The biology of giant ostracods (Crustacea, Cyprididae), a review focusing on the Mytilocypridinae from Australian inland waters
Mahabubur Rahman A B * , Jennifer Chaplin A and Adrian Pinder C
A B * , Jennifer Chaplin A and Adrian Pinder C
A Murdoch University, Centre for Sustainable Aquatic Ecosystems, Environmental and Conservation Sciences, 90 South Street, Murdoch, WA 6150, Australia.
B Department of Fisheries and Marine Science, Noakhali Science and Technology University, Noakhali 3814, Bangladesh.
C Department of Biodiversity Conservation and Attractions, 17 Dick Perry Avenue, Kensington, WA 6151, Australia.
Marine and Freshwater Research 74(1) 1-19 https://doi.org/10.1071/MF22092
Submitted: 21 April 2022 Accepted: 20 October 2022 Published: 16 November 2022
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
This study uses published and unpublished data to create a comprehensive and up-to-date synthesis of available information on a little-known group of invertebrates, namely, non-marine giant ostracods (cypridids ≥3 mm long). Approximately 8% of the ∼1000 living cypridid species are identified as ‘giant’. They occur in a range of subfamilies, in all zoogeographic regions, except Antarctica, and mainly in small standing-water ecosystems. Only those in the subfamily Mytilocypridinae are reasonably well studied. This subfamily is endemic to Australia and comprises a diverse range of giant species (∼29 species in 6 genera), mainly in temporary habitats, mostly salt lakes, including in extreme conditions. We evaluate the current taxonomy of the Mytilocypridinae, analyse patterns in the field distributions and abiotic tolerances of species, and review the general biology of these ostracods. We also identify those species most in need of consideration in conservation planning, highlight critical gaps in knowledge and show how these ostracods could serve as useful models for testing ecological and evolutionary theories. The results of this study can be used to inform conservation planning for giant ostracods and direct further study of these unique invertebrates, which are an important component of the biodiversity of small standing-water ecosystems.
Keywords: aquatic invertebrate, Australocypris, conservation, Mytilocypris, ostracod, salinity tolerance, salt lakes, taxonomy.
Introduction
Historically, conservation studies have tended to focus on conspicuous, charismatic, iconic or economically significant taxa or settings, resulting in a biased perspective on threats to biodiversity and on the structure and function of biological systems in general (e. g. Di Marco et al. 2017; Troudet et al. 2017). Taxa and settings that have been identified as overlooked include aquatic invertebrates (Collier et al. 2016), small standing-water ecosystems (e.g. wetlands, ponds and small lakes; Bolpagni et al. 2019) and inland saline waters (Saccò et al. 2021). It is important that conservation planning focuses more on these taxa and settings and is supported by scientific studies (e.g. Di Marco et al. 2017; Troudet et al. 2017; Bolpagni et al. 2019). Accordingly, this study synthesises available information on a little-known group of invertebrates, namely, ‘the giant ostracods’, especially those in the subfamily Mytilocypridinae, which are an important component of the communities of small standing-water ecosystems, both freshwater and saline, in Australia (see below).
The term ‘giant ostracod’ is typically used to describe those ostracod species in the family Cyprididae that are 3 mm or more in length (De Deckker 1977; Martens 1986; Halse and McRae 2004), although the term has also been applied to unusually large marine ostracods in other groups (e.g. Fenwick 1984). The information presented herein pertains only to the former, which comprise ∼8% of the ∼1000 living, non-marine species of Cyprididae (Martens 1986; Halse and McRae 2004; Karanovic 2012). It is likely that additional species of giant ostracod remain to be discovered, especially in regions where giant ostracods are common but poorly studied (e.g. the Afrotropical region, see below). Also, size data are not yet available for some species in taxa that include giant species, e.g. in the tribe Megalocypridini and the genus Chlamydotheca Saussure (Martens and Behen 1994; Karanovic 2012).
Giant ostracod species occur in a range of subfamilies and in all zoogeographic regions, except Antarctica (Fig. 1). The number of giant species is highest in southern parts of the Afrotropical region (Meisch et al. 2019), followed by the Australasian region, and lowest in the Palearctic and Oriental regions (Fig. 1). The subfamily Megalocypridinae has the most species, all but one of which are from the Afrotropical region (Fig. 1). The Mytilocypridinae is also diverse, but all species are from Australia (Fig. 1). Like the Mytilocypridinae, the Hungarocypridinae and Liocypridinae exclusively comprise giant species, but the total number of species in these two subfamilies is small (Fig. 1). The Liocypridinae is only found in the Afrotropical region (Fig. 1), whereas the Hungarocypridinae is represented in the Australasian (by Hungarocypris asymmetrica Victor & Fernando in Sulawesi; Victor and Fernando 1981), Palearctic and Oriental regions. Giant ostracods represent only a small minority of species in the other subfamilies in which they occur (Fig. 1). There are also giant species in the genera Cypriconcha Sars (Cole 1960; Delorme 1969) and Amphicypris Sars (Fontana and Ballent 2005), which have not yet been assigned to any subfamily (Meisch et al. 2019). The above taxonomic data show that gigantism has evolved on multiple occasions in the Cyprididae.
|
|
As is the case for the Cyprididae in general (Meisch et al. 2019), most giant ostracod species inhabit freshwater (Table S1), but some, including most mytilocypridinids, are from inland saline waterbodies (e.g. saline wetlands, coastal and inland salt lakes; De Deckker 1981a, 1983a; Halse and McRae 2004). Most species have been collected from small, shallow or temporary standing waterbodies, although some occur in large permanent lakes or rivers (see Supplementary Table S1). The evolution of gigantism in the Cyprididae has been linked to reduced predation pressure (e.g. due to a general absence of fish) in temporary (Martens et al. 2007) or high-salinity waters (De Deckker 1983a).
Information on the biology of giant ostracod species is mainly restricted to small amounts of morphological, distributional and habitat information in limited taxonomic studies, which has been summarised above and in Table S1. An exception is the Mytilocypridinae ostracods, which are well studied, especially with respect to their taxonomy (De Deckker 1974, 1976, 1978, 1981b, 1982; Finston 2002; Halse and McRae 2004; Finston 2007) and some aspects of their ecology (De Deckker and Geddes 1980; De Deckker 1981a, 1983a; Martens 1985a, 1985b; Martens et al. 1985; Radke et al. 2003; Pinder et al. 2005). This subfamily has no known close relatives (i.e. its recent evolutionary history is unknown) and consists of a diverse array of giant species, which are found only in Australia (Halse and McRae 2004). A few species are restricted to temporary fresh or low-salinity waterbodies, but most are found in salt lakes (defined herein as enclosed bodies of water with a salinity normally >3 g L−1), where they are a key component of the ecology (see De Deckker 1981a, 1983a). Salt lakes are innumerable in Australia; >80% of lakes and wetlands are saline, and most are shallow and temporary (Timms 2005). Many giant ostracod species show a tolerance to, or even an affinity for, hypersalinity (>50 g L−1; Hammer 1986) and other extreme conditions (Halse and McRae 2004; Lawrie et al. 2021). For example, Australocypris bennetti Halse & McRae has been collected from a salinity range of 25–282.1 g L−1 (Halse and McRae 2004; A. Pinder, unpubl. data) and a pH range of 2.7–9.06 (Halse and McRae 2004; Supplementary Table S2). Like several other invertebrate taxa (e.g. Parartemia Sayce, some Daphnia Müller and Coxiella E. A. Smith), the Mytilocypridinae appears to have undergone a significant radiation in inland saline environments (or their precursors) in Australia (Lawrie et al. 2021).
The aquatic habitats of Mytilocypridinae (and other) ostracods are being affected by a range of human-related activities (e.g. see Davis et al. 2003; Timms 2005; Kirono et al. 2012). Temporary waterbodies, both freshwater and saline, in southern Australia are typically holding water less often and may fill less predictably as precipitation decreases and evaporation increases in association with anthropogenic climate change (Timms 2005; Jellison et al. 2008; Atkinson et al. 2021). The salinity of waterbodies is also increasing (Williams 1995; Halse et al. 2003; Kirono et al. 2012; Lawrie et al. 2021), even in deep permanent lakes because water levels are receding (Kirono et al. 2012). Secondary salinisation, which is caused by a rise in saline ground water (e.g. as a result of the clearing of deep-rooted trees or irrigation) is another major threat (Timms 2005). Secondary salinisation has dramatically altered the physical and biological properties of waterbodies in some areas (Jellison et al. 2008), particularly in south-western Australia, where up to 30% of the landscape is predicted to be severely affected (Taylor and Hoxley 2003; Timms 2005), placing many freshwater and halophilic invertebrate species at risk of regional extinction (Davis et al. 2003; Halse et al. 2003; Lyons et al. 2007; Jolly et al. 2008). Mining is a threat to some salt lakes, either because of physical disturbance of dry lakebeds or the discharge of highly acidic or highly saline water into the lake (Williams 2002; Timms 2005). Mernagh et al. (2016) assessed the mineral potential of Australian salt lakes and identified six regions with a high potential for potash, lithium or boron mining. They noted that some of the lakes in these regions included sites of cultural and conservation significance. Other habitat threats include fish introductions in low-salinity lakes (Khan et al. 2002; Khan 2003; P. De Deckker, pers. comm., August, 2022), various types of pollution, groundwater extraction and diversion of surface flows (Williams 2002; Timms 2005; Gregory 2007).
The implications of the above-described habitat degradation for Mytilocypridinae ostracods have not been assessed. To make such an assessment, it is important to evaluate what is known about the biology of these ostracods, but currently relevant information is spread over many sources, including some unpublished material (see Methods for details). This study therefore synthesises published and unpublished information on the biology of Mytilocypridinae ostracods. The findings are used to (1) evaluate the current taxonomy of this group, (2) document important patterns in the geographic and environmental distributions of species, (3) review the general biology of these ostracods, (4) evaluate the conservation status of each species, and (5) highlight gaps in knowledge and recommend directions for future studies. The study also highlights the value of these ostracods for studying ecological and evolutionary theories.
Methods
The information used in this study comes from the following three main sources: (1) peer reviewed published articles, government reports and PhD theses; (2) a dataset held by the Department of Biodiversity, Conservation and Attractions (DBCA), Government of Western Australia; and (3) previously unpublished data held by the first author (M. Rahman), which are presented in Table S2.
Relevant articles on Mytilocypridinae ostracods were identified using Google Scholar, Scopus, and Science Direct databases, with various combinations of the following search terms: ‘ostracod’, ‘ostracoda’, ‘giant ostracod’, ‘large ostracod’, ‘non-marine ostracod’, ‘saline lake’, ‘salt lake’, ‘salt lake Australia’, ‘Australocypris’, ‘Mytilocypris’, ‘Trigonocypris’, ‘Caboncypris’, ‘Repandocypris’, ‘Lacrimicypris’. Mytilocyprinid species names, and their synonyms, compiled from Halse and McRae (2004), were also used as search terms. Multiple searches were conducted between June 2019 and December 2020. These searches identified 60 peer-reviewed journal articles, 25 government reports and 4 PhD theses with relevant information. Although Geddes et al. (1981) was found in the search, their data were excluded because the species identifications for Australocypris De Deckker in Western Australia are uncertain in view of the taxonomic revision of Halse and McRae (2004).
The DBCA dataset pertains to giant ostracods collected from sites in Western Australia between July 1994 and June 2020. It contains information on the location (latitude and longitude) of collection sites and associated environmental data, including water salinity and pH. Some of the data are unpublished but some are included in articles that were found in the above searches. The latter were used only once in this study. Unidentified species in the database were excluded from analyses. Raw data on the co-occurrences and salinity range of species in the Eyre Peninsula, summarised in Timms (2009a), were kindly made available by Brian Timms. The sources of geographical and environmental data for specific species are given in the Supplementary Table S3.
Maps showing the distribution of giant ostracod species across states, drainage basins and climatic regions in Australia were constructed using QGIS (ver. 3.22.0, see https://qgis.org/en/site/index.html) software. Information on drainage divisions (see http://www.bom.gov.au/water/about/riverBasinAuxNav.shtml) and climate zones (see http://www.bom.gov.au/climate/how/newproducts/images/zones.shtml) was obtained from the Bureau of Meteorology of Australia. Graphs and figures showing geographic and environmental data were prepared using Microsoft Office 365 and R (ver. 4.2.1, see https://cran.r-project.org/bin/windows/base/).
In this article, salinity data are expressed as grams of inorganic dissolved salt per litre of water. The following conversions were used to generate salinity data in these units when the source article reported a related measure. (1) Salinity data in grams per kilogram or parts per thousand were regarded as the same as grams per litre, although it is recognised that they are not identical (Williams and Sherwood 1994). (2) Total dissolved solids (TDS) data were multiplied by a correction factor of 0.91 (Bayly and Williams 1966) to take into account the fact that the former include dissolved organic matter as well as salts (Williams and Sherwood 1994). (3) Conductivity data were converted into salinity (g L−1) using the equation developed by Williams (1986). Although this formula is recommended only for data <100 mS cm−1 (Williams 1986), it was also used to convert measurements exceeding >100 mS cm−1 when there was no other way to obtain salinity data (e.g. because TDS data were not reported). In such cases, this would have resulted in underestimates of the actual salinity values.
For simplicity, unless stated otherwise, the term ‘salt lake’ refers to inland waterbodies and coastal lakes with a salinity normally in excess of 3 g L−1, and the term ‘giant ostracod’ refers specifically to Mytilocypridinae ostracods in the remainder of this article unless stated otherwise. All data for Australocypris hypersalina De Deckker have been reported under A. insularis (Chapman) De Deckker, because the former is a synonym of the latter (see below). Similarly, data for Mytilocypris minuta De Deckker and M. tasmanica chapmani McKenzie are reported under M. mytiloides (Brady) McKenzie.
Taxonomy and phylogeny
The Australian endemic subfamily Mytilocypridinae contains 21 described species in 6 genera (Halse and McRae 2004; Fig. 2). De Deckker conducted much of the initial taxonomic work on this group, identifying 15 species in 4 genera (De Deckker 1974, 1976, 1978, 1981b, 1982). Halse and McRae (2004) conducted the most recent taxonomic revision of the group, adding two new genera and eight new species (Supplementary Table S4). In that revision, M. praenuncia and M. tasmanica tasmanica were separated on the basis of only subtle differences in the bursa copulatrix and might represent the same species (see Finston 2000; Halse and McRae 2004). Recent species checklists, i.e. Martens and Savatenalinton (2011), Karanovic (2012) and Meisch et al. (2019), have essentially followed Halse and McRae’s (2004) revision, except for some minor details (see Table S4 for details). Because the overall morphological features of A. dispar more closely match those described for Caboncypris than Australocypris (see Table 1), we suggest that A. dispar is a type of Caboncypris.

|
The following new or undescribed species have been discovered since the revision of Halse and McRae (2004): (1) two undescribed species of Mytilocypris, namely, Mytilocypris ‘moojari’ from the Goldfields region (Quinlan et al. 2016) and Pilbara region (Pinder et al. 2010) and ‘M. n. sp. 1’ from Lake Carnegie (M. Rahman and A. Pinder, unpubl. data); (2) Lacrimicypris sp. nov. from a seasonal claypan in the Wheatbelt region (Pinder et al. 2013) and a wetland in the Perth Metropolitan region (A. Pinder, unpubl. data); (3) two undescribed species of Caboncypris, namely, ‘C. n. sp. 1’ from the Esperance region (Table S2) and ‘C. n. sp. 2’ from the Eyre Peninsula in South Australia (specimen provided by Patrick De Deckker); and (4) three undescribed species of Australocypris, namely, ‘A. n. sp. 1’ from the Wheatbelt region (Table S2), ‘A. n. sp. 2’ from the Esperance region (Table S2) and ‘A. n. sp. 3’ from the Great Southern (A. Pinder, unpubl. data, and Table S2).
Thus far, genetic assessment of species boundaries in giant ostracods is available only for Mytilocypris. Of the eight species that were recognised at the time, Finston (2000) found fixed allelic differences at allozyme loci for five (M. ambiguosa, M. henricae, M. praenuncia, M. splendida, M. tasmanica tasmanica), but no fixed differences among the other three (M. mytiloides, M. minuta and M. tasmanica chapmani). Also, the overall genetic differences between M. praenuncia and M. tasmanica tasmanica were small and may reflect geographic variation within a single species (see Finston 2000). Subsequent field (Finston 2004) and laboratory (Finston 2007) studies strongly suggest that M. mytiloides, M. minuta and M. tasmanica chapmani are a single morphologically variable species. The three morphotypes can be distinguished by differences in carapace size and shape, but these differences are largely determined by the salinity of the environment (Finston 2004). On this basis, Halse and McRae (2004) synonymised M. minuta and M. tasmanica chapmani to M. mytiloides.
Neither the broader phylogenetic relationships of the Mytilocypridinae nor those among the composite genera and species have been studied. Establishing these relationships is needed to validate the current taxonomy and develop our understanding of the origin and evolutionary history of these unique ostracods.
Habitat type
Giant ostracods are found mainly in seasonal (fill seasonally) and episodic (fill irregularly) waterbodies (Halse and McRae 2004). They also occur in permanent waterbodies, but essentially only in those that have salinities greater than the tolerance level of fish predators (De Deckker 1983a). For example, A. robusta is found in some of the large permanent crater lakes in western Victoria (De Deckker 1983a). Although some species are strictly or partly freshwater species (see below), giant ostracods are a conspicuous and diverse component of the fauna of salt lakes in Australia (De Deckker 1981a, 1983b). Of the species that occur in salt lakes, most occur only in naturally saline waterbodies, but Australocypris insularis (Pinder et al. 2005) and unspecified Mytilocypris species (Pinder et al. 2002) also occur in waterbodies affected by secondary salinisation.
Diversity and distribution
The most diverse genera of giant ostracod are Australocypris and Mytilocypris, which have seven described species and respectively three and two undescribed species, notwithstanding that A. dispar may really be a species of Caboncypris (see above). Caboncypris is the next-most diverse with two described and two undescribed species, and possibly also A. dispar. The other three genera (Lacrimicypris, Repandocypris and Trigonocypris) each have only two known species. These data could change as more species are discovered and information on phylogenetic relationships of genera and species becomes available. The remaining information in this section considers only described species, i.e. the undescribed species mentioned in the taxonomy and phylogeny section are excluded.
Giant ostracods are mainly known from southern Australia (Halse and McRae 2004), probably because most salt lakes occur in this region (Williams 2002; Halse and McRae 2004). On a state-by-state basis, the total number of species of giant ostracods progressively declines from Western Australia to South Australia to Victoria to Tasmania and is lowest in New South Wales and Queensland (Fig. 3c). Six of the species in Western Australia are endemic to this state, compared with only one or no endemic species in other states (Fig. 3c). Small ostracods, Parartemia brine shrimp, Coxiella gastropods show similar decreases in species richness in salt lakes as one moves eastward from Western Australia (see Lawrie et al. 2021). There are no reports of giant ostracods from the Northern Territory or the Australian Capital Territory.
We have also considered the breakdown of species richness across drainage divisions (Fig. 3a, 4), which may be the foci of evolution for these ostracods. The highest numbers of species occur in the South West Coast (13 species) and South Western Plateau drainage divisions (11 species; Fig. 3a), which are located fully (former) or partly (latter) in Western Australia (Fig. 4). The next-highest occurs in the Murray–Darling Basin drainage division (10 species; Fig 3a), mainly owing to a high number of species recorded from the well-studied Paroo River wetlands (Timms 1993; Watts 1999). The South West Coast drainage division also has the highest number of endemic species (three species; Fig. 3a). The Lake Eyre Basin and Carpentaria Coast are the only other drainage divisions with endemic species and they have one species each (Fig. 3a).
|
|
Finally, from the perspective of climate zones (Fig. 4), temperate and grassland zones have more species than desert and subtropical areas (Fig. 3b). There are one or two endemic species in each of the temperate, grassland and desert zones, but there is none in the subtropical zone (Fig. 3b). The desert zone is reasonably unsampled and so could contain a high proportion of undescribed species (Halse and McRae 2004), although waterbodies in this zone usually only fill episodically (Roshier et al. 2001), which may limit species richness (see Williams and Geddes 1991).
The basis for high species richness and endemism in giant ostracods in southern Western Australia is likely to be multi-faceted. A range of other taxa with desiccation-resistant life stages from small standing waterbodies also have overall more species and more endemic species in Western Australia than they do in other parts of southern Australia. These taxa include anostracans (Timms 2012, 2014), cladocerans (Hebert and Wilson 2000), copepods (Maly and Bayly 1991), Spinicaudata (Timms 2006) and Coxiella gastropods (Lawrie et al. 2021).
The opportunities for speciation in giant ostracods and other taxa with desiccation-resistant life stages may be high in southern Western Australia because there are vast numbers of temporary waterbodies that collectively encompass multiple climate zones and show considerable variation in salt content and hydrological cycle (see Geddes et al. 1981, who promoted these ideas as a part of the explanation for high species richness in Parartemia in this region). Also, Western Australia has a long history of geological stability with repeated climatic variations, which may have promoted speciation in these ostracods and other invertebrates inhabiting temporary waterbodies (Geddes et al. 1981; Timms 2006, 2013; Timms et al. 2009). Phylogeographic studies are needed to deduce how giant ostracod species have responded to palaeoclimatic events (see Byrne 2008).
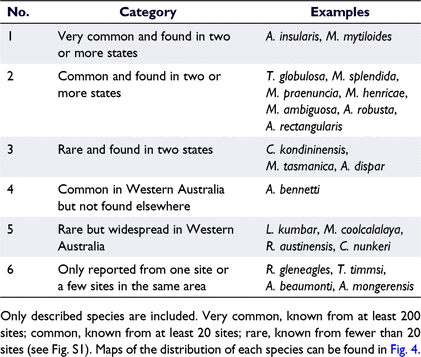
There is considerable variation in the ubiquity and abundance of giant ostracod species. We have identified six distributional categories, ranging from species that are widespread and very common to species that are known only from one site or a few sites in the same area (see Table 2, see also Fig. 4 for maps of species distributions). Australocypris insularis and Mytilocypris mytiloides are, by far, the most commonly encountered species, with each being recorded from more than 200 sites (Table 2, Supplementary Fig. S1) from throughout southern Australia, although only the latter species has been found in Tasmania (Table 3, Fig. 4, S1). Trigonocypris globulosa is notable because it is very geographically widespread, occurs in all four climatic zones and is the only species that is widespread in central Australia (Table 3, Fig. 4). Repandocypris gleneagles Halse & McRae and T. timmsi De Deckker are at the other extreme, each having been reported from a single site only (Table 3, Fig. 4, S1).
|
|
The reasons why some species are more common and widespread than other species in the same genus is not known but similar variation has been reported for some other invertebrate taxa from inland Australian waters (see Lawrie et al. 2021). When considering this pattern in Parartemia, Williams and Geddes (1991) suggested that the evolution of adaptations that support colonisation might be favoured in some species (resulting in widespread species), whereas selection for local adaptation might be favoured in others (resulting in narrowly distributed species). Williams (1984) suggested that a relatively wide distribution of Parartemia in some species was related to broader ecological tolerance and relaxed selection rather than a greater dispersal ability. It would be worthwhile to test these ideas by using giant ostracod species.
Information on the distributions of giant ostracod species is more detailed than that for most invertebrate species in inland Australian waters (e.g. see Lawrie et al. 2021, for a recent review of salt-lake taxa). Nevertheless, sampling is incomplete, particularly for rare species and remote regions. Also, most information is based on ad hoc sampling, which may not have captured the full complement of species that inhabit a waterbody, such as, if the abundance of a species varies with time. Some information may also be based on outdated taxonomy. For example, A. robusta De Deckker has been reported from two sites in Western Australia, Lake De Courcy and Lake King (Geddes et al. 1981); however, these reports predate Halse and McRae’s (2004) taxonomic revision. Recent data indicate that A. bennetti is the species in Lake King (Halse and McRae 2004), and probably also Lake De Courcy (M. Rahman, unpubl. data). Reports of A. robusta in Western Australia are, therefore, unconfirmed and so have not been included in the diversity and distributional data presented herein (see Fig. 4). Similarly, our data also exclude an unconfirmed report of A. rectangularis De Deckker from Lake Dundas (see Halse and McRae 2004), which is the only report of this species in Western Australia.
Ecology
Salinity range
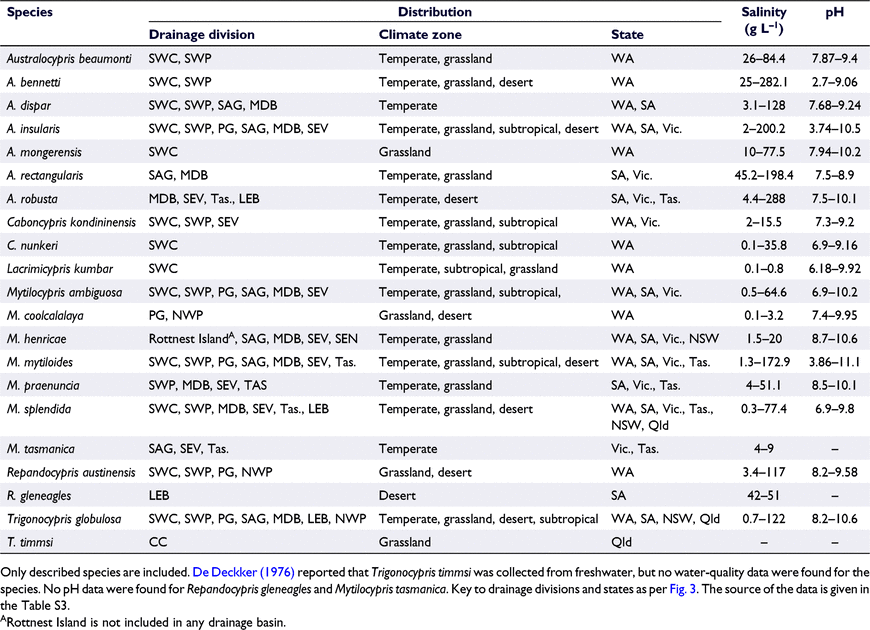
Lacrimicypris is known only from freshwater (Halse and McRae 2004; Table 3). Trigonocypris has one species (T. timmsi) reported from one freshwater site (De Deckker 1976) and another (T. globulosa) that occurs in sites ranging from freshwater to hypersaline water (Table 3). Caboncypris kondininensis Halse & McRae and C. nunkeri have been found in freshwater to low-salinity water respectively (up to 15.5 g L−1) and freshwater to moderately saline water (up to 35.8 g L−1; Table 3). Six of the seven Mytilocypris species are found mainly or exclusively in salt lakes (see Table 3); the remaining species, M. coolcalalaya Halse & McRae, is known only from freshwater and low-salinity water (Halse and McRae 2004; DBCA dataset). Australocypris (six or seven species, depending on the status of A. dispar) and Repandocypris (two species) are found only in salt lakes (see Table 3). These data suggest that the giant ostracods have transitioned from freshwater to saline water on multiple independent occasions.
Most salinity records for giant ostracods are based on field measurements and suggest that most species have very broad and often overlapping salinity tolerances (Table 3). Eight species have been found at salinities greater than 100 g L−1 and two at salinities greater than 200 g L−1 (Table 3), placing them among the most salt-tolerant ostracods in the world (see De Deckker 1981a; Lawrie et al. 2021). At the other extreme, aside from the strictly freshwater Lacrimicypris, there are four giant ostracod species that have been found only at salinities less than 20 g L−1 (Table 3). The overall salinity range in giant ostracods, particularly in the more salt-tolerant species, is large, for example, extending from 4.4 to 288 g L−1 in A. robusta (Table 3).
Estimates based on multiple individual data points provide more nuanced information about the usual salinity distributions of species than do those based on maximum and minimum values. Such estimates show that Australocypris species tend to occur at higher salinities than do species in other genera (Fig. 5). The median salinity record is highest (∼100 g L−1) for A. rectangularis and A. bennetti (Fig. 5). The median salinity for ‘A. dispar’ (22.8 g L−1) is the lowest of the Australocypris species (Fig. 5), which is interesting in view of the possibility that this could be a species of Caboncypris (see above). Among the Mytilocypris, the common and widespread species, M. mytiloides has the highest median salinity record at ∼19 g L−1 (range 1.3–172.9 g L−1; Fig. 5). By contrast, median salinity (∼48 g L−1, range 2–200.2 g L−1) for the common and widespread A. insularis is among the lowest for Australocypris (see Fig. 5).
Aladin and Potts (1996) investigated osmoregulation in a broad range of ostracod species, including Mytilocypris praenuncia, which has been recorded from salinities of 4–51 g L−1 in the field (Table 3). This species was capable of hyperosmotic regulation when raised in water with salinity of <8 g L−1 and of hypo-osmotic regulation when raised in water within the salinity range of 20–48 g L−1. The salinity of haemolymph and the water medium was near isosmotic at salinities in between. The mechanisms of osmoregulation were not fully resolved but mitochondria-rich cells located in the non-calcified zone of the inner carapace layer appear to be important. These cells are responsible for excreting salt in hypo-osmotic regulation and may be the site of salt uptake in hyperosmotic regulation (Aladin and Potts 1996). Osmoregulation in other species of giant ostracod is probably similar to that in M. praenuncia.
pH range
The pH of freshwater ecosystems in Australia generally varies between 6.5 and 8 (Hobday and Lough 2011), with some exceptions (Pinder et al. 2013). The pH of most of Australian salt lakes is also near neutral (Mernagh et al. 2016), although, overall, it varies from 1.4 (Benison and Bowen 2015) to 11.0 (Williams 1998). A high proportion of lakes in the Wheatbelt and Goldfields–Esperance regions of Western Australia are classified as moderately acidic (pH 4–6) or extremely acidic (pH 1.5–3.9), reflecting the acidic groundwaters in these regions (Benison and Bowen 2015). Acidic lakes tend to have their own unique but depauperate fauna compared with neutral–alkaline lakes (Pinder et al. 2004; Timms 2009b). Some have only recently become acidic as a result of rising groundwater (Pinder et al. 2005).
Most giant ostracod species are found only in neutral–alkaline water (Table 3, Fig. 6). Four species, namely C. nunkeri, M. ambiguosa, M. splendida and the freshwater L. kumbar, occur mainly in neutral–alkaline waters but are occasionally also found in slightly acidic conditions (Table 3, Fig. 6). Australocypris insularis and M. mytiloides also occur mainly in neutral–alkaline waters, but overall are distributed over a very broad range of pH, including highly acidic waters (Table 3, Fig. 6). Australocypris bennetti is common in both acidic and alkaline waters (Fig. 6) and is represented by different morphological forms in each (Halse and McRae 2004). Whether these different morphs reflect genetic or ecophenotypic effects is unclear. In general, ostracod species are rarely found in a pH of <5 (e.g. see Ruiz et al. 2013), and the occurrence of giant (and other) ostracods in low pH waters is surprising, because the acidic water is expected to dissolve the calcite carapace (De Deckker 2002). The mechanisms that giant ostracods use to survive in such extreme environments are not known.
|
|
The above-mentioned salinity and pH data for giant ostracods are based on field records. These records may overestimate the salinity or pH tolerance of some species because they do not consider whether individuals can survive long term or reproduce in the conditions. They are also based mainly on the adult stage of the life-cycle, which may be tolerant of a broader range of conditions than are other life stages (see Geddes 1976, who suggested this for Parartemia). Conversely, the field data are likely to underestimate the true extent of variation in other species that have been subject to limited spatial or temporal sampling. For example, prior to this study, A. mongerensis Halse & McRae was known only from a single site at a salinity of 11 g L−1 (Halse and McRae 2004) but we have found this species at another three sites in salinities ranging from 21 to 78 g L−1 (Table S2).
Experimental data indicate that salinity tolerance in M. henricae is almost twice as high under laboratory conditions as field records (Martens (1985b), indicating that the relationship between the physiological tolerances and field distributions of giant ostracods is probably complex. Outside of a critical range, salinity is probably not a crucial factor in determining the field distributions of species (Williams et al. 1990). Radke et al. (2003) undertook a detailed analysis of the relationship between the distributions of a range of giant and small ostracods species and the ionic composition of salt lakes in south-eastern Australia. They concluded that ostracods could be separated into four groups according to their preferred water chemistry, which in part reflected the salt content and pathway of solute evolution (e.g. ratios of Ca2+ to alkalinity) of the water. Giant ostracod species were represented in all four groups. The three species of Australocypris that were included in the study (A. rectangularis, A. insularis, A. robusta) all fell into different groups, whereas the five species of Mytilocypris were placed into two groups, one including M. mytiloides and M. ambiguosa and the other including M. henricae, M. splendida, and M. praenuncia (which also included A. robusta). Bayly (1969) proposed that salinity tolerance in some species of calanoid copepods from Australian salt lakes increased at a higher pH and was potentially linked to ionic proportions, particularly carbonate and bicarbonate, as well as salinity. The same might apply to giant ostracods.
Co-occurrence of species
Co-occurrence of giant ostracod species, particularly congeners, appears to be relatively rare (Table 4), although a mix of different species from up to three different genera has been collected from some sites (Cale et al. 2004; Table S2). Australocypris and Mytilocypris are the most relevant genera for understanding patterns of co-occurrence because they include a range of species with overlapping geographic distributions. Species of both genera, especially M. mytiloides and A. insularis, have been collected from many of the same sites across southern Australia (e.g. De Deckker and Geddes 1980; De Deckker and Williams 1982; Radke 2000; Cale et al. 2004, Timms 2009a, 2009b; Pinder et al. 2012; Cale and Pinder 2018). However, there are very few reports of co-occurring Australocypris species (De Deckker 1983a; Table 4), except that A. insularis and A. rectangularis were often found together on the Eyre Peninsula in South Australia (B. V. Timms, unpubl. data). Mytilocypris species, especially M. mytiloides and M. ambiguosa, appear to co-occur more often than Australocypris species, but less often than Mytilocypris species do with other genera (Table 4, Fig. 7). Congeneric species of some other types of salt-lake invertebrates (e.g. Parartemia) also rarely coexist (Timms 2012).

|
Co-occurrence of giant ostracod species may sometimes be overlooked, especially because the relative abundance (and therefore the collectability) of different species in a waterbody can vary through time (Halse and McRae 2004). Nevertheless, the rarity of reports for well-sampled taxa such as Mytilocypris and especially Australocypris is too low to be explained entirely by missed data. Because many giant ostracod species have very broad and overlapping physicochemical tolerances (see above), the rarity of co-occurrence is unlikely to be fully explained by differences in the physicochemical properties of waterbodies. Dispersal limitations are also an unlikely cause, given that many congeneric species have overlapping geographic distributions and co-occurrence of species from different genera is more common. Predation has been identified as a major factor excluding co-occurrence between congeners in the copepods Boeckella Guerne & Richard and Calamoecia Brady in Western Australia (Maly and Maly 1997). Predation and competition could be important in explaining the low rate of co-occurrence between congeneric giant ostracods (De Deckker 1983a).
General biology
Locomotion
Giant ostracods are active swimmers and use antennae for propulsion (De Deckker 1983a). They usually swim near the bottom and some species of Australocypris have been observed to burrow a few millimetres into soft sediment on lake floors (De Deckker 1983a). Anecdotal observations on specimens in aquaria suggest that individuals of A. robusta may prefer to burrow in ‘clayey’ rather than sandy sediment (De Deckker 1974). They also suggest that M. henricae and M. splendida swim less, and with the posterior well below the anterior, than do A. robusta, A. insularis (=A. hypersalina) and T. globulosa (De Deckker 1978). De Deckker (1978) proposed that swimming is more energetically expensive in Mytilocypris species because the tapering of the posterior of the carapace is more extreme and so adds weight without fitting soft tissue (except possibly for testes). Smith et al. (2015) indicated that swimming is energetically expensive for ostracods in general; the increased buoyancy from the saline water may reduce the energetic costs of swimming in salt lakes.
Trophic interactions
The diets of giant ostracods have not been well studied and may vary with the stage of life. In a series of laboratory experiments, Campbell (1995) showed that Australocypris insularis is an important predator on zooplankton, such as the calanoid copepods Calamoecia clitellata and C. salina, the small ostracods Diacypris compacta and D. dietzi, and juvenile ostracods. Large numbers of the copepod Calamoecia have been found in the gut and faeces of several Australocypris species (De Deckker 1983a). In the laboratory, A. robusta has been observed to feed mainly on dead crustaceans (mainly isopods) or filamentous algae if the former is absent (De Deckker 1974) and A. insularis has been seen congregating on and consuming living and dead individuals of Parartemia brine shrimp (M. Rahman, unpubl. data). Such predatory behaviour, where large numbers of ostracods attack animals much larger than themselves, has also been reported for a small number of marine and non-marine species (e.g. Wilkinson et al. 2007). Overall, the available evidence indicates that the diet of Australocypris species is broad and opportunistic and that some Australocypris species are important predators in salt-lake systems. There is no published information on the diets of other types of giant ostracod.
Very little is also known about the organisms that feed on giant ostracods. There are reports of Mytilocypris in the gut contents of an introduced fish species from salt lakes in Victoria (De Deckker 1977; Khan et al. 2002; Khan 2003) and in the gut contents of a native fish species from salt lakes in Western Australia (Halse 1981). Fish could be important predators of giant ostracods except that they are rarely found in giant ostracod habitats (see Halse and McRae 2004). Birds that feed in these habitats are known to consume larger invertebrates such as gastropods (Weston 2007) and brine shrimp (Pedler et al. 2018) and probably also feed on giant ostracods; for example, banded stilts have been seen feeding on ‘small black ostracods’ at Lake King (Bougher 1988), but we are not aware of any published records that specifically mention birds feeding on giant ostracods. Adults and larvae of dytiscid beetles, which have been reported from salinity ranging from 0 to 130 g L−1 in Australia (e.g. Pinder et al. 2002), are potential invertebrate predators.
Life history and reproduction
Specific information on the life history and reproduction of giant ostracods is sparse and restricted mainly to Mytilocypris species. The life cycle of M. henricae in Lake Bathurst comprises nine instars, eight larval stages and the adult (Martens et al. 1985), which is typical for ostracods (Smith et al. 2015). Giant ostracod populations invariably contain both males and females (De Deckker 1983a; Finston 2000, 2002; Smith et al. 2016; M. Rahman, unpubl. data) and allozyme data have confirmed that five Mytilocypris species use sexual reproduction (Finston 2002). Thus, although the Family Cyprididae includes a broad range and high incidence of parthenogenetic lineages, Mytilocypridinae ostracods are likely to be exclusively sexually reproducing. Because is the case with other ostracods, fertilisation in giant ostracods is internal, and males have an elaborate (and taxonomically informative) copulatory apparatus and giant aflagellate sperm (Matzke-Karasz et al. 2014; Smith et al. 2016). Sperm size has been measured in three species of Mytilocypridinae, namely, A. robusta, M. praenuncia and M. mytiloides, with sizes of ∼11 787, 4800 and 4675 μm respectively (Smith et al. 2016). The sperm of A. robusta is among the longest recorded for any animal (Smith et al. 2016).
Giant ostracods are sexually dimorphic, i.e. where structures not directly involved in reproduction show differences in form between the males and females of a species (Cox and Calsbeek 2010). The carapace or body size is larger in females (to accommodate large number of eggs) and has a posterodorsal hump in males (to accommodate the rotation of the hemipenis during copulation). The shape of the carapace also differs between males and females; for example, it is strongly triangular in T. timmsi males but subtriangular in females (De Deckker 1978; Halse and McRae 2004). The number and arrangement of claws and setae on the second antennae is also sex specific (De Deckker 1978; Halse and McRae 2004). The first leg is described as strongly sexually dimorphic because it has a prehensile palp in males that is used to clasp the female during copulation (De Deckker 1978; Halse and McRae 2004). Similar patterns in sexual dimorphism are seen in other cypridids (Karanovic 2012; Smith et al. 2015). Although sexual dimorphism in animals is often attributed to sexual selection, the basis or bases for sexual dimorphism in giant ostracods is not known and could have a variety of causes (see Punzalan and Hosken 2010).
Female-biased adult sex ratios appear to be common at least in Mytilocypris (Finston 2002) and Australocypris (J. Chaplin, unpubl. data). Such biases are common in sexually reproducing ostracods in general (reviewed by Chaplin et al. 1994; Martins 2019). It is uncertain whether these biases are related to mechanisms of sex determination or subsequent life history or ecological modifications of sex ratio (Ladle and Foster 1992; Martins 2019). Experimental studies using giant ostracods could be used to help resolve these questions.
Growth rate and adult size in Mytilocypris mytiloides, a common and widespread species, vary with salinity (Finston 2004, 2007), with individuals tending to grow faster but reaching a smaller size in high-salinity environments and vice versa. Finston (2007) suggested that salinity might act as a cue for habitat duration, such that M. mytiloides grows faster in ephemeral waterbodies to ensure that eggs can be laid before conditions become unfavourable. De Deckker (1983a) mentioned that adult sizes in Australocypris in waterbodies in the Coorong Lagoon system of South Australia were larger during the winter than in spring and summer when temperatures and salinities were higher and water depth was rapidly receding. He suggested that environmental factors, such as temperature, may have a direct controlling effect on the size, shape and ornamentation of the carapace. These ostracod data fit with suggestions that populations and species from more variable environments show high levels of phenotypic plasticity (Chevin and Hoffmann 2017).
All species of giant ostracod are likely to produce desiccation-resistant eggs because they all have populations in temporary waterbodies (e.g. see De Deckker 1983a; Halse and McRae 2004). This has been confirmed for Australocypris insularis (Williams 1991; Campbell 1995; Campagna 2007), A. bennetti (M. Rahman, unpubl. data), A. beaumonti (M. Rahman, unpubl. data), Trigonocypris globulosa (Timms 1998), Mytilocypris ambiguosa (Strachan et al. 2014, 2016; M. Rahman, unpubl. data), M. mytiloides (M. Rahman, unpubl. data), and Caboncypris kondininensis (M. Rahman, unpubl. data), which have been raised in the laboratory from sediment samples collected from dry waterbodies. Desiccation-resistant eggs are a general feature of non-marine ostracods (De Deckker 1983a; Rossi et al. 2012). These eggs play a key role in re-establishing populations after unfavourable or dry conditions (De Deckker 1977; De Stasio 1989; Strachan et al. 2014), preventing local extinctions (Rossi et al. 2012), and also in dispersal (Green et al. 2008). Because there are giant ostracod populations in permanent waterbodies, such as, A. robusta in Lake Keliambete, it appears that the resting eggs of at least some species do not need to undergo a period of desiccation to hatch (De Deckker 1983a).
The factors that stimulate hatching in eggs after a period of desiccation are largely unknown, but salinity is likely to play a role. De Deckker (1983a) reported that unspecified species of ostracod in athalassic ephemeral lakes in the Coorong Lagoon system tended to hatch, not immediately after rainfall when salinities were still high, but later when salinities where lower. He also mentioned that different species were present in Lake Buchanan in central Queensland in different years when salinity was different, which implies that salinity cues for hatching might be species specific. Finally, he noted that some species, such as A. robusta, can hatch at high salinities. Our knowledge of hatching cues in giant ostracods has advanced very little since De Deckker (1983a).
Dispersal
Very little is known about the mechanisms and extent of dispersal in giant ostracods, although it seems likely that the desiccation-resistant eggs are the main dispersal propagules (see De Deckker 1977). These eggs could be transported in a number of ways, such as, being attached to the external surface or in the coeca of water birds (Sandberg and Plusquellec 1974; De Deckker 1977; Figuerola and Green 2002; Green et al. 2008; Sánchez et al. 2012), by egestion from water birds (Proctor 1964; Green et al. 2008), by wind (Brendonck and Riddoch 1999) or by water flow during flooding (Finston 2002). De Deckker (1977) hypothesised that migrating waterfowls are a key dispersal vector for the giant ostracods, largely on the basis that these birds inhabit waterbodies similar to those inhabited by the ostracods. Ultimately, the distribution of ostracod species will depend not only on their capacity for dispersal, but also on the factors that determine the survival and growth of propagules or individuals at the destination waterbody.
Finston (2002) used allozyme data to investigate the population structures of five species of Mytilocypris. Conspecific populations were genetically distinguished from each other, suggesting that the amount of gene flow in a species is negligible and populations are self-sustaining (Finston 2002). However, the very common and widespread species, M. mytiloides, undergoes more (although still limited) gene flow over larger distances than do the other species (Finston 2002). It is not clear whether this is because this species has a greater dispersal ability per se or because it has broader ecological tolerances (Finston 2002). This begs the question as to whether phenotypic plasticity is generally more pronounced in the more broadly distributed species. Although the amount of gene flow in a giant ostracod species is unlikely to be sufficient to directly affect population dynamics, it will influence a species’ chances of colonising new waterbodies and regions, buffer against local extinctions and determine the breakdown of genetic variation within and among populations (Butlin and Menozzi 2000).
Conservation status
The Mytilocypridinae ostracods warrant attention in conservation plans in view of their unique evolutionary history and ecology and ongoing threats to their habitats, which are outlined in the introduction. Species that are rare and geographically restricted (Category 6 in Table 2) are seemingly most in need of consideration. These include R. gleneagles, which has been collected only from Lake Eyre South, South Australia (Halse and McRae 2004), and T. timmsi, which is known only from Pine Tree Lagoon, Queensland, and has not been found since its original description (De Deckker 1976). Although recent sampling has increased the number of known populations, both Australocypris mongerensis and A. beaumonti are still known only from a few sites in the northern edge of the Wheatbelt and Esperance regions in Western Australia respectively (see Fig. 4, Table S2). Species that are restricted to low-salinity habitats (see Table 3, Fig. 5) may be particularly vulnerable to a drying climate because waterbodies fill less often, hold water for shorter periods of time and contain higher-salinity water (Lawrie et al. 2021). Many giant ostracod populations occur in nature reserves, which is important because habitat protection is the only way to conserve giant ostracod species (see Halse and McRae 2004). However, although nature reserves offer some degree of protection, they can still be affected by increasing aridity, secondary salinisation by a regional rise in groundwater, and other disturbances within their catchment (Williams 2002; Atkinson et al. 2021).
We do not know of any confirmed loss of a giant ostracod population from a waterbody. Populations in some of the very large salt lakes in Western Australia that were once more or less seasonal but now rarely fill may be particularly susceptible to extirpation. For example, despite repeated attempts, we have been unable to find Australocypris sp. in Lake De Courcy since ∼2011 (J. Chaplin, unpubl. data). The desiccation-resistant eggs of giant ostracods should help prevent extirpation, but the details will depend on the factors that influence the viability and hatching of these eggs, which are currently unknown.
Conclusions and directions for future research
Approximately 8% of the ∼1000 species of non-marine cypridid ostracods are ‘giant’. Giant species are found in at least seven subfamilies and in all zoogeographic regions, except Antarctica. Most species are unstudied, except for taxonomy and accompanying notes on distribution and habitat. The giant ostracods occur mainly in small standing-water ecosystems and are potentially an important component of the fauna of these systems. The subfamily Mytilocypridinae, which is endemic to Australia, is relatively well studied. A few mytilocypridinids occur in freshwater or low-salinity water, but most occur in salt lakes, sometimes in extreme conditions. Approximately one-third of known Mytilocypridinae species have been discovered in the past 20 years. The highest levels of diversity occur in south-western Australia. Most species have broad and often overlapping physicochemical tolerances. Nevertheless, there is considerable variation in the ubiquity and abundance of species and congeners rarely co-occur in the same waterbody.
Studies that generate basic information about the biology of non-Mytilocypridinae giant ostracods are urgently required. Future studies of Mytilocypridinae ostracods should prioritise the following:
-
incorporating molecular data into taxonomic research to test for the presence of cryptic species and plastic morphological characters;
-
conducting phylogeographic analyses to help understand critical events in the evolution of these ostracods and how these relate to the past and present Australian ‘landscape’;
-
ascertaining species’ tolerances to salinity and other environmental stresses across all stages of their life cycles;
-
determining the mechanism that species use to survive in acid lakes;
-
clarifying the abiotic and biotic factors that determine species’ distributions, including why congeneric species rarely co-occur;
-
elucidating the long-term viability and hatching stimuli for desiccation-resistant eggs and whether this varies among species;
-
comparing patterns and mechanisms of dispersal and gene flow in broadly distributed and geographically restricted species;
-
documenting the trophic roles of giant ostracods in their communities;
-
using laboratory experiments to test ecological (e.g. about species co-existence) and evolutionary (e.g. about sex ratios) predictions;
-
assessing the levels of anthropogenic impacts, including secondary salinisation and mining activities, on giant ostracods;
-
identifying any critical habitats, such as sites that may serve as refugia during unfavourable conditions.
Supplementary material
Supplementary material is available online.
Data availability
The data that support the findings of this study are available from the references identified in figures and tables, Supplementary Table S2 and a database held by the Department of Biodiversity, Conservation and Attractions of Western Australia. The DBCA dataset may be obtained from the authority upon reasonable request.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Declarations of funding
Mahabubur Rahman was supported by a Commonwealth-supported Research Training Program (RTP) Scholarship at Murdoch University while conducting this research.
Acknowledgements
We thank Koen Martens for providing information on African giant ostracods. We are grateful to Farhan Bokhari and Volker Framenau of the Invertebrate Diagnostics Laboratory, Harry Butler Institute, for providing facility to capture high-resolution images of giant ostracods, and to Angus Lawrie for sharing valuable information on Australian salt-lake invertebrates. We are indebted to the Department of Biodiversity, Conservation and Attractions (DBCA) of Western Australia and Brian Timms for providing water-quality and geographical data. We also thank Stuart Halse, Patrick De Deckker and an anonymous reviewer for their insight, useful discussions, and generous advice, which has helped improve the paper and Patrick De Deckker for kindly providing some ostracod specimens from South Australia and Tasmania.
References
Aladin, NV, and Potts, WTW (1996). The osmoregulatory capacity of the Ostracoda. Journal of Comparative Physiology B 166, 215–222.| The osmoregulatory capacity of the Ostracoda.Crossref | GoogleScholarGoogle Scholar |
Atkinson, ST, Cale, D, Pinder, A, Chambers, JM, Halse, SA, and Robson, BJ (2021). Substantial long-term loss of alpha and gamma diversity of lake invertebrates in a landscape exposed to a drying climate. Global Change Biology 27, 6263–6279.
| Substantial long-term loss of alpha and gamma diversity of lake invertebrates in a landscape exposed to a drying climate.Crossref | GoogleScholarGoogle Scholar |
Bayly, IAE (1969). The occurrence of calanoid copepods in athalassic saline waters in relation to salinity and anionic proportions. Internationale Vereinigung für theoretische und angewandte Limnologie: Verhandlungen 17, 449–455.
| The occurrence of calanoid copepods in athalassic saline waters in relation to salinity and anionic proportions.Crossref | GoogleScholarGoogle Scholar |
Bayly, IAE, and Williams, WD (1966). Chemical and biological studies on some saline lakes of south-east Australia. Marine and Freshwater Research 17, 177–228.
| Chemical and biological studies on some saline lakes of south-east Australia.Crossref | GoogleScholarGoogle Scholar |
Benison, KC, and Bowen, BB (2015). The evolution of end-member continental waters: the origin of acidity in southern Western Australia. GSA Today 25, 4–10.
| The evolution of end-member continental waters: the origin of acidity in southern Western Australia.Crossref | GoogleScholarGoogle Scholar |
Bolpagni, R, Poikane, S, Laini, A, Bagella, S, Bartoli, M, and Cantonati, M (2019). Ecological and conservation value of small standing-water ecosystems: a systematic review of current knowledge and future challenges. Water 11, 402.
| Ecological and conservation value of small standing-water ecosystems: a systematic review of current knowledge and future challenges.Crossref | GoogleScholarGoogle Scholar |
Bougher, A (1988). After 50 years Banded Stilt breeding at Lake King. Western Australian Bird Notes 48, 1.
Brendonck, L, and Riddoch, BJ (1999). Wind-borne short-range egg dispersal in anostracans (Crustacea: Branchiopoda). Biological Journal of the Linnean Society 67, 87–95.
| Wind-borne short-range egg dispersal in anostracans (Crustacea: Branchiopoda).Crossref | GoogleScholarGoogle Scholar |
Butlin RK, Menozzi P (2000) Open questions in evolutionary ecology: do ostracods have the answers? In ‘Evolutionary biology and ecology of Ostracoda’. (Eds DJ Horne, K Martens) Developments in Hydrobiology, vol 148, pp. 1–14. (Springer: Dordrecht, Netherlands) https://doi.org/
| Crossref |
Byrne, M (2008). Evidence for multiple refugia at different time scales during Pleistocene climatic oscillations in southern Australia inferred from phylogeography. Quaternary Science Reviews 27, 2576–2585.
| Evidence for multiple refugia at different time scales during Pleistocene climatic oscillations in southern Australia inferred from phylogeography.Crossref | GoogleScholarGoogle Scholar |
Cale D, Pinder A (2018) Wheatbelt wetland biodiversity monitoring: fauna monitoring at Coyrecup Lake 1997-2012. Report number WWBM-FR02, Department of Biodiversity, Conservation and Attractions, WA, Australia.
Cale, DJ, Halse, SA, and Walker, CD (2004). Wetland monitoring in the Wheatbelt of south-west Western Australia: site descriptions, waterbird, aquatic invertebrate and groundwater data. Conservation Science Western Australia 5, 20–135.
Campagna V (2007) Limnology and biota of Lake Yindarlgooda: an inland salt lake in Western Australia under stress. PhD thesis, Curtin University, WA, Australia. Available at http://hdl.handle.net/20.500.11937/1883
Campbell, CE (1995). The influence of a predatory ostracod, Australocypris insularis, on zooplankton abundace and species composition in a saline lake. Hydrobiologia 302, 229–239.
| The influence of a predatory ostracod, Australocypris insularis, on zooplankton abundace and species composition in a saline lake.Crossref | GoogleScholarGoogle Scholar |
Chaplin, JA, Havel, JE, and Hebert, PDN (1994). Sex and ostracods. Trends in Ecology & Evolution 9, 435–439.
| Sex and ostracods.Crossref | GoogleScholarGoogle Scholar |
Chevin, L-M, and Hoffmann, AA (2017). Evolution of phenotypic plasticity in extreme environments. Philosophical Transactions of the Royal Society of London – B. Biological Sciences 372, 20160138.
| Evolution of phenotypic plasticity in extreme environments.Crossref | GoogleScholarGoogle Scholar |
Cole, GA (1960). The cyprid ostracod genus, Cypriconcha Sars. Transactions of the American Microscopical Society 79, 333–339.
| The cyprid ostracod genus, Cypriconcha Sars.Crossref | GoogleScholarGoogle Scholar |
Collier, KJ, Probert, PK, and Jeffries, M (2016). Conservation of aquatic invertebrates: concerns, challenges and conundrums. Aquatic Conservation: Marine and Freshwater Ecosystems 26, 817–837.
| Conservation of aquatic invertebrates: concerns, challenges and conundrums.Crossref | GoogleScholarGoogle Scholar |
Cox, RM, and Calsbeek, R (2010). Sex-specific selection and intraspecific variation in sexual size dimorphism. Evolution: International Journal of Organic Evolution 64, 798–809.
| Sex-specific selection and intraspecific variation in sexual size dimorphism.Crossref | GoogleScholarGoogle Scholar |
Davis, JA, McGuire, M, Halse, SA, Hamilton, D, Horwitz, P, McComb, AJ, Froend, RH, Lyons, M, and Sim, L (2003). What happens when you add salt: predicting impacts of secondary salinisation on shallow aquatic ecosystems by using an alternative-states model. Australian Journal of Botany 51, 715–724.
| What happens when you add salt: predicting impacts of secondary salinisation on shallow aquatic ecosystems by using an alternative-states model.Crossref | GoogleScholarGoogle Scholar |
De Deckker, P (1974). Australocypris, a new ostracod genus from Australia. Australian Journal of Zoology 22, 91–104.
| Australocypris, a new ostracod genus from Australia.Crossref | GoogleScholarGoogle Scholar |
De Deckker, P (1976). Trigonocypris, a new ostracod genus from Queensland. Australian Journal of Zoology 24, 145–157.
| Trigonocypris, a new ostracod genus from Queensland.Crossref | GoogleScholarGoogle Scholar |
De Deckker P (1977) The distribution of the ‘giant’ ostracods (family: Cyprididae Baird, 1845) endemic to Australia. In ‘Aspects of ecology and zoogeography of recent and fossil Ostracoda’. (Eds H Löffler, DL Danielopol) pp. 285–294. (W. Junk: The Hague, Netherlands)
De Deckker, P (1978). Comparative morphology and review of Mytilocyprinid ostracods (Family Cyprididae). Australian Journal of Zoology Supplementary Series 26, 1–62.
| Comparative morphology and review of Mytilocyprinid ostracods (Family Cyprididae).Crossref | GoogleScholarGoogle Scholar |
De Deckker, P (1981a). 10. Ostracods of athalassic saline lakes. Hydrobiologia 81, 131–144.
| 10. Ostracods of athalassic saline lakes.Crossref | GoogleScholarGoogle Scholar |
De Deckker, P (1981b). Taxonomy and ecology notes of some ostracods from Australian inland waters. Transactions of the Royal Society of South Australia 105, 91–138.
De Deckker, P (1982). On Caboncypris nunkeri De Decker gen. et sp. nov. Stereo-Atlas of Ostracod Shells 9, 125–132.
De Deckker, P (1983a). Notes on the ecology and distribution of non-marine ostracods in Australia. Hydrobiologia 106, 223–234.
| Notes on the ecology and distribution of non-marine ostracods in Australia.Crossref | GoogleScholarGoogle Scholar |
De Deckker, P (1983b). Australian salt lakes: their history, chemistry, and biota: a review. Hydrobiologia 105, 231–244.
| Australian salt lakes: their history, chemistry, and biota: a review.Crossref | GoogleScholarGoogle Scholar |
De Deckker P (2002) Ostracod palaeoecology. In ‘The Ostracoda: applications in quaternary research’. (Eds JA Holmes, AR Chivas) Vol. 131, pp. 121–134. (American Geophysical Union: Washington, DC, USA) https://doi.org/
| Crossref |
De Deckker, P, and Geddes, MC (1980). Seasonal fauna of ephemeral saline lakes near the Coorong lagoon, South Australia. Marine and Freshwater Research 31, 677–699.
| Seasonal fauna of ephemeral saline lakes near the Coorong lagoon, South Australia.Crossref | GoogleScholarGoogle Scholar |
De Deckker, P, and Williams, WD (1982). Chemical and biological features of Tasmanian salt lakes. Marine and Freshwater Research 33, 1127–1132.
| Chemical and biological features of Tasmanian salt lakes.Crossref | GoogleScholarGoogle Scholar |
Delorme, LD (1969). On the identity of the ostracode genera Cypriconcha and Megalocypris. Canadian Journal of Zoology 47, 271–281.
| On the identity of the ostracode genera Cypriconcha and Megalocypris.Crossref | GoogleScholarGoogle Scholar |
De Stasio, BT (1989). The seed bank of a freshwater Crustacean: copepodology for the plant ecologist. Ecology 70, 1377–1389.
| The seed bank of a freshwater Crustacean: copepodology for the plant ecologist.Crossref | GoogleScholarGoogle Scholar |
Di Marco, M, Chapman, S, Althor, G, Kearney, S, Besancon, C, Butt, N, Maina, JM, Possingham, HP, Rogalla von Bieberstein, K, Venter, O, and Watson, JEM (2017). Changing trends and persisting biases in three decades of conservation science. Global Ecology and Conservation 10, 32–42.
| Changing trends and persisting biases in three decades of conservation science.Crossref | GoogleScholarGoogle Scholar |
Fenwick, GD (1984). Life history and population biology of the giant ostracod Leuroleberis zealandica (Baird, 1850) (Myodocopida). Journal of Experimental Marine Biology and Ecology 77, 255–289.
| Life history and population biology of the giant ostracod Leuroleberis zealandica (Baird, 1850) (Myodocopida).Crossref | GoogleScholarGoogle Scholar |
Figuerola, J, and Green, AJ (2002). Dispersal of aquatic organisms by waterbirds: a review of past research and priorities for future studies. Freshwater Biology 47, 483–494.
| Dispersal of aquatic organisms by waterbirds: a review of past research and priorities for future studies.Crossref | GoogleScholarGoogle Scholar |
Finston, T (2000). Morphology and molecules conflict to confound species boundaries in salt lake ostracodes of the genus Mytilocypris (Crustacea: Ostracoda). Australian Journal of Zoology 48, 393–409.
| Morphology and molecules conflict to confound species boundaries in salt lake ostracodes of the genus Mytilocypris (Crustacea: Ostracoda).Crossref | GoogleScholarGoogle Scholar |
Finston, T (2002). Geographic patterns of population genetic structure in Mytilocypris (Ostracoda: Cyprididae): interpreting breeding systems, gene flow and history in species with differing distributions. Molecular Ecology 11, 1931–1946.
| Geographic patterns of population genetic structure in Mytilocypris (Ostracoda: Cyprididae): interpreting breeding systems, gene flow and history in species with differing distributions.Crossref | GoogleScholarGoogle Scholar |
Finston, TL (2004). Effect of a temporally heterogeneous environment on size and shape of the giant ostracods Mytilocypris (Ostracoda: Cyprididae) from Australian salt lakes. Marine and Freshwater Research 55, 499–507.
| Effect of a temporally heterogeneous environment on size and shape of the giant ostracods Mytilocypris (Ostracoda: Cyprididae) from Australian salt lakes.Crossref | GoogleScholarGoogle Scholar |
Finston, T (2007). Size, shape and development time are plastic traits in salt lake ostracods of the Mytilocypris mytiloides (Ostracoda: Cyprididae) species complex. Marine and Freshwater Research 58, 511–518.
| Size, shape and development time are plastic traits in salt lake ostracods of the Mytilocypris mytiloides (Ostracoda: Cyprididae) species complex.Crossref | GoogleScholarGoogle Scholar |
Fontana, SL, and Ballent, S (2005). A new giant cypridid ostracod (Crustacea) from southern Buenos Aires Province, Argentina. Hydrobiologia 533, 187–197.
| A new giant cypridid ostracod (Crustacea) from southern Buenos Aires Province, Argentina.Crossref | GoogleScholarGoogle Scholar |
Geddes, MC (1976). Seasonal fauna of some ephemeral saline waters in westren Victoria with particular reference to Parartemia zietziana Sayce (Crustacea: Anostraca). Marine and Freshwater Research 27, 1–22.
| Seasonal fauna of some ephemeral saline waters in westren Victoria with particular reference to Parartemia zietziana Sayce (Crustacea: Anostraca).Crossref | GoogleScholarGoogle Scholar |
Geddes, MC, De Deckker, P, Williams, WD, Morton, DW, and Topping, M (1981). 17. On the chemistry and biota of some saline lakes in Western Australia. Hydrobiologia 81, 201–222.
| 17. On the chemistry and biota of some saline lakes in Western Australia.Crossref | GoogleScholarGoogle Scholar |
Green, AJ, Jenkins, KM, Bell, D, Morris, PJ, and Kingsford, RT (2008). The potential role of waterbirds in dispersing invertebrates and plants in arid Australia. Freshwater Biology 53, 380–392.
| The potential role of waterbirds in dispersing invertebrates and plants in arid Australia.Crossref | GoogleScholarGoogle Scholar |
Gregory SJ (2007) The classification of inland salt lakes in Western Australia. PhD thesis, Curtin University, WA, Australia. Available at http://hdl.handle.net/20.500.11937/609
Halse, SA (1981). Faunal assemblages of some saline lakes near Marchagee, Western Australia. Marine and Freshwater Research 32, 133–142.
| Faunal assemblages of some saline lakes near Marchagee, Western Australia.Crossref | GoogleScholarGoogle Scholar |
Halse, SA, and McRae, JM (2004). New genera and species of ‘giant’ ostracods (Crustacea: Cyprididae) from Australia. Hydrobiologia 524, 1–52.
| New genera and species of ‘giant’ ostracods (Crustacea: Cyprididae) from Australia.Crossref | GoogleScholarGoogle Scholar |
Halse, SA, Ruprecht, JK, and Pinder, AM (2003). Salinisation and prospects for biodiversity in rivers and wetlands of south-west Western Australia. Australian Journal of Botany 51, 673–688.
| Salinisation and prospects for biodiversity in rivers and wetlands of south-west Western Australia.Crossref | GoogleScholarGoogle Scholar |
Hammer UT (1986) ‘Saline lake ecosystems of the world.’ (Dr W. Junk Publishers: Dordrecht, Netherlands)
Hebert, PDN, and Wilson, CC (2000). Diversity of the genus Daphniopsis in the saline waters of Australia. Canadian Journal of Zoology 78, 794–808.
| Diversity of the genus Daphniopsis in the saline waters of Australia.Crossref | GoogleScholarGoogle Scholar |
Hobday, AJ, and Lough, JM (2011). Projected climate change in Australian marine and freshwater environments. Marine and Freshwater Research 62, 1000–1014.
| Projected climate change in Australian marine and freshwater environments.Crossref | GoogleScholarGoogle Scholar |
Jellison R, Williams WD, Timms B, Alcocer J, Aladin NV (2008) Salt lakes: values, threats and future. In ‘Aquatic ecosystems: trends and global prospects’. (Ed. NVC Polunin) pp. 94–110. (Cambridge University Press) https://doi.org/
| Crossref |
Jolly, ID, McEwan, KL, and Holland, KL (2008). A review of groundwater–surface water interactions in arid/semi-arid wetlands and the consequences of salinity for wetland ecology. Ecohydrology 1, 43–58.
| A review of groundwater–surface water interactions in arid/semi-arid wetlands and the consequences of salinity for wetland ecology.Crossref | GoogleScholarGoogle Scholar |
Karanovic I (2012) ‘Recent freshwater ostracods of the world: Crustacea, Ostracoda, Podocopida.’ (Springer: Heidelberg, Germany) https://doi.org/
| Crossref |
Khan, TA (2003). Dietary studies on exotic carp (Cyprinus carpio L.) from two lakes of western Victoria, Australia. Aquatic Sciences 65, 272–286.
| Dietary studies on exotic carp (Cyprinus carpio L.) from two lakes of western Victoria, Australia.Crossref | GoogleScholarGoogle Scholar |
Khan, TA, Wilson, ME, and Bhise, MP (2002). Dietary studies on carp and other fish species in western Victorian lakes. Internationale Vereinigung für theoretische und angewandte Limnologie: Verhandlungen 28, 1191–1198.
| Dietary studies on carp and other fish species in western Victorian lakes.Crossref | GoogleScholarGoogle Scholar |
Kirono, DGC, Kent, DM, Jones, RN, and Leahy, PJ (2012). Assessing climate change impacts and risks on three salt lakes in western Victoria, Australia. Human and Ecological Risk Assessment: An International Journal 18, 152–167.
| Assessing climate change impacts and risks on three salt lakes in western Victoria, Australia.Crossref | GoogleScholarGoogle Scholar |
Ladle, RJ, and Foster, E (1992). Are giant sperm copulatory plugs? Acta Oecologica 13, 635–638.
Lawrie, AD, Chaplin, J, and Pinder, A (2021). Biology and conservation of the unique and diverse halophilic macroinvertebrates of Australian salt lakes. Marine and Freshwater Research 72, 1553–1576.
| Biology and conservation of the unique and diverse halophilic macroinvertebrates of Australian salt lakes.Crossref | GoogleScholarGoogle Scholar |
Lyons, MN, Halse, SA, Gibson, N, Cale, DJ, Lane, JAK, Walker, CD, Mickle, DA, and Froend, RH (2007). Monitoring wetlands in a salinizing landscape: case studies from the Wheatbelt region of Western Australia. Hydrobiologia 591, 147–164.
| Monitoring wetlands in a salinizing landscape: case studies from the Wheatbelt region of Western Australia.Crossref | GoogleScholarGoogle Scholar |
Maly, EJ, and Bayly, IAE (1991). Factors influencing biogeographic patterns of Australasian centropagid copepods. Journal of Biogeography 18, 455–461.
| Factors influencing biogeographic patterns of Australasian centropagid copepods.Crossref | GoogleScholarGoogle Scholar |
Maly, EJ, and Maly, MP (1997). Predation, competition, and co-occurrences of Boeckella and Calamoecia (Copepoda: Calanoida) in Western Australia. Hydrobiologia 354, 41–50.
| Predation, competition, and co-occurrences of Boeckella and Calamoecia (Copepoda: Calanoida) in Western Australia.Crossref | GoogleScholarGoogle Scholar |
Martens, K (1985a). Effects of temperature and salinity on postembryonic growth in Mytilocypris henricae (Chapman) (Crustacea, Ostracoda). Journal of Crustacean Biology 5, 258–272.
| Effects of temperature and salinity on postembryonic growth in Mytilocypris henricae (Chapman) (Crustacea, Ostracoda).Crossref | GoogleScholarGoogle Scholar |
Martens, K (1985b). Salinity tolerance of Mytilocypris henricae (Chapman) (Crustacea, Ostracoda). Hydrobiologia 124, 81–83.
| Salinity tolerance of Mytilocypris henricae (Chapman) (Crustacea, Ostracoda).Crossref | GoogleScholarGoogle Scholar |
Martens K (1986) Taxonomic revision of the subfamily Megalocypridinae Rome, 1965 (Crustacea, Ostracoda). Report number 9065696814, Verhandelingen van de Koninklijke Academie voor Wetenschappen, Letteren en Schone Kunsten van België, Klasse der Wetenschappen, Paleis der Academiën, Brussel, Belgium.
Martens K, Behen F (1994) ‘A checklist of the recent non-marine ostracods (Crustacea, Ostracoda) from the inland waters of South America and adjacent islands.’ (Ministère des Affaires Culturelles, Musée National d’Histoire Naturelle: Luxembourg)
Martens, K, and Savatenalinton, S (2011). A subjective checklist of the recent, free-living, non-marine Ostracoda (Crustacea). Zootaxa 2855, 1–79.
| A subjective checklist of the recent, free-living, non-marine Ostracoda (Crustacea).Crossref | GoogleScholarGoogle Scholar |
Martens, K, De Deckker, P, and Marples, TG (1985). Life history of Mytilocypris henricae (Chapman) (Crustacea: Ostracoda) in Lake Bathurst, New South Wales. Marine and Freshwater Research 36, 807–819.
| Life history of Mytilocypris henricae (Chapman) (Crustacea: Ostracoda) in Lake Bathurst, New South Wales.Crossref | GoogleScholarGoogle Scholar |
Martens K, Schön I, Meisch C, Horne DJ (2007) Global diversity of ostracods (Ostracoda, Crustacea) in freshwater. In ‘Freshwater animal diversity assessment. Vol. 198’. (Eds EV Balian, C Lévêque, H Segers, K Martens) pp. 185–193. (Springer: Dordrecht, Netherlands) https://doi.org/
| Crossref |
Martins, MJF (2019). Adult sex-ratio in ostracods and its implications for sexual selection. Invertebrate Reproduction & Development 63, 178–188.
| Adult sex-ratio in ostracods and its implications for sexual selection.Crossref | GoogleScholarGoogle Scholar |
Matzke-Karasz, R, Neil, JV, Smith, RJ, Symonová, R, Mořkovský, L, Archer, M, Hand, SJ, Cloetens, P, and Tafforeau, P (2014). Subcellular preservation in giant ostracod sperm from an early Miocene cave deposit in Australia. Proceedings of the Royal Society of London – B. Biological Sciences 281, 20140394.
| Subcellular preservation in giant ostracod sperm from an early Miocene cave deposit in Australia.Crossref | GoogleScholarGoogle Scholar |
Meisch, C, Smith, RJ, and Martens, K (2019). A subjective global checklist of the extant non-marine Ostracoda (Crustacea). European Journal of Taxonomy 492, 1–135.
| A subjective global checklist of the extant non-marine Ostracoda (Crustacea).Crossref | GoogleScholarGoogle Scholar |
Mernagh, TP, Bastrakov, EN, Jaireth, S, de Caritat, P, English, PM, and Clarke, JDA (2016). A review of Australian salt lakes and associated mineral systems. Australian Journal of Earth Sciences 63, 131–157.
| A review of Australian salt lakes and associated mineral systems.Crossref | GoogleScholarGoogle Scholar |
Pedler, RD, Ribot, RFH, and Bennett, ATD (2018). Long-distance flights and high-risk breeding by nomadic waterbirds on desert salt lakes. Conservation Biology 32, 216–228.
| Long-distance flights and high-risk breeding by nomadic waterbirds on desert salt lakes.Crossref | GoogleScholarGoogle Scholar |
Pinder, AM, Halse, SA, Shiel, RJ, Cale, DJ, and McRae, JM (2002). Halophile aquatic invertebrates in the Wheatbelt region of south-western Australia. Internationale Vereinigung für theoretische und angewandte Limnologie: Verhandlungen 28, 1687–1694.
| Halophile aquatic invertebrates in the Wheatbelt region of south-western Australia.Crossref | GoogleScholarGoogle Scholar |
Pinder, AM, Halse, SA, McRae, JM, and Shiel, RJ (2004). Aquatic invertebrate assemblages of wetlands and rivers in the Wheatbelt region of Western Australia. Records of the Western Australian Museum Supplement 67, 7–37.
| Aquatic invertebrate assemblages of wetlands and rivers in the Wheatbelt region of Western Australia.Crossref | GoogleScholarGoogle Scholar |
Pinder, AM, Halse, SA, McRae, JM, and Shiel, RJ (2005). Occurrence of aquatic invertebrates of the Wheatbelt region of Western Australia in relation to salinity. Hydrobiologia 543, 1–24.
| Occurrence of aquatic invertebrates of the Wheatbelt region of Western Australia in relation to salinity.Crossref | GoogleScholarGoogle Scholar |
Pinder, AM, Halse, SA, Shiel, RJ, and McRae, JM (2010). An arid zone awash with diversity: patterns in the distribution of aquatic invertebrates in the Pilbara region of Western Australia. Records of the Western Australian Museum, Supplement 78, 205–246.
| An arid zone awash with diversity: patterns in the distribution of aquatic invertebrates in the Pilbara region of Western Australia.Crossref | GoogleScholarGoogle Scholar |
Pinder, AM, Quinlan, K, Cale, DJ, and Leung, AE (2012). Aquatic invertebrates of the Hutt River and Hutt Lagoon catchments, Western Australia. Journal of the Royal Society of Western Australia 95, 29–51.
Pinder A, Quinlan K, Cale D, Shiel R (2013) Invertebrate communities and hydrological persistence in seasonal claypans of Drummond Nature Reserve, Western Australia. Report, Department of Parks and Wildlife, Government of Western Australia, WA, Australia.
Proctor, VW (1964). Viability of crustacean eggs recovered from ducks. Ecology 45, 656–658.
| Viability of crustacean eggs recovered from ducks.Crossref | GoogleScholarGoogle Scholar |
Punzalan, D, and Hosken, DJ (2010). Sexual dimorphism: why the sexes are (and are not) different. Current Biology 20, R972–R973.
| Sexual dimorphism: why the sexes are (and are not) different.Crossref | GoogleScholarGoogle Scholar |
Quinlan, K, Pinder, A, Coppen, R, and Jackson, J (2016). An opportunistic survey of aquatic invertebrates in the Goldfields region of Western Australia. Conservation Science Western Australia 10, 1–21.
Radke LC (2000) Solute divides and chemical facies in southeastern Australian salt lakes and the response of ostracods in time (Holocene) and space. PhD thesis, Australian National University, Canberra, ACT, Australia.
Radke, LC, Juggins, S, Halse, SA, De Deckker, P, and Finston, T (2003). Chemical diversity in south-eastern Australian saline lakes II: biotic implications. Marine and Freshwater Research 54, 895–912.
| Chemical diversity in south-eastern Australian saline lakes II: biotic implications.Crossref | GoogleScholarGoogle Scholar |
Roshier, DA, Whetton, PH, Allan, RJ, and Robertson, AI (2001). Distribution and persistence of temporary wetland habitats in arid Australia in relation to climate. Austral Ecology 26, 371–384.
| Distribution and persistence of temporary wetland habitats in arid Australia in relation to climate.Crossref | GoogleScholarGoogle Scholar |
Rossi, V, Albini, D, Benassi, G, and Menozzi, P (2012). To rest in hydration: hatching phenology of resting eggs of Heterocypris incongruens (Crustacea: Ostracoda). Fundamental and Applied Limnology/Archiv für Hydrobiologie 181, 49–58.
| To rest in hydration: hatching phenology of resting eggs of Heterocypris incongruens (Crustacea: Ostracoda).Crossref | GoogleScholarGoogle Scholar |
Ruiz, F, Abad, M, Bodergat, AM, Carbonel, P, Rodríguez-Lázaro, J, González-Regalado, ML, Toscano, A, García, EX, and Prenda, J (2013). Freshwater ostracods as environmental tracers. International Journal of Environmental Science and Technology 10, 1115–1128.
| Freshwater ostracods as environmental tracers.Crossref | GoogleScholarGoogle Scholar |
Saccò, M, White, NE, Harrod, C, Salazar, G, Aguilar, P, Cubillos, CF, Meredith, K, Baxter, BK, Oren, A, Anufriieva, E, Shadrin, N, Marambio-Alfaro, Y, Bravo-Naranjo, V, and Allentoft, ME (2021). Salt to conserve: a review on the ecology and preservation of hypersaline ecosystems. Biological Reviews 96, 2828–2850.
| Salt to conserve: a review on the ecology and preservation of hypersaline ecosystems.Crossref | GoogleScholarGoogle Scholar |
Sánchez, MI, Hortas, F, Figuerola, J, and Green, AJ (2012). Comparing the potential for dispersal via waterbirds of a native and an invasive brine shrimp. Freshwater Biology 57, 1896–1903.
| Comparing the potential for dispersal via waterbirds of a native and an invasive brine shrimp.Crossref | GoogleScholarGoogle Scholar |
Sandberg, PA, and Plusquellec, PL (1974). Notes on the anatomy and passive dispersal of Cyprideis (Cytheracea, Ostracoda). Geoscience and Man 6, 1–26.
Smith AJ, Horne DJ, Martens K, Schön I (2015) Class Ostracoda. In ‘Thorp and Covich’s freshwater invertebrates’, 4th edn. (Eds JH Thorp, DC Rogers) pp. 757–780. (Elsevier: New York, NY, USA) https://doi.org/
| Crossref |
Smith, RJ, Matzke-Karasz, R, Kamiya, T, and De Deckker, P (2016). Sperm lengths of non-marine cypridoidean ostracods (Crustacea). Acta Zoologica 97, 1–17.
| Sperm lengths of non-marine cypridoidean ostracods (Crustacea).Crossref | GoogleScholarGoogle Scholar |
Strachan, SR, Chester, ET, and Robson, BJ (2014). Microrefuges from drying for invertebrates in a seasonal wetland. Freshwater Biology 59, 2528–2538.
| Microrefuges from drying for invertebrates in a seasonal wetland.Crossref | GoogleScholarGoogle Scholar |
Strachan, SR, Chester, ET, and Robson, BJ (2016). Habitat alters the effect of false starts on seasonal-wetland invertebrates. Freshwater Biology 61, 680–692.
| Habitat alters the effect of false starts on seasonal-wetland invertebrates.Crossref | GoogleScholarGoogle Scholar |
Taylor, RJ, and Hoxley, G (2003). Dryland salinity in Western Australia: managing a changing water cycle. Water Science and Technology 47, 201–207.
| Dryland salinity in Western Australia: managing a changing water cycle.Crossref | GoogleScholarGoogle Scholar |
Timms, BV (1993). Saline lakes of the Paroo, inland New South Wales, Australia. Hydrobiologia 267, 269–289.
| Saline lakes of the Paroo, inland New South Wales, Australia.Crossref | GoogleScholarGoogle Scholar |
Timms, BV (1998). Further studies on the saline lakes of the eastern Paroo, inland New South Wales, Australia. Hydrobiologia 381, 31–42.
| Further studies on the saline lakes of the eastern Paroo, inland New South Wales, Australia.Crossref | GoogleScholarGoogle Scholar |
Timms, BV (2005). Salt lakes in Australia: present problems and prognosis for the future. Hydrobiologia 552, 1–15.
| Salt lakes in Australia: present problems and prognosis for the future.Crossref | GoogleScholarGoogle Scholar |
Timms, BV (2006). The large branchiopods (Crustacea: Branchiopoda) of gnammas (rock holes) in Australia. Journal of the Royal Society of Western Australia 89, 163–173.
Timms, BV (2009a). A study of the salt lakes and salt springs of Eyre Peninsula, South Australia. Hydrobiologia 626, 41–51.
| A study of the salt lakes and salt springs of Eyre Peninsula, South Australia.Crossref | GoogleScholarGoogle Scholar |
Timms, BV (2009b). Study of the saline lakes of the Esperance hinterland, Western Australia, with special reference to the roles of acidity and episodicity. Natural Resources and Environmental Issues 15, 44.
Timms, BV (2012). An appraisal of the diversity and distribution of large branchiopods (Branchiopoda: Anostraca, Laevicaudata, Spinicaudata, Cyclestherida, Notostraca) in Australia. Journal of Crustacean Biology 32, 615–623.
| An appraisal of the diversity and distribution of large branchiopods (Branchiopoda: Anostraca, Laevicaudata, Spinicaudata, Cyclestherida, Notostraca) in Australia.Crossref | GoogleScholarGoogle Scholar |
Timms, BV (2013). Aquatic invertebrates of pit gnammas in southwest Australia. Journal of the Royal Society of Western Australia 96, 55–67.
Timms, B (2014). A review of the biology of Australian halophilic anostracans (Branchiopoda: Anostraca). Journal of Biological Research-Thessaloniki 21, 21.
| A review of the biology of Australian halophilic anostracans (Branchiopoda: Anostraca).Crossref | GoogleScholarGoogle Scholar |
Timms, BV, Pinder, AM, and Campagna, VS (2009). The biogeography and conservation status of the Australian endemic brine shrimp Parartemia (Crustacea, Anostraca, Parartemiidae). Conservation Science Western Australia 7, 413–427.
Troudet, J, Grandcolas, P, Blin, A, Vignes-Lebbe, R, and Legendre, F (2017). Taxonomic bias in biodiversity data and societal preferences. Scientific Reports 7, 9132.
| Taxonomic bias in biodiversity data and societal preferences.Crossref | GoogleScholarGoogle Scholar |
Victor, R, and Fernando, CH (1981). Description of a new species of the genus Hungarocypris Vavra, 1906 (Crustacea: Ostracoda) from Sulawesi, Indonesia, with a discussion on the distribution of the genus. Hydrobiologia 77, 145–154.
| Description of a new species of the genus Hungarocypris Vavra, 1906 (Crustacea: Ostracoda) from Sulawesi, Indonesia, with a discussion on the distribution of the genus.Crossref | GoogleScholarGoogle Scholar |
Watts RJ (1999) Biodiversity in the Paroo River and its wetlands. In ‘A free-flowing river: the ecology of the Paroo River’. (Ed. RT Kingsford) pp. 13–22. (NSW National Parks and Wildlife Service: Sydney, NSW, Australia)
Weston, M (2007). The foraging and diet of non-breeding hooded plovers Thinornis rubricollis in relation to habitat type. Journal of the Royal Society of Western Australia 90, 89–95.
Wilkinson I, Wilby P, Williams P, Siveter D, Vannier J (2007) Ostracod carnivory through time. In ‘Predation in organisms’. (Ed. AMT Elewa) pp. 39–57. (Springer) https://doi.org/
| Crossref |
Williams, WD (1984). Chemical and biological features of salt lakes on the Eyre Peninsula, South Australia, and an explanation of regional differences in the fauna of Australian salt lakes. Internationale Vereinigung für theoretische und angewandte Limnologie: Verhandlungen 22, 1208–1215.
| Chemical and biological features of salt lakes on the Eyre Peninsula, South Australia, and an explanation of regional differences in the fauna of Australian salt lakes.Crossref | GoogleScholarGoogle Scholar |
Williams, WD (1986). Conductivity and salinity of Australian salt lakes. Marine and Freshwater Research 37, 177–182.
| Conductivity and salinity of Australian salt lakes.Crossref | GoogleScholarGoogle Scholar |
Williams, WD (1991). Saline lake microcosms (microecosystems) as a method of investigating ecosystem attributes. Internationale Vereinigung für theoretische und angewandte Limnologie: Verhandlungen 24, 1134–1138.
| Saline lake microcosms (microecosystems) as a method of investigating ecosystem attributes.Crossref | GoogleScholarGoogle Scholar |
Williams, WD (1995). Lake Corangamite, Australia, a permanent saline lake: conservation and management issues. Lakes & Reservoirs: Research & Management 1, 55–64.
| Lake Corangamite, Australia, a permanent saline lake: conservation and management issues.Crossref | GoogleScholarGoogle Scholar |
Williams, WD (1998). Salinity as a determinant of the structure of biological communities in salt lakes. Hydrobiologia 381, 191–201.
| Salinity as a determinant of the structure of biological communities in salt lakes.Crossref | GoogleScholarGoogle Scholar |
Williams, WD (2002). Environmental threats to salt lakes and the likely status of inland saline ecosystems in 2025. Environmental Conservation 29, 154–167.
| Environmental threats to salt lakes and the likely status of inland saline ecosystems in 2025.Crossref | GoogleScholarGoogle Scholar |
Williams WD, Geddes MC (1991) Anostracans of Australian salt lakes, with particular reference to a comparison of Parartemia and Artemia. In ‘Artemia biology’. (Eds RA Browne, P Sorgeloos, CNA Trotman) pp. 351–368. (CRC Press: Boca Raton, FL, USA)
Williams, WD, and Sherwood, JE (1994). Definition and measurement of salinity in salt lakes. International Journal of Salt Lake Research 3, 53–63.
| Definition and measurement of salinity in salt lakes.Crossref | GoogleScholarGoogle Scholar |
Williams, WD, Boulton, AJ, and Taaffe, RG (1990). Salinity as a determinant of salt lake fauna: a question of scale. Hydrobiologia 197, 257–266.
| Salinity as a determinant of salt lake fauna: a question of scale.Crossref | GoogleScholarGoogle Scholar |


