Noisy neighbours: effects of construction noises on nesting seabirds
Larissa Iasiello A and Diane Colombelli-Négrel A *
A *
A College of Science and Engineering, Flinders University, Adelaide, SA, Australia.
Marine and Freshwater Research 74(7) 573-585 https://doi.org/10.1071/MF22138
Submitted: 14 July 2022 Accepted: 21 March 2023 Published: 20 April 2023
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Context: Seabirds are important bio-indicators that play an important role in nutrient cycling within coastal communities. Yet, the impact of anthropogenic noises produced from coastal developments across seabird species has received little attention. To create more refined and effective mitigation strategies, a better understanding of how different seabird species and individuals respond to anthropogenic noise is required.
Aims: This study aimed to assess how individual seabirds respond to noises resulting from coastal development (construction noises).
Methods: We investigated the behavioural (vigilance, distress) and physiological (heart rate) responses of little penguins (Eudyptula minor) to experimental playback of construction noises and the potential impacts of construction noises on breeding success.
Key results: Little penguins spent significantly more time in vigilance (but showed no increase in heart rate) during the construction noise playback than they did during the control. Nests exposed to the noise experiment were more likely to produce at least one fledgling compared with those that were not.
Conclusions and implications: Our results support the distracted prey hypothesis, which over long periods may reduce the time individuals spend performing biologically important behaviours and increase predation risk.
Keywords: coastal management, declining populations, noise pollution, Phalacrocorax fuscescens, seabirds, sensory disturbance, stress response, Thalasseus bergii.
Introduction
Anthropogenic noise produced from coastal developments (e.g. buildings, infrastructure, transportation, and marine industries), as well as tourism and recreational activities, is continuously expanding and altering the sensory environment, thereby causing adverse effects on wildlife (Jefferson et al. 2009; Lai et al. 2015). Sensory disturbance, such as from coastal developments, can invoke a stress response in animals, where their attention is consumed by the introduced stimulus, which reduces the time they spend performing biologically important behaviours, such as foraging, communicating, and breeding (Chan and Blumstein 2011; Derose-Wilson et al. 2015; Buxton et al. 2017a; Franks 2017; Bevan et al. 2018). Distracted animals may also be more susceptible to fatal attacks from predators as their preoccupied state of mind reduces their ability to detect and avoid threats (Chan et al. 2010). Considering the continued expansion of human developments along coastlines, it is essential to investigate how different coastal species and individuals respond to anthropogenic noise for future mitigation strategies.
Seabirds are the most vulnerable group of birds (reviewed in Dias et al. 2019) and play vital roles in ecosystem-functioning processes within coastal habitats, such as trophic regulation, nutrient transportation, and community shaping (Graham et al. 2018). Owing to the integral role seabirds play within ecosystems, they are often used as monitors of environmental impacts caused by anthropogenic activities, both on land and at sea (Henny et al. 1982; Cifuentes et al. 2003; Votier et al. 2011; Voulgaris 2017; Ryan 2018; Thushari and Senevirathna 2020; Bianchini et al. 2022). Although all animals use their senses to navigate through their environment, acquire resources, avoid threats or for social activities (Niven and Laughlin 2008), seabirds’ senses are highly adapted to their environment (Boyle and Samson 1985; Tablado and Jenni 2015; Blackwell et al. 2016; Blumstein et al. 2017; Buxton et al. 2017a), making them highly sensitive to environmental sensory pollution (Vander Pol et al. 2009; Piña-Ortiz et al. 2016; Rodríguez et al. 2017; Ryan 2018; Mooney et al. 2019). Yet, the impacts of anthropogenic noise pollution from coastal developments on seabirds has received little attention, with most studies focusing on the effects of human voices (Australian pelican, Pelecanus conspicillatus; crested tern, Thalasseus bergii; brown noddy, Anous stolidus, see Melanie 2004; storm petrel, Hydrobates pelagicus, see Watson et al. 2014; Brandt’s cormorant, Phalacrocorax penicillatus, see Buxton et al. 2017a), boat or shipping traffic noises (27 artic seabird species, see Humphries and Huettmann 2014; storm petrel, see Soldatini et al. 2015), or aircraft noises (crested tern, see Brown 1990; king penguin, Aptenodytes patagonicus, see Hughes et al. 2008; Wilson’s plover, Charadrius wilsonia, see Derose-Wilson et al. 2015; crested tern, see Bevan et al. 2018), and only one study investigating the relationship between ambient anthropogenic noise produced from coastal developments and vigilance response in Eurasian oystercatchers (Haematopus ostralegus; Franks 2017). Because the distribution of seabird species largely overlaps with coastal and offshore anthropogenic activities, it is important to understand how introduced anthropogenic noise can affect these highly sensitive birds.
Across seabirds species, long-term fitness implications at the individual level may result from short-term exposure to introduced anthropogenic noise (Barnett and Hemsworth 1990; Calow and Forbes 1998), which in turn can lead to population declines (Melanie 2004; MacDougall et al. 2013; Scopel and Diamond 2017; Pink and Abrahams 2018) or abandonment of disturbed sites (Bennie et al. 2018; Thompson 2021). For example, repeated human visitation deterred mature Brandt’s cormorants from breeding in their regular breeding site (e.g. Buxton et al. 2017a), and a single short-term event caused an entire breeding colony of elegant terns (Thalasseus elegans) to abandon their nesting site (e.g. Thompson 2021). During sensitive lifecycle processes, such as breeding or moulting in seabirds, an individual’s stress response to a disturbance can be intensified and lead to reduced breeding success (Buxton et al. 2017a; Liu et al. 2020). For example, Brisson-Curadeau et al. (2017) found that experimental drone flights increased predation potential on thick-billed murre (Uria lomvia) eggs as a result of incubating adults initiating avoidance behaviours in response to drone proximity, and thus leaving their nest vulnerable to aerial predators. Repeated reduction in breeding success can halt population growth and potentially lead to population collapse (Scopel and Diamond 2017; Pink and Abrahams 2018). Identifying at which point an individual initially becomes stressed by an introduced stimulus, before it experiences long-term fitness costs, is vital to inform effective mitigation strategies.
When investigating how individuals respond to disturbance, there is increasing evidence that both behavioural and physiological responses need to be considered. Indeed, some species or individuals may not display any anti-predator behaviours but still produce an internal stress response (Weimerskirch et al. 2002; Ellenberg et al. 2006; Soldatini et al. 2015). For example, Adelie penguins (Pygoscelis adeliae) increase their heart rate when approached within 15 m by a human, without showing any modification to their behaviour (Giese 1998). Similarly, incubating king penguins showed no behavioural response to drones flying 3 m above their head, whereas their heart rates increased when drones were 10 m above their head (Weimerskirch et al. 2018). In their review on animal welfare, Barnett and Hemsworth (1990) showed that individual pigs (Sus scrofa domesticus) experiencing regular stress may be subjected to ulcers, hypertension, arteriosclerosis, and suppression of their immune system, all of which reduce an individual’s fitness and ability to perform biologically important tasks to an optimal standard. Undiagnosed periods of stress in seabirds has also been found to lead to increased energy expenditure that may have long-term negative fitness consequences for the individuals (see Weimerskirch et al. 2002; Ellenberg et al. 2006; Larcombe 2016).
Responses to human-related disturbance can vary among seabird species (Culik et al. 1990; Buxton et al. 2017a) and sexes (Weimerskirch et al. 2002; Ellenberg et al. 2009). For example, Brandt’s cormorants increased their vigilance in response to human voices (Buxton et al. 2017a), whereas Adelie penguins did not (Culik et al. 1990). Similarly, great cormorants (Phalacrocorax carbo) did not avoid offshore wind turbine sites and instead used the stationary structures as roosting sites, whereas northern gannets (Morus bassanus) showed strong avoidance behaviour in sites with wind turbines (Dierschke et al. 2016). Seabird stress response can also vary at the individual level depending on their sex. For example, a study by Weimerskirch et al. (2002) found that male wandering albatross (Diomedea exulans) exhibited a more intense response than did females prior to handling. Similarly, Ellenberg et al. (2009) found that female yellow-eyed penguins (Megaduptes antipodes) exposed to human presence exhibited lower heart rates, but longer recovery times, than did males, suggesting that males are more defensive but less stressed than are females. Male birds tend to display more intense defensive behaviours, maybe because of higher testosterone production (Kazama et al. 2011), whereas females tend to be more sensitive to disturbance at the nest owing to already existing pressures linked to storing body reserves and laying eggs (Goutte et al. 2010). Therefore, species- and sex-specific studies are important to better understand seabird response to human-related disturbances.
This study investigated the behavioural and physiological responses of little penguins (Eudyptula minor) to introduced anthropogenic noises resulting from coastal development (specifically, construction noises) v. rainfall noises (our control) and the potential impacts of construction noises on little penguin breeding success. On the basis of previous literature in birds and their response to anthropogenic noises (Brown 1990; Melanie 2004; Hughes et al. 2008; Humphries and Huettmann 2014; Watson et al. 2014; Derose-Wilson et al. 2015; Soldatini et al. 2015; Buxton et al. 2017a, 2017b; Franks 2017; Bevan et al. 2018), we hypothesised that individuals would display higher vigilance and distressed behaviours in response to the construction noises than to the rainfall noises. We also hypothesised that sexes will not differ in their behavioural and physiological response on the basis of previous studies in little penguins (Carroll et al. 2016; Schaefer and Colombelli-Négrel 2021). Finally, we hypothesised that breeding success will be lower for the nests exposed to the noise experiments than for those that were not (Klomp et al. 1991, Weerheim et al. 2003; Giling et al. 2008).
We tested the short-term response of little penguins to experimental playback of construction and rainfall (control) noises on Lipson Island (South Australia). This colony was selected because of the scheduled construction of a new port facility in 2022, 1.5 km away from Lipson Island (Ports Peninsula 2022). We chose to use construction noises as our disturbance stimulus to mimic the noises that would be created from the development of the new port. Because the study site is remote, surrounded by agricultural lands, and has not been previously exposed to major developments, it is important to investigate the impact of introduced anthropogenic noise on the locally breeding seabirds that were previously undisturbed by this stimulus. To better understand the impacts of the development on the seabird community (see Taylor et al. 2013; Scopel and Diamond 2017; Pink and Abrahams 2018), we originally planned to conduct playback experiments and monitor the breeding success of the following three seabirds species that are closely associated on Lipson Island and nest in the same habitat: little penguins, black-faced cormorants (Phalacrocorax fuscescens) and crested terns (T. bergii). However, experiments on cormorants and terns were not possible because of unforeseen circumstances. The black-faced cormorants, for example, started breeding at an unusual time (in February instead of July) and all breeding had finished by the time we started our experiments. In the case of the crested terns, video recordings at night (L. Iasiello and D. Colombelli-Négrel, unpubl. data) showed that all crested terns abandoned their nests and eggs for an unknown reason and, as a result, all eggs were predated on by silver gulls (Chroicocephalus novaehollandiae). Hence, in this study, we focused on the little penguins only and present the results of our monitoring on black-faced cormorants and crested terns in the Supplementary material (see also Fig. 1).
Materials and methods
Study location
All our experiments and observations were conducted on Lipson Island Conservation Park (34°26′S, 136°26′E) located in Lipson Cove, 70 km north of Port Lincoln, South Australia (Fig. 1). Lipson Island is a sandy island with a rocky coastline proclaimed as a Fauna Conservation Reserve in 1967, then renamed as a Conservation Park in 1972 (Birds SA 2021). The island is 336 m long, with a maximum width of 100 m, and is located 250 m from the mainland beach of Lipson Cove (Fig. 1). The island is accessible to the public by foot at low tide during the summer months, yet no terrestrial predators (e.g. dogs, Canis lupus familiaris; cats, Felis catus; foxes, Vulpes vulpes) have been witnessed to access the island (Robinson et al. 1996). The island is dominated by nitre bbush (Nitraria billardierei) and common iceplant (Mesembryanthemum crystallinum) (Robinson et al. 1996), which covers the majority of the island during the winter months and dies off during the summer months (L. Iasiello, pers. obs.). Numerous bird species have been recorded on Lipson Island, but the only species known to breed on the island include black-faced cormorants, pied cormorants (Phalacrocorax varius), silver gulls (C. novaehollandiae), feral pigeons (Columba livia domestica), crested terns, sooty oystercatchers (Haematopus fuliginosus), pacific gulls (Larus pacificus), and little penguins (Bird SA 2021; L. Iasiello and D. Colombelli-Négrel, pers. obs.). Other bird species of importance that have been recorded, but do not breed on the island, include rock parrots (Neophema petrophila), red-capped plovers (Charadrius ruficapillus), Australian pelicans (P. conspicillatus), white-bellied sea eagles (Haliaeetus leucogaster), and Caspian terns (Hydroprogne caspia) (Bird SA 2021; L. Iasiello, pers. obs.).
Study species
Little penguins in Australia can breed anytime between April and March (Reilly and Cullen 1981; Johannesen et al. 2003; Colombelli-Négrel 2015; Johnson and Colombelli-Négrel 2021). Breeding little penguins are central-place foragers, foraging up to 20–30 km away from their breeding site during the day (Collins et al. 1999; Hoskins et al. 2008). Each breeding pair lays up to two eggs per clutch and up to two clutches per breeding season (Reilly and Cullen 1981; Kemp and Dann 2001; Johannesen et al. 2003; Colombelli-Négrel 2015). During incubation and the first 2 weeks of chick-rearing, the male and female take turns to remain in the nest incubating the eggs or guarding the chicks while the other is out foraging (Saraux et al. 2011a, 2011b). After the chick guarding period, both adults forage during the day, leaving the chicks unattended, and return after dark to feed their chicks until the chicks are 8–9 weeks old (Numata et al. 2004). The incubation period lasts 33–44 days, and chicks normally fledge ~8–9 weeks after hatching (Kemp and Dann 2001; Colombelli-Négrel 2015). Little penguins are known to breed in a variety of nest types, such as surface nests (scrapes under an open bush), sand nests (nests dug in soft sand), bush nests (scrapes deep under a thick bush), rock nests (burrows under boulder or in rock crevices), or artificial nests (plastic boxes with rocks, metal drums or concrete structure) (Marker 2016; Colombelli-Négrel 2019). The most common nest type on Lipson Island was sand nests (which represented 86% of the nests found in this study), but some penguins also bred in rock nests (which represented 14% of the nests found). In May 2011, a 2-day survey as part of Port Spencer’s public environment report recorded only 29 little penguins on Lipson Island (Madden-Hallet et al. 2011).
Playback experiment
In total, 33 adult little penguins from 26 different nests were tested with the experimental broadcast of (1) construction and (2) rainfall noises. All playback experiments were conducted during the day (between 08:00 and 14:00 hours local time) between May and August 2021; all experiments lasted 2 h once started. None of the tested individuals had previously been captured for research purposes, but all individuals potentially had some past exposure to human presence because of the proximity of Lipson Island to the mainland and the fact that locals have been previously recorded on the island during summer (C. Berryman, pers. comm.). Therefore, all tested individuals were expected to have a similar experience with humans, and differences in stress responses were not likely to be related to any prior manipulation. At the time of the experiment, only one adult was present in each of the tested nests.
For each tested nest, the sex of the adult penguin present in the nest was confirmed using photographic identification, as previously described for little penguins (Colombelli-Négrel and Smale 2018; Wasiak 2020; Schaefer and Colombelli-Négrel 2021); the fact that the bird was incubating eggs (and not sitting on young chicks) was also established. A dummy egg was then added to the clutch without removing any of the natural eggs to record their physiological response (heart rate) following Schaefer and Colombelli-Négrel (2021). The dummy egg contained an internal omnidirectional lavalier condenser microphone (WL183, Shure Inc., USA) connected to either a Zoom H4n (Zoom North America, USA) or a Tascam DR-05 (TEAC Corporation, USA) as described in Schaefer and Colombelli-Négrel (2021). Once the dummy egg was placed, a Sony AS20 Action Camera (Sony Corporation, Australia) was set up directly at the entrance of the nest to record the adult behavioural responses. A small Moshi Bass burger speaker (Moshi Corporation, USA; sensitivity: >80 dB; frequency response: 280–16 kHz) and iPod (Apple Inc., USA) were also placed ~50 cm away from the targeted nest and a randomly chosen playback track that contained both the construction and rainfall playbacks was started. Once the experiment ended, the acceptance (or not) of the dummy egg by the incubating bird was noted and all equipment collected.
Each track began with 45 min of silence to allow individuals to recover from the deployment of the equipment. Both Larcombe (2016) and Schaefer and Colombelli-Négrel (2021) showed that little penguin heart-rate values returned to baseline levels within half an hour of deployment; hence, we are confident that this interval was sufficient. The 45 min of silence included periodic short bursts (of 1 s) of environmental noises (noises caused by the wind and the ocean and obtained from previous recordings, played at ~65 dB at 1 m every 5 min) to prevent the iPod from shutting down. This was followed by (1) 5 min of either continuous construction or rainfall noises, (2) another 45 min of silence (to allow recovery from the first playback), (3) another 5 min of playback (if construction noises were played first, then the rainfall noises were played second, and vice versa), and (4) another 30 min of silence. Ten different tracks (five starting with the construction noises and five starting with the rainfall noises) were created. To avoid pseudo-replication, different noises were used for each of the playback tracks. Rainfall and construction noises were obtained as wave files from different websites (rainfall noises: https://mixkit.co/free-sound-effects/rain and https://www.shockwave-sound.com; construction noises: http://free-loops.com/4598-construction-sounds.html, https://www.wavsource.com/sfx/sfx.htm, and https://www.mediacollege.com/downloads/sound-effects/machinery). All the construction noises included general construction noises, such as vehicles, banging and a jackhammer. The rainfall noises were played at ~60–65 dB (measured at 1 m) and the construction sounds were played at ~70 dB (measured at 1 m), with peaks of ~75–80 dB when a jackhammer was heard.
From the video recordings, our field observation that the individual had accepted the dummy egg (or not) was confirmed and the following behaviours were measured according to the ethogram presented in Table 1: (1) ‘behavioural recovery from deployment’; (2) ‘baseline vigilance’; (3) ‘baseline distress’; (4) ‘latency’; (5) ‘playback vigilance’; (6) ‘playback distress’; (7) ‘behavioural recovery from playback’; and (8) ‘vigilance intensity’ (for a visual reference, see Schaefer and Colombelli-Négrel 2021 and supplementary fig. S1 therein). To avoid biases, the same observer scored all penguin behaviours.

|
From the heart-rate recordings, the following were measured according to the ethogram presented in Table 1: (1) ‘heart-rate recovery from deployment’; (2) ‘baseline heart rate’; (3) ‘heart-rate playback’; (4) ‘heart rate recovery from playback’; and (5) ‘heart rate intensity’. To avoid biases, the same observer manually scored all heart rates. Heart rate was manually counted for each full minute or for 30 s and extrapolated into a minute when recordings were interrupted following Ellenberg et al. (2006, 2012), Larcombe (2016) and Schaefer and Colombelli-Négrel (2021). Heart-rate values were averaged for 5 min during baseline and 5 min during playback. If an individual did not accept the dummy eggs, only its behavioural response was recorded.
Breeding success
To determine the number of active penguin nests in little penguins, Lipson Island was visited approximately every 3 weeks between April and November 2021. During each visit, the island was actively searched in its entirety for active little penguin nests. Penguin nests were identified as active if they contained egg(s), chick(s) or adult penguin(s) (Schumann et al. 2013; Colombelli-Négrel 2015, 2017). Each active penguin nest found was marked with a small flagging tape attached to existing vegetation next to the nest and given a unique nest ID. The GPS coordinates for each nest were also recorded using a Garmin GPS 64 (Garmin Ltd, Australia); however, because of the poor accuracy of the GPS data points obtained, it was more reliable to mark the nests manually.
To determine the potential impact of the playback experiments on hatching success and breeding success, 66 nests were monitored (26 used in the noise experiments and an additional 40 not used in the noise experiments) every 3 weeks and the number of eggs, chicks and adults present in each nest during each visit was noted. Following Colombelli-Négrel (2015), hatching success was defined as the number of eggs that hatched compared with the total number of eggs that had been laid. Eggs were considered abandoned if they felt cold to touch and were unattended for two consecutive visits. A nest was recorded as successful (breeding success) if at least one egg hatched and one chick fledged (i.e. it disappeared from the nest at ~8 weeks of age and was not found depredated nor in any of the other nests). Predation was scored as suspected if chicks were found dead outside the entrance of the nest with clear signs, such as torn off appendages (Colombelli-Négrel 2015; L. Iasiello, pers. obs.). Chicks that disappeared before the age of 7 weeks were considered to have died as a result of poor growth or reduced food supply from parents (Colombelli-Négrel 2015).
Statistical analysis
SPSS (ver. 25.0 for Windows, SPSS Inc., Chicago, IL, USA) was used for all statistical analyses. Data are shown as means ± standard deviations unless otherwise stated. Prior to analysis, collinearity was assessed between (1) continuous predictors by using the variance inflation factor (VIF) analysis or (2) categorical predictors by using χ2 and Pearson’s correlations. In all final models, VIF values were <2, χ2 values were >0.05, and Pearson’s coefficients were close to zero, confirming no collinearity between predictor variables (Zuur et al. 2009; Fox et al. 2015).
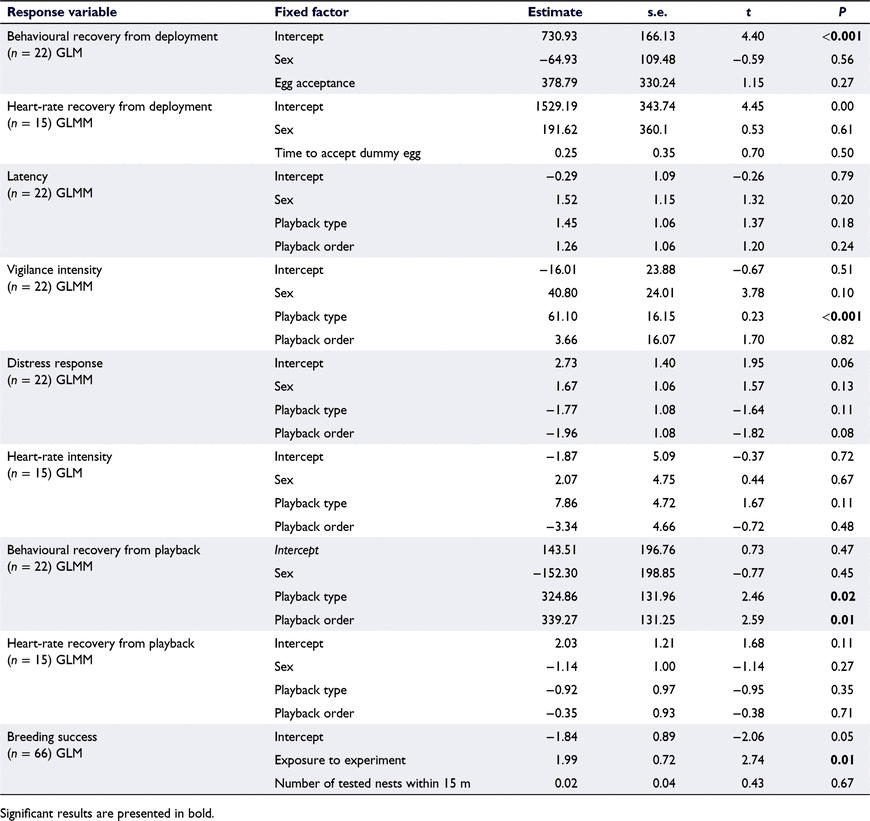
‘Behavioural recovery from deployment’ was analysed using a general linear model (GLM) with a normal distribution and an identity link function, using ‘sex’ (male, female) and ‘egg acceptance’ (whether the individual accepted the dummy egg or not) as fixed factors. The model did not converge when ‘individual ID’ was used as a random factor for this analysis. ‘Latency’ (time taken for individual to show first signs of vigilant or distress behaviour from start of playback) was categorised into a binary variable as fast (responded to playback within <5 s) or slow (responded to playback within >5 s) and analysed with a generalised linear mixed model (GLMM) with a binomial distribution and a logit-link function. ‘Vigilance intensity’ (the difference in the time spent in vigilance between the playback period and the baseline period) and ‘behavioural recovery from playback’ were analysed using GLMMs, with normal distributions and identity link functions. As very few individuals showed signs of distress during the playback, a categorical ‘distress response’ was created on the basis of whether the individuals’ showed signs of distress during the playback (yes, no). ‘Distress response’ was analysed with a GLMM with a binomial distribution with a logit link function. For each of these models, ‘sex’, ‘playback type’ (construction, rainfall), ‘playback order’ (first, second) were used as fixed factors and ‘individual ID’ as a random factor.
‘Heart-rate recovery from deployment’ was analysed using a GLMM with a normal distribution and an identity link function, using ‘sex’ and ‘time to accept dummy egg’ (time taken by the individual to accept the dummy egg and sit directly on top of the egg) as fixed factors and ‘individual ID’ as a random factor. ‘Heart-rate intensity’ was analysed using a GLM with a normal distribution and an identity link function, using ‘sex’, ‘playback type’, and ‘playback order’ as fixed factors. The model did not converge when ‘individual IDs’ were used as random factors and therefore and ‘individual IDs’ were removed from these analyses. ‘Heart-rate recovery from playback’ was categorised into fast (within 5 min) or slow (over 5 min) and analysed using a GLMM with a binomial distribution and logit link function, using ‘sex’, ‘playback type’ and ‘playback order’ as fixed factors and ‘individual ID’ as a random factor. The correlation between exposure to the playback experiment and ‘breeding success’ was explored using a GLM with a binomial distribution and a logit link function and using ‘exposure to experiment’ (whether a nest was exposed to the playback experiment or not) and ‘number of tested nests within 15 m’ (number of experimentally tested nests within a 15-m radius) as fixed factors. Models did not converge with ‘nest ID’ as a random factor and therefore ‘nest ID’ was removed from the analyses.
Ethics statement
This research was approved by Flinders University Animal Welfare Committee (AEB-A/FR1530 and BIOL AR4825) and supported by a scientific permit (Y26040) to conduct the research.
Results
Playback experiment
Not all of the tested 33 individuals could be used in the analysis because of equipment failures, poor visibility, birds not accepting the dummy egg or not sitting on the egg for the total length of the experiment. Therefore, the final sample sizes for the behavioural and physiological response were 22 individuals and 15 individuals respectively.
Recovery from deployment
Mean ‘behavioural recovery from deployment’ was 12 ± 9 min (range of 2–35 min) and mean ‘heart-rate recovery from deployment’ was 27 ± 7 min (range of 18–38 min). None of the fixed factors significantly correlated with ‘behavioural recovery from deployment’ or with ‘heart-rate recovery from deployment’ (Table 2). However, the random factor ‘individual ID’ significantly correlated with ‘heart-rate recovery from deployment’ (Wald’s Z = 2.72, P = 0.007).
Response to playback
Mean ‘latency’ to respond to the playback was 7 ± 14 s (construction noises: 3 ± 4 s, n = 22; rainfall noises: 11 ± 21 s, n = 17). None of the fixed or random factors correlated with ‘latency’ (‘individual ID’: Wald’s Z = 0.68, P = 0.50; Table 2) or the occurrence of distress behaviour (‘individual ID’: Wald’s Z = 0.81, P = 0.42; Table 2). Individuals spent significantly more time in vigilance (55 s more) during the construction noise playback than they did during the rainfall playback (Table 2, Fig. 2). None of the other factors correlated with ‘vigilance intensity’ (‘individual ID’: Wald’s Z = 1.68, P = 0.09; Table 2). Mean ‘heart-rate response’ to the playback was 2 ± 11 beats min−1 (construction noises: 5 ± 14 beats min−1, n = 13; rainfall: 2 ± 7 s, n = 12). None of the fixed factors correlated with ‘heart-rate response’ to playbacks (Table 2).
Recovery from playback
Mean ‘behavioural recovery from playback’ was 7 ± 9 min (range: 3 s–36 min) and mean ‘heart-rate recovery from playback’ was 7 ± 9 min (range: 5–28 min). Individuals took significantly longer time to recovery (4 min longer) from the construction playback than from the rainfall playback (Table 2, Fig. 3). ‘Playback order’ also significantly correlated with ‘behavioural recovery from playback’, with individuals taking significantly longer to recover (4 min longer) when exposed to the construction playback first (F1,35 = 6.68, P = 0.01; Table 2). The remaining factors did not correlate with ‘behavioural recovery from playback’ (‘individual ID’: Wald’s Z = 1.64, P = 0.10; Table 2). None of the factors correlated with ‘heart-rate recovery from playback’ (‘individual ID’: Wald’s Z = 0.12, P = 0.91; Table 2).
Breeding success
A total of 66 active little penguin nests was found between April 2021 and November 2021, which brings the population size to 132 breeding individuals. Nests were aggregated within the vegetated centre of the island (see Fig. 1), where 58 sand burrows and eight rock nests were recorded. In total, 148 eggs were laid, from which 88 chicks hatched, and 73 chicks fledged. Of these 66 nests, at least one failed breeding attempt was recorded for 34 nests and breeding pairs; for 9 nests (26%), the eggs were abandoned; for 10 nests (29%), only one chick hatched; and for 3 nests (9%), chick(s) were found dead outside the nest. Of these 66 nests, 32% showed evidence of a second clutch.
Exposure to the noise experiment significantly correlated with ‘breeding success’, with nests exposed to the noise experiment being more likely to be successful (to produce at least one fledgling) than those not exposed to the noise experiment (Table 2, Fig. 4). The ‘number of nests tested within 15 m’ did not correlate with ‘breeding success’ (Table 2).
|
|
Discussion
Animals generally first respond to human-induced disturbances (such as coastal development) by changing their behaviours, which determines which individuals survive and reproduce under changed conditions (Sih et al. 2011; Chapple et al. 2012) and thus have long-term consequences for the persistence of the populations. Yet, the effects of noise pollution from coastal developments on seabirds have received little attention (but see Melanie 2004; Watson et al. 2014; Buxton et al. 2017a; Franks 2017). This study showed that little penguins spent significantly more time in vigilance (but showed no increase in heart rate) during the construction noise playback than during the rainfall playback, supporting the distracted prey hypothesis (Chan et al. 2010). Our study adds to the growing literature investigating seabird response to anthropogenic noise pollution (Ainley et al. 2001; Melanie 2004; Dann and Chambers 2013; Franks 2017; Gineste et al. 2017; Bevan et al. 2018; Thompson 2021) to help improve our ability to predict the effects of human-induced environmental changes on populations and biodiversity.
This study showed that little penguins increased their vigilance and took longer to stop exhibiting such vigilance when exposed to construction noises than did those in our control (exposed to rainfall noises). However, little penguins did not increase their hear rate in response to the disturbance. This result aligns with a study by Derose-Wilson et al. (2015) showing that Wilson’s plovers (C. wilsonia) increased their vigilance, but not their heart rate, when aircrafts flew overhead, but contrasts with other studies in seabirds (e.g. Weimerskirch et al. 2002; Schaefer and Colombelli-Négrel 2021). This supports the distracted prey hypothesis (Chan et al. 2010) because the stimulus still caused individuals to become distracted, with a clear deviation in attention and potentially brain function (Laughlin 2001; Chan and Blumstein 2011), but not the anti-predator hypothesis that suggests that humans (and related disturbances) should elicit a response similar to that to predators (Frid and Dill 2002). One hypothesis to explain these results is that little penguins did not perceive the construction noises as a substantial threat, and responded to the noise with increased vigilance only because of its novelty. Alternatively, our stimulus was too short to exhibit a stress response. A study by Larcombe (2016) found that little penguins displayed a more intense response when approached by researchers than to playback recordings. Therefore, the lack of visual threat could also have led to the low response observed in our study (Ellenberg et al. 2006; Derose-Wilson et al. 2015; Larcombe 2016; but see Schaefer and Colombelli-Négrel 2021). Another potential conflicting factor in our playback experiments may be the choice of rainfall noises as our control because penguin burrows are prone to flooding during heavy rainfall or wave inundation (Stokes and Dee Boersma 1991; Yorio and Boersma 1994; Dann and Chambers 2009). Therefore, little penguins exposed to the rainfall noise could have experienced some distress or increase in heart rate, which could have nullified any playback effect. Future studies should consider alternative for control, such as wind or ambient noises.
Whereas studies on other seabird species have documented sex differences in response to disturbance (Weimerskirch et al. 2002; Ellenberg et al. 2009), this study found no correlation between the sex of the tested individuals and their behavioural or physiological responses, which supports previous work on little penguins (Carroll et al. 2016; Scheafer and Colombelli-Négrel 2021). It has been suggested that other factors, such as individual personality as well as previous experience with human disturbance (Ellenberg et al. 2009; Larcombe 2016; Colombelli-Négrel and Katsis 2021; Schaefer and Colombelli-Négrel 2021) may play a more important role than sex in little penguins when responding to disturbances. For example, Schaefer and Colombelli-Négrel (2021) found that little penguins breeding in colonies located in highly disturbed areas had more intense behavioural and physiological responses to playback experiments than did those breeding in less disturbed colonies. Therefore, future studies should incorporate several factors that may influence an individual’s response to disturbance, within their experimental design.
Disturbance at such a small time-scale is not likely to affect little penguins daily energy budgets. Indeed, Larcombe (2016) showed that an extreme physiological stress response in little penguins, initiated by researchers weighing individuals, took more than 31 min for their heart rate to recover and used 2.8% of their daily energy budget. The results from our study showed that individuals recovered within less than 16 min (within 7 min on average), suggesting that our experiments did not significantly decrease individuals fitness. However, repeated reoccurrence of an introduced stimulus, such as construction noises, is likely to reduce the time an individual spends performing self-care behaviours, such as resting or preening (Chan and Blumstein 2011; Fanning et al. 2020), and to distract them from other threats, such as predation (Ellison and Cleary 1978; Buxton et al. 2017a). Combined with other pressures from their environment, it can, in the long term, reduce or inhibit energy-intake activities and, thus, fitness (Faeth et al. 2005; Chace and Walsh 2006; Lowry et al. 2011; Samia et al. 2015; Vincze et al. 2016; Buxton et al. 2017a; Liu et al. 2020).
Alternatively, little penguins may habituate to the construction noises (Blumstein 2016). A lot of research has been conducted on the impacts of urbanisation on wildlife (Faeth et al. 2005; Chace and Walsh 2006; Lowry et al. 2011) and some species may be able to tolerate urban environments or habituate to a new stimulus so long as there is no known physical threat to the individual (Lowry et al. 2011; Samia et al. 2015; Vincze et al. 2016). On Vancouver Island (Canada), for example, researchers found that breeding colonies of glaucous-winged gulls (Larus glaucescens), double-crested (Phalacrocorax auritus) and pelagic (P. pelagicus) cormorants, black oystercatchers (Haematopus bachmani), and pigeon guillemots (Cepphus columba) exposed to high or moderate boat traffic initiated flushing or alert response at shorter distances than did colonies exposed to lower traffic (Chatwin et al. 2013). Species often remain in these altered areas because their distribution is limited and because they cannot relocate or find a suitable habitat elsewhere (Strasser and Heath 2013; Blumstein 2016). Yet, it is unclear whether individuals habituate to human disturbance or whether the introduced stimuli select for human-tolerant phenotypes (Lowry et al. 2011; Viblanc et al. 2012; see also Colombelli-Négrel and Katsis 2021). Future studies using repeated experiments may be able to answer this question.
Our study found that nests exposed to the noise experiment had a higher breeding success than did those that were not. Although this suggests that exposure to construction noises may not have a negative effect on breeding success, this result needs to be taken with caution because there could have been a bias in the nests chosen for the noise experiment towards nests that were more easily accessible, hence making them easier to monitor breeding success as well. It should also be noted that our exposure to construction noises lasted only 5 min (owing to constraints regarding timing, access to the island and battery capacity of the equipment), and thus may not have lasted long enough to affect breeding. In addition, although little penguins showed limited response to our playback, our study highlighted that the little penguin population on Lipson Island was larger than was previously estimated (132 in 2021, compared with 29 in 2011; Madden-Hallet et al. 2011), thereby increasing the number of individuals potentially affected. Additional studies should investigate the impact of construction noises on breeding success for longer periods, as well as across breeding stages, as parents experience higher energetic demands during the chicks’ provisioning period (Numata et al. 2004), and thus may exhibit higher stress responses during this time.
Conclusions
Given the rapid rates of environmental changes as a result of human activities, there is an urgent need to understand to what extent individuals respond to such changes and how these changing conditions affect their population, so as to focus efforts on the most vulnerable species and the most appropriate sites. This research helps fill this knowledge gap and further our understanding of species response to human-induced environmental changes. Coastal developments, such as the proposed new grain-export facility, bring increased human presence, such as traffic (both terrestrial and marine), pollution (i.e. grain, debris, light, and noise), and potentially lead to the attraction of more predators and invasive species within the area (see Thayer et al. 1999; Rodríguez et al. 2015, 2017; Buxton et al. 2017a; Franks 2017; Plan SA 2020; Syposz et al. 2021). As the breeding seabirds on Lipson Island have not been monitored for more than two consecutive years, and no official population trends can be sourced, differentiating between the impacts of the construction and operation of Port Spencer and previous underlying conditions may prove difficult. Our results also support the distracted prey hypothesis, which, over long periods, may reduce the time individuals spend preforming biologically important behaviours, and increase predation risk. Future studies, especially experimental studies over long periods, are recommended across species and locations to understand the long-term fitness impacts of anthropogenic noise pollution.
Supplementary material
Supplementary material is available online.
Data availability
Data are available on the Flinders University data repository at https://doi.org/10.25451/flinders.22493176.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Declaration of funding
Funding from Nature Foundation was a student grant allocated to L. Iasiello and the funding form Flinders University was part of the standard university funding allocation for students.
Author contributions
L. Iasiello and D. Colombelli-Négrel designed the study; L. Iasiello collected and analysed the data and wrote the first draft of the manuscript. All authors commented on the manuscript.
Acknowledgements
We thank and acknowledge the essential volunteers who helped with fieldwork and data collection for this research. We also thank Ryan Baring and Sonia Kleindorfer for their comments on the manuscript.
References
Ainley, DG, Podolsky, R, Deforest, L, Spencer, G, and Nur, N (2001). The status and population trends of the Newell’s shearwater on Kaua‘i: insights from modelling. Studies in Avian Biology 22, 108–123.Barnett, JL, and Hemsworth, PH (1990). The validity of physiological and behavioural measures of animal welfare. Applied Animal Behaviour Science 25, 177–187.
| The validity of physiological and behavioural measures of animal welfare.Crossref | GoogleScholarGoogle Scholar |
Bennie, J, Davies, TW, Cruse, D, Bell, F, Gaston, KJ, and James, J (2018). Artificial light at night alters grassland vegetation species composition and phenology. Journal of Applied Ecology 55, 442–450.
| Artificial light at night alters grassland vegetation species composition and phenology.Crossref | GoogleScholarGoogle Scholar |
Bevan, E, Whiting, S, Tucker, T, Guinea, M, Raith, A, and Douglas, R (2018). Measuring behavioral responses of sea turtles, saltwater crocodiles, and crested terns to drone disturbance to define ethical operating thresholds. PLoS ONE 13, e0194460.
| Measuring behavioral responses of sea turtles, saltwater crocodiles, and crested terns to drone disturbance to define ethical operating thresholds.Crossref | GoogleScholarGoogle Scholar |
Bianchini, K, Mallory, ML, Braune, BM, Muir, DCG, and Provencher, JF (2022). Why do we monitor? Using seabird eggs to track trends in Arctic environmental contamination. Environmental Reviews 30, 245–267.
| Why do we monitor? Using seabird eggs to track trends in Arctic environmental contamination.Crossref | GoogleScholarGoogle Scholar |
Birds SA (2021) Lipson Island Conservation Park. Available at https://birdssa.asn.au/location/lipson-island-conservation-park/#:~:text=History%3A%20Lipson%20Island%20and%20Lipson,on%2016%20March%201967%204%20 [Verified 9 September 2021]
Blackwell, BF, Devault, TL, Fernández-Juricic, E, Gese, EM, Gilbert-Norton, L, and Breck, SW (2016). No single solution: application of behavioural principles in mitigating human–wildlife conflict. Animal Behaviour 120, 245–254.
| No single solution: application of behavioural principles in mitigating human–wildlife conflict.Crossref | GoogleScholarGoogle Scholar |
Blumstein, DT (2016). Habituation and sensitization: new thoughts about old ideas. Animal Behaviour 120, 255–262.
| Habituation and sensitization: new thoughts about old ideas.Crossref | GoogleScholarGoogle Scholar |
Blumstein DT, Geffory B, Samia D, Bessa E (2017) ‘Ecotourism’s promise and peril.’ (Springer International Publisher)
Boyle, SA, and Samson, FB (1985). Effects of non-consumptive recreation on wildlife: a review. Wildlife Society Bulletin 13, 110–116.
Brisson-Curadeau, E, Bird, D, Burke, C, Fifield, DA, Pace, P, Sherley, RB, and Elliott, KH (2017). Seabird species vary in behavioural response to drone census. Scientific Reports 7, 17884.
| Seabird species vary in behavioural response to drone census.Crossref | GoogleScholarGoogle Scholar |
Brown, AL (1990). Measuring the effect of aircraft noise on sea birds. Environment International 16, 587–592.
| Measuring the effect of aircraft noise on sea birds.Crossref | GoogleScholarGoogle Scholar |
Buxton, RT, Galvan, R, McKenna, MF, White, CL, and Seher, V (2017a). Visitor noise at a nesting colony alters the behavior of a coastal seabird. Marine Ecology Progress Series 570, 233–246.
| Visitor noise at a nesting colony alters the behavior of a coastal seabird.Crossref | GoogleScholarGoogle Scholar |
Buxton, RT, McKenna, MF, Mennitt, D, Fristrup, K, Crooks, K, Angeloni, L, and Wittemyer, G (2017b). Noise pollution is pervasive in US protected areas. Science 356, 531–533.
| Noise pollution is pervasive in US protected areas.Crossref | GoogleScholarGoogle Scholar |
Calow, P, and Forbes, VE (1998). How do physiological responses to stress translate into ecological and evolutionary processes? Comparative Biochemistry and Physiology – A. Molecular & Integrative Physiology 120, 11–16.
| How do physiological responses to stress translate into ecological and evolutionary processes?Crossref | GoogleScholarGoogle Scholar |
Carroll, G, Turner, E, Dann, P, and Harcourt, R (2016). Prior exposure to capture heightens the corticosterone and behavioural responses of little penguins (Eudyptula minor) to acute stress. Conservation Physiology 4, cov061.
| Prior exposure to capture heightens the corticosterone and behavioural responses of little penguins (Eudyptula minor) to acute stress.Crossref | GoogleScholarGoogle Scholar |
Chace, JF, and Walsh, JJ (2006). Urban effects on native avifauna: a review. Landscape and Urban Planning 74, 46–69.
| Urban effects on native avifauna: a review.Crossref | GoogleScholarGoogle Scholar |
Chan, AAY-H, and Blumstein, DT (2011). Attention, noise, and implications for wildlife conservation and management. Applied Animal Behaviour Science 131, 1–7.
| Attention, noise, and implications for wildlife conservation and management.Crossref | GoogleScholarGoogle Scholar |
Chan, AAY-H, Giraldo-Perez, P, Smith, S, and Blumstein, DT (2010). Anthropogenic noise affects risk assessment and attention: the distracted prey hypothesis. Biology Letters 6, 458–461.
| Anthropogenic noise affects risk assessment and attention: the distracted prey hypothesis.Crossref | GoogleScholarGoogle Scholar |
Chapple, DG, Simmonds, SM, and Wong, BBM (2012). Can behavioral and personality traits influence the success of unintentional species introductions? Trends in Ecology & Evolution 27, 57–64.
| Can behavioral and personality traits influence the success of unintentional species introductions?Crossref | GoogleScholarGoogle Scholar |
Chatwin, TA, Joy, R, and Burger, AE (2013). Set-back distances to protect nesting and roosting seabirds off Vancouver Island from boat disturbance. Waterbirds 36, 43–52.
| Set-back distances to protect nesting and roosting seabirds off Vancouver Island from boat disturbance.Crossref | GoogleScholarGoogle Scholar |
Cifuentes, JM, Becker, PH, Sommer, U, Pacheco, P, and Schlatter, R (2003). Seabird eggs as bioindicators of chemical contamination in Chile. Environmental Pollution 126, 123–137.
| Seabird eggs as bioindicators of chemical contamination in Chile.Crossref | GoogleScholarGoogle Scholar |
Collins, M, Cullen, JM, and Dann, P (1999). Seasonal and annual foraging movements of little penguins from Phillip Island, Victoria. Wildlife Research 26, 705–721.
| Seasonal and annual foraging movements of little penguins from Phillip Island, Victoria.Crossref | GoogleScholarGoogle Scholar |
Colombelli-Négrel, D (2015). Low survival rather than breeding success explains little penguin population decline on Granite Island. Marine and Freshwater Research 66, 1057–1065.
| Low survival rather than breeding success explains little penguin population decline on Granite Island.Crossref | GoogleScholarGoogle Scholar |
Colombelli-Négrel D (2018) Penguin monitoring and conservation activities in the Gulf St Vincent: July 2017–June 2018. Report to the Adelaide and Mount Lofty NRM Board. Flinders University. Adelaide, SA, Australia.
Colombelli-Négrel, D, and Katsis, AC (2021). Little penguins are more aggressive on islands that experience greater unregulated human disturbance. Animal Behaviour 182, 195–202.
| Little penguins are more aggressive on islands that experience greater unregulated human disturbance.Crossref | GoogleScholarGoogle Scholar |
Colombelli-Négrel, D, and Smale, R (2018). Habitat explained microgeographic variation in little penguin agonistic calls. Auk 135, 44.
| Habitat explained microgeographic variation in little penguin agonistic calls.Crossref | GoogleScholarGoogle Scholar |
Culik B, Adelung D, Woakes AJ (1990) The effect of disturbance on the heart rate and behaviour of Adélie penguins (Pygoscelis adeliae) during the breeding season. In ‘Antarctic ecosystems’. (Eds KR Kerry, G Hempel) pp. 177–182. (Springer)
Dann P, Chambers L (2009) Climate change and little penguins. Report for Western Port Greenhouse Alliance. Published by the authors, Melbourne, Vic., Australia.
Dann, P, and Chambers, L (2013). Ecological effects of climate change on little penguins Eudyptula minor and the potential economic impact on tourism. Climate Research 58, 67–79.
| Ecological effects of climate change on little penguins Eudyptula minor and the potential economic impact on tourism.Crossref | GoogleScholarGoogle Scholar |
Derose-Wilson, A, Fraser, JD, Karpanty, SM, and Hillman, MD (2015). Effects of overflights on incubating Wilson’s plover behavior and heart rate. The Journal of Wildlife Management 79, 1246–1254.
| Effects of overflights on incubating Wilson’s plover behavior and heart rate.Crossref | GoogleScholarGoogle Scholar |
Dias, MP, Martin, R, Pearmain, EJ, Burfield, IJ, Small, C, Phillips, RA, Yates, O, Lascelles, B, Borboroglu, PG, and Croxall, JP (2019). Threats to seabirds: a global assessment. Biological Conservation 237, 525–537.
| Threats to seabirds: a global assessment.Crossref | GoogleScholarGoogle Scholar |
Dierschke, V, Furness, RW, and Garthe, S (2016). Seabirds and offshore wind farms in European waters: avoidance and attraction. Biological Conservation 202, 59–68.
| Seabirds and offshore wind farms in European waters: avoidance and attraction.Crossref | GoogleScholarGoogle Scholar |
Ellenberg, U, Mattern, T, Seddon, PJ, and Jorquera, GL (2006). Physiological and reproductive consequences of human disturbance in Humboldt penguins: the need for species-specific visitor management. Biological Conservation 133, 95–106.
| Physiological and reproductive consequences of human disturbance in Humboldt penguins: the need for species-specific visitor management.Crossref | GoogleScholarGoogle Scholar |
Ellenberg, U, Mattern, T, and Seddon, PJ (2009). Habituation potential of yellow-eyed penguins depends on sex, character and previous experience with humans. Animal Behaviour 77, 289–296.
| Habituation potential of yellow-eyed penguins depends on sex, character and previous experience with humans.Crossref | GoogleScholarGoogle Scholar |
Ellenberg, U, Mattern, T, Houston, DM, Davis, LS, and Seddon, PJ (2012). Previous experiences with humans affect responses of snares penguins to experimental disturbance. Journal of Ornithology 153, 621–631.
| Previous experiences with humans affect responses of snares penguins to experimental disturbance.Crossref | GoogleScholarGoogle Scholar |
Ellison, LN, and Cleary, L (1978). Effects of human disturbance on breeding of double-crested cormorants. Auk 95, 510–517.
Faeth, SH, Warren, PS, Shochat, E, and Marussich, WA (2005). Trophic dynamics in urban communities. BioScience 55, 399–407.
| Trophic dynamics in urban communities.Crossref | GoogleScholarGoogle Scholar |
Fanning, L, Larsen, H, and Taylor, PS (2020). A preliminary study investigating the impact of musical concerts on the behavior of captive Fiordland penguins (Eudyptes pachyrhynchus) and collared peccaries (Pecari tajacu). Animals 10, 2035.
| A preliminary study investigating the impact of musical concerts on the behavior of captive Fiordland penguins (Eudyptes pachyrhynchus) and collared peccaries (Pecari tajacu).Crossref | GoogleScholarGoogle Scholar |
Fowler, GS (1999). Behavioral and hormonal responses of Magellanic penguins (Spheniscus magellanicus) to tourism and nest site visitation. Biological Conservation 90, 143–149.
| Behavioral and hormonal responses of Magellanic penguins (Spheniscus magellanicus) to tourism and nest site visitation.Crossref | GoogleScholarGoogle Scholar |
Fox GA, Negrete-Yankelevich S, Sosa VJ (2015) ‘Ecological statistics.’ (Oxford University Press)
Franks RS (2017) A preliminary investigation into the effect of anthropogenic noise on the vigilance of Eurasian oystercatchers, Haematopus ostralegus. BSc(Zool) third-year research project thesis, Bangor University, Bangor, UK.
Frid, A, and Dill, LM (2002). Human-caused disturbance stimuli as a form of predation risk. Conservation Ecology 6, 11.
| Human-caused disturbance stimuli as a form of predation risk.Crossref | GoogleScholarGoogle Scholar |
Giese, M (1998). Guidelines for people approaching breeding groups of Adelie penguins (Pygoscelis adeliae). Polar Record 34, 287–292.
| Guidelines for people approaching breeding groups of Adelie penguins (Pygoscelis adeliae).Crossref | GoogleScholarGoogle Scholar |
Giling, D, Reina, RD, and Hogg, Z (2008). Anthropogenic influence on an urban colony of the little penguin Eudyptula minor. Marine and Freshwater Research 59, 647–651.
| Anthropogenic influence on an urban colony of the little penguin Eudyptula minor.Crossref | GoogleScholarGoogle Scholar |
Gineste, B, Souquet, M, Couzi, F-X, Giloux, Y, Philippe, J-S, Hoarau, C, Tourmetz, J, Potin, G, and Le Corre, M (2017). Tropical shearwater population stability at Reunion Island, despite light pollution. Journal of Ornithology 158, 385–394.
| Tropical shearwater population stability at Reunion Island, despite light pollution.Crossref | GoogleScholarGoogle Scholar |
Goutte, A, Andelier, F, Chastel, CC, Trouve, C, Moe, B, Bech, C, Gabrielsen, GW, and Chastel, O (2010). Stress and the timing of breeding: glucocorticoid-luteinizing hormones relationships in an arctic seabird. General and Comparative Endocrinology 169, 108–116.
| Stress and the timing of breeding: glucocorticoid-luteinizing hormones relationships in an arctic seabird.Crossref | GoogleScholarGoogle Scholar |
Graham, NAJ, Wilson, SK, Carr, P, Hoey, AS, Jennings, S, and MacNeil, MA (2018). Seabirds enhance coral reef productivity and functioning in the absence of invasive rats. Nature 559, 250–253.
| Seabirds enhance coral reef productivity and functioning in the absence of invasive rats.Crossref | GoogleScholarGoogle Scholar |
Henny, CJ, Blus, LJ, and Prouty, RM (1982). Organochlorine residues and shell thinning in Oregon seabird eggs. The Murrelet 63, 15–21.
| Organochlorine residues and shell thinning in Oregon seabird eggs.Crossref | GoogleScholarGoogle Scholar |
Holmes, N, Giese, M, and Kriwoken, LK (2005). Testing the minimum approach distance guidelines for incubating royal penguins Eudyptes schlegeli. Biological Conservation 126, 339–350.
| Testing the minimum approach distance guidelines for incubating royal penguins Eudyptes schlegeli.Crossref | GoogleScholarGoogle Scholar |
Hoskins, AJ, Dann, P, Ropert-Coudert, Y, Kato, A, Chiaradia, A, Costa, DP, and Arnould, JPY (2008). Foraging behaviour and habitat selection of the little penguin Eudyptula minor during early chick rearing in Bass Strait, Australia. Marine Ecology Progress Series 366, 293–303.
Hughes, KA, Waluda, CM, Stone, RE, Ridout, MS, and Shears, JR (2008). Short-term responses of king penguins Aptenodytes patagonicus to helicopter disturbance at South Georgia. Polar Biology 31, 1521–1530.
| Short-term responses of king penguins Aptenodytes patagonicus to helicopter disturbance at South Georgia.Crossref | GoogleScholarGoogle Scholar |
Humphries, GRW, and Huettmann, F (2014). Putting models to a good use: a rapid assessment of Arctic seabird biodiversity indicates potential conflicts with shipping lanes and human activity. Diversity and Distributions 20, 478–490.
| Putting models to a good use: a rapid assessment of Arctic seabird biodiversity indicates potential conflicts with shipping lanes and human activity.Crossref | GoogleScholarGoogle Scholar |
Jefferson, TA, Hung, SK, and Würsig, B (2009). Protecting small cetaceans from coastal development: impact assessment and mitigation experience in Hong Kong. Marine Policy 33, 305–311.
| Protecting small cetaceans from coastal development: impact assessment and mitigation experience in Hong Kong.Crossref | GoogleScholarGoogle Scholar |
Johannesen, E, Houston, D, and Russell, J (2003). Increased survival and breeding performance of double breeders in little penguins Eudyptula minor, New Zealand: evidence for individual bird quality? Journal of Avian Biology 34, 198–210.
| Increased survival and breeding performance of double breeders in little penguins Eudyptula minor, New Zealand: evidence for individual bird quality?Crossref | GoogleScholarGoogle Scholar |
Johnson, B, and Colombelli-Négrel, D (2021). Breeding success in Southern Australian little penguins is negatively correlated with high wind speeds and sea surface temperatures. Ornithological Applications 123, duaa062.
| Breeding success in Southern Australian little penguins is negatively correlated with high wind speeds and sea surface temperatures.Crossref | GoogleScholarGoogle Scholar |
Kazama, K, Niizuma, Y, Sakamoto, KQ, and Watanuki, Y (2011). Factors affecting individual variation in nest-defense intensity in colonially breeding black-tailed gulls (Larus crassirostris). Canadian Journal of Zoology 89, 938–944.
| Factors affecting individual variation in nest-defense intensity in colonially breeding black-tailed gulls (Larus crassirostris).Crossref | GoogleScholarGoogle Scholar |
Kemp, A, and Dann, P (2001). Egg size, incubation periods and hatching success of little penguins, Eudyptula minor. Emu – Austral Ornithology 101, 249–253.
| Egg size, incubation periods and hatching success of little penguins, Eudyptula minor.Crossref | GoogleScholarGoogle Scholar |
Klomp, NI, Meathrel, CE, Wienecke, BC, and Wooller, RD (1991). Surface nesting by little penguins on Penguin Island, Western Australia. Emu – Austral Ornithology 91, 190–193.
| Surface nesting by little penguins on Penguin Island, Western Australia.Crossref | GoogleScholarGoogle Scholar |
Lai, S, Loke, LHL, Hilton, MJ, Bouma, TJ, and Todd, PA (2015). The effects of urbanisation on coastal habitats and the potential for ecological engineering: a Singapore case study. Ocean & Coastal Management 103, 78–85.
| The effects of urbanisation on coastal habitats and the potential for ecological engineering: a Singapore case study.Crossref | GoogleScholarGoogle Scholar |
Larcombe S (2016) Little penguins (Eudyptula minor) and human disturbance. MSc(Zool) thesis, University of Otago, Dunedin, New Zealand.
Laughlin SB (2001) The metabolic cost of information – a fundamental factor in visual ecology. In ‘Ecology of sensing’. (Eds FG Barth, A Schmid) pp. 169–185. (Springer)
Liu, Q, Slabbekoorn, H, and Riebel, K (2020). Zebra finches show spatial avoidance of near but not far distance traffic noise. Behaviour 157, 333–362.
| Zebra finches show spatial avoidance of near but not far distance traffic noise.Crossref | GoogleScholarGoogle Scholar |
Lowry, H, Lill, A, and Wong, BB (2011). Tolerance of auditory disturbance by an avian urban adapter, the noisy miner. Ethology 117, 490–497.
| Tolerance of auditory disturbance by an avian urban adapter, the noisy miner.Crossref | GoogleScholarGoogle Scholar |
MacDougall, AS, McCann, KS, Gellner, G, and Turkington, R (2013). Diversity loss with persistent human disturbance increases vulnerability to ecosystem collapse. Nature 494, 86–89.
| Diversity loss with persistent human disturbance increases vulnerability to ecosystem collapse.Crossref | GoogleScholarGoogle Scholar |
Madden-Hallet D, Hammer M, Gursansky W, Donato DB (2011) Lipson Island baseline flora and fauna report and assessment of risk. Report to Golder Associates. Donato Environmental Services, Darwin, NT, Australia.
Marker PF (2016) Spatial scale and nest distribution of little penguins (Eudyptula minor). PhD thesis, University of Tasmania, Hobart, Tas., Australia.
Melanie B (2004) Human disturbance to colonially breeding seabirds and guidelines for visitor management on islands off the coast of Western Australia. Honours thesis, Murdoch University, Perth, WA, Australia.
Mooney, TA, Smith, A, Hansen, KA, Larsen, ON, Wahlberg, M, and Rasmussen, M (2019). Birds of a feather: hearing and potential noise impacts in puffins (Fratercula arctica). Proceedings of Meetings on Acoustics 37, 010004.
| Birds of a feather: hearing and potential noise impacts in puffins (Fratercula arctica).Crossref | GoogleScholarGoogle Scholar |
Niven, JE, and Laughlin, SB (2008). Energy limitation as a selective pressure on the evolution of sensory systems. Journal of Experimental Biology 211, 1792–1804.
| Energy limitation as a selective pressure on the evolution of sensory systems.Crossref | GoogleScholarGoogle Scholar |
Numata, M, Davis, LS, and Renner, M (2004). Growth and survival of chicks in relation to nest attendance patterns of little penguins (Eudyptula minor) at Oamaru and Motuara Island, New Zealand. New Zealand Journal of Zoology 31, 263–269.
| Growth and survival of chicks in relation to nest attendance patterns of little penguins (Eudyptula minor) at Oamaru and Motuara Island, New Zealand.Crossref | GoogleScholarGoogle Scholar |
Pink, M, and Abrahams, MV (2018). In shallow water ecosytems the abiotic environment is more important than prey abundance for foraging terns. Environmental Biology of Fishes 101, 355–362.
| In shallow water ecosytems the abiotic environment is more important than prey abundance for foraging terns.Crossref | GoogleScholarGoogle Scholar |
Piña-Ortiz, A, Covantes-Rosales, CE, Ceyca, JP, Castillo-Guerrero, JA, Aguilar-Zarate, G, and Betancourt-Lozano, M (2016). Organochlorine pesticide residues in blood of three seabird species in the coast of Sinaloa, Mexico: a comparison of trophic level. Toxicology Letters 259, S222–S222.
| Organochlorine pesticide residues in blood of three seabird species in the coast of Sinaloa, Mexico: a comparison of trophic level.Crossref | GoogleScholarGoogle Scholar |
Plan SA (2020) Port Spencer – grain export facility. Government of South Australia. Available at https://plan.sa.gov.au/state_snapshot/development_activity/major_projects/majors/port_spencer_deep_water_port_facility [Verified 24 November 2021]
Ports Peninsula (2022) Peninsula Ports secures funding to ensure grain exports will go ahead at Port Spencer. Available at https://peninsulaports.com.au/2022/05/17/peninsula-ports-secures-funding-to-ensure-grain-exports-will-go-ahead-at-port-spencer/ [Verified 24 November 2021]
Reilly, PN, and Cullen, JM (1981). The little penguin Eudyptula minor in Victoria, II: breeding. Emu – Austral Ornithology 81, 1–19.
| The little penguin Eudyptula minor in Victoria, II: breeding.Crossref | GoogleScholarGoogle Scholar |
Robinson T, Canty P, Mooney T, Rudduck P (1996) ‘South Australia’s offshore Islands.’ (Australian Government Publishing Service: Canberra, ACT, Australia)
Rodríguez, A, García, D, Rodríguez, B, Cardona, E, Parpal, L, and Pans, P (2015). Artificial lights and seabirds: is light pollution a threat for the threatened Balearic petrels? Journal of Ornithology 156, 893–902.
| Artificial lights and seabirds: is light pollution a threat for the threatened Balearic petrels?Crossref | GoogleScholarGoogle Scholar |
Rodríguez, A, Dann, P, and Chiaradia, A (2017). Reducing light-induced mortality of seabirds: high pressure sodium lights decrease the fatal attraction of shearwaters. Journal for Nature Conservation 39, 68–72.
| Reducing light-induced mortality of seabirds: high pressure sodium lights decrease the fatal attraction of shearwaters.Crossref | GoogleScholarGoogle Scholar |
Ryan, PG (2018). Entanglement of birds in plastics and other synthetic materials. Marine Pollution Bulletin 135, 159–164.
| Entanglement of birds in plastics and other synthetic materials.Crossref | GoogleScholarGoogle Scholar |
Samia, DSM, Nakagawa, S, Nomura, F, Rangel, TF, and Blumstein, DT (2015). Increased tolerance to humans among disturbed wildlife. Nature Communications 6, 8877.
| Increased tolerance to humans among disturbed wildlife.Crossref | GoogleScholarGoogle Scholar |
Saraux, C, Chiaradia, A, Le Maho, Y, and Ropert-Coudert, Y (2011a). Everybody needs somebody: unequal parental effort in little penguins. Behavioral Ecology 22, 837–845.
| Everybody needs somebody: unequal parental effort in little penguins.Crossref | GoogleScholarGoogle Scholar |
Saraux, C, Robinson-Laverick, SM, Le Maho, Y, Ropert-Coudert, Y, and Chiaradia, A (2011b). Plasticity in foraging strategies of inshore birds: how little penguins maintain body reserves while feeding offspring. Ecology 92, 1909–1916.
| Plasticity in foraging strategies of inshore birds: how little penguins maintain body reserves while feeding offspring.Crossref | GoogleScholarGoogle Scholar |
Schaefer, R, and Colombelli-Négrel, D (2021). Behavioural and heart rate responses to stressors in two populations of little penguins that differ in levels of human disturbance and predation risk. Ibis 163, 858–874.
| Behavioural and heart rate responses to stressors in two populations of little penguins that differ in levels of human disturbance and predation risk.Crossref | GoogleScholarGoogle Scholar |
Schumann, N, Dann, P, and Arnould, JPY (2013). Use of terrestrial habitats by burrow-nesting seabirds in south-eastern Australia. Emu – Austral Ornithology 113, 135–144.
| Use of terrestrial habitats by burrow-nesting seabirds in south-eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Scopel, LC, and Diamond, AW (2017). Predation and food–weather interactions drive colony collapse in a managed metapopulation of Arctic Terns (Sterna paradisaea). Canadian Journal of Zoology 96, 13–22.
| Predation and food–weather interactions drive colony collapse in a managed metapopulation of Arctic Terns (Sterna paradisaea).Crossref | GoogleScholarGoogle Scholar |
Sherwen, SL, Magarth, MJL, Butler, KL, and Hemsworth, PH (2015). Little penguins, Eudyptula minor, show increased avoidance, aggression and vigilance in response to zoo visitors. Applied Animal Behaviour Science 168, 71–76.
| Little penguins, Eudyptula minor, show increased avoidance, aggression and vigilance in response to zoo visitors.Crossref | GoogleScholarGoogle Scholar |
Sih, A, Ferrari, MCO, and Harris, DJ (2011). Evolution and behavioural responses to human-induced rapid environmental change. Evolutionary Applications 4, 367–387.
| Evolution and behavioural responses to human-induced rapid environmental change.Crossref | GoogleScholarGoogle Scholar |
Soldatini, C, Albores-Barajas, YV, Tagliavia, M, Massa, B, Fusani, L, and Canoine, V (2015). Effects of human disturbance on cave-nesting seabirds: the case of the storm petrel. Conservation Physiology 3, cov041.
| Effects of human disturbance on cave-nesting seabirds: the case of the storm petrel.Crossref | GoogleScholarGoogle Scholar |
Stokes, DL, and Dee Boersma, P (1991). Effects of substrate on the distribution of Magellanic penguin (Spheniscus magellanicus) burrows. Auk 108, 932–933.
Strasser, EH, and Heath, JA (2013). Reproductive failure of a human-tolerant species, the American kestrel, is associated with stress and human disturbance. Journal of Applied Ecology 50, 912–919.
| Reproductive failure of a human-tolerant species, the American kestrel, is associated with stress and human disturbance.Crossref | GoogleScholarGoogle Scholar |
Syposz, M, Padget, O, Willis, J, Van Doren, BM, Gillies, N, Fayet, AL, Wood, MJ, Alejo, A, and Guilford, T (2021). Avoidance of different durations, colours and intensities of artificial light by adult seabirds. Scientific Reports 11, 18941.
| Avoidance of different durations, colours and intensities of artificial light by adult seabirds.Crossref | GoogleScholarGoogle Scholar |
Tablado, Z, and Jenni, L (2017). Determinants of uncertainty in wildlife responses to human disturbance. Biological Reviews 92, 216–233.
| Determinants of uncertainty in wildlife responses to human disturbance.Crossref | GoogleScholarGoogle Scholar |
Taylor, AR, Dann, P, and Arnould, JP (2013). Timing of breeding and diet of the black-faced cormorant Phalacrocorax fuscescens. Marine Ornithology 41, 23–27.
Thayer, JA, Sydeman, WJ, Fairman, NP, and Allen, SG (1999). Attendance and effects of disturbance on coastal Common Murre colonies at Point Reyes, California. Waterbirds: The International Journal of Waterbird Biology 22, 130–139.
| Attendance and effects of disturbance on coastal Common Murre colonies at Point Reyes, California.Crossref | GoogleScholarGoogle Scholar |
Thompson J (2021) A drone crash caused thousands of elegant terns to abandon their nests. In Audubon Magazine, 11 June 2021. Available at https://www.audubon.org/news/a-drone-crash-caused-thousands-elegant-terns-abandon-their-nests#:~:text=On%20the%20ground%20was%20an,reserve%20manager%20at%20Bolsa%20Chica [Verified 24 November 2021]
Thushari, GGN, and Senevirathna, JDM (2020). Plastic pollution in the marine environment. Heliyon 6, e04709.
| Plastic pollution in the marine environment.Crossref | GoogleScholarGoogle Scholar |
Vander Pol SS, Becker PR, Day RD, Ellisor MB, Guichard A, Moors AJ, Point D, Pugh RS, Roseneau DG (2009) Seabird tissue archival and monitoring project: egg collections and analytical results for 2002-2005. US Department of Commerce, National Institute of Standards and Technology.
Viblanc, VA, Smith, AD, Gineste, B, and Groscolas, R (2012). Coping with continuous human disturbance in the wild: insights from penguin heart rate response to various stressors. BMC Ecology 12, 10.
| Coping with continuous human disturbance in the wild: insights from penguin heart rate response to various stressors.Crossref | GoogleScholarGoogle Scholar |
Vincze, E, Papp, S, Preiszner, B, Seress, G, Bokony, V, and Liker, A (2016). Habituation to human disturbance is faster in urban than rural house sparrows. Behavioral Ecology 27, 1304–1313.
| Habituation to human disturbance is faster in urban than rural house sparrows.Crossref | GoogleScholarGoogle Scholar |
Votier, SC, Archibald, K, Morgan, G, and Morgan, L (2011). The use of plastic debris as nesting material by a colonial seabird and associated entanglement mortality. Marine Pollution Bulletin 62, 168–172.
| The use of plastic debris as nesting material by a colonial seabird and associated entanglement mortality.Crossref | GoogleScholarGoogle Scholar |
Voulgaris M-D (2017) Metal concentrations in Scopoli’s shearwater (Calonectris diomedea) seabird in Strofades island complex, Greece; Strofades Island. MaCS - SIMCO thesis, University of Algarve, Faro, Portugal.
Wasiak P (2020) Fieldwork procedures for working with little penguins. Document prepared on behalf of Philip Island Nature Parks, Vic., Australia.
Watson, H, Bolton, M, and Monaghan, P (2014). Out of sight but not out of harm’s way: human disturbance reduces reproductive success of a cavity-nesting seabird. Biological Conservation 174, 127–133.
| Out of sight but not out of harm’s way: human disturbance reduces reproductive success of a cavity-nesting seabird.Crossref | GoogleScholarGoogle Scholar |
Weerheim, MS, Klomp, NI, Brunsting, AMH, and Komdeur, J (2003). Population size, breeding habitat and nest site distribution of little penguins (Eudyptula minor) on Montague Island, New South Wales. Wildlife Research 30, 151–157.
| Population size, breeding habitat and nest site distribution of little penguins (Eudyptula minor) on Montague Island, New South Wales.Crossref | GoogleScholarGoogle Scholar |
Weimerskirch, H, Shaffer, SA, Mabille, G, Martin, J, Boutard, O, and Rouanet, JL (2002). Heart rate and energy expenditure of incubating wandering albatrosses: basal levels, natural variation, and the effects of human disturbance. Journal of Experimental Biology 205, 475–483.
| Heart rate and energy expenditure of incubating wandering albatrosses: basal levels, natural variation, and the effects of human disturbance.Crossref | GoogleScholarGoogle Scholar |
Weimerskirch, H, Prudor, A, and Schull, Q (2018). Flights of drones over sub-Antarctic seabirds show species- and status-specific behavioural and physiological responses. Polar Biology 41, 259–266.
| Flights of drones over sub-Antarctic seabirds show species- and status-specific behavioural and physiological responses.Crossref | GoogleScholarGoogle Scholar |
Yorio, P, and Boersma, PD (1994). Causes of nest desertion during incubation in the Magellanic penguin (Spheniscus magellanicus). Condor 96, 1076–1083.
| Causes of nest desertion during incubation in the Magellanic penguin (Spheniscus magellanicus).Crossref | GoogleScholarGoogle Scholar |
Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM (2009) ‘Mixed effects models and extensions in ecology with R.’ (Springer)