Reproductive biology of female blue swimmer crabs in the temperate estuaries of south-eastern Australia
Samuel E. F. Nolan A D , Daniel D. Johnson B , Roshan Hanamseth
A D , Daniel D. Johnson B , Roshan Hanamseth  A , Iain M. Suthers A C and Matthew D. Taylor
A , Iain M. Suthers A C and Matthew D. Taylor  A B
A B
A Centre for Marine Science and Innovation, School of Biological, Earth and Environmental Sciences, University of New South Wales, NSW 2052, Australia.
B Port Stephens Fisheries Institute, New South Wales Department of Primary Industries, Taylors Beach Road, Taylors Beach, NSW 2316, Australia.
C Sydney Institute of Marine Science, Chowder Bay Road, Mosman, NSW 2088, Australia.
D Corresponding author. Email: samuelenolan@gmail.com
Marine and Freshwater Research 73(3) 366-376 https://doi.org/10.1071/MF21191
Submitted: 25 June 2021 Accepted: 8 November 2021 Published: 2 December 2021
Journal Compilation © CSIRO 2021 Open Access CC BY
Abstract
The blue swimmer crab (BSC, Portunus armatus) is an economically and culturally important species distributed throughout the coastal waters of the Indo-Pacific region. Reproduction of BSC is poorly understood in south-eastern Australia, a region that is experiencing substantial tropicalisation from global warming. We examined gonadal development, egg–mass relationships, and the influence of temperature on gonadal development and egg production within five different estuaries spanning ~2.5° of latitude. A negative correlation between the gonadosomatic index (GSI, an index of gonadal development and reproductive investment) and hepatosomatic index (HSI, an index of energy storage) was observed in only the final stages of ovarian development. The weight of the egg mass increased logarithmically with body mass, accounting for up to 55% of total body mass, which was significantly larger than observed in other studies. Thermal performance curves showed a peak in individual reproductive output at a mean monthly temperature of ~24°C, at which the individual egg mass weight reached a maximum and the HSI reached a minimum. Environmentally driven variation in BSC reproduction has implications for population productivity and inter-annual variation in recruitment.
Keywords: Portunidae, reproduction, egg production, fecundity, fisheries, allometry.
Introduction
Portunidae is the family of swimming crabs, characterised by the modified pair of posterior thoracic limbs that are flattened into paddles for swimming (Stephenson and Campbell 1959). Portunid crabs constitute valuable commercial and recreational fisheries globally (West et al. 2015; Tweedley et al. 2017; Johnston et al. 2018) and have experienced rapid growth in harvest rates in recent decades (Food and Agriculture Organization of the United Nations 1992, 2019). Habitat loss and overexploitation increase the susceptibility of populations to climate change and associated events, such as marine heatwaves (Chandrapavan et al. 2019). High fishing pressure coupled with declines in recruitment arising from adverse environmental conditions have contributed to declines in several portunid fisheries globally (Tweedley et al. 2017), including blue swimmer crabs (Portunus spp. complex) in Western Australia (Johnston et al. 2011; Chandrapavan et al. 2019) and Callinectes sapidus in Chesapeake Bay, USA (Lipcius and Stockhausen 2002; Zohar et al. 2008). The C. sapidus fishery in Chesapeake Bay is still recovering (Huang et al. 2015), and only one of the major P. armatus fisheries in Western Australia, Shark Bay, has rebounded to historic levels (Chandrapavan et al. 2019), whereas other fisheries such as Cockburn Sound are showing minimal signs of recovery (Johnston et al. 2021a). Measuring the links between environmental variability and recruitment will aid data-driven management of portunid fisheries (Caputi et al. 2014; Tweedley et al. 2017).
A key component of fisheries recruitment is the reproductive output of exploited populations. Gonadal development and egg production are energetically expensive processes, and are known to respond to environmental drivers (Tropea et al. 2015; Baliña et al. 2018). Gonadal development within a population is often measured using a gonadosomatic index (GSI, unit mass of gonad per unit mass of bodyweight). The GSI is a useful indicator of gonadal development, such that a high GSI indicates reproductive maturity and greater investment into reproduction (Liu et al. 2014). The hepatosomatic index (HSI, unit mass of hepatopancreas per unit mass of bodyweight) is a useful measure of relative energy storage, as the hepatopancreas acts as a major store of organic and inorganic reserves in crustaceans (Passano 1960; Magalhães et al. 2012). The egg mass index (EMI, unit mass of eggs per unit mass of bodyweight) is a measure of relative reproductive output standardised to animal size. This may be used as a proxy for fecundity to evaluate factors affecting variability in reproductive output (Sukumaran and Neelakantan 1997; Hisam et al. 2018), where egg count data are not available. The relationships between the GSI, HSI and EMI can inform patterns in female reproductive condition throughout the breeding season. Therefore, quantifying these relationships alongside potential abiotic influences may aid examination of recruitment variability.
The blue swimmer crab (BSC), Portunus armatus (Lai et al. 2010), is distributed throughout the coastal and estuarine waters of tropical and temperate Australia, supporting valuable commercial and recreational fisheries (de Lestang et al. 2003; Johnston et al. 2021b). The reproductive development of BSC is strongly influenced by water temperature (de Lestang et al. 2003), which varies among estuaries and therefore causes significant variations in reproductive output (Shields and Wood 1993; de Lestang et al. 2003; Kumar et al. 2003; Johnson et al. 2010; Johnston and Yeoh 2021). Whereas BSC in tropical waters tend to exhibit little seasonality in their reproductive biology (de Lestang et al. 2003), the increased temperature variability in temperate estuaries may drive strong seasonal variation in reproduction (Kumar et al. 2003). New South Wales includes the southernmost extent of the main BSC range on the eastern Australian coast, where spawning is generally confined to summer, from November to February (Johnston et al. 2018). In the only comprehensive study of a BSC population in south-eastern Australia, Johnson et al. (2010) found substantial variation in size-at-maturity and fecundity in a NSW estuary, compared with other populations around Australia. Specifically, BSC had larger batch fecundities than did similarly sized individuals in Western Australia and larger sizes at maturity than did females in South Australia (Johnson et al. 2010).
Estuaries in south-eastern Australia are warming an order of magnitude faster than predicted by current global ocean and atmospheric models, with water temperatures increasing at a rate of 0.2°C year–1 (Scanes et al. 2020a). Continued warming presents a challenge to the health of estuarine ecosystems and the profitability of associated aquaculture and wild fisheries (Madeira et al. 2012; Food and Agriculture Organization of the United Nations 2018). Within estuaries, these changes may cause increased energetic demands and altered development in some species (Parker et al. 2013), reduced fecundity and egg viability (Foo and Byrne 2017) and behavioural changes (Madeira et al. 2012). Shifting temperature regimes vary according to the estuary geomorphology (Scanes et al. 2020a), which means that predicting the reproductive consequences for commercially important species may be complex. Here, we considered patterns of reproduction across estuaries in the southern part of the latitudinal range for eastern Australian BSC. Specifically, we aimed to (1) describe the interaction between the GSI (index of gonadal development and reproductive investment) and the HSI (index of energy storage) during gonadal development, (2) characterise EMI trends across south-eastern Australian BSC, and (3) assess the potential influence of temperature on gonadal development and egg mass production.
Materials and methods
Sampling locations
This research focused on BSC within the following five estuaries in south-eastern Australia: Wallis Lake (32°16′S, 152°29′E), Port Stephens (32°42′S, 152°01′E) Lake Macquarie (33°04′S, 151°35′E), Botany Bay (33°59′S, 151°11′E), and Lake Illawarra (34°31′S 150°50E; Fig. 1). These estuaries exhibit distinct morphological differences (Table 1) that contribute to different environmental conditions (Scanes et al. 2020a), allowing us to study the reproductive development of BSC under a range of temperature regimes. The majority of commercial BSC harvested within south-eastern Australia is extracted from three of these estuaries (Wallis Lake, Port Stephens and Lake Illawarra), of which 70% of total commercial landings are harvested from Wallis Lake (e.g. 99 of the 144 t harvested in NSW during 2016–2017, Johnston et al. 2018). Lake Macquarie and Botany Bay have no commercial harvest (Ochwada-Doyle et al. 2014).

|

|
Sampling design and collection
Sampling was conducted at monthly intervals from October 2019 to February 2020, covering the peak spawning period (Johnson et al. 2010). Crabs were sampled using large-mesh (55-mm diamond mesh) crab traps (900-mm diameter by 300 mm high) in Lake Illawarra and Botany Bay and small-mesh (24-mm diamond mesh) crab traps (900 × 300 mm) in Wallis Lake, Port Stephens and Lake Macquarie. The small-mesh traps were found to be more effective at catching smaller, juvenile crabs, but did not appear to affect catch rates of larger, mature crabs (Hanamseth, Johnson, Suthers and Taylor, unpubl.data). Because juvenile (immature) crabs were excluded from our analysis, differences in gear type used among estuaries did not affect our findings. In each of the five estuaries, four sites were surveyed, with six replicate crab traps deployed per site, per sampling event. Crab traps were deployed along the sand–seagrass ecotone, 50 m apart, in water less than 4 m deep at high tide and greater than 1 m deep at low tide. Each crab trap was baited with two sea mullets (Mugil cephalus), cut in half and held within a bait bag clipped inside the traps. All crab traps were set overnight and retrieved the following day, with an immersion (soak-time) time between 18 and 24 h. A data logger (HOBO U24-002-C) was deployed in each estuary to provide a continuous time-series of water temperature. Although a single logger is not able to capture the entire range of temperatures within the estuaries, the logger was centrally located among our sampling locations, and located at roughly similar distances to the estuary mouth in different estuaries (to allow for comparison among estuaries).
On capture, the carapace length (CL, mm), sex and reproductive stage (immature, mature and berried) were recorded (Johnson et al. 2010). The reproductive stage of crabs was categorised on the basis of the carapace length (<50 mm, generally immature), shape and stiffness of the pleonal flap (angular and stiff when immature or rounded and loose when mature) and the presence of an egg mass (berried; Ryan 1967; Ingles and Bkaum 1989; Fisher 1999). For each monthly sampling event in each estuary, 10 randomly selected mature (non-egg bearing) females were retained for examination of gonadal development. Additionally, from each monthly sampling event in each estuary, up to 10 randomly selected berried (egg bearing) females with Stage 1 eggs (Johnson et al. 2010) were retained for examination of gonadal development and egg mass quantification. Only fully intact females were retained because missing appendages affect GSI, HSI and EMI calculations.
Laboratory procedures
At the laboratory, retained mature crabs were weighed (nearest 0.01 g), measured (CL, to the nearest 0.1 mm) and the ovaries were staged (Liu et al. 2014; n = 259; WLL = 83; PST = 43; LMQ = 50; BGR = 37; LIL = 46). The ovaries and hepatopancreas were then dissected and weighed (wet weight) to the nearest 0.01 g to calculate GSI (Liu et al. 2014) and HSI (Hismayasari et al. 2015). The same dissection procedures as detailed above were repeated for the berried female crabs (n = 113; WLL = 25; PST = 18; LMQ = 38; BGR = 8; LIL = 24), but extra steps were performed to process eggs. The pleonal flap containing the fresh egg mass was removed from each crab and immersed in 400 mL of 1-M potassium hydroxide (KOH) solution for 12 h to dissolve the funiculae that hold the eggs to the setae (Johnson et al. 2010). Following separation, eggs were washed and strained thoroughly using a 60 µm sieve, then the total egg mass was weighed to the nearest 0.01 g. Using the total egg mass weight and total bodyweight, we then calculated the EMI (Sukumaran and Neelakantan 1997; Hisam et al. 2018), as follows:

Statistical analysis
Analysis was conducted using the program R (ver. 3.6.3, R Foundation for Statistical Computing, Vienna, Austria) and the integrated development environment RStudio (RStudio, Inc., Boston, MA, USA, see http://www.rstudio.com). The GSI and HSI are ratios of organ weight to bodyweight. In this study, these proportions correlated with the size of the crab, and therefore the comparison of ratios among estuaries could be confounded by the distribution of body size. To remove the influence of total bodyweight on the relationship between GSI and HSI, linear models were fit to log-organ weight and log-bodyweight, with the slope of the resulting fit used as the exponent in the following formulae (see Fig. S1, S2, available as Supplementary material to this paper).
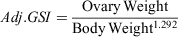

Linear models were used to assess changes in the relationship between the adjusted GSI and adjusted HSI across the stages of ovarian development in mature females. This relationship was analysed by ovary stage, with Stages 2–3 and 4–5 being grouped together owing to morphological similarities and to account for potential errors in distinguishing these visually similar stages.
Linear models were also used to assess the relationship between crab weight and egg mass weight (for all tests, significance was based on a value of α = 0.05). Crab weight and egg mass weight followed a logarithmic relationship, so a natural logarithm transformation was used on bodyweight in the linear model. An analysis of covariance (ANCOVA) was used to test for significant differences in this relationship among estuaries, where the weight of the egg mass was the dependent variable, total bodyweight was the covariate, and estuary was a fixed factor. A post hoc Tukey’s test was used to test for differences across all combinations of estuaries. A two-way ANOVA was used to test for significant differences in the EMI among estuaries and sampling periods and any significant interaction between the two factors, where the EMI was the dependent variable and the estuaries and sampling period were fixed factors. Post hoc Tukey’s tests were used to evaluate differences within fixed effects or interactions.
Generalised additive models (GAMs) were used to examine potential non-linear relationships between mean monthly temperature and the adjusted GSIs, adjusted HSIs and total egg mass weights. GAMs are a non-parametric extensions of generalised linear models (GLMs), and can be used to examine complex non-linear relationships between response variables and predictor variables by fitting complex smoothing functions to the data (Hastie and Tibshirani 1990). For each model, a smoothing function (s) was fitted to mean monthly temperature and the estuaries were included as parametric coefficients, where the intercept is denoted β0, as depicted in the formulae bellow.
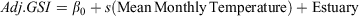
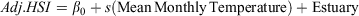

Mean monthly temperatures ranged from 18.57 (Wallis Lake, October 2019) to 26.96°C (Port Stephens, February 2020). Data points were evenly distributed between 20 and 27°C; however, the single data point at 18.57°C was ~1.5°C below the next data point (20.14°C). This outlying data point introduced uncertainty and was therefore excluded, with the GAMs only modelling within the temperature range (~20–27°C).
Results
Patterns in reproductive development
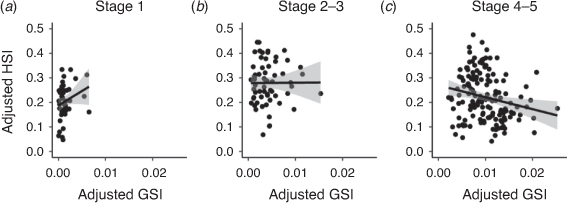
Overall, 1904 adult female (1538 mature; 366 egg-bearing) BSC were caught across five estuaries during the 5-month sampling period. Of these 1904 adult females, we retained 259 mature and 122 berried female crabs for laboratory analysis. Mature females ranged in size from 51 to 93 mm CL, whereas berried females ranged in size from 57 to 89 mm CL. Only female BSC in the later stages of ovarian development (4–5) showed a significant negative linear correlation between the adjusted GSI and adjusted HSI (F1,146 = 7.042, P = 0.009, Table 2, Fig. 2). There was no significant interaction between the GSI and HSI in females with Stage 1 ovaries (F1,45 = 2.767, P = 0.103, Table 2) or Stage 2–3 ovaries (F1,60 = 0.002, P = 0.969, Table 2)

|
|
Egg mass weight increased logarithmically with bodyweight (F1,111 = 61.430, P < 0.001, Table 3). There was variability among estuaries (Fig. 3), with BSC in Lake Macquarie having the smallest coefficient and therefore the smallest increase in egg mass weight relative to bodyweight (Table 3, Tables S1, S2, available as Supplementary material to this paper). Female crabs in Wallis Lake had the largest coefficient and therefore the largest increase in egg mass with bodyweight; however, because of a lower intercept than in Botany Bay, the Wallis Lake crabs tended to have smaller egg masses than those in Botany Bay (Tables 3, S1, S2, Fig. 3). The EMI varied significantly among estuaries (F4,93 = 6.477, P < 0.001) and sampling periods (F4,93 = 6.994, P < 0.001), with a significant interaction between the two factors (F11,93 = 3.575, P < 0.001; Fig. 4). EMI was as large as 55% and was significantly larger in Botany Bay than in Wallis Lake (P = 0.031), Port Stephens (P < 0.001) and Lake Macquarie (P = 0.001; Fig. 4). Lake Illawarra had a smaller mean EMI than did Botany Bay; however, this difference was not statistically significant (P = 0.054).

|
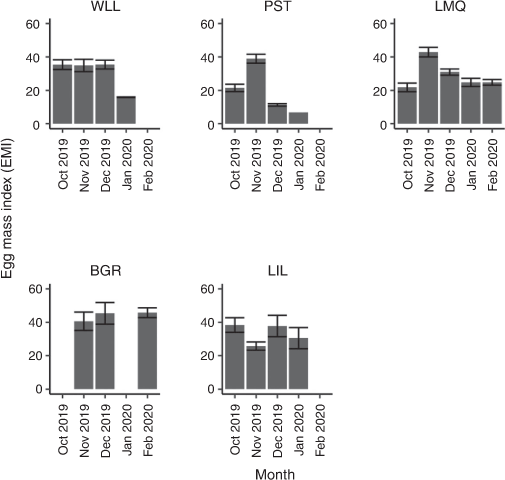
|
Water temperature as a driver of variability in reproduction
The seasonal warming was evident within all five estuaries (Fig. 5), reaching a peak in the January and February sampling periods. The two more southern estuaries (Botany Bay and Lake Illawarra) never exceeded the mean monthly water temperatures of the other three more northern estuaries (Fig. 5). Generalised additive models showed that there was a non-linear relationship between mean monthly temperature and GSI, HSI and EMI (Fig. 6). The GAM for adjusted GSI explained 14.5% of the deviance in the data and showed a rapid decline in adjusted GSI above ~24°C (n = 229, e.d.f = 2.812, F = 7.390, P < 0.001, Fig. 6); the GAM for adjusted HSI explained 13.3% of the deviance in the data and appeared to reach a minimum at ~24°C (n = 228, e.d.f = 3.675, F = 6.26, P < 0.001, Fig. 6). The GAM for EMI explained 66% of the deviance in the data, with a maxima occurring at ~23°C (n = 80, e.d.f = 5.662, F = 9.892, P < 0.001, Fig. 6).

|
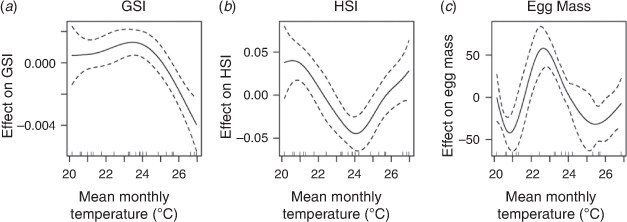
|
Discussion
Our data showed that gonadal development was related to the mobilisation of reserves from the hepatopancreas during the final stages of ovarian development in BSC. We found that the weight of the egg mass increased logarithmically with total bodyweight and was strongly influenced by water temperature. In the cooler waters of Botany Bay, there was a stronger relationship between size and egg mass weight, with larger females producing proportionally larger egg masses. We observed a peak in gonadal development and egg production at ~24°C, with a decline in reproductive development either side of this optimum temperature.
Relationship between the GSI and HSI
The hepatopancreas is the largest store of energy reserves in crustaceans and is crucial in the absorption, storage and metabolism of nutrients for physiological processes (Wang et al. 2014). The significant negative relationship between the adjusted GSI and HSI in the final stages of ovarian development (Fig. 2) reflects the high energy demands of this process (Wang et al. 2014). Only in the final stages of ovarian development are the reserves of the hepatopancreas significantly drawn on, and therefore ovarian development through Stages 1–3 is presumably directly sustained by foraging. During the final stage of ovarian development, the reproductive output of a female crab is therefore likely to be determined by the quantity of reserves mobilised from the hepatopancreas to the ovaries. Increased stress as a result of fluctuations in temperature may increase energetic demand from other physiological processes, limiting the resources available for ovarian development and egg production (Tropea et al. 2015; Baliña et al. 2018). In crustaceans such as Neocaridina davidi and N. heteropoda, ovarian maturation and spawning were found to be inhibited because of stress at high temperatures (33 and 32°C respectively), because of reduced mobilisation of biochemical reserves to the ovaries (Tropea et al. 2015; Baliña et al. 2018). Temperature is evidently a key driver of variability in gonadal development and energy expenditure in crustaceans (Tropea et al. 2015; Baliña et al. 2018), with suboptimal environmental conditions potentially reducing investment into reproduction, as depicted in Fig. 6 and discussed below.
Relationship between crab size and egg mass weight
Crabs in Botany Bay showed a larger increase in egg mass weight per unit of total bodyweight than did crabs in other estuaries (Fig. 3). Botany Bay is a drowned river valley, with a deep, wide entrance facilitating oceanic exchange with the Tasman Sea (Kingsford and Suthers 1994), which maintains cooler average temperatures than do the other estuaries (Fig. 5). In Western Australia, batch fecundity has been shown to increase with latitude from the subtropical estuaries (Shark Bay) towards the temperate estuaries (Geographe Bay; Johnston and Yeoh 2021). Warmer environments may facilitate the production of smaller and more frequent egg batches (Johnston and Yeoh 2021), a reproductive strategy that may reduce the risk of overall recruitment failure by spawning over a longer timeframe (McEvoy and McEvoy 1992). This may explain why BSC in tropical and subtropical environments can achieve rapid batch production and year-round spawning (up to 3 batches per year; de Lestang et al. 2003), whereas BSC in temperate environments are constrained in the production of fewer batches, and a shorter spawning season in which to produce them (up to 3 batches, but only in larger individuals, during the period October–January; Kumar et al. 2000; Kumar et al. 2003).
The egg mass index was as high as 55% in the berried females and was, on average, 34% in females with 70–80 mm CL (equivalent to ~144–189-mm carapace width), indicating significant reproductive investment. By contrast, Portunus pelagicus in the tropical waters of southern Thailand had a maximum EMI of only 29% (Hisam et al. 2018). Another study of Portunus sanguinolentus and P. pelagicus in the south-western coast of India found that the EMI peaked at an average of 15% in females with 100–110-mm carapace width and at an average of 12% in females between 130- and 140-mm carapace width respectively (Sukumaran and Neelakantan 1997). Portunus pelagicus in south-eastern India showed similarly low EMIs to these, with a maximum average of ~17% in females with 120–129-mm carapace width (Josileen 2013). With a much larger EMI, BSC in temperate south-eastern Australia appear to be investing more resources per brood than do portunid crabs in other more tropical environments (Sukumaran and Neelakantan 1997; Josileen 2013; Hisam et al. 2018). Such a difference in reproductive strategy, likely owing to cooler water, would lead to the production of fewer, but larger, egg masses by larger females in temperate environments (de Lestang et al. 2003; Hines et al. 2010; Johnston and Yeoh 2021).
Water temperature as a driver of variability in reproduction
Temperature variability can significantly affect individual reproduction (de Lestang et al. 2003) and contribute to year-on-year variation in recruitment and fisheries productivity (Johnston et al. 2011). Gonadal development and egg production are energetically expensive for ectotherms, and the GSI may be used as an index of thermal performance (Payne et al. 2016). The total egg mass weight produced by crabs within a single batch peaked during months with a mean temperature between ~23 and 24°C (Fig. 6). Conditions above 24°C approach a critical temperature for BSC, at which their reproductive functions begin to slow and lose efficiency, as indicated by the rapid decline in GSI above 24°C (Fig. 6; Shelford 1931; Frederich and Pörtner 2000; Jost et al. 2012). Concurrently, the adjusted HSI, as an indicator of the energy reserves within the female crab, reached a minimum within the same temperature range (Fig. 6), indicating that under these optimal conditions, the crabs were drawing more resources from the hepatopancreas to maximise reproductive output.
In their study of climate change within south-eastern Australian estuaries, Scanes et al. (2020a) observed an average warming of 2.16°C across a 12-year period (0.2°C year–1). The average summer temperature during this period (2007–2018) was 24.8°C in Wallis Lake, 23.5°C in Port Stephens, 25.2°C in Lake Macquarie and 23.3°C in Lake Illawarra; there were no data for Botany Bay from this study (Scanes et al. 2020b). The estuaries in the present study currently maintain average summer temperatures close to the observed optimal temperature range for BSC reproduction; however, as the warming trend observed in Scanes et al. (2020a) continues, average temperatures in the spawning season may exceed the optimal range and begin to impede reproduction. Incorporating temperature-based reproduction modelling into fisheries assessment may aid in the prediction of variation in fisheries productivity, which can subsequently support adaptation of management arrangements (e.g. de Lestang et al. 2010). An extreme heatwave event in Western Australia during 2011 led to major reductions in recruitment within several invertebrate fisheries, including BSC, and highlighted the susceptibility of coastal invertebrate stocks to extreme environmental events (Caputi et al. 2016; Chandrapavan et al. 2019). This event also highlighted the importance of early detection of changes in the temperature–reproduction–recruitment dynamic, to allow for management to protect vulnerable spawning stock (Caputi et al. 2016; Chandrapavan et al. 2019).
Potential impacts of tropicalisation
Anthropogenic climate change is driving global ocean warming, with accelerated warming in temperate regions with poleward-flowing western boundary currents (Wu et al. 2012; Vergés et al. 2014). The accelerated warming and ‘tropicalisation’ of temperate marine environments is particularly rapid in estuaries on the south-eastern Australian coastline (Scanes et al. 2020a). It is still uncertain how tropicalisation may affect the reproduction of BSC, and subsequently influence fisheries productivity in south-eastern Australia. The study of latitudinal clines in population size structure, EMI, and batch fecundity may represent a useful proxy for the effect of changing temperatures, and aid prediction of potential climate impacts in the future. In a study examining the effects of climate change on C. sapidus in Chesapeake Bay, a temperate estuary in the USA, Hines et al. (2010) predicted that warming waters may promote rapid growth and brood production in smaller females, with increased juvenile mortality and a reduced size at maturity. Although warming in temperate waters was predicted to potentially increase the reproductive output of this population, it was noted that the complex interactive effects of multiple stressors associated with climate change, alongside exploitation, make it difficult to accurately predict and act on future circumstances (Hines et al. 2010). In Western Australia, the reproductive biology in P. armatus was found to exhibit high plasticity in response to spatial and temporal variations in temperature (Johnston and Yeoh 2021). For example, size at maturity decreased with latitude (Johnston and Yeoh 2021), whereas batch fecundity was found to increase with latitude. Considering the findings of our study alongside other recent reports on the effect of temperature on reproduction (e.g. Johnston and Yeoh 2021), tropicalisation of temperate estuaries within south-eastern Australia may lead to a shift towards the production of smaller batches, more frequently throughout a longer spawning season, by females with a larger size at maturity.
Study limitations
It is prudent to consider several additional factors that may affect the relationships reported in our study. The use of baited traps has been shown to introduce a bias towards large, sexually mature crabs, potentially causing the over-representation of mature females in each size class and an underestimation of the size at maturity of female crabs (Smith et al. 2004); sampling methods such as seine-netting and otter trawling may provide more representative size structures for BSC (Smith et al. 2004). It is also important to acknowledge that this study was conducted only over a 5-month period (October 2019 to February 2020), with no replication across successive years. Year-round sampling over multiple successive years would allow us to better understand the influence of temperature on year-to-year recruitment and fisheries productivity, as well as the broader impact of climate change and tropicalisation on BSC reproduction. Finally, our study compared only gonad development (ovary weight), energy reserves (hepatopancreas weight) and egg mass weight (as a proxy for egg production) relative to total body mass, whereas fecundity (the number of offspring produced) is also commonly used as a measure of reproductive output. Future studies examining fecundity (i.e. egg production) across similar scales will further strengthen our understanding of the impacts of temperature, climate change, and tropicalisation on BSC reproduction.
Conclusions
In conclusion, we found the most fecund females in Botany Bay, the estuary with the largest exposure to the ocean and the lowest summer water temperature (Fig. 5). Thermal performance curves showed a peak in individual reproductive output at a mean monthly temperature of ~24°C. Above 24°C, the GSI (an index of gonadal development) began to decline. Temperature is a clear driver of spatial and temporal variation in reproduction in female BSC. Further investigation that examines similar relationships over broader temporal scales will improve our understanding of how environmental variability and coastal warming influence reproduction, recruitment and fisheries productivity in south-eastern Australia. Modelling of environment–reproduction–recruitment relationships will also support fisheries management practices, to ensure that harvest levels remain sustainable alongside the influence of environmental change.
Data availability
Data will be made available on reasonable request.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Declaration of funding
This project was supported by the Fisheries Research and Development Corporation on behalf of the Australian Government through a grant to M. D. Taylor, D. D. Johnson and I. M. Suthers (2017/006), and cofounded by the NSW Recreational Fishing Saltwater Trust.
Acknowledgements
Field work sampling was conducted under permit number P01/0059. The collection and handling of biological samples were conducted under the ethics approval ACE13-08. Special thanks go to DPI technicians B. Leach, M. Burns, L. Bidgood, A. Wiltshire, A. Pidd, and T. New and UNSW staff and students R. Hanamseth, D. Hewitt, H. Schilling, C. Hinchliffe, T. Mesaglio, S. Gorta, M. Holland, S. Liddy, A. Puckeridge, D. Cruz, C. Cao, A. Ingall, R. North, V. Tzoumis and J. Redmond for contributing their time and expertise to various aspects of the project. We also thank the two anonymous reviewers for their valuable editorial comments and suggestions for improving the manuscript.
References
Baliña, S., Temperoni, B., Lopez Greco, L. S., and Tropea, C. (2018). Losing reproduction: effect of high temperature on female biochemical composition and egg quality in a freshwater crustacean with direct development, the red cherry shrimp, Neocaridina davidi (Decapoda, Atyidae). The Biological Bulletin 234, 139–151.| Losing reproduction: effect of high temperature on female biochemical composition and egg quality in a freshwater crustacean with direct development, the red cherry shrimp, Neocaridina davidi (Decapoda, Atyidae).Crossref | GoogleScholarGoogle Scholar | 29949439PubMed |
Caputi, N., de Lestang, S., Hart, A., Kangas, M., Johnston, D., and Penn, J. (2014). Catch predictions in stock assessment and management of invertebrate fisheries using pre-recruit abundance – case studies from Western Australia. Reviews in Fisheries Science & Aquaculture 22, 36–54.
| Catch predictions in stock assessment and management of invertebrate fisheries using pre-recruit abundance – case studies from Western Australia.Crossref | GoogleScholarGoogle Scholar |
Caputi, N., Kangas, M., Denham, A., Feng, M., Pearce, A., Hetzel, Y., and Chandrapavan, A. (2016). Management adaptation of invertebrate fisheries to an extreme marine heat wave event at a global warming hot spot. Ecology and Evolution 6, 3583–3593.
| Management adaptation of invertebrate fisheries to an extreme marine heat wave event at a global warming hot spot.Crossref | GoogleScholarGoogle Scholar | 28725352PubMed |
Chandrapavan, A., Caputi, N., and Kangas, M. I. (2019). The decline and recovery of a crab population from an extreme marine heatwave and a changing climate. Frontiers in Marine Science 6, 510.
| The decline and recovery of a crab population from an extreme marine heatwave and a changing climate.Crossref | GoogleScholarGoogle Scholar |
de Lestang, S., Hall, N. G., and Potter, I. C. (2003). Reproductive biology of the blue swimmer crab (Portunus pelagicus, Decapoda: Portunidae) in five bodies of water on the west coast of Australia. Fishery Bulletin 101, 745–757.
de Lestang, S., Bellchambers, L. M., Caputi, N., Thomson, A. W., Pember, M. B., Johnston, D. J., and Harris, D. C. (2010). Stock–recruitment–environment relationship in a Portunus pelagicus fishery in Western Australia. In ‘Biology and Management of Exploited Crab Populations under Climate Change’. (Eds G. H. Kruse, G. L. Eckert, R. J. Foy, R. N. Lipcius, B. Sainte-Marie, D. L. Stram, and D. Woodby.) pp. 443–460. (Alaska Sea Grant, University of Alaska: Fairbanks, AK, USA.)
Fisher, M. R. (1999). Effect of temperature and salinity on size at maturity of female blue crabs. Transactions of the American Fisheries Society 128, 499–506.
| Effect of temperature and salinity on size at maturity of female blue crabs.Crossref | GoogleScholarGoogle Scholar |
Foo, S. A., and Byrne, M. (2017). Marine gametes in a changing ocean: impacts of climate change stressors on fecundity and the egg. Marine Environmental Research 128, 12–24.
| Marine gametes in a changing ocean: impacts of climate change stressors on fecundity and the egg.Crossref | GoogleScholarGoogle Scholar | 28237403PubMed |
Food and Agriculture Organization of the United Nations (1992). ‘FAO Yearbook. Fishery Statistics: Catches and Landings, 1990.’ (FAO: Rome, Italy.)
Food and Agriculture Organization of the United Nations (2018). The State of World Fisheries and Aquaculture 2018: Meeting the Sustainable Development Goals.’ (FAO: Rome, Italy.)
Food and Agriculture Organization of the United Nations (2019). ‘FAO Yearbook. Fishery and Aquaculture Statistics 2017.’ (FAO: Rome, Italy.)
Frederich, M., and Pörtner, H. O. (2000). Oxygen limitation of thermal tolerance defined by cardiac and ventilatory performance in spider crab, Maja squinado. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology 279, R1531–R1538.
| Oxygen limitation of thermal tolerance defined by cardiac and ventilatory performance in spider crab, Maja squinado.Crossref | GoogleScholarGoogle Scholar | 11049833PubMed |
Hastie, T. J., and Tibshirani, R. J. (1990). ‘Generalized Additive Models.’ (Routledge.)
Hines, A. H., Johnson, E. G., Darnell, M. Z., Rittschof, D., Miller, T. J., Bauer, L. J., Rodgers, P., and Aguilar, R. (2010). Predicting effects of climate change on blue crabs in Chesapeake Bay. In ‘Biology and Management of Exploited Crab Populations Under Climate Change’. (Eds G. H. Kruse, G. L. Eckert, R. J. Foy, R. N. Lipcius, B. Sainte-Marie, D. L. Stram, and D. Woodby.) pp. 109–127. (Alaska Sea Grant, University of Alaska Fairbanks: Fairbanks, AK. USA.)
Hisam, F., Hajisamae, S., Ikhwanuddin, M., Aziz, N. A., Naimullah, M., and Hassan, M. (2018). Study on the reproductive biology of the blue swimming crab, Portunus pelagicus females from Pattani coastal waters, Thailand. Aquaculture, Aquarium, Conservation & Legislation 11, 1776–1791.
Hismayasari, I. B., Marhendra, A. P. W., Rahayu, S., Saidin, S. D., and Supriyadi, D. (2015). Gonadosomatic index (GSI), hepatosomatic index (HSI) and proportion of oocytes stadia as an indicator of rainbowfish Melanotaenia boesemani spawning season. International Journal of Fisheries and Aquatic Studies 2, 359–362.
Huang, P., Woodward, R. T., Wilberg, M. J., and Tomberlin, D. (2015). Management evaluation for the Chesapeake Bay blue crab fishery: an integrated bioeconomic approach. North American Journal of Fisheries Management 35, 216–228.
| Management evaluation for the Chesapeake Bay blue crab fishery: an integrated bioeconomic approach.Crossref | GoogleScholarGoogle Scholar |
Ingles, J. A., and Bkaum, E. (1989). Reproduction and larval ecology of the blue swimming crab Portunus pelagicus in Ragay Gulf, Philippines. Internationale Revue der Gesamten Hydrobiologie und Hydrographie 74, 471–490.
| Reproduction and larval ecology of the blue swimming crab Portunus pelagicus in Ragay Gulf, Philippines.Crossref | GoogleScholarGoogle Scholar |
Johnson, D. D., Gray, C. A., and Macbeth, W. G. (2010). Reproductive biology of Portunus pelagicus in a south-east Australian estuary. Journal of Crustacean Biology 30, 200–205.
| Reproductive biology of Portunus pelagicus in a south-east Australian estuary.Crossref | GoogleScholarGoogle Scholar |
Johnston, D. J., and Yeoh, D. E. (2021). Temperature drives spatial and temporal variation in the reproductive biology of the blue swimmer crab Portunus armatus A. Milne-Edwards, 1861 (Decapoda: Brachyura: Portunidae). Journal of Crustacean Biology 41, ruab032.
| Temperature drives spatial and temporal variation in the reproductive biology of the blue swimmer crab Portunus armatus A. Milne-Edwards, 1861 (Decapoda: Brachyura: Portunidae).Crossref | GoogleScholarGoogle Scholar |
Johnston, D., Harris, D., Caputi, N., and Thomson, A. (2011). Decline of a blue swimmer crab (Portunus pelagicus) fishery in Western Australia – history, contributing factors and future management strategy. Fisheries Research 109, 119–130.
| Decline of a blue swimmer crab (Portunus pelagicus) fishery in Western Australia – history, contributing factors and future management strategy.Crossref | GoogleScholarGoogle Scholar |
Johnston, D., Chandrapavan, A., Garland, A., Beckmann, C., and Johnson, D. (2018). Blue swimmer crab Portunus armatus. In ‘Status of Australian Fish Stocks Reports’. (Fisheries Research and Development Corporation.)
Johnston, D., Chandrapavan, A., Walton, L., Beckmann, C., Johnson, D., and Garland, A. (2021a). ‘Status of Australian fish stocks report blue swimmer crab (2020).’ (Fisheries Research and Development Corporation.)
Johnston, D. J., Yeoh, D. E., and Harris, D. C. (2021b). Environmental drivers of commercial blue swimmer crab (Portunus armatus) catch rates in Western Australian fisheries. Fisheries Research 235, 105827.
| Environmental drivers of commercial blue swimmer crab (Portunus armatus) catch rates in Western Australian fisheries.Crossref | GoogleScholarGoogle Scholar |
Josileen, J. (2013). Fecundity of the blue swimmer crab, Portunus pelagicus (Linnaeus, 1758) (Decapoda, Brachyura, Portunidae) along the coast of Mandapam, Tamil Nadu, India. Crustaceana 86, 48–55.
| Fecundity of the blue swimmer crab, Portunus pelagicus (Linnaeus, 1758) (Decapoda, Brachyura, Portunidae) along the coast of Mandapam, Tamil Nadu, India.Crossref | GoogleScholarGoogle Scholar |
Jost, J. A., Podolski, S. M., and Frederich, M. (2012). Enhancing thermal tolerance by eliminating the pejus range: a comparative study with three decapod crustaceans. Marine Ecology Progress Series 444, 263–274.
| Enhancing thermal tolerance by eliminating the pejus range: a comparative study with three decapod crustaceans.Crossref | GoogleScholarGoogle Scholar |
Kingsford, M., and Suthers, I. (1994). Dynamic estuarine plumes and fronts: importance to small fish and plankton in coastal waters of NSW, Australia. Continental Shelf Research 14, 655–672.
| Dynamic estuarine plumes and fronts: importance to small fish and plankton in coastal waters of NSW, Australia.Crossref | GoogleScholarGoogle Scholar |
Kumar, M., Ferguson, G., Xiao, Y., Hooper, G., and Venema, S. (2000). Studies on reproductive biology and distribution of blue swimmer crab (Portunus pelagicus) in South Australian Waters. Research Report Series 47, South Australian Research and Development Institute, Adelaide, SA, Australia.
Kumar, M. S., Xiao, Y., Venema, S., and Hooper, G. (2003). Reproductive cycle of the blue swimmer crab, Portunus pelagicus, off southern Australia. Journal of the Marine Biological Association of the United Kingdom 83, 983–994.
| Reproductive cycle of the blue swimmer crab, Portunus pelagicus, off southern Australia.Crossref | GoogleScholarGoogle Scholar |
Lai, J. C., Ng, P. K., and Davie, P. J. (2010). A revision of the Portunus pelagicus (Linnaeus, 1758) species complex (Crustacea: Brachyura: Portunidae), with the recognition of four species. The Raffles Bulletin of Zoology 58, 199–273.
Lipcius, R. N., and Stockhausen, W. T. (2002). Concurrent decline of the spawning stock, recruitment, larval abundance, and size of the blue crab Callinectes sapidus in Chesapeake Bay. Marine Ecology Progress Series 226, 45–61.
| Concurrent decline of the spawning stock, recruitment, larval abundance, and size of the blue crab Callinectes sapidus in Chesapeake Bay.Crossref | GoogleScholarGoogle Scholar |
Liu, Z., Wu, X., Wang, W., Yan, B., and Cheng, Y. (2014). Size distribution and monthly variation of ovarian development for the female blue swimmer crab, Portunus pelagicus in Beibu Gulf, off south China. Scientia Marina 78, 257–268.
| Size distribution and monthly variation of ovarian development for the female blue swimmer crab, Portunus pelagicus in Beibu Gulf, off south China.Crossref | GoogleScholarGoogle Scholar |
Madeira, D., Narciso, L., Cabral, H. N., and Vinagre, C. (2012). Thermal tolerance and potential impacts of climate change on coastal and estuarine organisms. Journal of Sea Research 70, 32–41.
| Thermal tolerance and potential impacts of climate change on coastal and estuarine organisms.Crossref | GoogleScholarGoogle Scholar |
Magalhães, T., Mossolin, E. C., and Mantelatto, F. L. (2012). Gonadosomatic and hepatosomatic indexes of the freshwater shrimp Macrobrachium olfersii (Decapoda, Palaemonidae) from São Sebastião Island, southeastern Brazil. Pan-American Journal of Aquatic Sciences 7, 1–9.
McEvoy, L., and McEvoy, J. (1992). Multiple spawning in several commercial fish species and its consequences for fisheries management, cultivation and experimentation. Journal of Fish Biology 41, 125–136.
| Multiple spawning in several commercial fish species and its consequences for fisheries management, cultivation and experimentation.Crossref | GoogleScholarGoogle Scholar |
Ochwada-Doyle, F. A., McLeod, J., Barrett, G., Clarke, G., and Gray, C. (2014). Assessment of recreational fishing in three recreational fishing havens in New South Wales. RFT Project number DPID3338-1, Fisheries Final Report Series 139. (NSW Department of Primary Industries.) Available at https://www.dpi.nsw.gov.au/__data/assets/pdf_file/0008/545750/FFRS-139_Ochwada-Doyle-et-al-2014.pdf
Parker, L. M., Ross, P. M., O’Connor, W. A., Pörtner, H. O., Scanes, E., and Wright, J. M. (2013). Predicting the response of molluscs to the impact of ocean acidification. Biology 2, 651–692.
| Predicting the response of molluscs to the impact of ocean acidification.Crossref | GoogleScholarGoogle Scholar | 24832802PubMed |
Passano, L. M. (1960). Metabolism and growth. In ‘The Physiology of Crustacea’. (Ed. T. H. Waterman.) pp. 473–536. (Academic Press: New York, NY, USA.)
Payne, N. L., Smith, J. A., van der Meulen, D. E., Taylor, M. D., Watanabe, Y. Y., Takahashi, A., Marzullo, T. A., Gray, C. A., Cadiou, G., and Suthers, I. M. (2016). Temperature dependence of fish performance in the wild: links with species biogeography and physiological thermal tolerance. Functional Ecology 30, 903–912.
| Temperature dependence of fish performance in the wild: links with species biogeography and physiological thermal tolerance.Crossref | GoogleScholarGoogle Scholar |
Ryan, E. P. (1967). Structure and function of the reproductive system of the crab Portunus sanguinolentus (Herbst) (Brachyura: Portunidae) II. The female system. In ‘Proceedings of the Symposium on Crustacea’, 12–15 January 1965, Ernakulam, India. pp. 522–544. (Marine Biological Association of India.)
Scanes, E., Scanes, P. R., and Ross, P. M. (2020a). Climate change rapidly warms and acidifies Australian estuaries. Nature Communications 11, 1803.
| Climate change rapidly warms and acidifies Australian estuaries.Crossref | GoogleScholarGoogle Scholar | 32286277PubMed |
Scanes, E., Scanes, P. R., and Ross, P. M. (2020b). NSW estuary temperature, pH and salinity data. Available at https://datasets.seed.nsw.gov.au/dataset/nsw-estuary-temperature-ph-and-salinity-data
Shelford, V. E. (1931). Some concepts of bioecology. Ecology 12, 455–467.
| Some concepts of bioecology.Crossref | GoogleScholarGoogle Scholar |
Shields, J. D., and Wood, F. E. (1993). Impact of parasites on the reproduction and fecundity of the blue sand crab Portunus pelagicus from Moreton Bay, Australia. Marine Ecology Progress Series 92, 159–170.
| Impact of parasites on the reproduction and fecundity of the blue sand crab Portunus pelagicus from Moreton Bay, Australia.Crossref | GoogleScholarGoogle Scholar |
Smith, K. D., Hall, N. G., de Lestang, S., and Potter, I. C. (2004). Potential bias in estimates of the size of maturity of crabs derived from trap samples. ICES Journal of Marine Science 61, 906–912.
| Potential bias in estimates of the size of maturity of crabs derived from trap samples.Crossref | GoogleScholarGoogle Scholar |
Stephenson, W., and Campbell, B. (1959). The Australian portunids (Crustacea: Portunidae). III. The genus Portunus. Marine and Freshwater Research 10, 84–123.
| The Australian portunids (Crustacea: Portunidae). III. The genus Portunus.Crossref | GoogleScholarGoogle Scholar |
Sukumaran, K., and Neelakantan, B. (1997). Sex ratio, fecundity and reproductive potential in two marine portunid crabs, Portunus (Portunus) sanguinolentus (Herbst) and Portunus (Portunus) pelagicus (Linnaeus) along the Karnataka coast. Indian Journal of Geo-Marine Sciences 26, 43–48.
Taylor, M. D., Payne, N. L., Becker, A., and Lowry, M. B. (2017). Feels like home: homing of mature large-bodied fish following translocation from a power-station canal. ICES Journal of Marine Science 74, 301–310.
| Feels like home: homing of mature large-bodied fish following translocation from a power-station canal.Crossref | GoogleScholarGoogle Scholar |
Tropea, C., Stumpf, L., and Greco, L. S. L. (2015). Effect of temperature on biochemical composition, growth and reproduction of the ornamental red cherry shrimp Neocaridina heteropoda heteropoda (Decapoda, Caridea). PLoS One 10, e0119468.
| Effect of temperature on biochemical composition, growth and reproduction of the ornamental red cherry shrimp Neocaridina heteropoda heteropoda (Decapoda, Caridea).Crossref | GoogleScholarGoogle Scholar | 25768918PubMed |
Tweedley, J. R., Campbell, T. I., Loneragan, N. R., and Johnstone, D. (2017). Aspects of the biology and husbandry of portunid crabs relevant to aquaculture-based enhancement and fisheries management. Centre for Fish and Fisheries Research, Murdoch University, Perth, WA, Australia.
Vergés, A., Steinberg, P. D., Hay, M. E., Poore, A. G., Campbell, A. H., Ballesteros, E., Heck, K. L., Booth, D. J., Coleman, M. A., and Feary, D. A. (2014). The tropicalization of temperate marine ecosystems: climate-mediated changes in herbivory and community phase shifts. Proceedings of the Royal Society of London – B. Biological Sciences 281, 20140846.
| The tropicalization of temperate marine ecosystems: climate-mediated changes in herbivory and community phase shifts.Crossref | GoogleScholarGoogle Scholar |
Wang, W., Wu, X., Liu, Z., Zheng, H., and Cheng, Y. (2014). Insights into hepatopancreatic functions for nutrition metabolism and ovarian development in the crab Portunus trituberculatus: gene discovery in the comparative transcriptome of different hepatopancreas stages. PLoS One 9, e84921.
| Insights into hepatopancreatic functions for nutrition metabolism and ovarian development in the crab Portunus trituberculatus: gene discovery in the comparative transcriptome of different hepatopancreas stages.Crossref | GoogleScholarGoogle Scholar | 24454766PubMed |
West, L. D., Stark, K. E., Murphy, J. J., Lyle, J. M., and Ochwada-Doyle, F. A. (2015). Survey of recreational fishing in New South Wales and the ACT, 2013/14. NSW Department of Primary Industries, Wollongong, NSW, Australia.
Wu, L., Cai, W., Zhang, L., Nakamura, H., Timmermann, A., Joyce, T., McPhaden, M. J., Alexander, M., Qiu, B., and Visbeck, M. (2012). Enhanced warming over the global subtropical western boundary currents. Nature Climate Change 2, 161–166.
| Enhanced warming over the global subtropical western boundary currents.Crossref | GoogleScholarGoogle Scholar |
Zohar, Y., Hines, A. H., Zmora, O., Johnson, E. G., Lipcius, R. N., Seitz, R. D., Eggleston, D. B., Place, A. R., Schott, E. J., and Stubblefield, J. D. (2008). The Chesapeake Bay blue crab (Callinectes sapidus): a multidisciplinary approach to responsible stock replenishment. Reviews in Fisheries Science 16, 24–34.
| The Chesapeake Bay blue crab (Callinectes sapidus): a multidisciplinary approach to responsible stock replenishment.Crossref | GoogleScholarGoogle Scholar |



