Long-term acoustic telemetry reveals limited movement of fish in an unregulated, perennial river
Luke Carpenter-Bundhoo A D , Gavin L. Butler B , Nick R. Bond C , Stuart E. Bunn A and Mark J. Kennard A
A D , Gavin L. Butler B , Nick R. Bond C , Stuart E. Bunn A and Mark J. Kennard A
A Australian Rivers Institute, Griffith University, 170 Kessels Road, Nathan, Qld 4111, Australia.
B NSW Department of Primary Industries, Grafton Fisheries Centre, PMB 2, Grafton, NSW 2460, Australia.
C Centre for Freshwater Ecosystems, La Trobe University, Wodonga, Vic. 3690, Australia.
D Corresponding author. Email: l.carpenter-bundhoo@griffith.edu.au
Marine and Freshwater Research - https://doi.org/10.1071/MF21004
Submitted: 13 January 2021 Accepted: 5 May 2021 Published online: 25 June 2021
Journal Compilation © CSIRO 2021 Open Access CC BY-NC-ND
Abstract
Anthropogenic changes to river flows can alter hydrological connectivity and cues necessary for the movement of fish to complete their life cycles. Quantifying flow-related movement ecology of fish and understanding how this varies between species and river systems is important for effective environmental flow management. This study aimed to determine hydroecological factors that influence fish movements in an unregulated, perennial river and to compare these findings to fish from regulated river systems. Broad-scale movements of the endangered Maccullochella ikei and Tandanus tandanus were recorded over 3 years in the unregulated, perennial Nymboida River, Australia. The limited movements both species exhibited were infrequent and over short distances. Although M. ikei movements appeared mostly unrelated to environmental changes, T. tandanus moved on flow pulse peaks and were more likely to move during the breeding season. These findings contrast with previous studies of the same or similar species in differing flow regimes, suggesting that fish in perennial, highly connected rivers may not need to move as frequently as those in more regulated or intermittent systems. Should these disparate behaviours be present in other species occurring among contrasting flow regimes, it will be challenging to define generalisable environmental flow rules to inform river management.
Keywords: acoustic telemetry, fish movement, flow regime, river regulation.
Introduction
Movement is a key component in the life history of most organisms. The ability to move allows organisms to locate new resources, escape unfavourable biotic or abiotic conditions and to avoid inbreeding (Nathan et al. 2008). In riverine environments, animal movements are often cued or facilitated by changes in environmental conditions, such as increases in flow. For riverine fish, flow pulses can cue both fine-scale activity (Cocherell et al. 2011; Capra et al. 2017; Carpenter-Bundhoo et al. 2020a) and broad-scale movements (Simpson and Mapleston 2002; Koehn 2004; Young et al. 2010). Elevated river flows also enhance longitudinal connectivity, allowing fish to disperse from dry season refuges, access feeding and breeding sites and, for some species, facilitate downstream dispersal of pelagic eggs and larvae (Chapman and Kramer 1991; Koster et al. 2014; Lechner et al. 2016). In addition to flow magnitude, the frequency, timing, duration and rates of change of flow events have been shown to influence the movements of riverine fish (Reinfelds et al. 2013; Marshall et al. 2016).
Anthropogenic changes to river flows caused by the construction of dams, and the diversion and extraction of water, can severely affect important hydrological cues for movement, as well as hydrologic connectivity (Poff and Zimmerman 2010; Pelicice et al. 2015; Rahel and McLaughlin 2018). To mitigate the ecological effects of these hydrological changes, there is considerable effort and investment now being directed towards attempting to maintain or restore critical aspects of flow variability in rivers, including those relating to facilitating or triggering movement (Arthington 2012; Bond 2016).
Environmental flows are often released from water storages with the intention of benefitting multiple species but, for riverine fish in particular, these flows are often aimed at maintaining or increasing longitudinal connectivity, and stimulating reproduction (Arthington 2012). To deliver environmental flows effectively, knowledge is required about the life histories of targeted species and how variations in the magnitude, timing, frequency and duration of flow events affect fish movement behaviour over a range of spatiotemporal scales. This knowledge may be obtained by long-term studies assessing fish movement behaviours in response to variations in river flow (Konrad et al. 2011; Olden et al. 2014).
An important question for such studies is whether flow-related movement responses quantified in regulated river systems with modified flow regimes are representative of movement behaviours in more natural systems and vice versa. Over evolutionary time scales, long-term variability and predictability of critical flow regime characteristics (e.g. magnitude, frequency, duration, timing and rate of change in low- and high-flow events) impose strong selective filters on morphological, life history and behavioural adaptation of species (Poff et al. 1997; Lytle and Poff 2004). Over ecological time scales (i.e. the life span of an individual), variations in these flow regime characteristics are also important determinants of fish movement behaviour, within the constraints of evolutionary history. Fish populations in regulated systems may exhibit atypical behaviours in response to flow events and therefore provide inadequate benchmarks for developing environmental flow recommendations. For example, regulated rivers may lack critical flows to stimulate spawning movements (Bunn and Arthington 2002) and fish occupying degraded habitats in regulated rivers may be less likely to establish a local home range (e.g. Albanese et al. 2004; Crook 2004). Understanding the generality and transferability of flow–ecology response relationships is critical for establishing robust and defensible environmental flow rules for rivers (Chen and Olden 2018).
This study focused on the movement behaviour of two species of freshwater fish, namely the eastern freshwater cod Maccullochella ikei and freshwater catfish Tandanus tandanus. Both species are endemic to eastern Australia, with M. ikei restricted to two coastal rivers in northern New South Wales (NSW) and T. tandanus widespread in eastern Australian coastal rivers and in the Murray–Darling Basin (MDB). Maccullochella ikei is an internationally and nationally listed threatened species (Butler 2019). It has undergone a marked decline in population size since European settlement, due in part to habitat alteration and river fragmentation (Rowland 1993). Tandanus tandanus remains relatively abundant in coastal rivers, but it has declined in the MDB and is currently listed as an Endangered Population in the NSW portion of the MDB (Threatened Species Scientific Committee 2008). In some catchments, T. tandanus is considered a target species for environmental flow deliveries (Southwell et al. 2019). Relatively little is known about the movement biology of both species. Neither T. tandanus nor M. ikei exhibit obligate large-scale migrations for reproduction, and both typically inhabit confined home ranges (Reynolds 1983; Butler et al. 2014; Carpenter-Bundhoo et al. 2020a). However, M. ikei has been recorded making occasional long-distance movements in response to increases in river discharge (Butler et al. 2014).
Using acoustic telemetry, this study examined the movements of T. tandanus and M. ikei in response to variations in river flows and other biotic and abiotic factors in an unregulated perennial river. The aims of this study were to: (1) quantify movement behaviours; (2) determine hydroecological factors that influence movements in a perennial system where connectivity is largely unconstrained; and (3) compare these movement behaviours to similar studies undertaken in regulated, intermittent rivers involving like species. The findings of this study will contribute important knowledge about how fish subject to different flow regimes respond to environmental variability. Information gained from this study will help inform better restoration and management of flow regimes in regulated rivers tailored to specific species.
Materials and methods
Study area
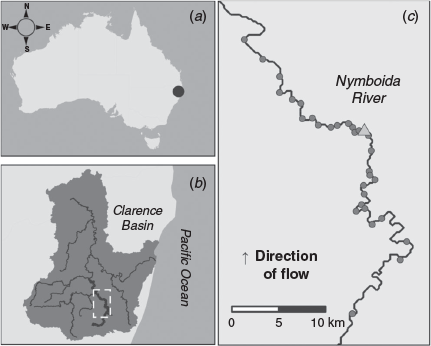
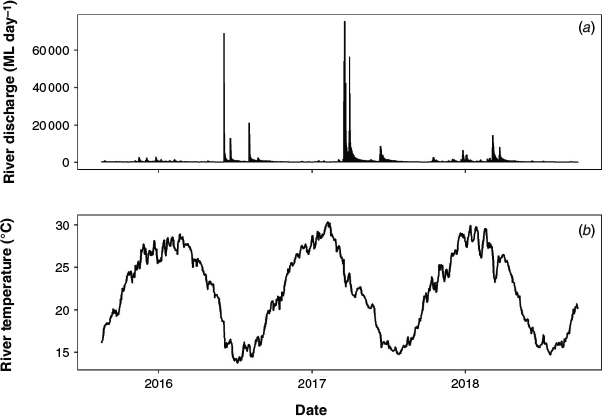
The Nymboida River has a catchment area of 1732 km2 and is a largely unregulated, perennial river located in the Clarence Basin, NSW, Australia (Fig. 1). The study reach spanned a 68-km section of the river commencing ~30 km upstream of the confluence of the Mann and Nymboida rivers (Fig. 1b). There were no major natural barriers to fish movement (e.g. waterfalls, rock bars) in the study reach; however, several shallow riffle areas may restrict fish movements during low-flow periods. In addition, the reach encompasses the Nymboida Weir, the only artificial barrier in the Nymboida River (Fig. 1c). The Nymboida Weir (wall height 4.25 m) forms a barrier to fish movements during low-flow periods, but has little effect on the flow regime downstream. Within the study reach, wetted channel width ranged from 20 to 95 m, and depth ranged from 1 to 5 m under base flow conditions. River discharge during the study period (May 2016–December 2018) usually remained <1000 ML day–1, with periodic small flow pulses (up to 10 000 ML day–1) and three large flow events (>50 000 ML day–1) that were sufficient to drown-out the weir (Fig. 2a). The weir drown-out events typically lasted 1–3 days. Daily average water temperature ranged from 13.7 to 30.3°C (Fig. 2b).
|
|
Linear acoustic telemetry array and explanatory environmental data
Broad-scale fish movements were recorded from May 2016 to December 2018 using an extensive linear array of 31 Vemco VR2W 69-kHz acoustic telemetry receivers. These were deployed at varying intervals (mean 2.3 km) along the 68-km study reach (Fig. 1). The receiver array recorded binary presence or absence data when a tagged fish entered the reception range (maximum range ~400 m) of a given receiver. Total daily river discharge data were sourced from Water NSW stream gauge #204069, situated approximately at the midpoint of the telemetry array. Mean daily water temperature data were sourced from the closest NSW Water gauge (#204400 at Grafton), situated ~170 km downstream (or 37 km north-east) of the telemetry array in the Clarence River. Over the duration of the study, data from all receivers in the array were downloaded approximately every 3–6 months and uploaded into a purpose-built database.
Fish collection, surgical tagging and recording
In all, 58 adult fish were collected, tagged and released over three trips conducted 17–24 July 2015, 23–29 July 2016 and 26–27 July 2017 (27 M. ikei and 31 T. tandanus; for full details of the fish tagged and detected during the study, see Table S1of the Supplementary material). Fish were sampled in a 1-km reach of the river spanning the Nymboida Weir and were collected using a boat-mounted electrofisher (Smith-Root GPP 2.5 KVA unit).
Upon capture, fish were anaesthetised, measured to the nearest millimetre (total length) and surgically implanted with acoustic transmitters as described in Carpenter-Bundhoo et al. (2020b). Transmitters were either a Vemco V9 or a V13 69-KHz acoustic telemetry transmitter, with selection based on keeping under the recommended 2.25% of bodyweight (Jepsen et al. 2002; Butler et al. 2009; Wagner et al. 2011). During surgery, each fish was sexed by internal examination of the gonads (M. ikei) or by external examination of the genitalia (T. tandanus). After the completion of surgery, each fish was held in one of several open-mesh floating cages in the river and kept under observation for ~1 h until normal behaviour resumed. Fish were then released close to the point of original capture.
All studies were performed in accordance with the relevant guidelines and regulations, reviewed and approved by the NSW Department of Primary Industries Animal Care and Ethics Committee (Permit numbers 06/06 and 98/14).
Data analysis
The distance of each acoustic receiver location from the most downstream receiver was calculated by digitising satellite images of the study reach into a spatial object in ArcGIS (ver. 10.4, ESRI, Redlands, CA, USA). The spatial object was then converted into a distance matrix using the V-Track package (ver. 1.2.1, see https://github.com/RossDwyer/VTrack; Campbell et al. 2012) in R (ver. 3.4.4, R Foundation for Statistical Computing, Vienna, Austria; see www.r-project.org/). Individual fish detections were then matched with the distance matrix and the distance between detections was calculated. A single movement was defined as an individual fish consecutively detected on two receivers. A daily sum of distance moved and displacement upstream or downstream was then calculated for each individual fish. Total linear range was calculated as the river distance between the most upstream and downstream detections for each fish for the entire study period, and cumulative distance moved was calculated as the sum of all upstream and downstream movements for each fish for the entire study period.
For each day of the study period, total daily river discharge (ML day–1), limb of the hydrograph (rising, receding, peak or base flow), absolute rate of change in discharge events and frequency of antecedent flow pulses over a 30-day period were calculated. Each day was assigned as either a base flow, rising, peak or receding limb. A base flow magnitude threshold was calculated for the study period using the Hydrostats package in R (ver. 0.2.7, N. R. Bond, see https://cran.r-project.org/web/packages/hydrostats/index.html). Rising limbs were above base flow and increased from the previous day, and receding limbs were above base flow and decreased from the previous day. Peaks were above the base flow, increased on the previous day, decreased on the following day and were independent of a larger peak 7 days before or after. The nominal breeding period of each species was designated as the first time water temperature reached 18°C in late winter and lasted 63 days for M. ikei (Butler and Rowland 2009) and 24°C in spring and lasted 70 days for T. tandanus (as reported in Carpenter-Bundhoo et al. 2020a).
We examined how the likelihood of movement varied in response to environmental and biological variables using a mixed-effect logistic regression model in the lme4 package in R (ver. 1.1-23, https://cran.r-project.org/web/packages/lme4/index.html; Bates et al. 2015). In this model, a binary response of movement (the presence or absence of movement) was the dependent variable and daily river discharge, average daily water temperature, fish size, sex and breeding period were the independent variables. Continuous independent variables were centred and scaled to ensure model convergence. Fish identity (ID) and year of release were included as random effects in the model. Temporal autocorrelation was apparent when examining model residuals at various time lags. We then introduced Lag-1 into the models, a variable characterising the movement state of a fish on the previous day. This would account for a possible Markov process in which future movement states depend on the preceding state and temporal autocorrelation in movement observations (Bestley et al. 2010). Temporal autocorrelation was no longer present when re-examining the residuals after the inclusion of Lag-1. All possible models and interactions were examined, and the best model was selected using the Akaike information criterion (AIC). Following the protocol of Zuur et al. (2010), data were checked for outliers and collinearity among predictor variables was assessed using variance inflation factors (VIF) in R.
Unless indicated otherwise, data are presented as the mean ± s.e.m.
Results
Scale of fish movements
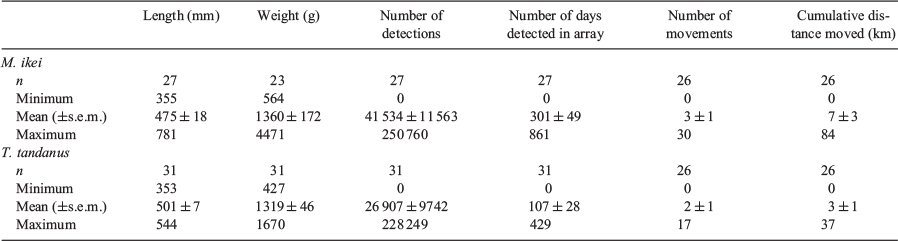
No fish were detected by the terminal receivers, implying that all fish remained within the array boundaries for the duration of the study. On average, M. ikei individuals were detected in the array for 301 ± 49 days and T. tandanus individuals were detected in the array for 107 ± 28 days (Table 1). One tagged M. ikei (ID 30198) and five T. tandanus (ID 23054, 23059, 23061, 23062 and 30192) were not detected during the study. In addition, all but two T. tandanus individuals released in 2015 stopped being detected within 15 months, well before the expected 24-month battery life of the acoustic tags.
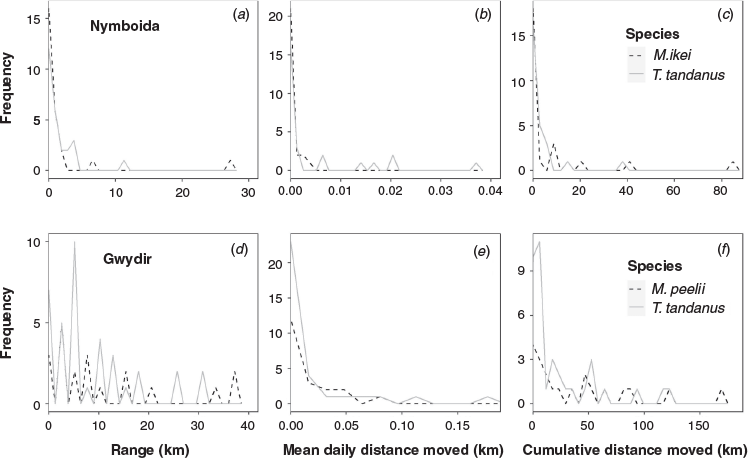
M. ikei inhabited an average total linear range of 1.7 ± 1.1 km. Of the 26 individuals detected, most were highly sedentary, with no movement detected between receivers for 18 individuals. Seven individuals were detected inhabiting a range of between 1 and 6 km, and one individual ranged over 27 km (Fig. S1a of the Supplementary material). T. tandanus was also generally sedentary, inhabiting a similar average range as M. ikei (1.5 ± 0.5 km). Of the 26 individuals detected, no movement was recorded for 14 individuals, 11 individuals were detected inhabiting a range of between 1 and 4 km and the remaining individual ranged over 11 km (Fig. S1b). M. ikei moved a mean cumulative distance of 7.5 ± 3.6 km and a maximum of 83.5 km, and T. tandanus moved a mean cumulative distance of 3.4 ± 1.7 km and a maximum of 37.1 km (Fig. S1).
Effects of environmental and biological factors on fish movements
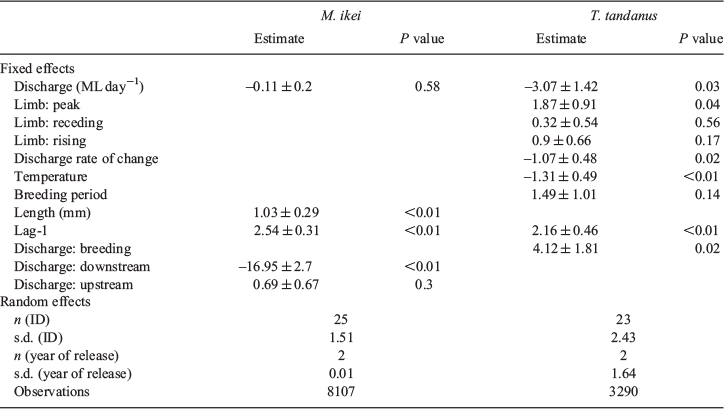
Of the 52 fish detected over the 3-year study period, movements occurred very infrequently, with individual fish detected moving on only 90 days for M. ikei and on 45 days for T. tandanus (Fig. 3a, b). During the study, one M. ikei (ID 15781) and one T. tandanus (ID 23035) crossed the weir, all in the upstream direction. These crossings all coincided with large drown-out flow events occurring in June 2016 or March 2017 (Fig. 3a–c). The GLMM revealed that larger individuals of M. ikei were more likely to move than smaller individuals (estimate = 1.03 ± 0.29; P < 0.01; Table 2). High-magnitude flows events were significantly less likely to elicit a downstream movement instead of upstream or no movements (estimate = –16.95 ± 2.7l P < 0.01; Table 2).
Overall, T. tandanus were significantly less likely to move during periods of higher discharge (estimate = –3.07 ± 1.42; P = 0.03; Table 2), but were more likely to move on higher discharge events during the breeding season (estimate = 4.12 ± 1.81; P < 0.01; Table 2). T. tandanus were more likely to move on the peak of the hydrograph than on rising and receding limbs or during base flow periods (estimate = 1.87 ± 0.91; P = 0.04; Table 2), and were less likely to move on flow pulses with greater rates of change (estimate = –1.07 ± 0.48; P = 0.02; Table 2).
Discussion
Movement ecology of M. ikei and T. tandanus
This study presents the most extensive assessment of the movement ecology of M. ikei to date; in addition, T. tandanus movements have been studied for the first time in an unregulated coastal river system. Considering the duration of recording and the large number of detections, the extent and frequency of fish movements throughout this study was generally low, with most fish confined to relatively small ranges. This finding accords with prior knowledge of the movement biology of these species, with both M. ikei and T. tandanus thought to be largely sedentary species (Pusey et al. 2004; Butler et al. 2014; Koster et al. 2015). Using radiotelemetry, Koster et al. (2015) found T. tandanus in a temperate river system to mostly use a linear range of <1 km, occasionally making larger movements of up to 1.5 km. Burndred et al. (2018) used acoustic telemetry to document T. tandanus movements in a subtropical river system and found that the mean maximum distance moved by 138 fish was 0.75 km. Using radiotelemetry, Butler et al. (2014) found that male and female M. ikei occupied an average stream length of 6.9 and 2.3 km respectively over a 12-month period. In that study, M. ikei were also found to occasionally make larger-scale movements, particularly during the breeding season, but these movements were atypical. Importantly, these studies were undertaken in perennial river systems, where habitat quality is high and riverine connectivity is largely maintained year-round.
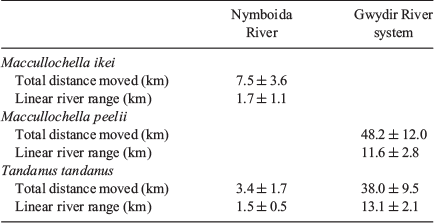
Although M. ikei only occur in the Clarence and Richmond catchments of coastal eastern Australia (Rowland 1996), the congeneric Maccullochella peelii have been recorded making far larger movements. In addition, studies in intermittent rivers have reported larger movements by T. tandanus. Carpenter-Bundhoo (2020) reported both M. peelii and T. tandanus moved higher cumulative distances, and over longer linear ranges, in the regulated Gwydir River system than their counterparts in the Nymboida River (Table 3; Fig. 4). In the Nymboida River, we recorded generally low linear movements and range, with a smaller proportion of individuals undertaking higher levels of movement (Fig. 4a–c). This closely matches what is described by Rodríguez (2002), who suggests it is likely that a population of fish comprises movers and non-movers, with the mobile proportion of the population being only a small subset. The movement behaviours of fish in the Gwydir River system did not match this pattern (Carpenter-Bundhoo 2020; Fig. 4d–f); however, similar behaviours have been recorded in other systems with limited connectivity. Marshall et al. (2016) reported T. tandanus in an intermittent river moved an average of 25 km upstream, and 13 km downstream after flow events. In a study of M. peelii in the heavily regulated Murray River and surrounding anabranches, fish were observed making numerous movements >50 km, and individuals showed far higher site fidelity in the anabranches that contained hydraulic conditions similar to the preregulation main channel Murray River (Leigh and Zampatti 2013). Reynolds (1983) recorded M. peelii moving up to 214 km upstream, but also found that many of the individuals in the study did not move. Both these studies were noted to have occurred during or immediately after prolonged periods of low flow, which may be a stimulus for M. peelii to go in search of higher-quality habitats (Reynolds 1983; Leigh and Zampatti 2013).
|
During our 3-year study period, the Nymboida River experienced a wide variation in environmental conditions. Fish were exposed to several large flow events, which have been documented to coincide with increased fine-scale activity (Carpenter-Bundhoo et al. 2020b) and stimulate broad-scale movements in both M. ikei and T. tandanus (Butler et al. 2014; Marshall et al. 2016; Burndred et al. 2018). The disparity between fish movement in the Nymboida River and that in regulated, often intermittent, systems like those in the northern MDB may be due to the greater availability of high-quality habitat found in unregulated, perennial rivers. This disparity in movement behaviours has also been observed in other species of Tandanus for which movements varied between flow regimes (Beatty et al. 2010; Storer et al. 2021) and levels of river regulation (Storer et al. 2021). Such differing movement patterns may reflect behavioural plasticity (Sih et al. 2011; Wong and Candolin 2015) in response to flow characteristics. In intermittent rivers, the cessation of flow creates impassable sections of dry or shallow riverbed interspersed with dry season refuge waterholes (Larned et al. 2010). In such systems, the need for opportunistic movements to take advantage of temporary connectivity to neighbouring habitats (e.g. for feeding and breeding) is likely to be greater than in perennial systems in which connectivity is not impeded.
Effects of environmental and biological factors on fish movements
Although both species exhibited relatively low rates of movement during the study period, there were several environmental and biological factors that were associated with periodic movement events. For M. ikei, larger individuals were more likely to move than small individuals. Although previous studies found no relationship between fish size and movement likelihood in M. ikei (Butler et al. 2014), larger individuals of the closely related M. peelii were more likely to undertake broad-scale movements (Leigh and Zampatti 2013). We also found M. ikei to be less likely to move in a downstream direction, compared with upstream or not moving at all, during periods of higher discharge. This may be explained by individuals actively trying to maintain their longitudinal position within the river network during high-flow events. M. ikei are known to exhibit high site fidelity (Butler et al. 2014). Maintaining longitudinal position during a flow event (e.g. by moving close to the riverbank) is likely energetically less costly than returning to a home range after downstream movement during high flows.
Several factors coincided with an increase in the likelihood of T. tandanus movement. For example, although T. tandanus were found to be less likely to move during high-discharge events, on the flows in which they did move, they were most likely to do so on peaks of the hydrograph, rather than on the rising or receding limbs or at base flow. T. tandanus were less likely to move during warmer temperatures, but more likely to move during elevated flows in the breeding season. Although T. tandanus are not known to make breeding-related movements (Lake 1967; Davis 1977), similar behavioural patterns have been noted in the congeneric Tandanus bostocki (Beesley et al. 2019). Being a species that possesses demersal eggs, it is unlikely that T. tandanus undertakes movements to counteract downstream larval and egg drift, as seen in many species with pelagic eggs (Koehn and Crook 2013). As suggested of T. bostocki (Beesley et al. 2019), greater movement activity during the breeding period may allow T. tandanus access to areas of gravel substrate for egg deposition. Although the Nymboida River is perennial, increased river discharge would likely result in further inundation of these shallow gravel beds.
Conclusions
The findings of this study indicate that fish movement behaviours can vary between populations of a species (T. tandanus) and between closely related species (M. ikei and M. peelii) in rivers with contrasting flow regimes. This has important implications for developing environmental flow rules for river management because the transferability of flow–ecology relationships through space and time may be limited for some species and river systems (Chen and Olden 2018). Furthermore, this study confirms the absence of an obligate breeding-related migration for T. tandanus and M. ikei and suggests that, in the case of T. tandanus, it may not need to move as frequently in perennial, highly connected rivers compared with intermittent river systems. Smaller home range sizes and lower movement frequencies and scales in perennial systems may be explained, in part, by the continuous availability of local resources necessary for survival, growth and reproduction. Despite there being several major flow events during the study period, these rarely triggered or facilitated large-scale movements by either species. For M. ikei, T. tandanus and, potentially closely related species such as M. peelii, our findings suggest that environmental flows in regulated systems should be used to maintain base flows and local connectivity.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Declaration of funding
Funding for this project was provided by the Holsworth Wildlife Research Endowment and the North Coast Local Land Service (formerly Northern Rivers Catchment Management Authority), Clarence Valley Council, Coffs Harbour Water, Essential Energy, Caring for Country, NSW Recreational Fishing Trust (Freshwater), NSW Department of Industry – Lands & Water and Fisheries NSW (Industries), as part of the Clarence River Fishtrack project.
Acknowledgements
The authors thank John St Vincent Welch, Tony Broderick, Nigel Blake and Leo Cameron for their help with fish collection and surgery.
References
Albanese, B., Angermeier, P. L., and Dorai-Raj, S. (2004). Ecological correlates of fish movement in a network of Virginia streams. Canadian Journal of Fisheries and Aquatic Sciences 61, 857–869.| Ecological correlates of fish movement in a network of Virginia streams.Crossref | GoogleScholarGoogle Scholar |
Arthington, A. H. (2012) ‘Environmental Flows: Saving Rivers in the Third Millennium.’ (University of California Press: Berkeley, CA, USA.)
Bates, D., Mächler, M., Bolker, B., and Walker, S. (2015). Fitting linear mixed-effects models using lme4. Journal of Statistical Software 67, 1–48.
| Fitting linear mixed-effects models using lme4.Crossref | GoogleScholarGoogle Scholar |
Beatty, S., Morgan, D., McAleer, F., and Ramsay, A. (2010). Groundwater contribution to baseflow maintains habitat connectivity for Tandanus bostocki (Teleostei: Plotosidae) in a south‐western Australian river. Ecology Freshwater Fish 19, 595–608.
| Groundwater contribution to baseflow maintains habitat connectivity for Tandanus bostocki (Teleostei: Plotosidae) in a south‐western Australian river.Crossref | GoogleScholarGoogle Scholar |
Beesley, L., Close, P. G., Gwinn, D. C., Long, M., Moroz, M., Koster, W. M., and Storer, T. (2019). Flow-mediated movement of freshwater catfish, Tandanus bostocki, in a regulated semi-urban river, to inform environmental water releases. Ecology Freshwater Fish 28, 434–445.
| Flow-mediated movement of freshwater catfish, Tandanus bostocki, in a regulated semi-urban river, to inform environmental water releases.Crossref | GoogleScholarGoogle Scholar |
Bestley, S., Patterson, T. A., Hindell, M. A., and Gunn, J. S. (2010). Predicting feeding success in a migratory predator: integrating telemetry, environment, and modelling techniques. Ecology 91, 2373–2384.
| Predicting feeding success in a migratory predator: integrating telemetry, environment, and modelling techniques.Crossref | GoogleScholarGoogle Scholar | 20836459PubMed |
Bond, N. R. (2016) Environmental flows: environmental watering. In ‘The Wetland Book: I: Structure and Function, Management, and Methods’. (Eds C. M. Finlayson, M. Everard, K. Irvine, R. J. McInnes, B. A. Middleton, A. A. van Dam, and N. C. Davidson.) pp. 1–4. (Springer: Dordrecht, Netherlands.)
Bunn, S. E., and Arthington, A. H. (2002). Basic principles and ecological consequences of altered flow regimes for aquatic biodiversity. Environmental Management 30, 492–507.
| Basic principles and ecological consequences of altered flow regimes for aquatic biodiversity.Crossref | GoogleScholarGoogle Scholar | 12481916PubMed |
Burndred, K. R., Cockayne, B. J., Donaldson, J. A., and Ebner, B. C. (2018). Natural flow events influence the behaviour and movement patterns of eel-tailed catfish (Tandanus tandanus) in a subtropical Queensland river. Australian Journal of Zoology 66, 185–194.
| Natural flow events influence the behaviour and movement patterns of eel-tailed catfish (Tandanus tandanus) in a subtropical Queensland river.Crossref | GoogleScholarGoogle Scholar |
Butler, G. (2019) Maccullochella ikei. The ‘IUCN Red List of Threatened Species 2019’. e.T12573A123378170. (International Union for Conservation of Nature and Natural Resources.) Available at https://www.iucnredlist.org/species/12573/123378170 [Verified 4 January 2021].
Butler, G. L., and Rowland, S. J. (2009). Using underwater cameras to describe the reproductive behaviour of the endangered eastern freshwater cod Maccullochella ikei. Ecology Freshwater Fish 18, 337–349.
| Using underwater cameras to describe the reproductive behaviour of the endangered eastern freshwater cod Maccullochella ikei.Crossref | GoogleScholarGoogle Scholar |
Butler, G. L., Mackay, B., Rowland, S. J., and Pease, B. C. (2009). Retention of intra-peritoneal transmitters and post-operative recovery of four Australian native fish species. Marine and Freshwater Research 60, 361–370.
| Retention of intra-peritoneal transmitters and post-operative recovery of four Australian native fish species.Crossref | GoogleScholarGoogle Scholar |
Butler, G., Rowland, S., Baverstock, P., and Brook, L. (2014). Movement patterns and habitat selection of the Endangered eastern freshwater cod Maccullochella ikei in the Mann River, Australia. Endangered Species Research 23, 35–49.
| Movement patterns and habitat selection of the Endangered eastern freshwater cod Maccullochella ikei in the Mann River, Australia.Crossref | GoogleScholarGoogle Scholar |
Campbell, H. A., Watts, M. E., Dwyer, R. G., and Franklin, C. E. (2012). V-Track: software for analysing and visualising animal movement from acoustic telemetry detections. Marine and Freshwater Research 63, 815–820.
| V-Track: software for analysing and visualising animal movement from acoustic telemetry detections.Crossref | GoogleScholarGoogle Scholar |
Capra, H., Plichard, L., Bergé, J., Pella, H., Ovidio, M., McNeil, E., and Lamouroux, N. (2017). Fish habitat selection in a large hydropeaking river: Strong individual and temporal variations revealed by telemetry. The Science of the Total Environment 578, 109–120.
| Fish habitat selection in a large hydropeaking river: Strong individual and temporal variations revealed by telemetry.Crossref | GoogleScholarGoogle Scholar | 27839764PubMed |
Carpenter-Bundhoo, L. (2020) Quantifying multi-scaled movements of Australian riverine fish to inform environmental flow management and conservation. Ph.D. Thesis, Griffith University, Brisbane, Qld, Australia.
Carpenter-Bundhoo, L., Butler, G. L., Bond, N. R., Bunn, S. E., Reinfelds, I. V., and Kennard, M. J. (2020a). Effects of a low-head weir on multi-scaled movement and behavior of three riverine fish species. Scientific Reports 10, 6817.
| Effects of a low-head weir on multi-scaled movement and behavior of three riverine fish species.Crossref | GoogleScholarGoogle Scholar | 32321932PubMed |
Carpenter-Bundhoo, L., Butler, G. L., Espinoza, T., Bond, N. R., Bunn, S. E., and Kennard, M. J. (2020b). Reservoir to river: quantifying fine scale fish movements after translocation. Ecology Freshwater Fish 29, 89–102.
| Reservoir to river: quantifying fine scale fish movements after translocation.Crossref | GoogleScholarGoogle Scholar |
Chapman, L. J., and Kramer, D. L. (1991). The consequences of flooding for the dispersal and fate of poeciliid fish in an intermittent tropical stream. Oecologia 87, 299–306.
| The consequences of flooding for the dispersal and fate of poeciliid fish in an intermittent tropical stream.Crossref | GoogleScholarGoogle Scholar | 28313849PubMed |
Chen, W., and Olden, J. D. (2018). Evaluating transferability of flow–ecology relationships across space, time and taxonomy. Freshwater Biology 63, 817–830.
| Evaluating transferability of flow–ecology relationships across space, time and taxonomy.Crossref | GoogleScholarGoogle Scholar |
Cocherell, S. A., Cocherell, D. E., Jones, G. J., Miranda, J. B., Thompson, L. C., Cech, J. J., and Klimley, A. P. (2011). Rainbow trout Oncorhynchus mykiss energetic responsesto pulsed flows in the American River, California, assessed by electromyogram telemetry. Environmental Biology of Fishes 90, 29–41.
| Rainbow trout Oncorhynchus mykiss energetic responsesto pulsed flows in the American River, California, assessed by electromyogram telemetry.Crossref | GoogleScholarGoogle Scholar |
Crook, D. A. (2004). Is the home range concept compatible with the movements of two species of lowland river fish? Journal of Animal Ecology 73, 353–366.
| Is the home range concept compatible with the movements of two species of lowland river fish?Crossref | GoogleScholarGoogle Scholar |
Davis, T. (1977). Reproductive biology of the freshwater catfish, Tandanus tandanus, in the Gwydir River, Australia. II. Gonadal cycle and fecundity. Marine and Freshwater Research 28, 159–169.
| Reproductive biology of the freshwater catfish, Tandanus tandanus, in the Gwydir River, Australia. II. Gonadal cycle and fecundity.Crossref | GoogleScholarGoogle Scholar |
Jepsen, N., Koed, A., Thorstad, E. B., and Baras, E. (2002). Surgical implantation of telemetry transmitters in fish: how much have we learned? Hydrobiologia 483, 239–248.
| Surgical implantation of telemetry transmitters in fish: how much have we learned?Crossref | GoogleScholarGoogle Scholar |
Koehn, J. D. (2004). Carp (Cyprinus carpio) as a powerful invader in Australian waterways. Freshwater Biology 49, 882–894.
| Carp (Cyprinus carpio) as a powerful invader in Australian waterways.Crossref | GoogleScholarGoogle Scholar |
Koehn, J. D., and Crook, D. A. (2013) Movements and migration. In ‘Ecology of Australian Freshwater Fishes’. (Eds P. Humphries and K. Walker.) pp. 105–130. (CSIRO Publishing: Melbourne, Vic., Australia.)
Konrad, C. P., Olden, J. D., Lytle, D. A., Melis, T. S., Schmidt, J. C., Bray, E. N., Freeman, M. C., Gido, K. B., Hemphill, N. P., and Kennard, M. J. (2011). Large-scale flow experiments for managing river systems. Bioscience 61, 948–959.
| Large-scale flow experiments for managing river systems.Crossref | GoogleScholarGoogle Scholar |
Koster, W. M., Dawson, D. R., O’Mahony, D. J., Moloney, P. D., and Crook, D. A. (2014). Timing, frequency and environmental conditions associated with mainstem-tributary movement by a lowland river fish, golden perch (Macquaria ambigua). PLoS One 9, e96044.
| Timing, frequency and environmental conditions associated with mainstem-tributary movement by a lowland river fish, golden perch (Macquaria ambigua).Crossref | GoogleScholarGoogle Scholar | 24788137PubMed |
Koster, W. M., Dawson, D. R., Clunie, P., Hames, F., McKenzie, J., Moloney, P. D., and Crook, D. A. (2015). Movement and habitat use of the freshwater catfish (Tandanus tandanus) in a remnant floodplain wetland. Ecology Freshwater Fish 24, 443–455.
| Movement and habitat use of the freshwater catfish (Tandanus tandanus) in a remnant floodplain wetland.Crossref | GoogleScholarGoogle Scholar |
Lake, J. S. (1967). Rearing experiments with five species of Australian freshwater fishes. I. Inducement to spawning. Marine and Freshwater Research 18, 137–154.
| Rearing experiments with five species of Australian freshwater fishes. I. Inducement to spawning.Crossref | GoogleScholarGoogle Scholar |
Larned, S. T., Datry, T., Arscott, D. B., and Tockner, K. (2010). Emerging concepts in temporary-river ecology. Freshwater Biology 55, 717–738.
| Emerging concepts in temporary-river ecology.Crossref | GoogleScholarGoogle Scholar |
Lechner, A., Keckeis, H., and Humphries, P. (2016). Patterns and processes in the drift of early developmental stages of fish in rivers: a review. Reviews in Fish Biology and Fisheries 26, 471–489.
| Patterns and processes in the drift of early developmental stages of fish in rivers: a review.Crossref | GoogleScholarGoogle Scholar |
Leigh, S. J., and Zampatti, B. P. (2013). Movement and mortality of Murray cod, Maccullochella peelii, during overbank flows in the lower River Murray, Australia. Australian Journal of Zoology 61, 160–169.
| Movement and mortality of Murray cod, Maccullochella peelii, during overbank flows in the lower River Murray, Australia.Crossref | GoogleScholarGoogle Scholar |
Lytle, D. A., and Poff, N. L. (2004). Adaptation to natural flow regimes. Trends in Ecology & Evolution 19, 94–100.
| Adaptation to natural flow regimes.Crossref | GoogleScholarGoogle Scholar |
Marshall, J. C., Menke, N., Crook, D. A., Lobegeiger, J. S., Balcombe, S. R., Huey, J. A., Fawcett, J. H., Bond, N. R., Starkey, A. H., Sternberg, D., Linke, S., and Arthington, A. H. (2016). Go with the flow: the movement behaviour of fish from isolated waterhole refugia during connecting flow events in an intermittent dryland river. Freshwater Biology 61, 1242–1258.
| Go with the flow: the movement behaviour of fish from isolated waterhole refugia during connecting flow events in an intermittent dryland river.Crossref | GoogleScholarGoogle Scholar |
Nathan, R., Getz, W. M., Revilla, E., Holyoak, M., Kadmon, R., Saltz, D., and Smouse, P. E. (2008). A movement ecology paradigm for unifying organismal movement research. Proceedings of the National Academy of Sciences of the United States of America 105, 19052–19059.
| A movement ecology paradigm for unifying organismal movement research.Crossref | GoogleScholarGoogle Scholar | 19060196PubMed |
Olden, J. D., Konrad, C. P., Melis, T. S., Kennard, M. J., Freeman, M. C., Mims, M. C., Bray, E. N., Gido, K. B., Hemphill, N. P., and Lytle, D. A. (2014). Are large‐scale flow experiments informing the science and management of freshwater ecosystems? Frontiers in Ecology and the Environment 12, 176–185.
| Are large‐scale flow experiments informing the science and management of freshwater ecosystems?Crossref | GoogleScholarGoogle Scholar |
Pelicice, F. M., Pompeu, P. S., and Agostinho, A. A. (2015). Large reservoirs as ecological barriers to downstream movements of Neotropical migratory fish. Fish and Fisheries 16, 697–715.
| Large reservoirs as ecological barriers to downstream movements of Neotropical migratory fish.Crossref | GoogleScholarGoogle Scholar |
Poff, N. L., and Zimmerman, J. K. H. (2010). Ecological responses to altered flow regimes: a literature review to inform the science and management of environmental flows. Freshwater Biology 55, 194–205.
| Ecological responses to altered flow regimes: a literature review to inform the science and management of environmental flows.Crossref | GoogleScholarGoogle Scholar |
Poff, N. L., Allan, J. D., Bain, M. B., Karr, J. R., Prestegaard, K. L., Richter, B. D., Sparks, R. E., and Stromberg, J. C. (1997). The natural flow regime. Bioscience 47, 769–784.
| The natural flow regime.Crossref | GoogleScholarGoogle Scholar |
Pusey, B., Kennard, M. J., and Arthington, A. H. (2004) ‘Freshwater Fishes of North-eastern Australia.’ (CSIRO Publishing: Melbourne, Vic., Australia.)
Rahel, F. J., and McLaughlin, R. L. (2018). Selective fragmentation and the management of fish movement across anthropogenic barriers. Ecological Applications 28, 2066–2081.
| Selective fragmentation and the management of fish movement across anthropogenic barriers.Crossref | GoogleScholarGoogle Scholar | 30168645PubMed |
Reinfelds, I. V., Walsh, C. T., van der Meulen, D. E., Growns, I. O., and Gray, C. A. (2013). Magnitude, frequency and duration of instream flows to stimulate and facilitate catadromous fish migrations: Australian bass (Macquaria novemaculeata Perciformes, Percichythidae). River Research and Applications 29, 512–527.
| Magnitude, frequency and duration of instream flows to stimulate and facilitate catadromous fish migrations: Australian bass (Macquaria novemaculeata Perciformes, Percichythidae).Crossref | GoogleScholarGoogle Scholar |
Reynolds, L. (1983). Migration patterns of five fish species in the Murray–Darling River system. Marine and Freshwater Research 34, 857–871.
| Migration patterns of five fish species in the Murray–Darling River system.Crossref | GoogleScholarGoogle Scholar |
Rodríguez, M. A. (2002). Restricted movement in stream fish: the paradigm is incomplete, not lost. Ecology 83, 1–13.
| Restricted movement in stream fish: the paradigm is incomplete, not lost.Crossref | GoogleScholarGoogle Scholar |
Rowland, S. J. (1993). Maccullochella ikei, an endangered species of freshwater cod (Pisces: Percichthyidae) from the Clarence River system, NSW and M. peelii mariensis, a new subspecies from the Mary River system, Qld. Records of the Australian Museum 45, 121–145.
| Maccullochella ikei, an endangered species of freshwater cod (Pisces: Percichthyidae) from the Clarence River system, NSW and M. peelii mariensis, a new subspecies from the Mary River system, Qld.Crossref | GoogleScholarGoogle Scholar |
Rowland, S. J. (1996). Threatened fishes of the world: Maccullochella ikei Rowland, 1985 (Percichthyidae). Environmental Biology of Fishes 46, 350.
| Threatened fishes of the world: Maccullochella ikei Rowland, 1985 (Percichthyidae).Crossref | GoogleScholarGoogle Scholar |
Sih, A., Ferrari, M. C., and Harris, D. J. (2011). Evolution and behavioural responses to human‐induced rapid environmental change. Evolutionary Applications 4, 367–387.
| Evolution and behavioural responses to human‐induced rapid environmental change.Crossref | GoogleScholarGoogle Scholar | 25567979PubMed |
Simpson, R. R., and Mapleston, A. J. (2002). Movements and habitat use by the endangered Australian freshwater Mary River cod, Maccullochella peelii mariensis. Environmental Biology of Fishes 65, 401–410.
| Movements and habitat use by the endangered Australian freshwater Mary River cod, Maccullochella peelii mariensis.Crossref | GoogleScholarGoogle Scholar |
Southwell, M., Hill, R., Burch, L., Henderson, T., Mace, N., Ryder, D., Ying Tsoi, W., Butler, G. L., Carpenter-Bundhoo, L., Davis, T., Frazier, P., Spencer, J., Bowen, S., and Humphries, J. (2019) Commonwealth Environmental Water Office Long Term Intervention Monitoring Project, Gwydir River System Selected Area Five Year Evaluation Report.
Storer, T., Bannister, J., Bennett, K., Byrnes, E., Crook, D. A., Morgan, D. L., Gleiss, A., and Beatty, S. J. (2021). Influence of discharge regime on the movement and refuge use of a freshwater fish in a drying temperate region. Ecohydrology 14, e2253.
| Influence of discharge regime on the movement and refuge use of a freshwater fish in a drying temperate region.Crossref | GoogleScholarGoogle Scholar |
Threatened Species Scientific Committee (2008) The Tandanus tandanus – Eel tailed catfish in the Murray/Darling Basin as an endangered population. Department of Primary Industries, Port Stephens Fisheries Centre, Nelsons Bay, NSW, Australia.
Wagner, G. N., Cooke, S. J., Brown, R. S., and Deters, K. A. (2011). Surgical implantation techniques for electronic tags in fish. Reviews in Fish Biology and Fisheries 21, 71–81.
| Surgical implantation techniques for electronic tags in fish.Crossref | GoogleScholarGoogle Scholar |
Wong, B. B. M., and Candolin, U. (2015). Behavioral responses to changing environments. Behavioral Ecology 26, 665–673.
| Behavioral responses to changing environments.Crossref | GoogleScholarGoogle Scholar |
Young, R. G., Hayes, J. W., Wilkinson, J., and Hay, J. (2010). Movement and mortality of adult brown trout in the Motupiko River, New Zealand: effects of water temperature, flow, and flooding. Transactions of the American Fisheries Society 139, 137–146.
| Movement and mortality of adult brown trout in the Motupiko River, New Zealand: effects of water temperature, flow, and flooding.Crossref | GoogleScholarGoogle Scholar |
Zuur, A. F., Ieno, E. N., and Elphick, C. S. (2010). A protocol for data exploration to avoid common statistical problems. Methods in Ecology and Evolution 1, 3–14.
| A protocol for data exploration to avoid common statistical problems.Crossref | GoogleScholarGoogle Scholar |