Genetic diversity and restricted genetic connectivity in an endangered marine fish (Brachionichthys hirsutus) provides a model for conservation management in related and data-deficient species
Sharon A. Appleyard A D , Tim P. Lynch B , Mark A. Green B and Francisco Encinas-Viso C
A D , Tim P. Lynch B , Mark A. Green B and Francisco Encinas-Viso C
A CSIRO Australian National Fish Collection, National Research Collections Australia, Castray Esplanade, Hobart, Tas. 7004, Australia.
B CSIRO Oceans and Atmosphere, Castray Esplanade, Hobart, Tas. 7004, Australia.
C CSIRO Australian National Herbarium, National Research Collections Australia, Clunies Ross Street, Canberra, ACT 2601, Australia.
D Corresponding author. Email: sharon.appleyard@csiro.au
Marine and Freshwater Research 72(12) 1735-1745 https://doi.org/10.1071/MF21169
Submitted: 8 June 2021 Accepted: 22 July 2021 Published: 26 August 2021
Journal Compilation © CSIRO 2021 Open Access CC BY-NC-ND
Abstract
Determining the genetic diversity and differentiation among populations is a critical element of conservation biology, but for many aquatic, data-deficient species with small population sizes, this is not possible. Closely related species may therefore provide a model. For the first time, using over 4000 single-nucleotide polymorphism loci, we characterise the population genetic diversity and structure of one of the world’s rarest marine fish, the spotted handfish (Brachionichthys hirsutus), a species which is also a member of the most threatened marine bony fish family (Brachionichthyidae). Fin clips were taken from 170 live spotted handfish across seven disjunct sites within the only known estuary (in Tasmania, Australia) where multiple populations of the species are found. Spatially discrete populations clustered into three genetic groupings and a significant variance in allele frequencies among populations (overall FST = 0.043), even at the small scale of the estuary, was observed. Furthermore, low contemporary migration rate estimates suggest low genetic homogeneity between locations. Because of the low genetic connectivity, population clusters of spotted handfish within the estuary should be considered as separate conservation management units. This insight should be considered for management and conservation strategies of other data-deficient and threatened species in the family.
Keywords: spotted handfish, SNP, population structure, threatened species.
Introduction
The successful management of aquatic biodiversity, including endangered species, requires an understanding of the factors that influence range and connectivity (Waples and Punt 2008; Lowe and Allendorf 2010; Beheregaray et al. 2017; Padovan et al. 2020). Obtaining this knowledge is challenging for many aquatic species, because of habitats that lack physical barriers, varying dispersal potentials of different life stages, ecological differences among species and populations and logistical difficulties of tracking or tracing individuals both spatially and temporally (Ward et al. 1994; Avise 1998; Waples 1998; Bargelloni et al. 2000; Lowe and Allendorf 2010; Junge et al. 2019). Additionally, determining genetic diversity (in species where the number of individuals in the wild may have been negatively affected or heavily reduced) for the purposes of understanding a species recovery potential is challenging because of limited sampling opportunities. This is particularly the case for rare, endangered and or data-deficient species and means conservation management decisions often need to be made using proxy data from similar species (Jamieson et al. 2008; Coates et al. 2018).
Although methods such as capture–mark–recapture can track demographic animal movements, only genetics can detect whether movement has resulted in reproductive contribution into the subsequent population (Lowe and Allendorf 2010; Ovenden 2013). Molecular techniques and population-wide data can be used to assess genetic and genomic diversity, estimate effective population sizes, track movements of individuals and determine genetic connectivity among populations (Lowe and Allendorf 2010; Ovenden 2013). These techniques can therefore determine the degree to which gene flow affects evolutionary processes within populations (Waples and Gaggiotti 2006; Lowe and Allendorf 2010; Ovenden 2013). Importantly, genetic connectivity is not demographic connectivity, with the two differing primarily in the degree on which population growth rates are affected by dispersal (Lowe and Allendorf 2010; Ovenden 2013).
Rapid advancements in genomic analyses and high-throughput sequencing now enable the simultaneous identification and screening of high-density genome single-nucleotide polymorphism (SNP) markers in individuals of non-model organisms such as threatened species, without the need for a reference genome (Baird et al. 2008; Elshire et al. 2011; Peterson et al. 2012; Paris et al. 2017). These new methods have allowed for insights into the conservation and management of various aquatic species including the critically endangered Australian freshwater fish, the Murray cod (Maccullochella peelii). SNPs were used to examine population structure and adaptation potential to inform a risk assessment framework, which suggested controlled mixing between genetically distinct populations for enhancing resilience of the species (Harrisson et al. 2017). Dresser et al. (2018) used population SNP data in southern bog turtles (Glyptemys muhlenbergii) to determine that genetic population structure corresponded with political boundaries of administrative units in the United States, whereas conservation management of two species of anadromous alosine fishes (alewife, Alosa pseudoharengus, and blueback herring, Alosa aestivalis) from the Atlantic coast of North America is now based on genetic SNP assignments of marine captured individuals back to their regional stock of origin (Baetscher et al. 2017).
Our genomic investigation was undertaken to gain the first insights into diversity, genetic differentiation, and connectivity of a member of the most endangered family of bony marine fishes, the Brachionichthyidae (Stuart-Smith et al. 2020). The spotted handfish (Brachionichthys hirsutus) is a critically endangered shallow-water anglerfish, which is endemic to south-eastern Tasmania (Bruce et al. 1998; Last and Gledhill 2009). It is a member of a diverse family, consisting of 14 species, and which includes the only exclusively marine bony fish to be recognised as Extinct (in modern time), namely, the smooth handfish (Sympterichthys unipennis). Seven of the other species in the family are listed as Critically Endangered or Endangered, and five species are listed as Data Deficient and are known from fewer than five specimens, and seven species have not been seen for between 15 and 36 years (Stuart-Smith et al. 2020). Of the extant species, the spotted handfish is the most intensively studied and has been used as a model to guide International Union for Conservation of Nature (IUCN) Red List of Threatened Species assessment updates for other members of the family (Stuart-Smith et al. 2020).
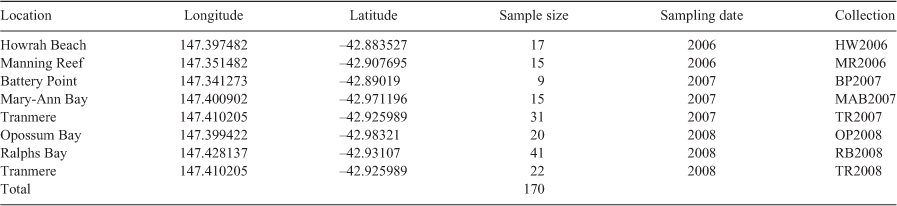
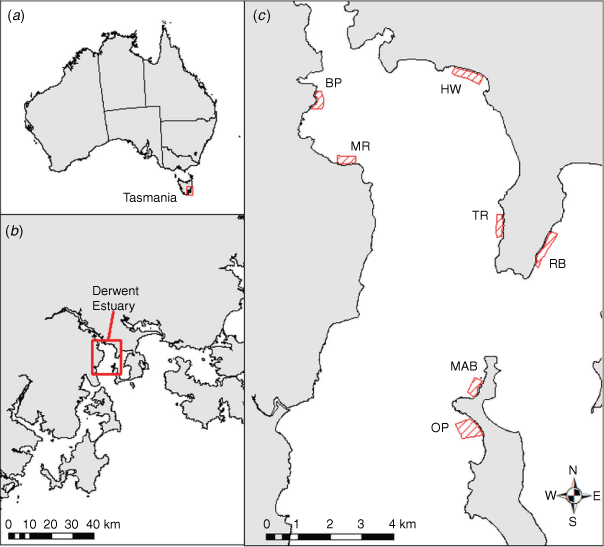
The spotted handfish is a small (individuals grow up to ~135 mm; Bessell 2018), rare and cryptic species found in the cool, temperate coastal marine waters in Tasmania (Bruce et al. 1998; Lynch et al. 2015; Wong et al. 2018). Between the 1980s and 1990s, spotted handfish experienced significant population declines (Barrett 1996), resulting in the species being the first marine fish to be listed as Critically Endangered under the Australian Environment Protection and Biodiversity Conservation Act (1999); it is also listed as Endangered in Tasmania (under the Threatened Species Protection Act 1995) and as Critically Endangered by IUCN (Bruce and Last 1996).
Once widespread from the eastern coast of Tasmania to the D’Entrecasteaux Channel in southern Tasmania and locally common (Last et al. 1983), studies and underwater surveys in the past 10 years have indicated the presence of only nine known ‘hotspots’ of spotted handfish in the Derwent estuary (Green 2005, 2007; Last et al. 2007; Last and Gledhill 2009) and one recently re-discovered location in the D’Entrecasteaux Channel (Wong and Lynch 2017). Locations and boundaries were initially set by exploratory surveys in 1996 (Barrett 1996), 1998–1999 (Green and Bruce 2000), 2006–2007 (Green 2007) and further refined by surveys lead by M. Green between 2007 and 2014. These hotspots are the only known locations of spotted handfish populations (as at 2021, following extensive surveys, by the authors T. Lynch and M. Green, National Handfish Recovery Team (NHRT) divers and consultants undertaking environmental impact assessments). In these areas, spotted handfish are habitat specialists that live on soft sediments, at depths from 1 to 60 m (Last and Gledhill 2009), with the location of the hotspots likely being a result of particular micro-habitat features for which spotted handfish shows a strong preference (Lynch et al. 2015; Lynch 2018; Wong et al. 2018).
Natural dispersal of handfish generally appears to be restricted, both for juveniles and adults. Capture–mark–recapture analyses of spotted handfish movements have shown that individuals move within, but not between, hotspot locations (Bessell 2018). The maximum recorded (net) distance moved by a spotted handfish is ~570 m, with this individual taking 585 days to move this distance (Bessell 2018). As is suggested by the name, all handfishes have modified fins and walk across the bottom rather than swim. This lack of adult movement may be common or even more extreme for reef-based handfish species. Reproductive knowledge of several species in this family (e.g. spotted handfish; Ziebell’s handfish, Brachiopsilus ziebelli; red handfish, Thymichthys politus) is limited. However, it is thought that all have direct recruitment, with female handfish laying benthic clutches of eggs (60–250 eggs) attached to small structures formed by sponges, seaweed, seagrass or stalked ascidians (Bruce et al. 1998). Handfish parents then guard the clutches till fertilised eggs hatch as fully metamorphised juveniles (Hormann 2019); the species thereby lacks a dispersive planktonic phase and directly recruits to the adult habitat (Bruce et al. 1998). It is thought that egg guarding and direct recruitment are common across species, with these life-history characteristics having also been observed in Ziebell’s and red handfish.
Spotted handfish appear to then have a juvenile growth period of approximately 2 years (Bruce et al. 1998). The extent of genetic or genomic connectivity of spotted handfish among the hotspot locations in the Derwent estuary is unknown, but with these general life-history characteristics of habitat specialisation and low dispersal, the potential for population fragmentation could be high.
Here, we use genomic markers deployed in DNA extracted from small fin clips taken from spotted handfish individuals from the only known currently existing hotspot locations within the Derwent estuary to investigate genetic connectivity. A genotype-by-sequencing (GBS, Elshire et al. 2011) approach was deployed, which, for the first time, generated SNPs in this endangered anglerfish. We refer herein to genetic diversity, although, as thousands of SNPs across the genome were used, this is sometimes referred to as ‘genomic diversity’. Genetic and genomic diversity are therefore used interchangeably. Our objectives were to (1) document the level of genetic diversity in spotted handfish individuals and (2) test the null hypothesis of genetic homogeneity across the remnant populations (termed herein ‘collections’) of spotted handfish in the Derwent estuary, thereby facilitating a better understanding of connectivity throughout the species range and establishing a model for connectivity for species within the threatened Family Brachionichthyidae.
Materials and methods
Sampling of spotted handfish
Fin clip tissue samples from individual fish were obtained during population surveys by divers in the Derwent estuary during 2006–2008. Tissue samples were sourced from seven known spatial locations in the estuary (Table 1, Fig. 1). One site (Tranmere) was sampled twice in 2007 and 2008; fish at all other locations were sampled once.
|
|
Population surveys, along 100 m transect lines, occurred at each location using underwater visual census methods while SCUBA diving (Green 2001, 2007; Green et al. 2012). Each fish encountered within the 3 m wide search zone was measured (total length by using callipers), photographed (to identify individuals using their unique spot patterns) and an anterior 3–4 mm of the second dorsal fin was removed from individuals (with clean dissection scissors) and placed into vials underwater that were later refilled with 100% ethanol on the surface. Fin clips in ethanol were stored at –20°C (with ethanol topped up) until DNA extraction.
DNA extraction, SNP library development and genotyping
Up to 25 mg of fin clip tissue per individual was DNA extracted using the Wizard SV 96 Genomic Purification System (Promega, Australia), as per the manufacturer’s instructions, with the additions that fin clips were digested overnight with Proteinase K (Promega) and DNA was eluted in 160 µL of DNAse free water (Promega). DNA was subaliquoted and stored at –80°C. An aliquot of each DNA sample was sent to the Australian Genome Research Facility (AGRF, Melbourne Australia) by courier (at room temperature) for SNP genotyping (by GBS).
At AGRF, DNA was digested with two restriction enzymes (EcoRI and MspI) and an in-house library preparation was undertaken (see the ‘SNP processing’ section in the Supplementary material). Sequencing of libraries was undertaken on an Illumina NextSeq platform (Illumina Inc., USA) according to AGRF in-house GBS methodologies. AGRF then processed the sequencing reads using their in-house bioinformatic pipeline and Stacks software (ver. 1.47-2, see http://catchenlab.life.illinois.edu/stacks/; Catchen et al. 2011, 2013). AGRF provided the post-processed SNPs in a variant call file (VCF).
SNP filtering and data analyses
Individual indexing information was removed using bcftools reheader (ver. 1.10, see http://samtools.github.io/bcftools/bcftools.html#reheader; Li et al. 2009) and the SNPs were filtered initially by using VCFtools (ver. 0.1.14, https://github.com/vcftools/vcftools; Danecek et al. 2011), with preliminary high-level filtering undertaken by treating all individuals as belonging to one group and not applying collection filtering. The pipeline for filtering is outlined in the ‘High performance computing SNP filtering’ section of the Supplementary material. The original VCF file from AGRF (from an overarching spotted handfish study with individuals from 1998 to 2019, see Lynch et al. 2020), a renamed and filtered VCF file (based on individuals from the overarching study, Lynch et al. 2020), the spotted handfish strata collection data from the overarching study and a genepop (Rousset 2008) input file (for the eight spotted handfish collections from 2006 to 2008) are lodged on the CSIRO Data Access Portal, https://doi.org/10.25919/jekk-e341.
Subsequent collection level filtering on the resulting VCF file within the R software environment was undertaken (on a per collection basis, with duplicates removed) using R (ver. 3.5.1, R Foundation for Statistical Computing, Vienna, Austria, see https://www.R-project.org/) and R-Studio (ver. 1.1.463, RStudio, Inc., Boston, MA, USA, see http://www.rstudio.com/). Additional population genomics packages in R and stand-alone programs were used for file conversions and genetic diversity analyses. The ‘R filtering’ and ‘Population genetic analyses – diversity, proximity and structure’ sections of the Supplementary material outline these programs.
In the absence of a spotted handfish genome, because environmental parameters were not collected at the time and with small collection sample sizes, analyses were not undertaken for candidate loci or historical demographic modelling. Average genetic diversity estimates (including allelic richness, mean observed and expected heterozygosity (HO and HE respectively), estimates of the inbreeding coefficient (FIS) per collection (calculated across all loci in each collection; as per Robertson and Hill 1984; Weir and Cockerham 1984, with collection FIS values assessed for significance by Fisher’s exact tests) and pairwise population differentiation (based on FST; Wright 1943, and as implemented by Weir and Cockerham 1984), were undertaken. Significance for all pairwise FST tests was assessed following 10 000 permutations and P-values for each pairwise comparison were corrected following the Benjamini and Yekutieli (2001) false discovery rate correction approach.
The overall structure and observed genetic clusters in the spotted handfish SNP data were determined using a combination of stepwise approaches (as recommended by Perez et al. 2018), starting with an Analysis of Molecular Variance (AMOVA; Excoffier et al. 1992).
Redundancy Analysis (RDA; a linear, multivariate constrained ordination technique) was then used to analyse regression between genetic distance (FST values; the response variable) and environmental data (i.e. the geographic distances from the only known locations of spotted handfish in the Derwent Estuary; the explanatory variable) for Identity by Distance (IBD) testing. The resulting Principal-Component Analysis matrix of fitted values produced canonical axes, which were considered linear combinations of the predictor (Legendre and Legendre 2012), with the significance of the linear model tested following 10 000 permutations. RDA was undertaken in the R package, vegan (ver. 2.5-7, J. Oksanen, F. G. Blanchet, M. Friendly, R. Kindt, P. Legendre, D. McGlinn, P. R. Minchin, R. B. O’Hara, G. L. Simpson, P. Solymos, M. H. H. Stevens, E. Szoecs, and H. Wagner, see https://cran.r-project.org/web/packages/vegan/index.html).
Since the collection of the samples outlined in this study, the Howrah Beach local population has been extended west to the adjacent beach and the local Opossum Bay population has been extended south to an adjacent beach. A further isolated population was discovered in a different estuary, the Huon estuary, which is 40 km south-west from the closest Derwent estuary population. This additional local population does not affect the overall spatial scale of the Derwent estuary sampling (two of the authors, T. Lynch and M. Green are on the spotted handfish recovery team).
Additionally, the Bayesian model in STRUCTURE (ver. 2.3.4, see https://web.stanford.edu/group/pritchardlab/structure_software/release_versions/v2.3.4/html/structure.html; Pritchard et al. 2000) was deployed. Because the small number of spotted handfish individuals sampled from the collections may invalidate the model assumptions of STRUCURE (e.g. the model assumes Hardy–Weinberg Equilibrium and no linkage among markers; Pritchard et al. 2000), a model-free (Jombart et al. 2010) Discriminant Analysis of Principal Components (DAPC) was also deployed.
Recent migration rates (i.e. the proportion of immigrants per collection) were estimated using BayesAss (ver. 3.0.4, https://github.com/brannala/BA3/releases; Wilson and Rannala 2003). This Bayesian analysis estimated the rates and direction of recent gene flow among the sampled collections. The Markov Chain Monte Carlo (MCMC) mixing parameters of migration rates, allele frequencies and inbreeding coefficients were adjusted as recommended by Rannala (2007) (for BayesAss). Five independent runs, using different seed numbers with a burn-in of 1 000 000 iterations followed by 10 000 000 MCMC iterations (sampling every 100 iterations) was undertaken. Convergence and mixing of chains were checked by plotting trace files using Tracer (ver. 1.7.1, A. Rambaut, M. A. Suchard, D. Xie, and A. J. Drummond, see http://beast.bio.ed.ac.uk/Tracer).
The ‘Population genetic analyses – diversity, proximity and structure’ section of the Supplementary material outlines the details for the above analyses and gives additional background as to the deployment of these tests.
Results
The GBS produced 65.2 GB of data and 431 852 810 reads (average 2 219 351 reads per individual). Following analysis in Stacks and filtering (for informative and polymorphic SNPs across individuals in at least one collection, and filtering out individuals with low numbers of RAD-tags and depth), a final dataset of 4172 SNPs in 153 individuals from the eight spotted handfish collections was obtained.
Genetic diversity (measured as the percentage of polymorphic SNP loci in each collection) and heterozygosity varied across the eight collections. As Table 2 outlines, the percentage of polymorphic loci ranged from 71% in BP2007 and HW2006 to >90% in OP2008, RB2008 and TR2007. The average gene diversity within the collections was HS = 0.224, with the mean observed heterozygosity in the spotted handfish collections being 0.250. Observed and expected heterozygosity were similar among the collections, with observed heterozygosity in each of the collections varying slightly, although HO in the largest collection, RB2008, was the lowest. Samples from the two smallest collections (BP2007 and HW2006) showed the largest average inbreeding estimates (FIS = 0.098 and 0.139 respectively) and the FIS estimates for the two Tranmere samplings (2007 and 2008) were very similar (0.073 and 0.080). Overall, average inbreeding estimates, which as a reflection of the proportion of variance in the subcollections contained in individuals, was not high at FIS = 0.080.

|
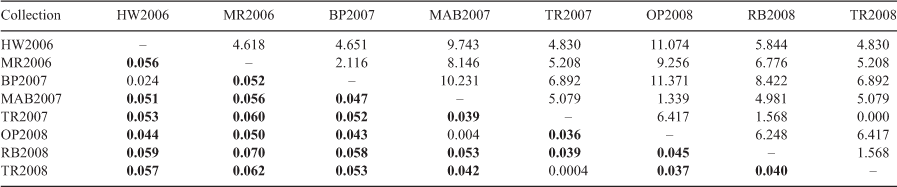
Genetic differentiation (based on overall FST, and which reflects variance among the collections) across the eight collections was significant at 0.043 (P = 0.001). On further inspection, pairwise FST values among the eight collections (Table 3) also demonstrated significant genetic differentiation between most collections, aside from HW2006 and BP2007 (FST = 0.024, P > 0.002); MAB2007 and OP2008 (FST = 0.004, P > 0.002) and the two temporal collections from Tranmere (TR2007 and TR2008, FST = 0.0004, P > 0.002). The significance of differentiation among collections was irrespective of the correction factor applied, because all other P-values were 0.000.
When collections that were not significantly different were combined (i.e. HW2006 and BP2007; TR2007 and TR2008; MAB2007 and OP2008) and the FST analysis was repeated, significant differentiation (FST 0.037 – 0.070, P < 0.001) was still observed among all comparisons. On the basis of the eight (non-combined) collections, the constrained ordination RDA showed that 100% of the variation in FST (genetic distance) was explained by geographic distance (adjusted R2 = 1.000, P < 0.05; see also Fig. S2 and S3 of the Supplementary material).
AMOVA analyses, based on multi-locus genotypes and squared Euclidean distances between individuals (calculated from the within-individual allele frequencies) indicated a similar significant differentiation of allele frequency covariances (ΦST = 0.045, P < 0.001) across the eight collections; this outcome was the same when the AMOVA was repeated and based on the five collections (ΦST = 0.045, P < 0.001). On the basis of the SNP data from the eight collections, although K > 2.5 was chosen by the Evanno et al. (2005) method as the most likely number of spotted handfish genetic clusters in the Derwent estuary (see Fig. S4 of the Supplementary material), we cautiously (see also Cullingham et al. 2020) observed additional substructuring, as detected by the STRUCTURE analyses K = 3 (see Fig. 2b).

|
Similarly, the model-free DAPC plot (Fig. 3) highlighted a defined pattern of genetic clustering across the collections, with separation being observed across three main groupings. Individuals from the top part of the estuary at Howrah Beach (HW2006), Manning Reef (MR2006) and Battery Point (BP2007) formed one group of genetic proximal individuals. A second overlapping group of individuals were from the middle part of the estuary from the two collections at Tranmere (TR2007, TR2008) and the Ralphs Bay (RB2008) collection. For this cluster, as Fig. 3 highlights, there was admixture between the two temporal samplings from Tranmere (TR). Individual fish had been photographed when fin clipped at both sampling times and were not recognised as recaptures in 2008; therefore, it is unlikely that there is a subsample of individuals fin-clipped twice at Tranmere. A third proximal grouping of individuals (again overlapping) appears in the lower half of the plot, which are from individuals from the lower sections of the estuary at Mary Anne Bay (MAB2007) and Opossum Bay (OP2008).

|
The Battery Point (BP) individuals, although part of the proximal genetic grouping in the top left of the plot, were more separated in plot space from the Manning Reef (MR) individuals, even though these two sites are adjacent. This is likely to reflect the small but significant FST value between these two collections. Two of the 40 Ralphs Bay individuals had SNP profiles that were closer to those observed in individuals outside of the second cluster. The DAPC analysis, along with the assignment of the spotted handfish individuals to the three main clusters, also provided a visual assessment of the between-population patterns (Jombart et al. 2010). The spatial placement of the spotted handfish groups in the DAPC somewhat mimics that of the sampling locations in the Derwent estuary, with the Battery Point, Manning Reef and Howrah Beach locations being northerly to those of Tranmere, Ralphs Bay and the more southerly locations of Mary-Ann Bay and Opossum Bay.
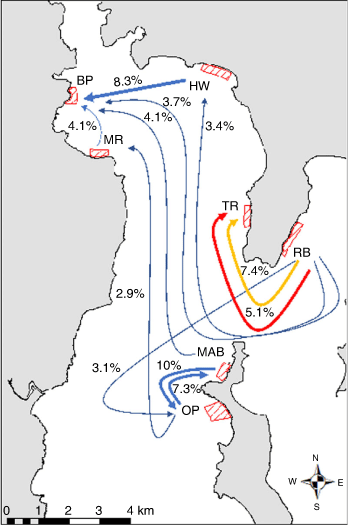
Migration analyses showed asymmetric estimated gene flow among the different locations in the Derwent estuary, e.g. between BP2007 and HW2006; HW2006 and RB2008; TR2007 and RB2008; MR2006 and OP2008; and OP2008 and MAB2007 (see Table S1). Battery Point (BP2007) particularly seemed to be a location that was estimated to receive a higher proportion of individuals from other locations (2.0–8.3%), thereby representing a potential sink collection. However, the overall estimated migration rates among collections were very low (0.6–10.2%; see Fig. 4 and Table S1). This finding corroborated the FST and STRUCTURE analyses, whereby there was a higher gene flow (i.e. proportion of immigrants) estimated between near (e.g. MAB2007 and OP2008) than distant collections (e.g. HW2006 and OP2008). The RB2008 collection was the most isolated in terms of proportion of immigrants (0.6–2.7%), which confirmed the findings of STRUCTURE (i.e. showing a distinctive genetic cluster for RB2008 from other locations; Fig. 2).
|
Discussion
This is the first SNP population genomics study undertaken in the Family Brachionichthyidae and in any handfish species. This is also the first-time multi-locus nuclear markers of any sort have been deployed for genetic diversity, differentiation, and structure analyses among spotted handfish populations. On the basis of SNP loci that were polymorphic in at least one collection, spotted handfish individuals showed a consistent (observed heterozygosity of >0.220) level of genomic diversity across the locations, with an estimated global FIS value suggesting that there was no strong indications of inbreeding, despite these small, discrete populations. Our seven spatial and two temporal collections (with the most recent samplings from 2008) indicated significant genetic structuring of spotted handfish collections within the estuary. Although we recognise that it is important to note that low sample sizes can affect a population genomics study (as outlined in Meirmans 2015), more robust sampling of this fish is difficult because of its threatened, endangered and protected species status.
Genomic diversity, structure and proximity analyses suggested that spotted handfish individuals (at least until 2008) from spatially differentiated locations in the Derwent estuary should not be considered panmictic. Hence, we were not able to accept the hypothesis that mating was considered random across all locations; all spotted handfish individuals were not potential partners, because mating depends on spatial locations and distances between. However, the observed genomic heterogeneity, and the contemporary migration rate estimations among the locations, implied that adults in the north and south of the river undertake low migrations (followed by reproduction), even within the confines of the estuary, and that eggs or juveniles also do not disperse. Restricted movement, even among locations is suggested, with reproduction and replenishment of locations being reliant mostly on recruitment within locations. This direct recruitment (Bruce et al. 1998) and micro-habitat preference (Wong et al. 2018) characteristic of spotted handfish, alongside anthropogenic impacts, may have contributed to this once ‘common’ species in south-eastern Tasmanian waters (Last and Gledhill 2009) now present as fragmented populations. Importantly, this lack of movement between fragmented populations may be relevant for other species of handfish, including T. politus, which is known from only two small locations, and B. ziebelli, which has not been sighted since ~2005, as well as other data-deficient species (Stuart-Smith et al. 2020).
Our results have given strong support to the ‘hotspot’ location concept (as outlined in Green 2005, 2007; Last et al. 2007; Last and Gledhill 2009) for spotted handfish in the Derwent estuary. Although spotted handfish individuals may occur between the collection locations throughout the estuary (albeit in much lower densities; Bessell 2018) and extensive ongoing visual surveys have indicated that they likely do not, the genetic results here indicated a lack of genetic contribution from one hotspot to another. The pairwise FST values were smallest (indicating more homogeneity) among individuals from spatial locations more geographically close (i.e. between Mary-Ann Bay and Tranmere; Tranmere and Ralphs Bay; Tranmere and Opossum Bay) than those among collections further apart in the estuary (i.e. Manning Reef and Ralphs Bay; these FST values were larger and significant). This is also supported by the estimation of contemporary migration rates among locations, particularly Ralphs Bay, which was the most isolated in terms of proportion of immigrants and represented a distinctive genetic cluster from other locations. Genetic differentiation among collections was generally consistent with geographic distance between locations. The smallest (and non-significant) FST values were between the two Tranmere temporal samplings and between geographically close Mary-Ann Bay and Opossum Bay. The Manning Reef individuals were shown to be somewhat genetically different from other spotted handfish individuals, even from those at nearby Battery Point. These two locations are separated by just over 2 km, but are within some of the most heavily urbanised parts of the estuary.
The genetic results support previous conclusions that, generally, individuals do not move widely among locations, rather once hatchlings settle on the benthos, individuals stay in these areas (Bruce et al. 1998; Bessell 2018). Individual spotted handfish movements within locations have been recorded (such as within Battery Point and Mary-Ann Bay); however, movement among locations in the Derwent estuary has not (Bessell 2018). Although undetected movement of individuals among locations may occur (Bessell 2018), microhabitat preferences (including preferred complex habitats rather than open sand flats; Wong et al. 2018), the distances among habitats and lack of contemporary continuous connecting habitat in the river will affect breeding opportunities among locations in the estuary. Reduced breeding opportunities among locations are likely to reduce demographic connectivity and, hence, result in reduced connectivity and higher genetic differentiation among locations.
Wong et al. (2018) documented spotted handfish populations in the Derwent estuary as 1.58–43.0 fishes per hectare. Although no contemporary samples were screened in this study, diversity could have declined after the 2006–2008 sampling documented here. A recent analysis of all survey data from 1997 to 2019 suggested that the estuary-wide population continued to decline between 1997 and 2014, but has since stabilised (Stuart-Smith et al. 2021). The analysis of local population dynamics also suggested high variation among local populations, with rapid declines and, in a few cases, rapid expansions in densities of observed fish. We do not have pre-1997 estimates of the population size of spotted handfish in the estuary, although a range reduction (on the basis of a geographic contraction of individuals over time to south-eastern Tasmania; Last and Gledhill 2009), and a major decline in spotted handfish population numbers, occurred in the 1980s and 1990s (Barrett 1996; Green and Bruce 2000; before our tissue sampling). With a generation time estimated at 8–10 years (Bessell 2018) and because significant conservation efforts for spotted handfish have been undertaken in the past 15 years (Lynch 2018), it is likely that contemporary genetic variation would be similar to that observed in this study, and reasonable to suggest that the location differentiation persists.
Genetic diversity (as detected by the SNPs) at each location appears to be consistent, and although not formally tested, is likely to reflect the breeding adults at each location. Although our genome-wide SNP data did not indicate a complete lack of connectivity among spatially differentiated individuals in the estuary, the SNP data suggested that gene flow maybe highly restricted among some locations, thereby resulting in closer genetic proximity of individuals that are more geographically close. We note that there is limited possibility of continuous sampling of spotted handfish individuals among the current sampling sites, because these are the only known sites in the estuary (following extensive dive and visual surveys) with no other intervening populations.
Alongside the diversity and connectivity findings, the study also showed that small, non-destructively sampled fin clips from this small anglerfish species provided suitable high-quality genomic DNA for SNP genotyping. This highlights the potential to use this sampling method for other species of handfishes to enable multi-locus SNP analyses, thereby supporting broader genomic investigations into this most threatened marine bony fish Family Brachionichthyidae (Stuart-Smith et al. 2020). Our study has also bridged the gap between time of sampling of individuals and the deployment of contemporary genomic analyses. Our samples were collected in the mid-2000s, when genomic technologies were first gaining traction. Prior to SNP analyses, population genetic studies were based on tens of microsatellite loci or from a couple of mitochondrial DNA gene fragments. By carefully and appropriately storing tissue samples, we were able to screen for genome-wide markers when SNP technology matured, over 10 years after sample collection. Moreover, the archival DNA from these 2006–2008 spotted handfish collections is stored at –80°C, therefore provisioning future new genetic and genomic assays that may include future genome screening, or the development of molecular sex markers. Alongside future molecular research in spotted handfish, population viability assessments are being considered. However, these future research avenues require resourcing, Commonwealth approval and support, and karyotyping or transcriptomic screening will require new, specifically targeted samples that may be difficult to source given the species listing status.
Our findings represent a significant development for understanding the conservation requirements of this critically endangered species and, potentially, for species in the wider family of Brachionichthyidae, many of which share life-history traits similar to those of B. hirsutus. Generically, it appears that these fish are more like threatened terrestrial or freshwater species, rather than other marine species such as many fish and coral that have juveniles dispersing planktonically, or viviparous or oviparous reproductive system species such as sharks, rays, turtles and cetaceans, which have wide-ranging adults. Like for terrestrial or freshwater species, barriers to movements and recruitment by spotted handfish, including habitat degradation and anthropogenic impacts, have magnified the downsides of their direct recruitment of juveniles, limited adult movements and now low population sizes. Owing to the family’s reproductive behaviours and restricted movements, a lack of pelagic larval phase and without outside replenishment, handfish species may, therefore, be at a high risk of stochastic events leading to local extinctions. This insight should be considered for the other data-deficient and threatened species in the family and for other aquatic species with similar life-history restrictions to juvenile and or adult dispersal.
If higher levels of homogeneity among the spotted handfish populations were present as a result of either juvenile or adult interconnectivity, the species may be able to mitigate the impact of losses of one or two populations, while still maintaining the diversity of the overall gene pool. However, as each spotted handfish population here is genetically structured within the estuary, each location needs specific consideration for management and conservation, rather than considering all as a single interconnected population. Local extinctions would represent a decrease in the species genetic make-up and a loss of overall genetic diversity. It is also unlikely for an extinct local population to re-establish through natural processes.
For declining species with small, fragmented populations, each of these distinct populations need to be carefully conserved because they represent distinct gene pools and also a greater risk of sequential local extinction. Each local population needs to be individually monitored and then conserved through intervention if it starts to decline. The NHRT currently is guided by research that states that each population should be (a) annually monitored and individually considered for interventions such as planting of artificial spawning habitats (ASH) and (b) that locations where the species has recently gone locally extinct should be considered for reintroduction if habitat has recovered and animals are available for re-stocking (Lynch et al. 2020; Stuart-Smith et al. 2020).
For both existing local populations and for any reintroduction efforts, further work is needed to consider artificial gene flow to avoid inbreeding depression or, alternatively, maintaining isolation so as not to dilute gene pools. Importantly, however, Stuart-Smith et al. (2020) highlighted the impacts that increasing water temperatures, associated with climate change, may have on handfishes. For these cool temperate and coastally adapted handfish, the lack of fast dispersal means they may not be able to naturally migrate south in response to warming water. Even if they could, because they are in south-eastern Tasmania, the species has, at best, <100 km of available coastal habitat left to the south of the estuary.
Overall, when we integrate the sometimes highly variable local population dynamics data (Lynch et al. 2020; Stuart-Smith et al. 2021) from spotted handfish with our genomic findings, the data suggest that spatial locations in which spotted handfish are found should continue to be monitored and managed or considered as separate conservation units, even within the Derwent estuary. Furthermore, these results will guide future conservation strategies, particularly when establishing founders for re-introduction breeding, determining the number of captive releases and identifying future translocation sources if required, with the aim of maximising diversity across the spotted handfish populations.
Supplementary material
Additional supporting information on spotted handfish sampling, SNP processing, filtering and population analyses is outlined in the Supplementary material.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Declaration of funding
This research was partly funded by the Australian Government’s National Environmental Science Programme (NESP) Marine Biodiversity Hub (https://www.nespmarine.edu.au) Project A10 – Conservation of spotted handfish and their habitat.
Acknowledgements
The authors thank the divers on the current spotted handfish breeding program. The 2006–2008 handfish sampling was permitted under Permits 6054 and 7099 (Tasmanian Department of Primary Industries, Water and Environment) following approvals from the Tasmanian Department of Primary Industries and Water Animal Ethics Committee (27/2005-6 & 15/2007-08). The authors also thank Miles Lawler and the ANFC for providing archival spotted handfish tissue samples, which were used to develop the SNP libraries that were initially funded by a sequencing grant to SA provided by BioPlatforms Australia. Tyson Bessell, Safia Maher and Skip Woolley provided insights and assistance with statistical approaches, and Bruce Deagle and Jemina Stuart-Smith provided comments and reviews on a draft version of the manuscript. Dr Chris Burridge and a second reviewer provided valuable recommendations and feedback, which were incorporated into the paper.
References
Avise, J. C. (1998). Conservation genetics in the marine realm. The Journal of Heredity 89, 377–382.| Conservation genetics in the marine realm.Crossref | GoogleScholarGoogle Scholar |
Baetscher, D. S., Hasselman, D. J., Reid, K., Palkovacs, E. P., and Carlos Garza, J. (2017). Discovery and characterisation of single nucleotide polymorphisms in two anadromous alosine fishes of conservation concern. Ecology and Evolution 7, 6638–6648.
| Discovery and characterisation of single nucleotide polymorphisms in two anadromous alosine fishes of conservation concern.Crossref | GoogleScholarGoogle Scholar | 28904746PubMed |
Baird, N. A., Etter, P. D., Atwood, T. S., Currey, M. C., Shiver, A. L., Lewis, Z. A., Selker, E. U., Cresko, W. A., and Johnson, E. A. (2008). Rapid SNP discovery and genetic mapping using sequenced RAD markers. PLoS One 3, e3376.
| Rapid SNP discovery and genetic mapping using sequenced RAD markers.Crossref | GoogleScholarGoogle Scholar | 18852878PubMed |
Bargelloni, L., Marcato, S., Zane, L., and Patarnello, T. (2000). Mitochondrial phylogeny of Notothenioids: a molecular approach to Antarctic fish evolution and biogeography. Systematic Biology 49, 114–129.
| Mitochondrial phylogeny of Notothenioids: a molecular approach to Antarctic fish evolution and biogeography.Crossref | GoogleScholarGoogle Scholar | 12116475PubMed |
Barrett, N. (1996). Spotted handfish survey. Report to Endangered Species Unit, ANCA. CSIRO Division of Fisheries, Hobart, Tas., Australia.
Beheregaray, L. B., Pfeiffer, L. V., Attard, C. R. M., Sandoval-Castillo, J., Domingos, F. M. C. B., Faulks, L. K., Gilligan, D. M., and Unmack, P. J. (2017). Genome-wide data delimits multiple climate-determined species ranges in a widespread Australian fish, the golden perch (Macquaria ambigua). Molecular Phylogenetics and Evolution 111, 65–75.
| Genome-wide data delimits multiple climate-determined species ranges in a widespread Australian fish, the golden perch (Macquaria ambigua).Crossref | GoogleScholarGoogle Scholar | 28347889PubMed |
Benjamini, Y., and Yekutieli, D. (2001). The control of the false discovery rate in multiple testing under dependency. Annals of Statistics 29, 1165–1188.
| The control of the false discovery rate in multiple testing under dependency.Crossref | GoogleScholarGoogle Scholar |
Bessell, T. J. (2018). Using autonomous photo-identification systems and otoliths to estimate age, growth and movement of the spotted handfish. B.Sc.(Hons) Thesis, Institute for Marine and Antarctic Studies, University of Tasmania, Hobart, Tas., Australia.
Bruce, B., and Last, P. (1996). Brachionichthys hirsutus. In ‘1996 IUCN Red List of Threatened Animals 1996’. (Eds J. Baillie and B. Groombridge.) p. 78. (International Union for Conservation of Nature and Natural Resources: Gland, Switzerland.) Available at https://portals.iucn.org/library/sites/library/files/documents/RL-1996-001.pdf
Bruce, B. D., Green, M. A., and Last, P. R. (1998). Threatened fishes of the world: Brachionichthys hirsutus (Lacepede, 1804) (Brachionichthyidae). Environmental Biology of Fishes 52, 418.
| Threatened fishes of the world: Brachionichthys hirsutus (Lacepede, 1804) (Brachionichthyidae).Crossref | GoogleScholarGoogle Scholar |
Catchen, J. M., Amores, A., Hohenlohe, W. C., and Postlethwait, J. H. (2011). Stacks: building and genotyping loci de novo from short read sequences. G3 – Genes Genomes Genetics 1, 171–182.
| Stacks: building and genotyping loci de novo from short read sequences.Crossref | GoogleScholarGoogle Scholar | 22384329PubMed |
Catchen, J., Hohenlohe, P. A., Bassham, S., Amores, A., and Cresko, W. A. (2013). Stacks: an analysis tool set for population genomics. Molecular Ecology 22, 3124–3140.
| Stacks: an analysis tool set for population genomics.Crossref | GoogleScholarGoogle Scholar | 23701397PubMed |
Coates, D. J., Byrne, M., and Moritz, C. (2018). Genetic diversity and conservation units: dealing with the species-population continuum in the age of genomics. Frontiers in Ecology and Evolution 6, 165.
| Genetic diversity and conservation units: dealing with the species-population continuum in the age of genomics.Crossref | GoogleScholarGoogle Scholar |
Cullingham, C. I., Miller, J. M., Peery, R. M., Dupuis, J. R., Malenfant, R. M., Gorrell, J. C., and Janes, J. K. (2020). Confidently identifying the correct K value when using the ΔK method: when does K = 2? Molecular Ecology 29, 862–869.
| Confidently identifying the correct K value when using the ΔK method: when does K = 2?Crossref | GoogleScholarGoogle Scholar | 32034821PubMed |
Danecek, P., Auton, A., Abecasis, G., Albers, C., Cornelis, A., Banks, E., DePristo, M. A., Handsaker, R. E., Lunter, G., Marth, G. T., Sherry, S. T., McVean, G., Durbin, R., 1000 Genomes Project Analysis Group (2011). The variant call format and VCFtools. Bioinformatics 27, 2156–2158.
| The variant call format and VCFtools.Crossref | GoogleScholarGoogle Scholar | 21653522PubMed |
Dresser, C. M., Pierson, T. W., and Fitzpatrick, B. M. (2018). Isolation by distance, local adaptation, and fortuitous coincidence of geo-political boundaries with spatial-genetic clusters in southern Bog turtles. Global Ecology and Conservation 16, e00474.
| Isolation by distance, local adaptation, and fortuitous coincidence of geo-political boundaries with spatial-genetic clusters in southern Bog turtles.Crossref | GoogleScholarGoogle Scholar |
Elshire, R. J., Glaubitz, J. C., Sun, Q., Poland, J. A., Kawamoto, K., Buckler, E. S., and Mitchell, S. E. (2011). A robust, simple Genotyping-by-Sequencing (GBS) approach for high diversity species. PLoS One 6, e19379.
| A robust, simple Genotyping-by-Sequencing (GBS) approach for high diversity species.Crossref | GoogleScholarGoogle Scholar | 21573248PubMed |
Evanno, G., Regnaut, S., and Goudet, J. (2005). Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Molecular Ecology 14, 2611–2620.
| Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study.Crossref | GoogleScholarGoogle Scholar | 15969739PubMed |
Excoffier, L., Smouse, P. E., and Quattro, J. M. (1992). Analysis of molecular variance inferred from metric distances among DNA haplotypes: application to human mitochondrial DNA restriction data. Genetics 131, 479–491.
| Analysis of molecular variance inferred from metric distances among DNA haplotypes: application to human mitochondrial DNA restriction data.Crossref | GoogleScholarGoogle Scholar | 1644282PubMed |
Green, M. A. (2001). Spotted handfish recovery plan 2002–2006. CSIRO Marine Research, Hobart, Tas., Australia.
Green, M. A. (2005). Marine habitat rehabilitation and threatened fish investigation. Report to Biodiversity Conservation branch of the Department of Primary Industries, Water and Environment. Department of Primary Industries, Water and Environment, Hobart, Tas., Australia.
Green, M. A. (2007). Implementing Handfish Recovery Plan 2006/7. Report to Biodiversity Conservation branch of the Department of Primary Industries, Water and Environment. Department of Primary Industries, Water and Environment, Hobart, Tas., Australia.
Green, M., and Bruce, B. (2000). Spotted Handfish Recovery Plan: Final Report: Year 1 (1999). Environment Australia, Canberra, ACT, Australia.
Green, M. A., Stuart, R. D., Valentine, J. P., Einoder, L. D., Barrett, N. S., Cooper, A. T., and Stalker, M. D. (2012). Spotted handfish monitoring and recovery actions – 2011–2012. Report for Derwent Estuary Program and Caring for Our Country. CSIRO Marine and Atmospheric Research/ Institute of Marine and Antarctic Studies, Hobart, Tas., Australia.
Harrisson, K. A., Amish, S. J., Pavlova, A., Narum, S. R., Telonis-Scott, M., Rourke, M. L., Lyon, J., Tonkin, Z., Gilligan, D. M., Ingram, B. A., Lintermans, M., Gan, H. M., Austin, C. M., Luikart, G., and Sunnucks, P. (2017). Signatures of polygenic adaptation associated with climate across the range of a threatened fish species with high genetic connectivity. Molecular Ecology 26, 6253–6269.
| Signatures of polygenic adaptation associated with climate across the range of a threatened fish species with high genetic connectivity.Crossref | GoogleScholarGoogle Scholar | 28977721PubMed |
Hormann, A. (2019). A behavioural study of the spotted handfish (Brachionichthys hirsutus) and the species use of artificial spawning habitat. M.A.Sc. Thesis, Institute for Marine and Antarctic Studies, University of Tasmania, Hobart, Tas., Australia.
Jamieson, I. G., Grueber, C. E., Waters, J. M., and Gleeson, D. M. (2008). Managing genetic diversity in threatened populations: a New Zealand perspective. New Zealand Journal of Ecology 32, 130–137.
Jombart, T., Devillard, S., and Balloux, F. (2010). Discriminant analysis of principal components: a new method for the analysis of genetically structured populations. BMC Genetics 11, 94.
| Discriminant analysis of principal components: a new method for the analysis of genetically structured populations.Crossref | GoogleScholarGoogle Scholar | 20950446PubMed |
Junge, C., Donnellan, S. C., Huveneers, C., Bradshaw, C. J. A., Simon, A., Drew, M., Duffy, C., Johnson, G., Cliff, G., Braccini, M., Cutmore, S. C., Butcher, P., McAuley, R., Peddemors, V., Rogers, P., and Gillanders, B. M. (2019). Comparative population genomics confirms little population structure in two commercially targeted carcharhinid sharks. Marine Biology 166, 16.
| Comparative population genomics confirms little population structure in two commercially targeted carcharhinid sharks.Crossref | GoogleScholarGoogle Scholar |
Last, P. R., and Gledhill, D. C. (2009). A revision of the Australian handfishes (Lophiiformes: Brachionichthyidae), with descriptions of three new genera and nine new species. Zootaxa 2252, 1–77.
| A revision of the Australian handfishes (Lophiiformes: Brachionichthyidae), with descriptions of three new genera and nine new species.Crossref | GoogleScholarGoogle Scholar |
Last, P. R., Scott, E. O. G., and Talbot, F. H. (1983). ‘Fishes of Tasmania.’ (Tasmania Fisheries Development Authority: Hobart, Tas., Australia.)
Last, P. R., Gledhill, D. C., and Holmes, B. H. (2007). A new handfish, Brachionichthys australis sp. nov. (Lophiiformes: Brachionichthyidae), with a redescription of the critically endangered spotted handfish, B. hirsutus (Lacepede). Zootaxa 1666, 53–68.
Legendre, P., and Legendre, L. (2012). ‘Numerical Ecology’, 3rd edn. (Elsevier: Amsterdam, Netherlands.)
Li, H., Handsaker, B., Wysoker, A., Fennell, T., Ruan, J., Homer, N., Marth, G., Abecasis, G., and Durbin, R. (2009). The sequence alignment/map format and SAMtools. Bioinformatics 25, 2078–2079.
| The sequence alignment/map format and SAMtools.Crossref | GoogleScholarGoogle Scholar | 19505943PubMed |
Lowe, W. H., and Allendorf, F. W. (2010). What can genetics tell us about population connectivity. Molecular Ecology 19, 3038–3051.
| What can genetics tell us about population connectivity.Crossref | GoogleScholarGoogle Scholar | 20618697PubMed |
Lynch, T. (2018). NESP Project A10 – Conservation of spotted handfish and their habitat. Project update, February 2018. Department of Agriculture, Water and Environment, Canberra, ACT, Australia.
Lynch, T., Green, M., and Davies, C. (2015). Diver towed GPS to estimate densities of a critically endangered fish. Biological Conservation 191, 700–706.
| Diver towed GPS to estimate densities of a critically endangered fish.Crossref | GoogleScholarGoogle Scholar |
Lynch, T., Appleyard, S. A., Wong, L., Martini, A., Bessesll, T., Stuart-Smith, J., Soo, L., Faber, S., and Devine, C. (2020). Conservation of handfish and their habitats – annual report. Report to the National Environmental Science Program, Marine Biodiversity Hub, Canberra, ACT, Australia.
Meirmans, P. G. (2015). Seven common mistakes in population genetics and how to avoid them. Molecular Ecology 24, 3223–3231.
| Seven common mistakes in population genetics and how to avoid them.Crossref | GoogleScholarGoogle Scholar | 25974103PubMed |
Ovenden, J. (2013). Crinkles in connectivity: combining genetics and other types of biological data to estimate movement and interbreeding between populations. Marine and Freshwater Research 64, 201–207.
| Crinkles in connectivity: combining genetics and other types of biological data to estimate movement and interbreeding between populations.Crossref | GoogleScholarGoogle Scholar |
Padovan, A., Chick, R. C., Cole, V. J., Dutoit, L., Hutchings, P. A., Cameron, J., and Fraser, C. I. (2020). Genomic analyses suggest strong population connectivity over large spatial scales of the commercially important baitworm, Australonuphis teres (Onuphidae). Marine and Freshwater Research 71, 1549–1556.
| Genomic analyses suggest strong population connectivity over large spatial scales of the commercially important baitworm, Australonuphis teres (Onuphidae).Crossref | GoogleScholarGoogle Scholar |
Paris, J. R., Stevens, J. R., and Catchen, J. M. (2017). Lost in parameter space: a road map for STACKS. Methods in Ecology and Evolution 8, 1360–1373.
| Lost in parameter space: a road map for STACKS.Crossref | GoogleScholarGoogle Scholar |
Perez, M. F., Franco, F. F., Bombonato, J. R., Bonatelli, I. A. S., Khan, G., Romeiro-Brito, M., Fegies, A. C., Ribeiro, P. M., Silva, G. A. R., and Moraes, E. M. (2018). Assessing population structure in the face of isolation by distance: are we neglecting the problem. Diversity & Distributions 24, 1883–1889.
| Assessing population structure in the face of isolation by distance: are we neglecting the problem.Crossref | GoogleScholarGoogle Scholar |
Peterson, B. K., Weber, J. N., Kay, E. K., Fisher, H. S., and Hoekstra, H. E. (2012). Double digest RADseq: an inexpensive method for de novo SNP discovery and genotyping in model and non-model species. PLoS One 7, e37135.
| Double digest RADseq: an inexpensive method for de novo SNP discovery and genotyping in model and non-model species.Crossref | GoogleScholarGoogle Scholar | 22675423PubMed |
Pritchard, J. K., Stephens, M., and Donnelly, P. (2000). Inference of population structure using multilocus genotype data. Genetics 155, 945–959.
| Inference of population structure using multilocus genotype data.Crossref | GoogleScholarGoogle Scholar | 10835412PubMed |
Rannala, B. (2007). BayesAss Edition 3.0 User’s Manual. Available at https://manualzz.com/doc/7334907/bayesass-edition-3.0-user-s-manual
Robertson, A., and Hill, W. G. (1984). Deviations from Hardy–Weinberg proportions: sampling variances and use in estimation of inbreeding coefficients. Genetics 107, 703–718.
| Deviations from Hardy–Weinberg proportions: sampling variances and use in estimation of inbreeding coefficients.Crossref | GoogleScholarGoogle Scholar | 6745643PubMed |
Rousset, F. (2008). GENEPOP’007: a complete re-implementation of the GENEPOP software for Windows and Linux. Molecular Ecology Resources 8, 103–106.
| GENEPOP’007: a complete re-implementation of the GENEPOP software for Windows and Linux.Crossref | GoogleScholarGoogle Scholar | 21585727PubMed |
Stuart-Smith, J., Edgar, G. J., Last, P., Linardich, C., Lynch, T., Barrett, N., Bessell, T., Wong, L., and Stuart-Smith, R. D. (2020). Conservation challenges for the most threatened family of marine bony fishes (handfishes: Brachionichthyidae). Biological Conservation 252, 108831.
| Conservation challenges for the most threatened family of marine bony fishes (handfishes: Brachionichthyidae).Crossref | GoogleScholarGoogle Scholar |
Stuart-Smith, J., Lynch, T., McEnnulty, F., Green, M., Devine, C., Trotter, A., Bessell, T., Wong, L., Hale, P., Martini, A., Stuart-Smith, R., and Barrett, N. (2021). Conservation of handfishes and their habitats – final report 2020. Report to the National Environmental Science Program, Marine Biodiversity Hub. University of Tasmania, Hobart, Tas., Australia.
Waples, R. S. (1998). Separating the wheat from the chaff: patterns of genetic differentiation in high gene flow species. The Journal of Heredity 89, 438–450.
| Separating the wheat from the chaff: patterns of genetic differentiation in high gene flow species.Crossref | GoogleScholarGoogle Scholar |
Waples, R. S., and Gaggiotti, O. E. (2006). What is a population? An empirical evaluation of some genetic methods for identifying the number of gene pools and their degree of connectivity. Molecular Ecology 15, 1419–1439.
| What is a population? An empirical evaluation of some genetic methods for identifying the number of gene pools and their degree of connectivity.Crossref | GoogleScholarGoogle Scholar | 16629801PubMed |
Waples, R., and Punt, A. (2008). Integrating genetic data into management of marine resources: how can we do it better? Fish and Fisheries 9, 423–449.
| Integrating genetic data into management of marine resources: how can we do it better?Crossref | GoogleScholarGoogle Scholar |
Ward, R. D., Woodwark, M. D., and Skibinski, D. O. F. (1994). A comparison of genetic diversity levels in marine, freshwater, and anadromous fishes. Journal of Fish Biology 44, 213–232.
| A comparison of genetic diversity levels in marine, freshwater, and anadromous fishes.Crossref | GoogleScholarGoogle Scholar |
Weir, B. S., and Cockerham, C. C. (1984). Estimating F-statistics for the analysis of population structure. Evolution 38, 1358–1370.
| 28563791PubMed |
Wilson, G. A., and Rannala, B. (2003). Bayesian inference of recent migration rates using multilocus genotypes. Genetics 163, 1177–1191.
| Bayesian inference of recent migration rates using multilocus genotypes.Crossref | GoogleScholarGoogle Scholar | 12663554PubMed |
Wong, L. S. C., and Lynch, T. P. (2017). Monitoring of spotted handfish (Brachionichthys hirsutus) populations and on ground conservation actions. Project: A10 Monitoring and conservation of spotted handfish. Milestone report to the National Environmental Science Program, Marine Biodiversity Hub, Canberra, ACT, Australia.
Wong, L. S. C., Lynch, T. P., Barrett, N. S., Wright, J. T., Green, M. A., and Flynn, D. J. H. (2018). Local densities and habitat preference of the critically endangered spotted handfish (Brachionichthys hirsutus): large scale field trial of GPS parameterised underwater visual census and diver attached camera. PLoS One 13, e0201518.
| Local densities and habitat preference of the critically endangered spotted handfish (Brachionichthys hirsutus): large scale field trial of GPS parameterised underwater visual census and diver attached camera.Crossref | GoogleScholarGoogle Scholar |
Wright, S. (1943). Isolation by distance. Genetics 28, 114–138.
| Isolation by distance.Crossref | GoogleScholarGoogle Scholar | 17247074PubMed |