Small-scale movement and migration cues of Australian bass (Percalates novemaculeata) in an urbanised river
Culum Brown A and Evan E. Byrnes
A and Evan E. Byrnes  A B C *
A B C *
A Department of Biological Sciences, Macquarie University, Sydney, NSW 2109, Australia.
B Centre for Sustainable Aquatic Ecosystems, Harry Butler Institute, Murdoch University, Perth, WA 6150, Australia.
C School of Environmental Science, Simon Fraser University, Burnaby, BC, V5A 1S6, Canada.
Marine and Freshwater Research 73(6) 742-753 https://doi.org/10.1071/MF21238
Submitted: 14 August 2021 Accepted: 21 January 2022 Published: 3 March 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Urban river catchments are often severely affected by human activities but may still retain significant biodiversity. Surprisingly little is known about the behaviour of urban fishes, even those popular with anglers. Key environmental variables that trigger fish behaviour, such as river flow, are highly affected by instream structures including weirs and changes in the natural flow regime. Here, we used acoustic telemetry to examine the movements of Australian bass in a river located in suburban Sydney, Australia. We found that fish tended to be nocturnally active, however, small-scale movements were highly idiosyncratic and less associated with river flow than other factors. Larger-scale movements associated with spawning migrations were strongly correlated with winter floods. Half of the tagged fish migrated to the confluence of the river with Sydney Harbour to breed. Their return migration was hampered by a weir, but the provision of a new fish way facilitated successful return to freshwater at high tide. Despite occupying a highly affected, urban catchment fish behaviour showed similarity to those occupying more natural drainages. Our results highlight the importance of maintaining natural river flows in urban catchments and sustaining fish movement and migration capabilities through the installation of appropriate fish passage devices.
Keywords: activity pattern, animal movement, biotelemetry, fish, freshwater, migration, teleost, urban environments.
Introduction
Freshwater fish movements are often related to environmental cues and tend to occur on two scales. First, many fish make large-scale migrations, primarily for reproductive purposes and often at a certain time of the year (Brönmark et al. 2014; Secor 2015). Second, smaller-scale movements occur within a defined home range as part of a daily routine. For example, fish respond to daily rhythms in environmental factors, including light and temperature, showing crepuscular escalation in activity levels when environmental conditions may enhance foraging success and mitigate predation risk (Løkkeborg et al. 2000; Myers et al. 2016). The environmental triggers of movement are many and varied, but flow is particularly important for large-scale mating migrations for many species (Sykes et al. 2009; Brönmark et al. 2014). Periods of high rainfall and associated flood waters provide access to, or generate, productive nursery areas and may help migrating adults overcome obstacles, both natural and man-made (Baumgartner et al. 2014; Harris et al. 2017). River flow is heavily affected by human activities such as water extraction and dam building, and in urban environments, impervious surfaces further disrupt natural flow regimes. Furthermore, climate change is inducing large shifts in weather patterns, particularly in Australia where rainfall is becoming more infrequent and unpredictable (Lough and Hobday 2011).
Many fish species migrate between marine and freshwater habitats as part of their life cycle (diadromy; Potter et al. 2015). In Australia, for example, there are ~42 species of diadromous fish species (Harris et al. 2017). Given the importance of migration in the life-history of diadromous fish species, any obstacle to migration can have a severe effect on both local and broad-scale population viability and species diversity. Following dam building in three rivers in the US, diadromous species suffered population declines of between 95 and 99% (Limburg and Waldman 2009). Dams and weirs present a physical barrier to migration, but also interfere with important migration cues, such as water flow and temperature (Harris 1983; Walsh et al. 2012). Such structures are extremely common in urban areas, where fish face additional problems because the entire catchment may be heavily modified and urban runoff contains high levels of pollutants and nutrients. In addition, high proportions of impermeable surfaces within urban river catchments cause water to be delivered to the local streams far more quickly than in natural systems, thus flash flooding is commonplace (Li and Wang 2009). This altered hydrology can interfere with migratory behaviour. As such, urban streams are among the most heavily affected environments from anthropogenic activities and modifications (Angermeier 1995; Jonsson et al. 1999; Walsh et al. 2005).
The problems associated with barriers to fish migration have been known for a long time. In response to this, fish ladders are commonly installed to facilitate fish passage (Larinier 2001). In addition, river flow management strategies have started to incorporate scheduled water releases into the river from impoundments to mimic natural flow regimes and stimulate natural fish migration cues (so called environmental flows; e.g. Kiernan et al. 2012). In Australia, many of the early fish ladder designs were based on northern hemisphere models used for salmon. However, these northern hemisphere designs were largely ineffective for native Australian fish, owing to the steep inclines and high water velocities and turbulence characteristic of these designs (Mallen-Cooper and Brand 2007; Harris et al. 2017). As such, these ladders are gradually being replaced with systems better suited to Australian species (Baumgartner et al. 2014).
The Lane Cove River in Sydney, Australia is one such example where initial improper fish ladder design appeared to be impassable to native fish. The Lane Cove River is a relatively small catchment (95 km2) which drains into the northern side of Sydney Harbour (Fig. 1). Most of the catchment is highly urbanised, despite much of the river being surrounded by national park. Nevertheless, a resident population of Australian bass (Percalates novemaculeata, Family: Percichthyidae) inhabits the river. At the upper limits of the estuarine influence, a large weir was constructed in 1938 and likely presents a considerable barrier for fish migration. The weir was initially fitted with a steep sided, stepladder fish passageway of northern hemisphere design, but local fisherman reported continued declines in the local Australian bass population (Bass Sydney Fishing Club, pers. comm.). In 2009, this fish ladder was replaced by a ramp with a more moderate incline and instream boulders to provide flow refuges. However, it remains unconfirmed whether this updated design allows fish to migrate upstream past the weir.
Australian bass are catadromous and found along the east coast of Australia. Adults typically inhabit the upper freshwater reaches of coastal rivers and make winter migrations to estuaries to spawn (Harding et al. 2017). Larvae develop in estuaries and juveniles return to upstream freshwater habitats (Harris 1983). Most of Australia’s human population also occurs in the south-east of the continent and has heavily affected the rivers in the region (van Dijk et al. 2013; Mayer-Pinto et al. 2015). Numerous populations of Australian bass have declined over recent decades primarily due to dams, weirs and insufficient river flow disrupting their annual breeding migrations and limiting access to freshwater habitats for juvenile recruitment (Jerry and Cairns 1998). Harris (1983) estimated that as much as half of the available freshwater habitat within the species range is impeded by artificial barriers. Australian bass are highly prized by recreational anglers and can reach 60 cm long. They are aggressive predators and, therefore, likely play important roles in maintaining ecosystem health through mediating prey population sizes and behaviour (Dill et al. 2003; Pusey et al. 2004). Breeding occurs from May to September in the Sydney area (Harris 1983). The timing of breeding in south-east Queensland to the north appears to be somewhat later (June–September; Harding et al. 2017), whereas to the south, in Victoria, breeding can continue into December (McCarraher 1986). In all cases, the downstream migration is initiated by flood events and spawning occurs in the estuary. Little is known about the general activity patterns of this species, but it also likely that small-scale movements in the upper reaches of the catchment occur in response to rainfall outside of the breeding season. Although anecdotal evidence from fishermen has suggested that this species is crepuscular, it has never been verified empirically.
There has been considerable recent research resulting in significant new information on movements of Australian fish, though for many species there are still large knowledge gaps (Koehn and Crook 2013). In particular, little is known about the movements and migration of endemic Australian freshwater fish species in the context of urban environments. Here, we use acoustic biotelemetry tags to monitor the movements of adult Australian bass within the Lane Cove River in relation to various environmental cues. Specifically, we aimed to ascertain whether downstream spawning migrations and daily small-scale movements were influenced by river flow and assess if these movements were affected by the fish ladder installed on the weir.
Materials and methods
Fish tagging and tracking
All work with animals was conducted under a Macquarie University Animal Ethics Committee permit (2015/015). Wild Australian bass were captured using barbless lures by members of the Bass Sydney Fishing club in the upper reaches of the Lane Cove River. Upon capture, fish were held in a 20-L live well, supplied with fresh water and transported (up to 2 km) to researchers on the riverbank for tag implantation. Fish fork length and total length were measured, and fish were anaesthetised in a 150 mg L−1 bath of ethyl 3-aminobenzoate methane sulfonate 98% (MS-222) until they lost buoyancy control. They were then placed in a V-shaped holder and running freshwater was passed across their gills during surgery. A small incision (~10 mm) was made on the ventral surface anterior to the anal fin, and an acoustic tag (Vemco V7, Innovasea Systems, Inc., Nova Scotia, Canada) was inserted into the abdominal cavity. The incision was sealed with a single Monosyn absorbable monofilament suture (Q463 MonoWeb, Patterson Veterinary, Devens, MA, USA) and cyanoacrylate (Loctite Super Glue, Henkel, Düsseldorf, Germany), and fish were allowed to recover in fresh river water before being released at the point of capture. We targeted fish with a total length greater than 25 cm knowing they would be sexually mature and likely to breed (Llewellyn and MacDonald 1980; McDowall 1996). Vemco V7 tags weigh just 0.7 g in water and have a battery life of ~220 days. The fish were tagged and released at the site of capture on 11 December 2015 providing several months of movement data leading up to and including the expected winter migration.
Prior to fish tagging, eight acoustic receivers (Vemco VR2W) were installed throughout the river (Fig. 1), approximately 1 m from the bottom by securing them to a star picket driven into the sediment. Four were placed upstream of the weir in the freshwater reach, and four in the estuary (downstream of the weir). Receiver detection ranges were determined above and below the weir by submerging a V7 acoustic tag a known distance from receivers. Receiver detection range exceeded 100 m both above and below the weir. In addition, receivers were placed in narrow sections of the river so that fish could not pass receivers at distances greater than 70 m, making it unlikely for fish to pass receivers undetected. Due to physical barriers (e.g. waterfall), fish were unable to pass further than 200 m upstream of the most upstream receiver. These receivers complemented an existing array of receivers in Sydney Harbour maintained by IMOS (Integrated Marine Observing System) (Fig. 1).
Receivers were downloaded 4 months after tagging and again in December 2016, by which time all tags had ceased to function. The maximum tag battery life was 238 days (last detection 5 August 2015); thus, the data covered the Austral summer, autumn and winter periods.
Data processing
False detections were removed from the dataset and data was combined into hourly detections at each receiver before analysis. False detections were defined as single detections within a 24-h period, or when two detections obtained by different receivers were too close in time (e.g. seconds to a few minutes) for an individual to reasonably travel the distance separating the receivers. In addition, the first 24 h of data for each fish were removed to allow fish to recover from the tagging process (Jepsen et al. 2015).
To quantify fish activity patterns, detections were transformed into binarily coded movements and migration events. Movements were deemed to occur when individual fish were detected by one receiver and then by another. Migration events were deemed to occur when fish were detected by receivers downstream of the weir, excluding receiver five (Fig. 1), for at least 24 h. This ensured that fish had to expand their activity space at least 1 km downstream of the weir for the movement to be considered a migration, thus decreasing the likelihood that exploratory or non-volitional movements (e.g. being washed over the weir) were included as migrations. To confirm the timing of migration events, cumulative linear activity spaces were estimated and assessed for timing of rapid expansions of space use. Cumulative activity spaces were estimated by determining the shortest distance along the river channel between the outermost location coordinates for each individual fish. Fish locations for each acoustic detection were assumed to be within 150 m of the receivers, and as such receiver locations were used for fish location coordinates. The distances between receiver locations were estimated by measuring the shortest path along the river channel using Google Earth Pro (Google, USA).
To determine if the time of return from migrations was associated with tidal cycles, we established the time the fish moved from receiver five (below the weir; Fig. 1) to receiver four (above the weir; Fig. 1) and compared it to local tide timetables. Our focus was on the high tide since this is when the bottom of the fish ladder was underwater, and thereby accessible to the fish. Thus, we calculated the difference between the movement of fish over the weir and high tide. River tidal data was obtained from Willy Weather (http://www.willyweather.com.au).
Drivers of activity, movements and migration
Three different models were fit to determine the influence of environmental and temporal factors on the activity and migrations of Australian bass. For each model, a set of candidate models containing all possible combinations of covariates was assessed (Supplementary Tables S1–S3). All models were then ranked based on comparison of corrected Akaike’s Information Criterion (∆AICc), and the importance of each variable was determined by summing the AICc weight across all models that the variable occurred within the top ranked models (i.e. models within Δ4 AICc of the top model), giving the probability that the covariate would occur in the top ranked model (Burnham et al. 2011). A reduced model was then constructed including all covariates that had a greater than 50% probability of being selected for in the top AICc ranked model. Model fit was further assessed using log-likelihood estimates, with maximum log-likelihood indicating a more parsimonious fit. All models were fit in R (ver. 3.5. 2, R Foundation for Statistical Computing, Vienna, Austria) and using α value of 0.05.
To examine the fine-scale (hourly) activity patterns of fish, we used a generalised additive mixed model (GAMM; ‘mgcv’ package, ver. 1–7, S. Wood and M. S. Wood, see https://cran.r-project.org/web/packages/mgcv/index.html). Occurrence of movements within an hour were modelled as a function of hour of the day, date, moon phase (i.e. new, waxing, full, waning), and an interaction between time of the day and moon phase, with individual ID included as a random effect. Time of the day and date were fit using a cyclic and p-spline respectively. Moon phase was fit as a categorical variable. The model was fit assuming a binomial distribution.
To determine how daily movements varied temporally and were influenced by environmental and temporal factors, we used a generalised linear mixed-effect model (GLMM; lme4 package, ver. 1.1-27.1, see https://cran.r-project.org/web/packages/lme4/index.html; Bates et al. 2015). Movements were pooled into daily bins and modelled as a function of daily rainfall (mm), day of the year (Julian day), and moon phase, with individual ID included as a random effect. Daily rainfall data was obtained from the Australian Bureau of Meteorology (http://www.bom.gov.au) from station 660011, located ~2.5 km from the weir. The GLMM was fit assuming a Gaussian error distribution.
To examine the influence of environmental factors on the timing of migrations, we applied a logistic regression by employing a GLMM with a binomial distribution and logit link function. The onset of migration events were modelled as a function of daily rainfall, day of the year (Julian day), moon phase, with individual ID included as a random effect. Given that migrations and spawning have been shown to be influenced by high flow during the winter, initial models also included an interaction between date and moon phase and between date and daily rainfall. After fitting preliminary models, inclusion of the interaction between date and moon phase resulted in models predicting unrealistic absolute probabilities (i.e. 0 or 100%). Therefore, to avoid overfitting, this interaction was removed, and the model selection was conducted as per above.
Results
Detections and home range
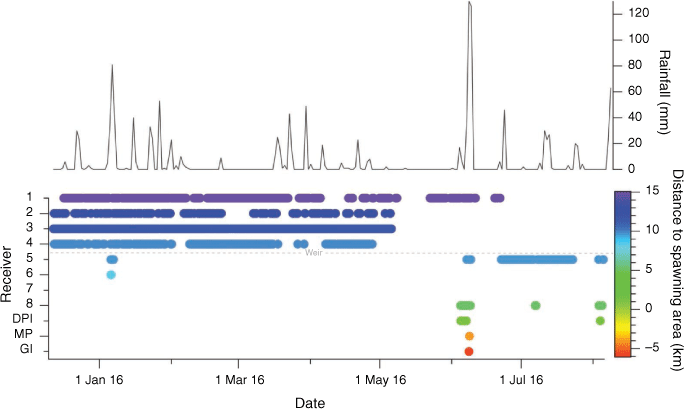
Tagged fish were detected for periods ranging from 39 to 236 days (mean ± s.d.: 153.40 ± 60.77 days), producing a total of 167 424 detections (16 742.40 ± 21 666.84) that were combined into 8705 hourly detections (870.50 ± 911.59) (Table 1). Receiver seven did not record any detections, despite no apparent gear malfunctions (Fig. 2). Fish remained in the freshwater reaches (i.e. upriver of the weir) from tagging until early June, except when a single fish was briefly detected below the weir for 10 h (Fig. 2). The home ranges of fish for the first 6 months of the study (outside the spawning season) showed that fish varied in their spatial habitat use, with spatial use ranging from remaining in the vicinity of just two receivers to use of the full freshwater reach of the river (Fig. 3). Four fish had a core home range (≤50% KUD) around receiver three, one fish had a core home range only consisting of the pool immediately underneath a large waterfall (marking the uppermost navigable portion of the river), two fish around receiver two and two fish around receiver four, just above the weir (Fig. 3). Fish 36742 used the entire length of the freshwater reach fairly equally, with a core home range including all four receivers above the weir (Fig. 3).

|
Activity patterns
The most parsimonious GAMM describing activity included effects of time of the day (i.e. hour; F = 5.02, P < 0.01), month (F = 16.26, P < 0.01) and moon phase (t = −10.287, P < 0.01) (Table 2, Supplementary Table S1). Fish demonstrated a nocturnal activity pattern, increasing activity in the evening and decreasing activity after early morning until early afternoon (Fig. 4a). Fish also demonstrated a seasonal activity pattern, with higher activity in the austral summer, and activity decreasing through the autumn to a low during winter (Fig. 4a). Lastly, activity of fish varied between moon phases but only significantly differed between new moon and waning moon phases, when activity was highest and lowest respectively (Fig. 4a).

|
Daily movement drivers
Daily movements were significantly influenced by daily rainfall (t = 4.94, P < 0.01) and moon phase (t = 4.58, P < 0.01) (Table 2, Supplementary Table S2). Rainfall had a positive, albeit minor effect on fish movement frequency, with the number of movements increasing from approximately one movement every other day at low rainfall amounts to fish moving slightly more than once per day at high rainfall amounts (Fig. 4b). Fish tended to move the least during waning moons but moved similarly through other moon phases (Fig. 4b). The number of daily movements between or away from receivers did not show a seasonal pattern (i.e. model selection did not support inclusion of time of the year) (Table 2, Supplementary Table S2).
Migration (timing, drivers and location)
Individual cumulative space use remained relatively stable until early June, when dramatic increases were observed, indicating migratory movements. Of the six fish that were monitored through early June, five fish demonstrated migratory movements into the estuary (Table 1, Fig. 2). Timing of migrations were significantly influenced by moon phase (z = −3.40, P < 0.01) and were marginally influenced by an interaction between time of the year and daily rainfall (z = 1.80, P = 0.07) (Table 2, Supplementary Table S3, Fig. 4c). All except one migratory movement occurred during new moons (Fig. 5). Interestingly, the one other migration event occurred in January during a waning moon, however, this migration event was particularly short, with the fish only moving downstream to receiver six and returning upstream of the weir 10 h later (Fig. 2). The probability of migrations increased with increased rainfall, however, the probability was dependent on time of the year (Fig. 5). For instance, during a new moon in early January, the probability of migrating increased from 3.07 to 5.37% with daily rainfall increasing from 0 to 25 mm. Comparatively, during a new moon in early June, the probability of migrating increased from 7.50 to 61.65% with daily rainfall increasing from 0 to 25 mm.

|
Most of detections in the estuarine reaches of the river (~85%) occurred at the receiver directly below the weir (receiver five; Fig. 1), but there were also detections at receiver one (8%) and at the DPI receiver (5%) suggesting that the spawning ground for this population is in the lower reaches of the Lane Cove River. One fish was detected at the Goat Island receiver adjacent to the Sydney Harbour Bridge before returning to the river.
Fish returning from movements into the estuarine reaches of the river successfully navigated past the weir, as indicated by detections at the receiver immediately below the weir (receiver 5), shortly followed by detections above the weir (Fig. 2). None of the movements upstream over the weir were associated with rainfall events. Rather the timing of return to the freshwater section of the river appeared to be associated with tidal cycles. The mean time between high tide and passage through the fish way was 0.17 min (s.d.: 16.45 min); ~10 s after peak high tide.
Discussion
Australian bass in the urbanised catchment of the Lane Cove River undertook migrations that were consistent with previous studies of this species in more natural and other catchments (Brown 2011; Harding et al. 2017). The fish were able to overcome the weir in both downstream and upstream directions. Their downstream migration was associated with large rainfall events during winter, with the majority of migrations extending to the lower estuarine portion of the river, indicating the spawning location was likely near the confluence with Sydney Harbour. On the return migration, fish spent considerable time at the base of the weir but passed through the fishway shortly after high tide. In the freshwater reaches of the river, Australian bass also showed small-scale movements in response to light levels and rainfall, but their response to these environmental variable where highly individualistic. Australian bass were most active in the late afternoons and early evenings. Four of the ten acoustically tagged fish disappeared from the upper reaches of the river before the winter migration, and we assume they were taken by anglers.
Many freshwater fish around the world migrate in response to a range of environmental cues (e.g. Lucas and Baras 2008; Sykes et al. 2009; Skov et al. 2010; Harris et al. 2017). Here, we found that the timing of migration for Australian bass occurred following high river flows associated with large rainfall events (greater than ~25 mL), but the likelihood of migrating was much higher during the winter, particularly during a new moon. These results suggest fish rely on a complex interaction of multiple environmental cues (i.e. temperature, flow rate and moon phase) to coordinate their spawning run. The finding that migration timing is associated with lunar phase in a catadromous fish is not unexpected. The new moon means that light levels are low at night, but importantly tidal range is much greater (spring tides) which might facilitate movement over obstacles or reduce the costs of long-distance instream movements. Female tupong (Pseudophritis urvillii), for example, undertook rapid downstream migrations through the estuary in response to high river flows, and movement from the estuary to the sea was associated with moon phase (Crook et al. 2010). Similarly, migratory activity in brown trout occurred during the new moon (Slavík et al. 2012) and the movement of anadromous herring from rivers to the ocean occurred during the summer on the new moon during periods of low rainfall (Yako et al. 2002). Atlantic salmon migration timing has also been linked with water flow, temperature and moon phase (Thorstad et al. 2008). A wide range of fish species spawn on specific phases of the lunar cycle (Taylor 1984; Colin 1992; Donahue et al. 2015). Japanese eels, for example, likewise spawn on the new moon (Tsukamoto et al. 2003). This is the first study to suggest that the migratory behaviour of Australian bass is also cued into lunar cycles despite occupying an urban stream.
Migration timing of Australian bass varies across the species distribution, with migrations occurring later in the winter at lower latitudes (May, Brown 2011; June, this study; July, Harding et al. 2017). These differences likely reflect differences in river discharge and water temperatures, which are widely considered the most important environmental factors for triggering migrations in most diadromous species (Davies and Sloane 1987; Quinn et al. 1997; Sims et al. 2004; Taylor and Cooke 2012). For catadromous species, increased water levels and river discharge can be crucial for accessing spawning grounds, as it may influence the ability of fish to overcome obstacles, such as shallow riffles, submerged objects (i.e. boulders and trees) and weirs (Reinfelds et al. 2010; Koehn and Crook 2013; Storer et al. 2021). In addition, increased discharge could decrease energy expenditure by facilitating more passive downstream transport during migrations (Silva et al. 2020), in turn allowing a greater proportion of energy intake and stores to be allocated to gamete production. Notably, one fish in this study moved into the estaurine section of river, a movement normally characteristic of a migration, following a large rainfall event in the summer, supporting that discharge is the primary trigger of migrations in Australian bass. Of course, increased discharge may also increase productivity in downstream spawning areas through increased nutrient influx (Harris 1986). Coupling measurements of productivity at spawning sites throughout the year with migration timing data would help to understand the relative importance of spawning site condition (e.g. productivity) v. spawning site access for shaping timing of migrations. Extending such studies to compare between species that display different migration timings, within and across different diadromous types would be particularly informative for understanding the evolutionary pressures shaping the timing of migrations in fishes.
The acoustic data from receivers placed in the estuary suggest that the spawning site of the bass was in the lower reaches of the Lane Cove River estuary a few kilometres from where it joins Sydney Harbour. Similarly, Harding et al. (2017) suggested that the Logan River population also used the lower estuary for spawning. Although it is known that salinity in these areas are important for successful spawning and corresponds with peak sperm motility (Harris 1986), it is unclear if other habitat variables are important in spawning site selection (e.g. flow, substrate, woody debris). Identification of the spawning location provides opportunities to better manage this population, such as for informing seasonal closures of fishing within the lower estuarine reaches of the river, however, further tagging of this species would be useful for elucidating the environmental factors critical to their spawning success.
It is well established that water flow is a key environmental cue for instream movement generally, including on short (hourly to daily) temporal scales (Taylor and Cooke 2012). High flows may aid movement over obstructions and different flows can be utilised to minimise energy expenditure while moving. As such, it was surprising that rainfall (i.e. river discharge), had little influence on the daily movement patterns of fish within the upstream reaches. Moreover, the direction that fish moved in response to rainfall was highly variable, where some fish moved upstream while others moved downstream, indicating highly individualised movement and habitat use strategies. Differences in environmental conditions (e.g. temperature regimes, resource density, landscape of fear) and timing of environmental cues (e.g. seasonal precipitation), may lead to natural variation in behavioural strategies between populations of animals, such as activity and foraging patterns (Nardi et al. 2003; Cruz-Font et al. 2019; Gámez-Brunswick and Rojas-Soto 2020). However, as all fish here were similarly sized and within a single river, it is unclear what caused such wide variation. Nonetheless, such variation has been noted before and poses a significant problem for management (Bolnick et al. 2003). Koehn et al. (2009), for example, reported that movement per se in Murray cod is reasonably predictable, but both the timing and the extent of movement is highly individualistic within a single river system. Mechanistic movement studies quantifying sexual and individual differences in metabolic rate, diet and predation risk would be useful to disentangle the variation in movement and behavioural strategies attributed to physiological and environmental processes v. variation owing to personality differences.
Most of the fish showed discrete preferences for parts of the river, although one fish utilised the full length of the freshwater reaches. This observation is generally consistent with many fish throughout the world which tend to have relatively small home ranges, but their behaviour can be punctuated with occasional long distance movements (Rodríguez 2002). Mary River cod, for example, occupy relatively small home ranges for most of the year, but then undertake rapid, large scale movements up to 35 km (Simpson and Mapleston 2002). Given that all bass in our study were of similar size, they likely competed for similar prey species and thus by distributing throughout the freshwater reach, home range overlap and resource competition could be reduced (Jetz et al. 2004). Given the lack of any obvious environmental variability (e.g. light levels, depth, prey species) and few barriers to movement throughout the freshwater reaches, it would be expected that the fish home-range areas would be evenly distributed.
The movement data of the acoustically tagged Australian bass also provided insights into daily activity patterns. Fish were most active in the late afternoon and evening until approximately midnight. This pattern of activity is reasonably common for large predatory species, which possess morphological adaptations (e.g. their senses such as vision and lateral line) that are well adapted for detecting and ambushing prey during low light periods, when detectability of predators by prey decreases (Howick and O’Brien 1983; Liang et al. 1998). For example, large mouth bass (Micropterus salmoides), which also demonstrate crepuscular and early night foraging had the highest prey capture success in low light levels similar to sunset and early night (McMahon and Holanov 1995). Trout cod (Maccullochella macquariensis), which are also from the family Percichthyidae, are also crepuscular or nocturnal in their patterns of activity (Thiem et al. 2008). In addition, small freshwater fish and crustacean species, the primary prey items of Australian bass rest at night, likely making them easier to capture, further aiding foraging success overnight (Bishop et al. 1995).
In river barriers, including low-head weirs are the most common impediment to freshwater fish movement, especially during periods of low flow (Crook et al. 2015; de Leaniz et al. 2019). Here, we show that Australian bass successfully overcame the weir on their downstream migration. This is not surprising because during flood events, the Lane Cove River weir can be under several metres of water. In 2009, a new fishway was installed to help Australian bass on their return, upstream migration. Although fish successfully navigated the ramp to access the freshwater upstream reach of the river, the base of the ramp was only accessible during high tide. Fish spent a considerable amount of time sitting and waiting for an opportunity to return upstream, during which they may have been concentrated directly below the weir, potentially subjecting fish to unfavourable environmental conditions (de Leaniz et al. 2019) or making them easier to target by predators, including anglers. Despite these risks, the success of the redesigned fishway shows that, with appropriate design, many of the obstacles preventing fish movement can be overcome. The remaining issue is to understand how these designs might benefit a wide range of fish species, not just iconic angling and economically important species, such as bass and salmon.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Declaration of funding
We are grateful to the Kuringgai Council for generously funding the research through an environment grant.
Supplementary material
Supplementary material is available online.
Acknowledgements
This work was conducted on land traditionally owned by the Guringai people; we extend our gratitude and pay respects to the Guringai elders past, present and emerging. We thank IMOS (Integrated Marine Observing System) for supplying the acoustic receivers from their receiver pool. Thanks also to the members of the Bass Sydney Fishing Club for donating their time to catch the fish, and to Dr Catarina Vila-Pouca for assisting with receiver maintenance and download. We also thank reviewers for their constructive comments, which helped to substantially improve the manuscript.
References
Angermeier, PL (1995). Ecological attributes of extinction‐prone species: loss of freshwater fishes of Virginia. Conservation Biology 9, 143–158.| Ecological attributes of extinction‐prone species: loss of freshwater fishes of Virginia.Crossref | GoogleScholarGoogle Scholar |
Bates, D, Mächler, M, Bolker, B, and Walker, S (2015). Fitting linear mixed-effects models using lme4. Journal of Statistical Software 67, 1–48.
| Fitting linear mixed-effects models using lme4.Crossref | GoogleScholarGoogle Scholar |
Baumgartner, LJ, Conallin, J, Wooden, I, Campbell, B, Gee, R, Robinson, WA, and Mallen-Cooper, M (2014). Using flow guilds of freshwater fish in an adaptive management framework to simplify environmental flow delivery for semi‐arid riverine systems. Fish and Fisheries 15, 410–427.
| Using flow guilds of freshwater fish in an adaptive management framework to simplify environmental flow delivery for semi‐arid riverine systems.Crossref | GoogleScholarGoogle Scholar |
Bishop, KA, Pidgeon, RWJ, and Walden, DJ (1995). Studies on fish movement dynamics in a tropical floodplain river: prerequisites for a procedure to monitor the impacts of mining. Australian Journal of Ecology 20, 81–107.
| Studies on fish movement dynamics in a tropical floodplain river: prerequisites for a procedure to monitor the impacts of mining.Crossref | GoogleScholarGoogle Scholar |
Bolnick, DI, Svanbäck, R, Fordyce, JA, Yang, LH, Davis, JM, Hulsey, CD, and Forister, ML (2003). The ecology of individuals: incidence and implications of individual specialization. American Naturalist 161, 1–28.
| The ecology of individuals: incidence and implications of individual specialization.Crossref | GoogleScholarGoogle Scholar |
Brönmark, C, Hulthén, K, Nilsson, P, Skov, C, Hansson, L-A, Brodersen, J, and Chapman, B (2014). There and back again: migration in freshwater fishes. Canadian Journal of Zoology 92, 467–479.
| There and back again: migration in freshwater fishes.Crossref | GoogleScholarGoogle Scholar |
Brown P (2011) Australian bass movement, migration and habitat suitability in the Snowy River. Freshwater fish resources in the Snowy River, Victoria. Fisheries Victoria Research Report Series 25, Department of Primary Industries, Victoria, Australia.
Burnham, KP, Anderson, DR, and Huyvaert, KP (2011). AIC model selection and multimodel inference in behavioral ecology: some background, observations, and comparisons. Behavioral Ecology and Sociobiology 65, 23–35.
| AIC model selection and multimodel inference in behavioral ecology: some background, observations, and comparisons.Crossref | GoogleScholarGoogle Scholar |
Colin, PL (1992). Reproduction of the Nassau grouper, Epinephelus striatus (Pisces: Serranidae) and its relationship to environmental conditions. Environmental Biology of Fishes 34, 357–377.
| Reproduction of the Nassau grouper, Epinephelus striatus (Pisces: Serranidae) and its relationship to environmental conditions.Crossref | GoogleScholarGoogle Scholar |
Crook, DA, Koster, WM, Macdonald, JI, Nicol, SJ, Belcher, CA, Dawson, DR, O’mahony, DJ, Lovett, D, Walker, A, and Bannam, L (2010). Catadromous migrations by female tupong (Pseudaphritis urvillii) in coastal streams in Victoria, Australia. Marine and Freshwater Research 61, 474–483.
| Catadromous migrations by female tupong (Pseudaphritis urvillii) in coastal streams in Victoria, Australia.Crossref | GoogleScholarGoogle Scholar |
Crook, DA, Lowe, WH, Allendorf, FW, Erős, T, Finn, DS, Gillanders, BM, Hadwen, WL, Harrod, C, Hermoso, V, Jennings, S, and Kilada, RW (2015). Human effects on ecological connectivity in aquatic ecosystems: integrating scientific approaches to support management and mitigation. The Science of the Total Environment 534, 52–64.
| Human effects on ecological connectivity in aquatic ecosystems: integrating scientific approaches to support management and mitigation.Crossref | GoogleScholarGoogle Scholar | 25917446PubMed |
Cruz-Font, L, Shuter, BJ, Blanchfield, PJ, Minns, CK, and Rennie, MD (2019). Life at the top: lake ecotype influences the foraging pattern, metabolic costs and life history of an apex fish predator. Journal of Animal Ecology 88, 702–716.
| Life at the top: lake ecotype influences the foraging pattern, metabolic costs and life history of an apex fish predator.Crossref | GoogleScholarGoogle Scholar |
Davies, PE, and Sloane, RD (1987). Characteristics of the spawning migrations of brown trout, Salmo trutta L., and rainbow trout, S. gairdneri Richardson, in Great Lake, Tasmania. Journal of Fish Biology 31, 353–373.
| Characteristics of the spawning migrations of brown trout, Salmo trutta L., and rainbow trout, S. gairdneri Richardson, in Great Lake, Tasmania.Crossref | GoogleScholarGoogle Scholar |
de Leaniz, CG, Berkhuysen, A, and Belletti, B (2019). Beware small dams, they can do damage, too. Nature 570, 164.
| Beware small dams, they can do damage, too.Crossref | GoogleScholarGoogle Scholar | 31186570PubMed |
Dill, LM, Heithaus, MR, and Walters, CJ (2003). Behaviorally mediated indirect interactions in marine communities and their conservation implications. Ecology 84, 1151–1157.
| Behaviorally mediated indirect interactions in marine communities and their conservation implications.Crossref | GoogleScholarGoogle Scholar |
Donahue, MJ, Karnauskas, M, Toews, C, and Paris, CB (2015). Location isn’t everything: timing of spawning aggregations optimizes larval replenishment. PLoS One 10, e0130694.
| Location isn’t everything: timing of spawning aggregations optimizes larval replenishment.Crossref | GoogleScholarGoogle Scholar | 26103162PubMed |
Gámez-Brunswick, C, and Rojas-Soto, O (2020). The effect of seasonal variation on the activity patterns of the American black bear: an ecological niche modeling approach. Mammalia 84, 315–322.
| The effect of seasonal variation on the activity patterns of the American black bear: an ecological niche modeling approach.Crossref | GoogleScholarGoogle Scholar |
Harding, DJ, Dwyer, RG, Mullins, TM, Kennard, MJ, Pillans, RD, and Roberts, DT (2017). Migration patterns and estuarine aggregations of a catadromous fish, Australian bass (Percalates novemaculeata) in a regulated river system. Marine and Freshwater Research 68, 1544–1553.
| Migration patterns and estuarine aggregations of a catadromous fish, Australian bass (Percalates novemaculeata) in a regulated river system.Crossref | GoogleScholarGoogle Scholar |
Harris, JH (1983). Impoundment of coastal drainages of southeastern Australia, and a review of its relevance to fish migrations. Australian Zoologist 21, 235–250.
Harris, JH (1986). Reproduction of the Australian bass, Macquaria novemaculeata (Perciformes: Percichthyidae) in the Sydney basin. Marine and Freshwater Research 37, 209–235.
| Reproduction of the Australian bass, Macquaria novemaculeata (Perciformes: Percichthyidae) in the Sydney basin.Crossref | GoogleScholarGoogle Scholar |
Harris, JH, Kingsford, RT, Peirson, W, and Baumgartner, LJ (2017). Mitigating the effects of barriers to freshwater fish migrations: the Australian experience. Marine and Freshwater Research 68, 614–628.
| Mitigating the effects of barriers to freshwater fish migrations: the Australian experience.Crossref | GoogleScholarGoogle Scholar |
Howick, GL, and O’Brien, WJ (1983). Piscivorous feeding behavior of largemouth bass: an experimental analysis. Transactions of the American Fisheries Society 112, 508–516.
| Piscivorous feeding behavior of largemouth bass: an experimental analysis.Crossref | GoogleScholarGoogle Scholar |
Jepsen, N, Thorstad, EB, Havn, T, and Lucas, MC (2015). The use of external electronic tags on fish: an evaluation of tag retention and tagging effects. Animal Biotelemetry 3, 49–23.
| The use of external electronic tags on fish: an evaluation of tag retention and tagging effects.Crossref | GoogleScholarGoogle Scholar |
Jerry, DR, and Cairns, SC (1998). Morphological variation in the catadromous Australian bass, from seven geographically distinct riverine drainages. Journal of Fish Biology 52, 829–843.
| Morphological variation in the catadromous Australian bass, from seven geographically distinct riverine drainages.Crossref | GoogleScholarGoogle Scholar |
Jetz, W, Carbone, C, Fulford, J, and Brown, JH (2004). The scaling of animal space use. Science 306, 266–268.
| The scaling of animal space use.Crossref | GoogleScholarGoogle Scholar | 15472074PubMed |
Jonsson, B, Waples, RS, and Friedland, K (1999). Extinction considerations for diadromous fishes. ICES Journal of Marine Science 56, 405–409.
| Extinction considerations for diadromous fishes.Crossref | GoogleScholarGoogle Scholar |
Kiernan, JD, Moyle, PB, and Crain, PK (2012). Restoring native fish assemblages to a regulated California stream using the natural flow regime concept. Ecological Applications 22, 1472–1482.
| Restoring native fish assemblages to a regulated California stream using the natural flow regime concept.Crossref | GoogleScholarGoogle Scholar | 22908707PubMed |
Koehn JD, Crook DA (2013) Movement and migration. In ‘Ecology of Australian Freshwater Fishes’. (Eds P Humphries, KF Walker) pp. 105–130. (CSIRO Publishing: Melbourne, Vic., Australia)
Koehn, JD, McKenzie, JA, O’Mahony, DJ, Nicol, SJ, O’Connor, JP, and O’Connor, WG (2009). Movements of Murray cod (Maccullochella peelii peelii) in a large Australian lowland river. Ecology Freshwater Fish 18, 594–602.
| Movements of Murray cod (Maccullochella peelii peelii) in a large Australian lowland river.Crossref | GoogleScholarGoogle Scholar |
Larinier, M (2001). Environmental issues, dams and fish migration. FAO Fisheries Technical Paper 419, 45–89.
Li, Y, and Wang, C (2009). Impacts of urbanization on surface runoff of the Dardenne Creek watershed, St Charles County, Missouri. Physical Geography 30, 556–573.
| Impacts of urbanization on surface runoff of the Dardenne Creek watershed, St Charles County, Missouri.Crossref | GoogleScholarGoogle Scholar |
Liang, X, Kiu, J, and Huang, B (1998). The role of sense organs in the feeding behaviour of Chinese perch. Journal of Fish Biology 52, 1058–1067.
| The role of sense organs in the feeding behaviour of Chinese perch.Crossref | GoogleScholarGoogle Scholar |
Limburg, KE, and Waldman, JR (2009). Dramatic declines in North Atlantic diadromous fishes. Bioscience 59, 955–965.
| Dramatic declines in North Atlantic diadromous fishes.Crossref | GoogleScholarGoogle Scholar |
Llewellyn LC, MacDonald MC (1980) Family Percichthyidae. Australian freshwater basses and cods. In ‘Freshwater fishes of south-eastern Australia’. pp. 142–149. (Reed Books)
Løkkeborg, S, Skajaa, K, and Fernö, A (2000). Food-search strategy in ling (Molva L.): crepuscular activity and use of space. Journal of Experimental Marine Biology and Ecology 247, 195–208.
| Food-search strategy in ling (Molva L.): crepuscular activity and use of space.Crossref | GoogleScholarGoogle Scholar | 10742504PubMed |
Lough, JM, and Hobday, AJ (2011). Observed climate change in Australian marine and freshwater environments. Marine and Freshwater Research 62, 984–999.
| Observed climate change in Australian marine and freshwater environments.Crossref | GoogleScholarGoogle Scholar |
Lucas M, Baras E (2008) ‘Migration of freshwater fishes.’ (Wiley)
Mallen-Cooper, M, and Brand, DA (2007). Non-salmonids in a salmonid fishway: what do 50 years of data tell us about past and future fish passage? Fisheries Management and Ecology 14, 319–332.
| Non-salmonids in a salmonid fishway: what do 50 years of data tell us about past and future fish passage?Crossref | GoogleScholarGoogle Scholar |
Mayer-Pinto, M, Johnston, EL, Hutchings, PA, Marzinelli, EM, Ahyong, ST, Birch, G, Booth, DJ, Creese, RG, Doblin, MA, Figueira, W, and Gribben, PE (2015). Sydney Harbour: a review of anthropogenic impacts on the biodiversity and ecosystem function of one of the world’s largest natural harbours. Marine and Freshwater Research 66, 1088–1105.
| Sydney Harbour: a review of anthropogenic impacts on the biodiversity and ecosystem function of one of the world’s largest natural harbours.Crossref | GoogleScholarGoogle Scholar |
McCarraher DB (1986) Observations on the distribution, spawning, growth and diet of Australian bass (Macquaria novemaculeata) in Victorian waters. Technical Report Series, Arthur Rylah Institute for Environmental Research, Australia.
McDowall RM (1996) ‘Freshwater fishes of south-eastern Australia.’ (Reed)
McMahon, TE, and Holanov, SH (1995). Foraging success of largemouth bass at different light intensities: implications for time and depth of feeding. Journal of Fish Biology 46, 759–767.
| Foraging success of largemouth bass at different light intensities: implications for time and depth of feeding.Crossref | GoogleScholarGoogle Scholar |
Myers, EM, Harvey, ES, Saunders, BJ, and Travers, MJ (2016). Fine‐scale patterns in the day, night and crepuscular composition of a temperate reef fish assemblage. Marine Ecology 37, 668–678.
| Fine‐scale patterns in the day, night and crepuscular composition of a temperate reef fish assemblage.Crossref | GoogleScholarGoogle Scholar |
Nardi, M, Morgan, E, and Scapini, F (2003). Seasonal variation in the free-running period in two Talitrus saltator populations from Italian beaches differing in morphodynamics and human disturbance. Estuarine, Coastal and Shelf Science 58, 199–206.
| Seasonal variation in the free-running period in two Talitrus saltator populations from Italian beaches differing in morphodynamics and human disturbance.Crossref | GoogleScholarGoogle Scholar |
Potter, IC, Tweedley, JR, Elliott, M, and Whitfield, AK (2015). The ways in which fish use estuaries: a refinement and expansion of the guild approach. Fish and Fisheries 16, 230–239.
| The ways in which fish use estuaries: a refinement and expansion of the guild approach.Crossref | GoogleScholarGoogle Scholar |
Pusey B, Kennard M, Arthington A (2004) ‘Freshwater fishes of north-eastern Australia.’ (CSIRO Publishing: Melbourne, Vic., Australia)
Quinn, TP, Hodgson, S, and Peven, C (1997). Temperature, flow, and the migration of adult sockeye salmon (Oncorhynchus nerka) in the Columbia River. Canadian Journal of Fisheries and Aquatic Sciences 54, 1349–1360.
| Temperature, flow, and the migration of adult sockeye salmon (Oncorhynchus nerka) in the Columbia River.Crossref | GoogleScholarGoogle Scholar |
Reinfelds, I, Lincoln‐Smith, M, Haeusler, T, Ryan, D, and Growns, I (2010). Hydraulic assessment of environmental flow regimes to facilitate fish passage through natural riffles: Shoalhaven River below Tallowa Dam, New South Wales, Australia. River Research and Applications 26, 589–604.
| Hydraulic assessment of environmental flow regimes to facilitate fish passage through natural riffles: Shoalhaven River below Tallowa Dam, New South Wales, Australia.Crossref | GoogleScholarGoogle Scholar |
Rodríguez, MA (2002). Restricted movement in stream fish: the paradigm is incomplete, not lost. Ecology 83, 1–13.
| Restricted movement in stream fish: the paradigm is incomplete, not lost.Crossref | GoogleScholarGoogle Scholar |
Secor DH (2015) ‘Migration ecology of marine fishes.’ (JHU Press)
Silva, AT, Bærum, KM, Hedger, RD, Baktoft, H, Fjeldstad, H-P, Gjelland, KØ, Økland, F, and Forseth, T (2020). The effects of hydrodynamics on the three-dimensional downstream migratory movement of Atlantic salmon. The Science of the Total Environment 705, 135773.
| The effects of hydrodynamics on the three-dimensional downstream migratory movement of Atlantic salmon.Crossref | GoogleScholarGoogle Scholar | 31972933PubMed |
Simpson, RR, and Mapleston, AJ (2002). Movements and habitat use by the endangered Australian freshwater Mary River cod, Maccullochella peelii mariensis. Environmental Biology of Fishes 65, 401–410.
| Movements and habitat use by the endangered Australian freshwater Mary River cod, Maccullochella peelii mariensis.Crossref | GoogleScholarGoogle Scholar |
Sims, DW, Wearmouth, VJ, Genner, MJ, Southward, AJ, and Hawkins, SJ (2004). Low‐temperature‐driven early spawning migration of a temperate marine fish. Journal of Animal Ecology 73, 333–341.
| Low‐temperature‐driven early spawning migration of a temperate marine fish.Crossref | GoogleScholarGoogle Scholar |
Skov, C, Aarestrup, K, Baktoft, H, Brodersen, J, Brönmark, C, Hansson, L-A, Nielsen, EE, Nielsen, T, and Anders Nilsson, P (2010). Influences of environmental cues, migration history, and habitat familiarity on partial migration. Behavioral Ecology 21, 1140–1146.
| Influences of environmental cues, migration history, and habitat familiarity on partial migration.Crossref | GoogleScholarGoogle Scholar |
Slavík, O, Horký, P, Randák, T, Balvín, P, and Bílý, M (2012). Brown trout spawning migration in fragmented central European headwaters: effect of isolation by artificial obstacles and the moon phase. Transactions of the American Fisheries Society 141, 673–680.
| Brown trout spawning migration in fragmented central European headwaters: effect of isolation by artificial obstacles and the moon phase.Crossref | GoogleScholarGoogle Scholar |
Storer, T, Bannister, J, Bennett, K, Byrnes, E, Crook, DA, Morgan, DL, Gleiss, A, and Beatty, SJ (2021). Influence of discharge regime on the movement and refuge use of a freshwater fish in a drying temperate region. Ecohydrology 14, e2253.
| Influence of discharge regime on the movement and refuge use of a freshwater fish in a drying temperate region.Crossref | GoogleScholarGoogle Scholar |
Sykes, GE, Johnson, CJ, and Shrimpton, JM (2009). Temperature and flow effects on migration timing of Chinook salmon smolts. Transactions of the American Fisheries Society 138, 1252–1265.
| Temperature and flow effects on migration timing of Chinook salmon smolts.Crossref | GoogleScholarGoogle Scholar |
Taylor, MH (1984). Lunar synchronization of fish reproduction. Transactions of the American Fisheries Society 113, 484–493.
| Lunar synchronization of fish reproduction.Crossref | GoogleScholarGoogle Scholar |
Taylor, MK, and Cooke, SJ (2012). Meta-analyses of the effects of river flow on fish movement and activity. Environmental Reviews 20, 211–219.
| Meta-analyses of the effects of river flow on fish movement and activity.Crossref | GoogleScholarGoogle Scholar |
Thiem, JD, Ebner, BC, and Broadhurst, BT (2008). Diel activity of the endangered trout cod (Maccullochella macquariensis) in the Murrumbidgee River. Proceedings of the Linnean Society of New South Wales 129, 167–173.
Thorstad, EB, Økland, F, Aarestrup, K, and Heggberget, TG (2008). Factors affecting the within-river spawning migration of Atlantic salmon, with emphasis on human impacts. Reviews in Fish Biology and Fisheries 18, 345–371.
| Factors affecting the within-river spawning migration of Atlantic salmon, with emphasis on human impacts.Crossref | GoogleScholarGoogle Scholar |
Tsukamoto, K, Otake, T, Mochioka, N, Lee, T-W, Fricke, H, Inagaki, T, Aoyama, J, Ishikawa, S, Kimura, S, and Miller, MJ (2003). Seamounts, new moon and eel spawning: the search for the spawning site of the Japanese eel. Environmental Biology of Fishes 66, 221–229.
| Seamounts, new moon and eel spawning: the search for the spawning site of the Japanese eel.Crossref | GoogleScholarGoogle Scholar |
van Dijk, AI, Beck, HE, Crosbie, RS, de Jeu, RA, Liu, YY, Podger, GM, Timbal, B, and Viney, NR (2013). The Millennium Drought in southeast Australia (2001–2009): natural and human causes and implications for water resources, ecosystems, economy, and society. Water Resources Research 49, 1040–1057.
| The Millennium Drought in southeast Australia (2001–2009): natural and human causes and implications for water resources, ecosystems, economy, and society.Crossref | GoogleScholarGoogle Scholar |
Walsh, CJ, Roy, AH, Feminella, JW, Cottingham, PD, Groffman, PM, and Morgan, RP (2005). The urban stream syndrome: current knowledge and the search for a cure. Journal of the North American Benthological Society 24, 706–723.
| The urban stream syndrome: current knowledge and the search for a cure.Crossref | GoogleScholarGoogle Scholar |
Walsh, CT, Reinfelds, IV, Gray, CA, West, RJ, van der Meulen, DE, and Craig, JR (2012). Seasonal residency and movement patterns of two co‐occurring catadromous percichthyids within a south‐eastern Australian river. Ecology Freshwater Fish 21, 145–159.
| Seasonal residency and movement patterns of two co‐occurring catadromous percichthyids within a south‐eastern Australian river.Crossref | GoogleScholarGoogle Scholar |
Yako, LA, Mather, ME, and Juanes, F (2002). Mechanisms for migration of anadromous herring: an ecological basis for effective conservation. Ecological Applications 12, 521–534.
| Mechanisms for migration of anadromous herring: an ecological basis for effective conservation.Crossref | GoogleScholarGoogle Scholar |