Drivers restricting biodiversity in Australian saline lakes: a review
Brian V. Timms
Centre for Ecosystem Science, School of Biological, Earth and Environmental Sciences, University of New South Wales, Kensington, NSW 2052, Australia. Email: brian.timms@unsw.edu.au
Marine and Freshwater Research 72(4) 462-468 https://doi.org/10.1071/MF20205
Submitted: 30 June 2020 Accepted: 8 September 2020 Published: 13 November 2020
Journal Compilation © CSIRO 2021 Open Access CC BY-NC-ND
Abstract
Inland saline lakes are well known to be less biodiverse than fresh waters. In Australia, the most important driver affecting biodiversity is salinity that imposes an inverse linear relationship. However, in detailed studies across a wide salinity spectrum, the relationship is scale dependent. This is mediated in part by the range of salinity tolerated becoming broader as the maximum tolerated salinity increases. Other factors of importance sometimes include hydrology, habitat heterogeneity, season, pH and oxygen, but these are usually not easy to quantify. Even rarer is the influence of colonisation by marine organisms, which is applicable only at some sites near the coastline and the influence of ionic proportions on the presence of some species and, hence, diversity. The contribution of predation or competition on diversity, reported in some overseas salinas, is suspected but yet to be proved in Australia. The crustacean component in saline lakes is more influenced by these drivers than is most of the insect fraction.
Keywords: crustaceans, habitat heterogeneity, hydrology, insects, oxygen, pH, salinity, season.
Introduction
Diversity is a basic ecological characteristic of every community. It is particularly relevant in drier climates where species are restricted by many adverse environmental conditions, as in Australian inland waters of dryland regions (Davis et al. 2018). These authors consider quantitatively various drivers in 10 types of aquatic habitats, but omit saline waters (those >3 g L–1). For this later grouping, Williams et al. (1990) provided some data on the role of salinity and, later, Williams (1998) considered the influence of various factors but without quantification. It is now appropriate to consider these drivers and others in more detail and, where possible, to quantify them.
Early investigations in Australian salinas (Bayly and Williams 1966; Bayly 1970) emphasised water chemistry and the unusual fauna but did not consider the role of a salinity scale or any other factors on diversity. It took a few decades for researches to consider these factors and to try to quantify them (Bayly 1969, 1970, 1976; Timms 1973, 1981, 1987, 1993, 1996, 1998a, 1998b, 2001a, 2001b, 2007, 2008, 2009a, 2009b, 2018; Geddes 1976; De Deckker and Geddes 1980; Geddes et al. 1981; Williams 1981, 1984, 1998; Williams and Kokkinn 1988; Williams et al. 1990; Pinder et al. 2002, 2004, 2005). In many of these studies, the conclusions are clearly enunciated, but in others a re-analysis of the data is necessary. This review will consider these drivers arguably in their order of importance. The location of sites included in these various studies are shown in Fig. 1.

|
Materials and methods
Even though this is a review paper and, thus, utilises already published information, data presented in some papers are re-analysed. This applies to fig. 4 and 5 in Geddes (1976), table 17.2 in Geddes et al. (1981), various tables in Timms (1973, 1981), figures designated as fig. 2 in Timms (1993) and also fig. 2 in Williams (1990). Fortunately, in these studies, species richness in various sites arranged by salinity can be ascertained from these figures. Note that in choosing sites, a wider salinity range was used at the higher salinity, in keeping with the statement by Williams (1990) that tolerances were greater at higher salinities. Serendipitously, this approach enabled enough sites to be chosen to make the comparisons more robust.
The following three aspects of diversity were used: α diversity, which is the momentary species richness; β diversity, which is used in the sense of cumulative species richness over time at a nominated site; and γ diversity, which is the total list of species recorded in many sites within a defined district. All quoted salinities refer to the total concentration of inorganic ions.
Results
Salinity
In Australia, the earliest published role of the proportional influence of salinity is seen in Geddes et al. (1981) and Timms (1973, 1981), both being made clearer by a re-analysis of their data. Timms (1981) made a comprehensive study of just three Victorian lakes, namely, Purrumbete (0.4 g L–1), Bullenmerri (8 g L–1) and Gnotuk (58 g L–1). In all five communities studied, there was a marked difference between the fresh and the two saline lakes and then a decrease with an increased salinity in the latter (Table 1a). No mathematical relationship was suggested (Timms 1973). The study of Geddes et al. (1981) of 54 Western Australian lakes showed a significant negative relationship between α diversity and salinity (r = 0.695, significant at P < 0.01; calculated from table 17.2 of Geddes et al. 1981), but of variable intensity among regions (see later in the paper). The aquatic fauna of saline Lake Buchanan and associated pools in northern Queensland showed a significant negative relationship between α diversity and salinity (Timms 1987). Again, Timms (1993, 1998a) showed a linear relationship between salinity and α diversity for a series of lakes in the Paroo in north-western New South Wales, but of different slope under different rainfall senarios (see later in the paper; Fig. 2). In a thorough study of many Western Australian sites, Pinder et al. (2005) proposed a complicated mathematical relationship between diversity and salinity; the equations varying for various salinity ranges. Contrary to the above studies, Timms (2009a) on Eyre Peninsula lakes failed to find a significant relationship between diversity and salinity as (1) there were few hyposaline lakes to give high diversity at low salinities, (2) there were many marine springs at ~35 g L–1, thus increasing mesosaline species and (3) there were many (66%) hypersaline lakes so that their euryhaline species did not show much decrease in diversity over a wide salinity range. Finally, a study of reasonably homogenous lakes in the Esperance area of Western Australia had a more classical significant relationship between salinity and β diversity (Timms 2009b). The overall conclusion is inescapable, namely, that generally the salinity factor is dominant in determining diversity in saline lakes across Australia.
|
|
However, the relationship is not necessarily linear as implied in the most of the above studies. In a study of 79 lakes in the western district of Victoria, Williams et al. (1990) reported variable levels of correlation between salinity and diversity over different salinity ranges and a hand-drawn sigma-shaped curve between the two. They concluded that ‘an appreciation of scale is fundamental.’ Furthermore, they stated that species living at low salinities have the narrowest range of salinity tolerance, those at intermediate salinities, a broader range and those at the highest salinities, the widest tolerance to salinity. Pinder et al. (2002, 2005) reported similarly for their detailed Western Australia studies, but found no relationship between salinity and richness at salt concentrations of <3 g L–1.
Hydrology
There are indications that permanent saline lakes have more species at similar specific salinities than do seasonal waters, and that among the latter with shorter hydroperiods, species richness is even lower.
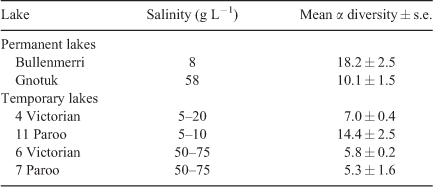
A re-analysis of the data for two permanent lakes, namely, Bullenmerri and Gnotuk, and sets of seasonal lakes showed that the former were more species rich than were the latter (Table 1). This difference is an expression in the permanent lakes of the greater depth and, hence, more benthic species and also the presence of vegetated littorals and rocky shorelines in the permanent lakes vis-à-vis seasonal lakes; i.e. habitat heterogeneity is the basic reason for the differences (see later), but other explanations are possible, such as in permanent lakes species have more time to accumulate. Divergence between permanent and seasonal lakes is less at higher salinities, no doubt being associated with the restricted species pool. One example is the dominance of ‘giant’ ostracods in Australian higher-salinity lakes (Halse 2002; Halse and McRae 2004) and the presence of just a few species in most hypersaline communities simplifying whole-lake diversity.
Two sets of data are available on hydrological influence in seasonal lakes. The study of Geddes et al. (1981) of 54 lakes across a large area of Western Australia referred to above (Fig. 1) showed differences among regions, the wetlands of the far northern area (α diversity of 2.9) being less diverse than those of southern areas (α diversity of 6.2–7.5), with the assumed shortest water presence in the northern salinas in a zone of lower rainfall and higher evaporation. This hydrological briefness is confirmed by a study on rock pools (gnammas) across much the same area (Timms 2012; Brendonck et al. 2015), thus providing the environmental data needed to support the conclusion. However, also influencing diversity in these northern sites could be less frequent filling and, so, a less diverse egg bank, although eggs can remain viable in sediments for many years, commonly a decade, and so, mitigate for infrequent fillings (Boulton and Lloyd 1992; Katajisto 1996).
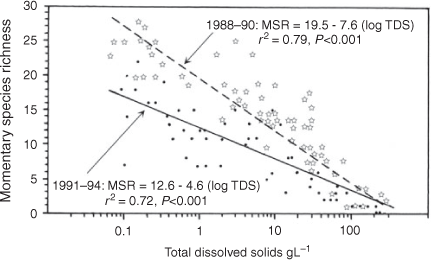
The graphs for two separate studies in the Paroo (Timms 1993, 1998a) show different slopes for the relationship in wet years v. dry years, with a lower diversity at low to moderate salinities in dry years (Fig. 2). There were eight wettings in the 3 dry years v. 12 in the 3 wet years, almost all wettings being of shorter duration in the dry years as suggested by rainfall records. The difference is due largely to the lack of insects in the dry years, probably indicating their lower resilience to generally dry conditions than for crustaceans, which hatch easily from resistant eggs and, generally, need a shorter time to complete their life cycles (Timms 1998a).
Habitat heterogeneity
This subject is not often raised in saline-lake studies, no doubt owing to the difficulty to quantify it. However, sometimes the influence of a varied structured habitat, as in a large inlet to a lake, can be compared with the homogeneity of wave-washed shores in large lakes. Such an example is the Werewilka Inlet to Lake Wyara in south-western Queensland (Timms 1998b, 2001a). The inlet has much greater variation in its sediment than do the shores of the lake (see fig. 3 in Timms 2001a). The invertebrate data from both lake and inlet were reworked (Table 2) to show the clear difference in diversity between the two sites. This table was then further extended by the analysis of diversity in some large saline lakes compared with that in small salinas in inland Australia. There was an adverse effect on the diversity of wave-washed, relatively homogeneous shores of large lakes, compared with somewhat heterogeneous shores of small lakes (Table 2; Timms 2001a). For organisms with tolerance to low salinity (<5 g L–1), the difference was significant at P = 0.08 (r = 0.084); however, for the saline species (salinity of >20 g L–1) and of >50 g L–1), the difference was not significant (r = 0.4687 and r = 0.5269 respectively). The difference is explained by low-tolerance insects being more common among littoral rocks and debris than on pure muddy sand, whereas there was little difference between saline organisms (mainly crustaceans) inhabiting largely the pelagic zone of both types of waters.

|
An adjunct to habitat heterogeneity is the presence of macrophytes. Generally, these are not eaten (a notable herbivorous exception is moth larvae), but plants (e.g. Ruppia, Lepileana, Chara) provide physical habitat and are particularly attractive to damsel flies and microfauna. No doubt the presence of macrophytes at lower salinities enhances diversity, but there are no Australian studies.
Season
Generally, saline lakes are more biodiverse in summer than in winter, the difference largely being due to the abundance of insects in summer. This is most apparent in inland salinas in the Paroo where one study recorded 38 species in summer but only 24 species in winter (Timms 2018). Summer and winter crustacean species were almost identical, but very few insects were found in winter. Two seasonal studies in southern Australia did not record insects where water was present only in winter and spring (Geddes 1976; De Deckker and Geddes 1980). In a study at Esperance, the insect and crustacean lists were of similar length (30 v. 36 taxa respectively); however, a re-examination of their seasonality showed that insects were recorded mainly, but not exclusively, in non-winter collections (Timms 2009b; and B. V. Timms, unpubl. data). Most winter presences were of dipteran larvae.
pH
Not many Australian saline lakes are acidic, but there are some in Western Australia (Pinder et al. 2002, 2004, 2005; Timms 2009b), and on Eyre Peninsula, South Australia (P. Hudson, pers. comm., July 2016). Pinder et al. (2004) found that pH was strongly correlated with the composition of salt-lake communities. Most such lakes have Parartemia spp. characteristic of such waters. For P. contracta, Conte and Geddes (1988) showed that it is able to live at a low pH because of a unique biochemical ability to access CO2 from sources other than HCO3 at pH of <6.3. In their study, it was the only invertebrate in localities of low pH. The Esperance area has many lakes of pH <7 (for these, there was a positive relationship with a decreasing pH of r = 0.367, P < 0.05; Timms 2009b). At a pH of <4, the only invertebrates present were Parartemia acidiphila (noted as Parartemia sp. F in the study) and the ostracod Australocypris bennetti (acid form).
Oxygen
Given that the solubility of oxygen in water decreases linearly with an increasing salinity, its effect on diversity is likely to be similar to that of salinity. However, it is not likely to affect air-breathing insects, nor small crustaceans relying on diffusion for gas exchange, just larger crustaceans and, then, more probably at the lower oxygen tensions characteristic of higher salinities. The only documented Australian example was reported by Mitchell and Geddes (1977) for solar salt ponds near Adelaide. In these, Artemia was the only brine shrimp in the most saline ponds because of its haemoglobin, whereas the ecologically similar Parartemia, with no or very little haemoglobin, was restricted to ponds of lower salinity and, hence, higher oxygen tensions.
Marine influence
It is rare to find marine-derived species in inland saline lakes, no doubt owing to the lack of resistant stages in marine species to withstand seasonal or episodic desiccation. However, examples are known in lakes close to the coast, but physically separated, such as along the Beachport–Robe coast of south-eastern South Australia (Bayly 1970), on Eyre Peninsula, South Australia (Timms 2009a), in north-western Western Australia (Halse et al. 2000) and also in south-western Australia (A. Pinder, pers. comm., May 2020). At least in the case of polychaetes and copepods in the Beachport–Robe series, existence is precarious and recolonisation, possibly by birds, is probably necessary (Bayly 1970). In the Eyre Peninsula series, the presence of 18 species (of 88 in total) is apparently relictal and relies on permanent near-seawater salinities (Timms 2009a). There are no quantitative data on any effect on α diversity, but γ diversity for a region is raised a little.
Ionic composition
Australian saline lakes are somewhat homogeneous in their ionic composition, almost all being dominated by sodium (Na) and chloride (Cl) ions. At very high salinities (at >~300 g L–1), microorganisms can be negatively affected by high magnesium concentrations (Baas-Becking 1931; Borowitza 1981); however, almost no multicellular organisms live at such salinities. At more common hypersaline and mesosaline conditions, Radke et al. (2003) showed that ionic composition can influence ostracod communities, and in hyposaline waters (~22 g L–1), the presence of carbonates can increase the tolerance to salinity by the copepod Boeckella triarticultata (Bayly 1969). No data are available on the effect on diversity, but such would be minor.
Discussion
Studies on world saline lakes usually report a generalised inverse relationship between salinity and species richness (Hammer 1986); however, the relationship has been quantified only recently by some studies, much like the Australian studies mentioned above. Examples include McCulloch et al. (2008) on the Sua Pan, Botswana, Waterkeyn et al. (2008) on the Camargue wetlands in southern France, Horváth et al. (2014) on soda pans of the Carpathian Basin of eastern Europe, and Anufriineua and Shadrin (2018) on Crimean lakes. These authors try to quantify other factors too such as ionic composition, pH and hydroperiod.
The eight abiotic factors affecting diversity in saline lakes itemised above are not a complete list, nor have all the factors been quantified. Biotic factors such as predation and competition have not been recorded in Australia and could be influential. Perhaps the biggest omission is how the presence of predators at middle salinities influences diversity. The example of the corixid Trichocorixa verticalis in Great Salt Lake is pertinent. It reduced Artemia populations and allowed two copepods and a rotifer, thus overall increasing diversity (Wurtsbaugh 1992). The effect of fish predation on Artemia is well known, so that should fish be removed, Artemia thrives and as do a few other invertebrates, so that diversity is increased. Although corixids (Knowles and Williams 1973) and the taxonomically similar Parartemia to Artemia occur widely in Australian saline lakes (Timms 2014), no study has recorded any biological influence. Campbell (1993) noted that the ostracod Australocypris insularis is a predator in some Australian salt lakes; however, its influence on diversity is also unknown.
The list of factors enunciated above is not the same as the ones mentioned by Williams (1998). His list is more inclusive and includes salinity, oxygen, ionic composition, pH, hydrological patterns, geographical position, palaeoclimatic events, chance, human interventions and biological interactions. His approach is more focussed on community structure rather than species diversity per se, and so is complementary to the present study. The Williams et al. (1990) paper is most relevant as it was the first to point out that the relationship between salinity and species richness is not linear but depends on the salinity scale. The linear relationships reported above are simplistic, and for a real understanding, scale should be involved. The same probably applies to other linear relationships reported for other factors. Obviously, factors influencing species composition and diversity are numerous and complex. Salinity is the easiest to quantify and, so, is often referred in saline-lake studies; however, it should be kept in mind that other factors are variously relevant.
In a comparison to other waters in the drylands of Australia (Davis et al. 2018), drivers of diversity in salt lakes are almost entirely different. Latitude and hydrologic connectivity so important in the waters Davis et al. (2018) studied, play no primary role in salinas; in fact, some can be in contradiction. For instance, the increase in diversity in saline lakes north to south in Western Australia (Geddes et al. 1981) is the antithesis of the situation in other aquatic communities in which diversity decreases with an increasing latitude (Davis et al. 2018); in this case, the explanation lies in rainfall differences, not in factors directly associated with latitude. However, as in other dryland aquatic communities, ecogeographic factors also influence community composition in saline lakes (Williams 1984; Timms 2007) but any diversity differences are explained by the factors enunciated above, not by the drivers for all of ecosystems in Davis et al. (2018). The hydrologically disconnected communities (springs and rockholes) of the arid biome have some drivers in common with saline lakes, particularly those pertaining to dispersal; however, again, salt lakes are so distinctive from the norm because of the dominant role of just one driver, salinity.
Another aspect is clear. The insect component reacts differently to the drivers than do the crustaceans. Generally, insect diversity is more adversely affected by winter season and adverse hydrology than is the diversity of crustaceans; however, it is advantaged by heterogeneous habitat. The latter is partly explained by most crustaceans being planktonic and most insects being littoral. For the influence of salinity on diversity, chance plays a bigger role among insects than crustaceans, many insect occurrences in saline waters are haphazard, often involving only few dispersing individuals of a species (Timms 1993, 1998a). Adult aquatic insects are apparently not so much affected by the lesser factors of pH and oxygen, and certainly not by marine influence and ionic composition (Gillott 2005).
Conflicts of interest
The author declares that he has no conflicts of interest.
Declaration of funding
This research was self-funded and did not receive any specific other funding.
Acknowledgements
Adrian Pinder and two anonymous referees suggested many improvements to the manuscript for which I am most appreciative.
References
Anufriineua, E. V., and Shadrin, N. V. (2018). Diversity of fauna in Crimean hypersaline water bodies. Journal of Siberian Federal University. Biology (Basel) 11, 294–305.| Diversity of fauna in Crimean hypersaline water bodies.Crossref | GoogleScholarGoogle Scholar |
Baas-Becking, L. G. M. (1931). Salt effects on swarmers of Dunaliella viridis Teodoresco. The Journal of General Physiology 14, 765–779.
| Salt effects on swarmers of Dunaliella viridis Teodoresco.Crossref | GoogleScholarGoogle Scholar |
Bayly, I. A. E. (1969). The occurrence of calanoid copepods in athalassic saline waters in relation to salinity and anionic proportions. Verhandlungen Internationale Vereinigung Limnologie 17, 449–455.
| The occurrence of calanoid copepods in athalassic saline waters in relation to salinity and anionic proportions.Crossref | GoogleScholarGoogle Scholar |
Bayly, I. A. E. (1970). Further studies on same saline lakes of south-east Australia. Australian Journal of Marine and Freshwater Research 21, 117–129.
| Further studies on same saline lakes of south-east Australia.Crossref | GoogleScholarGoogle Scholar |
Bayly, I. A. E. (1976). The plankton of Lake Eyre. Australian Journal of Marine and Freshwater Research 27, 661–665.
| The plankton of Lake Eyre.Crossref | GoogleScholarGoogle Scholar |
Bayly, I. A. E., and Williams, W. D. (1966). Chemical and biological studies on some saline lakes in south-east Australia. Australian Journal of Marine and Freshwater Research 17, 177–228.
| Chemical and biological studies on some saline lakes in south-east Australia.Crossref | GoogleScholarGoogle Scholar |
Borowitza, L. J. (1981). The microflora. Adaptations to life in extremely saline waters. Hydrobiologia 81, 33–46.
Boulton, A., and Lloyd, L. N. (1992). Flooding frequency and invertebrate emergence from dry floodplain sediments of the River Murray, Australia. Regulated Rivers: Research and Management 7, 137–151.
| Flooding frequency and invertebrate emergence from dry floodplain sediments of the River Murray, Australia.Crossref | GoogleScholarGoogle Scholar |
Brendonck, L., Jocqué, M., Tuytens, K., Timms, B. V., and Vanschoenwinkel, B. (2015). Hydrological stability drives both local and regional diversity patterns in rock pool metacommunities. Oikos 124, 741–749.
| Hydrological stability drives both local and regional diversity patterns in rock pool metacommunities.Crossref | GoogleScholarGoogle Scholar |
Campbell, E. (1993). Planktivory by planktonic ostracods in Australian salt lakes. Verhandlungen Internationale Vereinigung Limnologie 25, 887–889.
Conte, F. P., and Geddes, M. C. (1988). Acid brine shrimps: metabolic strategies in osmotic and ionic adaptation. Hydrobiologia 158, 191–200.
| Acid brine shrimps: metabolic strategies in osmotic and ionic adaptation.Crossref | GoogleScholarGoogle Scholar |
Davis, J., Sim, L., Thomson, R. M., Pinder, A., Brim Box, J., Murphy, N. P., Morán-Ordóňez, A., and Sunnucks, P. (2018). Patterns and drivers of aquatic invertebrate diversity across an arid biome. Ecography 41, 375–387.
| Patterns and drivers of aquatic invertebrate diversity across an arid biome.Crossref | GoogleScholarGoogle Scholar |
De Deckker, P., and Geddes, M. C. (1980). Seasonal fauna of the ephemeral saline lakes near the Coorong Lagoon, South Australia. Australian Journal of Marine and Freshwater Research 31, 677–699.
| Seasonal fauna of the ephemeral saline lakes near the Coorong Lagoon, South Australia.Crossref | GoogleScholarGoogle Scholar |
Geddes, M. C. (1976). Seasonal fauna of some ephemeral saline lakes in western Victoria with particular reference to Parartemia zietziana Sayce (Crustacea: Anostraca). Australian Journal of Marine and Freshwater Research 27, 1–22.
| Seasonal fauna of some ephemeral saline lakes in western Victoria with particular reference to Parartemia zietziana Sayce (Crustacea: Anostraca).Crossref | GoogleScholarGoogle Scholar |
Geddes, M. C., De Deckker, P., Williams, W. D., Morton, D. W., and Topping, M. (1981). On the chemistry and biota of some saline lakes in Western Australia. Hydrobiologia 81–82, 201–222.
| On the chemistry and biota of some saline lakes in Western Australia.Crossref | GoogleScholarGoogle Scholar |
Gillott, C. (2005). ‘Entomology’, 3rd edn. (Springer: Heidelberg, Germany.)
Halse, S. A. (2002). Diversity of Ostracoda (Crustacea) in inland waters of Western Australia. Verhandlungen Internationale Vereinigung Limnologie 28, 914–918.
| Diversity of Ostracoda (Crustacea) in inland waters of Western Australia.Crossref | GoogleScholarGoogle Scholar |
Halse, S. A., and McRae, J. M. (2004). New genera and species of giant ostracods (Crustacea: Cyprididae) from Western Australia. Hydrobiologia 524, 1–52.
| New genera and species of giant ostracods (Crustacea: Cyprididae) from Western Australia.Crossref | GoogleScholarGoogle Scholar |
Halse, S. A., Shiel, R. S., Storey, A. W., Edward, D. H. D., Lansbury, I., Cale, D. J., and Harvey, M. S. (2000). Aquatic invertebrates and waterbirds of wetlands and rivers of the southern Carnarvon Basin, Western Australia. Records of the Western Australian Museum 60, 217–267.
| Aquatic invertebrates and waterbirds of wetlands and rivers of the southern Carnarvon Basin, Western Australia.Crossref | GoogleScholarGoogle Scholar |
Hammer, U. T. (1986). ‘Saline Lakes Ecosystems of the World.’ (Junk: Dordrecht, Netherlands.)
Horváth, Z., Vad, C. F., Tóth, A., Zsuga, K., Boris, E., Vöröx, L., and Ptacnik, R. (2014). Zooplankton diversity and functioning along a natural stress gradient: when the going gets tough the tough get going. Oikos 123, 461–471.
| Zooplankton diversity and functioning along a natural stress gradient: when the going gets tough the tough get going.Crossref | GoogleScholarGoogle Scholar |
Katajisto, T. (1996). Copepod eggs survive a decade in the sediment of the Baltic Sea. Hydrobiologia 320, 153–159.
| Copepod eggs survive a decade in the sediment of the Baltic Sea.Crossref | GoogleScholarGoogle Scholar |
Knowles, J. N., and Williams, W. D. (1973). Salinity range and osmoregulation of corixids (Hemiptera: Heteroptera) in south-east Australian waters. Australian Journal of Marine and Freshwater Research 24, 297–302.
| Salinity range and osmoregulation of corixids (Hemiptera: Heteroptera) in south-east Australian waters.Crossref | GoogleScholarGoogle Scholar |
McCulloch, G. P., Irvine, K., Eckardt, F. D., and Bryant, R. (2008). Hydrochemical fluctuations and crustacean community composition in an ephemeral saline lake (Sua Pan, Makgadikgadi, Botswana). Hydrobiologia 596, 31–46.
| Hydrochemical fluctuations and crustacean community composition in an ephemeral saline lake (Sua Pan, Makgadikgadi, Botswana).Crossref | GoogleScholarGoogle Scholar |
Mitchell, B. D., and Geddes, M. C. (1977). Distribution of the brine shrimps Parartemia zietziana (Sayce) and Artemia salina (Linneaus) along a salinity and oxygen gradient in a South Australian salt field. Freshwater Biology 7, 461–467.
| Distribution of the brine shrimps Parartemia zietziana (Sayce) and Artemia salina (Linneaus) along a salinity and oxygen gradient in a South Australian salt field.Crossref | GoogleScholarGoogle Scholar |
Pinder, A. M., Halse, S. A., McRae, J. M., and Shiel, R. J. (2002). Halophile aquatic invertebrates in the wheatbelt region of south-western Australia. Verhandlungen Internationale Vereinigung Limnologie 28, 1687–1694.
| Halophile aquatic invertebrates in the wheatbelt region of south-western Australia.Crossref | GoogleScholarGoogle Scholar |
Pinder, A. M., Halse, S. A., McRae, J. M., and Shiel, R. J. (2004). Aquatic invertebrate assemblages of wetlands and rivers in the wheatbelt region of Western Australia. Records of the Western Australian Museum 67, 7–37.
| Aquatic invertebrate assemblages of wetlands and rivers in the wheatbelt region of Western Australia.Crossref | GoogleScholarGoogle Scholar |
Pinder, A. M., Halse, S. A., McRae, J. M., and Shiel, R. J. (2005). Occurrence of aquatic invertebrates of the Wheatbelt region of Western Australia in relation to salinity. Hydrobiologia 543, 1–24.
| Occurrence of aquatic invertebrates of the Wheatbelt region of Western Australia in relation to salinity.Crossref | GoogleScholarGoogle Scholar |
Radke, L. C., Juggins, S., Halse, S. A., and de Deckker, P. (2003). Chemical diversity in south-eastern Australian saline lakes II: Biotic implications. Marine and Freshwater Research 54, 895–912.
| Chemical diversity in south-eastern Australian saline lakes II: Biotic implications.Crossref | GoogleScholarGoogle Scholar |
Timms, B. V. (1973). A comparative study of the limnology of three maars in western Victoria. Ph.D. Thesis, Monash University, Melbourne, Vic., Australia.
Timms, B. V. (1981). Animal communities in three Victorian lakes of differing salinity. Hydrobiologia 81–82, 181–193.
| Animal communities in three Victorian lakes of differing salinity.Crossref | GoogleScholarGoogle Scholar |
Timms, B. V. (1987). Limnology of Lake Buchanan, a tropical saline lake, and associated pools, of north Queensland. Australian Journal of Marine and Freshwater Research 38, 877–884.
| Limnology of Lake Buchanan, a tropical saline lake, and associated pools, of north Queensland.Crossref | GoogleScholarGoogle Scholar |
Timms, B. V. (1993). Saline lakes of the Paroo, inland New South Wales, Australia. Hydrobiologia 267, 269–289.
| Saline lakes of the Paroo, inland New South Wales, Australia.Crossref | GoogleScholarGoogle Scholar |
Timms, B. V. (1996). A comparison between freshwater and saline wetlands on Bloodwood Station, the Paroo, Australia, with special reference to their use by waterbirds. International Journal of Salt Lake Research 5, 287–313.
| A comparison between freshwater and saline wetlands on Bloodwood Station, the Paroo, Australia, with special reference to their use by waterbirds.Crossref | GoogleScholarGoogle Scholar |
Timms, B. V. (1998a). Further studies on the saline lakes of the eastern Paroo, inland New South Wales, Australia. Hydrobiologia 381, 31–42.
| Further studies on the saline lakes of the eastern Paroo, inland New South Wales, Australia.Crossref | GoogleScholarGoogle Scholar |
Timms, B. V. (1998b). A study of Lake Wyara, and episodically filled saline lake in southwest Queensland, Australia. International Journal of Salt Lake Research 7, 113–132.
| A study of Lake Wyara, and episodically filled saline lake in southwest Queensland, Australia.Crossref | GoogleScholarGoogle Scholar |
Timms, B. V. (2001a). A study of the Werewilka Inlet of the saline Lake Wyara, Australia: a harbour of diversity for a sea of simplicity. Hydrobiologia 466, 245–254.
| A study of the Werewilka Inlet of the saline Lake Wyara, Australia: a harbour of diversity for a sea of simplicity.Crossref | GoogleScholarGoogle Scholar |
Timms, B. V. (2001b). Limnology of the intermittent pools of Bells Creek, Paroo, arid Australia, with special reference to biodiversity of invertebrates and succession. Proceedings of the Linnean Society of New South Wales 123, 193–213.
Timms, B. V. (2007). The biology of the saline lakes of central and eastern inland of Australia: a review with special reference to their biogeographic affinities. Hydrobiologia 576, 27–37.
| The biology of the saline lakes of central and eastern inland of Australia: a review with special reference to their biogeographic affinities.Crossref | GoogleScholarGoogle Scholar |
Timms, B. V. (2008). The ecology of episodic saline lakes of inland eastern Australia, as exemplified by a ten year study of the Rockwell–Wombah Lakes of the Paroo. Proceedings of the Linnean Society of New South Wales 129, 1–16.
Timms, B. V. (2009a). A study of the salt lakes and salt springs of Eyre Peninsula, South Australia. Hydrobiologia 626, 41–51.
| A study of the salt lakes and salt springs of Eyre Peninsula, South Australia.Crossref | GoogleScholarGoogle Scholar |
Timms, B. V. (2009b). A study of the salt lakes inland of Esperance, Western Australia, with special reference to the role of ground water acidity and episodicity. In ‘Saline Lakes around the World; Unique Systems with Unique Values’. (Eds A. Oren, D. Natfz, P. Palacois, and W. A. Wurstbaugh.) pp. 215–224. Natural Resources and Environmental Issues, Vol. XV. (S. J. and Jessie E Quincey Natural Resource Research Library: Logan, UT, USA.)
Timms, B. V. (2012). Seasonal study of aquatic invertebrates in five sets of latitudinally separated gnammas in southern Western Australia. Journal of the Royal Society of Western Australia 95, 13–28.
Timms, B. V. (2014). A review of the biology of Australian halophilic anostracans (Branchiopoda; Anostraca). Journal of Biological Research (Thessaloniki) 21, 21.
| A review of the biology of Australian halophilic anostracans (Branchiopoda; Anostraca).Crossref | GoogleScholarGoogle Scholar |
Timms, B. V. (2018). On the influence of season and salinity on the phenology of invertebrates in Australian saline lakes, with special reference to those of the Paroo in the semiarid inland. Journal of Oceanography and Limnology 36, 1907–1916.
| On the influence of season and salinity on the phenology of invertebrates in Australian saline lakes, with special reference to those of the Paroo in the semiarid inland.Crossref | GoogleScholarGoogle Scholar |
Waterkeyn, A., Gillas, P., Vanschoenwinkel, B., and Brendonck, L. (2008). Invertebrate community patterns in Mediterranean temporary wetlands along hydroperiod and salinity gradients. Freshwater Biology 53, 1808–1822.
| Invertebrate community patterns in Mediterranean temporary wetlands along hydroperiod and salinity gradients.Crossref | GoogleScholarGoogle Scholar |
Williams, W. D. (1981). The limnology of saline lakes in western Victoria. Hydrobiologia 81–82, 233–259.
| The limnology of saline lakes in western Victoria.Crossref | GoogleScholarGoogle Scholar |
Williams, W. D. (1984). Chemical and biological features of salt lakes on the Eyre Peninsula, South Australia, and an explanation of regional differences in the fauna of Australian salt lakes. Verhandlungen Internationale Vereinigung Limnologie 22, 1208–1215.
| Chemical and biological features of salt lakes on the Eyre Peninsula, South Australia, and an explanation of regional differences in the fauna of Australian salt lakes.Crossref | GoogleScholarGoogle Scholar |
Williams, W. D. (1990). Salt lakes: the limnology of Lake Eyre. In ‘Natural History of the North East Deserts’. (Eds M. J. Tyler, C. R. Twidale, M. Davies, and C. B. Wells.) pp. 85–99. (Royal Society of South Australia: Adelaide, SA, Australia.)
Williams, W. D. (1998). Salinity as a determinant of the structure of biological communities in salt lakes. Hydrobiologia 381, 191–201.
| Salinity as a determinant of the structure of biological communities in salt lakes.Crossref | GoogleScholarGoogle Scholar |
Williams, W. D., and Kokkinn, M. J. (1988). The biological affinities of the fauna in episodically filled salt lakes: a study of Lake Eyre, South Australia. Hydrobiologia 158, 227–236.
| The biological affinities of the fauna in episodically filled salt lakes: a study of Lake Eyre, South Australia.Crossref | GoogleScholarGoogle Scholar |
Williams, W. D., Boulton, A. J., and Taaffe, R. G. (1990). Salinity as a determinant of salt lake fauna: a question of scale. Hydrobiologia 197, 257–266.
| Salinity as a determinant of salt lake fauna: a question of scale.Crossref | GoogleScholarGoogle Scholar |
Williams, W. D., De Deckker, P., and Shiel, R. J. (1998). The limnology of Lake Torrens, and episodic salt lake of central Australia, with particular reference to unique events in 1989. Hydrobiologia 384, 101–110.
| The limnology of Lake Torrens, and episodic salt lake of central Australia, with particular reference to unique events in 1989.Crossref | GoogleScholarGoogle Scholar |
Wurtsbaugh, W. A. (1992). Food-web modification by an invertebrate predator in the Great Salt Lake (USA). Oecologia 89, 168–175.
| Food-web modification by an invertebrate predator in the Great Salt Lake (USA).Crossref | GoogleScholarGoogle Scholar | 28312870PubMed |


