Conservation value of a subtropical reef in south-eastern Queensland, Australia, highlighted by citizen-science efforts
Monique G. G. Grol A B C M , Julie Vercelloni A D , Tania M. Kenyon A D E , Elisa Bayraktarov A F , Cedric P. van den Berg A G H , Daniel Harris I J , Jennifer A. Loder A C K , Morana Mihaljević A J L , Phebe I. Rowland A and Chris M. Roelfsema A I J
A B C M , Julie Vercelloni A D , Tania M. Kenyon A D E , Elisa Bayraktarov A F , Cedric P. van den Berg A G H , Daniel Harris I J , Jennifer A. Loder A C K , Morana Mihaljević A J L , Phebe I. Rowland A and Chris M. Roelfsema A I J
A UniDive, The University of Queensland Underwater Club, 159 Sir William MacGregor Drive, Saint Lucia, Qld 4072, Australia.
B CoralWatch, Queensland Brain Institute, The University of Queensland, QBI Building 79, Research Road, Saint Lucia, Qld 4072, Australia.
C Reef Citizen Science Alliance, Conservation Volunteers Australia, Ballarat, PO Box 423, Vic 3353, Australia.
D Australian Research Council Centre of Excellence for Coral Reef Studies, James Cook University, Sir George Fisher Research Building, Townsville, Qld 4811, Australia.
E Marine Spatial Ecology Lab, School of Biological Sciences, The University of Queensland, Goddard Building 8, University Dr, Saint Lucia, Qld 4072, Australia.
F Centre for Biodiversity and Conservation Science, The University of Queensland, Goddard Building 8, University Dr, Saint Lucia, Qld 4072, Australia.
G Visual Ecology Lab, School of Biological Sciences, The University of Queensland, Goddard Building 8, University Dr, Saint Lucia, Qld 4072, Australia.
H Sensory Neurophysiology Lab, Queensland Brain Institute, The University of Queensland, QBI Building 79, Research Road, Saint Lucia, Qld 4072, Australia.
I Remote Sensing Research Centre, School of Earth and Environmental Sciences, The University of Queensland, Chamberlain Building 35, Campbell Rd, Saint Lucia, Qld 4072, Australia.
J School of Earth and Environmental Sciences, The University of Queensland, Chamberlain Building 35, Campbell Rd, Saint Lucia, Qld 4072, Australia.
K Reef Check Australia, Brisbane, 1/377 Montague Road, West End, Qld 4101, Australia.
L Science Lab UZH, University of Zurich, Winterthurerstrasse 190, CH-8057 Zurich, Switzerland.
M Corresponding author. Email: mgggrol@hotmail.com
Marine and Freshwater Research 72(1) 1-13 https://doi.org/10.1071/MF19170
Submitted: 13 May 2019 Accepted: 9 April 2020 Published: 22 May 2020
Journal Compilation © CSIRO 2021 Open Access CC BY-NC-ND
Abstract
Subtropical reefs are important habitats for many marine species and for tourism and recreation. Yet, subtropical reefs are understudied, and detailed habitat maps are seldom available. Citizen science can help fill this gap, while fostering community engagement and education. In this study, 44 trained volunteers conducted an ecological assessment of subtropical Flinders Reef using established Reef Check and CoralWatch protocols. In 2017, 10 sites were monitored to provide comprehensive information on reef communities and to estimate potential local drivers of coral community structure. A detailed habitat map was produced by integrating underwater photos, depth measurements, wave-exposure modelling and satellite imagery. Surveys showed that coral cover ranged from 14% to 67%. Site location and wave exposure explained 47% and 16% respectively, of the variability in coral community composition. Butterflyfishes were the most abundant fish group, with few invertebrates being observed during the surveys. Reef impacts were three times lower than on other nearby subtropical reefs. These findings can be used to provide local information to spatial management and Marine Park planning. To increase the conservation benefits and to maintain the health of Flinders Reef, we recommend expanding the current protection zone from 500- to a 1000-m radius.
Additional keywords: benthic substrate mapping, coral composition, CoralWatch, ecological assessment, Moreton Bay, Reef Check Australia, subtropical reefs, wave exposure.
Introduction
Subtropical reefs occur along the tropical-to-temperate transition zone and support unique assemblages of tropical, subtropical and temperate marine species (Harriott and Banks 2002; Harrison and Booth 2007; Davie et al. 2011; McPhee 2017). Although subtropical reefs may have lower coral diversity than do tropical reefs and do not rapidly form an accreting reef structure (McIlroy et al. 2019), the live coral cover forming subtropical reefs can be comparable to that of tropical reefs in some locations (Harrison et al. 1998; Wallace and Rosen 2006; Dalton and Roff 2013). Subtropical reefs offer important ecological habitat for migratory marine life such as humpback whales and recruiting coral reef fish (Booth et al. 2018; Noad et al. 2019). They also have social, cultural and economic value through activities such as fishing and tourism (Ross et al. 2019; Ruhanen et al. 2019).
Subtropical reefs are commonly promoted as potential refuges for the conservation of tropical reef species moving poleward as a result of climate change (Beger et al. 2011, 2014; Baird et al. 2012; Makino et al. 2014). Like their tropical counterparts, these subtropical reefs are subject to the effects of climate change, such as changes in water temperature and chemistry (Beger et al. 2014; Sommer et al. 2014; Kim et al. 2019), as well as more localised anthropogenic stressors including pollution, eutrophication, overfishing and physical habitat damage (Gibbes et al. 2014; McPhee 2017). In some instances, these issues may have even more profound and immediate effects on subtropical reefs because of the innate transitional nature of their environments (Beger et al. 2011). Research studies along the tropical-to-temperate transition in eastern Australia focus mainly on the ecological understanding of subtropical reefs at a regional or subregional spatial scale (Sommer et al. 2018; Kim et al. 2019). Detailed information of changes in community composition at finer spatial scales is often limited, which may hinder the development of management strategies for these unique ecosystems.
South-eastern Queensland subtropical reefs, including reefs in Moreton Bay Marine Park, are recognised as ecological, diving and fishing hotspots (Smith et al. 2008; McPhee 2017). The many subtropical patch reefs in Moreton Bay feature high-latitude coral communities and are dominated by generalist, stress-tolerant species that are well adapted to marginal environmental conditions (Sommer et al. 2014). Like for other subtropical reefs, their habitat structure at local scales is heavily influenced by wave energy and exposure (Dollar 1982; Jokiel et al. 2004; Wallace and Rosen 2006; Dalton and Roff 2013). Pressures from rapid urbanisation and population growth beyond the 2.3 million people (Australian Bureau of Statistics 2017) in south-eastern Queensland have been highlighted for the semi-enclosed embayment area of Moreton Bay (Gibbes et al. 2014; Saunders et al. 2019) and are expected to increase in coming decades (Saunders et al. 2019). The collection of ecological-monitoring and habitat-mapping data in this region is, therefore, important to understand the condition and potential impacts on subtropical reef habitats and associated wildlife (Smith et al. 2008; Done et al. 2017).
One of the most biodiverse and popular reefs in the region is Flinders Reef, which is located just outside the embayment of Moreton Bay. Previous studies on Flinders Reef have mostly focussed on monitoring specific taxonomic groups such as fish (Johnson 2010), corals (Wells 1955; Harrison et al. 1998; Wallace et al. 2009; Dalton and Roff 2013; Sommer et al. 2017), sponges (Hooper and Kennedy 2002; Hooper and Ekins 2004) and molluscs (DeVantier et al. 2010). Reef health-impact surveys were restricted to a small portion of reef area and without consistency among sampling methodologies (Beeden et al. 2014). Thus, despite these efforts, detailed information on benthic community composition at Flinders Reef and explicit habitat maps are limited. For instance, the current map of Flinders Reef is restricted to a simple outline of the exposed sandstone platform and includes ecological information at a reef scale. Citizen-science programs are emerging as non-traditional sources of data contribution that engage the community in collecting, analysing and reporting on ecosystem health (Branchini et al. 2015a; Schläppy et al. 2017; Fritz et al. 2019). Citizen-generated data can complement traditional research and management programs, with a higher frequency of surveys, covering a large spatial extent and accessing remote areas not commonly visited, with lower associated costs (Teleki 2012). Citizen science has been recently included into the international agenda for sustainable development goals of the United Nations (Fritz et al. 2019). Global citizen-science coral-reef programs including Reef Check (http://www.reefcheck.org, verified 17 April 2020) and CoralWatch (https://www.coralwatch.org, verified 17 April 2020) have been active in Moreton Bay since 2007 (Siebeck et al. 2006; Marshall et al. 2012; Loder et al. 2015). The data and information currently generated by Reef Check inform the annual report cards of Healthy Land and Water, which assess the health of subtropical reefs in south-eastern Queensland (https://hlw.org.au/report-card/, verified 17 April 2020).
In the present study, citizen scientists monitored different sites at Flinders Reef, filling in gaps in data collection, providing relevant information for local management planning and producing a detailed reef habitat map. The objectives of this study, hereafter referred to as the Flinders Reef Ecological Assessment (FREA), were to (1) develop a detailed benthic habitat map for Flinders Reef, (2) provide a detailed spatial characterisation of the community composition at a reef site scale, including benthic communities, reef impacts, abundance of fish and invertebrates and coral health status, and (3) estimate potential drivers of the coral community structure across the reef. Findings associated with our ecological assessment support ongoing science, management and conservation efforts, and highlight the efficacy of citizen science.
Materials and methods
Study location and site selection
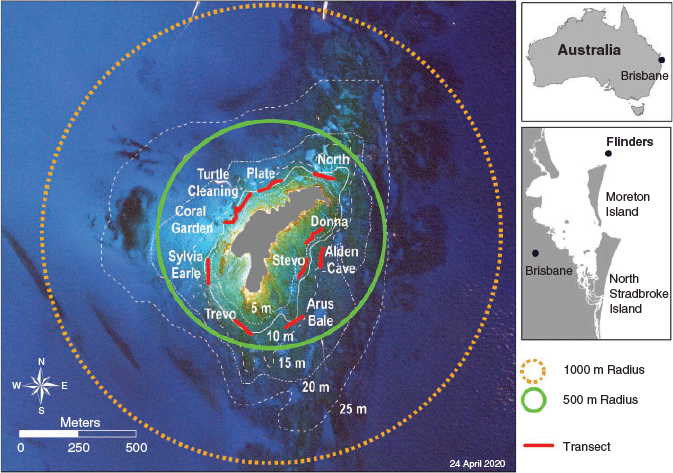
Flinders Reef is located on a small sandstone platform (6.5 ha), three nautical miles north of Moreton Island in the northern part of Moreton Bay Marine Park, south-eastern Queensland, Australia (26°58.715′S, 153°29.150′E; Fig. 1). The location hosts a rich coral community with 125 documented species (Harrison et al. 1998; Harriott and Banks 2002; Wallace et al. 2009; Sommer et al. 2014). It is considered to be one of the most southern distribution ranges of many tropical coral and fish species, including Acropora spp. and Labridae (Dalton and Roff 2013; McPhee 2017; Sommer et al. 2017). Since 2009, the reef has been a designated protected green zone under Marine Park management, which prohibits harvesting, fishing and anchoring within a 500-m radius from the centre of the reef platform (Fig. 1). The conservation park zone has a 2-km radius from the centre of Flinders Reef and a total of eight public moorings are available for activities allowed within this zone. As such, Flinders Reef is afforded some protection from human influences because of zoning status, and its distance from the mainland, which limits both visitation and land-based influences such as poor water quality (McPhee 2017). However, the reef remains subject to potential climate-change influences and pressures from direct use.
|
To set up a representative monitoring and habitat-mapping framework around Flinders Reef, 10 survey sites were established at 5–10-m depth within the green-zone area (Fig. 1). The 10 sites were selected around the sandstone platform to capture representative areas with characteristic differences in exposure to wind speed and wave height, where prevailing wind and wave direction is east–south-east. The following four of the 10 sites are long-term Reef Check Australia monitoring sites: Alden’s Cave, Coral Gardens, Turtle Cleaning Station and Plateland. Surveys were conducted in Austral spring (March) and autumn (September) in 2017, so as to capture potential seasonal changes in marine communities. One site, Arus Bale, was surveyed only in autumn because of adverse weather conditions.
Citizen-science expertise and training
Approximately 100 members of the university dive club, The University of Queensland Underwater Club (UniDive), participated in the development of the FREA citizen-science project and contributed over 10 000 volunteer hours. The participants were mostly students, staff or alumni within the university. UniDive has a long history of award-winning citizen-science projects in south-eastern Queensland (McMahon et al. 2002; Ford et al. 2003; Roelfsema et al. 2016, 2017). Ecological survey protocols were based on globally standardised Reef Check and CoralWatch survey methods. Prior to field surveys, participants completed theoretical and practical training on ecological survey methods (Reef Check Australia and CoralWatch) and mapping survey methods, facilitated by experienced researchers. To take part in field activities, participants were required to hold a rescue-diver certification (or equivalent) and successfully complete Reef Check Australia training by achieving a score of ≥85% on a theory test, ≥95% on an in-water species-identification test and passing a practical in-water survey skills test.
Data collection
A total of 44 divers conducted a cumulative 500 survey dives over 23 day-trips, surveying and mapping Flinders Reef. Ongoing training and quality control were overseen by qualified trainers and researchers throughout the project duration. Recorded data were compared for errors and inconsistencies via reviews of datasheets in the field and during data entry. If discrepancies were identified, recorded data were compared with survey photographs taken by the divers.
Baseline benthic-habitat mapping
A preliminary benthic-habitat map of Flinders Reef was created by applying an established protocol that involved delineating features visible in high spatial-resolution satellite imagery by using visual differences in colour and texture (Roelfsema et al. 2016, 2017). Habitat types were then further defined by overlaying the georeferenced field data onto satellite images for validation. The georeferenced field data included (1) water-depth measurements collected by boat echo sounder or diver, (2) maps of significant geological or ecological features identified through spatially referenced visual census by divers, and (3) georeferenced benthic images collected by a diver towing a surface GPS and photographing 1-m2 benthic quadrats every 1–2 m along the survey area (Roelfsema et al. 2013).
Baseline ecological- and reef-impact surveys (Reef Check Australia)
Ecological- and reef-impact surveys using standardised Reef Check protocols (Hill and Wilkinson 2004; Hill 2005) were undertaken by conducting visual surveys of the benthos, reef health impacts, and selected invertebrate and fish indicator categories (see Supplementary Material table S1, available at journal’s website). Reef Check surveys collect information on biological indicators that have a functional role on the reef. They serve individually as indicators of specific types of human impacts and, collectively, as a proxy for ecosystem health, based on the economic and ecological value, their sensitivity to human impacts and ease of identification (Hill and Loder 2013). The term ‘category’ is used to describe an individual species, family or group (see table S1). At each site, surveys were conducted along a transect that comprised four 20-m-long segments used as replicates (hereafter, referred to as surveyed segments). A 5-m gap was left between each replicate segment to avoid pseudo-replication. Benthic surveys documented living and non-living benthic categories by using a point-intercept sampling method to record the observed benthic category at 0.5-m intervals along each transect segment. The data were used to calculate a mean percentage cover of each category per site. Along the same transect as benthic surveys, divers recorded indicator-invertebrate abundance and signs of reef impacts in a 5-m-wide belt transect (covering four 100-m2 segments). To search the area, the divers swam 2.5 m perpendicular from the centre transect line and then switched back to cross the line and search the area on the other side, continually searching the survey area swimming in an S-shaped pattern. Visual census surveys for indicator fishes were undertaken on the same belt-transect area to record nominated fish categories. Divers conducting the fish surveys swam slowly along the transect line while searching within an imaginary 5-m-wide and 5-m-high tunnel. To ensure standardisation of data-collection effort, the reef health-impact, invertebrate and fish surveyors spent 7–10 min in each segment. Recognising the subtropical nature of Flinders Reef, existing Reef Check Australia protocols were modified by adding the indicator group ‘corallimorphs’ to the benthic surveys. Additional fish species were also incorporated into the fish surveys, including blue groper (Achoerodus viridis), spangled emperor (Lethrinus nebulosus), other emperors (Lethrinidae) and morwongs (Cheilodactylus fuscus and C. vestitus). For further analysis and visualisation purposes, indicator categories were consolidated into larger groups (see table S1).
Coral-health surveys (CoralWatch)
Coral health was surveyed using CoralWatch protocols (Siebeck et al. 2006; Marshall et al. 2012). The CoralWatch coral-health chart was used to compare the colour of living coral colonies to a pre-calibrated 6-point colour scale as a proxy for coral health; i.e. healthier corals are darker in colour. The coral-health surveyor swam along the same 5-m-wide belt transect and, for five randomly selected coral colonies per segment, the growth form, the lightest colour score and darkest colour score were recorded, totalling 20 coral colonies being assessed per site.
Wave exposure
Wave height at Flinders Reef was determined using a third-generation nearshore wave model Simulating WAves Nearshore (SWAN) version 41.31 (Booij et al. 2001). The SWAN model, and models such as XBeach (Roelvink et al. 2009), have been used extensively in coastal and coral-reef environments to propagate offshore deep-water wave heights to shallow water environments (Harris et al. 2018; Baldock et al. 2019). This provides wave-exposure estimates for reef environments that have been used in previous ecological mapping and monitoring (e.g. Chollett and Mumby 2012). Wave inputs for the SWAN model were based on the 1976–2017 wave record from the Brisbane wave-rider buoy, operated by the Queensland Department of Environment and Science. The wave-rider buoy is deployed in deep water, east of North Stradbroke Island and south-east of the field site. The average wave conditions during the 41-year period had a significant wave height (Hs) of 1.67 m, a wave period (T) of 9.43 s, and a south-eastern wave direction (Dir) of 120.7°. Bathymetry for the SWAN model was generated from the Australian bathymetry and topography 2009 dataset (Ausbathy) produced by Geoscience Australia (Whiteway 2009). A nearest-neighbour interpolation method was used to convert the 9-arc second Ausbathy grid to a 50 × 50 m bathymetric grid for Flinders Reef and surrounding region, including the northern and eastern coast of Moreton Island. The default parameters in SWAN were selected for wave modelling. For more information, refer to the SWAN website and user manual (http://swanmodel.sourceforge.net, verified 17 April 2020). Values of significant wave heights for each site were extracted on the basis of the centre coordinates of each transect in a Universal Transverse Mercator (UTM) coordinate system.
Data and statistical analysis
Data manipulation
Differences between autumn and spring surveys were assessed using a Student’s t-test on the basis of the overall mean of measurements for the four survey types, i.e. benthos, reef impacts, invertebrates and fish. The assumptions of normality were met for these data. Because no significant differences were found, measurements were averaged between seasons (see table S2 and Fig. 1). At each site, impact, invertebrate and fish surveys were calculated per 100 m2 and benthic surveys were calculated as percentage cover. Many of the reef-impact categories are coral specific; hence, areas with high coral cover may have a disproportionate number of impacts when compared with areas of low coral cover. To allow for direct comparison among sites of varying coral cover, the abundance of reef impacts was divided by the percentage hard coral cover for that area.
Statistical analyses to estimate variability and drivers of coral community composition
A hierarchical clustering analysis was used to determine the spatial variability in the structure of coral communities among survey sites. Coral community structure was composed of seven hard-coral and four soft-coral categories (see table S1) and coverage was square-root transformed to satisfy analysis assumptions. Clusters were estimated using a Bray–Curtis dissimilarity matrix, by using a complete-linkage cluster-aggregation method. Non-metric multidimensional scaling (nMDS) ordination was then used to visualise the structure of coral communities within sites, on the basis of four segments being surveyed per site.
The influence of site location and wave exposure on coral community composition was estimated using a permutational multivariate analysis of variance (PERMANOVA) based on Bray–Curtis dissimilarity distances with surveyed segments nested within sites, and wave exposure formulated as a fixed effect. Analyses were performed using the R packages ‘clustsig’ (https://cran.r-project.org/web/packages/clustsig/index.html, verified 27 April 2020) and ‘vegan’ (www.cran.r-project.org/web/packages/vegan/index.html, verified on 27 April 2020) within R version 3.2.2 software (www.r-project.org/, verified 27 April 2020). Significance of clusters and size effects in the PERMANOVA were tested using permutation approaches based on 999 permutations and a 5% error level.
Wave exposure expressed as low and high categories was correlated with coral community structure by using the Pearson product-moment correlation coefficient. Values of these coefficients and associated P-values indicated the strength and direction of the correlation at a 5% error level. The two wave-exposure categories were calculated using the median values of wave height across all sites.
Coral health-chart analysis (CoralWatch)
CoralWatch coral-heath scores were recorded for a total of 378 coral colonies. The average colour score at Flinders Reef and per site (±standard error, s.e.) was calculated by pooling the two seasons (autumn and spring).
Results
Baseline benthic-habitat mapping
The georeferenced habitat map created for Flinders Reef depicts substrate types, water depth and significant features (Fig. 2; Roelfsema et al. 2018). Prominent mapped features included vast branching hard-coral beds at Coral Garden and large plate corals with diameters up to ~2 m at a depth of 10–15 m near Plate, and in the deeper water south of Alden Cave and Trevo. Encrusting and plate corals were observed mostly on the south-eastern side, with branching hard corals and soft corals on the western side. Asparagopsis sp. was the dominant macroalga observed at Flinders Reef, while macroalgae in the genus Laurencia were more abundant in deeper waters (>15 m). Rock and rubble surfaces not covered by coral were covered by macroalgae or turf algae. Sandy areas were predominantly found in deeper waters (>15 m).
Ecological baseline
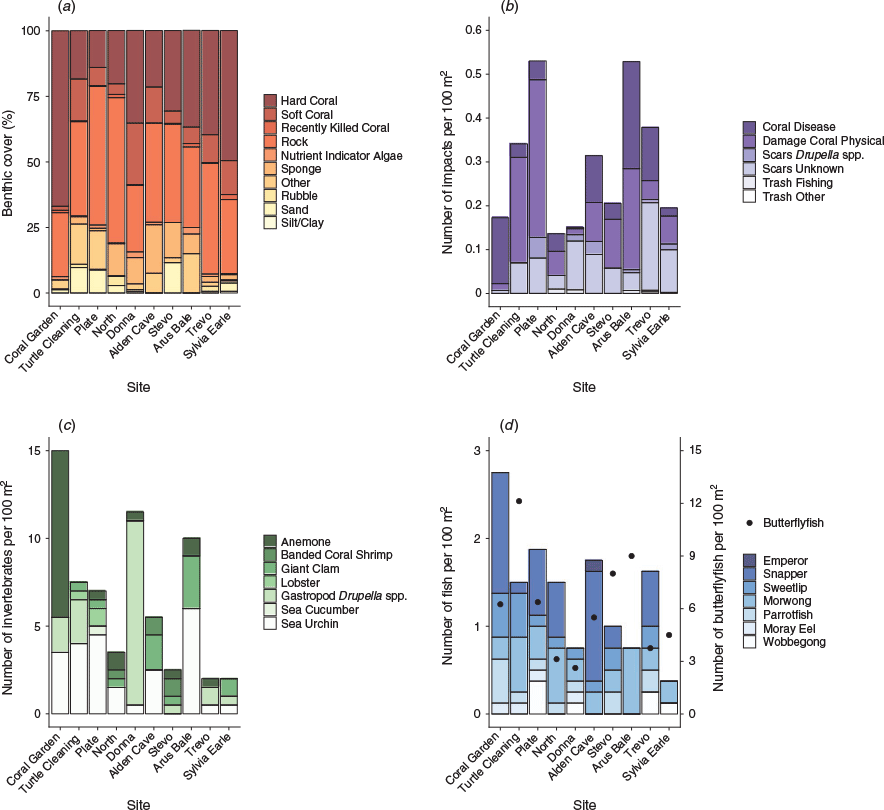
For the benthic surveys, rock was the most common benthic category, with an average cover across sites estimated to be 37.0% (±3.38% s.e.) followed by hard coral (33.3 ± 5.12% s.e.) and soft coral (10.0 ± 2.10% s.e.). The sites with the highest and lowest hard-coral cover were Coral Garden (66.9%) and Plate (14.1%) respectively (Fig. 3a).
Overall, the number of reef impacts detected was low, with an average of 0.05 (±0.01 s.e.) per 100 m2 (Fig. 3b). The most common impacts observed were physical coral damage, with an average of 0.12 (±0.04 s.e.) occurrences per 100 m2, followed by coral disease and unknown coral scars, which both averaged 0.08 (±0.02 and ± 0.01 s.e. respectively) occurrences per 100 m2. Turtle Cleaning and Arus Bale sites had the greatest prevalence of impacts, driven by coral physical damage and, at Arus Bale, also coral disease. The following three reef-impact categories were not observed: crown-of-thorns starfish (Acanthaster planci) scars and coral damage owing to boat anchor or dynamite. The pooled results from the CoralWatch coral health-chart colour-indicator surveys showed an average colour score of 3.9 ± 0.07 s.e. The highest average colour score was recorded at Trevo (4.4 ± 1.80 s.e.) and lowest average score at Arus Bale (2.7 ± 0.22 s.e.).
The average abundance of reported invertebrates was 6.65 (±1.40 s.e.) individuals per 100 m2 (Fig. 3c). The presence and abundance of indicator invertebrate categories varied among survey sites, with the most diverse site being Plate, with 5 of 14 recorded taxa being observed (Fig. 3c). Coral Garden had the highest number of invertebrates per 100 m2 (2.14 ± 1.33 s.e.), being primarily made up of anemones (9.50 per 100 m2). Trevo and Sylvia Earle had the lowest abundance of invertebrates, with an abundance of 0.29 (±1.50 s.e.) invertebrates per 100 m2. The most abundant invertebrate groups were sea urchins (especially Diadema spp.), gastropods (Drupella spp.) and anemones with, on average, 2.35 (±0.65 s.e.), 1.70 (±1.02 s.e.) and 1.35 (±0.91 s.e.) individuals per 100 m2 respectively. The highest abundance of Drupella spp. was found at Donna (10.50 per 100 m2).
Fish community composition was largely dominated by butterflyfishes, which were recorded at each of the 10 sites (Fig. 3d). In total, 524 butterflyfish individuals were counted during all surveys, representing 81.53% of the total counted fishes. On average, 6.12 (±0.93 s.e.) butterflyfishes were recorded per 100 m2, ranging from 2.62 at Donna to 12.10 at Turtle Cleaning. The second-most dominant fish group was snapper, with 40 individuals being recorded (6.65% of total counted fishes) at seven sites and an average of 0.50 (±0.16 s.e.) fish per 100 m2, followed by morwong (0.39 ± 0.06 s.e.), sweetlip (0.20 ± 0.05 s.e.) and parrotfish (0.15 ± 0.05 s.e.) per 100 m2.
Coral community analysis
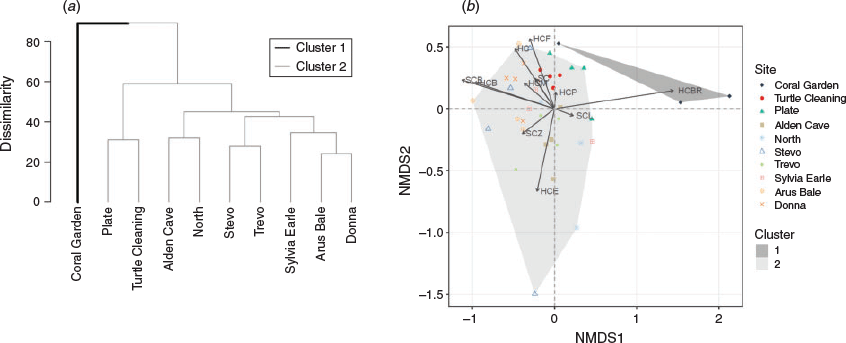
Coral community composition at Coral Garden was distinct (89% dissimilarity, Cluster 1) from the remaining sites (Cluster 2, P = 0.016, Fig. 4a). The north-western sites (Turtle Cleaning and Plate) were different from the others (58% dissimilarity, Cluster 2); however, this clustering pattern was not significant. Cluster 1 was dominated by branching corals (Fig. 4b) representing 64.0% (±13.45% s.e.) of the benthic cover at Coral Garden according to the ecological surveys. Cluster 2 comprised a mix of coral indicator groups. Coral community composition at the north-western sites, Turtle Cleaning and Plate, was characterised by plating and foliose hard corals. In comparison, sites on the eastern side of Flinders Reef, i.e. Alden Cave, North and Trevo, were characterised by encrusting hard coral (Fig. 4b).
Wave exposure and community composition
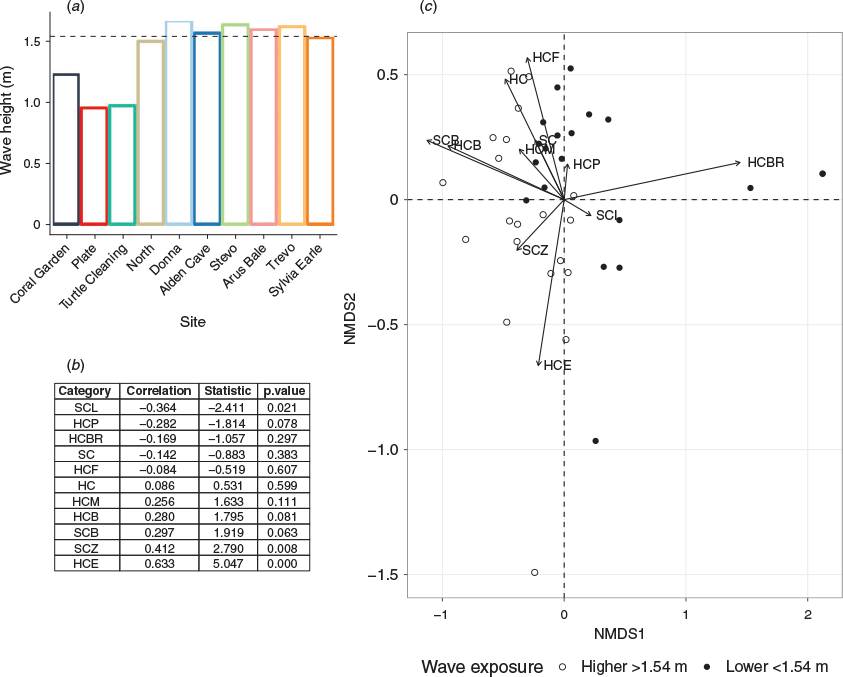
Sites located on the north-western side of Flinders Reef were the least exposed to waves. Significant wave height was 0.9 m for Turtle Cleaning and Plate, and 1.2 m at Coral Garden (Fig. 5a). Wave height for the seven remaining sites varied between 1.5 and 1.6 m (Fig. 5a). The median significant wave height across all sites was 1.54 m, separating the less exposed sites (located west to north of Flinders Reef) from the more exposed sites (east to south). There was a positive relationship between wave exposure and the proportion of encrusting corals (HCE, P < 0.001) and zoanthids (SCZ, P = 0.008), and a negative relationship between wave exposure and leathery soft coral (SCL, P = 0.021) (Fig. 5b). Fragile hard corals (HCF, HCP and HCBR) and soft corals (SC) were associated with a lower wave exposure, whereas more robust hard coral types (HCM, HC and HCE) were associated with a higher wave exposure (Fig. 5c).
|
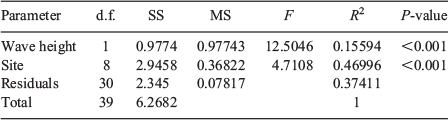
Site and wave exposure both had a significant (P < 0.001) effect on the coral community composition and explained 47% and 15.6% of the variability in hard corals respectively (Table 1).
|
Discussion
The study provided a detailed ecological assessment of the community composition structure, a baseline benthic-habitat map for Flinders Reef, and estimated the role of wave exposure in driving spatial heterogeneity in coral community structure. This information can be used to select long-term monitoring sites and shape management recommendations for future re-zoning plans.
Baseline benthic-habitat mapping
Habitat maps form the basis and inventory of any decision-making process for Marine Park management, and this process will improve with increasing levels of spatial and thematic map detail (Roelfsema et al. 2013). This study presents the first highly detailed habitat map, highlighting the importance of a citizen-science approach providing this information. The habitat-mapping approach can be accessed by marine citizen-science projects to provide valuable maps, using basic mapping training, open source software, off-the-shelf low-cost compact underwater cameras, a hand-held GPS and publicly available satellite imagery. As such, we hope this method will become more widely applied in coral reef surveys by providing detailed methodology protocols (this study; Roelfsema et al. 2017).
Baseline ecological assessment
This study has highlighted a remarkably high hard-coral cover on a subtropical reef, with some sites having comparable coral cover to the Great Barrier Reef (De’ath et al. 2012). Although physical coral damage, unknown coral scars and coral disease were recorded at all sites, overall impacts at Flinders Reef were three times lower than those observed for more accessible reef locations in Moreton Bay such as Point Lookout (Roelfsema et al. 2016). Coral health-chart surveys indicated scores within the healthy range and suggested that corals were unaffected by coral bleaching at the time the surveys were conducted. Previous studies before the establishment of the green zone have reported anchor damage at Flinders Reef (Harrison et al. 1998). The lack of anchor damage in the present study suggests that the installation of moorings and establishment of a green zone (with no anchoring) may be effective in protecting the reef from damage. Yet, higher levels of coral damage were recorded at the most popular dive locations around Flinders Reef, which have the highest cover of branching coral. This could reflect damage by SCUBA divers but also the fragility of branching coral compared with other coral morphologies (Woodley et al. 1981). Further observational studies would be required to understand potential drivers of this damage. The effectiveness of the green zone is also supported by the lack of fishing lines recorded during our impact surveys. However, there are anecdotal reports of fishing within the protected area, and close surveillance of poaching activities can be made difficult by the remoteness of the location.
The distribution and abundance of targeted invertebrates varied spatially, which is consistent with long-term Reef Check Australia findings (Loder et al. 2010; Mulloy et al. 2018). During the surveys, many closely related and functionally equivalent yet non-target invertebrates were observed, including burrowing sea urchin (Echinostrephus aciculatus), blackfish (Holothuria atra), and black teatfish (Holothuria whitmaei). The abundance of corallivorous gastropods (Drupella spp.) was not related with the cover of hard coral nor with the recorded abundance of Drupella scars; however, further data collection is needed to confirm this trend. Corallivorous gastropods formed isolated aggregations in a few surveyed sites (e.g. Donna and Turtle Cleaning), but the overall distribution of gastropods (Drupella spp.) was low; as observed in other coastal waters (Morton and Blackmore 2009). The high abundance of anemones at Coral Garden may be facilitated by the low wave exposure at this site relative to the other sites. A previous survey of subtropical anemones found that abundance was significantly higher on leeward reef sites than on more exposed sites (Richardson et al. 1997).
Butterflyfishes were observed at all survey sites and were most abundant at Turtle Cleaning. High butterflyfish abundance has been observed in other locations in south-eastern Queensland on offshore as well as inshore reefs (Loder et al. 2010; Mulloy et al. 2018). Many butterflyfishes are corallivores that mainly target hard coral, and some species prefer soft coral polyps as a food source (Cole et al. 2008). They have distinct prey preferences that can be specific to one coral species, genus or growth form (Cole et al. 2008), which can limit their abundance and distribution. Fish community composition and abundance is often influenced by cover and structural complexity of live coral (Jennings et al. 1996; Grol et al. 2011), which may explain the high fish abundance observed at Coral Garden. However, aside from butterflyfishes, fish abundances were comparable to the low abundances recorded at other subtropical reefs in south-eastern Queensland (Mulloy et al. 2018). Whereas fish surveys were limited to a confined survey area near the sandstone platform, many of the surveyed fish groups may prefer deeper areas away from currents, surge and exposure. Additionally, parrotfish and grouper abundance may have been underestimated owing to the inclusion of only larger-sized individuals (surveys included only parrotfish >20 cm and grouper >30 cm); smaller parrotfishes were observed during the surveys, but were not included in the data collection (M. Grol, pers. obs.). Smaller juvenile fishes are known to use shallower reef areas as nurseries and have smaller home ranges than do adult fish (Dahlgren et al. 2006; Huijbers et al. 2008).
Wave exposure and coral community composition
There was a clear zonation in coral community composition, notably being influenced by site location and wave exposure. Soft corals and more fragile hard-coral morphologies such as branching corals were associated with the north-western reef sites, characterised by a lower wave exposure. More robust hard-coral morphologies were found at the exposed eastern and south-eastern sites. Branching hard corals are more susceptible to damage from waves and storm events (Woodley et al. 1981), which may explain the dominance of fragile branching coral on the sheltered side of Flinders Reef, at sites such as Coral Garden. The observed zonation patterns and coral cover align with previous studies at Flinders Reef (Harrison et al. 1998 and Dalton and Roff 2013 respectively), as well as broader studies on the influence of wave exposure on high-latitude coral assemblages (Bradbury and Young 1981; Dollar 1982). In addition to wave exposure, coral community composition may be influenced by the intensity and regularity of disturbances, including recurring bleaching events (Spalding and Brown 2015; Hughes et al. 2018; Kim et al. 2019) or storms (Cheal et al. 2017), patterns of coral-recruit settlement (Done 1982) and the depth at which the coral community is located (Roberts et al. 2015). In the present study, depth was considered equal because transects fell within the same depth range.
Recommendations for monitoring
The habitat mapping and ecological-survey results showed that Sylvia Earle has habitat characteristics distinctly different from those of the other sites surveyed at Flinders Reef. Sylvia Earle is located on the western, more sheltered side of Flinders Reef but is still exposed to the pre-dominant east–south-eastern wind and wave direction. This site is less rugose than all other sites, and has a steep slope. It may be beneficial to review long-term monitoring sites with Reef Check Australia to consider the feasibility of expanding representational monitoring locations, such as, for example, to include Sylvia Earle in future monitoring. Furthermore, the indicator species included in the Reef Check Australia protocol were selected for broad geographic coverage with a focus on tropical species (Hodgson 2000). Including additional survey categories for benthos, fish and invertebrates relevant to subtropical regions in future surveys, as well as smaller size classes of parrotfish and groupers, may improve ecosystem-health monitoring of subtropical reefs. The continued inclusion of tropical species in these surveys will be increasingly important so as to detect ‘tropicalisation’ of subtropical marine environments, i.e. the movement of tropical species poleward (Burrows et al. 2011; Baird et al. 2012; Poloczanska et al. 2013; Beger et al. 2014; Sommer et al. 2014).
Recommendations for management
The current Flinders Reef green zone includes a 500-m-radius circle from the centre of the Flinders Reef sandstone platform. The ecological assessment and habitat mapping provide a detailed description of the benthic composition of Flinders Reef and highlight deeper reef habitats that are excluded from the green zone. This may prompt consideration for expansion of the green zone to a circular area of a 1000-m radius. Such an expansion would result in inclusion of all areas mapped with coral communities to a depth of 25 m, a two-fold increase in protected area of benthic categories that include corals, and a three-fold increase in protected area that include rocky substrate (Figs 1, 2, dotted polygon), which is required for coral settlement and post-settlement survival (Yadav et al. 2016). Furthermore, green zones have been shown to enhance recreational fishing opportunities outside of the protected area through exports of increased fish biomass and abundance (Emslie et al. 2015), benefiting both fishermen and the ecosystem.
The role of citizen science and relevance for subtropical reefs
This study has showcased the value of citizen science as an approach that can complement traditional scientific and management approaches and engage local community members to learn about and take active steps to care for local environments (Fritz et al. 2019). In addition to generating data, citizen-science programs improve community knowledge about ecosystem functions and threats and, subsequently, enhance public stewardship of those ecosystems (Marshall et al. 2012; Teleki 2012; Branchini et al. 2015b). The FREA citizen-science project brought together more than 100 local divers and created many opportunities for them to learn about the ecology of subtropical reefs. Moreover, the project enhanced broader community support and understanding of subtropical reefs through a range of communication tools, including a technical report, coffee-table photo book, posters, television segments and community events. The study offered a platform for constructive discussions and applications around the monitoring, management and stewardship of Flinders Reef into the future. The FREA project also characterised the fine-scale structure of coral communities on subtropical reefs, to better understand their dynamics and inform best-practice management.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Acknowledgements
We acknowledge the 44 core survey divers and many additional UniDive volunteers who spent more than 10 000 h on the FREA project. Financial support was given by the Queensland Parks and Wildlife Services, Honourable Dr Steven Miles the former Minister for Environment and the Great Barrier Reef at the time of the research, Solar School, Healthy Land and Water, and those who supported the ING Dreamstarter crowd funder. In-kind support provided by Point Lookout Scuba Dive Charters (Ken Holzheimer); The University of Queensland Boating and Diving; Moreton Island Adventures; Tangatours; Queensland Parks and Wildlife Service; Geoimage; Dr Ian Tibbets, The University of Queensland; Aquatic Centre, The University of Queensland; Reef Check Australia and CoralWatch. At last, we thank our independent reviewers JAK and MGE.
References
Australian Bureau of Statistics (2017). Census 2016. Available at http://www.abs.gov.au/websitedbs/D3310114.nsf/Home/census [verified 22 February 2019].Baird, A., Sommer, B., and Madin, J. (2012). Pole-ward range expansion of Acropora spp. along the east coast of Australia. Journal of the International Society for Reef Studies 31, 1063.
| Pole-ward range expansion of Acropora spp. along the east coast of Australia.Crossref | GoogleScholarGoogle Scholar |
Baldock, T. E., Shabani, B., and Callaghan, D. P. (2019). Open access Bayesian belief networks for estimating the hydrodynamics and shoreline response behind fringing reefs subject to climate changes and reef degradation. Environmental Modelling & Software 119, 327–340.
| Open access Bayesian belief networks for estimating the hydrodynamics and shoreline response behind fringing reefs subject to climate changes and reef degradation.Crossref | GoogleScholarGoogle Scholar |
Beeden, R., Turner, M., Dryden, J., Merida, F., Goudkamp, K., Malone, C., Marshall, P., Birtles, A., and Maynard, J. (2014). Rapid survey protocol that provides dynamic information on reef condition to managers of the Great Barrier Reef. An International Journal Devoted to Progress in the Use of Monitoring Data in Assessing Environmental Risks to Man and the Environment 186, 8527–8540.
| Rapid survey protocol that provides dynamic information on reef condition to managers of the Great Barrier Reef.Crossref | GoogleScholarGoogle Scholar |
Beger, M., Babcock, R., Booth, D. J., Bucher, D., Condie, S. A., Creese, B., Cvitanovic, C., Dalton, S., Harrison, P., Hoey, A., Jordan, A., Loder, J., Malcolm, H., Purcell, S., Roelfsema, C., Sachs, P., Smith, S., Sommer, B., Stuartsmith, R., Thomson, D., Wallace, C., Zann, M., and Pandolfi, J. (2011). Research challenges to improve the management and conservation of subtropical reefs to tackle climate change threats. (Findings of a workshop conducted in Coffs Harbour, Australia on 13 September 2010). Ecological Management & Restoration 12, e7–e10.
| Research challenges to improve the management and conservation of subtropical reefs to tackle climate change threats. (Findings of a workshop conducted in Coffs Harbour, Australia on 13 September 2010).Crossref | GoogleScholarGoogle Scholar |
Beger, M., Sommer, B., Harrison, P. L., Smith, S. D. A., and Pandolfi, J. M. (2014). Conserving potential coral reef refuges at high latitudes. Diversity & Distributions 20, 245–257.
| Conserving potential coral reef refuges at high latitudes.Crossref | GoogleScholarGoogle Scholar |
Booij, N., Holthuijsen, L. H., and Battjes, J. A. (2001). Ocean to near-shore wave modelling with SWAN. In ‘4th International Conference on Coastal Dynamics 2001’. Lund, Sweden, pp. 335–344.
Booth, D. J., Beretta, G. A., Brown, L., and Figueira, W. F. (2018). Predicting success of range-expanding coral reef fish in temperate habitats using temperature–abundance relationships. Frontiers in Marine Science 5, 31.
| Predicting success of range-expanding coral reef fish in temperate habitats using temperature–abundance relationships.Crossref | GoogleScholarGoogle Scholar |
Bradbury, R. H., and Young, P. C. (1981). The effects of a major forcing function, wave energy, on a coral reef ecosystem. Marine Ecology Progress Series 5, 229–241.
| The effects of a major forcing function, wave energy, on a coral reef ecosystem.Crossref | GoogleScholarGoogle Scholar |
Branchini, S., Pensa, F., Neri, P., Tonucci, B., Mattielli, L., Collavo, A., Sillingardi, M., Piccinetti, C., Zaccanti, F., and Goffredo, S. (2015a). Using a citizen science program to monitor coral reef biodiversity through space and time. Biodiversity and Conservation 24, 319–336.
| Using a citizen science program to monitor coral reef biodiversity through space and time.Crossref | GoogleScholarGoogle Scholar |
Branchini, S., Meschini, M., Covi, C., Piccinetti, C., Zaccanti, F., and Goffredo, S. (2015b). Participating in a citizen science monitoring program: implications for environmental education. PLoS One 10, e0131812.
| Participating in a citizen science monitoring program: implications for environmental education.Crossref | GoogleScholarGoogle Scholar | 26200660PubMed |
Burrows, M. T., Schoeman, D. S., Buckley, L. B., Moore, P., Poloczanska, E. S., Brander, K. M., Brown, C., Bruno, J. F., Duarte, C. M., Halpern, B. S., Holding, J., Kappel, C. V., Kiessling, W., O’Connor, M. I., Pandolfi, J. M., Parmesan, C., Schwing, F. B., Sydeman, W. J., and Richardson, A. J. (2011). The pace of shifting climate in marine and terrestrial ecosystems. Science 334, 652–655.
| The pace of shifting climate in marine and terrestrial ecosystems.Crossref | GoogleScholarGoogle Scholar | 22053045PubMed |
Cheal, A. J., MacNeil, M. A., Emslie, M. J., and Sweatman, H. (2017). The threat to coral reefs from more intense cyclones under climate change. Global Change Biology 23, 1511–1524.
| The threat to coral reefs from more intense cyclones under climate change.Crossref | GoogleScholarGoogle Scholar | 28139035PubMed |
Chollett, I., and Mumby, P. (2012). Predicting the distribution of Montastraea reefs using wave exposure. Coral Reefs 31, 493–503.
| Predicting the distribution of Montastraea reefs using wave exposure.Crossref | GoogleScholarGoogle Scholar |
Cole, A. J., Pratchett, M. S., and Jones, G. P. (2008). Diversity and functional importance of coral feeding fishes on tropical coral reefs. Fish and Fisheries 9, 286–307.
| Diversity and functional importance of coral feeding fishes on tropical coral reefs.Crossref | GoogleScholarGoogle Scholar |
Dahlgren, C., Kellison, G., Adams, A., Gillanders, B., Kendall, M., Layman, C., Ley, J., Nagelkerken, I., and Serafy, J. (2006). Marine nurseries and effective juvenile habitats: concepts and applications. Marine Ecology Progress Series 312, 291–295.
| Marine nurseries and effective juvenile habitats: concepts and applications.Crossref | GoogleScholarGoogle Scholar |
Dalton, S. J., and Roff, G. (2013). Spatial and temporal patterns of eastern Australia subtropical coral communities. PLoS One 8, e75873.
| Spatial and temporal patterns of eastern Australia subtropical coral communities.Crossref | GoogleScholarGoogle Scholar | 24058705PubMed |
Davie, P., Cranitch, G., Wright, J., and Cowell, B. (2011). ‘Wild Guide to Moreton Bay and Adjacent Coasts’, Vol. 1, 2nd edn. (The Queensland Museum: Brisbane, Qld, Australia.)
De’ath, G., Fabricius, K. E., Sweatman, H., and Puotinen, M. (2012). The 27-year decline of coral cover on the Great Barrier Reef and its causes. Proceedings of the National Academy of Sciences of the United States of America 109, 17995.
| The 27-year decline of coral cover on the Great Barrier Reef and its causes.Crossref | GoogleScholarGoogle Scholar | 23027961PubMed |
DeVantier, L., Williamson, D., and Willan, R. (2010). Nearshore marine biodiversity of the Sunshine coast, south-east Queensland: inventory of molluscs, corals and fishes, July 2010. Australia. Available at
Dollar, S. (1982). Wave stress and coral community structure in Hawaii. Journal of the International Society for Reef Studies 1, 71–81.
| Wave stress and coral community structure in Hawaii.Crossref | GoogleScholarGoogle Scholar |
Done, T. (1982). Patterns in the distribution of coral communities across the central Great Barrier Reef. Journal of the International Society for Reef Studies 1, 95–107.
| Patterns in the distribution of coral communities across the central Great Barrier Reef.Crossref | GoogleScholarGoogle Scholar |
Done, T., Roelfsema, C., Harvey, A., Schuller, L., Hill, J., Schläppy, M.-L., Lea, A., Bauer-Civiello, A., and Loder, J. (2017). Reliability and utility of citizen science reef monitoring data collected by Reef Check Australia, 2002–2015. Marine Pollution Bulletin 117, 148–155.
| Reliability and utility of citizen science reef monitoring data collected by Reef Check Australia, 2002–2015.Crossref | GoogleScholarGoogle Scholar | 28162251PubMed |
Emslie, M. J., Logan, M., Williamson, D. H., Ayling, A. M., Macneil, A. M., Ceccarelli, D., Cheal, A. J., Evans, R. D., Johns, K. A., Jonker, M. J., Miller, I. R., Osborne, K., Russ, G. R., and Sweatman, H. P. A. (2015). Expectations and outcomes of reserve network performance following re-zoning of the Great Barrier Reef Marine Park. Current Biology 25, 983–992.
| Expectations and outcomes of reserve network performance following re-zoning of the Great Barrier Reef Marine Park.Crossref | GoogleScholarGoogle Scholar | 25819564PubMed |
Ford, S., Langridge, M., Roelfsema, C., Bansemer, C., Pierce, S., Cabrera, K. G., Fellegrada, I., McMahon, K., Keller, M., Joyce, K., Aurish, N., and Prebble, C. (2003). ‘Surveying Habitats Critical to the Survival of Grey Nurse Sharks in South-east Queensland.’ (Unidive: Brisbane, Qld, Australia.)
Fritz, S., See, L., Carlson, T., Haklay, M., Oliver, J. L., Fraisl, D., Mondardini, R., Brocklehurst, M., Shanley, L. A., Schade, S., Wehn, U., Abrate, T., Anstee, J., Arnold, S., Billot, M., Campbell, J., Espey, J., Gold, M., Hager, G., He, S., Hepburn, L., Hsu, A., Long, D., Masó, J., McCallum, I., Muniafu, M., Moorthy, I., Obersteiner, M., Parker, A. J., Weisspflug, M., and West, S. (2019). Citizen science and the United Nations sustainable development goals. Nature Sustainability 2, 922–930.
| Citizen science and the United Nations sustainable development goals.Crossref | GoogleScholarGoogle Scholar |
Gibbes, B., Grinham, A., Neil, D., Olds, A., Maxwell, P., Connolly, R., Weber, T., Udy, N., and Udy, J. (2014). Moreton Bay and its estuaries: a sub-tropical system under pressure from rapid population growth. In ‘Estuaries of Australia in 2050 and Beyond’. (Ed. E. Wolanski.) pp. 203–222. (Springer: Dordrecht, Netherlands.)
Grol, M. G. G., Nagelkerken, I., Bosch, N., and Meesters, E. H. (2011). Preference of early juveniles of a coral reef fish for distinct lagoonal microhabitats is not related to common measures of structural complexity. Marine Ecology Progress Series 432, 221–233.
| Preference of early juveniles of a coral reef fish for distinct lagoonal microhabitats is not related to common measures of structural complexity.Crossref | GoogleScholarGoogle Scholar |
Harriott, V., and Banks, S. (2002). Latitudinal variation in coral communities in eastern Australia: a qualitative biophysical model of factors regulating coral reefs. Journal of the International Society for Reef Studies 21, 83–94.
| Latitudinal variation in coral communities in eastern Australia: a qualitative biophysical model of factors regulating coral reefs.Crossref | GoogleScholarGoogle Scholar |
Harris, D. L., Rovere, A., Casella, E., Power, H., Canavesio, R., Collin, A., Pomeroy, A., Webster, J. M., and Parravicini, V. (2018). Coral reef structural complexity provides important coastal protection from waves under rising sea levels. Science Advances 4, eaao4350.
| Coral reef structural complexity provides important coastal protection from waves under rising sea levels.Crossref | GoogleScholarGoogle Scholar | 29503866PubMed |
Harrison, P. L., and Booth, D. J. (2007). Coral reefs: naturally dynamic and increasingly disturbed ecosystems. In ‘Marine Ecology’. (Eds S. D. Connell and B. M. Gillanders.) pp. 316–377. (Oxford University Press: Melbourne, Vic, Australia.)
Harrison, P. L., Harriott, V. J., Banks, S. A., and Holmes, N. J. (1998). The coral communities of Flinders Reef and Myora Reef in the Moreton Bay Marine Park, Queensland, Australia. In ‘Moreton Bay and Catchment’. (Eds L. R. Tibbetts, N. J. Hall, and W. C. Dennison.) pp. 525–536. (Moreton Bay and Catchment, School of Marine Science, The University of Queensland: Brisbane, Qld, Australia.)
Hill, J. (2005). ‘Reef Check Australia Training Manual.’ (Reef Check Foundation: Townsville, Qld, Australia.)
Hill, J., and Loder, J. (2013). ‘Reef Check Australia Survey Methods.’ (Reef Check Foundation: Townsville, Qld, Australia.)
Hill, J., and Wilkinson, C. (2004). ‘Methods for Ecological Monitoring of Coral Reefs.’ (Australian Institute of Marine Science: Townsville, Qld, Australia.)
Hodgson, G. (2000). Coral reef monitoring and management using Reef Check. Integrated Coastal Zone Management 1, 169–179.
Hooper, J. N. A., and Ekins, M. (2004). Collation and validation of museum collection databases related to the distribution of marine sponges in northern Australia. Technical reports of the Queensland Museum, No. 002, Brisbane, Qld, Australia.
Hooper, J. N. A., and Kennedy, J. A. (2002). Small-scale patterns of sponge biodiversity (Porifera) from the Sunshine Coast reefs, eastern Australia. Invertebrate Systematics 16, 637–653.
| Small-scale patterns of sponge biodiversity (Porifera) from the Sunshine Coast reefs, eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Hughes, T. P., Anderson, A. D., Connolly, S. R., Heron, S. F., Kerry, J. T., Lough, J. M., Baird, A. H., Baum, J. K., Berumen, M. L., Bridge, T. C., Claar, D. C., Eakin, C. M., Gilmour, J. P., Graham, N. A. J., Harrison, H., Hobbs, J.-P. A., Hoey, A. S., Hoogenboom, M., Lowe, R. J., McCulloch, M. T., Pandolfi, J. M., Pratchett, M., Schoepf, V., Torda, G., and Wilson, S. K. (2018). Spatial and temporal patterns of mass bleaching of corals in the Anthropocene. Science 359, 80–83.
| Spatial and temporal patterns of mass bleaching of corals in the Anthropocene.Crossref | GoogleScholarGoogle Scholar | 29302011PubMed |
Huijbers, C. C. M., Grol, M. G. G., and Nagelkerken, I. (2008). Shallow patch reefs as alternative habitats for early juvenile of some mangrove/seagrass-associated fish species in Bermuda. Revista de Biología Tropical 56, 161–169.
Jennings, S., Boullé, D., and Polunin, N. (1996). Habitat correlates of the distribution and biomass of Seychelles’ reef fishes. Environmental Biology of Fishes 46, 15–25.
| Habitat correlates of the distribution and biomass of Seychelles’ reef fishes.Crossref | GoogleScholarGoogle Scholar |
Johnson, J. W. (2010). Fishes of the Moreton Bay Marine Park and adjacent continental shelf waters, Queensland, Australia. Memoirs of the Queensland Museum 54, 299–353.
Jokiel, P. L., Brown, E. K., Friedlander, A., Rodgers, S. K. U., and Smith, W. R. (2004). Hawaii coral reef assessment and monitoring program: spatial patterns and temporal dynamics in reef coral communities. Pacific Science 58, 159–174.
| Hawaii coral reef assessment and monitoring program: spatial patterns and temporal dynamics in reef coral communities.Crossref | GoogleScholarGoogle Scholar |
Kim, S. W., Sampayo, E. M., Sommer, B., Sims, C. A., Gomez-Cabrera, M. D. C., Dalton, S. J., Beger, M., Malcolm, H. A., Ferrari, R., Fraser, N., Figueira, W. F., Smith, S. D. A., Heron, S. F., Baird, A. H., Byrne, M., Eakin, C. M., Edgar, R., Hughes, T. P., Kyriacou, N., Liu, G., Matis, P. A., Skirving, W. J., and Pandolfi, J. M. (2019). Refugia under threat: mass bleaching of coral assemblages in high-latitude eastern Australia. Global Change Biology 25, 3918–3931.
| Refugia under threat: mass bleaching of coral assemblages in high-latitude eastern Australia.Crossref | GoogleScholarGoogle Scholar | 31472029PubMed |
Loder, J., Bauer, A., Byrne, C., Lea, A., and Salmond, J. (2010). Reef Check Australia, south east Queensland, survey season summary report. Reef Check Foundation, Townsville, Qld, Australia.
Loder, J., Done, T., Lea, A., Bauer, A., Salmond, J., Hill, J., Galway, L., Kovacs, E., Roberts, J., Walker, M., Mooney, S., Pribyl, A., and Schläppy, M. L. (2015). ‘Citizens & Reef Science: a Celebration of Reef Check Australia’s Volunteer Reef Monitoring, Education and Conservation Programs 2001–2014.’ (Reef Check Foundation: Townsville, Qld, Australia.)
Makino, A., Yamano, H., Beger, M., Klein, C. J., Yara, Y., and Possingham, H. P. (2014). Spatio-temporal marine conservation planning to support high-latitude coral range expansion under climate change. Diversity & Distributions 20, 859–871.
| Spatio-temporal marine conservation planning to support high-latitude coral range expansion under climate change.Crossref | GoogleScholarGoogle Scholar |
Marshall, N. J., Kleine, D. A., and Dean, A. J. (2012). CoralWatch: education, monitoring, and sustainability through citizen science. Frontiers in Ecology and the Environment 10, 332–334.
| CoralWatch: education, monitoring, and sustainability through citizen science.Crossref | GoogleScholarGoogle Scholar |
McIlroy, S. E., Thompson, P. D., Yuan, F. L., Bonebrake, T. C., and Baker, D. M. (2019). Subtropical thermal variation supports persistence of corals but limits productivity of coral reefs. Proceedings. Biological Sciences 286, 20190882.
| Subtropical thermal variation supports persistence of corals but limits productivity of coral reefs.Crossref | GoogleScholarGoogle Scholar | 31311470PubMed |
McMahon, K., Bansemer, C., Fellegara, I., Keller, M., Kerswell, A., Kwik, J., Longstaff, B., Roelfsema, C. M., Thomas, J., and Stead, J. (2002). ‘A Baseline Assessment of the Flora and Fauna of North Stradbroke Island Dive Sites, Queensland.’ (Unidive: Brisbane, Qld, Australia.)
McPhee, D. P. (2017). ‘Environmental History and Ecology of Moreton Bay.’ (CSIRO Publishing: Melbourne, Vic, Australia.)
Morton, B., and Blackmore, G. (2009). Seasonal variations in the density of and corallivory by Drupella rugosa and Cronia margariticola (Caenogastropoda: Muricidae) from the coastal waters of Hong Kong: ‘plagues’ or ‘aggregations’? Journal of the Marine Biological Association of the United Kingdom 89, 147–159.
| Seasonal variations in the density of and corallivory by Drupella rugosa and Cronia margariticola (Caenogastropoda: Muricidae) from the coastal waters of Hong Kong: ‘plagues’ or ‘aggregations’?Crossref | GoogleScholarGoogle Scholar |
Mulloy, R., Salmond, J., Passenger, J., and Loder, J. (2018). Reef Check Australia 2017–18 south east Queensland season summary report. Reef Check Foundation, Brisbane, Qld, Australia.
Noad, M. J., Kniest, E., and Dunlop, R. A. (2019). Boom to bust? Implications for the continued rapid growth of the eastern Australian humpback whale population despite recovery. Population Ecology 61, 198–209.
| Boom to bust? Implications for the continued rapid growth of the eastern Australian humpback whale population despite recovery.Crossref | GoogleScholarGoogle Scholar |
Poloczanska, E. S., Brown, C. J., Sydeman, W. J., Kiessling, W., Schoeman, D. S., Moore, P. J., Brander, K., Bruno, J. F., Buckley, L. B., Burrows, M. T., Duarte, C. M., Halpern, B. S., Holding, J., Kappel, C. V., O’Connor, M. I., Pandolfi, J. M., Parmesan, C., Schwing, F., Thompson, S. A., and Richardson, A. J. (2013). Global imprint of climate change on marine life. Nature Climate Change 3, 919.
| Global imprint of climate change on marine life.Crossref | GoogleScholarGoogle Scholar |
Richardson, D. L., Harriott, V. J., and Harrison, P. L. (1997). Distribution and abundance of giant sea anemones (Actiniaria) in subtropical eastern Australian waters. Marine and Freshwater Research 48, 59–66.
| Distribution and abundance of giant sea anemones (Actiniaria) in subtropical eastern Australian waters.Crossref | GoogleScholarGoogle Scholar |
Roberts, T., Moloney, J., Sweatman, H., and Bridge, T. (2015). Benthic community composition on submerged reefs in the central Great Barrier Reef. Journal of the International Society for Reef Studies 34, 569–580.
| Benthic community composition on submerged reefs in the central Great Barrier Reef.Crossref | GoogleScholarGoogle Scholar |
Roelfsema, C., Phinn, S., Jupiter, S., Comley, J., and Albert, S. (2013). Mapping coral reefs at reef to reef-system scales, 10s–1000s km2, using object-based image analysis. International Journal of Remote Sensing 34, 6367–6388.
| Mapping coral reefs at reef to reef-system scales, 10s–1000s km2, using object-based image analysis.Crossref | GoogleScholarGoogle Scholar |
Roelfsema, C., Thurstan, R., Beger, M., Dudgeon, C., Loder, J., Kovacs, E., Gallo, M., Flower, J., Cabrera, K. G., Ortiz, J., Lea, A., and Kleine, D. (2016). A citizen science approach: a detailed ecological assessment of subtropical reefs at Point Lookout, Australia. PLoS One 11, e0163407.
| A citizen science approach: a detailed ecological assessment of subtropical reefs at Point Lookout, Australia.Crossref | GoogleScholarGoogle Scholar | 27706182PubMed |
Roelfsema, C., Bayraktarov, E., Van den Berg, C., Breeze, S., Grol, M., Kenyon, T., de Kleermaeker, S., Loder, J., Mihaljević, M., Passenger, J., Rowland, P., Vercelloni, J., and Wingerd, J. (2017). ‘Ecological Sssessment of the Flora and Fauna of Flinders Reef.’ (Unidive: Brisbane, Qld, Australia.)
Roelfsema, C. M., Andersen, R., Arlow, P., Barrenger, T., Bray, P., Grol, M. G. G., Kunze, J., O’Hagen, A., Pheasant, M., Pollard, L., Stenhouse, M., and Stetner, D. (2018). 2017 habitat maps derived from Flinders Reef Ecological Assessment (FREA) surveys, Queensland, Australia in ArcGIS (shapefile) format. Available at https://doi.org/10.1594/PANGAEA.890756 [verified 17 April 2020].
Roelvink, D., Reniers, A., van Dongeren, A., van Thiel de Vries, J., McCall, R., and Lescinski, J. (2009). Modelling storm impacts on beaches, dunes and barrier islands. Coastal Engineering 56, 1133–1152.
| Modelling storm impacts on beaches, dunes and barrier islands.Crossref | GoogleScholarGoogle Scholar |
Ross, H., Jones, N., Witt, K., Pinner, B., Shaw, S., Rissik, D., and Udy, J. (2019). Values towards Moreton Bay and catchments. In ‘Moreton Bay Quandamooka & Catchment: Past, Present, and Future’. (Eds I. R. Tibbetts, P. C. Rothlisberg, D. T. Neil, T. A. Homburg, D. T. Brewer, and A. H. Arthington.) (The Moreton Bay Foundation: Brisbane, Qld, Australia.) Available at https://moretonbayfoundation.org/articles/values-towards-moreton-bay-and-catchments/ (verified 27 April 2020)
Ruhanen, L., Orams, M., and Whitford, M. (2019). Tourism in the Moreton Bay Region. In ‘Moreton Bay Quandamooka & Catchment: Past, Present, and Future’. (Eds I. R. Tibbetts, P. C. Rothlisberg, D. T. Neil, T. A. Homburg, D. T. Brewer, and A. H. Arthington.) (The Moreton Bay Foundation: Brisbane, Qld, Australia.) Available at https://moretonbayfoundation.org/articles/tourism-in-the-moreton-bay-region/ (verified 27 April 2020)
Saunders, M., Runting, R., Charles-Edwards, E., Syktus, J., and Leon, J. (2019). Projected changes to population, climate, sea-level and ecosystems. In ‘Moreton Bay Quandamooka & Catchment: Past, Present, and Future’. (Eds I. R. Tibbetts, P. C. Rothlisberg, D. T. Neil, T. A. Homburg, D. T. Brewer, and A. H. Arthington.) (The Moreton Bay Foundation: Brisbane, Qld, Australia.) Available at https://moretonbayfoundation.org/articles/projected-changes-to-population-climate-sea-level-and-ecosystems/ (verified 27 April 2020)
Schläppy, M.-L., Loder, J., Salmond, J., Lea, A., Dean, A. J., and Roelfsema, C. M. (2017). Making waves: marine citizen science for impact. Frontiers in Marine Science 4, 146.
| Making waves: marine citizen science for impact.Crossref | GoogleScholarGoogle Scholar |
Siebeck, U., Marshall, N., Klüter, A., and Hoegh-Guldberg, O. (2006). Monitoring coral bleaching using a colour reference card. Journal of the International Society for Reef Studies 25, 453–460.
| Monitoring coral bleaching using a colour reference card.Crossref | GoogleScholarGoogle Scholar |
Smith, S. D. A., Rule, M. J., Harrison, M., and Dalton, S. J. (2008). Monitoring the sea change: preliminary assessment of the conservation value of nearshore reefs, and existing impacts, in a high-growth, coastal region of subtropical eastern Australia. Marine Pollution Bulletin 56, 525–534.
| Monitoring the sea change: preliminary assessment of the conservation value of nearshore reefs, and existing impacts, in a high-growth, coastal region of subtropical eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Sommer, B., Harrison, P. L., Beger, M., and Pandolfi, J. M. (2014). Trait mediated environmental filtering drives assembly at biogeographic transition zones. Ecology 95, 1000–1009.
| Trait mediated environmental filtering drives assembly at biogeographic transition zones.Crossref | GoogleScholarGoogle Scholar | 24933818PubMed |
Sommer, B., Sampayo, E. M., Beger, M., Harrison, P. L., Babcock, R. C., and Pandolfi, J. M. (2017). Local and regional controls of phylogenetic structure at the high-latitude range limits of corals. Proceedings of the Royal Society. Biological Sciences Series B 284, 20170915.
| Local and regional controls of phylogenetic structure at the high-latitude range limits of corals.Crossref | GoogleScholarGoogle Scholar |
Sommer, B., Beger, M., Harrison, P. L., Babcock, R. C., and Pandolfi, J. M. (2018). Differential response to abiotic stress controls species distributions at biogeographic transition zones. Ecography 41, 478–490.
| Differential response to abiotic stress controls species distributions at biogeographic transition zones.Crossref | GoogleScholarGoogle Scholar |
Spalding, M. D., and Brown, B. E. (2015). Warm-water coral reefs and climate change. Science 350, 769–771.
| Warm-water coral reefs and climate change.Crossref | GoogleScholarGoogle Scholar | 26564846PubMed |
Teleki, K. A. (2012). Power of the people?. Aquatic Conservation 22, 1–6.
| Power of the people?.Crossref | GoogleScholarGoogle Scholar |
Wallace, C. C., and Rosen, B. R. (2006). Diverse staghorn corals (Acropora) in high-latitude Eocene assemblages: implications for the evolution of modern diversity patterns of reef corals. Proceedings of the Royal Society B. Biological Sciences 273, 975–982.
| Diverse staghorn corals (Acropora) in high-latitude Eocene assemblages: implications for the evolution of modern diversity patterns of reef corals.Crossref | GoogleScholarGoogle Scholar |
Wallace, C. C., Fellegara, I., Muir, P. R., and Harrison, P. L. (2009). The scleractinian corals of Moreton Bay, eastern Australia: high latitude, marginal assemblages with increasing species richness. Memoirs of the Queensland Museum 54, 1–118.
Wells, J. W. (1955). ‘Recent and Subfossil Corals of Moreton Bay, Queensland.’ (University of Queensland Press: Brisbane, Qld, Australia.)
Whiteway, T. G. (2009). Australian bathymetry and topography grid, June 2009. Geoscience Australia record 2009/21, Australia.
Woodley, J. D., Chornesky, E. A., Clifford, P. A., Jackson, J. B. C., Kaufman, L. S., Knowlton, N., Lang, J. C., Pearson, M. P., Porter, J. W., Rooney, M. C., Rylaarsdam, K. W., Tunnicliffe, V. J., Wahle, C. M., Wulff, J. L., Curtis, A. S. G., Dallmeyer, M. D., Jupp, B. P., Koehl, M. A. R., Niegel, J., and Sides, E. M. (1981). Hurricane Allen’s impact on Jamaican coral reefs. Science 214, 749–755.
| Hurricane Allen’s impact on Jamaican coral reefs.Crossref | GoogleScholarGoogle Scholar | 17744383PubMed |
Yadav, S., Rathod, P., Alcoverro, T., and Arthur, R. (2016). ‘Choice’ and destiny: the substrate composition and mechanical stability of settlement structures can mediate coral recruit fate in post-bleached reefs. Journal of the International Society for Reef Studies 35, 211–222.
| ‘Choice’ and destiny: the substrate composition and mechanical stability of settlement structures can mediate coral recruit fate in post-bleached reefs.Crossref | GoogleScholarGoogle Scholar |