Temperature dependency equation for chub mackerel (Scomber japonicus) identified by a laboratory rearing experiment and microscale analysis
Masahiro Nakamura A E , Michio Yoneda A , Toyoho Ishimura B , Kotaro Shirai C , Masaki Tamamura B and Kozue Nishida B D
A E , Michio Yoneda A , Toyoho Ishimura B , Kotaro Shirai C , Masaki Tamamura B and Kozue Nishida B D
A National Research Institute of Fisheries and Environment of Inland Sea, Fisheries Research Agency, 2780, Kinourakou, Hakata-Cho, Imabari, Ehime 794-2305, Japan.
B National Institute of Technology, Ibaraki College, 866, Nakane, Hitachinaka, Ibaraki 312-0011, Japan.
C Atmosphere and Ocean Research Institute, The University of Tokyo, 5-1-5, Kashiwanoha, Kashiwa, Chiba 277-0882, Japan.
D Japan Society for the Promotion of Science (JSPS), 5-3-1, Kojimachi, Chiyoda-ku, Kojimachi Business Center Building, Tokyo 102-0083, Japan.
E Corresponding author. Email: mnakamura@affrc.go.jp
Marine and Freshwater Research 71(10) 1384-1389 https://doi.org/10.1071/MF19313
Submitted: 29 September 2019 Accepted: 22 January 2020 Published: 21 February 2020
Journal Compilation © CSIRO 2020 Open Access CC BY-NC-ND
Abstract
In this study, juveniles of chub mackerel (Scomber japonicus) were reared from eggs in six different temperature treatments, and their otoliths were subjected to micromilling and microvolume stable oxygen isotope (δ18O) analysis. We determined the δ18O values of otoliths (δ18Ootolith) formed at mean temperatures of 16.3, 17.6, 18.3, 20.0, 24.0 and 26.5°C and identified a linear relationship between rearing water temperature (T, °C) and δ18Ootolith as follows: δ18Ootolith (VPDB) – δ18Owater (VSMOW) = –0.25 (±0.01)T + 4.46 (±0.21) (R2 = 0.96, P < 0.01), where VPDB is Vienna Peedee Belemnite, VSMOW is Vienna Standard Mean Ocean Water and the error values in parentheses are standard deviations. This species-specific temperature dependency equation for chub mackerel will enable accurate reconstruction of individual thermal histories and provide essential information for effective resource management.
Additional keywords: otolith, pelagic fish, population structure, recruitment abundance, stable oxygen isotope.
Introduction
Chub mackerel (Scomber japonicus) is a pelagic fish species that has a cosmopolitan distribution in subtropical and temperate waters in the Indo-Pacific Ocean (Scoles et al. 1998). In the north-west Pacific, this species is distributed from Japan, along the south coast of China to the Malay Archipelago (Collette and Nauen 1983) as a complex assembly of weakly diverged genetic populations (Cheng et al. 2015). The abundance of the Pacific stock of chub mackerel went into sharp decline around the early 1980s (Yukami et al. 2019) and was kept at extremely low levels in the 1990s because of heavy fishing of immature fish (Kawai et al. 2002). However, the stock size has been increasing since around 2005 and had recovered to its pre-decline level in 2013 (Yukami et al. 2019). This increase in stock size is considered to have been caused by strong year classes, which have occurred frequently since 2013 (Hashimoto et al. 2019). However, the reason why these strong year classes have occurred remains unclear. To keep chub mackerel resources in good condition and maintain productive fisheries, it is important to know the mechanism underlying the recent increase in recruitment abundance.
Recent studies have suggested that the recruitment abundance of chub mackerel is related to their early growth rate, which primarily depends on ambient water temperature (Kamimura et al. 2015; Kaneko et al. 2019). One of these studies was a population-level otolith study that assumed the sea surface temperature (SST) of an area abundant in chub mackerel larvae as the temperature that the larvae had experienced (Kamimura et al. 2015). The other study was a particle tracking study that regarded the SSTs to which particles were subjected in simulations as the thermal histories of larvae (Kaneko et al. 2019). Although these two studies provided important insights into the mechanism of recruitment fluctuation of chub mackerel, more studies are needed to determine the relationship between the thermal histories of chub mackerel larvae and their growth rates directly at the individual level, not indirectly at the population level as in the previous studies.
Stable oxygen isotope ratio (δ18O) analysis with fish otoliths, which are calcium carbonate structures in the endolymphatic sac of teleost fish that are usually composed of aragonite, is a reliable method for estimating the temperature experience of individuals because δ18O can be used as a proxy of ambient water temperature (Høie et al. 2004; Storm-Suke et al. 2007; Godiksen et al. 2010; Sakamoto et al. 2017). The relationship between ambient water temperature and otolith δ18O has been quantified in various fish species and is often expressed using the following simplified formula:
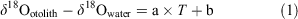
where δ18Ootolith is the δ18O value of the otolith, δ18Owater is the δ18O value of ambient water and T is the temperature of ambient water, whereas a and b are constants. This type of equation shows the dependency of δ18O on ambient water temperature and is herein referred to as a temperature dependency equation. The constants in the temperature dependency equation for otoliths are generally close to those in the temperature dependency equation for inorganic aragonite reported by Kim et al. (2007; see Kitagawa et al. 2013; Sakamoto et al. 2017).
However, the constants obtained in the δ18O analysis of several species differed slightly from those of the equation for inorganic aragonite (Kitagawa et al. 2013; Sakamoto et al. 2017; Willmes et al. 2019). Several other studies have also suggested that the constants of temperature dependency equations differ among species (Høie et al. 2004; Storm-Suke et al. 2007; Godiksen et al. 2010; Kitagawa et al. 2013; Sakamoto et al. 2017). Thus, species-specific temperature dependency equations are required for precise and accurate estimates of the ambient water temperatures experienced by target species.
The aim of this study was to empirically establish a species-specific temperature dependency equation for chub mackerel. Chub mackerel juveniles were reared under six different temperature treatments. In order to minimise potential errors that could be caused by environmental fluctuations during the experimental period and indirect estimation, we used a microscale sampling technique and a microvolume carbonate analytical system proposed in previous studies (Ishimura et al. 2004, 2008) and analysed δ18O data from otolith areas formed under stable water temperature and δ18Owater conditions. This system allowed us to obtain a δ18O value of as little as 0.2 μg CaCO3 with an analytical precision of better than ±0.10‰.
Materials and methods
Egg hatching
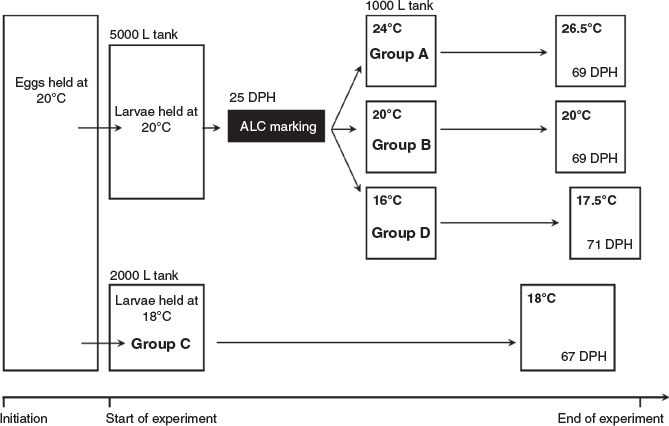
Eggs were obtained from induced spawning of captive broodstock maintained at the Hakatajima Station, National Research Institute of Fisheries and Environment of Inland Sea (Imabari, Japan) using the procedure reported by Nyuji et al. (2012). A group of suitable 2-year-old chub mackerel (53–59 individuals of each sex) was injected intramuscularly with 400 μg kg–1 of bodyweight gonadotropin-releasing hormone analogue (GnRHa) on 28 May 2018 and was maintained in a 20 000-L square tank with circulating seawater. The first spawning was observed ~36 h after injection, and subsequent daily spawning occurred. Eggs from the first and second spawns were placed and incubated in 5000- and 2000-L fibre-reinforced plastic tanks at mean temperatures of 20 and 18°C respectively under a 14-h light–10-h dark cycle (Fig. 1). The larvae were fed with a mixture of rotifers (Brachionus) and planktonic algae (Isochrysis galbana or Nannochloropsis oculata) once daily until 16 days post-hatching (DPH) (18°C tank) or 12 DPH (20°C tank). From these timings onward, larvae were also fed with newly hatched brine shrimp (Artemia salina) once per day. After 27 DPH (18°C tank) and 21 DPH (20°C tank), larvae were fed with ~3% of their bodyweight (g) of commercial dry pellets composed of 52% protein, 11% oil, 18% ash and 3% fibre (New Arteck, Marubeni Nisshin Feed, Tokyo, Japan).
|
Rearing in different temperature treatments
Fish were reared in four different temperature treatments, hereafter referred to as Groups A, B, C and D (Fig. 1). The fish in Groups A, B and D were reared in the 20°C tank until they grew into juveniles. All individuals metamorphosed into juveniles by 25 DPH. At this point, the individuals in these groups were immersed in 15-ppt alizarin complexone (ALC), which deposited visibly distinguishable fluorescent marks on their otoliths to facilitate precise dating. Then, the individuals were evenly divided and introduced into three different 1000-L tanks (80 individuals in each tank). The water temperature of Group A was kept at ~24.0°C for 17 days, then raised to ~26.5°C and maintained at this temperature until the end of the rearing experiment. The water temperature of Group B was maintained at 20.0°C throughout the experimental period. The water temperature of Group D was kept at ~16.0°C for 20 days, then raised to ~17.5°C and maintained at this temperature until the end of the rearing experiment. Fish in Group C were kept at ~18.0°C from the egg stage until the end of the rearing experiment in the 2000-L tank. At the end of the rearing experiment, 45–60 individuals were randomly sampled from each tank. The fork length (FL) and bodyweight of the sampled individuals were measured before freezing. Rearing water samples for δ18O analysis were taken from all the tanks twice a week throughout the experimental period. The water samples were placed in 50-mL sealed glass vials and refrigerated before isotopic analysis to prevent evaporation.
δ18O analysis of otoliths and rearing water
Sagittal otoliths were dissected out from 17 individuals from the different temperature treatments ranging in size from 58.4 to 135.6 mm FL; adherent bits of tissue were removed using a needle and a thin paintbrush under a stereomicroscope. The otoliths were rinsed with Milli-Q water and air dried. The otoliths were then embedded in epoxy resin (Polyester Solidifier; Nichika, Kyoto, Japan) and polished with sandpaper (2000 grit) and lapping film (4000 grit).
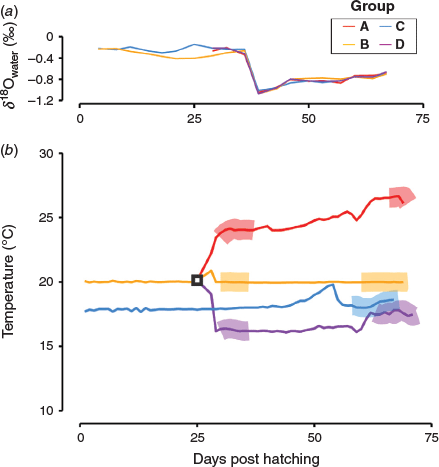
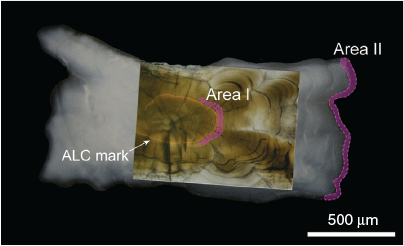
Values of δ18Owater decreased markedly on approximately Day 35 of the experiment (Fig. 2a) because the salinity of the sea water around the Hakatajima Station dropped significantly due to heavy rain. In addition, extremely hot days caused some fluctuations in water temperature (Fig. 2b). To avoid periods of such unstable δ18Owater and temperatures, otoliths were milled using a high-precision micromilling system (GEOMILL326; Izumo-web, Izumo, Japan) and two areas formed under stable conditions were extracted: Area I, adjacent to ALC marks, and Area II near the otolith edges (coloured areas in Fig. 3). These areas were formed under relatively stable temperature and δ18Owater conditions (standard deviations <0.25°C and <0.07‰ for temperature and δ18Owater respectively) and were easy to extract because of their visible boundaries, namely the ALC marks and the otolith edges (Fig. 3). As shown in Fig. 2b, Areas I and II in Group A were formed at mean temperatures of 24.0 and 26.5°C respectively. Areas I and II in group B were formed at a mean temperature of 20.0°C. Area II in Group C was formed at a mean temperature of 18.0°C. Areas I and II in Group D were formed at mean temperatures of 16.0 and 17.5°C respectively. The width of each area was determined using the daily otolith growth rate. The daily growth rate of each otolith was calculated by dividing the otolith radius by DPH (Group C) or days after ALC marking (Groups A, B and D). These areas were extracted along daily increments, the daily deposition of which was verified in a previous study (Takahashi et al. 2014). Irregular otolith microstructure, which is a typical signature of vaterite formation, was not observed. The weight and width of the extracted otolith areas were 0.5–3.6 µg and 40–160 µm respectively. The milling depth was 50 µm for all extracted otolith areas. The δ18Ootolith value of each otolith area (n = 10 for Area I, n = 14 for Area II) was determined using a continuous-flow isotope ratio mass spectrometry system proposed in previous studies (Ishimura et al. 2004, 2008) at the National Institute of Technology, Ibaraki College (Hitachitanaka, Japan) and δ18Ootolith values are reported in delta notation relative to Vienna Peedee Belemnite (VPDB). Detailed analytical procedures have been reported by Nishida and Ishimura (2017) and Sakamoto et al. (2017). The otolith samples were reacted with phosphoric acid at 25.0°C. Most of the isotope values of carbonate in previous studies (e.g. Kim et al. 2007; Kitagawa et al. 2013; Sakamoto et al. 2017) were calculated using isotope fractionation factors for calcite. Therefore, to facilitate comparison with these studies, we used a commonly accepted calcite acid fractionation factor of 1.01025 (Sharma and Clayton 1965). We prepared a 24-point dataset of δ18Ootolith from the 17 otoliths; two data points (i.e. otolith Areas I and II) obtained from 7 individuals and one data point (i.e. otolith Area I or II) obtained from the remaining 10 individuals. Detailed information about all the otolith areas analysed is given in Table S1, available as Supplementary material to this paper. The δ18O value of each rearing water sample was determined using a Picarro L2130-i Analyzer (Sanyo Trading, Tokyo, Japan) at the National Institute of Technology, Ibaraki College, with a long-term analytical precision of ±0.05‰ for seawater; these δ18Owater values are reported relative to Vienna Standard Mean Ocean Water (VSMOW).
|
|
All experimental procedures followed the guidelines for animal welfare of the Fisheries Research and Education Agency, Japan (50322001) and were approved by the Committee of Animal Welfare of the National Research Institute of Fisheries and Environment of Inland Sea (Number 2016-3).
Statistical analysis
Because no significant effect of otolith areas on δ18Ootolith – δ18Owater was detected with multiple regression analyses (P = 0.743), all 24 data points for δ18Ootolith obtained from Areas I and II were pooled. Multiple regression analysis also showed that different widths and different weights did not have any significant effects on δ18Ootolith (P = 0.64 and P = 0.47 respectively). A linear regression model was applied to the pooled data to determine the relationship between rearing water temperature and δ18Ootolith – δ18Owater for chub mackerel. The response variable was δ18Ootolith – δ18Owater, where δ18Ootolith was the δ18O value of each otolith area and δ18Owater was the mean δ18O value of rearing water during the formation of the otolith area. The explanatory variable was the mean water temperature at which each otolith area was formed. All statistical procedures were conducted using R (ver. 3.4.1, R Foundation for Statistical Computing, Vienna, Austria, see http://www.R-project.org/, accessed 7 September 2018). Figures were generated using ggplot2 (ver. 3.0.0, see https://cloud.r-project.org/package=ggplot2), and multiple regression analysis was conducted using the lm function.
Unless indicated otherwise, data are given as the mean ± s.d.
Results
During the period of formation of otolith Area I, the mean water temperatures in Groups A, B and D were 24.0 ± 0.2, 20.0 ± 0.0 and 16.3 ± 0.1°C respectively, and the δ18Owater values of Groups A, B and D were –0.27 ± 0.07, –0.33 ± 0.04 and –0.21 ± 0.00‰ respectively. During the period of formation of otolith Area II, the mean water temperatures in Groups A, B, C and D were 26.5 ± 0.22, 20.0 ± 0.01, 18.3 ± 0.25 and 17.6 ± 0.15°C respectively, whereas the mean δ18Owater values were –0.69 (single piece of data), –0.75 ± 0.05, –0.76 ± 0.05 and –0.71 ± 0.07‰ respectively.
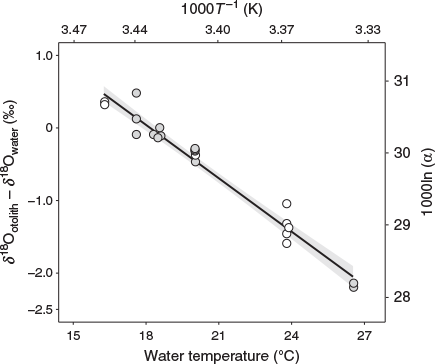
For Area I, mean δ18Ootolith values were +0.07 ± 0.02‰ at 16.3°C, and –0.61 ± 0.07‰ at 20.0°C, –1.58 ± 0.20‰ at 24.0°C; for Area II, mean δ18Ootolith values were –0.52 ± 0.23‰ at 17.6°C, –0.84 ± 0.07‰ at 18.3°C –1.09 ± 0.07‰ at 20.0°C, and –2.85 ± 0.01‰ at 26.5°C. Using the least squares method, the linear regression relationship between water temperatures in the range 16.3–26.5°C and δ18O was expressed in the form of a temperature dependency equation:
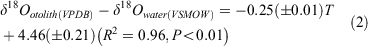
where T is temperature (°C) and the errors in parentheses are standard deviations (Fig. 4).
|
Fractionation expressed as 1000ln(α) was calculated as follows:
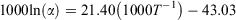
where T is temperature (K) and α is the fractionation factor between otolith and water.
Discussion
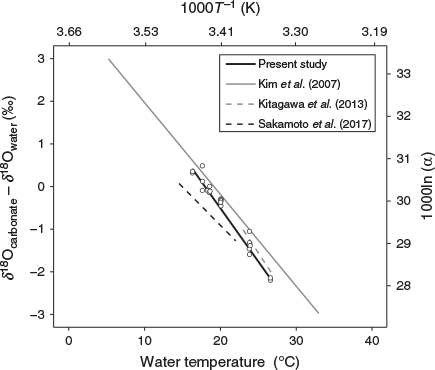
We succeeded in obtaining a reliable temperature dependency equation with a small standard deviation as the δ18O thermometer of chub mackerel (Fig. 4). The equation obtained in this study showed a lower temperature than the equation for inorganic aragonite reported by Kim et al. (2007) at every δ18Ootolith – δ18Owater value within the temperature range tested. Therefore, the application of the equation for inorganic aragonite to chub mackerel would lead to errors in the estimation of their temperature experience, as suggested by previous studies (e.g. Kitagawa et al. 2013; Sakamoto et al. 2017; Willmes et al. 2019). For example, suppose δ18Ootolith – δ18Owater is –2.0‰. In this case, the temperature experienced by the fish is estimated to be 28.5°C using the equation for inorganic aragonite of Kim et al. (2007), whereas it is estimated to be 26.0°C using the species-specific equation for chub mackerel developed in the present study (Eqn 2). This difference is not negligible in identifying the migration routes of chub mackerel.
Chub mackerel and Japanese sardine are pelagic species that inhabit approximately the same area and use similar migration routes. However, the slope of the species-specific temperature dependency equation for chub mackerel is steeper than that of the equation for Japanese sardine reported by Sakamoto et al. (2017; Fig. 5). In addition, the equation for bluefin tuna proposed by Kitagawa et al. (2013) shows a 0.6–0.9°C higher temperature than our equation for chub mackerel at every δ18Ootolith – δ18Owater value within the temperature range tested for bluefin tuna (Fig. 5), even though these species belong to the same family Scombridae. Given the fact that these three otolith studies were conducted under the same analytical settings and using the same data processing methods, it is unlikely that these interspecies differences were caused by systematic errors arising from differences in methodology. Therefore, neither the ecological similarity nor the phylogenetic distance between species seems to be a reliable criterion for predicting the similarity of the equations. Thus, we agree with the suggestion made previously by some authors that species-specific temperature dependency equations should be empirically determined for an accurate reconstruction of individual thermal histories (Kalish 1991; Thorrold et al. 1997; Høie et al. 2004; Godiksen et al. 2010).
|
Unpredictable fluctuations in rearing environments are inherent in any long-term rearing experiment unless it is conducted in a closed recirculating system such as the one used by Kikuchi et al. (2006). In the present experiment, δ18Owater fluctuations associated with salinity fluctuations were observed. This study accomplished precise dating by reading daily increments of the ALC-marked otoliths of hatchery-reared individuals. This allowed us to pinpoint the otolith areas formed under stable environmental conditions. In addition, the aforementioned micromilling technique and microvolume analysis enabled us to precisely extract the targeted otolith areas and determine the δ18Ootolith value of each area. In this way, we successfully obtained a reliable equation even from fish that had experienced unexpected environmental changes. The robustness against environmental fluctuations would be another advantage of this methodology, in addition to its applicability to small fish species pointed out by Sakamoto et al. (2017).
For precise estimation of the temperature history of chub mackerel, the salinity of ambient water should also be considered because it affects δ18Owater, as does water temperature. Most chub mackerel belonging to the Pacific stock inhabit offshore areas, which are not subjected to freshwater input and are unlikely to undergo large fluctuations in δ18Owater associated with salinity change. Nevertheless, a minor portion of the Pacific stock migrates to inshore areas (Yukami et al. 2019), where δ18Owater can possibly be affected by freshwater input. Thus, some caution is required when applying the equation obtained in this study to individuals captured from inshore areas.
The equation for chub mackerel obtained from this study provides essential information for effective resource management through an accurate reconstruction of individual thermal histories. For example, a comparison of the thermal histories of fast- and slow-growing individuals can facilitate further understanding of the thermal mechanism that accounts for the difference in growth rate between the individuals, which is a possible cause of the significant annual variation in recruitment abundance and the recent occurrence of strong year classes (Kamimura et al. 2015; Kaneko et al. 2019).
In addition, a recent study proposed a new method that combines microscale δ18O analysis with numerical simulation to reconstruct the early migration routes of fishes at the individual level (Sakamoto et al. 2019). The simulation in this method is based on ocean data assimilation models that include both temperature and salinity data. Thus, by combining the δ18O thermometer presented in this study with the new method, the possible migration history of chub mackerel can be estimated. This will contribute to an understanding of the interaction among chub mackerel populations in the north-west Pacific and of the ecological processes that shape the contemporary genetic population structure of chub mackerel in this region.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Declaration of funding
This study was funded by the research fund KAKENHI Grants from the Japan Society for the Promotion of Science to Michio Yoneda (JP18H04924), Toyoho Ishimura (16H02944, 18H04921), Kozue Nishida (17J11417, 17K14413) and Kotaro Shirai (JP15H05823).
Acknowledgements
The authors sincerely thank the members of the Hakatajima Station for technical support in rearing fish, and N. Izumoto (Atmosphere and Ocean Research Institute, The University of Tokyo) for helping with sample distribution. The authors are also grateful to Y. Nakamura for improving the manuscript.
References
Cheng, J., Yanagimoto, T., Song, N., and Gao, T. X. (2015). Population genetic structure of chub mackerel Scomber japonicus in the northwestern Pacific inferred from microsatellite analysis. Molecular Biology Reports 42, 373–382.| Population genetic structure of chub mackerel Scomber japonicus in the northwestern Pacific inferred from microsatellite analysis.Crossref | GoogleScholarGoogle Scholar | 25366174PubMed |
Collette, B. B., and Nauen, C. E. (1983). ‘FAO Species Catalogue Vol. 2. Scombrids of the World: An Annotated and Illustrated Catalogue of Tunas, Mackerels, Bonitos and Related Species Known to Date.’ FAO Fisheries Synopsis 125. (Food and Agriculture Organization of the United Nations: Rome, Italy.)
Godiksen, J., Svenning, M. A., Dempson, J., Marttila, M., Storm-Suke, A., and Power, M. (2010). Development of a species-specific fractionation equation for Arctic charr (Salvelinus alpinus (L.)): an experimental approach. Hydrobiology 650, 67–77.
| Development of a species-specific fractionation equation for Arctic charr (Salvelinus alpinus (L.)): an experimental approach.Crossref | GoogleScholarGoogle Scholar |
Hashimoto, M., Nishijima, S., Yukami, R., Watanabe, C., Kamimura, Y., Furuichi, S., Ichinokawa, M., and Okamura, H. (2019). Spatiotemporal dynamics of the Pacific chub mackerel revealed by standardized abundance indices. Fisheries Research 219, 105315.
| Spatiotemporal dynamics of the Pacific chub mackerel revealed by standardized abundance indices.Crossref | GoogleScholarGoogle Scholar |
Høie, H., Otterlei, E., and Folkvord, A. (2004). Temperature-dependent fractionation of stable oxygen isotopes in otoliths of juvenile cod (Gadus morhua L.). ICES Journal of Marine Science 61, 243–251.
| Temperature-dependent fractionation of stable oxygen isotopes in otoliths of juvenile cod (Gadus morhua L.).Crossref | GoogleScholarGoogle Scholar |
Ishimura, T., Tsunogai, U., and Gamo, T. (2004). Stable carbon and oxygen isotopic determination of sub-microgram quantities of CaCO3 to analyze individual foraminiferal shells. Rapid Communications in Mass Spectrometry 18, 2883–2888.
| Stable carbon and oxygen isotopic determination of sub-microgram quantities of CaCO3 to analyze individual foraminiferal shells.Crossref | GoogleScholarGoogle Scholar | 15517527PubMed |
Ishimura, T., Tsunogai, U., and Nakagawa, F. (2008). Grain-scale heterogeneities in the stable carbon and oxygen isotopic compositions of the international standard calcite materials (NBS 19, NBS 18, IAEA-CO-1, and IAEA-CO-8). Rapid Communications in Mass Spectrometry 22, 1925–1932.
| Grain-scale heterogeneities in the stable carbon and oxygen isotopic compositions of the international standard calcite materials (NBS 19, NBS 18, IAEA-CO-1, and IAEA-CO-8).Crossref | GoogleScholarGoogle Scholar | 18484681PubMed |
Kalish, J. M. (1991). Oxygen and carbon stable isotopes in the otoliths of wild and laboratory-reared Australian salmon (Arripis trutta). Marine Biology 110, 37–47.
| Oxygen and carbon stable isotopes in the otoliths of wild and laboratory-reared Australian salmon (Arripis trutta).Crossref | GoogleScholarGoogle Scholar |
Kamimura, Y., Takahashi, M., Yamashita, N., Watanabe, C., and Kawabata, A. (2015). Larval and juvenile growth of chub mackerel Scomber japonicus in relation to recruitment in the western North Pacific. Fisheries Science 81, 505–513.
| Larval and juvenile growth of chub mackerel Scomber japonicus in relation to recruitment in the western North Pacific.Crossref | GoogleScholarGoogle Scholar |
Kaneko, H., Okunishi, T., Seto, T., Kuroda, H., Itoh, S., Kouketsu, S., and Hasegawa, D. (2019). Dual effects of reversed winter–spring temperatures on year-to-year variation in the recruitment of chub mackerel (Scomber japonicus). Fisheries Oceanography 28, 212–227.
| Dual effects of reversed winter–spring temperatures on year-to-year variation in the recruitment of chub mackerel (Scomber japonicus).Crossref | GoogleScholarGoogle Scholar |
Kawai, H., Yatsu, A., Watanabe, C., Mitani, T., Katsukawa, T., and Matsuda, H. (2002). Recovery policy for chub mackerel stock using recruitment-per-spawning. Fisheries Science 68, 963–971.
| Recovery policy for chub mackerel stock using recruitment-per-spawning.Crossref | GoogleScholarGoogle Scholar |
Kikuchi, K., Iwata, N., Furuta, T., Kawabata, T., and Yanagawa, T. (2006). Growth of tiger puffer Takifugu rubripes in closed recirculating culture system. Fisheries Science 72, 1042–1047.
| Growth of tiger puffer Takifugu rubripes in closed recirculating culture system.Crossref | GoogleScholarGoogle Scholar |
Kim, S. T., O’Neil, J. R., Hillaire-Marcel, C., and Mucci, A. (2007). Oxygen isotope fractionation between synthetic aragonite and water: influence of temperature and Mg2+ concentration. Geochimica et Cosmochimica Acta 71, 4704–4715.
| Oxygen isotope fractionation between synthetic aragonite and water: influence of temperature and Mg2+ concentration.Crossref | GoogleScholarGoogle Scholar |
Kitagawa, T., Ishimura, T., Uozato, R., Shirai, K., Yosuke, A., Shinoda, A., Otake, T., Tsunogai, U., and Kimura, S. (2013). Otolith δ18O of Pacific bluefin tuna Thunnus orientalis as an indicator of ambient water temperature. Marine Ecology Progress Series 481, 199–209.
| Otolith δ18O of Pacific bluefin tuna Thunnus orientalis as an indicator of ambient water temperature.Crossref | GoogleScholarGoogle Scholar |
Nishida, K., and Ishimura, T. (2017). Grain-scale stable carbon and oxygen isotopic variations of the international reference calcite, IAEA-603. Rapid Communications in Mass Spectrometry 31, 1875–1880.
| Grain-scale stable carbon and oxygen isotopic variations of the international reference calcite, IAEA-603.Crossref | GoogleScholarGoogle Scholar | 28833709PubMed |
Nyuji, M., Selvaraj, S., Kitano, H., Ohga, H., Yoneda, M., Shimizu, A., Kaneko, K., Yamaguchi, A., and Matsuyama, M. (2012). Changes in the expression of pituitary gonadotropin subunits during reproductive cycle of multiple spawning female chub mackerel Scomber japonicus. Fish Physiology and Biochemistry 38, 883–897.
| Changes in the expression of pituitary gonadotropin subunits during reproductive cycle of multiple spawning female chub mackerel Scomber japonicus.Crossref | GoogleScholarGoogle Scholar | 22109677PubMed |
Sakamoto, T., Komatsu, K., Yoneda, M., Ishimura, T., Higuchi, T., Shirai, K., Kamimura, Y., Watanabe, C., and Kawabata, A. (2017). Temperature dependence of δ18O in otolith of juvenile Japanese sardine: laboratory rearing experiment with micro-scale analysis. Fisheries Research 194, 55–59.
| Temperature dependence of δ18O in otolith of juvenile Japanese sardine: laboratory rearing experiment with micro-scale analysis.Crossref | GoogleScholarGoogle Scholar |
Sakamoto, T., Komatsu, K., Shirai, K., Higuchi, T., Ishimura, T., Setou, T., Kamimura, Y., Watanabe, C., and Kawabata, A. (2019). Combining microvolume isotope analysis and numerical simulation to reproduce fish migration history. Methods in Ecology and Evolution 10, 59–69.
| Combining microvolume isotope analysis and numerical simulation to reproduce fish migration history.Crossref | GoogleScholarGoogle Scholar |
Scoles, D. R., Collette, B. B., and Graves, J. E. (1998). Global phylogeography of mackerels of the genus Scomber. Fishery Bulletin 96, 823–842.
Sharma, T., and Clayton, R. N. (1965). Measurement of O18/O16 ratios of total oxygen of carbonates. Geochimica et Cosmochimica Acta 29, 1347–1353.
| Measurement of O18/O16 ratios of total oxygen of carbonates.Crossref | GoogleScholarGoogle Scholar |
Storm-Suke, A., Dempson, J. B., Reist, J. D., and Power, M. (2007). A field-derived oxygen isotope fractionation equation for Salvelinus species. Rapid Communications in Mass Spectrometry 21, 4109–4116.
| A field-derived oxygen isotope fractionation equation for Salvelinus species.Crossref | GoogleScholarGoogle Scholar | 18022960PubMed |
Takahashi, M., Yoneda, M., Kitano, H., Kawabata, A., and Saito, M. (2014). Growth of juvenile chub mackerel Scomber japonicus in the western North Pacific Ocean: with application and validation of otolith daily increment formation. Fisheries Science 80, 293–300.
| Growth of juvenile chub mackerel Scomber japonicus in the western North Pacific Ocean: with application and validation of otolith daily increment formation.Crossref | GoogleScholarGoogle Scholar |
Thorrold, S. R., Campana, S. E., Jones, C. M., and Swart, P. K. (1997). Factors determining δ13C and δ18O fractionation in aragonitic otoliths of marine fish. Geochimica et Cosmochimica Acta 61, 2909–2919.
| Factors determining δ13C and δ18O fractionation in aragonitic otoliths of marine fish.Crossref | GoogleScholarGoogle Scholar |
Willmes, M., Lewis, L. S., Davis, B. E., Loiselle, L., James, H. F., Denny, C., Baxter, R., Conrad, J. L., Fangue, N. A., Hung, T., Armstrong, R. A., Williams, I. S., Holden, P., and Hobbs, J. A. (2019). Calibrating temperature reconstructions from fish otolith oxygen isotope analysis for California’s critically endangered delta smelt. Rapid Communications in Mass Spectrometry 33, 1207–1220.
| Calibrating temperature reconstructions from fish otolith oxygen isotope analysis for California’s critically endangered delta smelt.Crossref | GoogleScholarGoogle Scholar | 30993783PubMed |
Yukami, R., Nishijima, S., Isu, S., Watanabe, C., Kamimura, Y., and Furuichi, S. (2019). Stock assessment and evaluation for the Pacific stock of chub mackerel (fiscal year 2018). In ‘Marine Fisheries Stock Assessment and Evaluation for Japanese Waters (Fiscal Year 2018/2019)’. pp. 163–208. (Fisheries Research and Education Agency of Japan: Yokohama, Japan.) Available at http://abchan.fra.go.jp/digests2018/details/201805.pdf [In Japanese, verified 10 January 2020].
1 These authors contributed equally to this work.


