Bomb radiocarbon age validation for the long-lived, unexploited Arctic fish species Coregonus clupeaformis
John M. Casselman A , Cynthia M. Jones B and Steven E. Campana C DA Queen’s University, Department of Biology, Kingston, ON, K7L 3N6, Canada.
B Center for Quantitative Fisheries Ecology, Old Dominion University, Norfolk, VA 23508, USA.
C Life and Environmental Science, University of Iceland, IS-101 Reykjavik, Iceland.
D Corresponding author. Email: scampana@hi.is
Marine and Freshwater Research 70(12) 1781-1788 https://doi.org/10.1071/MF18354
Submitted: 13 September 2018 Accepted: 15 January 2019 Published: 9 April 2019
Journal Compilation © CSIRO 2019 Open Access CC BY-NC-ND
Abstract
The growth rates of freshwater fish in the Arctic would be expected to be very low, but some previous studies of lake whitefish (Coregonus clupeaformis) have reported relatively rapid growth and longevity estimates of less than 15 years. We used bomb radiocarbon chronologies to validate an ageing method based on otolith sections for lake whitefish in both an unexploited Arctic lake (MacAlpine Lake; longevity 50 years) and a lightly exploited temperate population (Lake Simcoe; longevity 49 years). Our results confirm previous suggestions that other ageing methods can seriously underestimate lake whitefish age after ~5–8 years. A Chapman–Robson estimate of instantaneous natural mortality rate (M) of 0.12 in the unfished Arctic lake was one-quarter of that measured in other Arctic lake whitefish populations, and one-third of that predicted by Pauly’s (1980) growth–temperature equation. The high estimates of M reported in other whitefish studies and by Pauly’s equation are almost certainly due to their being based on (incorrect) scale or surface otolith ages. Radiocarbon dating confirms that any attempt at predicting sustainable production for long-lived freshwater fishes like lake whitefish will need to be based on accurate ages derived from otolith sections.
Additional keywords: age determination, carbon-14, lake whitefish, mortality rate, otolith.
Introduction
The atmospheric testing of atomic bombs in the 1950s and 1960s resulted in a rapid and well-documented increase in atmospheric radiocarbon, which first appeared c. 1952 and peaked in 1964 (Nydal 1993). The exchange of radiocarbon between the atmosphere and precipitation was also rapid, resulting in riverine and shallow freshwater radiocarbon values that peaked shortly after that of the atmosphere, albeit at lower concentrations (Peng and Broecker 1980; Spiker 1980). In contrast, peak values of bomb radiocarbon in the world’s oceans were delayed until the late 1960s or 1970s as riverine input, precipitation and atmospheric exchange of CO2 gradually increased the concentration in surface marine waters (Druffel and Linick 1978). The time series of first appearance of bomb radiocarbon in marine and temperate environments is so well understood that it has proven very useful as a dated marker in any structures that form annual growth bands, such as trees (Worbes and Junk 1989), and calcified structures, such as corals, whale teeth and fish otoliths (Druffel and Linick 1978; Kalish 1993; Campana 1997; Stewart et al. 2006). However, the first options for validating the age and growth of long-lived, subpolar freshwater fish became available only when the bomb radiocarbon chronology in the freshwater Arctic environment was quantified (Campana et al. 2008).
The growth rates of fish in the Arctic would be expected to be very low, due both to low temperatures and low nutrient levels (Power 1997). Lake trout (Salvelinus namaycush) have long been suspected to be long lived (Johnson 1976; Power 1978), with longevities of up to 63 years having been confirmed in the past decade (Campana et al. 2008). However, the growth rate and longevity of another abundant sub-Arctic and Arctic freshwater fish, namely lake whitefish (Coregonus clupeaformis), has been disputed. An early but comprehensive survey of numerous Arctic lake whitefish populations suggested that the growth rate was rapid but highly variable and not correlated with water temperature, with longevity estimates of less than 15 years (Healey 1975). Ages were based on scale interpretations. Shortly afterwards, Johnson (1976) reported that lake whitefish in the Arctic appear to be long lived, with modal ages of 13–19 years; however, his use of scales and surface otolith readings left open the possibility that the fish were even longer lived and slower growing than he suspected. This possibility was tentatively confirmed by Power (1978), whose use of otoliths broken transversely through the core and then charred (equivalent in view to that of an otolith transverse section) resulted in lake whitefish age estimates >50 years in some fish. Power (1978) also reported that ages from scales and whole otoliths increasingly underestimated ages from broken otoliths after age 15–20 years. Subsequent research on more southerly populations of lake whitefish has confirmed that broken or sectioned otolith ages can systematically exceed those of scales, fin rays and whole otoliths by more than 100% (12 years) after ages of 5–8 years (Mills and Beamish 1980; Raitaniemi and Heikinheimo 1998; Muir et al. 2008; Yule et al. 2008; Herbst and Marsden 2011). Indeed, age and growth interpretations based on traditional surface readings of scales or otoliths are now known to underestimate (sometimes grossly) the age of most long-lived fishes (Casselman 1987; Campana 2001; Cailliet and Andrews 2008). The availability of a bomb radiocarbon reference chronology for Arctic lakes allows the direct confirmation of the age interpretation and longevity of subpolar lake whitefish.
The objective of this study was to use bomb radiocarbon chronologies to validate an ageing method for lake whitefish in an unexploited Arctic (MacAlpine Lake) population, and to contrast these results with a bomb radiocarbon age validation for a more temperate population (Lake Simcoe). We then used this validated ageing method to determine the growth, longevity, age at maturation and mortality rate of the unexploited Arctic lake population, and contrasted it with published reports based on outdated methods that differ markedly from our results.
Materials and methods
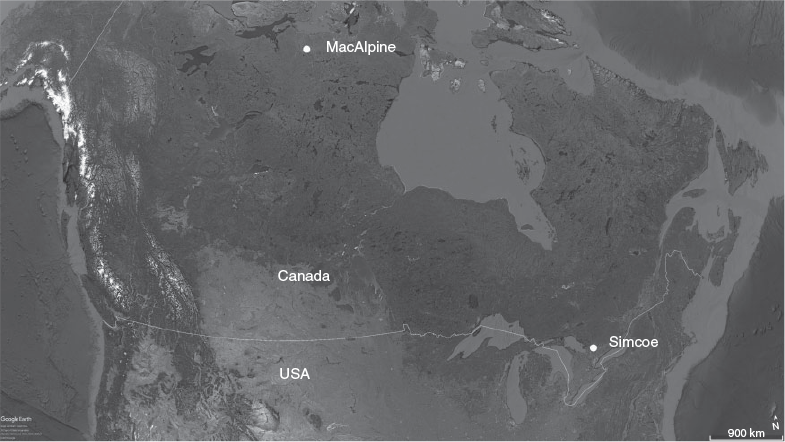
Lake whitefish from the Arctic were sampled from MacAlpine Lake (66°35′N, 102°47′W), a large Arctic lake 120 km south of the Beaufort Sea in the Barren Grounds of Nunavut, Canada (Fig. 1). The lake is located at an elevation of 176 m, has an area of 40 310 ha and an approximate mean and maximum depth of 7 and 15 m respectively. Mean annual air temperature is –11.6°C. The lake is isolated (150 km from the nearest small community) and can be reached only by floatplane. As a result, fishing effort is close to zero and is limited to occasional recreational fishing charters targeting lake trout (Salvelinus namaycush). There are reports of a short-lived gill net fishery for whitefish in the general area of MacAlpine Lake in the early 1970s, but it is unclear whether MacAlpine Lake itself was ever fished.
Arctic whitefish (n = 115) were caught at depths of 3–11 m over five sets in August 2003 using bottom gill nets of graded mesh (2.5- to 16.5-cm mesh at 1.3-cm intervals). The only other fish species caught were lake trout and small numbers of Arctic char (Salvelinus alpinus), cisco (Coregonus artedi), round whitefish (Prosopium cylindraceum) and burbot (Lota lota). All fish were measured (fork length (FL)) and weighed fresh before internal examination for sex, maturity state, gonad condition and weight and stomach contents. Both sagittal otoliths were removed and stored dry for later examination.
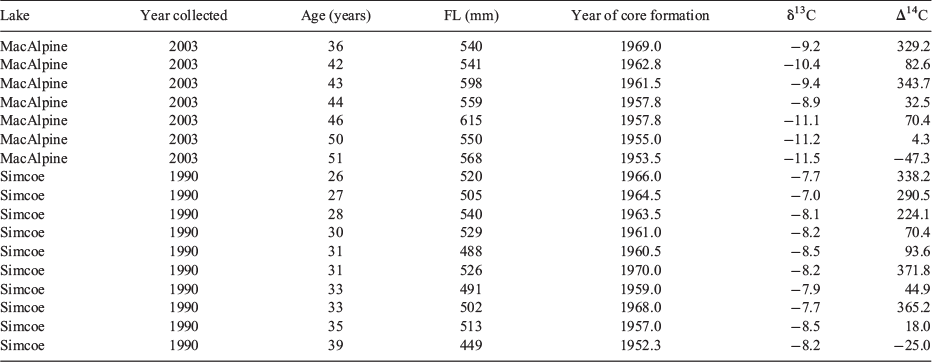
A second set of lake whitefish otoliths was available from a lightly exploited, southern population in Lake Simcoe (44°26′N, 79°20′W), ~70 km north of Lake Ontario and Toronto (Fig. 1). This lake has an area of 72 200 ha, an elevation of 219 m, a mean depth of 15 m, a maximum depth of 41 m and a mean air temperature of 7.0 C, characteristic of a modified continental climate. The lake has not had a commercial fishery, but does have a recreational fishery in both the summer and winter. Whitefish otoliths were collected and stored dry as part of spawning surveys in November 1990, but only those with otolith section ages of 25–39 years were assayed for bomb radiocarbon, so as to have been born between 1952 and 1970 (the most sensitive part of the bomb curve).
All otoliths were processed for age determination using modern, accurate embedding, sectioning and image analysis methods (Campana et al. 2008). Ages were based on counts of presumed annual growth increments that were visible in transverse sections of the sagittal otolith. Otoliths to be aged were first embedded in a slow-drying hard epoxy (Araldite epoxy GY502 and hardener HY956 (Brenntag Canada, Toronto, ON, Canada) in a 5 : 1 weight ratio). Sections (~450-µm thickness) through the core were prepared with a single cut using twin blades separated by a spacer on an Isomet low-speed diamond-bladed saw (Buehler World Wide Headquarters, Lake Bluff, IL, USA). The sections were subsequently mounted on a standard microscope slide with a thin coat of epoxy, then lightly polished to improve visibility. While under a binocular microscope at a magnification of 16–40× and using reflected light, the growth increment sequence was digitally photographed at a resolution of 1280 × 1024 pixels, then digitally enhanced for clarity and contrast using Adobe Photoshop CS2 (see http://www.adobe.com/products/photoshop.html) (Campana et al. 2016). Age interpretation was based on the enhanced images. A second set of digital photographs was prepared under transmitted light using the same microscope. Each reflected and transmitted light image was aged independently by three experienced age readers (J. M. Casselman, C. M. Jones and S. E. Campana). Bias between ageing methods was evaluated with age bias plots, whereas precision was quantified using the CV (Campana 2001).
Otolith cores for bomb radiocarbon age validation were isolated from three adjacent 1.2-mm transverse sections of the otolith, polished lightly in order to view the growth sequence. Otolith cores representing the first 2–4 years of life were isolated from the central section as a solid piece with a Merchantek computer-controlled micromilling machine (ESI, Portland, OR, USA) using 300-µm diameter steel cutting bits and burrs. Additional core material from the same otolith was isolated from the two adjacent sections, but restricted to the innermost two growth increments so as to allow for the offset of these lateral sections from the primordium. This procedure of obtaining material from multiple sections per otolith was necessary to maximise the amount of sample material available for assay from each otolith, bringing the weight of the isolated core material to 3–7 mg. The date of sample formation was calculated as the year of fish collection minus the age span of the fish from the edge of the otolith to the midpoint of the range of growth increments present in the extracted core. After sonification in Super Q water and drying, the sample was weighed to the nearest 0.1 mg in preparation for 14C assay with accelerator mass spectrometry (AMS). AMS assays also provided δ13C (‰) values, which were used to correct for isotopic fractionation effects and provide information on the source of the carbon. Radiocarbon values were subsequently reported as Δ14C, which is the per mille (‰) deviation of the sample from the radiocarbon concentration of 19th century wood, corrected for sample decay before 1950 according to methods outlined by Stuiver and Polach (1977). The mean s.d. of the individual radiocarbon assays was ~5‰.
The reference chronology for the freshwater environment in the Arctic was based on collections of juvenile (age 0–5) Arctic charr (S. alpinus), whose cores were formed between 1952 and 1967 (Campana et al. 2008). The reference chronology was extended by the addition of validated otolith cores from adult lake trout whose cores formed between 1942 and 1995 (Campana et al. 2008).
The feature of a bomb radiocarbon chronology that best serves as a stable dated reference mark is the year of initial increase above prebomb levels in response to the period of atmospheric testing of nuclear weapons. Comparison of this year of initial increase in the reference chronology with that of the species being tested (in this case whitefish) provides a good measure of age estimation accuracy, because consistent over- or underestimation of age will shift the calculated year of initial increase in the test chronology to earlier or more recent years (Francis et al. 2010). The year of initial appearance of bomb Δ14C (YT) was estimated as described by Campana et al. (2008). This calculation assumes that the samples with the earliest annulus-based hatch dates (1952–53) were formed prebomb. If, in fact, these were not prebomb radiocarbon values and the actual prebomb values were lower, the calculated YT would be earlier than reported. Because differences in Δ14C among shallow freshwater species are neither expected nor observed (Campana et al. 2008), the entire period of increasing bomb radiocarbon in the test (whitefish) chronology was also examined for phase shifting relative to the reference chronology.
Length (L50) and age (A50) at sexual maturity were estimated using logistic regression. Growth model parameters for lake whitefish from MacAlpine Lake were estimated using the von Bertalanffy model:
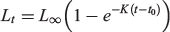
where Lt is the FL (mm) of whitefish at age t (years), L∞ is the asymptotic length, K is a growth coefficient (year–1) and t0 is the age at zero length.
Total instantaneous mortality rate (Z) was estimated from age composition data using the Chapman–Robson estimator, which has been shown to be superior to most other traditional catch-curve analyses for small sample sizes (Smith et al. 2012). In an unfished population like MacAlpine Lake, Z is equal to the instantaneous rate of natural mortality (M). For comparative purposes, M was also predicted from Pauly’s (1980) equation, which used von Bertalanffy growth parameters and temperature from many fish species to derive the equation parameters. When using Pauly’s (1980) equation, we also incorporated the empirical ‘adjustments’ for polar fish populations and ‘effective temperatures’ for waters of less than 5°C that he recommended.
Results
Otolith growth patterns of lake whitefish
Growth increments, presumed to be formed annually, were clearly visible under both reflected and transmitted light in at least some regions of all the otolith sections examined. Reflected light often gave the clearest view of the innermost ~30 increments, whereas transmitted light was most helpful in interpreting increments nearer the otolith margin in older fish. In most otoliths up to an apparent age of ~20 years, several growth axes appeared to contain complete growth sequences, particularly those leading from the core to the proximal surface on either side of the sulcus (Fig. 2). However, in older fishes, the number of increments visible along the growth axis often differed with the transect selected for ageing. In general, the clarity and spacing of the growth increment sequence was optimised in the thickened dorsal lobe (proximal surface) immediately adjacent to the sulcus (Fig. 2). Growth increments were also often interpretable (but were sometimes less distinct) in the ventral lobe (proximal surface) immediately adjacent to the sulcus. When both growth axes were interpretable, age bias plots showed no bias in count between the two axes to an age of 50 years. Growth increments did not appear to form consistently after an age of ~20 years towards the ventral or dorsal tips, despite the fact that these axes were often the longest in the section.

|
The ageing precision (CV) across the three primary (experienced) age readers for MacAlpine Lake whitefish was 6.4%; the CV doubled when ages from two less experienced age readers were included (CV = 13.6%). Ageing precision also differed significantly between lakes, with the CV for the Lake Simcoe otoliths (CV = 3.2%) being half that of Lake MacAlpine otoliths.
Age validation of lake whitefish
The date of formation of the lake whitefish otolith cores was estimated in two ways: (1) through age determination of the fish based on otolith growth increment counts; and (2) through comparison of otolith core Δ14C values with the values known to be present in the Arctic freshwater environment at the time (the Arctic freshwater reference chronology). Where the increment- and Δ14C-based dates are in agreement, the increment-based age interpretations must be (on average) correct.
The correspondence between the Arctic freshwater reference chronology and the chronology derived from the lake whitefish otolith cores was very close (Fig. 3). Both chronologies describe a curve that closely resembles an attenuated version of the atmospheric chronology, with a rapid increase after 1955, reaching an asymptote in the late 1960s. Estimates of YT in MacAlpine Lake (1955) and Lake Simcoe (1957) were very similar to that of the freshwater Arctic reference chronology (1957). In comparing the overall alignment of the whitefish and reference chronologies, the key samples for comparison were the cores formed between 1957 and 1965 (Table 1), because they were formed during the period when environmental 14C levels changed most rapidly. All 10 of these core samples, dated as having formed between 1957 and 1965 based on increment counts of 27–50 years, were closely aligned with the reference chronology, with a mean absolute uncertainty of ~2 years. The alignment was less close for the three cores believed to have been formed before 1955 (prebomb), suggesting either a different prebomb baseline or overageing by a few years. Because elevated prebomb baselines in freshwater fish otoliths have not been reported previously, it may be that these three fish were slightly overaged, even though the other fish do not appear to have been overaged. Whitefish from both lakes fit the reference chronology equally well, indicating no difference in accuracy between sub-Arctic (MacAlpine) and temperate (Simcoe) lakes. Therefore, these results validate the interpretation of the lake whitefish otolith increments as reasonably accurate age indicators, and confirm the longevity of the species to an age of 45–50 years.

|
Growth and maturity
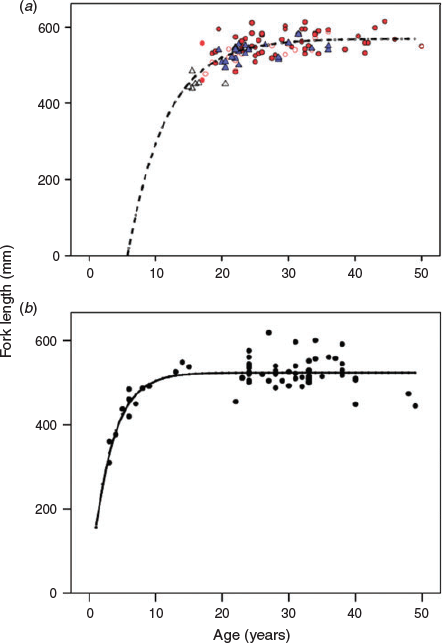
The growth of MacAlpine Lake whitefish was typical of long-lived fishes, with a rapidly growing juvenile stage and asymptotic adult growth after an age of 15–20 years (Fig. 4). The von Bertalanffy growth model resulted in estimates of asymptotic length (mean ± s.e.m., L∞ = 569 ± 7 mm), growth coefficient (K = 0.17 ± 0.04) and t0 (6.0 ± 2.4) that fit all stages well, despite the scarcity of small individuals. There were insufficient numbers of small females to properly define sex-specific growth curves. However, adult females appeared to reach both greater size and age than males (Fig. 4). Maximum observed longevity was ~50 years.
|
Sexual maturity was reasonably well defined for males in our sample of fish, but not in females (Fig. 4). L50 for males was 489 mm, whereas A50 was 18.5 years. Female L50 was (imprecisely) estimated at 495 mm, whereas female A50 could not be estimated at all. The difficulty in estimating female maturity can be attributed both to the scarcity of small and young females in the sample, as well as to the relatively large number of large, old females with small ovaries. Because ovary weight and the presence of mature or resorbed follicles were the main criteria for identifying mature females, it appears that 16% of the females of mature age (>25 years) had skipped spawning for that year.
The growth of Lake Simcoe whitefish was much more rapid before the onset of sexual maturity than that of MacAlpine whitefish, with estimates of asymptotic length (mean ± s.e.m., L∞ = 524 ± 4 mm), growth coefficient (K = 0.33 ± 0.06) and t0 (–0.1 ± 0.7) that fit the data well over the entire age range of 3–49 years. Maximum observed longevity was similar to that of MacAlpine Lake at 49 years, but the asymptotic length indicative of maturity appeared to correspond with a much younger age of ~12 years (Fig. 4).
Mortality
Estimates of Z for the unexploited MacAlpine Lake population are equivalent to estimates of M. The Chapman–Robson estimator of M for MacAlpine Lake whitefish was quite low at 0.12 year–1.
The predicted M from Pauly’s (1980) equation using both the ‘effective temperature’ and polar fish adjustments that he recommended was 0.35. M was predicted to be much lower (0.15) without the correction for effective temperature, and lower again (0.11) with no corrections for either effective temperature or polar fish.
Discussion
The close correspondence of otolith section age and the age indicated by the radiocarbon content of the adult otolith core indicates that our age estimates are realistic, and validates our age interpretation criteria for lake whitefish. These results demonstrate that lake whitefish is a very long-lived species (at least 50 years) in the Arctic. Otolith transverse sections are now routinely used to determine the age of many fishes, but such was not the case as recently as 30 years ago (Beamish and McFarlane 1983). With the realisation that surface readings of otoliths and scales often led to severe age underestimation in long-lived fishes (Beamish 1979), modern ageing methods often strive to prepare transverse sections of otoliths or their functional equivalent, charred otolith halves. This development in ageing methodology explains why some early reports of whitefish growth and longevity grossly underestimate actual age (Healey 1975). Power’s (1978) insightful examination of whitefish otoliths broken through the core resulted in much greater ages, which were very much in keeping with the age interpretations reported here. In light of our results and those of other studies clearly indicating that scale, fin ray, operculum and surface otolith readings can seriously underestimate lake whitefish age after ~age 5–8 (Mills and Beamish 1980; Raitaniemi and Heikinheimo 1998; Muir et al. 2008; Yule et al. 2008; Herbst and Marsden 2011), continued use of structures other than otolith sections (or charred halves) in this species is both unwarranted and unwise.
In principle, age validation should be performed not only at the species level, but for any population whose growth rate or otolith macrostructure differs substantially from the validated population (Campana 2001). In practice, however, such a level of validation is seldom considered warranted. Our study demonstrated that the same preparation method and age interpretation criteria resulted in similar levels of ageing accuracy in both a slow-growing (MacAlpine) and fast-growing (Simcoe) population. In contrast, the use of alternative, inaccurate methods can result in age-dependent accuracy; that is, the ageing inaccuracy first becomes obvious at an age that is approximately proportional to the inverse of growth rate. For example, scale ages begin to seriously underestimate otolith sections at ages of 6–8 years in fast-growing whitefish populations (Muir et al. 2008; Yule et al. 2008; Herbst and Marsden 2011), but at an age of 15 years in slow-growing Arctic populations (Power 1978). This implies that empirical age correction factors between whitefish scales and otoliths are not possible, or at least are not transferable across populations or year-classes with different growth rates. After comparing over a decade of paired striped bass (Morone saxatilis) otoliths and scales, Liao et al. (2013) reported that the ratios and variances were not consistent between years, and that corrections for the scale ageing error could not be made reliably. Thus, it may well be that scale–otolith ageing corrections are not possible in many fish species.
Our results indicate that a virgin (unfished), cold water population of whitefish can grow more slowly, and to a much greater age, than most temperate populations, even if they grow to a comparable asymptotic length. Our L∞ of 569 mm FL is comparable with that reported for both Arctic (Healey 1975; Beauchamp et al. 2004; Mills et al. 2004) and temperate (Muir et al. 2008) populations, and has been reported to be uncorrelated with either maturation age or prereproductive growth rate in whitefish (Beauchamp et al. 2004). Conversely, longevity and age of sexual maturation are clearly greater in slow-growing Arctic populations of whitefish compared with most warmer water or fished populations, with longevity estimates of 27–30 years in temperate populations (Mills et al. 2004; Muir et al. 2008; Herbst and Marsden 2011) and over 50 years in the Arctic (Power 1978; present study). Thus, our observation of a longevity of 49 years in the almost-unexploited Lake Simcoe population was quite surprising. Conversely, the much greater age of sexual maturation of Arctic populations relative to temperate populations has been observed consistently (Beauchamp et al. 2004; Wang et al. 2008).
Accurate estimates of M are seldom available, in part because of the scarcity of virgin populations (for which there is no confounding effect of fishing mortality, only M) and in part because of the prerequisite for accurate ageing data. Empirical prediction estimators of M are often used instead, most often that of Pauly (1980), which uses von Bertalanffy growth parameters and temperature. Our measured estimate of M = 0.12, based on a virgin population and accurate age composition data, is markedly lower than that measured in other Arctic lake whitefish populations for which only scale ages were available (M ~ 0.5; Healey 1975), and much lower than the total mortality of various exploited temperate populations (Z = 0.5–1.2; Muir et al. 2008; Wang et al. 2008). Our estimate of M is also markedly lower than the value of 0.35 predicted by Pauly’s (1980) estimator, using his corrections for polar fish populations in water of less than 5°C. Interestingly, use of Pauly’s (1980) equation without the two corrections produced a predicted M of 0.11, which is almost identical to our estimate. Given that many of the studies used to develop Pauly’s (1980) empirical estimator of M are now known to be based on (incorrect) scale or surface otolith ages, it is fair to question whether his ad hoc ‘corrections’ for effective temperature and polar fish were required simply to correct for inaccurate ageing data from long-lived cold water fish.
Much has been written about the challenges and failures in developing sustainable fisheries for long-lived marine animals (Musick 1999; Dulvy et al. 2014). In fact, the same principles apply to long-lived freshwater fishes, especially long-lived fishes in Arctic lakes that typically have a delayed age of sexual maturation and sporadic recruitment (Chavarie et al. 2016). The likelihood of stock collapse becomes even more acute if the ages are underestimated and growth rate overestimated, resulting in overestimated productivity. Indeed, the fishery collapse of cisco (C. artedi) in Lake Superior was attributed, in part, to scale-based ageing, which grossly overestimated stock productivity (Yule et al. 2008). Although fisheries targeting non-anadromous freshwater fish species in the Arctic are currently few in number, global warming is expected to increase fishing effort in the Arctic (Reist et al. 2006). Any attempt at sustainable fishing for long-lived freshwater fishes like lake whitefish will almost certainly need to be based on accurate ages derived from otolith sections.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Declaration of funding
Funding was provided by the Canadian Department of Fisheries and Oceans and US National Science Foundation Grant OCE-9985884.
Acknowledgements
Warren Joyce, Rob Slapkauskis and Joe Dibbits provided expert technical assistance. Lake whitefish otoliths from Lake Simcoe were provided by the staff of the Lake Simcoe Fisheries Assessment Unit; their assistance is much appreciated. Thanks to the staff of the National Ocean Sciences Accelerator Mass Spectrometry lab at Woods Hole Oceanographic Institution for their expertise in carrying out the radiocarbon assays. Allen Andrews provided constructive comments on the manuscript.
References
Beamish, R. J. (1979). New information on the longevity of Pacific ocean perch (Sebastes alutus). Journal of the Fisheries Research Board of Canada 36, 1395–1400.| New information on the longevity of Pacific ocean perch (Sebastes alutus).Crossref | GoogleScholarGoogle Scholar |
Beamish, R. J., and McFarlane, G. A. (1983). The forgotten requirement for age validation in fisheries biology. Transactions of the American Fisheries Society 112, 735–743.
| The forgotten requirement for age validation in fisheries biology.Crossref | GoogleScholarGoogle Scholar |
Beauchamp, K. C., Collins, N. C., and Henderson, B. A. (2004). Covariation of growth and maturation of lake whitefish (Coregonus clupeaformis). Journal of Great Lakes Research 30, 451–460.
| Covariation of growth and maturation of lake whitefish (Coregonus clupeaformis).Crossref | GoogleScholarGoogle Scholar |
Cailliet, G. M., and Andrews, A. H. (2008). Age-validated longevity of fishes: its importance for sustainable fisheries. In ‘Fisheries for Global Welfare and Environment’. (Eds K. Tsukamoto, T. Kawamura, T. Takeuchi, TD Beard Jr, and MJ Kaiser.) pp. 103-20. (Terrapub: Tokyo, Japan.)
Campana, S. E. (1997). Use of radiocarbon from nuclear fallout as a dated marker in the otoliths of haddock, Melanogrammus aeglefinus. Marine Ecology Progress Series 150, 49–56.
| Use of radiocarbon from nuclear fallout as a dated marker in the otoliths of haddock, Melanogrammus aeglefinus.Crossref | GoogleScholarGoogle Scholar |
Campana, S. E. (2001). Accuracy, precision and quality control in age determination, including a review of the use and abuse of age validation methods. Journal of Fish Biology 59, 197–242.
| Accuracy, precision and quality control in age determination, including a review of the use and abuse of age validation methods.Crossref | GoogleScholarGoogle Scholar |
Campana, S. E., Casselman, J. M., and Jones, C. M. (2008). Bomb radiocarbon chronologies in the Arctic, with implications for the age validation of lake trout (Salvelinus namaycush) and other Arctic species. Canadian Journal of Fisheries and Aquatic Sciences 65, 733–743.
| Bomb radiocarbon chronologies in the Arctic, with implications for the age validation of lake trout (Salvelinus namaycush) and other Arctic species.Crossref | GoogleScholarGoogle Scholar |
Campana, S. E., Valentin, A. E., MacLellan, S. E., and Groot, J. B. (2016). Image-enhanced burnt otoliths, bomb radiocarbon and the growth dynamics of redfish (Sebastes mentella and S. fasciatus) off the eastern coast of Canada. Marine and Freshwater Research 67, 925–936.
| Image-enhanced burnt otoliths, bomb radiocarbon and the growth dynamics of redfish (Sebastes mentella and S. fasciatus) off the eastern coast of Canada.Crossref | GoogleScholarGoogle Scholar |
Casselman, J. M. (1987). Determination of age and growth. In ‘The Biology of Fish Growth’. (Eds. A. H. Weatherley and H. S. Gill.) pp. 209–242. (Academic Press: London, UK.)
Chavarie, L., Howland, K., Venturelli, P., Kissinger, B. C., Tallman, R., and Tonn, W. (2016). Life-history variation among four shallow-water morphotypes of lake trout from Great Bear Lake, Canada. Journal of Great Lakes Research 42, 193–203.
| Life-history variation among four shallow-water morphotypes of lake trout from Great Bear Lake, Canada.Crossref | GoogleScholarGoogle Scholar |
Druffel, E. M., and Linick, T. W. (1978). Radiocarbon in annual coral rings of Florida. Geophysical Research Letters 5, 913–916.
| Radiocarbon in annual coral rings of Florida.Crossref | GoogleScholarGoogle Scholar |
Dulvy, N. K., Fowler, S. L., Musick, J. A., Cavanagh, R. D., Kyne, P. M., Harrison, L. R., Carlson, J. K., Davidson, L. N. K., Fordham, S. V., Francis, M. P., Pollock, C. M., Simpfendorfer, C. A., Burgess, G. H., Carpenter, K. E., Compagno, L. J. V., Ebert, D. A., Gibson, C., Heupel, M. R., Livingstone, S. R., Sanciangco, J. C., Stevens, J. D., Valenti, S., and White, W. T. (2014). Extinction risk and conservation of the world’s sharks and rays. eLife 3, e00590.
| Extinction risk and conservation of the world’s sharks and rays.Crossref | GoogleScholarGoogle Scholar | 24448405PubMed |
Francis, R. I. C. C., Campana, S. E., and Neil, H. L. (2010). Validation of fish ageing methods should involve bias estimation rather than hypothesis testing: a proposed approach for bomb radiocarbon validations. Canadian Journal of Fisheries and Aquatic Sciences 67, 1398–1408.
| Validation of fish ageing methods should involve bias estimation rather than hypothesis testing: a proposed approach for bomb radiocarbon validations.Crossref | GoogleScholarGoogle Scholar |
Healey, M. C. (1975). Dynamics of exploited whitefish populations and their management with special reference to the Northwest Territories. Journal of the Fisheries Research Board of Canada 32, 427–448.
| Dynamics of exploited whitefish populations and their management with special reference to the Northwest Territories.Crossref | GoogleScholarGoogle Scholar |
Herbst, S. J., and Marsden, J. E. (2011). Comparison of precision and bias of scale, fin ray, and otolith age estimates for lake whitefish (Coregonus clupeaformis) in Lake Champlain. Journal of Great Lakes Research 37, 386–389.
| Comparison of precision and bias of scale, fin ray, and otolith age estimates for lake whitefish (Coregonus clupeaformis) in Lake Champlain.Crossref | GoogleScholarGoogle Scholar |
Johnson, L. (1976). Ecology of Arctic populations of lake trout, Salvelinus namaycush, lake whitefish, Coregonus clupeaformis, Arctic char, S. alpinus, and associated species in unexploited lakes of the Canadian Northwest Territories. Journal of the Fisheries Research Board of Canada 33, 2459–2488.
| Ecology of Arctic populations of lake trout, Salvelinus namaycush, lake whitefish, Coregonus clupeaformis, Arctic char, S. alpinus, and associated species in unexploited lakes of the Canadian Northwest Territories.Crossref | GoogleScholarGoogle Scholar |
Kalish, J. M. (1993). Pre- and post-bomb radiocarbon in fish otoliths. Earth and Planetary Science Letters 114, 549–554.
| Pre- and post-bomb radiocarbon in fish otoliths.Crossref | GoogleScholarGoogle Scholar |
Liao, H., Sharov, A. F., Jones, C. M., and Nelson, G. A. (2013). Quantifying the effects of aging bias in Atlantic striped bass, Morone saxatilis, stock assessment. Transactions of the American Fisheries Society 142, 193–207.
| Quantifying the effects of aging bias in Atlantic striped bass, Morone saxatilis, stock assessment.Crossref | GoogleScholarGoogle Scholar |
Mills, K. H., and Beamish, R. J. (1980). Comparison of fin-ray and scale age determinations for Lake Whitefish (Coregonus clupeaformis) and their implications for estimates of growth and annual survival. Canadian Journal of Fisheries and Aquatic Sciences 37, 534–544.
| Comparison of fin-ray and scale age determinations for Lake Whitefish (Coregonus clupeaformis) and their implications for estimates of growth and annual survival.Crossref | GoogleScholarGoogle Scholar |
Mills, K. H., Gyselman, E. C., Chalanchuk, S. M., and Allan, D. J. (2004). Growth, annual survival, age and length frequencies for unexploited lake whitefish populations. Annales Zoologici Fennici 41, 263–270.
Muir, A. M., Ebener, M. P., He, J. X., and Johnson, J. E. (2008). A comparison of the scale and otolith methods of age estimation for lake whitefish in Lake Huron. North American Journal of Fisheries Management 28, 625–635.
| A comparison of the scale and otolith methods of age estimation for lake whitefish in Lake Huron.Crossref | GoogleScholarGoogle Scholar |
Musick, J. A. (1999). Ecology and conservation of long-lived marine animals. In ‘Life in the Slow Lane: Ecology and Conservation of Long-Lived Marine Animals’. (Ed. J. A. Musick.) American Fisheries Society Symposium 23, pp 1–10. (American Fisheries Society: Bethesda, MD, USA.)
Nydal, R. (1993). Application of bomb 14C as a tracer in the global carbon cycle. Trends in Geophysical Research 2, 355–364.
Pauly, D. (1980). On the relationships between natural mortality, growth parameters, and mean environmental temperature in 175 fish stocks. ICES Journal of Marine Science 39, 175–192.
| On the relationships between natural mortality, growth parameters, and mean environmental temperature in 175 fish stocks.Crossref | GoogleScholarGoogle Scholar |
Peng, T. H., and Broecker, W. (1980). Gas exchange rates for three closed-basin lakes. Limnology and Oceanography 25, 789–796.
| Gas exchange rates for three closed-basin lakes.Crossref | GoogleScholarGoogle Scholar |
Power, G. (1978). Fish population structure in Arctic lakes. Journal of the Fisheries Research Board of Canada 35, 53–59.
| Fish population structure in Arctic lakes.Crossref | GoogleScholarGoogle Scholar |
Power, G. (1997). A review of fish ecology in arctic North America. In ‘Fish Ecology in Arctic North America’. (Ed. J. B. Reynolds.) American Fisheries Society Symposium 19, pp. 13–39. (American Fisheries Society: Bethesda, MD, USA.)
Raitaniemi, J., and Heikinheimo, O. (1998). Variability in age estimates of whitefish (Coregonus lavaretus) from two Baltic populations – differences between methods and between readers. Nordic Journal of Freshwater Research 74, 101–109.
Reist, J. D., Wrona, F. J., Prowse, T. D., Power, M., Dempson, J. B., King, J. R., and Beamish, R. J. (2006). An overview of effects of climate change on selected Arctic freshwater and anadromous fishes. Ambio 35, 381–387.
| An overview of effects of climate change on selected Arctic freshwater and anadromous fishes.Crossref | GoogleScholarGoogle Scholar | 17256642PubMed |
Smith, M. W., Then, A. Y., Wor, C., Ralph, G., Pollock, K. H., and Hoenig, J. M. (2012). Recommendations for catch-curve analysis. North American Journal of Fisheries Management 32, 956–967.
| Recommendations for catch-curve analysis.Crossref | GoogleScholarGoogle Scholar |
Spiker, E. C. (1980). The behavior of C-14 and C-13 in estuarine water: effects of in situ CO2 production and atmospheric exchange. Radiocarbon 22, 647–654.
| The behavior of C-14 and C-13 in estuarine water: effects of in situ CO2 production and atmospheric exchange.Crossref | GoogleScholarGoogle Scholar |
Stewart, R. E. A., Campana, S. E., Jones, C. M., and Stewart, B. E. (2006). Bomb radiocarbon dating calibrates beluga (Delphinapterus leucas) age estimates. Canadian Journal of Zoology 84, 1840–1852.
| Bomb radiocarbon dating calibrates beluga (Delphinapterus leucas) age estimates.Crossref | GoogleScholarGoogle Scholar |
Stuiver, M., and Polach, H. A. (1977). Reporting of 14C data. Radiocarbon 19, 355–363.
| Reporting of 14C data.Crossref | GoogleScholarGoogle Scholar |
Wang, H.-Y., Hook, T. O., Ebener, M. P., Mohr, L. C., and Schneeberger, P. J. (2008). Spatial and temporal variation of maturation schedules of lake whitefish (Coregonus clupeaformis) in the Great Lakes. Canadian Journal of Fisheries and Aquatic Sciences 65, 2157–2169.
| Spatial and temporal variation of maturation schedules of lake whitefish (Coregonus clupeaformis) in the Great Lakes.Crossref | GoogleScholarGoogle Scholar |
Worbes, M., and Junk, W. J. (1989). Dating tropical trees by means of 14C from bomb tests. Ecology 70, 503–507.
| Dating tropical trees by means of 14C from bomb tests.Crossref | GoogleScholarGoogle Scholar |
Yule, D. L., Stockwell, J. D., Black, J. A., Cullis, K. I., Cholwek, G. A., and Myers, J. T. (2008). How systematic age underestimation can impede understanding of fish population dynamics: lessons learned from a Lake Superior cisco stock. Transactions of the American Fisheries Society 137, 481–495.
| How systematic age underestimation can impede understanding of fish population dynamics: lessons learned from a Lake Superior cisco stock.Crossref | GoogleScholarGoogle Scholar |