Flow variability and longitudinal characteristics of organic carbon in the Lachlan River, Australia
Nicholas P. Moran A B D , George G. Ganf A , Todd A Wallace A C and Justin D. Brookes AA School of Earth and Environmental Science, The University of Adelaide, SA 5000, Australia.
B School of Biological Sciences, Monash University, Clayton, Vic. 3800, Australia.
C The Murray–Darling Freshwater Research Centre, Mildura Laboratory, La Trobe University, Mildura, Vic. 3052, Australia.
D Corresponding author. Email: Nicholas.Moran@Monash.edu
Marine and Freshwater Research 65(1) 50-58 https://doi.org/10.1071/MF12297
Submitted: 16 October 2012 Accepted: 14 June 2013 Published: 20 September 2013
Abstract
Heterotrophic organic-carbon cycling is a major source of energy to aquatic food webs, yet there are few studies into patterns of heterotrophic productivity in large lowland rivers. The Lachlan River experienced a period of extreme flow variability from September 2010 to February 2011; for example, daily discharge (ML day–1) at one site reached >22 times its 10-year average. Heterotrophic cycling of dissolved organic carbon (DOC) and particulate organic carbon (POC) were assessed over this period at six sites on the Lachlan River. Concentrations of total organic carbon (TOC) ranged from 7 to 30 mg L–1, of which the majority was in dissolved form. Concentration of DOC was positively correlated with daily discharge. Biochemical oxygen demand of TOC over 5 days (BOD5) showed significant variability, ranging from 0.6 to 6.6 mg O2 L–1. BOD5 did not appear related to discharge, but instead to a range of other factors, including regulation via weirs, lateral and longitudinal factors. Partitioning of DOC and POC showed that POC had an influence on BOD5 comparable to DOC. This is relevant to environmental-flow management in the Lachlan River, the Murray–Darling Basin and rivers generally, by showing that flow variability influences a fundamental ecosystem characteristic, namely organic carbon.
Additional keywords: biogeochemistry, catchment management, flow regulation, Murray–Darling system, organic matter.
Introduction
Organic carbon as an energy source is a key component of riverine ecosystems whether this is via longitudinal and riparian inputs (Vannote et al. 1980), lateral and floodplain connectivity (Junk et al. 1989), vertical connectivity (Boulton et al. 2010) or in-stream autotrophic productivity (Thorp and Delong 1994). Studies conducted globally have reported that carbon dynamics and hydrological variability are closely linked because of interactions between flow rate and organic-matter exchanges across floodplains, riparian zones and sediments (e.g. South American, Petry et al. 2003; Australian, Westhorpe and Mitrovic 2012; North American, Dalzell et al. 2007). Therefore, to characterise patterns of organic carbon, their sources and variability should be analysed across a range of hydrological conditions (Tank et al. 2010).
Australian lowland rivers generally have high flow variability relative to river systems globally (Puckridge et al. 1998) and are intensely regulated (Thoms and Sheldon 2000). Given the hydrological variability, it has been hypothesised that floodplain-derived carbon is an important source of energy for aquatic food webs; however, regulation has limited the contribution of floodplain-derived carbon (Gawne et al. 2007; Robertson et al. 1999). Recent studies have found that autochthonous carbon plays a more significant role than predicted in riverine primary productivity (Oliver and Merrick 2006). For example, in three eastern Australian rivers, Hadwen et al. (2010) found that the heterotrophic community was DOC limited and the ambient DOC concentration was low and generally recalcitrant. In the regionally significant Murrumbidgee River, Vink et al. (2005) found that the ecosystem was predominantly heterotrophic during a period of in-channel flow but there was a trend of increasing autotrophic activity downstream. Additionally, Gawne et al. (2007) found that both heterotrophic and autotrophic productivity were significant contributors to the productivity of the Murray River but that this varied seasonally. These studies have largely taken place under extended low-flow conditions (Bond, Lake et al. 2008); however, recent floods in New South Wales, Australia, provided the opportunity to assess carbon dynamics under both low and high flows.
The influence of total organic carbon (TOC) is not only related to flow but also to its concentration, composition and bioavailability (Cleveland et al. 2004; Wallace et al. 2008). The dissolved fraction (DOC) is commonly the larger fraction by an ~10 : 1 ratio, so is considered a more important source of energy to heterotrophic bacteria than the particulate organic fraction (POC) (Volk et al. 1997; Wetzel 2001). Within the DOC fraction, bioavailability is related to the composition and the proportions of functional groups present, such as humic substances and hydrophilic acids, which are influenced by a broad range of factors (Qualls 2005). This includes the type and age of leaf litter, the rate of leaching DOC from litter (Baldwin 1999) and its bioavailability, the amount of litter and debris that has accumulated before flooding, whether or not the litter has undergone previous flooding and the extent and timing of rainfall or flooding (Whitworth et al. 2012). Interactive effects between organic matter and floodplain soil systems are also factors influencing DOC composition (Cleveland et al. 2004). Standardised bioassays can be used to assess the biochemical oxygen demand (BOD) and the biodegradability of DOC, POC and TOC generated by heterotrophic metabolism. The addition of nutrients and or labile carbon can help diagnose whether or not carbon metabolism is limited by nutrient availability and/or the presence of active heterotrophic bacteria. This technique was used to address the hypothesis that although DOC concentration will be related to flow, bioavailability will depend on the source of the carbon.
Recently, reinstating a natural (i.e. more variable) hydrograph through environmental flows has become a priority to achieve favourable ecosystem outcomes, such as supporting sustainable fisheries and ecologically significant wetlands (Walker et al. 1995; Kingsford 2011). This was recommended in the Murray–Darling Basin and specifically in the Lachlan River where a 44–67 GL year-1 increase in environmental allocations has been proposed (Murray–Darling Basin Authority 2010). However, environmental flows may also have a downside if the organic carbon is readily bioavailable, which may lead to hypoxia, fish deaths and other undesirable water-quality characteristics (Howitt et al. 2007; Hladyz et al. 2011).
Materials and methods
Catchment and site description
The Lachlan River catchment (85 532 km2) flows west from the Great Dividing Range in New South Wales (NSW), generally terminating in the Great Cumbung Swamp. The climate is temperate, with rainfall averaging 370 and 780 mm per year in the western and eastern reaches, respectively (CSIRO 2008). The rainfall occurs predominantly in winter (June–August), leading to highly variable seasonal flow (Kemp 2010). Flows are highly regulated with 14 weirs plus the Wyangala Dam (~301 GL), which is located in the upper catchment. The influence of Wyangala Dam often delays peak flows in the western lowlands to July–October (McMahon and Finlayson 2003).
Study sites (S1–S6) were distributed longitudinally across a ~225-km (point-to-point) stretch of the Lachlan River, from Condobolin (upstream) to Booligal (downstream) (Fig. 1). This western lowland region is characterised by a shallow gradient and majority agricultural land use (Kemp 2010). There is a trend of decreasing channel size and average flow from upstream to downstream sites; for example, bankfull width is 48 m at Condobolin (S1) and 27 m at Booligal (S6) (Kemp 2010). This is largely due to the natural diversion of water to distributaries that do not return to the main channel. At all sites, the riparian vegetation consists of sclerophyll forest dominated by river red gums (Eucalyptus camaldulensis) and river-adjacent land use is overwhelmingly agricultural.

|
Locations for sampling are natural pools that maintain a 3–5-m depth in low-flow (~150 ML day–1, ~1.74 m3 s–1) conditions, with shallow (~1.0–1.5 m) upstream and downstream runs. This ensured that sites did not completely dry up during the study period. Sites are evenly distributed between ‘weir pool’ sites immediately upstream of weir pools (S1, S3, S6) and free-flowing sites located outside of the direct influence of weirs (S2, S4, S5). The distribution of sample sites was designed to account for possible longitudinal variability in heterotrophic metabolism and the characteristics of the organic carbon pool (Kelly 2001; Stanford and Ward 2001).
Field sampling
Sampling occurred during 21–23 September 2010, 18–21 January 2011 and 21–23 February 2011. Water samples were collected with a Schindler trap (2 L) from a boat anchored at three locations per site (approximately midstream, 50–100 m apart), giving three replicate samples. At each location, three trap samples were mixed to give a depth-integrated composite sample, one each from the top (0.3 m below the air-water interface), middle (approximated from river depth) and bottom (0.3 m above the sediment–water interface) of the water column.
Samples (~1.2 L) in opaque jars were immediately transferred to an insulated cool box (esky) and stored in the dark until transferred (typically within 30 min of sample collection) to a portable refrigerator at ~4°C. Samples for filtered treatments were pre-filtered through pre-washed Whatman GF/C (Whatman International, Maidstone, Kent, UK) filters at the end of each sampling day. All sample jars were subsequently placed in salted ice-slurry for 3–4-day transport to the laboratory. At the laboratory, samples were subsequently filtered through pre-washed Millipore PVDF 0.45 µm membrane filters (Merck Millipore, Billerica, Massachusetts, USA). This excluded most particulate material but retained dissolved carbon and nutrients within the filtrate. The pore size, 0.45 μm, was chosen so as to maintain consistency with previous studies and datasets from regionally relevant river systems.
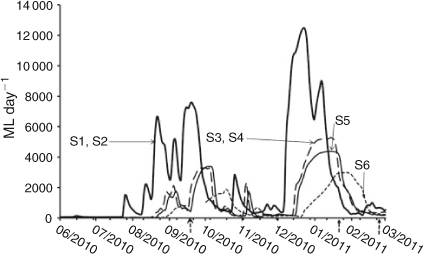
Discharge data (ML day–1, 1 ML day–1 = 0.01157 m3 s–1) were taken from the nearest gauging station to each site from the New South Wales State Government (State Water, http://waterinfo.nsw.gov.au, accessed 30th April 2011; Fig. 2). Under low flow conditions, the weirs between S1/S2 and S3/S4 would provide a significant hydrological disparity in water-level variability and flow rates between these sites, but their influence on discharge data was considered negligible, given the generally high flow and relatively strong (given the structural barrier) longitudinal connectivity during sampling. The ability to make direct comparisons between sites on the basis of discharge is limited because the sampling occurred at different positions on their respective hydrographs and because several sites share a gauging station.
|
The S6 gauging station was offline between 15 September 2010 and 1 October 2010. Discharge on the 23 September 2010 sampling period was approximated by averaging the daily discharge from the date immediately before (15 September 2010) and after (1 October 2010) the disruption.
Laboratory bioassays – nutrient, carbon addition
Standardised bioassays based on a modification of the American Public Health Association (APHA) Standard Method (5210B) 5-day test (BOD5; Eaton and Franson 2005) were used to assess the biodegradability of the DOC and TOC and the BOD generated by heterotrophic metabolism. A nitrification inhibitor, 2-chloro-6-(trichloro methyl) pyridine (10 µg L–1), was used to ensure that BOD was not influenced by nitrogenous oxygen demand from the biological oxidation of reduced forms of nitrogen (ammonia, organic nitrogen). Bioassays undertaken with this nitrification inhibitor are typically referred to as carbonaceous biochemical oxygen demand (CBOD). The influence of temperature and autotrophic metabolism were minimised by conducting bioassays at 20°C in the dark (standard conditions). To ensure the presence of a heterotrophic community, all samples were seeded with 20 mL L–1 of unfiltered water collected from the Lachlan River (S6) that had been stored in a jar in salted ice slurry for 2–3 days.
September 2010 samples were analysed using independent incubation bottles (310 mL Wheaton BOD bottles; Wheaton Industries, Millville, NJ, USA) for Day-0 and Day-5 time steps to minimise issues associated with pseudo-replication and repeated measures. Samples were diluted using reverse-osmosis (RO) water acclimatised for 24 h at 20°C to 60%, i.e. a sample to RO (including additions) ratio of 60 : 40. Samples were diluted to avoid reaching anoxia within the 5-day period and O2 (cf. organic carbon) becoming the limiting factor. The treatments included the following:
-
filtered with no nutrient or carbon addition,
-
filtered with addition of NaNO3 and K2HPO4 to 500 µg N L–1 and 50 µg P L–1,
-
filtered with addition of β-Glucose to 1000 µg C L–1, and
-
controls: seed/dilution water, dilution water.
The focus of the September bioassays was to test whether the availability of labile carbon or nutrient concentrations were limiting heterotrophic metabolism of DOC.
Laboratory bioassays – DOC/POC partitioning and leachate addition
Unlike the nutrient-addition bioassays, January and February 2011 treatments were designed to assess how the heterotrophic community would respond to allochthonous carbon inputs and also how BOD and carbon concentration were partitioned between POC and DOC fractions. A leachate was prepared from floodplain litter collected from six litter bins suspended above the floodplain adjacent (<10m) to the main channel at S4 and S5. Litter collected from January 2011 accumulated between 28 October 2010 and 19 January 2011, and litter from February 2011 accumulated between 19 January 2011 and 22 February 2011 at S4 and 19 January 2011 and 23 February 2011 at S5. The collected material was air-dried and 40 g of intact litter (including bark, twigs, leaves randomly selected by hand) was leached in 20 L of RO water for 6 h at 20°C. Leachate was screened with 45-µm mesh before use. Although this does not reflect all possible allochthonous carbon sources, this does reflect one typical allochthonous input that would occur across the study sites.
January and February samples were not diluted before the bioassays, given the relatively low BOD results of the September samples. The same bottle was sampled on Days 0, 1, 2, 3, 5. Treatments included the following:
-
filtered with no addition,
-
unfiltered with no addition,
-
unfiltered, 5% (15.5 mL) leachate addition, and
-
controls: dilution water; seed with dilution water; leachate and seed with dilution water.
BOD results of the filtered and unfiltered samples were related to the BOD of dissolved carbon fractions (BODDOC) and total carbon (BODTOC), respectively, such that the metabolism of DOC and POC (BODPOC = BODTOC – BODDOC) could be partitioned across sites and sampling periods. This is based on the assumption that the interactive effect between BODDOC and BODPOC is negligible.
Organic carbon concentrations and nutrient analysis
Thirty millilitre samples were taken from each bioassay bottles at Day 0 and analysed for DOC, to establish initial carbon concentrations (DOCi). Filtered and unfiltered samples were submitted to the Environmental Analysis Laboratory (EAL), Southern Cross University, NSW, Australia, for measurement of DOC and TOC. As the September samples underwent nutrient-addition treatment, the ambient nutrient concentrations were also obtained (total dissolved nitrogen (TDN), total dissolved phosphorus (TDP), orthophosphate, nitrate, nitrite, ammonia).
Data analysis
The program GraphPad Prism (Version 5.01, GraphPad Software, San Diego, CA, USA) was used for regressions and one-way and two-way ANOVA analyses. The specific ANOVA analyses conducted are outlined in the results below. A significance level of P = 0.05 was used for all analyses. Data transformations were not required.
Results
Discharge
Flow conditions included two catchment-derived flow pulses between September–October 2010 and December–January 2011 (Fig. 2). Flow conditions and relative positions on the hydrograph differed among sites because of longitudinal variations in the hydrograph and dates sampled. For example, in September 2010, S1 was sampled at the peak of a flow pulse, whereas S6 was sampled at the front end of that pulse. In addition, the magnitude of the flow pulses decreased among sites, such that the December–January flow pulse at S1 peaked on 22 December 2010 at 12 481 ML day–1 (~144.46 m3 s–1), whereas at S6, the pulse peaked on 28 January 2011 at 2988 ML day–1 (~34.58 m3 s–1). These pulses were exceptionally high flows, ~18 times the 10-year average at S1 and ~23 times that at S6. Nonetheless, flows did not exceed bankfull flows, which are ~15 000 ML day–1 (~172 m3 s–1) at S1 and 5000 ML day–1 (~60 m3 s–1) at S6 (Kemp 2010).
The relationship between discharge and DOC
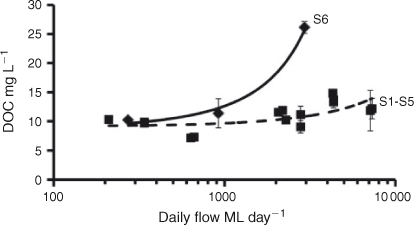
The initial DOC concentrations ([DOCi], Table 1) at each site were positively related with daily discharge at sampling (Fig. 3). Linear regression of relative discharge and [DOCi] gave slopes for S1–S5 that were not significantly (P = 0.6343) different from each other. Pooled data from these sites showed a significantly positive relationship, with a slope of 610.1 mg C ML–1 (ML day–1)–1. S6 varied from all other sites, with a significantly (P < 0.0001) higher slope. The slope at S6 was 6123 mg C ML–1 (ML day–1)–1.
|
|
BOD5 nutrient addition
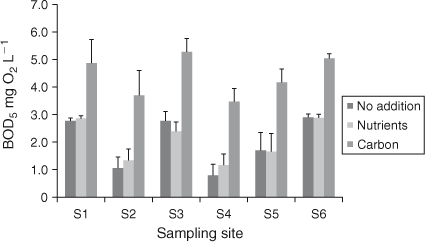
Addition of nitrogen and phosphorus (above ambient concentrations, Table 2) to September 2010 samples (Fig. 4) did not significantly influence BOD5 (two-way ANOVA, P > 0.05). In contrast, the addition of β-glucose led to a significant increase in BOD5 (two-way ANOVA, P < 0.0001, average increase in BOD5 = 2.4 mg O2 L–1). The non-significant interaction effect between site and addition treatment (P = 0.8993) indicated that the magnitude of the increase in BOD5 was independent of site. Direct assessment of the BOD increase as a result of carbon addition (BOD5 (+ Carbon) – BOD5 (No addition)) indicated that the magnitude of BOD increase was constant across all sites (one-way ANOVA, P = 0.9394).
|
|
Unlike carbon concentration, BOD5 did not show a relationship with discharge, nor did BOD5 show a statistically significant relationship with [DOCi] or site. Linear regression of BOD5 and discharge gave slopes that were not significantly different from zero at all sites (P > 0.05, r2 = 0.0014 (S2), 0.0001 (S3), 0.3761 (S4), 0.0521 (S5), 0.0620 (S6)). The exception was S1, which was significantly positive (slope = 2.675 ± 0.400 × 10–4, r2 = 0.8644) and was significantly (P = 0.0003) different from zero.
Leachate-addition bioassays
The change in BOD because of leachate addition was analysed using the difference between the BOD of leachate addition and no addition bioassays on Days 1, 2, 3 and 5 (i.e. ΔBODx = BODx (unfiltered + leachate) – BODx (unfiltered)). Leachate control bioassays were used to remove the BOD attributable to consumption of carbon from the leachate itself; therefore, ΔBODx reflects the oxygen demand attributable to the influence of the leachate on the organic carbon of the samples. BODx can be modelled using a first-order rate reaction (O’Connell et al. 2000); however, in this case, a linear model was used because linear regressions gave r2-values of >0.95 in all cases. This linear response may result from short bioassay duration and a high organic carbon concentration, where depletion of carbon does not cause BODx to asymptote within the bioassay period.
A response to leachate addition was detected in both sampling periods using linear regressions of ΔBODx and time at each site. For January 2011 samples, ΔBODx produced slopes significantly different from zero over 5 days at all sites (S1–S5, P < 0.0001; S6, P = 0.0007) and the relationship did not differ significantly (P = 0.1439) among sites. In February 2011 samples, ΔBODx were significantly non-zero over 5 days at all sites excluding S3 (S1–6 P = 0.0230, 0.0207, 0.0583, < 0.0001, 0.0409, 0.0030) and the relationship did not differ among sites (P = 0.6380).
The magnitude of the response differed between sampling periods, with the mean increase in BOD5 as a result of leachate in January 1.302 mg O2 L–1 (standard deviation = 0.230), whereas in February, this was 0.384 mg O2 L–1 (standard deviation = 0.161). It is relevant that the oxygen demand (BOD5) exerted by the leachate used in the January bioassays was ~1 mg O2 L–1, compared with 0.2 mg O2 L–1 in the February bioassays. This may have influenced the different responses observed between the two sampling times.
POC/DOC concentrations and BOD
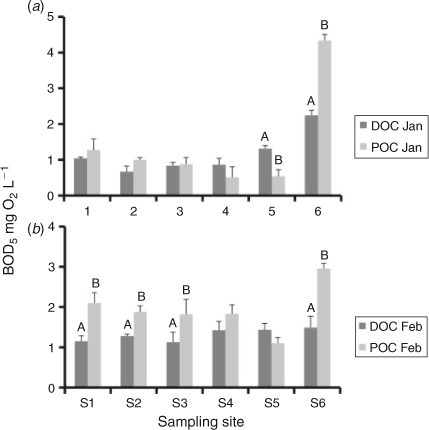
The DOC : POC ratio can be calculated from [TOC] and [DOC] concentrations (Table 1) as follows: [POC] = [TOC] – [DOC]). In January 2011, the initial DOC : POC ratios were >10 at all sites, excluding S6 which was slightly lower (8.9 : 1). In the February 2011 samples, ratios ranged from 7.4–11 : 1 at S1–S5, and were 5.2 : 1 at S6. The results of the BOD5 analysis demonstrated that for the majority of sites in January and February 2011, BODPOC was equal to or greater that BODDOC (Fig. 5).
|
Longitudinal patterns in BOD
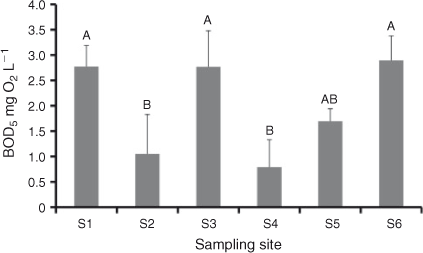
BOD5 from September 2011 showed no overall longitudinal trend but did differ between non-weir and weir pool sites. Sites could be divided into two groups with weir pool sites (S1, S3, S6) significantly higher than non-weir sites S2 and S4, with S5 being intermediate (Fig. 6). There was no significant difference between weir and non-weir sites at any other sampling period.
|
In January and February 2011, significant variations in BODDOC and BODPOC showed that S6 had the highest BOD5, except for BODDOC in February 2011 (Fig. 5).
BOD per unit carbon (i.e. BOD : TOC ratios) may be used as a measure of the relative bioavailability of the carbon pool (Wallace 2006). An assessment of BOD : TOC among sites and sampling periods demonstrated a longitudinal trend of BOD : TOC decreasing downstream for sites S1–S5 in January and February. However, site S6 displays a BOD : TOC similar to that observed at S1 (Fig. 7).

|
Discussion
Carbon limitation of heterotrophic metabolism
Leachate addition to samples stimulated BOD over a short period, i.e. 6-h leachate preparation and 5-day incubation, supporting the proposition that fresh input of leaf leachate will stimulate a rapid biological response (Baldwin 1999). Relative DOC : N and DOC : P ratios suggest that inorganic nutrients will limit BOD (Geider and La Roche 2002). Nonetheless, nutrient additions had no influence, whereas additions of bioavailable carbon (i.e. β-glucose) significantly increased BOD5. One way to account for this is if a significant proportion of the organic carbon pool is recalcitrant, causing heterotrophic metabolism to be limited by labile carbon subsidies. This is consistent with estimates of the recalcitrant nature of global riverine carbon (Søndergaard and Middelboe 1995).
Carbon utilisation rates vary among litter types (e.g. leaf, bark, twigs; O’Connell et al. 2000) and between aged and fresh leaf litter (Baldwin 1999). Watkins et al. (2010) showed that the timing of inundation was critical to Australian river floodplains as litter fall peaks over summer and aging of river red gum leaves increased their ability to be decomposed. The significantly lower response to leachate addition in February 2011 is likely to have been influenced by the carbon composition of leachate derived from litter fall collected at different times, which vary with age.
The uniform BOD response to leachate addition across sites suggested that there was no longitudinal variation in the microbial community’s ability to respond to organic carbon addition. Factors such as the nature and composition of the natural organic matter (NOM) inputs can cause variations in the abundance and composition of the microbial community and heterotrophic functional groups present (Foreman and Covert 2003; Gulis and Suberkropp 2003). It is unknown whether the heterotrophic community composition was uniform among sites, but the communities did respond to leachate addition uniformly. Differences in BOD among sites were therefore assumed to be the result of shifts in the composition and bioavailability of the organic carbon pool and not of variations in the microbial community (Volk et al. 1997).
The relationship between relative discharge and carbon
Across the Lachlan River, there was a positive relationship between discharge and DOC concentration at each site during the study period. Allochthonous carbon inputs are predicted to increase with catchment flows as a result of increased longitudinal inputs plus increased riparian and floodplain connectivity (Tockner et al. 2000). This occurs where flows exceed bankfull and interact directly with the floodplain, i.e. the ‘flood pulse’ concept (Junk et al. 1989), but also when increased flow is below bankfull, i.e. the ‘flow pulse’ (Puckridge et al. 1998).
The flow pulses during the present study were catchment derived as opposed to regulated dam-release flows. Impoundment of water because of weirs/dams is able to influence the composition of organic matter and reduce its bioavailability to downstream sites (Stanford and Ward 2001). As such, flow pulses that are sourced largely from storage releases are less likely to input carbon longitudinally but can release NOM from riparian sources, floodplain and sediment interactions (Walker et al. 1995; Robertson et al. 1999). Nonetheless, storage flows would not necessarily produce identical results as the flows observed in the study period, and the difference between these, would benefit from further study.
POC/DOC partitioning
In the Lachlan River, DOC : POC ratios largely reflected the 10 : 1 ratio suggested by Wetzel (2001), with some variation, such as S6 having a DOC : POC ratio of <10 at both periods. Contrasting this, the BODPOC was generally equal to or higher than BODDOC. Independent data from the Lachlan River (January 2011; data not shown) did not detect chlorophyll concentrations at high levels such that phytoplankton respiration would account for the high BODPOC. Because BODPOC was measured in the presence of DOC, interactive effects between BODPOC and BODDOC, such as increased substrate area, may have contributed to the observed elevated BODPOC.
Elevated BODPOC can contribute to water-column anoxia related to a high concentration of organic carbon. Anoxia is common in blackwater events and can cause widespread fish kills (Howitt et al. 2007). Although the results from the Lachlan River did not directly show that POC was more labile than DOC because BOD does not directly relate to the amount of carbon consumed (Wallace et al. 2008), they did show that POC and DOC can have a comparable influence on ecosystem characteristics. Furthermore, Cole et al. (2006) showed that POC can be an important source of carbon to high trophic-level species. As such, studies focusing on the DOC component of organic carbon may be excluding a critical part of the energy subsidy in a river system.
Longitudinal patterns in carbon cycling
Variations in BOD could not be clearly associated with any one factor, e.g. site, flow or [DOCi], but this is not unexpected, considering the BOD variation may result from multiple complex factors. Longitudinal position, flow conditions and seasonal factors all appeared to exert some influence on BOD.
Differences in BOD between weir and non-weir sites in September 2010 suggested that regulation and changes to retention time can cause variation in the carbon pool. On a catchment scale, regulatory structures fragment hydrological and ecological processes while limiting the lateral connectivity (Nilsson et al. 2005). Particularly, in dryland rivers, there is a significant difference in the hydrology of weir pools versus mid- and upper reaches (Davies et al. 1995). This may be seen as analogous with the serial discontinuity concept, which argues that major regulatory mechanisms, e.g. dams, influence longitudinal patterns in physical parameters, nutrient conditions and ultimately community composition in rivers (Ward and Stanford 1983). Because hydrology and aquatic metabolism appear to be linked (Bernot et al. 2010), the observed BOD differences between weir and non-weir sites may have been the result of regulation. As a weir effect was observed only once, making further conclusions is speculation and longer-term studies focusing on the influence of regulation on carbon composition and carbon cycling are needed.
The peak of an anoxic flow pulse coincided with January 2011 at S5 and S6, which corresponded to significantly elevated DOC and TOC concentrations and BODTOC. Blackwater events, where high flows cause increased carbon inputs, BOD stimulation, DO depletion and anoxia, make it difficult for managers who wish to institute patterns of floodplain inundation while avoiding fish kills. Howitt et al. (2007) modelled the occurrence of blackwater from two flooding events in the Barmah–Millewa river red gum forests. Temperature was one key factor, wherein summer high-flow periods were significantly more likely to lead to an anoxic water column because of the influence of temperature on microbial activity. Results from the Lachlan River supported this because the January 2011 flow pulse corresponded with anoxic conditions (DO = 3.00 mg O2 L–1 (S5), 0.49 mg O2 L–1 (S6)) and heightened water temperatures of 27.06°C (S5) and 25.37°C (S6). September 2010 sampling also coincided with a flow pulse but DO at S1–S6 ranged between 7.55 and 8.45 mg O2 L–1 and temperature was 14.98–16.27°C. Litter-fall rates of Australian sclerophyll vegetation are characteristically highest during summer months (Pressland 1982). This may also have contributed because litter fall was observed significantly higher in the November–January period than in September–October (data not shown). Nonetheless, there are a range of contributing factors to any given blackwater event and multi-factor models, as in Howitt et al. (2007), are the most useful predictive tool.
BODTOC/[TOC] may give an indication of longitudinal factors influencing observed variations in BOD. BODTOC/[TOC] can relate to carbon bioavailability because high BOD per unit carbon suggests that a higher proportion of carbon is being consumed, although it has been observed that BOD does not directly relate to the amount of carbon consumed (Wallace et al. 2008). As such, BODTOC/[TOC] relates to the relative influence of a particular pool of carbon on the BOD of the water column and can be only loosely related to bioavailability.
In the Lachlan River, the observed BODTOC/[TOC] decreased longitudinally between S1 and S5. This is consistent with one of the mechanisms underlying the River Continuum concept, i.e. that upstream communities consume the most bioavailable organic carbon fraction and predominantly recalcitrant carbon will be transported downstream (Vannote et al. 1980). This phenomenon may influence the characteristics of the allochthonous carbon pool along a river, resulting in a trend of increasing concentration downstream but decreasing bioavailability. This would be exacerbated by the concentrating effect of water losses via evaporation/transpiration. This trend was observed by Hadwen et al. (2010) in the Ovens and Logan Rivers. The accumulation of recalcitrant carbon as a package of water progresses downstream may influence organic carbon by increasing the concentration of organic carbon ([DOC]) but decreasing the influence on the water column (BOD), which may be due to lower bioavailability.
S6 defied the BODTOC/[TOC] trend. A hypothesis to explain this and the significant increases in BOD and carbon concentration from S5 to S6 incorporates differences in river-adjacent land use that can cause significant variations in organic carbon and disrupt longitudinal patterns in carbon (Jarvie et al. 2008; Neal et al. 2008). As the Lachlan River flows towards its terminus, it contracts, branches and anabranches (Kemp 2010). Unlike upstream where land use is overwhelmingly agricultural, the dominant landscape feature between S5 and S6 is a river red gum forest, the Moon Moon State Forest.
Unlike carbon concentrations, the observed BOD suggests that carbon composition and bioavailability are unlikely to be characterised by a single factor. It is clear that flow variability has an influence on organic carbon characteristics of the Lachlan River, but directly relating this to heterotrophic carbon cycling is more complex, let alone relating it to aquatic biota. Both the advantages of environmental flow pulses, such as carbon availability for the food web, and potential deleterious effects, such as blackwater events, need to be considered when managing flow regimes in regulated river systems.
Acknowledgements
We thank the Murray–Darling Freshwater Research Centre and the support staff for financial and logistical support. Also, thanks go to everyone from the School of Earth and Environmental Science who aided and contributed.
References
Baldwin, D. S. (1999). Dissolved organic matter and phosphorus leached from fresh and ‘terrestrially’ aged river red gum leaves: implications for assessing river-floodplain interactions. Freshwater Biology 41, 675–685.| Dissolved organic matter and phosphorus leached from fresh and ‘terrestrially’ aged river red gum leaves: implications for assessing river-floodplain interactions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXlslKjtLg%3D&md5=f100b9c2abd1642feadbfee80c19aaafCAS |
Bernot, M. J., Sobota, D. J., Hall, R. O., Mulholland, P. J., Dodds, W. K., Webster, J. R., Tank, J. L., Ashkenas, L. R., Cooper, L. W., Dahm, C. N., Gregory, S. V., Grimm, N. B., Hamilton, S. K., Johnson, S. L., McDowell, W. H., Meyer, J. L., Peterson, B., Poole, G. C., Valett, H. M., Arango, C., Beaulieu, J. J., Burgin, A. J., Crenshaw, C., Helton, A. M., Johnson, L., Merriam, J., Niederlehner, B. R., O'Brien, J. M., Potter, J. D., Sheibley, R. W., Thomas, S. M., and Wilson, K. (2010). Inter-regional comparison of land-use effects on stream metabolism. Freshwater Biology 55, 1874–1890.
| Inter-regional comparison of land-use effects on stream metabolism.Crossref | GoogleScholarGoogle Scholar |
Bond, N. R., Lake, P. S., and Arthington, A. H. (2008). The impacts of drought on freshwater ecosystems: an Australian perspective. Hydrobiologia 600, 3–16.
| The impacts of drought on freshwater ecosystems: an Australian perspective.Crossref | GoogleScholarGoogle Scholar |
Boulton, A. J., Datry, T., Kasahara, T., Mutz, M., and Stanford, J. A. (2010). Ecology and management of the hyporheic zone: stream-groundwater interactions of running waters and their floodplains. Journal of the North American Benthological Society 29, 26–40.
| Ecology and management of the hyporheic zone: stream-groundwater interactions of running waters and their floodplains.Crossref | GoogleScholarGoogle Scholar |
Cleveland, C. C., Neff, J. C., Townsend, A. R., and Hood, E. (2004). Composition, dynamics, and fate of leached dissolved organic matter in terrestrial ecosystems: results from a decomposition experiment. Ecosystems 7, 275–285.
| Composition, dynamics, and fate of leached dissolved organic matter in terrestrial ecosystems: results from a decomposition experiment.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXkvF2ktr4%3D&md5=3bbb5bf0a64ed5540ae0eb702d8a76bfCAS |
Cole, J. J., Carpenter, S. R., Pace, M. L., Van de Bogert, M. C., Kitchell, J. L., and Hodgson, J. R. (2006). Differential support of lake food webs by three types of terrestrial organic carbon. Ecology Letters 9, 558–568.
| Differential support of lake food webs by three types of terrestrial organic carbon.Crossref | GoogleScholarGoogle Scholar | 16643301PubMed |
CSIRO (2008). Water availability in the Lachlan: a report to the Australian Government from the CSIRO Murray–Darling Basin Sustainable Yields Project. CSIRO, Canberra, Australia.
Dalzell, B. J., Filley, T. R., and Harbor, J. M. (2007). The role of hydrology in annual organic carbon loads and terrestrial organic matter export from a midwestern agricultural watershed. Geochimica et Cosmochimica Acta 71, 1448–1462.
| The role of hydrology in annual organic carbon loads and terrestrial organic matter export from a midwestern agricultural watershed.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXis1Gjsrc%3D&md5=ad32643be3b9a00468a4f3d8ae1770f5CAS |
Davies, B. R., Thoms, M. C., Walker, K. F., O’Keeffe, J. H., and Gore, J. A. (1995). Dryland rivers: their ecology, conservation and management. In ‘The Rivers Handbook. Vol. 2’. (Eds. P. Calow and G. E. Petts.) pp. 484–511. (Blackwell Scientific: Oxford, UK.)
Eaton, A. D., and Franson, M. A. H. (2005). ‘Standard Methods for the Analysis of Water and Wastewater.’ 20th edn. (American Public Health Association: Washington, DC.)
Foreman, C. M., and Covert, J. S. (2003). Linkages between dissolved organic matter composition and bacterial community structure. In ‘Aquatic Ecosystems: Interactivity of Dissolved Organic Matter’. (Eds S. Findlay and R. L. Sinsabaugh.) pp. 343–362. (Academic Press: Amsterdam.)
Gawne, B., Merrick, C., Williams, D. G., Rees, G., Oliver, R., Bowen, P. M., Treadwell, S., Beattie, G., Ellis, I., Frankenberg, J., and Lorenz, Z. (2007). Patterns of primary and heterotrophic productivity in an arid lowland river. River Research and Applications 23, 1070–1087.
| Patterns of primary and heterotrophic productivity in an arid lowland river.Crossref | GoogleScholarGoogle Scholar |
Geider, R. J., and La Roche, J. (2002). Redfield revisited: variability of C:N:P in marine microalgae and its biochemical basis. European Journal of Phycology 37, 1–17.
| Redfield revisited: variability of C:N:P in marine microalgae and its biochemical basis.Crossref | GoogleScholarGoogle Scholar |
Gulis, V., and Suberkropp, K. (2003). Interactions between stream fungi and bacteria associated with decomposing leaf litter at different levels of nutrient availability. Aquatic Microbial Ecology 30, 149–157.
| Interactions between stream fungi and bacteria associated with decomposing leaf litter at different levels of nutrient availability.Crossref | GoogleScholarGoogle Scholar |
Hadwen, W. L., Fellows, C. S., Westhorpe, D. P., Rees, G. N., Mitrovic, S. M., Taylor, B., Baldwin, D. S., Silvester, E., and Croome, R. (2010). Longitudinal trends in river functioning: patterns of nutrient and carbon processing in three Australian rivers. River Research and Applications 26, 1129–1152.
| Longitudinal trends in river functioning: patterns of nutrient and carbon processing in three Australian rivers.Crossref | GoogleScholarGoogle Scholar |
Hladyz, S., Watkins, S. C., Whitworth, K. L., and Baldwin, D. S. (2011). Flows and hypoxic blackwater events in managed ephemeral river channels. Journal of Hydrology 401, 117–125.
| Flows and hypoxic blackwater events in managed ephemeral river channels.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXktF2hs7Y%3D&md5=8cbab28ca4c9c615dc952a0a86ddfdacCAS |
Howitt, J. A., Baldwin, D. S., Rees, G. N., and Williams, J. L. (2007). Modelling blackwater: predicting water quality during flooding of lowland river forests. Ecological Modelling 203, 229–242.
| Modelling blackwater: predicting water quality during flooding of lowland river forests.Crossref | GoogleScholarGoogle Scholar |
Jarvie, H. P., Haygarth, P. M., Neal, C., Butler, P., Smith, B., Naden, P. S., Joynes, A., Neal, M., Wickham, H., Armstrong, L., Harman, S., and Palmer-Felgate, E. J. (2008). Stream water chemistry and quality along an upland–lowland rural land-use continuum, south west England. Journal of Hydrology 350, 215–231.
| Stream water chemistry and quality along an upland–lowland rural land-use continuum, south west England.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXisFSru70%3D&md5=6925dc5a130031abad93c1f4e7997b1cCAS |
Junk, W. J., Bayley, P. B., and Sparks, R. E. (1989). The flood pulse concept in river-floodplain systems. Proceedings of the International Large River Symposium. Canadian Special Publication of Fisheries and Aquatic Sciences 106, 110–127.
Kelly, V. J. (2001). Influence of reservoirs on solute transport: a regional-scale approach. Hydrological Processes 15, 1227–1249.
| Influence of reservoirs on solute transport: a regional-scale approach.Crossref | GoogleScholarGoogle Scholar |
Kemp, J. (2010). Downstream channel changes on a contracting, anabranching river: the Lachlan, southeastern Australia. Geomorphology 121, 231–244.
| Downstream channel changes on a contracting, anabranching river: the Lachlan, southeastern Australia.Crossref | GoogleScholarGoogle Scholar |
Kingsford, R. T. (2011). Conservation management of rivers and wetlands under climate change – a synthesis. Marine and Freshwater Research 62, 217–222.
| Conservation management of rivers and wetlands under climate change – a synthesis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXjsVKksb0%3D&md5=884a294406cbf6b805d507b4f873f14dCAS |
McMahon, T. A., and Finlayson, B. L. (2003). Droughts and anti-droughts: the low flow hydrology of Australian rivers. Freshwater Biology 48, 1147–1160.
| Droughts and anti-droughts: the low flow hydrology of Australian rivers.Crossref | GoogleScholarGoogle Scholar |
Murray–Darling Basin Authority (2010). ‘Guide to the Proposed Basin Plan: Overview.’ (Murray–Darling Basin Authority: Canberra.)
Neal, C., Jarvie, H. P., Love, A., Neal, M., Wickham, H., and Harman, S. (2008). Water quality along a river continuum subject to point and diffuse sources. Journal of Hydrology 350, 154–165.
| Water quality along a river continuum subject to point and diffuse sources.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXisFSrurc%3D&md5=7e6334e35954bddbcd027811e3cf3b95CAS |
Nilsson, C., Reidy, C. A., Dynesius, M., and Revenga, C. (2005). Fragmentation and flow regulation of the world’s large river systems. Science 308, 405–408.
| Fragmentation and flow regulation of the world’s large river systems.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXjtFOnt7g%3D&md5=ecf82d0650128d6e22785d0b7650db88CAS | 15831757PubMed |
O’Connell, M., Baldwin, D. S., Robertson, A. I., and Rees, G. (2000). Release and bioavailability of dissolved organic matter from floodplain litter: influence of origin and oxygen levels. Freshwater Biology 45, 333–342.
| Release and bioavailability of dissolved organic matter from floodplain litter: influence of origin and oxygen levels.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXos1Omu7g%3D&md5=53e31e8c92c8c279f7581f19f752b2c4CAS |
Oliver, R. L., and Merrick, C. J. (2006). Partitioning of river metabolism identifies phytoplankton as a major contributor in the regulated Murray River (Australia). Freshwater Biology 51, 1131–1148.
| Partitioning of river metabolism identifies phytoplankton as a major contributor in the regulated Murray River (Australia).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XmvVymsrg%3D&md5=75b60d893232ebb387015c75c38747fcCAS |
Petry, P., Bayley, P. B., and Markle, D. F. (2003). Relationships between fish assemblages, macrophytes and environmental gradients in the Amazon River floodplain. Journal of Fish Biology 63, 547–579.
| Relationships between fish assemblages, macrophytes and environmental gradients in the Amazon River floodplain.Crossref | GoogleScholarGoogle Scholar |
Pressland, A. J. (1982). Litter production and decomposition from an overstorey of Eucalyptus spp. on two catchments in the New England region of New South Wales. Australian Journal of Ecology 7, 171–180.
| Litter production and decomposition from an overstorey of Eucalyptus spp. on two catchments in the New England region of New South Wales.Crossref | GoogleScholarGoogle Scholar |
Puckridge, J. T., Sheldon, F., Walker, K. F., and Boulton, A. J. (1998). Flow variability and the ecology of large rivers. Marine and Freshwater Research 49, 55–72.
| Flow variability and the ecology of large rivers.Crossref | GoogleScholarGoogle Scholar |
Qualls, R. G. (2005). Biodegradability of dissolved organic from decomposing fractions of carbon leached leaf litter. Environmental Science & Technology 39, 1616–1622.
| Biodegradability of dissolved organic from decomposing fractions of carbon leached leaf litter.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXlsFOmtQ%3D%3D&md5=7d3114d5b965c47bc534572056e7f849CAS |
Robertson, A. I., Bunn, S. E., Boon, P. I., and Walker, K. F. (1999). Sources, sinks and transformations of organic carbon in Australian floodplain rivers. Marine and Freshwater Research 50, 813–829.
| Sources, sinks and transformations of organic carbon in Australian floodplain rivers.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXks1ymug%3D%3D&md5=07059e89dc6f89ba3b2f8a69ff0ff1e2CAS |
Søndergaard, M., and Middelboe, M. (1995). A cross-system analysis of labile dissolved organic-carbon. Marine Ecology Progress Series 118, 283–294.
| A cross-system analysis of labile dissolved organic-carbon.Crossref | GoogleScholarGoogle Scholar |
Stanford, J. A., and Ward, J. V. (2001). Revisiting the serial discontinuity concept. Regulated Rivers: Research and Management 17, 303–310.
| Revisiting the serial discontinuity concept.Crossref | GoogleScholarGoogle Scholar |
Tank, J. L., Rosi-Marshall, E. J., Griffiths, N. A., Entrekin, S. A., and Stephen, M. L. (2010). A review of allochthonous organic matter dynamics and metabolism in streams. Journal of the North American Benthological Society 29, 118–146.
| A review of allochthonous organic matter dynamics and metabolism in streams.Crossref | GoogleScholarGoogle Scholar |
Thoms, M. C., and Sheldon, F. (2000). Lowland rivers: an Australian introduction. Regulated Rivers: Research and Management 16, 375–383.
| Lowland rivers: an Australian introduction.Crossref | GoogleScholarGoogle Scholar |
Thorp, J. H., and Delong, M. D. (1994). The Riverine productivity model – an heuristic view of carbon-sources and organic-processing in large river ecosystems. Oikos 70, 305–308.
| The Riverine productivity model – an heuristic view of carbon-sources and organic-processing in large river ecosystems.Crossref | GoogleScholarGoogle Scholar |
Tockner, K., Malard, F., and Ward, J. V. (2000). An extension of the flood pulse concept. Hydrological Processes 14, 2861–2883.
| An extension of the flood pulse concept.Crossref | GoogleScholarGoogle Scholar |
Vannote, R. L., Minshall, G. W., Cummins, K. W., Sedell, J. R., and Cushing, C. E. (1980). River continuum concept. Canadian Journal of Fisheries and Aquatic Sciences 37, 130–137.
| River continuum concept.Crossref | GoogleScholarGoogle Scholar |
Vink, S., Bormans, M., Ford, P. W., and Grigg, N. J. (2005). Quantifying ecosystem metabolism in the middle reaches of Murrumbidgee River during irrigation flow releases. Marine and Freshwater Research 56, 227–241.
| Quantifying ecosystem metabolism in the middle reaches of Murrumbidgee River during irrigation flow releases.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXjtlWrsrc%3D&md5=851ca7d385cca687970969908d36a947CAS |
Volk, C. J., Volk, C. B., and Kaplan, L. A. (1997). Chemical composition of biodegradable dissolved organic matter in streamwater. Limnology and Oceanography 42, 39–44.
| Chemical composition of biodegradable dissolved organic matter in streamwater.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXjtVCmu70%3D&md5=eb98d03fad9f9aaf591327ec77a0db48CAS |
Walker, K. F., Sheldon, F., and Puckridge, J. T. (1995). A perspective on dryland river ecosystems. River Research and Applications 11, 85–104.
Wallace, T. A. (2006). Composition and bioavailability of DOC across a rural–urban gradient. Ph.D. Thesis, The University of Adelaide, Adelaide.
Wallace, T. A., Ganf, G. G., and Brookes, J. D. (2008). A comparison of phosphorus and DOC leachates from different types of leaf litter in an urban environment. Freshwater Biology 53, 1902–1913.
| A comparison of phosphorus and DOC leachates from different types of leaf litter in an urban environment.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhtFKisLnF&md5=68a5c30876a6f04cd9cd7f0b758c7918CAS |
Ward, J. V., and Stanford, J. A. (1983) The serial discontinuity concept of lotic ecosystems. In ‘Dynamics of Lotic Ecosystems’. (Eds T. D. Fontaine and S. M. Bartell.) pp. 29–42. (Ann Arbor Science Publishers: Ann Arbor, MI.)
Watkins, S. C., Quinn, G. P., and Gawne, B. (2010). Changes in organic-matter dynamics and physicochemistry, associated with riparian vegetation loss and river regulation in floodplain wetlands of the Murray River, Australia. Marine and Freshwater Research 61, 1207–1217.
| Changes in organic-matter dynamics and physicochemistry, associated with riparian vegetation loss and river regulation in floodplain wetlands of the Murray River, Australia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXht1yjsrjO&md5=1ccfd1e430ae6953941b9d77279a53f1CAS |
Westhorpe, D. P., and Mitrovic, S. M. (2012). Dissolved organic carbon mobilisation in relation to variable discharges and environmental flows in a highly regulated lowland river. Marine and Freshwater Research 63, 1218–1230.
| Dissolved organic carbon mobilisation in relation to variable discharges and environmental flows in a highly regulated lowland river.Crossref | GoogleScholarGoogle Scholar |
Wetzel, R. G. (2001). Fundamental processes within natural and constructed wetland ecosystems: Short-term versus long-term objectives. Water Science and Technology 44, 1–8.
| 1:STN:280:DC%2BD38%2FnslKlsg%3D%3D&md5=d5f05783da699fd96d03bb8ab49a6d86CAS | 11804079PubMed |
Whitworth, K. L., Baldwin, D. S., and Kerr, J. L. (2012). Drought, floods and water quality: drivers of a severe hypoxic blackwater event in a major river system (the southern Murray–Darling Basin, Australia). Journal of Hydrology 450, 190–198.
| Drought, floods and water quality: drivers of a severe hypoxic blackwater event in a major river system (the southern Murray–Darling Basin, Australia).Crossref | GoogleScholarGoogle Scholar |


