using this style of tag, with varying results in PRS and spatiotemporal movements (French et al. 2015; Hutchinson et al. 2015; Hutchinson and Bigelow 2019; Sulikowski et al. 2020).
Sandbar sharks (Carcharhinus plumbeus) have a global distribution across temperate and tropical waters and are captured in commercial and recreational fisheries worldwide (McAuley et al. 2005). Historically, commercial shark fisheries in northern Western Australia (WA) targeted sandbar sharks, with a peak of >700 tonnes (Mg) landed in 2004–2005 (McAuley et al. 2005; Braccini et al. 2020). Annual scientific shark surveys in northern WA have been conducted for >20 years (commencing in 2001) to monitor sandbar shark abundance (Braccini et al. 2020). Since large spatial closures were implemented in 2005 and the cessation of operations in the remaining northern shark fisheries, these scientific surveys indicate that there has been an increase in the abundance of sandbar sharks along the north-western coast (stock statuses: over-exploited in 2005 and recovering in 2021) (Braccini et al. 2020; Newman et al. 2021). Sandbar sharks are routinely tagged-and-released as part of these surveys, but there are limited reports of recaptured individuals from either recreational or commercial fisheries, and little is known about the fate of tagged sandbar sharks on release in the survey area.
In the USA, sandbar sharks caught from demersal longlines exhibited variable PRS (29–96%) owing to differences in gear specifications, shark size, soak times and fishing depth (Marshall et al. 2015; Gulak and Carlson 2021; Whitney et al. 2021). Therefore, results from previous studies may not be representative of the PRS of individuals discarded from all line fisheries (Marshall et al. 2015; Gulak and Carlson 2021). Hence, further research on the fate of sandbar sharks tagged-and-released from longlines, including experiment survey gear, is required for a more thorough understanding of its potential deleterious effects, particularly here in WA, with a recovering population. The scope of this study was to assess whether experimental surveys influence the recovering population, considering that other forms of commercial fishing in the region may have less influence (i.e. owing to shark fishing regulations and cessation of the northern shark fisheries). We took an integrated approach using qualitative release condition, PSAT tagging, and analysis of blood physiological parameters (i.e. ACTH, GC and lactate) to assess the likelihood of survival of sandbar sharks caught on demersal longline surveys.
Materials and methods
Onboard sampling
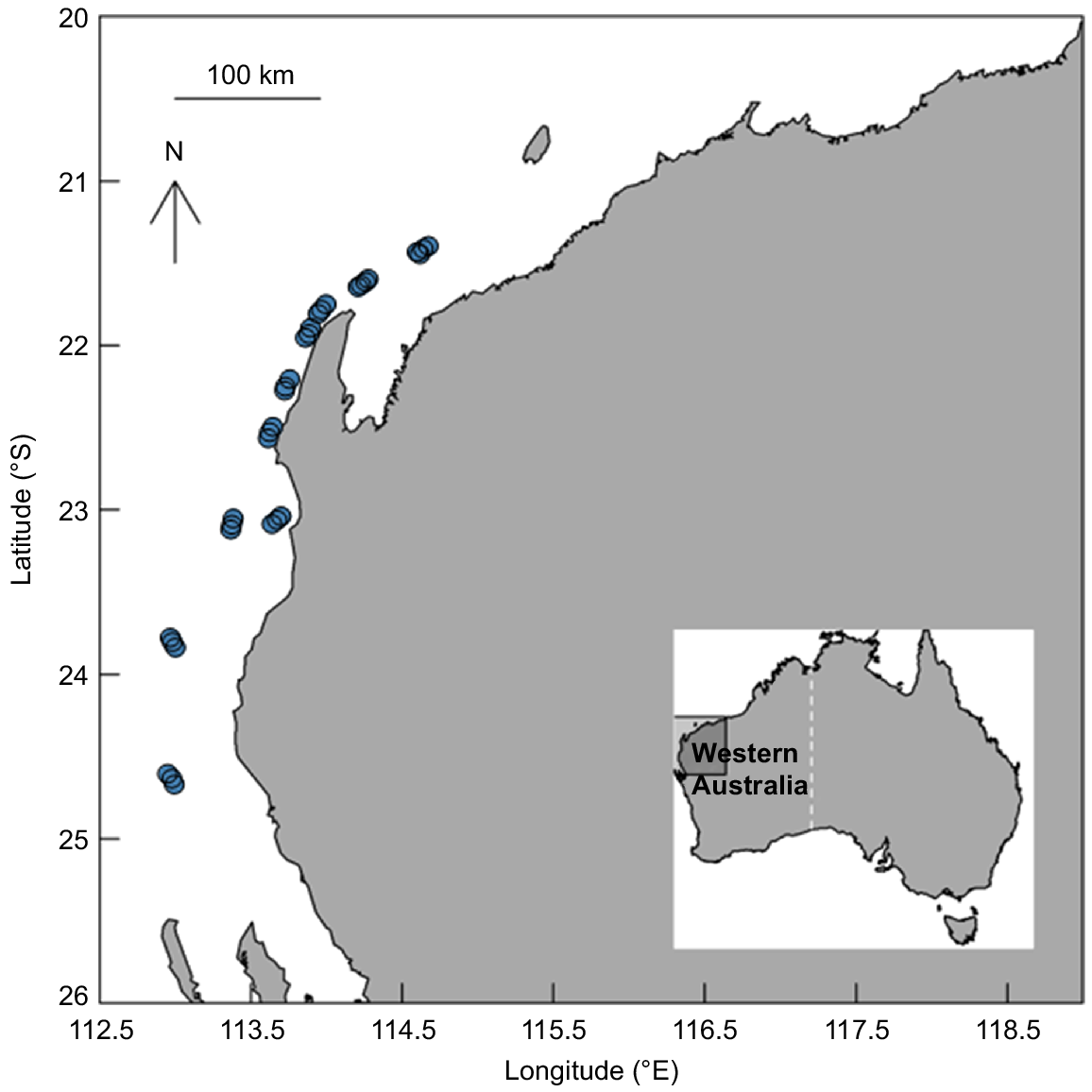
Scientific demersal longline surveys were conducted in 2020 and 2021 at 10 fixed stations along the continental shelf of northern WA (Fig. 1).
Map of demersal longline (blue) survey area in northern Western Australia. Each site has five replica longline sets.
Approximately 250 hooks were deployed each day. Each deployment comprised three to five longlines of ~500 m long each. Size 12/0 J-shaped hooks baited with sea mullet (Mugil cephalus) (cut into two or three pieces, ~20 cm) were attached to ~2-m metal snoods to the mainline. For further details on gear configuration and deployment, refer to Braccini et al. (2020). To determine time on hook (TOH, i.e. time spent on the line after hooking), hook timers (Lindgren-Pitman Inc.) were randomly attached to 16 snoods on each deployment. The start and end latitude and longitude, depth, water temperature and time of day were recorded for each longline deployment. Thirty-nine longline sets were conducted over 10 sites with soak times ranging from 3 to 4 h.
On capture, sandbar sharks were brought on deck and the hook was removed. The TOH was recorded, and blood samples (5 mL) were taken immediately from the caudal vein in 57 individuals randomly selected for PCS analysis by studying blood and plasma physiology, of which this process took ~1 min. Sharks were then measured (fork length, FL, cm) and sexed. Whole-blood lactate concentrations were measured in situ in all individuals, and the whole-blood samples from 29 of these sharks were centrifuged for 5 min at 7871g and room temperature, and plasma was separated and stored at −20°C for further analysis of ACTH and GC concentrations. Thirteen of the sharks sampled for further blood and plasma physiology were opportunistically fitted (with considerations of distributions across TOH) with survivorship pop-up satellite archival transmitting (PSAT) tags (Model sPAT, Wildlife Computers Inc., Redmond, WA, USA) to quantify PRS. Gills were not irrigated during handling to mimic commercial fishing operations. The PSAT tags were tethered to a nylon intramuscular anchor inserted at the base of the dorsal fin. All sharks captured were tagged with a plastic fin tag and released. At the time of release, the condition of each individual was classified as (1) alive and strong, (2) alive, but weak or disoriented, (3) moribund (eye and jaw response when stimulated, otherwise exhausted and unresponsive) or (0) dead (Braccini and Waltrick 2019).
Post-capture survival (PCS) – blood and plasma physiology
Plasma concentrations of ACTH were analysed using an ELISA kit (Product Code: CSB-E15926Fh, CUSABIO TECHNOLOGY LLC, Houston, TX, USA). Absorbance was measured with a microplate reader (Stat Fax 303 Plus Awareness Technology, Inc., Palm City, FL, USA) at a wavelength of 450 nm and converted to picograms per millilitre by using a four-parameter logistic curve. Plasma samples and assay standards (50 μL) were assayed in duplicate in a single kit. The assay kit was validated by assessment of the slope of serial dilutions of plasma samples against the assay standards. The standard curve consisted of five ACTH concentrations ranging from 75 to 1200 pg mL−1 and the serial dilutions consisted of two new standard curves spiked with two different 10-μL pools of plasma samples, each plasma pool was constructed by adding 5 μL of 10 individual samples (Fuller et al. 2020). Spiked standard curves showed good parallelism with the assay standard curve (Fig. S1 of the Supplementary material). For further information on calculating ACTH, refer to ‘Adrenocorticotropic hormone (ACTH)’ of the Supplementary material (Fig. S1).
Total GCs were analysed using a CORT ELISA Kit (Product code: 501320, Cayman Chemical, Ann Arbor, MI, USA). This kit was previously validated to quantify 1α-OH-B, by using the cross-reactivity of the CORT antibody with 1α-OH-B and excluding other GCs by mass spectrometry (Evans et al. 2010; Lyons and Wynne-Edwards 2019; Iki et al. 2020). The cross-reactivity between CORT antibody and 1α-OH-B was reported to be 1.49% (Iki et al. 2020) and 5.2% (Evans et al. 2010). As reported by Cayman Chemical, the CORT antibody has high cross-reactivity with CORT (100%), 11-deoxycorticosterone (15.8%), 11-dehydrocorticosterone (2.9%), and cortisol (2.5%). Because we did not exclude other GCs, we assumed the CORT assay would reflect total GC concentrations of all components that immunoreacted with the CORT antibody, expressed in CORT units. For further information on calculating GCs, refer to ‘Glucocorticosteroids’ of the Supplementary material (Fig. S2).
A hand-held Lactate Pro 2 analyser (Arkray LT-1730, ARKRAY USA, Inc., Miami, FL, USA) was used to measure whole-blood and plasma lactate concentrations in situ, following the manufacturer’s instructions. The Lactate Pro 2 reads in the range of 0.8–25 mmol L−1, displaying ‘high’ for values higher than the upper range limit.
Post-release survival (PRS) – satellite tagging
All PSAT tags were programmed to detach from the sharks after a 30-day monitoring period. The tags are designed to record depth, temperature, and light information, which were archived at 24-h intervals for the full 30-day deployment, and 10-min intervals for the last 5 days of deployment. The PSAT tags were programmed to activate once the individual swam at a depth >10 m and to prematurely release (i.e. before the 30-day specified period) if the individual remained at a constant depth (±2 m) for a consecutive 24 h. The tag transmits data to the ARGOS satellite system. Raw data were analysed by Wildlife Computers, which provided a report indicating the pop-off date, location, and daily values for temperature, light, and depth, as well as movement data at 10-min resolution for the final 5 days (Drymon and Wells 2017).
Data analyses
A two-way ANOVA was used to assess for differences in the blood and plasma metabolite concentrations between release condition and sex. Quantitative and semi-quantitative analytical methods such as Pearson’s correlations and means plots were used to assess how well the concentrations of blood metabolites can be used as a survival predictor. On the basis of previous work on changes in plasma/whole blood metabolites with time (Fuller et al. 2020; Iki et al. 2020), ACTH, GCs and lactate concentrations were binned in 15-min increments for the first hour on the hook, and then bins were expanded to 30-min increments through 4 h (i.e. the maximum time on the hook).
For the 13 PSAT-tagged sharks, PRS was estimated using the transmitted depth data for the full 30-day deployment, unless the tag detached prematurely. Premature PSAT tag release occurs in one of the following three situations: sharks that sink to the seafloor and remain below a certain depth (referred to as a ‘sinker’), sharks sit at a constant depth (‘sitter’), or if a tag was floating at the surface (maximum depth was ≤1 m) (‘floater’) (Hutchinson et al. 2015; Lynch et al. 2017). The following two mortality scenarios accounting for the five ‘floater’ tags were considered: ‘floaters’ were (S1) considered premature tag detachments from surviving sharks, or (S2) premature detachment was the result of mortality (and scavenging) or a predation event. Kaplan–Meier (KM) survival models fit the data under these two scenarios and were used as a non-parametric approach for estimating survival. For each PSAT-tagged shark, depth profiles were plotted for the last 5 days (one record per 10 min) of the deployment periods. Changes in light, temperature, and depth values in the final 5 days of deployment were reviewed to classify the fate of ‘floater’ tags as predation events or premature detachments.
All data analysis was performed using R software (ver. 4.2.3 (2023-03-15), R Foundation for Statistical Computing, Vienna, Austria, see https://www.R-project.org/, accessed 12 April 2023) with packages: car (ver. 3.1-2, see https://cran.r-project.org/package=car; Fox and Weisberg 2019), ggplot2 (ver. 3.4.0, see https://CRAN.R-project.org/package=ggplot2; Wickham 2016), tidyverse (ver. 2.0.0, H. Wickham, R. François, L. Henry et al., see https://github.com/tidyverse/dplyr; Wickham et al. 2019), survival (ver. 3.5-3, T. Therneau, see https://CRAN.R-project.org/package=survival), ggpubr (ver. 0.6.0, A. Kassambara, see https://rpkgs.datanovia.com/ggpubr/), rstatix (ver. 0.7.2, A. Kassambara, see https://rpkgs.datanovia.com/rstatix/), lubridate (ver. 1.9.3, see https://cran.r-project.org/package=lubridate; Grolemund and Wickham 2011), ggpmisc (ver. 0.5.5, P. J. Aphalo, see https://docs.r4photobiology.info/ggpmisc/), maptools (ver. 1.1-8, R. Bivand and N. Lewin-Koh, see http://maptools.r-forge.r-project.org/), RODBC (ver. 1.3-20, B. Ripley and M. Lapsley, see https://cran.r-project.org/package=RODBC), chron (ver. 2.3-61, D. James and K. Hornik, see https://cran.r-project.org/package=chron), stringr (ver. 1.5.1, H. Wickham, see https://github.com/tidyverse/stringr), data.table (ver. 1.14.9, M. Dowle and A. Srinivasan, see https://rdocumentation.org/packages/data.table/versions/1.14.8), mgcv (ver. 1.9-0, see https://cran.r-project.org/package=mgcv/; Wood 2017), mgcViz (ver. 1.9-0, see https://cran.r-project.org/package=mgcViz; Fasiolo et al. 2019), PBSmapping (ver. 2.73.4, J. T. Schnute, N. Boers and R. Haigh, see https://github.com/pbs-software/pbs-mapping) and geosphere (ver. 1.5-18, R. J. Hijmans, see https://cran.r-project.org/package=geosphere).
Ethics
Shark capture, tagging, and the taking of blood samples were conducted under exemptions of the Fish Resources Management Act 1994. Exemptions were granted to the Department of Primary Industries and Regional Development (DPIRD) for research. All aspects of this study conformed to the National Health and Medical Research Council (2014).
Results
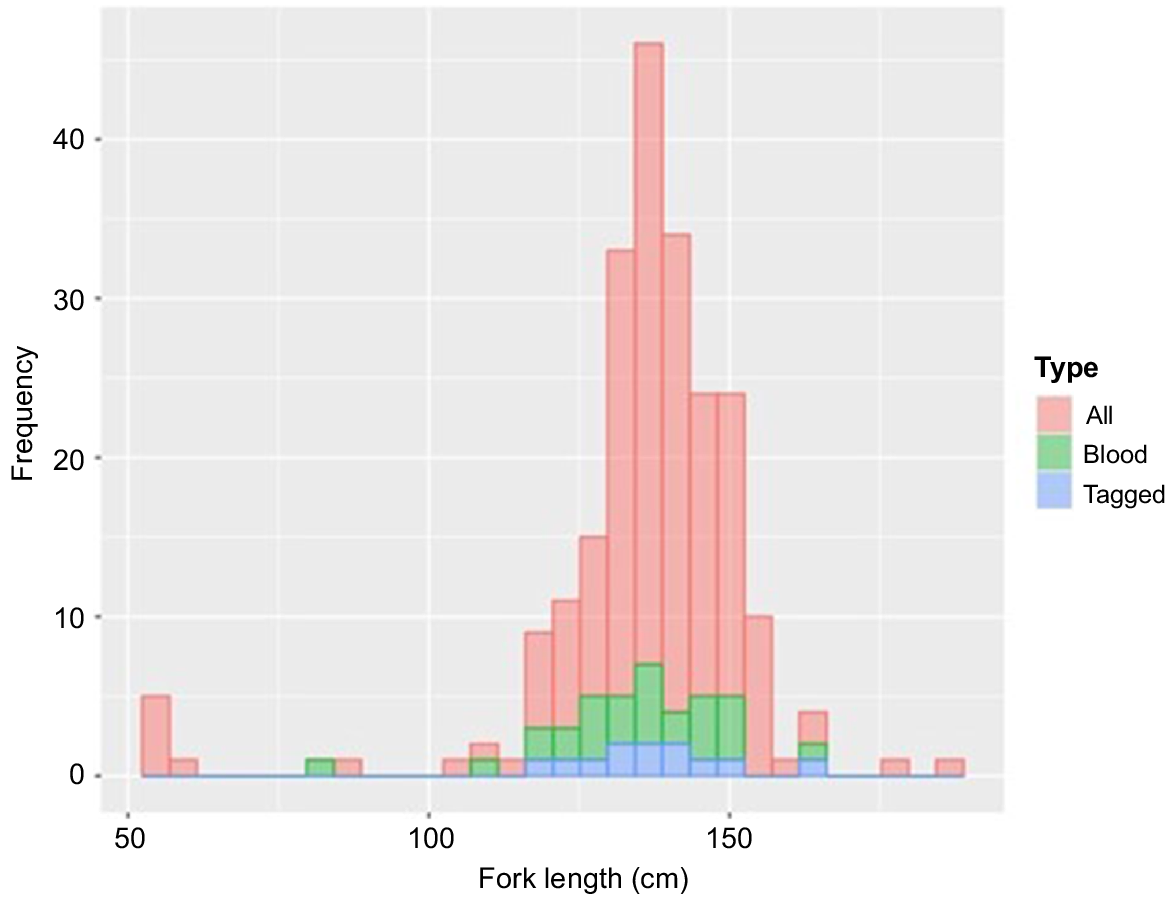
In total, 240 sandbar sharks (mean FL ± s.e. 136.7 ± 0.3 cm) were captured, with TOH ranging from 46 to 250 min (n = 28, mean TOH ± s.e. 105 ± 23 min). Most sharks (98.8%) were hooked cleanly (i.e. through the side or bottom jaw), with only three sharks being entangled in the line (all of which had blood samples taken). Two of these entangled sharks exhibited a physical injury to their gill slits. Fifty-seven sharks had blood samples (29 whole blood and 28 lactate only) collected (mean FL 138.2 ± 0.5 cm; range 82–162 cm), of which 13 were tagged with PSAT tags (mean FL 138.2 ± 1.0 cm; range 119–162 cm) (Fig. 2).
Length–frequency distribution (fork length, FL, cm) of all sandbar sharks (mean FL 136.7 ± 0.3 cm, n = 240), with sharks that were blood sampled (mean FL 138.2 ± 0.5 cm, n = 57) and tagged sharks (mean FL 138.2 ± 1.0 cm, n = 13).
Post-capture survival (PCS) – blood and plasma physiology
Of the 240 sandbar sharks caught in the surveys, 74% were assigned to Release category 1. Most of the blood-sampled sandbar sharks (n = 49) were assigned to Release category 1, three in Category 2, and five were in Category 3. Total PCS was observed in this study, and all animals were released after sampling. An ANOVA was not performed on release conditions because of a limited number of samples in Conditions 2 and 3 for all blood metabolites, resulting in little statistical relevance. Conditions 2 and 3 were combined to compare sharks released in good condition to those not in good conditions, although the sample size was still limited for the latter category.
No significant difference was detected between sexes for any blood-physiology metric (ACTH t = 0.6, d.f. = 10, P = 0.6; GCs t = 0.6, d.f. = 10, P = 0.6; and lactate t = 2.2, d.f. = 4, P = 0.1).
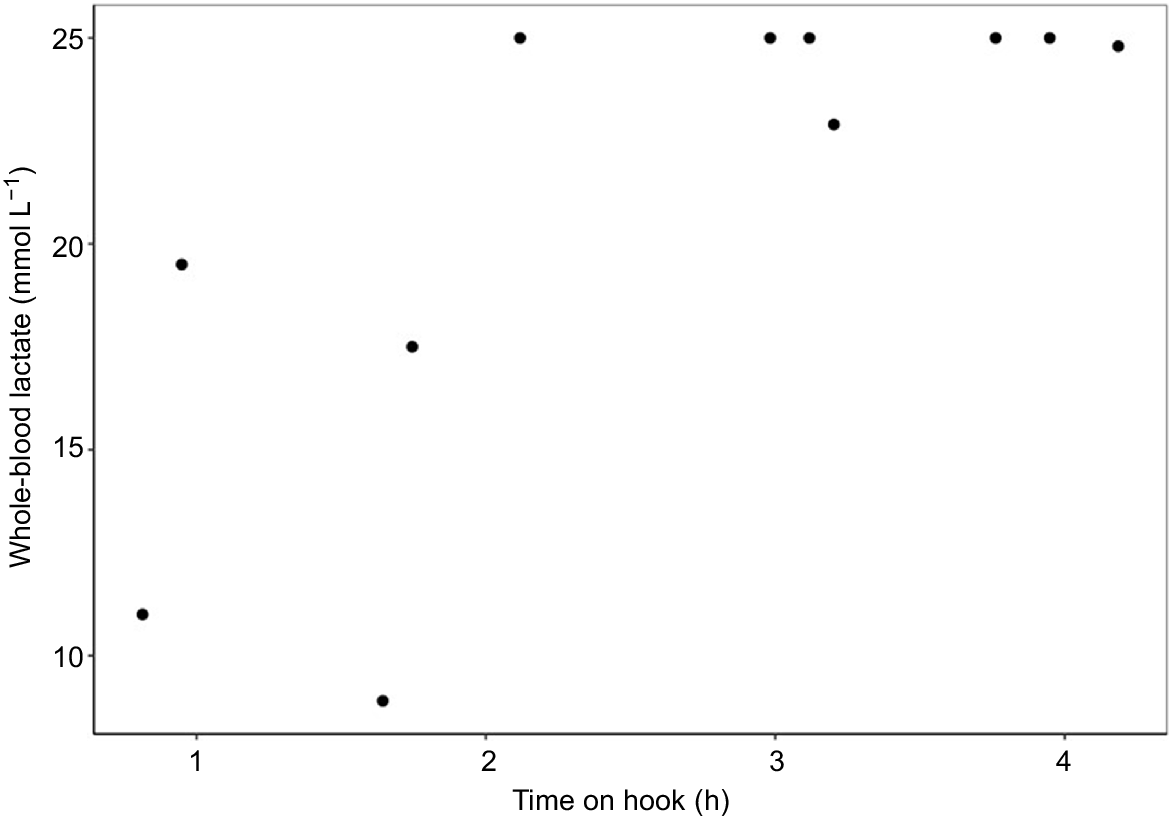
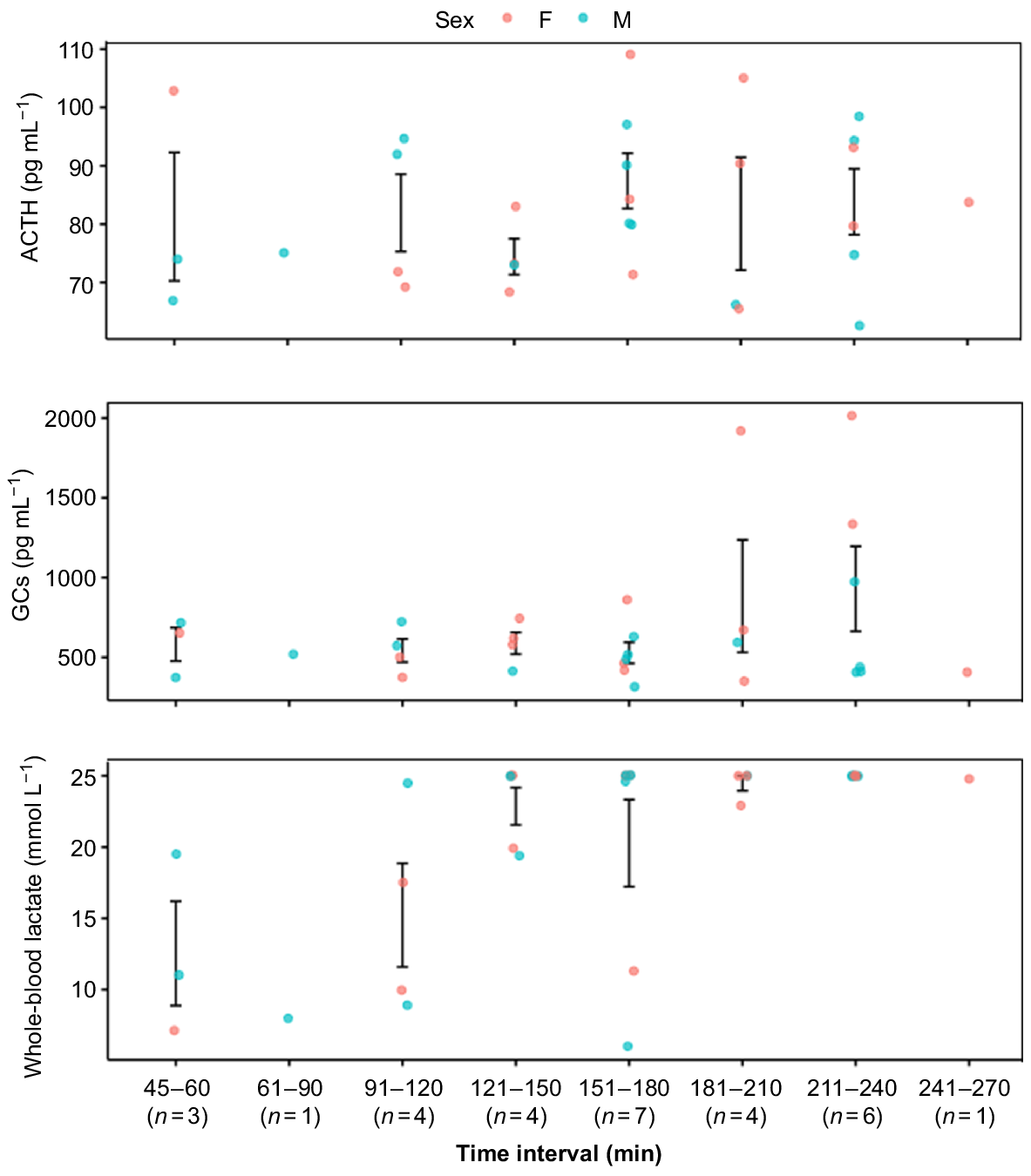
Adrenocorticotropic hormone values ranged from 62.6 to 109.0 pg mL−1, with variability being observed at each time interval. No significant (t = 0.54, d.f. = 23, P = 0.6) differences were observed in ACTH mean values through time. Glucocorticosteroid values ranged from 316.8 to 2013.7 pg mL−1, and no significant (t = 1.8, d.f. = 23, P = 0.1) differences were seen through time. Neither ACTH (χ2 = 0.03, P = 0.9) nor GCs (χ2 = 1.4, P = 0.2) showed differences with sex (Fig. 3). Whole-blood lactate continued to rise with an increasing TOH (t = 2.6, d.f. = 24, P = 0.02) (Fig. 3).
Mean whole-blood and plasma metabolite concentration of 29 subsampled sharks, with all three blood and plasma metabolite values for time intervals time on hook (TOH, min) (adrenocorticotropic hormone, ACTH; glucocorticoids, GCs). Means were calculated for sample sizes of ≥3. Error bars are standard errors. Lactate field values ranged from 0 to high (≥25 mmol L−1); high values were given a value of 25.
Whole-blood lactate was measured for all PSAT-tagged sharks (n = 13, mean 16.1 ± 7.2 mmol L−1), although only 11 tagged sharks had hook times available (Fig. 4). Whole-blood lactate had higher mean values after 2 h (before 2 h 13.9 ± 6.6 mmol L−1; after 2 h 21.9 ± 6.1 mmol L−1).
Satellite tags
Post-release survival varied from 100 to 61.5% (Scenarios #1 and 2 respectively) depending on the interpretation of premature PSAT-tag releases associated with the five ‘floater’ tags (Fig. S3). Under Scenario 1 (i.e. mortality equals ‘sinker’), there was no mortality after 30 days, so the KM model could not be fitted because of lack of contrast. Under Scenario 2, five of the tagged sharks had premature tag releases (i.e. mortality equals ‘floater’) and are assumed to be mortalities in KM models. The five tagged sharks that had premature tag releases were primarily released in Condition 1 (n = 4), with TOH ranging from 103 to 222 min (Table S1 of the Supplementary material).
For the last 5 days of deployment, tagged sandbar sharks moved in the water column between the surface (0 m) and 276-m depth (Fig. S4). There was a diel movement pattern, with some individuals moving deeper during the day (from 06:00 to 18:00 hours) and towards shallower waters at night (from 18:00 to 06:00 hours) (Fig. S4). Some sharks remained at a more constant depth during the day (e.g. SS #1) and others had larger vertical behaviours (e.g. SS #11). Shark SS #11 had the most visible diel patterns, with strong peaks in depth in the last 5 days of tag deployment (Fig. 5).
Three types of temporal patterns in depth (constant, floater, and diel) for the last 5 days of deployment for a subset of satellite-tagged sharks (day 06:00 to 18:00 hours; night 18:00 to 06:00 hours).
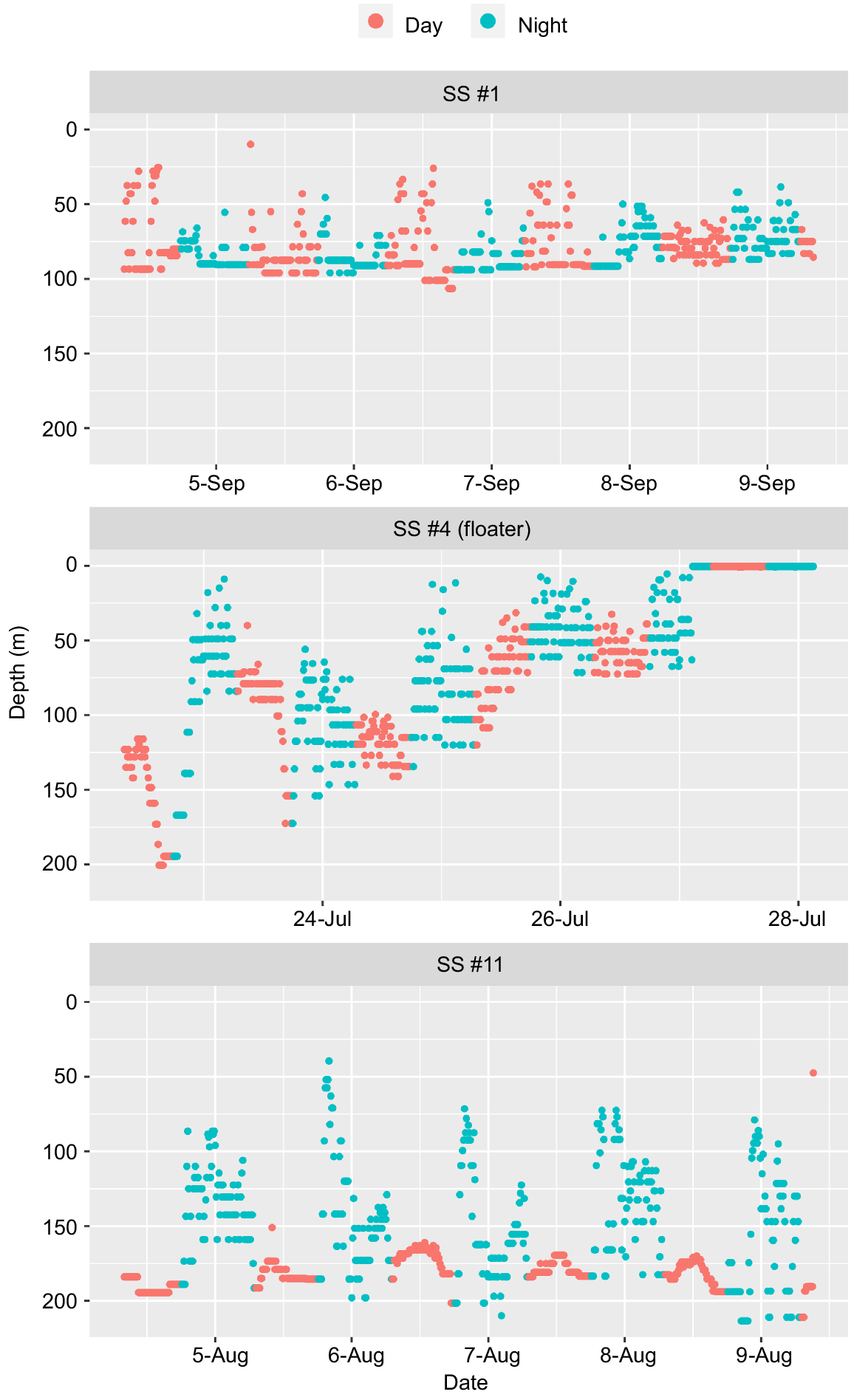
Five sharks (tag numbers SS #4, Fig. 5, and SS #2, 6, 8 and 13, Fig. S4) showed characteristics categorised as ‘floaters’, with depth being constant at 0.5 m for 24 h. All ‘floater’ tags’ temperature and light patterns remained consistent with tags at similar depth ranges that remained attached for the 30-day period. These patterns in light and temperature in the ‘floater’ tags did not conform to the values usually seen in predation events, such as an expected in changes in vertical movement profiles, and attenuation in temperature and light level variation from ingestion. There was also no evidence of a mortality event, followed by immediate scavenging and tag detachment (i.e. the animal sinking to the seafloor for a brief period (brief constant depth) before the tag detached.) For results on the minimum and maximum depth profiles of the 13 tagged individuals over the entire 30-day period, refer to Fig. S5.
Discussion
The scope of this study was to assess whether experimental surveys influence the recovering population of sandbar sharks and, subsequently, address whether the limited recapture reports from annual scientific surveys were due to capture-related mortality. We combined the use of satellite telemetry and stress physiology to assess whether the low recapture rate of sandbar sharks was due to a low PRS in the scientific surveys (8.5%) as hypothesised by Bartes et al. (2021). We found that all captured animals survived a maximum 4-h hooking time.
Post-capture survival (PCS) – blood and plasma physiology
Hormones are the first indication of stress responses in vertebrates (Armour et al. 1993; Fuller et al. 2020). In a controlled environment after an acute stress event, we would have expected to observe an early peak in ACTH, followed by an increase in the GCs concentraton on ACTH stimulation (Sapolsky et al. 2000). However, considering sharks were brought on board and sampled after at least 45 min on the hook, we were not able to register the initial hormonal behaviour at the beginning of the stress response, as shown in other shark studies performed in controlled conditions (Fuller et al. 2020; Iki et al. 2020). Although it is impossible to get baseline concentrations, we expect low concentrations in animals that are not stressed, as has been shown in Belanger et al. (2001). Previous work on ACTH as a stress parameter in the Atlantic sharpnose sharks (Rhizoprionodon terraenovae) showed concentrations (ACTH 50–120 pg mL−1) similar to those in our study (ACTH 62.64–109.04 pg mL−1) (Fuller et al. 2020). In our study, ACTH showed variable values over time, and like in the study of Fuller et al. (2020), there were no differences in ACTH with sex. The time response to GCs following the production of ACTH is variable among species, but in teleost fishes, it typically take minutes (Pankhurst 2011). In this study, no clear inhibition of ACTH concentrations was observed, likely because sharks remained stressed over the entire TOH or differing levels of energy expression in individuals (i.e. routinely swimming v. short bursts of escape behaviours) (Gallagher et al. 2017; Bouyoucos et al. 2018). Concentrations of GCs in elasmobranch species have been reported to increase less than 1 h after an acute stress event in Japanese banded houndshark (Triakis scyllium; Iki et al. 2020) and blacktip reef sharks (Carcharhinus melanopterus; Schoen et al. 2021) and up to 24 h after the initial stress event (cururu stingrays, Potamotrygon wallacei; Brinn et al. 2012). Variable PCS rates in sandbar sharks have yet to be explored concerning hormone production. The limitation of sample size in this study and other studies has reduced the ability to observe these hormonal differences among individuals and among different species. The number of samples in this study used for hormonal blood analysis was driven by equipment shortages and difficulty with taking blood (clotting of blood) and future studies should focus on larger sample sizes (n = 64) to avoid this limitation (α = 0.05, f = 0.25, power = 0.8).
During capture, increased energy expenditure or impaired respiration (e.g. restricted motility owing to length of gangion or net entanglement) leads to anaerobic metabolism, and its by-products, such as lactate (Brill et al. 2008; Skomal and Mandelman 2012). Although lactate measurements in situ cannot be used to forecast survival following a stress event by capture, the whole-blood lactate values in this study (16.1 ± 7.2 mmol L−1) are comparable to those of sandbar shark (11.5 ± 7 mmol L−1) and to those of other Carcharhinidae species (10.2 ± 11 mmol L−1) in Marshall et al.’s (2012) study. Marshall et al. (2012) compared haematological indicators in 11 species in both pelagic and demersal longlines with varying soak times of 2–12 h, although they did not use hook timers. Varying PCS was observed with 9% PCS in Atlantic sharpnose sharks and 93.2% in blue sharks (Prionace glauca) (Hutchinson and Bigelow 2019). With this study and the addition of hook timers, we have build on the understanding of the physiological effects of TOH and PCS of sandbar sharks (Mandelman and Skomal 2009; Gulak and Carlson 2021).
Most of the sandbar sharks swam strongly after release, with only a few being in worse-release conditions (i.e. Categories 2 or 3). Consequently, the elevated concentrations of GCs and ACTH over time reflect an adequate survival response for sandbar sharks caught in demersal longline surveys (Romero and Beattie 2022). Because of the small number of blood samples (n = 5, n = 3) and tagged sharks (n = 1, n = 0) in release conditions 2 or 3, release condition could not be assessed as an indicator of PCS or PRS. Release conditions vary among species and gear types, with dusky sharks having 52% in pelagic longline studies (Sulikowski et al. 2020) and Atlantic sharpnose sharks having 4% in rod and reel studies (Fuller et al. 2020) of catch recorded in release condition 1 (or equivalent rating), whereas sandbar sharks in the study of Marshall et al. (2015) had a total of 87.4%, and this study had 88% in release condition 1. Release conditions have been a useful predictor in PRS for other Carcharhinids, such as juvenile silky sharks (Carcharhinus falciformis), where larger sample sizes were available in each release-condition category (Hutchinson et al. 2015). Although qualitative metrics are difficult to compare, further research should aim to increase sample size (n = 30, d = 0.37, α = 0.05, power = 0.8) to assess whether release condition can explain the variation in PRS.
Satellite tags
Tagged individuals had 100% PRS. Sandbar sharks are known to be a robust species, with high rates of PRS (Marshall et al. 2015; Gulak and Carlson 2021; Whitney et al. 2021). Whitney et al. (2021) reported 96.4% PRS for sandbar sharks caught by longlines in a US fishery after TOH of 3 h. Further, Marshall et al. (2015) reported variable and lower PRS with a longer TOH (i.e. 100% survival with <3 h TOH; 80% survival with >4 h TOH), but this variability may also be attributed to differences in gear, handling practices, sex, and shark size, from those in other sandbar shark PRS studies (Marshall et al. 2015; Lynch et al. 2017). Our study also resulted in a higher number of adult sharks (136.7 ± 0.3 cm FL) than in the study of Marshall et al. (2015) (99.5 ± 21 cm FL). For some species, such as silky sharks, mature sharks are more robust, with 84.8% PRS in longline fisheries (Schaefer et al. 2021) compared with juveniles with 16% in purse seine fisheries (Hutchinson et al. 2015). However, as stated previously, further research would have to be conducted to fully understand the species-specific relationship between different factors and PRS (Knotek et al. 2022). Post-release survival rates of sandbar sharks are high compared with the 3 and 12% survival of dusky sharks (Carcharhinus obscurus) and 25% survival of Atlantic sharpnose sharks after 3 h TOH in other longline studies (Morgan and Burgess 2007; Marshall et al. 2012, 2015; Sulikowski et al. 2020; Whitney et al. 2021).
Previous PSAT PRS studies have shown that constant-depth signatures reflecting a dead animal on the seafloor can be used to infer the fate of PSAT-tagged sharks (French et al. 2015; Hutchinson et al. 2015; Drymon and Wells 2017; Hutchinson and Bigelow 2019). However, in the current study, released individuals remained alive until the PSAT released from the anchor. Of these, five tags released prematurely and floated on the surface as a result of improper anchorage into the tissue or tag malfunction (Kohler and Turner 2001; Hammerschlag et al. 2011; Musyl et al. 2011). Timing of post-release mortality across studies is consistent (Ellis et al. 2017), with most mortalities occurring within hours of release. Although these five individuals had a premature tag detachment days after release, which can indicate mortality or predation, it is more likely that these ‘floater’ tags are a result of tag-attachment failures. Although there was a chance for predation post-release, given predator abundance in the area, the vertical movement profiles of the five ‘floater’ tags were consistent with surviving sharks and did not display any clear evidence of a predation event or attempt (i.e. attenuated temperature and light levels or brief periods of constant seafloor depth) (Mitchell et al. 2018; Ryan et al. 2019; Braccini et al. 2021). The larger depth variances seen prior to the 24 h before tag detachment have previously been linked with surviving sandbar sharks (Marshall et al. 2015). This provides support to our assumption that 100% of the sharks tagged in this experiment survived the catch-and-release process under the longline fishing settings used as part of the survey.
The behaviour exhibited by the satellite-tagged sandbar sharks mirrors that of their natural hunting behaviour (Conrath and Musick 2008). This further supports that scientific surveys are not having negative effects on sandbar sharks. The observed day–night cycle is supported by sandbar sharks’ predominant prey item of squid, which has strong diel vertical movement patterns (Stevens and McLoughlin 1991; Last and Stevens 1994; Siwabessy et al. 2000). The presence of squid in the mouths of tagged sandbar sharks was also noted during data collection (Chris Dowling, pers. obs).
The maximum depth recorded for the entire 30-day deployment reflects the depth profile of the continental shelf in which the sharks were caught and released (Siwabessy et al. 2000; McAuley et al. 2007). Sandbar sharks are known to inhabit depths from intertidal down to 280 m (Stevens and McLoughlin 1991), with depths of 172 m having been recorded in studies by Conrath and Musick (2008). In our study, the data transmitted from the PSAT tags recorded depths of up to 307 m, deeper than previously recorded (Last and Stevens 1994; Conrath and Musick 2008; Andrzejaczek et al. 2018).
Conclusions
By combining the information on the immediate expression of ACTH, GCs and of whole-blood lactate concentrations continuing to rise with an increasing TOH (Hoffmayer and Parsons 2001; Fuller et al. 2020), we theorise that the sandbar sharks remained stressed throughout the capture process. However, the post-capture and post-release survival were high under current survey sampling practices, indicating that this species is resilient to capture and handling during longline surveys. The extended TOH of 4 h limited our ability to investigate the expression of ACTH and GCs to examine the relationship between the studied hormones and TOH. A shorter TOH would be required to fully explore this relationship through capturing the rapid expressions of these circulating hormones. To observe a more clearly defined relationship between lactate concentrations and TOH, lactate concentrations should be measured for a longer period of time after the initial stress event (McAuley et al. 2005; Gulak and Carlson 2021). Although a shorter TOH is recommended for testing primary stress responses for sandbar sharks, using whole-blood lactate as a secondary stress response could be an effective method of predicting mortality with longer exposure times, coupled with PRS monitoring to qualify modelling (Braccini et al. 2020; Gulak and Carlson 2021). The depth profiles of sandbar sharks in the last 5 days of tag deployment support the possibility of sharks following their prey through the water column, which could indicate the hardiness of the species after days returning to the ocean after the capture process, with some individuals exhibiting strong diel patterns of diving during the day and coming into shallower waters at night. Our results showed a clear indication that the current longline methods used in shark abundance surveys do not affect the post-capture and post-release survival of sandbar sharks. The combination of blood metabolites as stress parameters, release conditions and satellite tagging methods could be applied to other species or fisheries to develop predictive PRS modelling (Braccini et al. 2012). This could decrease the costs of survival studies as well as create an understanding of how each species reacts to fishing pressures.
Data availability
Data are available upon request to the authors. Please contact matias.braccini@dpird.wa.gov.au.
Declaration of funding
Funding for this research was provided by the Department of Primary Industries and Regional Development, State Government of Western Australia.
Acknowledgements
We greatly acknowledge D. Boddington, A. Tate, A. Harry, and the crew of the R.V. Naturaliste for their assistance with data collection, and C. Desfosses for aiding in proofreading. We also thank the editor and the anonymous reviewers for their valuable input.
References
Anderson WG (2012) The endocrinology of 1α-hydroxycorticosterone in elasmobranch fish: a review. Comparative Biochemistry and Physiology – A. Molecular & Integrative Physiology 162(2), 73-80.
| Crossref | Google Scholar |
Andrzejaczek S, Gleiss AC, Pattiaratchi CB, et al. (2018) First insights into the fine-scale movements of the sandbar shark, Carcharhinus plumbeus. Frontiers in Marine Science 5, 483.
| Crossref | Google Scholar |
Armour KJ, O’Toole LB, Hazon N (1993) Mechanisms of ACTH- and angiotensin II-stimulated 1α-hydroxycorticosterone secretion in the dogfish, Scyliorhinus canicula. Journal of Molecular Endocrinology 10(3), 235-244.
| Crossref | Google Scholar | PubMed |
Bartes S, Simpfendorfer C, Walker TI, et al. (2021) Conventional tagging of sharks in Western Australia: the main commercial species exhibit contrasting movement patterns. Marine and Freshwater Research 72(11), 1643-1656.
| Crossref | Google Scholar |
Belanger JM, Son JH, Laugero KD, Moberg GP, Doroshov SI, Lankford SE, Cech JJ, Jr (2001) Effects of short-term management stress and ACTH injections on plasma cortisol levels in cultured white sturgeon, Acipenser transmontanus. Aquaculture 203(1), 165-176.
| Crossref | Google Scholar |
Bouyoucos IA, Talwar BS, Brooks EJ, et al. (2018) Exercise intensity while hooked is associated with physiological status of longline-captured sharks. Conservation Physiology 6(1), coy074.
| Crossref | Google Scholar |
Braccini M, Waltrick D (2019) Species-specific at-vessel mortality of sharks and rays captured by demersal longlines. Marine Policy 99, 94-98.
| Crossref | Google Scholar |
Braccini M, Van Rijn J, Frick L (2012) High post-capture survival for sharks, rays and chimaeras discarded in the main shark fishery of Australia? PLoS ONE 7(2), e32547.
| Crossref | Google Scholar | PubMed |
Braccini M, Molony B, Blay N (2020) Patterns in abundance and size of sharks in northwestern Australia: cause for optimism. ICES Journal of Marine Science 77(1), 72-82.
| Crossref | Google Scholar |
Braccini M, Kangas M, Jaiteh V, et al. (2021) Quantifying the unreported and unaccounted domestic and foreign commercial catch of sharks and rays in Western Australia. Ambio 50(7), 1337-1350.
| Crossref | Google Scholar | PubMed |
Brill R, Bushnell P, Schroff S, et al. (2008) Effects of anaerobic exercise accompanying catch-and-release fishing on blood-oxygen affinity of the sandbar shark (Carcharhinus plumbeus, Nardo). Journal of Experimental Marine Biology and Ecology 354(1), 132-143.
| Crossref | Google Scholar |
Brinn RP, Marcon JL, McComb DM, et al. (2012) Stress responses of the endemic freshwater cururu stingray (Potamotrygon cf. histrix) during transportation in the Amazon region of the Rio Negro. Comparative Biochemistry and Physiology – A. Molecular & Integrative Physiology 162(2), 139-145.
| Crossref | Google Scholar |
Cardeñosa D, Fields AT, Babcock EA, et al. (2020) Species composition of the largest shark fin retail-market in mainland China. Scientific Reports 10(1), 12914.
| Crossref | Google Scholar | PubMed |
Clarke SC, McAllister MK, Milner-Gulland EJ, et al. (2006) Global estimates of shark catches using trade records from commercial markets. Ecology Letters 9(10), 1115-1126.
| Crossref | Google Scholar | PubMed |
Clarke S, Milner-Gulland EJ, Bjørndal T (2007) Social, economic, and regulatory drivers of the shark fin trade. Marine Resource Economics 22(3), 305-327.
| Crossref | Google Scholar |
Clementi GM, Babcock EA, Valentin-Albanese J, et al. (2021) Anthropogenic pressures on reef-associated sharks in jurisdictions with and without directed shark fishing. Marine Ecology Progress Series 661, 175-186.
| Crossref | Google Scholar |
Conrath CL, Musick JA (2008) Investigations into depth and temperature habitat utilization and overwintering grounds of juvenile sandbar sharks, Carcharhinus plumbeus: the importance of near shore North Carolina waters. Environmental Biology of Fishes 82(2), 123-131.
| Crossref | Google Scholar |
Dapp DR, Huveneers C, Walker TI, et al. (2016) Moving from measuring to predicting bycatch mortality: predicting the capture condition of a longline-caught pelagic shark. Frontiers in Marine Science 2, 126.
| Crossref | Google Scholar |
Davidson LNK, Krawchuk MA, Dulvy NK (2016) Why have global shark and ray landings declined: improved management or overfishing? Fish and Fisheries 17(2), 438-458.
| Crossref | Google Scholar |
Drymon JM, Wells RJD (2017) Double tagging clarifies post-release fate of great hammerheads (Sphyrna mokarran). Animal Biotelemetry 5(1), 28.
| Crossref | Google Scholar |
Dulvy NK, Pacoureau N, Rigby CL, et al. (2021) Overfishing drives over one-third of all sharks and rays toward a global extinction crisis. Current Biology 31(21), 4773-4787.E8.
| Crossref | Google Scholar |
Ellis JR, McCully Phillips SR, Poisson F (2017) A review of capture and post-release mortality of elasmobranchs. Journal of Fish Biology 90(3), 653-722.
| Crossref | Google Scholar | PubMed |
Evans AN, Rimoldi JM, Gadepalli RSV, Nunez BS (2010) Adaptation of a corticosterone ELISA to demonstrate sequence-specific effects of angiotensin II peptides and C-type natriuretic peptide on 1α-hydroxycorticosterone synthesis and steroidogenic mRNAs in the elasmobranch interrenal gland. The Journal of Steroid Biochemistry and Molecular Biology 120(4–5), 149-154.
| Crossref | Google Scholar |
Fasiolo M, Nedellec R, Goude Y, Wood SN (2019) Scalable visualisation methods for modern generalized additive models. arXiv 9 May 2019. [Preprint].
| Crossref | Google Scholar |
Fox J, Weisberg S (2019) ‘An R companion to applied regression.’ (Sage: Thousand Oaks, CA, USA) Available at https://socialsciences.mcmaster.ca/jfox/Books/Companion/
French RP, Lyle J, Tracey S, et al. (2015) High survivorship after catch-and-release fishing suggests physiological resilience in the endothermic shortfin mako shark (Isurus oxyrinchus). Conservation Physiology 3(1), cov044.
| Crossref | Google Scholar |
Fuller L, Stell E, Leary C, et al. (2020) Circulating adrenocorticotropic hormone levels, lactate levels, hematocrit and osmolality in relation to capture stress in Atlantic sharpnose sharks, Rhizoprionodon terraenovae. Comparative Biochemistry and Physiology – A. Molecular & Integrative Physiology 243, 110655.
| Crossref | Google Scholar |
Gallagher AJ, Staaterman ER, Cooke SJ, Hammerschlag N (2017) Behavioural responses to fisheries capture among sharks caught using experimental fishery gear. Canadian Journal of Fisheries and Aquatic Sciences 74, 1-7.
| Crossref | Google Scholar |
Grolemund G, Wickham H (2011) Dates and times made easy with lubridate. Journal of Statistical Software 40(3), 1-25.
| Crossref | Google Scholar |
Gulak SJB, Carlson JK (2021) Less soak time saves those upon the line: capture times and hooking mortality of sharks caught on bottom longlines. North American Journal of Fisheries Management 41(3), 791-808.
| Crossref | Google Scholar |
Hammerschlag N, Gallagher AJ, Lazarre DM (2011) A review of shark satellite tagging studies. Journal of Experimental Marine Biology and Ecology 398(1–2), 1-8.
| Crossref | Google Scholar |
Hammerschlag N, Meÿer M, Seakamela SM, Kirkman S, Fallows C, Creel S (2017) Physiological stress responses to natural variation in predation risk: evidence from white sharks and seals. Ecology 98, 3199-3210.
| Crossref | Google Scholar |
Heberer C, Aalbers SA, Bernal D, et al. (2010) Insights into catch-and-release survivorship and stress-induced blood biochemistry of common thresher sharks (Alopias vulpinus) captured in the southern California recreational fishery. Fisheries Research 106(3), 495-500.
| Crossref | Google Scholar |
Hoffmayer ER, Parsons GR (2001) The physiological response to capture and handling stress in the Atlantic sharpnose shark, Rhizoprionodon terraenovae. Fish Physiology and Biochemistry 25(4), 277-285.
| Crossref | Google Scholar |
Hueter RE, Manire CA, Tyminski JP, et al. (2006) Assessing mortality of released or discarded fish using a logistic model of relative survival derived from tagging data. Transactions of the American Fisheries Society 135(2), 500-508.
| Crossref | Google Scholar |
Hutchinson M, Bigelow K (2019) Quantifying post release mortality rates of sharks incidentally captured in Pacific tuna longline fisheries and identifying handling practices to improve survivorship. Working paper WP-19-003, Pacific Islands Fisheries Science Center, National Marine Fisheries Service, National Oceanic and Atmospheric Administration, USA. doi:10.25923/2SXY-S659
Hutchinson MR, Itano DG, Muir JA, et al. (2015) Post-release survival of juvenile silky sharks captured in a tropical tuna purse seine fishery. Marine Ecology Progress Series 521, 143-154.
| Crossref | Google Scholar |
Iki A, Anderson WG, Deck CA, et al. (2020) Measurement of 1α hydroxycorticosterone in the Japanese banded houndshark, Triakis scyllium, following exposure to a series of stressors. General and Comparative Endocrinology 292, 113440.
| Crossref | Google Scholar | PubMed |
Karsten AH (2000) Stress effects on fecal corticosterone levels in the epaulette shark, Hemiscyllium ocellatum. PhD dissertation, Medical College of Ohio. Available at https://search.proquest.com/openview/323873e556b1097b8cb93462d9a6ddf9/1?pq-origsite=gscholar&cbl=18750&diss=y
Kneebone J, Chisholm J, Bernal D, et al. (2013) The physiological effects of capture stress, recovery, and post-release survivorship of juvenile sand tigers (Carcharias taurus) caught on rod and reel. Fisheries Research 147, 103-114.
| Crossref | Google Scholar |
Knotek RJ, Frazier BS, Daly-Engel TS, et al. (2022) Post-release mortality, recovery, and stress physiology of blacknose sharks, Carcharhinus acronotus, in the Southeast US recreational shark fishery. Fisheries Research 254, 106406.
| Crossref | Google Scholar |
Kohler NE, Turner PA (2001) Shark tagging: a review of conventional methods and studies. In ‘The behavior and sensory biology of elasmobranch fishes: an anthology in memory of Donald Richard Nelson’. (Eds TC Tricas, SH Gruber) pp. 191–224. (Springer Netherlands: Dordrecht, Netherlands) doi:10.1007/978-94-017-3245-1_12
Last PR, Stevens JD (1994) ‘Sharks and rays of Australia.’ (CSIRO: Melbourne, Vic., Australia) Available at http://hdl.handle.net/102.100.100/239780?index=1
Lynch SD, Marcek BJ, Marshall HM, et al. (2017) The effects of pop-up satellite archival tags (PSATs) on the metabolic rate and swimming kinematics of juvenile sandbar shark Carcharhinus plumbeus. Fisheries Research 186, 205-215.
| Crossref | Google Scholar |
Lyons K, Wynne-Edwards KE (2019) Legacy environmental polychlorinated biphenyl contamination attenuates the acute stress response in a cartilaginous fish, the round stingray. Stress 22(3), 395-402.
| Crossref | Google Scholar |
Mandelman JW, Skomal GB (2009) Differential sensitivity to capture stress assessed by blood acid–base status in five carcharhinid sharks. Journal of Comparative Physiology – B. Biochemical, Systems, and Environmental Physiology 179(3), 267-277.
| Crossref | Google Scholar | PubMed |
Manire CA, Rasmussen LE, Maruska KP, Tricas TC (2007) Sex, seasonal, and stress-related variations in elasmobranch corticosterone concentrations. Comparative Biochemistry and Physiology – A. Molecular & Integrative Physiology 148(4), 926-935.
| Crossref | Google Scholar |
Marshall H, Field L, Afiadata A, et al. (2012) Hematological indicators of stress in longline-captured sharks. Comparative Biochemistry and Physiology – A. Molecular & Integrative Physiology 162(2), 121-129.
| Crossref | Google Scholar |
Marshall H, Skomal G, Ross PG, et al. (2015) At-vessel and post-release mortality of the dusky (Carcharhinus obscurus) and sandbar (C. plumbeus) sharks after longline capture. Fisheries Research 172, 373-384.
| Crossref | Google Scholar |
McAuley RB, Simpfendorfer CA, Hyndes GA, et al. (2007) Distribution and reproductive biology of the sandbar shark, Carcharhinus plumbeus (Nardo), in Western Australian waters. Marine and Freshwater Research 58(1), 116-126.
| Crossref | Google Scholar |
Mitchell JD, McLean DL, Collin SP, et al. (2018) Quantifying shark depredation in a recreational fishery in the Ningaloo Marine Park and Exmouth Gulf, Western Australia. Marine Ecology Progress Series 587, 141-157.
| Crossref | Google Scholar |
Mohan JA, Jones ER, Hendon JM, et al. (2020) Capture stress and post-release mortality of blacktip sharks in recreational charter fisheries of the Gulf of Mexico. Conservation Physiology 8(1), coaa041.
| Crossref | Google Scholar |
Morgan A, Burgess GH (2007) At-vessel fishing mortality for six species of sharks caught in the northwest Atlantic and Gulf of Mexico. Gulf and Caribbean Research 19, 123-129.
| Crossref | Google Scholar |
Musyl MK, Domeier ML, Nasby-Lucas N, et al. (2011) Performance of pop-up satellite archival tags. Marine Ecology Progress Series 433, 1-28.
| Crossref | Google Scholar |
National Health and Medical Research Council (2014) ‘Australian Code for the Care and Use of Animals for Scientific Purposes’, 8th edn. (NHMRC: Canberra, ACT, Australia) Available at https://www.nhmrc.gov.au/about-us/publications/australian-code-care-and-use-animals-scientific-purposes/australian-code-care-and-use-animals-scientific-purposes-code [Verified 17 November 2023]
Oliver S, Braccini M, Newman SJ, et al. (2015) Global patterns in the bycatch of sharks and rays. Marine Policy 54, 86-97.
| Crossref | Google Scholar |
Pacoureau N, Rigby CL, Kyne PM, Sherley RB, Winker H, Carlson JK, Fordham SV, Barreto R, Fernando D, Francis MP, Jabado RW, Herman KB, Liu K-M, Marshall AD, Pollom RA, Romanov EV, Simpfendorfer CA, Yin JS, Kindsvater HK, Dulvy NK (2021) Half a century of global decline in oceanic sharks and rays. Nature 589(7843), 567-571.
| Crossref | Google Scholar |
Pankhurst NW (2011) The endocrinology of stress in fish: an environmental perspective. General and Comparative Endocrinology 170(2), 265-275.
| Crossref | Google Scholar | PubMed |
Rasmussen LEL, Crow GL (1993) Serum corticosterone concentrations in immature captive whitetip reef sharks, Triaenodon obesus. Journal of Experimental Zoology 267(3), 283-287.
| Crossref | Google Scholar |
Romero LM, Beattie UK (2022) Common myths of glucocorticoid function in ecology and conservation. Journal of Experimental Zoology – A. Ecological and Integrative Physiology 337(1), 7-14.
| Crossref | Google Scholar | PubMed |
Ruiz-Jarabo I, Barragán-Méndez C, Jerez-Cepa I, et al. (2019) Plasma 1α-hydroxycorticosterone as biomarker for acute stress in catsharks (Scyliorhinus canicula). Frontiers in Physiology 10, 1217.
| Crossref | Google Scholar |
Rulifson RA (2007) Spiny dogfish mortality induced by gill-net and trawl capture and tag and release. North American Journal of Fisheries Management 27(1), 279-285.
| Crossref | Google Scholar |
Ryan KL, Taylor SM, McAuley R, et al. (2019) Quantifying shark depredation events while commercial, charter and recreational fishing in Western Australia. Marine Policy 109, 103674.
| Crossref | Google Scholar |
Sapolsky RM, Romero LM, Munck AU (2000) How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocrine Reviews 21(1), 55-89.
| Crossref | Google Scholar | PubMed |
Schaefer K, Fuller D, Castillo-Geniz JL, et al. (2021) Post-release survival of silky sharks (Carcharhinus falciformis) following capture by Mexican flag longline fishing vessels in the northeastern Pacific Ocean. Fisheries Research 234, 105779.
| Crossref | Google Scholar |
Schoen AN, Bouyoucos IA, Anderson WG, et al. (2021) Simulated heatwave and fishing stressors alter corticosteroid and energy balance in neonate blacktip reef sharks, Carcharhinus melanopterus. Conservation Physiology 9, coab067.
| Crossref | Google Scholar |
Siwabessy PJW, Penrose JD, Fox DR, Kloser RJ (2000) Bottom classification in the continental shelf: a case study for the north-west and south-east shelf of Australia. Joondalup, Australia. In ‘Acoustics 2000: Putting the Science and Technology to Work’, 15–17 November 2000, Joondalup, WA, Australia. (Eds T McMinn, G Yates) pp. 265–270. (Australian Acoustical Society) Available at https://acoustics.asn.au/conference_proceedings/2000-Putting%20Science%20and%20Technology%20to%20Work.pdf
Skomal GB, Mandelman JW (2012) The physiological response to anthropogenic stressors in marine elasmobranch fishes: a review with a focus on the secondary response. Comparative Biochemistry and Physiology – A. Molecular & Integrative Physiology 162(2), 146-155.
| Crossref | Google Scholar |
Snelson FF, Jr, Rasmussen LEL, Johnson MR, et al. (1997) Serum concentrations of steroid hormones during reproduction in the Atlantic stingray, Dasyatis Sabina. General and Comparative Endocrinology 108(1), 67-79.
| Crossref | Google Scholar | PubMed |
Stevens JD, McLoughlin KJ (1991) Distribution, size and sex composition, reproductive biology and diet of sharks from northern Australia. Marine and Freshwater Research 42(2), 151-199.
| Crossref | Google Scholar |
Sulikowski JA, Golet W, Hoffmayer ER, et al. (2020) Observing post-release mortality for dusky sharks, Carcharhinus obscurus, captured in the US pelagic longline fishery. Fisheries Research 221, 105341.
| Crossref | Google Scholar |
Whitney NM, Lear KO, Morris JJ, et al. (2021) Connecting post-release mortality to the physiological stress response of large coastal sharks in a commercial longline fishery. PLoS ONE 16(9), e0255673.
| Crossref | Google Scholar | PubMed |
Wickham H (2016) ‘ggplot2: elegant graphics for data analysis.’ (Springer-Verlag: New York, NY, USA) Available at https://ggplot2.tidyverse.org
Wickham H, Averick M, Bryan J, et al. (2019) Welcome to the tidyverse. Journal of Open Source Software 4(43), 1686.
| Crossref | Google Scholar |
Wood SN (2017) ‘Generalized additive models: an introduction with R’, 2nd edn. (Taylor and Francis) doi:10.1201/9781315370279