Diversity of cyanobacteria and cyanotoxins in Hartbeespoort Dam, South Africa
Andreas Ballot A B D , Morten Sandvik B , Thomas Rundberget A B , Christo J. Botha C and Christopher O. Miles BA Norwegian Institute for Water Research, N-0349 Oslo, Norway.
B Norwegian Veterinary Institute, N-0106 Oslo, Norway.
C Department of Paraclinical Sciences, Faculty of Veterinary Science, University of Pretoria, Onderstepoort, 0110, South Africa.
D Corresponding author. Email: andreas.ballot@niva.no
Marine and Freshwater Research 65(2) 175-189 https://doi.org/10.1071/MF13153
Submitted: 12 February 2013 Accepted: 10 July 2013 Published: 18 October 2013
Journal Compilation © CSIRO Publishing 2014 Open Access CC BY-NC-ND
Abstract
The South African Hartbeespoort Dam is known for the occurrence of heavy Microcystis blooms. Although a few other cyanobacterial genera have been described, no detailed study on those cyanobacteria and their potential toxin production has been conducted. The diversity of cyanobacterial species and toxins is most probably underestimated. To ascertain the cyanobacterial composition and presence of cyanobacterial toxins in Hartbeespoort Dam, water samples were collected in April 2011. In a polyphasic approach, 27 isolated cyanobacterial strains were classified morphologically and phylogenetically and tested for microcystins (MCs), cylindrospermopsin (CYN), saxitoxins (STXs) and anatoxin-a (ATX) by liquid chromatography–tandem mass spectrometry (LC–MS/MS) and screened for toxin-encoding gene fragments. The isolated strains were identified as Sphaerospermopsis reniformis, Sphaerospermopsis aphanizomenoides, Cylindrospermopsis curvispora, Raphidiopsis curvata, Raphidiopsis mediterrranea and Microcystis aeruginosa. Only one of the Microcystis strains (AB2011/53) produced microcystins (35 variants). Forty-one microcystin variants were detected in the environmental sample from Hartbeespoort Dam, suggesting the existence of other microcystin producing strains in Hartbeespoort Dam. All investigated strains tested negative for CYN, STXs and ATX and their encoding genes. The mcyE gene of the microcystin gene cluster was found in the microcystin-producing Microcystis strain AB2011/53 and in eight non-microcystin-producing Microcystis strains, indicating that mcyE is not a good surrogate for microcystin production in environmental samples.
Additional keywords: Cylindrospermopsis, Hartbeespoort Dam, microcystin, Microcystis, Raphidiopsis,Sphaerospermopsis.
Introduction
Periodic cyanobacterial blooms and dominance by cyanobacteria are a common phenomenon in many freshwater ecosystems worldwide and are caused by nutrient over-enrichment because of agricultural, urban and industrial activities (Paerl and Huisman 2009). Cyanobacteria found in such blooms are often able to produce a variety of hepatotoxic and neurotoxic secondary metabolites and are a limiting factor for the utilisation of water from these lakes and reservoirs as drinking water and for irrigation and recreational purposes (Hitzfeld et al. 2000; Carmichael 2001; Saqrane and Oudra 2009). Serious chronic human and acute animal health problems, in some cases even mortalities, have been related to the presence of hepatotoxic and neurotoxic metabolites produced by cyanobacteria (Carmichael 2001; Paerl and Huisman 2009).
Since the 1950s, Hartbeespoort Dam has been known for the occurrence of massive blooms of the potentially toxin-producing cyanobacterium Microcystis aeruginosa (Kützing) (Allanson and Gieskes 1961; Ashton et al. 1985; Zohary and Pais-Madeira 1990; Van Ginkel 2003; Oberholster and Botha 2010; Conradie and Barnard 2012). Occasionally, a few heterocytous cyanobacterial species, e.g. Anabaena sp. and Cylindrospermopsis sp., and a few non-heterocytous cyanobacterial species e.g. Oscillatoria maxima, Pseudanabaena sp., Aphanocapsa sp., Planktothrix sp., have been reported in the phytoplankton community in Hartbeespoort Dam in conjunction with M. aeruginosa (Allanson and Gieskes 1961; Zohary 1985; Hambright and Zohary 2000; Van Ginkel 2003; Janse van Vuuren and Kriel 2008; Conradie and Barnard 2012).
In the 1970s, cattle mortalities occurred on the shores of Hartbeespoort Dam and were related to toxins produced by blooms of M. aeruginosa (Toerien et al. 1976). The livestock mortalities lead to an intensive study of Microcystis colonies, toxin production and toxins in Hartbeespoort Dam (Toerien et al. 1976). A toxin called D-6 was isolated from a Microcystis bloom collected from Hartbeespoort Dam in 1974 (Botes et al. 1982a). Toxin D-6 was similar to a toxin BE-4 isolated from Microcystis strain WR 70 from Witbank Dam in South Africa. Toxin BE-4, now known as microcystin-LA, was the first microcystin to have its structure determined (Botes et al. 1982a, 1982b, 1984). Microcystins are cyclic heptapeptides with the common structure cyclo-(-D-Ala1-L-X2-D-isoMeAsp3-L-Z4-Adda5-D-isoGlu6-Mdha7). The position of amino acids is indicated by the superscripted number (Diehnelt et al. 2006). The most variable L-amino acids are found in the positions 2 and 4 (letters × and Z) in the microcystin molecule (Diehnelt et al. 2006). Typical amino acids in position 3 are either D-aspartic acid (Asp) or D-erythro- methylaspartic acid (MAsp). In position 7 either N-methyldehydroalanine (Mdha), dehydroalanine (Dha), or 2-amino-2-butenoic acid (Dhb) occur (Diehnelt et al. 2006).
Altogether, 10 microcystin (MC) variants have been described from Hartbeespoort Dam in different studies: MC-RR, MC-LR, MC-FR, MC-YR, MC-LA, MC-YA, MC-LAba, MC-WR, MC-(H4)YR and [Asp3, Dha7]MC-RR (Wicks and Thiel 1990; Van Ginkel 2003; Mbukwa et al. 2012). However, the number of microcystin variants found in Hartbeespoort Dam is low compared with the more than 100 microcystin variants that have been described worldwide (Neffling 2010). These microcystins are produced by Microcystis spp. and members of other cyanobacterial genera e.g. Planktothrix, Anabaena, and Nostoc (Sivonen and Jones 1999).
It is hypothesised that the number of cyanobacterial species and toxins present in Hartbeespoort Dam documented to date is underestimated because most former studies of Hartbeespoort Dam have focussed on Microcystis spp. only, and often utilised analytical methods with limited ability to discriminate microcystin analogues and detect other types of cyanobacterial toxins. This study therefore aimed to apply modern analytical methods in a polyphasic approach to elucidate in detail the cyanobacterial composition, phylogeny and toxicity of the cyanobacteria present in Hartbeespoort Dam, and their toxin profiles.
Material and methods
Study area, measurements and sampling
Hartbeespoort Dam is a manmade reservoir located near Pretoria, South Africa. Hartbeespoort Dam was completed in 1923 and filled with water in 1925 (Cochrane 1987). The reservoir has a surface area of around 20 km2 and a mean depth of 9.6 m (Ashton et al. 1985). Hartbeespoort Dam was originally planned as a water supply for Pretoria and Johannesburg but, after completion, was mainly used for irrigation and recreation (Cochrane 1987; Water Research Commission 2008). The initial oligotrophic conditions in Hartbeespoort Dam changed over the next 25 years to eutrophic because of excessive nutrient loading (Allanson and Gieskes 1961). Several studies conducted between 1970 and 2010 have confirmed a further change to hypertrophic conditions in Hartbeespoort Dam (Steyn et al. 1975; Ashton et al. 1985; Wicks and Thiel 1990; Van Ginkel 2003; Oberholster and Botha 2010).
The sampling point at Hartbeespoort Dam was close to the northern shore (25°44′05.34″S, 27°52′08.64″E). Samples for analysis of phytoplankton composition, cyanobacterial toxins and for the isolation of cyanobacterial strains were taken in April 2011. The growing season for cyanobacteria in Hartbeesport Dam is from January until April according to Conradie and Barnard (2012). For quantitative phytoplankton analysis, a 125 mL subsample was removed from a sample taken from the lake surface, and fixed with Lugol’s solution. A 50 mL water sample for isolation of cyanobacteria was taken and kept in a cool shady place and gently shaken twice per day before analysis in Norway.
For cyanotoxin analysis, 10 L of lake water from the surface was sampled in a plastic container, frozen, thawed and then shaken with 30 g of activated HP-20 resin (DIAION, Mitsubishi Chemical Corporation, Tokyo, Japan) overnight to extract microcystins (Miles et al. 2012). The sample was filtered through nylon netting (200 µm mesh) and the resin recovered and stored at 4°C until transportation to Norway. The resin was rinsed with distilled water and eluted slowly with methyl alcohol (MeOH) (3 × 50 mL), the eluates were evaporated to dryness in vacuo and dissolved in MeOH (5 mL). A specimen was diluted 10-fold for analysis.
Isolation of strains and morphological characterisation
Using a microcapillary, single colonies of Microcystis and filaments of Sphaerospermopsis, Cylindrospermopsis and Raphidiopsis were isolated. They were washed five times and placed in wells on microtiter plates containing 300 µL Z8 medium (Kotai 1972). After successful growth, the samples were placed in 50 mL Erlenmeyer flasks containing 20 mL Z8 medium and maintained at 22°C. Strains were classified on the basis of morphological traits according to Komárek and Anagnostidis (1998), Horecká and Komárek (1979), Komárek and Komárkova (2006) and Cronberg and Annadotter (2006). Morphological characterisations were conducted using an Olympus BX50 light microscope with an Olympus Dp72 camera and CellSense Digital Image software (Olympus, Oslo, Norway). The morphological identification was determined on the basis of the following criteria: (i) size of vegetative cells, heterocytes and akinetes and (ii) nature and shape of filaments or colonies. Length and width of 50–250 vegetative cells and of 20–50 heterocytes and akinetes were measured. All strains used in this study are maintained at the Norwegian Institute for Water Research, Oslo, Norway.
Genomic DNA extraction, PCR amplification and sequencing
Fresh culture material of all cyanobacterial strains was frozen and thawed three times and boiled for 5 min to break the cell walls and remove mucilage surrounding the filaments or colonies. After centrifugation (5 min, 16000 g) the supernatant was discarded. Autoclaved zirconium beads (0.5 g), 600 µL sodium phosphate buffer (pH 8) and 100 mL 25% sodium dodecylsulfate (SDS) were added to each pellet. After horizontal vortexing for 10 min, the sample was centrifuged (6 min, 14 000 g). The supernatant was transferred into a new 2 mL Eppendorf tube. The pellet was washed with 500 µL sodium phosphate buffer, mixed thoroughly and centrifuged (6 min, 14 000 g). The supernatants were combined and 200 µL lysozyme (10 mg/mL in TE buffer (Tris–EDTA)) was added. After incubation at 37°C for 15 min, 150 µL 25% SDS and 10 µL proteinase K (20 mg/mL) were added, followed by incubation at 60°C for 15 min. To separate the DNA from proteins, 600 µL ice-cold 7.5 M ammonium acetate was added and the sample centrifuged for 8 min (14 000 g). The supernatant was transferred to a new 2 mL Eppendorf tube, and 0.7 volumes of isopropanol was added. After centrifugation at 14 000 g for 60 min, the pellet was washed twice with 80% ethanol and centrifuged for 5 min (16 000 g). The pellet containing genomic cyanobacterial DNA was dissolved in 40 µL TE buffer and stored at –20°C.
All PCRs were performed on a Peltier thermal cycler PTC 200 (MJ Research, Inc., San Francisco, CA) using the Taq PCR core kit (Qiagen GmbH, Hilden, Germany). The reaction mixture contained 0.1 µL Taq DNA polymerase (5 U/µL), 0.5 µL deoxynucleoside triphosphate mix (10 mM), 2 µL Qiagen PCR buffer, 1 µL forward and reverse primer (10 µM), and 1 µL genomic DNA (total volume 20 µL). The primers PCβf and PCαr were used to amplify the intergenic spacer and flanking regions of the cpcB and cpcA genes of the phycocyanin operon (PC-IGS) (Neilan et al. 1995). PCR was also used to check whether the isolated strains were potential producers of ATX, CYN, MCs or STXs. A polyketide synthase (PKS) encoding gene (anaF) of the anatoxin gene cluster was amplified using the primer atxoaf (Ballot et al. 2010a) and the newly designed primer atxoar (acctccgactaaagctaggtcg). Amplification of the cyrJ gene fragment was conducted using the primers cynsulfF and cylnamR (Mihali et al. 2008). The primers sxtaf and sxtar were used to amplify a part of the sxtA gene of the saxitoxin gene cluster (Ballot et al. 2010b). A part of the mcyE gene of the microcystin gene cluster was investigated using the primers mcyEF2 and mcyER4 and the PCR program according to Rantala et al. (2004). The cycling protocol for the PC-IGS fragment was one cycle of 5 min at 94°C and then 35 cycles of 1 min at 94°C, 1 min at 55°C, and 1 min at 72°C with a final elongation step of 72°C for 5 min. PCR products were visualised by 1% agarose gel electrophoresis with GelRed staining and UV illumination.
Amplified PC-IGS and mcyE products were purified through Qiaquick PCR purification columns (Qiagen, Hilden, Germany). Sequencing of the purified PC-IGS and mcyE products was performed using the same primers as for PCR. For each PCR product, both strands were sequenced on an ABI 3130 XL genetic analyser using the BigDye terminator V.3.1 cycle sequencing kit (Applied Biosystems, Applera Deutschland GmbH, Darmstadt, Germany) according to the manufacturer’s instructions.
Phylogenetic analysis
Sequences of the PC-IGS locus in all Sphaerospermopsis, Cylindrospermopsis, Raphidiopsis and Microcystis strains were analysed using Bioedit (Hall 2007) and Align (version 03/2007) MS Windows-based manual sequence alignment editor (Hepperle 2008) to obtain DNA sequence alignments, which were then corrected manually. Segments with highly variable and ambiguous regions and gaps making proper alignment impossible were excluded from the analyses.
A PC-IGS set containing 443 positions was used in the Nostocales PC-IGS tree. Nostocaceae Cyanobiont (AY181211) was employed as the outgroup and 31 additional Nostocales sequences derived from GenBank were included in the PC-IGS analyses. A set containing 521 positions was used for the Microcystis PC-IGS analysis. Pseudanabaena mucicola (HQ662535) was employed as the outgroup and 35 additional African Microcystis sequences derived from GenBank were included in the PC-IGS analyses. Phylogenetic trees for PC-IGS were constructed using the maximum likelihood (ML) algorithm in PAUP* v.10b (Swofford 2002). In the ML analyses, evolutionary substitution models were evaluated using the AIC criterion in jModelTest v.0.1.1 (Guindon and Gascuel 2003; Posada 2008). The TIM2+G evolutionary model was found to be the best-fitting evolutionary model for the PC-IGS tree (Nostocales) and TrNef+G for the PC-IGS tree (Microcystis). ML analyses of both trees were performed with 1000 bootstrap replicates using PAUP* v.10b (Swofford 2002). The sequence data were submitted to the EMBL Nucleotide Sequence Database under the accession numbers listed in Table 1.

|
Toxin analysis
Fresh culture material of all cyanobacterial strains was frozen and thawed three times, ultrasonicated for 5 min and filtered through Spin-X centrifuge tube filters (Corning Inc., Corning USA), at 10 000 g. The filtrate was used for analysis of STXs. For analysis of MCs, the filtrate (100 μL) was mixed with MeOH (100 μL) (Miles et al. 2012), and for analysis of CYN and ATX the filtrate was mixed with acetonitrile (1 : 4).
Microcystin analysis
Standards
Microcystin (MC-RR, MC-LR, MC-YR, MC-WR, MC-LA, MC-LY, MC-LF, MC-LW) standards were purchased from Alexis Biochemicals (Grünberg, Germany), an NMR-quantitated standard of [Dha7]MC-LR was obtained from IMB NRC, Halifax, NS, Canada, and MC-RY was isolated from a cyanobacterial bloom (Miles et al. 2013b). [Asp3]MC-LY (Miles et al. 2012) isolated from M. aeruginosa CYA548, and with its structure confirmed by NMR and mass spectral analysis (C. O. Miles, H. E. Nonga, M. Sandvik, S. Chaudhry, A. L. Wilkins, F. Rise and A. Ballot, unpubl. data), was also used as a standard. Standards of MC-WR and MC-LW in 1 : 1 MeOH–water (1 mL) were each treated with 30% H2O2 (50 μL) and allowed to stand at room temperature for a week to cause partial oxidation of tryptophan (Puddick et al. 2013). The major oxidation product from MC-WR was identical by LC-MS2 to MC-NfkR identified in a Microcystis extract (Puddick 2012; Puddick et al. 2013), whilst the major oxidation product from from MC-LW showed LC-MS2 retention, mass and fragmentation pattern consistent with MC-LNfk.
Freeze-dried culture material of Nostoc 152 (containing [ADMAdda5]MC-LR, [ADMAdda5]MC-LHar and [Asp3, ADMAdda5]MC-LR as the major microcystins (Namikoshi et al. 1990)) was obtained from K. Sivonen (Helsinki University, Finland), and a specimen (8 mg) extracted with MeOH–H2O (1 : 1, 1.5 mL) as for the fresh culture material. Aliquots of the extract were treated with pH 9.7 carbonate buffer (Miles et al. 2012) (to produce [DMAdda5]-microcystins by hydrolysis) at 30°C, and progress of the reaction monitored by LC-MS2 for 2.5 days. Treatment of hydrolysed and unhydrolysed aliquots (in carbonate buffer) with mercaptoethanol (to derivatise the Mdha7-group), followed by LC-MS2 analysis (Miles et al. 2012), was used to confirm the identity of the major hydrolysis products ([DMAdda5]MC-LR, [DMAdda5]MC-LHar and [Asp3, DMAdda5]MC-LR) and the hydrolysed extract was then used as a qualitative standard for these microcystins.
LC-MS2 analysis
LC-MS2 analysis with and without mercaptoethanol derivatisation was performed as described by Miles et al. (2012). Briefly liquid chromatography was performed on a Symmetry C18 column (3.5 µm, 100 × 2.1 mm; Waters, Milford, MA, USA), using a Surveyor MS Pump Plus and a Surveyor Auto sampler Plus (Finnigan, Thermo Electron Corp., San Jose, CA, USA) eluted (0.3 mL min–1) with a linear gradient (300 μL min–1) of acetonitrile (A) and water (B) each containing 0.1% formic acid. The gradient was from 22.5% to 42.5% A over 4 min, then to 75% A at 10 min, to 95% A at 11 min (1 min hold) followed by a return to 22.5% A with a 3-min hold to equilibrate the column. The HPLC system was coupled to a Finnigan LTQ ion trap mass spectrometer (Finnigan Thermo Electron Corp., San Jose, CA, USA) operated in full-scan positive ion ESI mode (m/z 500–1600).
Microcystins were analysed by LC-MS2, and quantitated from their [M+H]+ ions in scan mode relative to the most closely related commercial standard available (e.g. MC-YR-analogues relative to MC-YR etc). Identities were considered confirmed when retention time and fragmentation pattern were identical to commercial standards or to analogues with, or derived from, authenticated structures (MC-RY, [Asp3]MC-LY, MC-NfkR, [DMAdda5]MC-LR and [Asp3, DMAdda5]MC-LR). Identification was considered tentative if peaks with appropriate retention times yielded appropriate fragmentation patterns (Miles et al. 2012). Oxidised MC-WR analogues in the samples were identified by comparison with MS2 spectra of related compounds (Puddick 2012).
Cylindrospermopsin and anatoxin-a analyses
Liquid chromatography was performed on a SeQuant ZIC-HILIC column (3.5 µm, 150 × 2.1 mm) (Merck, Darmstadt, Germany), using an Accela HPLC module (Thermo Scientific, San Jose, CA, USA). Separation was achieved using step gradient elution at 0.2 mL min–1 starting with 20% A (water containing 5 mM ammonium acetate and 0.1% acetic acid) and 80% B (95% MeCN containing 5 mM ammonium acetate and 0.1% acetic acid) for 8 min, then rising to 60% A over 15 min followed by a return to 20% A (8 min hold) before the next injection. The HPLC system was coupled to a TSQ Quantum Access triple-quadrupole mass spectrometer operating with an ESI interface (Thermo Scientific, San Jose, CA, USA). Typical ESI parameters were a spray voltage of 3.5 kV, heated capillary temperature at 250°C and nebulizer gas at 600 L h–1 of N2. The mass spectrometer was operated in MS/MS mode with argon as collision cell gas at 1.4 × 10–3 Torr. Ionisation and MS/MS collision energy settings (typically 25–30 eV) were optimised while continuously infusing (syringe pump) 200 ng/mL of CYN and ATX, at a flow rate of 5 µL min–1. Screening of CYN and ATX were performed with multiple-reaction monitoring (MRM) in positive ionisation mode using the following transitions: m/z CYN 416.1→176.0, 416.1→194.0, ATX m/z 166.1→131.1, 166.1→149.1. Certified cylindrospermopsin and anatoxin-a (NRC CRM) from National Research Council, Halifax, NS, Canada were used as standards. The detection limit for both toxins was 10 µg L–1.
Saxitoxin analysis
Analysis of STXs was conducted according to the HPLC method of Rourke et al. (2008), except that separation was achieved on a Waters T3 Atlantis column and the acetonitrile content of mobile phases A and B were 4% and 16%, respectively.
Results
Phytoplankton community
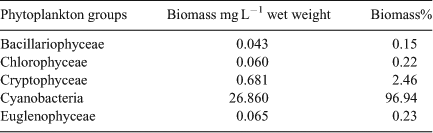
Cyanobacteria dominated the phytoplankton sample from Hartbeespoort Dam in April 2011 and comprised 96.9% of the total phytoplankton biomass of 27.7 mg L–1 (Table 2). The most dominant cyanobacterium was M. aeruginosa with a biomass of 26.3 mg L–1 wet weight, or 97.9% of the cyanobacterial biomass. Other cyanobacterial species present belonged to the genera Sphaerospermopsis, Cylindrospermopsis, Raphidiopsis, Pseudanabaena and Aphanocapsa which together comprised a biomass of 0.56 mg L–1 wet weight (2.1% of the cyanobacterial biomass). Other phytoplankton groups observed were Bacillariophyceae, Chlorophyceae, Cryptophyceae and Euglenophyceae with a total biomass of 0.85 mg L–1 or 3.1% of the total biomass (Table 2).
|
Morphological and phylogenetic characterisation
Twenty-seven potentially toxin producing cyanobacterial strains were isolated from Hartbeespoort Dam (Table 1).
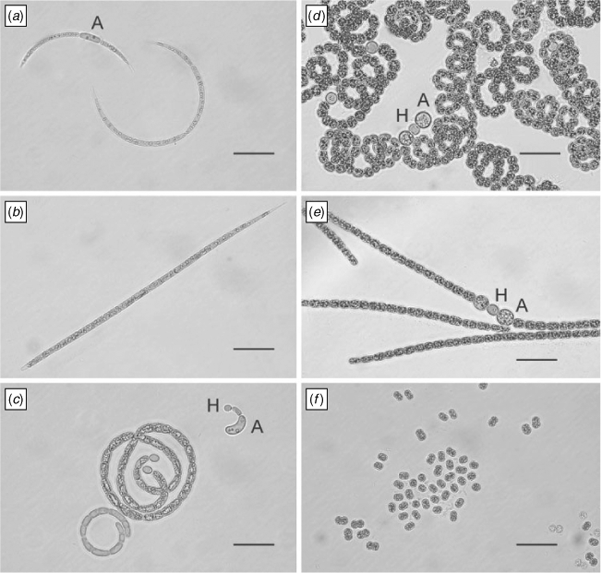
On the basis of morphological features e.g. presence and form of vegetative cells, heterocytes and akinetes, six of the isolated strains were identified as Sphaerospermopsis aphanizomenoides (Forti) Zapomelová, Jezberová, Hrouzek, Hisem, Reháková & Komárková, and two strains as Sphaerospermopsis reniformis (Lemmermann) Zapomelová, Jezberová, Hrouzek, Hisem, Reháková & Komárková. The S. aphanizomenoides strains were characterised by straight filaments and the S. reniformis strains by coiled filaments. The cell size of the vegetative cells varied between 2.2–13.2 × 1.8–6.8 µm in S. aphanizomenoides and 2.6–7.6 × 3.0–7.2 µm in S. reniformis. Round to ellipsoid heterocytes with a cell size of 3.7–8.2 × 2.8–6.6 µm and 4.4–7.6 × 4.6–7.8 µm were observed in strains of S. aphanizomenoides and S. reniformis, respectively. Round to slightly ellipsoid akinetes were observed adjacent to heterocytes in four S. aphanizomenoides strains and in both S. reniformis strains with cell sizes of 6.5–14.2 × 4.9–11.1 µm and 6.9–12.0 × 7.0–11.7 µm, respectively (Fig. 1, Table 3).
|

|
One strain was identified as Cylindrospermopsis curvispora M. Watanabe. It was characterised by coiled filaments, vegetative cells with a cell size of 2.4–10.4 × 1.9–3.6 µm. ellipsoid heterocytes with a cell size between 2.9–7.4 × 2.0–3.7 µm and kidney shaped akinetes with a cell size of 9.4–19.6 × 3.1–4.7 µm (Fig. 1, Table 3). One strain was determined as Raphidiopsis curvata F.E.Fritsch & M.F.Rich and one strain as Raphidiopsis mediterranea Skuja (Fig. 1, Table 3). The R. curvata strain was characterised by curved filaments and the R. mediterranea strain by straight filament. In both strains no heterocytes were observed. The size of the vegetative cell ranged from 3.8–17.3 × 1.4–2.8 µm in R. curvata and from 5.9–17.8 × 1.6–2.7 µm in R. mediterranea. Akinetes with a size of 6.7–12.5 × 2.4–4.0 µm were observed in R. curvata only (Fig. 1, Table 3).
Sixteen strains were identified as M. aeruginosa (Fig. 1). The mean cell diameter of the various Microcystis strains ranged from 3.2 µm (strain AB2011/53) to 5.4 µm (strain AB2011/42) (data not shown).
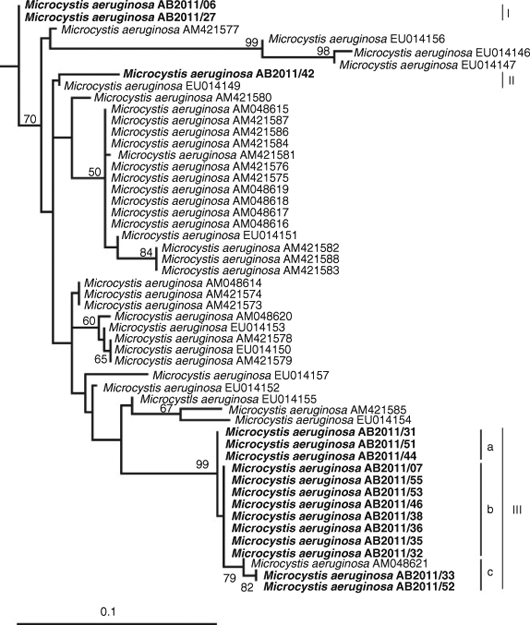
The morphological determination of the isolated strains was supported by phylogenetic features (Figs 2, 3, Table 1). Phylogenetic relationships of the investigated strains are presented in the ML tree of the PC-IGS region of Nostocales strains (Fig. 2) and a separate tree of African Microcystis strains (Fig. 3). In the ML-tree in Fig. 2 the Cylindrospermopsis and Raphidiopsis spp. were grouped in a distinct cluster (cluster I) which is supported by a bootstrap value of 100%. Cylindrospermospis spp. and Raphidiopsis spp. could not be distinguished phylogenetically and formed mixed subclusters. Cylindrospermopsis curvispora from Hartbeespoort Dam could not be distinguished from other C. raciborskii strains (Fig. 2).
|
All S. aphanizomenoides and S. reniformis strains were grouped in a separate cluster (cluster II) supported by a bootstrap value of 95%. They were grouped closer to Anabaena and Aphanizomenon strains than to Cylindrospermopsis and Raphidiopsis strains. Sphaerospermopsis reniformis formed mixed subclusters with S. aphanizomenoides and A. aphanizomenoides strains and could not be distinguished phylogenetically (Fig. 2).
The Microcystis strains from Hartbeespoort Dam were grouped in 3 clusters which were separated from other African Microcystis strains. The exception was cluster III, where a Microcystis strain from Lake Victoria, Uganda (AM048621), was included, forming a subcluster (Fig. 3). The microcystin producing strain AB2011/53 was located in cluster IIIb. Its PC-IGS sequence was characterised by a similarity of 100% to those of seven non-microcystin producing strains.
Identification of cyanobacterial toxins and toxin producing strains
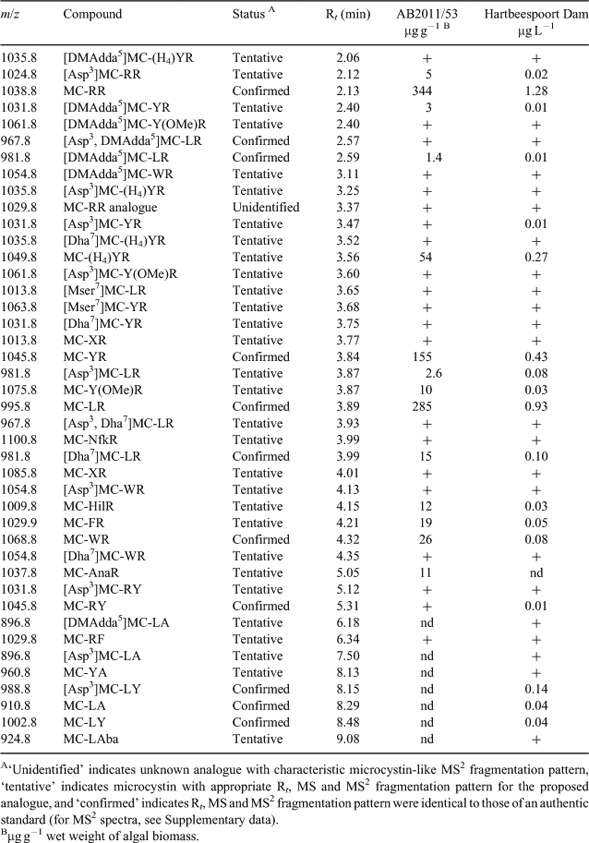
As determined by LC-MS2 analysis, 41 microcystin variants were found in the sample from Hartbeespoort Dam from April 2011 (Table 4). The most abundant variants were MC-RR, MC- LR, MC-YR and MC-(H4)YR (Fig. 4). The MC-LR concentration was 0.93 µg L–1 and the total microcystin concentration was ~3.6 µg L–1. For 23 of the 41 microcystins, the concentrations were below the limit of quantification (0.01 µg L–1). All microcystins in Table 4, with the exception of the [Mser7]-congeners, reacted with mercaptoethanol in the presence of carbonate buffer, indicating that they contained Mdha or Dha, rather than Mdhb or Dhb, as the amino acid at site-7 (Miles et al. 2012; Miles et al. 2013a).
|

|
Fifteen of the 16 M. aeruginosa strains isolated from Hartbeespoort Dam did not produce microcystins. However, one strain (AB2011/53) produced 35 microcystins as determined by LC-MS2 (Table 4), with a total microcystin concentration (extra- and intracellular) of 943 µg g–1 wet weight, equivalent to 0.024 pg cell–1.
All 27 cyanobacterial strains investigated in this study tested negative for CYN, ATX and STXs by LC-MS and HPLC analysis.
Amplification of toxin encoding genes
Amplification of the mcyE gene was observed in the MC-producing M. aeruginosa strain AB2011/53 and in 8 other non-MC producing Microcystis strains from Hartbeespoort Dam. None of the 27 strains exhibited amplification of the sxtA gene (saxitoxin gene cluster), cyrJ gene (cylindrospermopsin gene cluster) and the anaF gene (anatoxin-a encoding gene cluster).
Discussion
This study clearly demonstrated the presence of the potentially toxic Nostocales cyanobacteria C. curvispora, R. curvata, R. mediterranea, S. aphanizomenoides and S. reniformis in the phytoplankton community of Hartbeespoort Dam, South Africa. None of these species have previously been detected in Hartbeespoort Dam, but have been reported from tropical and subtropical regions of Africa (Cronberg and Komárek 2004; Cronberg and Annadotter 2006). Van Ginkel (2003) has detected Cylindrospermopsis spp. (later described as C. raciborskii by Janse van Vuuren and Kriel (2008) for the first time in South Africa in the Orange River in 2000, and later in low numbers in Hartbeespoort Dam. Cylindrospermopsis curvispora has been described only from a few countries in the world. It was initially detected in a Japanese reservoir by Watanabe (1995), and was later also found in Sri Lanka, in western Africa in Senegal, and in southern Africa in Zambia and Botswana (Cronberg and Komárek 2004; Thomazeau et al. 2010). McGregor and Fabbro (2000) have described coiled morphotypes of Australian C. raciborskii with a similar morphology to C. curvispora strain AB2011/30. Therefore, it cannot be excluded that C. curvispora is actually another morphotype of C. raciborskii. This is supported by a study from Thomazeau et al. (2010), who concluded that C. curvispora cannot be distinguished genetically from C. raciborskii using 16S rRNA gene sequences.
Cylindrospermopsis spp. and Raphidiopsis spp. are clearly distinguished morphologically by the possession or lack of heterocytes. Rhaphidiopsis curvata is characterised by short crescent filaments and R. mediterranea by short straight filaments (Cronberg and Annadotter 2006), features which could be clearly seen for filaments of both species in the environmental sample from Hartbeespoort Dam. However, in culture, both isolated Raphidiopsis strains AB2011/25 and AB2011/37 grew mostly as long straight, or slightly curved, filaments. Only a small proportion of the R. curvata culture AB2011/25 was observed growing as short crescent filaments. Such morphological variations between cyanobacterial strains growing in natural environments or under culture conditions were also reported in other studies (e.g. Ballot et al. 2008; Zapomělová et al. 2008). This demonstrates that a correct identification, using morphological traits only, in some cases is misleading or not even possible. An intensive study on the cyanobacterial composition should therefore always include a combination of classical methods (e.g. microscopy) and newer genetic methodologies.
Cylindrospermospsis curvispora, R. curvata and R. mediterranea strains can be clearly distinguished using morphological criteria. However, the mixed cluster (cluster I) of Cylindrospermopsis and Raphidiopsis sequences from Hartbeestpoort Dam and those derived from GenBank in the phylogentetic tree in (Fig. 2) confirms suggestions by McGregor and Fabbro (2000), Moustaka Gouni et al. (2009) and Stucken et al. (2010), that Raphidiopsis and Cylindrospermopsis in fact constitute a single genus. Cluster I in Fig. 2 also clearly indicates that C. curvispora from Hartbeespoort Dam is very closely related to other C. raciborskii strains and is closer to R. mediterranea and R. curvata from Hartbeespoort Dam than to Cylindrospermopsis and Raphidiopsis species from other locations. These findings raise the question of whether C. curvispora, R. curvata and R. mediterranea can be regarded as separate species or are most likely just rare morphotypes of C. raciborskii.
Strains of Cylindrospermopsis and Raphidiopsis from Australia, Brazil, China, Japan and Thailand produce CYN, STXs or ATX (Hawkins et al. 1997; Saker and Neilan 2001; Li et al. 2001; Namikoshi et al. 2003; Soto-Liebe et al. 2010). However, all the Cylindrospermopsis and Raphidiopsis strains isolated from Hartbeespoort Dam tested negative for production of cyanotoxins and their encoding genes. Interestingly, no CYN-, STX- or ATX-producing Cylindrospermopsis or Raphidiopsis strains have been located on the African continent to date although genetic data have suggested the colonisation of Australia by African Cylindrospermospis strains (Gugger et al. 2005; Haande et al. 2008). The only possible exception is in Egypt, where C. raciborskii strains with hepatotoxic effects and R. mediterranea strains with neurotoxic effects on mice were detected (Mohamed 2007). However, the findings by Mohamed (2007) were not supported by LC-MS analyses and the supposed toxins were not identified.
Sphaerospermopsis aphanizomenoides and S. reniformis have also not been described from Hartbeespoort Dam before. The filaments of both species were clearly visible among the dominant Microcystis colonies. Coiled and straight filaments of Sphaerospermopsis are readily confused with Anabaena spp. if akinetes and heterocytes are lacking. In culture, but not in the environmental sample from Hartbeespoort Dam, some of the Sphaerospermopsis filaments possessed heterocytes and akinetes. There is a possibility that Anabaena spp. observed in an earlier study by Van Ginkel (2003) were in fact Sphaerospermopsis spp. So far, only a few findings of S. reniformis or other coiled species with a similar morphology (S. torques reginae, A. eucompacta, A. oumiana) have been reported from water bodies in Africa, Asia, Europe and Central and South America (Li and Watanabe 1999; Cronberg and Annadotter 2006; Zapomělová et al. 2009; Werner et al. 2012). However, this dearth of reports could be attributed to misidentification of this morphospecies (Cronberg and Annadotter 2006; Werner et al. 2012). In the PC-IGS tree, all Sphaerospermopsis spp. from Hartbeespoort Dam are grouped together and are separated from other Nostocales cyanobacteria. This supports findings by Zapomělová et al. (2009, 2010), who reclassified former Aphanizomenon aphanizomenoides and Anabaena reniformis into the new genus Sphaerospermopsis according to their morphological and phylogenetic characteristics. Planktothrix spp. which was described by Conradie and Barnard (2012) as occurring in low numbers in samples preserved with Lugol's solution from Hartbeespoort Dam in 2005, was not observed in samples collected for the current study.
So far worldwide, no Sphaerospermopsis strains have been found to possess genes which encode for the biosynthesis of CYN, STXs, ATX, and MCs or producing these toxins, including in our study. However, the existence of toxin producing Sphaerospermopsis strains cannot be excluded because in many other Nostocales genera, e.g. Cylindrospermopsis, Aphanizomenon, Anabaena, non-toxin and toxin producing strains have been described (Ballot et al. 2010a, 2010b; Li et al. 2001; Haande et al. 2008).
Similar to other studies conducted at Hartbeespoort Dam (e.g. Allanson and Gieskes 1961; Zohary and Pais-Madeira 1990; Van Ginkel 2003; Conradie and Barnard 2012), the present study confirmed that M. aeruginosa is the dominant cyanobacterium. Blooms of M. aeruginosa in Hartbeespoort Dam have been recorded since the 1950s, and this species has continued to dominate the phytoplankton community of this reservoir (Allanson and Gieskes 1961; Wicks and Thiel 1990; Conradie and Barnard 2012). Harding et al. (2004) and Conradie and Barnard (2012) have described frequent Microcystis dominances of up to 100% of the phytoplankton biomass in Hartbeespoort Dam.
The difference between the 41 MC variants found in the water sample from Hartbeespoort Dam and the 35 variants produced by Microcystis strain AB2011/53 shows clearly that other MC producing cyanobacteria (most likely other MC producing Microcystis strains) must have been present in Hartbeespoort Dam at the time of investigation. The novel variant MC-AnaR (tentatively identified from its MS2 fragmentation pattern) found in Microcystis strain AB2011/53 was not detected in the water sample from Hartbeespoort Dam, probably because its concentration in the water sample was below the detection limit of the LC-MS analysis. The number of microcystins detected in this study is considerably higher than the 10 MC variants (MC-RR, MC-LR, MC-YR, MC-FR, MC-YA, MC-LA, MC-LAib, MC-WR, MC-(H4)YR, [Asp3, Dha7)]MC-RR) described in previous studies of Hartbeespoort Dam using HPLC analysis (Botes et al. 1984; Wicks and Thiel 1990; Van Ginkel 2003; Mbukwa et al. 2012). This is probably primarily because of the analysis method here. Use of thiol derivatisation permitted subtraction of chromatograms (Fig. 4) to assist in identifying minor components. Thiol reactivity also provided greater certainty in the identification of reacting components as putative microcystins, which could then be evaluated by examination of their MS2 spectra (Miles et al. 2012; Miles et al. 2013b). In the current investigation, MC-RR, MC-LR, MC-YR were the most prevalent microcystins, whereas Wicks and Thiel (1990) described MC-LR and MC-FR, and Van Ginkel (2003) MC-LA, as the most abundant microcystins in Hartbeespoort Dam. This suggests a varying dominance of different MC producing Microcystis strains.
LC-MS2 analysis revealed production of [DMAdda5]MC-LR, [DMAdda5]MC-LHar and [Asp3, DMAdda5]MC-LR (pseudo-first order kinetics, t1/2 ca 30 h) in carbonate buffer caused by hydrolysis of the acetate group from the major analogues in the Nostoc 152 extract ([ADMAdda5]MC-LR, [ADMAdda5]MC-LHar and [Asp3, ADMAdda5]MC-LR (Namikoshi et al. 1990)). Hydrolysed Nostoc 152 extract was used as a qualitative LC-MS standard to confirm the identities of [ADMAdda5]-microcystins in the extracts from Hartbeespoort Dam and M. aeruginosa culture AB2011/53. [DMAdda5]-analogues of the major microcystins in Hartbeespoort Dam and AB2011/53 extracts, including [DMAdda5]MC-LR and [Asp3, DMAdda5]MC-LR, were readily identified from their shorter retention times (by ~1.5–2-min) and prominent fragment ions at m/z 585 (rather than m/z 599 in their [Adda5]-congeners) and [MH–120]+ (rather than [MH–134]+) in their MS2 spectra (Supplementary data). [DMAdda5]-microcystins were typically present at ca 1% of the levels of the parent [Adda5]-analogues in the samples from Hartbeespoort Dam, suggesting that they are minor products of normal microcystin biosynthesis. Additionally, MC-NfkR, a tryptophan-oxidised congener of MC-WR, was identified at low levels by LC-MS2 in the extracts from Hartbeespoort Dam and M. aeruginosa culture AB2011/53 and its identity confirmed by oxidation of an authentic specimen of MC-WR using the method of Puddick et al. (2013). This appears to be the first report a tryptophan-oxidised microcystin congener in a field sample.
The water sample from Hartbeespoort Dam contained 0.93 µg L–1 of MC-LR, which is slightly below the World Health Organisation’s provisional guideline (1 µg L–1 MC-LR) for drinking-water (WHO 1998), although the total MC concentration (3.6 µg L–1) was considerably higher. However, Harding et al. (2004) measured a much higher median MC concentration of 580 µg L–1 (between 0 and 28 930 µg L–1) during a survey in 2003 and 2004, and Conradie and Barnard (2012) detected microcystin concentrations up to 3200 µg L–1 in Hartbeespoort Dam in 2005. In the studies by Harding et al. 2004 and Conradie and Barnard 2012, biomass was measured as chlorophyll-a and no correlation was found between the highest microcystin concentrations and the highest chlorophyll-a concentations. Conradie and Barnard (2012) used an ELISA for the detection of microcystins and could therefore not distinguish the microcystin variants in their study.
The low MC concentrations detected in this study, can be explained by the dominance of non-MC producing Microcystis in Hartbeespoort Dam. Of the 16 Microcystis strains isolated, only one (AB2011/53) produced microcystins. Interestingly, we identified the mcyE gene, a glutamate-activating adenylation domain which is part of the microcystin-encoding gene cluster (Tillett et al. 2000), not only in the MC-producing strain AB2011/53, but also in eight non-microcystin-producing strains from Hartbeespoort Dam. The presence of the mcyE gene in non-MC-producing cyanobacteria has also been described by Noguchi et al. (2009) and this raises a question as to the suitability of the mcyE gene to quantify toxin-producing Microcystis spp. in quantitative PCR investigations. Other genes of the microcystin encoding gene cluster, e.g. mcyA, mcyB, and mcyT, have been reported in non-MC-producing Microcystis and Planktothrix strains (Mikalsen et al. 2003; Kurmayer et al. 2004; Christiansen et al. 2008). Genes encoding the biosynthesis of other cyanobacterial toxins, e.g. CYN and STXs, have been detected in several non-toxin-producing cyanobacteria (Wood et al. 2007; Rasmussen et al. 2008; Ballot et al. 2010b). Various mechanisms, such as horizontal gene transfer, mutations, insertions and deletions, have been proposed as explanations for non-toxin-producing cyanobacteria possessing parts of toxin-encoding gene clusters (Christiansen et al. 2008; Tooming-Klunderud et al. 2008; Moustafa et al. 2009).
As depicted in the PC-IGS tree in Fig. 3, the toxin-producing Microcystis strain AB2011/53 cannot be distinguished from seven non-MC-producing strains which are grouped in subcluster IIIb. Microcystis strains with similar PC-IGS sequences are present worldwide in North America, Asia, and Europe when using NCBI Blast (NCBI). However, differences can be seen when comparing the mean cell sizes. Vegetative cells of MC-producing strain AB2011/53 measured only 3.2 µm, which was considerably smaller then the other Microcystis strains in subcluster IIIb (mean cell sizes between 3.8 and 5.2 µm). The other eight Microcystis strains of this study in cluster I, II, IIIa and IIIB possess PC-IGS sequences which are unique to Hartbeespoort Dam according to NCBI Blast and are distributed in different clusters. However, the number of PC-IGS sequences in GenBank is relatively low and further studies could reveal a wider distribution of Microcystis species with similar PC-IGS sequences.
By investigating more locations in Hartbeespoort Dam over a longer time period we probably could have found a higher cyanobacterial diversity and more diverse cyanotoxin composition. However, the current study shows clearly that a carefully conducted polyphasic approach even of samples taken at one selected date and at one location can result in a detailed overview about the cyanobacterial and cyanotoxin composition in a certain part of a lake. It is obvious that previous studies conducted at Hartbeespoort Dam did not reveal a similar diverse cyanobacterial community and cyanotoxin composition even though those studies were conducted over longer time periods and more locations were sampled. In the current study, the proportion of MC producing Microcystis strains was low in Hartbeesport Dam compared with non-MC producing Microcystis strains. A shift to the dominance of MC producing Microcystis strains could increase the MC concentrations in the water body considerably threatening the use of Hartbeespoort Dam for irrigation, fishing and water sports and increasing the risk to human and animal health during the growth season of cyanobacteria.
Furthermore, the massive Microcystis blooms could be curtailed by reducing nutrient loading in Hartbeespoort Dam. However, such measures could then promote the growth of the potential toxin producing Cylindrospermopsis, and Sphaerospermopsis or other heterocytous cyanobacteria. The ability of those heterocytous cyanobacteria to fix atmospheric nitrogen would be an advantvage and enable them to outcompete Microcystis spp. which are dependant on dissolved inorganic nitrogen compounds (Sukenik et al. 2012).
In conclusion, this is the first report of S. aphanizomenoides, S. reniformis, C. curvispora, R. mediterranea and R. curvata in Hartbeespoort Dam. None of the isolates of these species produced cyanobacterial toxins although Cylindrospermopsis and Raphidiopsis spp. are known toxin producers in Australia, Asia and South America. Forty-one MC variants were present in an environmental sample from Hartbeespoort Dam and 35 MC variants were detected in a Microcystis strain isolated from the same water sample. The majority of the isolated Microcystis strains did not produce MCs, which can explain the relatively low MC concentrations in the water sample from Hartbeespoort Dam.
Abbreviations Used
Aib, amino isobutyric acid; Adda, (2S,3S,8S,9S,4E,6E)-3-amino-9-methoxy-2,6,8-trimethyl-10-phenyl-4,6-decadienoic acid; Aba, aminobutyric acid (unspecified stereochemistry); ADMAdda, 9-O-acetyl-desmethylAdda; Ana, aminononanoic acid (unspecified stereochemistry); Ala, alanine; ATX, anatoxin-a; CYN, cylindrospermopsin; Dha, dehydroalanine; Dhb, dehydrobutyrine; DMAdda, 9-O-desmethylAdda; Glu, glutamic acid; (H4)Y, tetrahydrotyrosine; MC, microcystin; Mdha, N-methyldehydroalanine; Mdhb, N-methyldehydrobutyrine; ML, maximum likelihood; Nfk, N-formylkynurenine; PC-IGS, intergenic spacer and flanking regions of the cpcB and cpcA genes of the phycocyanin operon; SDS, sodium dodecylsulfate; STXs, saxitoxins; TE, tris(hydroxymethyl)aminomethane–EDTA; (H4)Y, tetrahydrotyrosine; Y(OMe), methoxytyrosine.
Supplementary data
Supplementary data (MS2 spectra extracted during LC-MS2 analysis of water from Hartbeespoort Dam, culture of M. aeruginosa strain AB2011/53, hydrolysed culture of Nostoc sp. strain 152, and microcystin standards) associated with this article can be found in the on-line version at http://dx.doi.org/10.1071/MF13153
Acknowledgements
This study was supported by grant 196085/V10 (Monitoring of Cyanotoxins in Southern Africa) from The Research Council of Norway. We thank K. Sivonen (Helsinki University, Helsinki, Finland) for providing Nostoc sp. strain 152, and IMB NRC (Halifax, NS, Canada) for the [Dha7]MC-LR standard, and J. Puddick for helpful discussions regarding oxidised analogues of MC-WR and for providing an extract containing MC-NfkR.
References
Allanson, B. R., and Gieskes, J. M. T. M. (1961). Investigations into the ecology of polluted inland waters in the Transvaal, Part II: An introduction to the limnology of Hartbeespoort Dam with special reference to the effect of industrial and domestic pollution. Hydrobiologia 18, 77–94.Ashton, P. J., Chutter, P. M., Cochrane, K. L., De Moor, F. C., Hely-Hutchinson, J. R., Jarvis, A. C., Robarts, R. D., Scott, W. E., Thornton, J. A., Twinch, A. J., Zohary, T., Bostock, L. B., Combrink, S., Fenn, T. A., Grimbeek, L. M., Herbst, H. M., Hills, M. J., Mitchell, R. F., Pais Madeira, A. M., and van Blommestein, S. D. (1985). Limnology of Hartbeespoort. National Scientific Programmes Unit: CSIR, SANSP Report 110, 1985, pp. 1– 279, http://hdl.handle.net/10204/2425.
Ballot, A., Dadheech, P. K., Haande, S., and Krienitz, L. (2008). Morphological and phylogenetic analysis of Anabaenopsis abijatae and Anabaenopsis elenkinii (Nostocales, Cyanobacteria) from tropical inland water bodies. Microbial Ecology 55, 608–618.
| Morphological and phylogenetic analysis of Anabaenopsis abijatae and Anabaenopsis elenkinii (Nostocales, Cyanobacteria) from tropical inland water bodies.Crossref | GoogleScholarGoogle Scholar | 17704858PubMed |
Ballot, A., Fastner, J., Lentz, M., and Wiedner, C. (2010a). First report of anatoxin-a-producing cyanobacterium Aphanizomenon issatschenkoi in northeastern Germany. Toxicon 56, 964–971.
| First report of anatoxin-a-producing cyanobacterium Aphanizomenon issatschenkoi in northeastern Germany.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtVyjtLvM&md5=a9ec1e9e0c9103aa9cdab9a875cdd613CAS | 20615427PubMed |
Ballot, A., Fastner, J., and Wiedner, C. (2010b). Paralytic shellfish poisoning toxin producing cyanobacterium Aphanizomenon gracile in northeast Germany. Applied and Environmental Microbiology 76, 1173–1180.
| Paralytic shellfish poisoning toxin producing cyanobacterium Aphanizomenon gracile in northeast Germany.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXisVKms78%3D&md5=e4c2cf36f4503ef2751f6408f013eab3CAS | 20048055PubMed |
Botes, D. P., Kauger, H., and Viljoen, C. C. (1982a). Isolation and characterization of four toxins from the blue-green alga, Microcystis aeruginosa. Toxicon 20, 945–954.
| Isolation and characterization of four toxins from the blue-green alga, Microcystis aeruginosa.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL3sXms1ygtw%3D%3D&md5=f4323d2346c6cb34a099cb75274f4d3aCAS | 6819659PubMed |
Botes, D. P., Viljoen, C. C., Kruger, H., Wessels, P. L., and Williams, D. H. (1982b). Configuration assignments of the amino acid residues and the presence of N-methyldehydroalanine in toxins from the blue–green alga, Microcystis aeruginosa. Toxicon 20, 1037–1042.
| Configuration assignments of the amino acid residues and the presence of N-methyldehydroalanine in toxins from the blue–green alga, Microcystis aeruginosa.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL3sXhtVaqtrc%3D&md5=0c078536135f738f0684f919d13d59adCAS | 6819658PubMed |
Botes, D. P., Tuinman, A. A., Wessels, P. L., Viljoen, C. C., and Kruger, H. (1984). The structure of cyanoginosin-LA, a cyclic heptapeptide toxin from the cyanobacterium Microcystis aeruginosa. Journal of the Chemical Society, Perkin Transactions 1: Organic and Bio-Organic Chemistry , 2311–2318.
| The structure of cyanoginosin-LA, a cyclic heptapeptide toxin from the cyanobacterium Microcystis aeruginosa.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2MXitVChtL8%3D&md5=b06386d809be80580e39f0360bd67fcfCAS |
Carmichael, W. W. (2001). Health effect of toxin-producing cyanobacteria: “The CyanoHABs” Human and Ecological Risk Assessment 7, 1393–1407.
| Health effect of toxin-producing cyanobacteria: “The CyanoHABs”Crossref | GoogleScholarGoogle Scholar |
Christiansen, G., Molitor, C., Philmus, B., and Kurmayer, R. (2008). Nontoxic strains of cyanobacteria are the result of major gene deletion events induced by a transposable element. Molecular Biology and Evolution 25, 1695–1704.
| Nontoxic strains of cyanobacteria are the result of major gene deletion events induced by a transposable element.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXpvFOjtrw%3D&md5=5081f65e68c99f08c8452f82f49917cfCAS | 18502770PubMed |
Cochrane, K. L. (1987). The biomass and yield of the dominant fish species in Hartbeespoort Dam, South Africa. Hydrobiologia 146, 89–96.
| The biomass and yield of the dominant fish species in Hartbeespoort Dam, South Africa.Crossref | GoogleScholarGoogle Scholar |
Conradie, K. R., and Barnard, S. (2012). The dynamics of toxic Microcystis strains and microcystin production in two hypertrofic South African reservoirs. Harmful Algae 20, 1–10.
| The dynamics of toxic Microcystis strains and microcystin production in two hypertrofic South African reservoirs.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhslGjsLvL&md5=6872d57c0adee7243b49d9949d74ca2aCAS |
Cronberg, G., and Annadotter, H. (2006). Manual on aquatic cyanobacteria: a photo guide and synopsis of their toxicology. International Society for the Study of Harmful Algae and United Nations Educational, Scientific and Cultural Organisation, Denmark, pp. 1–105.
Cronberg, G., and Komárek, J. (2004). Some nostocalean cyanoprokaryotes from lentic habitats of Eastern and Southern Africa. Nova Hedwigia 78, 71–106.
| Some nostocalean cyanoprokaryotes from lentic habitats of Eastern and Southern Africa.Crossref | GoogleScholarGoogle Scholar |
Diehnelt, C. W., Dugan, N. R., Peterman, S. M., and Budde, W. L. (2006). Identification of microcystin toxins from a strain of Microcystis aeruginosa by liquid chromatography introduction into a hybrid linear ion trap-fourier transform ion cyclotron resonance mass spectrometer. Analytical Chemistry 78, 501–512.
| Identification of microcystin toxins from a strain of Microcystis aeruginosa by liquid chromatography introduction into a hybrid linear ion trap-fourier transform ion cyclotron resonance mass spectrometer.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXht12rtb7F&md5=d2b945b828eda5041debe151bd769ab0CAS | 16408933PubMed |
Gugger, M., Molica, R., Le Berre, B., Dufour, P., Bernard, C., and Humbert, J. F. (2005). Genetic diversity of Cylindrospermopsis strains (Cyanobacteria) isolated from four continents. Applied and Environmental Microbiology 71, 1097–1100.
| Genetic diversity of Cylindrospermopsis strains (Cyanobacteria) isolated from four continents.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhsVKgtb8%3D&md5=84359879bff54f21b7c82b0faf6e48f1CAS | 15691973PubMed |
Guindon, S., and Gascuel, O. (2003). A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Systematic Biology 52, 696–704.
| A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood.Crossref | GoogleScholarGoogle Scholar | 14530136PubMed |
Haande, S., Rohrlack, T., Ballot, A., Røberg, K., Skulberg, R., Beck, M., and Wiedner, C. (2008). Genetic characterisation of Cylindrospermopsis raciborskii (Nostocales, Cyanobacteria) isolates from Africa and Europe. Harmful Algae 7, 692–701.
| Genetic characterisation of Cylindrospermopsis raciborskii (Nostocales, Cyanobacteria) isolates from Africa and Europe.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXovFemtLw%3D&md5=93dabd2deda28654d324ec7e09cbdd51CAS |
Hall, T. (2007). BioEdit: biological sequence alignment editor for Win95/98/NT/2K/XP [Online]. Website last modified on June 27, 2007 (accessed on September 13, 2011). Available at http://www.mbio.ncsu.edu/BioEdit/bioedit.html.
Hambright, K. D., and Zohary, T. (2000). Phytoplankton species diversity control through competitive exclusion and physical disturbances. Limnology and Oceanography 45, 110–122.
| Phytoplankton species diversity control through competitive exclusion and physical disturbances.Crossref | GoogleScholarGoogle Scholar |
Harding, W. R., Thornton, J. A., Steyn, G., Panuska, J., and Morrison, I. R. (2004). Hartbeespoort Dam Remediation Project (Phase 1) Final Report (Volume I). Available at http://www.dwaf.gov.za/Harties/documents/ActionPlanVol1Oct04full.pdf [accessed 5 November 2012]
Hawkins, P. R., Chandrasena, N. R., Jones, G. J., Humpage, A. R., and Falconer, I. R. (1997). Isolation and toxicity of Cylindrospermopsis raciborskii from an ornamental lake. Toxicon 35, 341–346.
| Isolation and toxicity of Cylindrospermopsis raciborskii from an ornamental lake.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXhsl2msb8%3D&md5=f8b55edac4d5c62f31cc8caa1fd23dedCAS | 9080590PubMed |
Hepperle, D. (2008). Align vers. 07/2008, multisequence alignment-editor and preparation/manipulation of phylogenetic datasets. Error!Hyperlink reference not valid. 2009]
Hitzfeld, B. C., Höger, S. J., and Dietrich, D. R. (2000). Cyanobacterial toxins: removal during drinking water treatment, and human risk assessment. Environmental Health Perspectives 108, 113–122.
| 1:CAS:528:DC%2BD3cXit12rsb0%3D&md5=470de4ded328f9ce7dfaf08e869d65f3CAS | 10698727PubMed |
Horecká, M., and Komárek, J. (1979). Taxonomic position of three planktonic blue–green algae from the genera Aphanizomenon and Cylindrospermopsis. Preslia 51, 289–312.
Janse van Vuuren, S., and Kriel, G. P. (2008). Cylindrospermopsis raciborskii, a toxic invasive cyanobacterium in South African fresh waters. African Journal of Aquatic Science 33, 17–26.
| Cylindrospermopsis raciborskii, a toxic invasive cyanobacterium in South African fresh waters.Crossref | GoogleScholarGoogle Scholar |
Komárek, J., and Anagnostidis, K. (1998) Cyanoprokaryota I. Teil: Chroococcales, In ‘Süsswasserflora von Mitteleuropa 19/1’. (Eds. H. Ettl, G. Gärtner G, H. Heynig and D. Mollenhauer.) (Spektrum Akademischer Verlag.)
Komárek, J., and Komárkova, J. (2006). Diversity of Aphanizomenon-like cyanobacteria. Czech Phycology 6, 1–32.
Kotai, J. 1972. Instructions for Preparation of Modified Nutrient Solution Z8 for Algae. Publication B-11/69. Norwegian Institute for Water Research, Oslo, Norway.
Kurmayer, R., Christiansen, G., Fastner, J., and Börner, T. (2004). Abundance of active and inactive microcystin genotypes in populations of the toxic cyanobacterium Planktothrix spp. Environmental Microbiology 6, 831–841.
| Abundance of active and inactive microcystin genotypes in populations of the toxic cyanobacterium Planktothrix spp.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXntVKisbw%3D&md5=98eed1205a07af05867df3747a00aecaCAS | 15250885PubMed |
Li, R., and Watanabe, M. M. (1999). Anabaena eucompacta sp. nov. (Nostocales, Cyanobacteria), a new planktonic species with tightly spiraled filaments from Japan. Bulletin of the National Science Museum, Tokyo Serie B 25, 89–94.
Li, R., Carmichael, W. W., Brittain, S., Eaglesham, G. K., Shaw, G. R., Mahakhant, A., Noparatnaraporn, N., Yongmanitchai, W., Kaya, K., and Watanabe, M. M. (2001). Isolation and identification of the cyanotoxin cylindrospermopsin and deoxy-cylindrospermopsin from a Thailand strain of Cylindrospermopsis raciborskii (Cyanobacteria). Toxicon 39, 973–980.
| Isolation and identification of the cyanotoxin cylindrospermopsin and deoxy-cylindrospermopsin from a Thailand strain of Cylindrospermopsis raciborskii (Cyanobacteria).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXit1ars74%3D&md5=3b9e18487cd1081e52b605e249dcc391CAS | 11223086PubMed |
Mbukwa, E. A., Msagati, T. A. M., and Mamba, B. B. (2012). Quantitative variations of intracellular microcystin-LR, -RR and -YR in samples collected from four locations in Hartbeespoort Dam in North West Province (South Africa) during the 2010/2011 summer season. International Journal of Environmental Research and Public Health 9, 3484–3505.
| Quantitative variations of intracellular microcystin-LR, -RR and -YR in samples collected from four locations in Hartbeespoort Dam in North West Province (South Africa) during the 2010/2011 summer season.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xhs1WhurrL&md5=7b42a7b21cb32c94ef921b35c21a2719CAS | 23202758PubMed |
McGregor, G. B., and Fabbro, L. D. (2000). Dominance of Cylindrospermopsis raciborskii (Nostocales, Cyanoprokaryota) in Queensland tropical and subtropical reservoirs: implications for monitoring and management. Lakes and Reservoirs: Research and Management 5, 195–205.
| Dominance of Cylindrospermopsis raciborskii (Nostocales, Cyanoprokaryota) in Queensland tropical and subtropical reservoirs: implications for monitoring and management.Crossref | GoogleScholarGoogle Scholar |
Mihali, T. K., Kellmann, R., Muenchhoff, J., Barrow, K. D., and Neilan, B. A. (2008). Characterization of the gene cluster responsible for cylindrospermopsin biosynthesis. Applied and Environmental Microbiology 74, 716–722.
| Characterization of the gene cluster responsible for cylindrospermopsin biosynthesis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhvVSqu7o%3D&md5=63bf24bb496bf0fde72e2b4d42f9d533CAS | 18065631PubMed |
Mikalsen, B., Boison, G., Skulberg, O. M., Fastner, J., Davies, W., Gabrielsen, T. M., Rudi, K., and Jakobsen, K. S. (2003). Natural variation in the microcystin synthetase operon mcy ABC and impact on microcystin production in Microcystis strains. Journal of Bacteriology 185, 2774–2785.
| Natural variation in the microcystin synthetase operon mcy ABC and impact on microcystin production in Microcystis strains.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXjt1Gmsrs%3D&md5=8e7a3ac8fe5698d62a2a0e16ba80f691CAS | 12700256PubMed |
Miles, C. O., Sandvik, M., Nonga, H. E., Rundberget, T., Wilkins, A. L., Rise, F., and Ballot, A. (2012). Thiol derivatization for LC-MS identification of microcystins in complex matrices. Environmental Science & Technology 46, 8937–8944.
| Thiol derivatization for LC-MS identification of microcystins in complex matrices.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhtFSju77K&md5=46df2fcb7ab60ff5c1bb04721d6cf561CAS |
Miles, C. O., Sandvik, M., Haande, S., Nonga, H. E., and Ballot, A. (2013a). LC-MS analysis with thiol derivatization to differentiate [Dhb7]- from [Mdha7]-microcystins: analysis of cyanobacterial blooms, Planktothrix cultures and European crayfish from Lake Steinsfjorden, Norway. Environmental Science & Technology 47, 4080–4087.
| LC-MS analysis with thiol derivatization to differentiate [Dhb7]- from [Mdha7]-microcystins: analysis of cyanobacterial blooms, Planktothrix cultures and European crayfish from Lake Steinsfjorden, Norway.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXkslahtrk%3D&md5=f1f134eaef2a8ff85c8bf83d6f80c1d6CAS |
Miles, C. O., Sandvik, M., Nonga, H. E., Rundberget, T., Wilkins, A. L., Rise, F., and Ballot, A. (2013b). Identification of microcystins in a Lake Victoria cyanobacterial bloom using LC-MS with thiol derivatization. Toxicon 70, 21–31.
| Identification of microcystins in a Lake Victoria cyanobacterial bloom using LC-MS with thiol derivatization.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXpsVCgsrk%3D&md5=c90fffcce16150bbb7b4970683162378CAS | 23567039PubMed |
Mohamed, Z. A. (2007). First report of toxic Cylindrospermopsis raciborskii and Raphidiopsis mediterranea (Cyanoprokaryota) in Egyptian freshwaters. FEMS Microbiology Ecology 59, 749–761.
| First report of toxic Cylindrospermopsis raciborskii and Raphidiopsis mediterranea (Cyanoprokaryota) in Egyptian freshwaters.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXjsV2jsrs%3D&md5=39555061f4c6af6e175cd77787fabbdbCAS | 17069621PubMed |
Moustafa, A., Loram, J. E., Hackett, J. D., Anderson, D. M., Plumley, F. G., and Bhattacharya, D. (2009). Origin of saxitoxin biosynthetic genes in cyanobacteria. PLoS ONE 4, e5758.
| Origin of saxitoxin biosynthetic genes in cyanobacteria.Crossref | GoogleScholarGoogle Scholar | 19484122PubMed |
Moustaka-Gouni, M., Kormas, K. A., Vardaka, E., Katsiapi, M., and Gkelis, S. (2009). Raphidiopsis mediterranea Skuja represents non-heterocytous life-cycle stages of Cylindrospermopsis raciborskii (Woloszynska) Seenayya et Subba Raju in Lake Kastoria (Greece), its type locality: evidence by morphological and phylogenetic analysis. Harmful Algae 8, 864–872.
| Raphidiopsis mediterranea Skuja represents non-heterocytous life-cycle stages of Cylindrospermopsis raciborskii (Woloszynska) Seenayya et Subba Raju in Lake Kastoria (Greece), its type locality: evidence by morphological and phylogenetic analysis.Crossref | GoogleScholarGoogle Scholar |
Namikoshi, M., Rinehart, K. L., Sakai, R., Sivonen, K., and Carmichael, W. W. (1990). Structures of three new cyclic heptapeptide hepatotoxins produced by the cyanobacterium (blue–green alga) Nostoc sp. strain 152. The Journal of Organic Chemistry 55, 6135–6139.
| Structures of three new cyclic heptapeptide hepatotoxins produced by the cyanobacterium (blue–green alga) Nostoc sp. strain 152.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3MXnt1Sj&md5=5bc869a3d7f469b39052ae367a48a0fbCAS |
Namikoshi, M., Murakamia, T., Watanabe, M. F., Oda, T., Yamada, J., Tsujimura, S., Nagaia, H., and Oishi, S. (2003). Simultaneous production of homoanatoxin-a, anatoxin-a, and a new non-toxic 4-hydroxyhomoanatoxin-a by the cyanobacterium Raphidiopsis mediterranea Skuja. Toxicon 42, 533–538.
| Simultaneous production of homoanatoxin-a, anatoxin-a, and a new non-toxic 4-hydroxyhomoanatoxin-a by the cyanobacterium Raphidiopsis mediterranea Skuja.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXnsF2ksrc%3D&md5=15d021c6a2eb49671dad82b46ec49bd0CAS | 14529735PubMed |
Neffling, M. R. (2010). Fast LC-MS detection of cyanobacterial peptide hepatotoxins-method development for determination of total contamination levels in biological materials. Ph.D. Thesis, Åbo Akademi University, Turku, Finland.
Neilan, B. A., Jacobs, D., and Goodman, A. (1995). Genetic diversity and phylogeny of toxic cyanobacteria determined by DNA polymorphisms within the phycocyanin locus. Applied and Environmental Microbiology 6, 3875–3883.
Noguchi, T., Shinohara, A., Nishizawa, A., Asayama, M., Nakano, T., Hasegawa, M., Harada, K., Nishizawa, T., and Shirai, M. (2009). Genetic analysis of the microcystin biosynthesis gene cluster in Microcystis strains from four bodies of eutrophic water in Japan. The Journal of General and Applied Microbiology 55, 111–123.
| Genetic analysis of the microcystin biosynthesis gene cluster in Microcystis strains from four bodies of eutrophic water in Japan.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXlsFyqsrw%3D&md5=0b32a64e9a6c1f2ba8c7a4e7e6863ff7CAS | 19436128PubMed |
Oberholster, P. J., and Botha, A. M. (2010). Use of remote sensing and molecular markers to detect toxic cyanobacterial hyperscum crust: a case study on Lake Hartbeespoort, South Africa. African Journal of Biotechnology 9, 8791–8799.
| 1:CAS:528:DC%2BC3MXhtlOktw%3D%3D&md5=043ee3e7fb3ddccf3d834eac1b9b6fcbCAS |
Paerl, H. W., and Huisman, J. (2009). Climate change: a catalyst for global expansion ofharmful cyanobacterial blooms. Environmental Microbiology Reports 1, 27–37.
| Climate change: a catalyst for global expansion ofharmful cyanobacterial blooms.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXotlaktrk%3D&md5=fee780fa191e2ec135e98b20f76fdc01CAS | 23765717PubMed |
Posada, D. (2008). jModelTest: phylogenetic model averaging. Molecular Biology and Evolution 25, 1253–1256.
| jModelTest: phylogenetic model averaging.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXotlKgsb4%3D&md5=6aff26d0e412326d9e17b06a56932ff6CAS | 18397919PubMed |
Puddick, J. (2012). Spectroscopic investigations of oligopeptides from aquatic cyanobacteria. Ph.D. Thesis, University of Waikato, Hamilton, New Zealand.
Puddick, J., Prinsep, M. R., Wood, S. A., Miles, C. O., Rise, F., Cary, S. C., Hamilton, D. P., and Wilkins, A. L. (2013). Structural characterization of 40 new microcystins containing tryptophan and oxidized tryptophan residues. Marine Drugs 11, 3025–3045.
| Structural characterization of 40 new microcystins containing tryptophan and oxidized tryptophan residues.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhsVWhs7nN&md5=f06a62c1824e2b2dc40bd9e5bc1d8107CAS | 23966035PubMed |
Rantala, A., Fewer, D. P., Hisbergues, M., Rouhiainen, L., Vaitomaa, J., Börner, T., Sivonen, K., and Sill, K. (2004). Phylogenetic evidence for the early evolution of microcystin synthesis. Proceedings of the National Academy of Sciences 101, 568–573.
| 1:CAS:528:DC%2BD2cXmsFGntQ%3D%3D&md5=78cdb19fdd080a7904947859b15a3f5eCAS |
Rasmussen, J. P., Giglio, S., Monis, P. T., Campbell, R. J., and Saint, C. P. (2008). Development and field testing of a real-time PCR assay for cylindrospermopsin producing cyanobacteria. Journal of Applied Microbiology 104, 1503–1515.
| Development and field testing of a real-time PCR assay for cylindrospermopsin producing cyanobacteria.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXmvVCntr4%3D&md5=7653fa0668bc52d6aa21c00fbd90c4d6CAS | 18179541PubMed |
Rourke, W. A., Murphy, C. J., Pitcher, G., van de Riet, J. M., Burns, B. G., Thomas, K. M., and Quilliam, M. A. (2008). Rapid postcolumn methodology for determination of paralytic shellfish toxins in shellfish tissue. Journal of AOAC International 91, 589–597.
| 1:CAS:528:DC%2BD1cXntlWqtLo%3D&md5=1edfaf3d32ced027702a5360924797d8CAS | 18567305PubMed |
Saker, M. L., and Neilan, B. A. (2001). Varied diazotrophies, morphologies, and toxicities of genetically similar isolates of Cylindrospermopsis raciborskii (Nostocales, Cyanophyceae) from Northern Australia. Applied and Environmental Microbiology 67, 1839–1845.
| Varied diazotrophies, morphologies, and toxicities of genetically similar isolates of Cylindrospermopsis raciborskii (Nostocales, Cyanophyceae) from Northern Australia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXis1egtb0%3D&md5=73d1285e857dedd7e89a41d8ac9969e8CAS | 11282641PubMed |
Saqrane, S., and Oudra, B. (2009). CyanoHAB occurrence and water irrigation cyanotoxin contamination: ecological impacts and potential health risks. Toxins 1, 113–122.
| CyanoHAB occurrence and water irrigation cyanotoxin contamination: ecological impacts and potential health risks.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXpsl2kug%3D%3D&md5=502c146361f67e73fa935e372a992ae1CAS | 22069535PubMed |
Sivonen, K., and Jones, G. (1999). Cyanobacterial toxins. In ‘Toxic Cyanobacteria in Water: a Guide to Public Health Significance, Monitoring and Management. The World Health Organization’. (Eds I. Chorus and J. Bertram.) pp. 41–111. (E. and F.N. Spon: London, UK.).
Soto-Liebe, K., Murillo, A., Krock, B., Stucken, K., Fuentes-Valdés, J. J., Trefault, N., Cembella, A., and Vásquez, M. (2010). Reassessment of the toxin profile of Cylindrospermopsis raciborskii T3 and function of putative sulfotransferases in synthesis of sulfated and sulfonated PSP toxins. Toxicon 56, 1350–1361.
| Reassessment of the toxin profile of Cylindrospermopsis raciborskii T3 and function of putative sulfotransferases in synthesis of sulfated and sulfonated PSP toxins.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtlGntb%2FJ&md5=eb53e1a3700d96538b72a3bc698abb1dCAS | 20692275PubMed |
Steyn, D. J., Toerien, D. F., and Visser, J. H. (1975). Eutrophication levels of some South African empoundments. II Hartbeespoort Dam. Water S.A. 1, 93–101.
| 1:CAS:528:DyaE2sXkt1ylu78%3D&md5=67188c071a809148e8da4d7ecd9f143fCAS |
Stucken, K., John, U., Cembella, A., Murillo, A. A., Soto-Liebe, K., Fuentes-Valdes, J. J., Friedel, M., Plominsky, A. M., Vasquez, M., and Glöckner, G. (2010). The smallest known genomes of multicellular and toxic cyanobacteria: comparison, minimal gene sets for linked traits and the evolutionary implications. PLoS ONE 5, e9235.
| The smallest known genomes of multicellular and toxic cyanobacteria: comparison, minimal gene sets for linked traits and the evolutionary implications.Crossref | GoogleScholarGoogle Scholar | 20169071PubMed |
Sukenik, A., Hadas, O., Kaplan, A., and Quesada, A. (2012). Invasion of Nostocales (cyanobacteria) to subtropical and temperate freshwater lakes–physiological, regional,and global driving forces. Frontiers in Microbiology 3, 86.
| Invasion of Nostocales (cyanobacteria) to subtropical and temperate freshwater lakes–physiological, regional,and global driving forces.Crossref | GoogleScholarGoogle Scholar | 22408640PubMed |
Swofford, D. L. (2002). ‘PAUP*: phylogenetic analysis using parsimony (* and other methods), version 4.0 b10.’ (Sinauer: Sunderland, MA.)
Thomazeau, S., Houdan-Fourmont, A., Couté, A., Duval, C., Couloux, A., Rousseau, F., and Bernard, C. (2010). The contribution of sub-saharan African strains to the phylogeny of cyanobacteria: focusing on the Nostocaceae (Nostocales, cyanobacteria). Journal of Phycology 46, 564–579.
| The contribution of sub-saharan African strains to the phylogeny of cyanobacteria: focusing on the Nostocaceae (Nostocales, cyanobacteria).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXovFCmu7w%3D&md5=1100ed3b627f2db94ed30a1ddc953f58CAS |
Tillett, D., Dittmann, E., Erhard, M., von Döhren, H., Börner, T., and Neilan, B. A. (2000). Structural organization of microcystin biosynthesis in Microcystis aeruginosa PCC7806: an integrated peptide-polyketide synthetase system. Chemistry & Biology 7, 753–764.
| Structural organization of microcystin biosynthesis in Microcystis aeruginosa PCC7806: an integrated peptide-polyketide synthetase system.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXotVWitrw%3D&md5=3c8dfce5a9ad5769950f7743391ee704CAS |
Toerien, D. F., Scott, W. E., and Pitout, M. J. (1976). Microcystis toxins, isolation, identification, implications. Water S.A. 2, 160–162.
| 1:CAS:528:DyaE2sXhvVChurw%3D&md5=c72cd5e331763330104646e13992b73eCAS |
Tooming-Klunderud, A., Fewer, D. P., Rohrlack, T., Jokela, J., Rouhiainen, L., Sivonen, K., Kristensen, T., and Jakobsen, K. S. (2008). Evidence for positive selection acting on microcystin synthetase adenylation domains in three cyanobacterial genera. BMC Evolutionary Biology 8, 256.
| Evidence for positive selection acting on microcystin synthetase adenylation domains in three cyanobacterial genera.Crossref | GoogleScholarGoogle Scholar | 18808704PubMed |
Van Ginkel, C. E. (2003). A National Survey of the incidence of cyanobacterial blooms and toxin production in major impoundments. Internal Report No. N/0000/00/DEQ/0503. Resource Quality Services, Department of Water Affairs and Forestry. Pretoria, South Africa.
Watanabe, M. (1995). Studies on planctonic blue–green algae 5. A new species of Cylindrospermopsis (Nostocaceae) from Japan. Bulletin of the National Science Museum. Tokyo Serie B 21, 45–48.
Water Research Commission (2008). ‘SA’s Water History—Taming the Poort.’ Water Research Commission. 2008–06. http://www.ewisa.co.za/misc/DamNWHartebeespoort/Harties%20history%20WW%20May-June%2008.pdf [accessed 12 December 2011]
Werner, V. R., Laughinghouse, H. D., Fiore, M. F., Sant’Anna, C. L., Hoff, C., Santos, K. R. D., Neuhaus, E. B., Molica, R. J. R., Honda, R. Y., and Echenique, R. O. (2012). Morphological and molecular studies of Sphaerospermopsis torques-reginae (Cyanobacteria, Nostocales) from South American water blooms. Phycologia 51, 228–238.
| Morphological and molecular studies of Sphaerospermopsis torques-reginae (Cyanobacteria, Nostocales) from South American water blooms.Crossref | GoogleScholarGoogle Scholar |
Wicks, R. J., and Thiel, P. G. (1990). Environmental factors affecting the production of peptide toxins in floating scums of the cyanobacterium Microcystis aeruginosa in a hypertrophic African reservoir. Environmental Science & Technology 24, 1413–1418.
| Environmental factors affecting the production of peptide toxins in floating scums of the cyanobacterium Microcystis aeruginosa in a hypertrophic African reservoir.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3cXlslOkur8%3D&md5=77a16fac51206811fd484fbcd23e16bfCAS |
Wood, S. A., Rasmussen, J. P., Holland, P. T., Campbell, R., and Crowe, A. L. M. (2007). First report of the cyanotoxin anatoxin-a from Aphanizomenon issatschenkoi (Cyanobacteria). Journal of Phycology 43, 356–365.
| First report of the cyanotoxin anatoxin-a from Aphanizomenon issatschenkoi (Cyanobacteria).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXltVKlsrs%3D&md5=fe042675391f5089786d500d2108f242CAS |
World Health Organization (1998). ‘Guidelines for Drinking-water Quality.’ 2nd edn. Addendum to volume 2, Health criteria and other supporting information. (World Health Organization: Geneva.)
Zapomělová, E., Hrouzek, P., Řeháková, K., Šabacká, M., Stibal, M., Caisová, L., Komárková, J., and Lukešová, A. (2008). Morphological variability in selected heterocystous cyanobacterial strains as a response to varied temperature, light intensity and medium composition. Folia Microbiologica 53, 333–341.
| Morphological variability in selected heterocystous cyanobacterial strains as a response to varied temperature, light intensity and medium composition.Crossref | GoogleScholarGoogle Scholar | 18759118PubMed |
Zapomělová, E., Jezberová, J., Hrouzek, P., Hisem, D., Řeháková, K., and Komárková, J. (2009). Polyphasic characterization of three strains of Anabaena reniformis and Aphanizomenon aphanizomenoides (Cyanobacteria) and their reclassification to Sphaerospermum gen. nov. (incl. Anabaena kisseleviana). Journal of Phycology 45, 1363–1373.
| Polyphasic characterization of three strains of Anabaena reniformis and Aphanizomenon aphanizomenoides (Cyanobacteria) and their reclassification to Sphaerospermum gen. nov. (incl. Anabaena kisseleviana).Crossref | GoogleScholarGoogle Scholar |
Zapomělová, E., Jezberová, J., Hrouzek, P., Hisem, D., Řeháková, K., and Komárková, J. (2010). Polyphasic characterization of three strains of Anabaena reniformis and Aphanizomenon aphanizomenoides (Cyanobacteria) and their reclassification to Sphaerospermum gen. nov. (incl. Anabaena kisseleviana). Journal of Phycology 46, 415.
| Polyphasic characterization of three strains of Anabaena reniformis and Aphanizomenon aphanizomenoides (Cyanobacteria) and their reclassification to Sphaerospermum gen. nov. (incl. Anabaena kisseleviana).Crossref | GoogleScholarGoogle Scholar |
Zohary, T. (1985). Hyperscums of the cyanobacterium Microcystis aeruginosa in a hypertrophic lake (Hartbeespoort Dam, South Africa). Journal of Plankton Research 7, 399–409.
| Hyperscums of the cyanobacterium Microcystis aeruginosa in a hypertrophic lake (Hartbeespoort Dam, South Africa).Crossref | GoogleScholarGoogle Scholar |
Zohary, T., and Pais-Madeira, A. M. (1990). Structural, physical and chemical characteristics of Microcystis aeruginosa hyperscums from a hypertrophic lake. Freshwater Biology 23, 339–352.
| Structural, physical and chemical characteristics of Microcystis aeruginosa hyperscums from a hypertrophic lake.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3cXlsVyrsrs%3D&md5=a10130710479afa360ef8c897909df1dCAS |



