Long-term monitoring of potamodromous migratory fish larvae in an undammed river
Rafael Rogério Rosa A B C , Jislaine Cristina Silva A B and Andréa Bialetzki A BA Laboratório de Ictioplâncton and Nupélia (Núcleo de Pesquisas em Limnologia, Ictiologia e Aquicultura), Universidade Estadual de Maringá, Avenida Colombo, 5790, Bloco G-80, CEP 87020-900, Maringá, Paraná, Brazil.
B Programa de Pós-graduação em Ecologia de Ambientes Aquáticos Continentais, Departamento de Biologia, Universidade Estadual de Maringá, Avenida Colombo, 5790, Bloco G-80, CEP 87020-900, Maringá, Paraná, Brazil.
C Corresponding author. Email: rafaelrogrosa@gmail.com
Marine and Freshwater Research 71(3) 384-393 https://doi.org/10.1071/MF18412
Submitted: 26 October 2018 Accepted: 7 May 2019 Published: 14 August 2019
Abstract
Freshwater fish reproduce annually in environments that provide favourable conditions for spawning and larval survival and growth. Thus, the aims of this study were to use long-term larval density data to evaluate the temporal distribution of the dourado Salminus brasiliensis, its habitat use for larval development and the effects of environmental variables on reproduction. S. brasiliensis larvae were mainly recorded in October and January in the Ivinheima River, and higher densities of larvae, primarily in the preflexion and flexion stages, were captured in the river and backwater biotopes. Water level, dissolved oxygen and temperature were the primary variables affecting the density of larvae, and this species can migrate over 200 km to reproduce in the Ivinheima River and its tributaries. Therefore, S. brasiliensis is reproducing annually, indicating favourable local conditions for migration and spawning, and exhibits differential use of biotopes for reproduction, including rivers and backwaters (spawning) and lagoons (refuge and growth). Therefore, considering that the dourado is a key species for conservation, ensuring its reproductive success means maintaining a balanced ecological structure.
Additional keywords: ichthyoplankton, larval ecology, migratory species, upper Paraná River basin.
Introduction
Studies on the reproductive dynamics of fish typically indicate relationships between environmental variables and the reproductive capacity of species (Winemiller 1989; Suzuki and Pompeu 2016). Teleost fish generally have a reproductive cycle in which spawning occurs in environmental conditions that are optimal for the development and survival of offspring, and that are the main characteristics that act on spatial and temporal distribution of larvae (Agostinho et al. 2004; Ziober et al. 2015). In addition, many fish species exhibit ontogenetic changes in habitat use related to growth, feeding and refuge, deviating from main channels to backwaters and lagoons (Copp 1990; King 2004).
Neotropical fish reproduction primarily occurs from October to March (Bialetzki et al. 2005), a period that is characterised by the rainy season and an increase in temperature and water levels (Baumgartner et al. 2008; Lopes et al. 2014). The increase in temperature facilitates gonadal maturation in the fish, and the rainy season and elevated water levels induce spawning and allow the eggs to drift in the main channel and find ideal habitats for feeding and growth (Agostinho et al. 2004; Baumgartner et al. 2008). However, although there is temporal variation in spawning in tropical river basins around the world, spawning always coincides with the general patterns described above (Humphries et al. 2002; Jiménez-Segura et al. 2010; King et al. 2016; López-Casas et al. 2016).
Various studies in river–flood plain systems have reported the importance of connectivity among floodplain habitats for all kinds of fish species, especially migratory species (Carolsfeld et al. 2003). Junk et al. (1989) proposed the ‘flood pulse concept’, which describes how seasonal flooding increases connectivity among aquatic environments and how alternating periods of drought and flood alter limnological characteristics, patterns of nutrient cycling and the structure of aquatic communities. Changes in the intensity, duration, amplitude and other characteristics of flood regimes affect the reproductive cycle of various fish species because they increase the flooded area and facilitate larval and juvenile development (Welcomme and Halls 2005; Reinfelds et al. 2013).
There are sixteen recorded long-distance migratory fish species the upper Paraná River flood plain (Carolsfeld et al. 2003), and these species exhibit reproductive patterns associated with longitudinal migration in the main river channels and depend on the flood regime as well as other environmental variables, such temperature, pH and dissolved oxygen, for spawning and development (Reynalte-Tataje et al. 2013; Barzotto et al. 2015). However, since the construction of the dam at the Engenheiro Sérgio Motta hydropower plant in Porto Primavera in 1998, the dam-free stretch of the flood plain has been reduced from 809 to 230 km (Agostinho et al. 2004). For migratory species that have specific requirements to complete their life cycle, the effects of the construction of the dam at Porto Primavera can be seen in population declines in recent decades in the Paraná River (Sanches et al. 2006; Suzuki et al. 2009).
As a measure to compensate for the construction of this dam, the Ivinheima River State Park was created in 1998, encompassing the lower portion of the Ivinheima River, an important right-bank tributary of the Paraná River that still has intact natural characteristics and considered an important breeding site for fish species in the region (Sanches et al. 2006; Reynalte-Tataje et al. 2011). Locally, this tributary has become a main route for migratory fish, including Salminus brasiliensis (Cuvier, 1816), known locally as the dourado (Nakatani et al. 1997; Reynalte-Tataje et al. 2013).
The potamodromous fish S. brasiliensis is a large species with a broad distribution in river basins in Paraná, Paraguay, Uruguay and several parts of South America. Its populations are vulnerable to overfishing and fluvial changes caused by anthropic activities (Barzotto and Mateus 2017), particularly in the upper Paraná River basin and its tributaries (Abilhoa and Duboc 2004; Agostinho et al. 2007) and the Uruguay River basin (Marques et al. 2002). S. brasiliensis is a top predator in South America and is highly appreciated in artisanal and sport fishing (Carolsfeld et al. 2003). In terms of reproduction, this species is characterised by external fertilisation, total spawning, no parental care (Godoy 1975) and long migrations during the reproductive period, which occur once a year towards the headsprings of large rivers (Carolsfeld et al. 2003; Hahn et al. 2011). After fertilisation, the eggs drift in the main channel of the river and are directed to backwaters and lagoons, where individuals remain until the end of the larval period before returning to the river channel to start a new cycle (Vega‐Orellana et al. 2006).
Because of its ecological relevance in trophic networks, importance in artisanal fishing and dependence on multiple environments for migration and reproduction, the dourado is a key species for the elaboration of management plans. Thus, the aim of the present study, performed in the Ivinheima River in the last dam-free section of the upper Paraná River basin, was to analyse the distribution of S. brasiliensis larvae using long-term data to understand interannual temporal and spatial variations, the effects of environmental variables on reproduction and likely areas of spawning and growth. Specifically, the study aimed to: (1) analyse the temporal distribution of S. brasiliensis larvae; (2) determine the biotopes used by each larval developmental stage to satisfy its specific requirements; (3) identify the environmental variables and flood regime that determine the reproductive patterns of S. brasiliensis; and (4) estimate the possible distance that the dourado migrates to spawning and natural nursery areas based on larval development time and current velocity.
Materials and methods
Study area
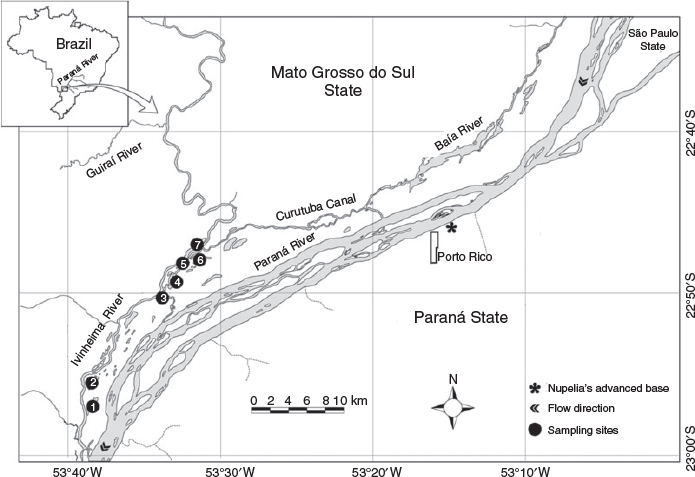
The Ivinheima River is located on the right bank of the Paraná River in the state of Mato Grosso do Sul, Brazil. The upper and middle stretches run in a north–south direction, and the lower stretch runs parallel to the Paraná River until all stretches converge. When it reaches the flood plain of the river, the Ivinheima River connects the mouth of the Curutuba Canal to the confluence with the Paraná River, has a sinuosity quotient of 1.26, a width : depth ratio of 22 : 1 and an average slope of 10.8 cm km–1 (Souza Filho and Stevaux 1997). The Ivinheima River is ~270 km long, and is one of the few tributaries of the Paraná River that still has lotic characteristics and exhibits no impoundment, which is different from the rest of the basin and emphasises the importance of conserving its ichthyofauna.
The study area was in the lower section of the Ivinheima River (22°48′00″S, 53°32′00″W and 22°59′10″S, 53°39′02″W) inside a permanent conservation unit known as Ivinheima River State Park. According to the hydrological characteristics of the sampling sites and the use of different microhabitats by the species, samples were taken from different biotopes, including the river (Ivinheima River II and Ivinheima River III), backwater (the mouths of the Finado Raimundo and Patos lagoons) and lagoon (the Finado Raimundo, Patos and Pintado lagoons; Fig. 1).
Sampling
To assess interannual variations, we used long-term data from samples collected monthly during seven consecutive reproductive periods (RP) that always comprised October–March (Bialetzki et al. 2005). The following periods were considered: 2008–09 (RP1), 2009–10 (RP2), 2010–11 (RP3), 2011–12 (RP4), 2012–13 (RP5), 2013–14 (RP6) and 2014–15 (RP7). In all, 80 samples were taken monthly in the biotopes for each reproductive period: river (Ivinheima River II and Ivinheima River III), backwater (the mouths of the Finado Raimundo and Patos lagoons) and lagoon (the Finado Raimundo, Patos and Pintado lagoons). Thus, there were 480 samples in total and 3360 samples during the seven reproductive periods.
Samples were taken with conical–cylindrical plankton nets with a mesh size of 0.5 mm; a flowmeter was attached to the mouth of the nets to measure the volume of filtered water. When sampling in the Ivinheima River, nets were fixed to an extended cable perpendicular to the water surface; three nets were installed at the surface (on the left and right banks as well as in the centre of the river) and two at the bottom. All nets were simultaneously exposed for 15 min. In lagoons and backwaters, nets were dragged for 10 min at the surface and at the bottom. All samples were taken over a diel cycle with a 6-h interval between sampling (at 0000, 0600, 1200 and 1800 hours), and the individuals captured were fixed in 4% formalin buffered with calcium carbonate.
In the laboratory, the collected material was sorted and the larvae were separated from the rest of the plankton. Identification followed Nakatani et al. (2001), after which the collected larvae were classified into the following developmental stages: larval yolk (LV), preflexion (PF), flexion (FL) and postflexion (FP), as proposed by Ahlstrom and Moser (1976) and later modified by Nakatani et al. (2001).
Concurrent with the ichthyoplankton sampling, the following environmental variables were measured: water temperature (°C), dissolved oxygen (mg L–1), pH and electrical conductivity (μS cm–1). Water level (m) and rainfall (mm) data were provided by Itaipu Binacional from the Ivinheima Hydrometeorological Station.
Data analysis
Interannual variation
Distribution temporal and spatial of S. brasiliensis larvae. A generalised linear model (GLM) was used to evaluate differences in larval density (response variable) in relation to the reproductive period (predictor variable) and differences in the density of each stage of larval development among biotopes (explanatory variable). Larval abundance was extremely patchy, with many zero values and occasional observations of high densities, so a negative binomial distribution best fit the data. Thus, a negative binomial distribution and a log-link function were used to model the distribution of density in R (R Foundation for Statistical Computing, Vienna, Austria) using the glm.nb function from the MASS package (ver. 7.3-49, see http://www.stats.ox.ac.uk/pub/MASS4/Software.html, accessed 8 August 2019; Venables and Ripley 2002). When the GLM result was significant, a post hoc test was performed using the lsmeans function, with Tukey adjustment from the lsmeans package (ver. 2.27-62, see https://cran.r-project.org/web/packages/lsmeans/index.html, accessed 8 August 2019; Lenth 2016). This function computes least-squares means for specified factors in a linear model and makes comparisons among them. The plot displays 95% confidence interval (CI) estimates of the adjusted mean from the GLM.
Relationship between larval density and environmental variables. Because water level is considered to be one of the environmental variables that most affects the reproduction of migratory fish (Agostinho et al. 2004), it was evaluated separately from the other variables. Thus, a Spearman correlation was first performed using larval density and water level values from the day of sampling to 15 days before, in addition to monthly mean values. This aim of this analysis was to relate the delay in the increase in water level to the reproduction of the species and, subsequently, larval density.
Possible relationships between environmental variables and the temporal distribution of S. brasiliensis larvae were analysed using principal component analysis (PCA), which summarises the variability in these factors. To this end, all variables (except pH) were first log-transformed (log10(x + 1)) to reduce the differences between the scales, and PCA axes with eigenvalues greater than 1 were retained for interpretation, according to the Kaiser–Guttman criterion (Jackson 1993). Environmental variables with eigenvectors (correlations) above 0.7 were considered important for the formation of the PCA axes (Comrey and Lee 1992). Later, to test the effects of environmental variables on larval density, a Spearman correlation was performed using the scores of the retained PCA axes and larval density (response variable).
Hydrological cycle
Floods were characterised according to the following attributes: (1) duration of flooding (number of days that the water level remained above 2.75 m; this threshold was previously set for the overflow of the Ivinheima River and its lowlands; Comunello et al. 2003); (2) intensity of flooding (highest level in each period); and (3) delay in flooding (the number of 15-day intervals between 1 October and the beginning of the floods; Oliveira et al. 2015). Daily water level data were used for this analysis.
A simple linear regression was used to evaluate the possible effects of flooding attributes (duration, intensity and delay) on the mean density of S. brasiliensis larvae. Before this analysis, we first tested the assumptions of normality, homoscedasticity and linearity.
PCA, GLM, simple linear regressions and correlations were performed using R software (ver. 1.1.463, R Foundation for Statistical Computing, Vienna, Austria, see http://www.R-project.org/, accessed 3 July 2018), and graphs were drawn using Statistica (ver. 7.1, see http://www.statsoft.com; Statsoft 2010). For all statistical tests, P ≤ 0.05 was considered significant.
Conceptual model
Based on the distributions of S. brasiliensis larval stages in different biotopes, we elaborated a conceptual model describing the reproductive dynamics (spawning) and the drift of the larvae along the Ivinheima River. For this, we only used information about the embryonic and larval development time of this species (in hours; Morais Filho and Schubart 1955; A. Bialetzki, unpubl. data) and the current velocity of the Ivinheima River (Souza Filho and Stevaux 1997) using the following equation:
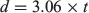
where d is the distance travelled between each larval development stage, 3.06 km h–1 is a mean constant estimate of the current velocity of the Ivinheima River (Souza Filho and Stevaux 1997) and t is the time (h) of development for each larval stage (Egg–LV: 14 h; LV–PF: 6 h; PF–FL: 12 h; FL–FP: 42 h at 26.5°C; A. Bialetzki, unpubl. data). We emphasise that the only purpose of this conceptual model was to check the possible distances that S. brasiliensis can travel to spawn and growth sites in this dam-free stretch based on the larval stage of life and some other parameters, without considering other environmental variables that act as triggers to synchronise reproduction.
Results
Interannual variation
Temporal and spatial distribution of S. brasiliensis larvae
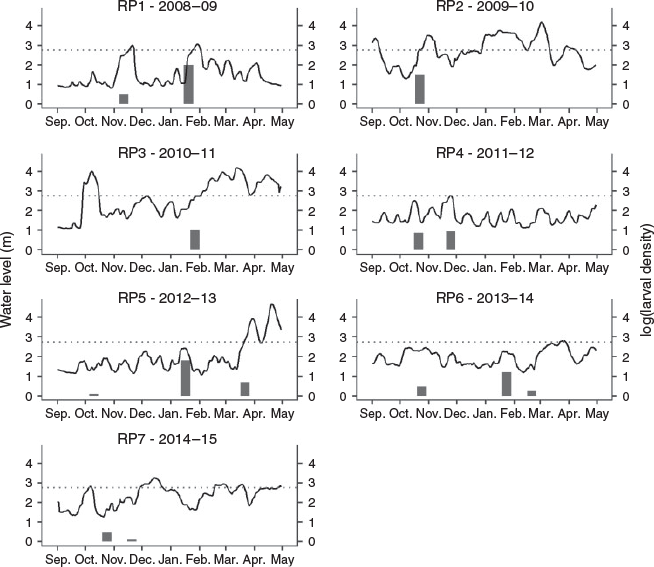
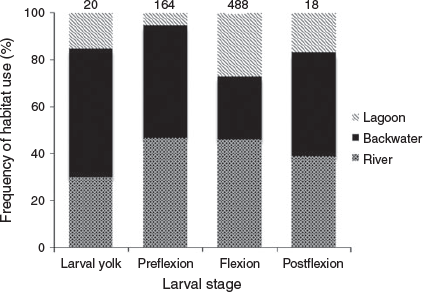
During the seven reproductive periods sampled, 690 S. brasiliensis larvae were captured, distributed among the LV (n = 20; 2.9%), PF (n = 164; 23.77%), FL (n = 488; 70.73%) and FP (n = 18; 2.6%) stages. The highest total densities were recorded in January 2009 (RP1) and 2013 (RP5), with 95.66 and 52.42 larvae per 10 m3 respectively (Fig. 2).
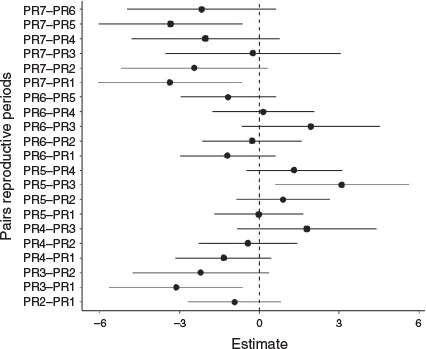
Significant differences in larval density were observed between reproductive periods (GLM; d.f. = 6, F = 5.49, P ≤ 0.001; Fig. 3). For relationships between stages of larval development and biotopes, there was only a significant difference for PF (GLM, d.f. = 2, F = 9.05 P ≤ 0.0001), where lagoon differed from backwater (P < 0.001) and backwater differed from river (P < 0.015; Fig. 4). No overdispersion was identified.
|
|
Relationship between larval density and environmental variables
The monthly variations in abiotic variables during the seven reproductive periods are given in Table S1, available as Supplementary material to this paper.
For the sites sampled, the highest Spearman correlation was obtained when considering water levels approximately 14 days before sampling (r = –0.51; P < 0.05), which demonstrates that the relationship between water level and larval density is stronger when such a delay is considered. Thus, the values from Day 14 before sampling were used to check for the association between water levels and larval density in subsequent analyses.
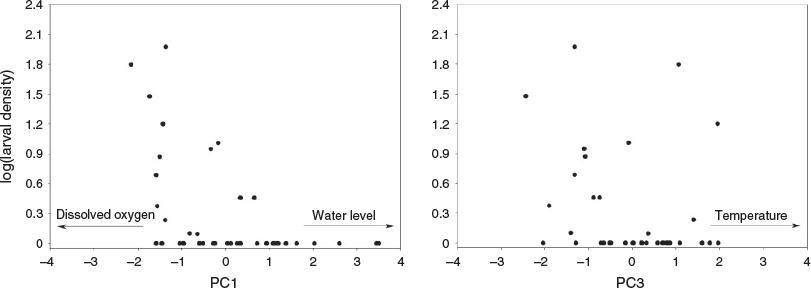
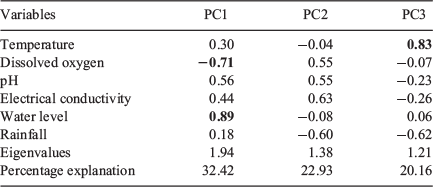
Axes 1, 2 and 3 of the PCA had eigenvalues greater than 1 and were retained for interpretation, explaining 75.51% of the variability in the data. The first axis (PC1) had an eigenvalue of 1.94 and explained 32.42% of the variability; water level contributed positively to the ordination (eigenvector = 0.89), whereas dissolved oxygen contributed negatively (eigenvector = –0.71). The second axis (PC2) had an eigenvalue of 1.38 and explained 22.93% of the variability in the data. The third axis (PC3) had an eigenvalue of 1.21 and explained 20.16% of the variability; temperature positively influenced this axis (eigenvector = 0.83; Table 1).
|
Correlations between the scores of the retained PCA axes and the density of S. brasiliensis larvae were only significant for PC1 (r = –0.57; P < 0.0001) and PC3 (r = –0.35; P < 0.03), indicating the influence of water level, dissolved oxygen and temperature on larval density (Fig. 5).
Hydrological cycle
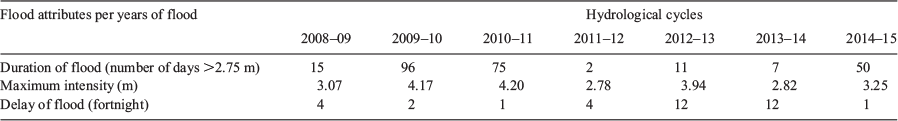
In the periods sampled, the longest flood duration was observed in 2009–10, followed by that in the 2010–11 cycle. Floods of high intensity and long duration (75–100 days above 2.75 m) were recorded in 2009–10 and 2010–11. During these periods, flood delays occurred in the second and first half of October respectively. In the 2012–13 and 2013–14 cycles, floods occurred only in March and were of low intensity and short duration (Table 2).
|
However, the results of the regression analysis did not indicate a significant relationship between flood attributes (duration, intensity and delay) and the density of S. brasiliensis larvae in the sampling periods (P > 0.05).
Conceptual model
Using larvae at the flexion stage, which were the most abundant in all the environments sampled (total density 156.52 larvae per 10 m3), and based on the development time (h) of each larval stage and river flow, it was possible to check that S. brasiliensis spawned ~96 km upstream from the sites where the individuals were caught, probably in tributaries of the Ivinheima River. Therefore, it can be inferred that FP larvae will be found ~122 km downstream from the site of initial establishment, possibly reaching the Paraná River and its lagoons or other secondary channels (Fig. 6).
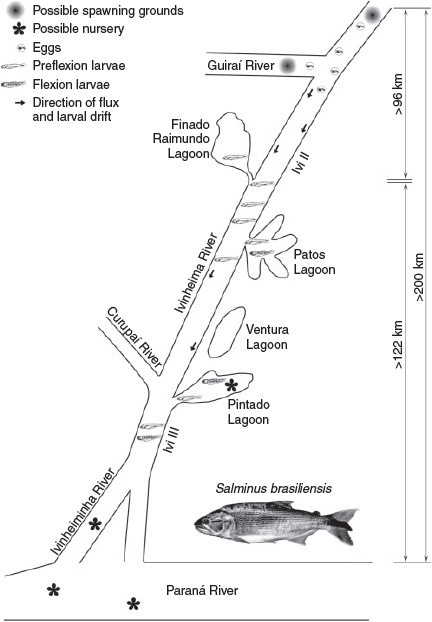
|
Discussion
The results of this study indicate that S. brasiliensis individuals migrate great distances upstream to spawn, and the larvae posteriorly drift in the river channel, reaching the backwaters and lagoons to grow in Ivinheima River or perhaps to drift in the Paraná River and lagoons, a pattern observed for migratory species (Baumgartner et al. 2004; Silva et al. 2017). Furthermore, in all reproductive periods, the larvae were mostly captured between October and January, which suggests that S. brasiliensis reproduction begins in October, when the water level and temperature begin to rise in the region. Most fish species reproduce annually, and spawning occurs under environmental conditions that are ideal for offspring survival, especially in terms of food availability, shelter and the habitat used for initial growth (de Castro et al. 2002; López-Casas et al. 2016).
In Neotropical rivers, the reproductive period usually occurs between October and March and is characterised by increases in temperature, rainfall and water level (Vazzoler 1996). These variables determine the distribution and abundance of fish eggs and larvae (Nakatani et al. 2001; Jiménez-Segura et al. 2010). However, the response is species specific (Baumgartner et al. 2008; Reynalte-Tataje et al. 2011); that is, different species respond to each of the environmental variables differently. The temporal variation in the density of S. brasiliensis larvae captured coincides with patterns observed in other Neotropical regions (see Vilizzi 2012; Zacardi et al. 2017; Rosa et al. 2018), with the highest densities primarily occurring in January, when the increase in water level and temperature was mainly observed.
The spatial distribution of S. brasiliensis larvae in the sampling sites is consistent with the results of various other studies; during the reproductive periods of long-distance migratory species, spawning primarily occurs in tributaries and eggs drift in the river until they hatch (Nakatani et al. 2004; Martin and Paller 2008; Jiang et al. 2010; Lopes et al. 2014). This longitudinal spatial pattern of reduced egg density and increased larval density from upstream to downstream in river–flood plain systems is due to the greater geographical incline of the rivers, which allows individuals to drift to the areas where embryo development occurs (Baumgartner et al. 2004). S. brasiliensis larvae were mainly captured in the river and backwater biotopes, confirming this downstream drifting pattern to refuges (lagoons) for growth and protection from predators. However, the low occurrence of S. brasiliensis larvae in the sampled lagoons suggests that the growth sites for S. brasiliensis are downstream of these locations, perhaps due to the swimming ability of the FL and FP developmental stages, development in other environments or problems with subsampling in the lagoons resulting in a lack of information. Daga et al. (2009) found larvae of S. brasiliensis and Pseudoplatystoma corruscans, both migratory species, in the Saraiva Lagoon, which is ~137 km downstream of the mouth of the Ivinheima River on the right bank of the Paraná River, suggesting that these larvae probably originated in spawning sites in the Amambai (89 km above) and Ivinheima rivers and used this site for growth and maintenance.
Effects of the environmental variables influencing the occurrence of S. brasiliensis larvae have also been reported for other migratory fish in the upper Paraná River flood plain (Baumgartner et al. 2008; Reynalte-Tataje et al. 2013), in tributaries of the upper Uruguay River basin (Hermes-Silva et al. 2009; Ávila-Simas et al. 2014), in the Magdalena River basin in Colombia (Jiménez-Segura et al. 2010; Kerguelén-Durango and Atencio-García 2015), in the Murray River in Australia (King et al. 2016) and in the Pearl River in southern China (Shuai et al. 2016). We observed an inverse correlation between the density of S. brasiliensis larvae and dissolved oxygen, water level and temperature, which suggests that the trigger for spawning in this species is high dissolved oxygen concentrations and moderately temperatures (~24–29°C) at the beginning of the increases in water levels. These results explain the absence or low frequency of S. brasiliensis larvae when the water level was at its peak because, under these conditions, there is an excessive input of organic matter to these environments with the inundation of the flood plain, resulting in a decrease in dissolved oxygen (Thomaz et al. 1992, 1997). A high dissolved oxygen concentration is necessary for the successful development of fish eggs (Werner 2002), so low dissolved oxygen concentrations prevent the growth and survival of S. brasiliensis larvae (De Leão Serafini et al. 2009). Similar results were reported by Reynalte-Tataje et al. (2011, 2013), who emphasised that increased flow, dissolved oxygen and temperature synchronously influence the reproduction of migratory and sedentary species.
Variations in temperature affect metabolism and physiological processes, and thus embryo and larvae development (Rombough 1997; Björnsson et al. 2001). In addition to the effects of temperature on larval density of S. brasiliensis reported herein, Barzotto et al. (2015) described the effects of temperature on dourado reproduction in river–flood plain systems, finding that it is possible that temperatures between 25 and 28°C are responsible for the timing of spawning and are necessary for the reproductive success of S. brasiliensis. In tropical regions, how temperature acts on the physiological and behavioural processes of fish reproduction is not completely clear, but temperature has been shown to be an important factor in the fish life cycle, increasing or decreasing the rates of metabolic processes and affecting the timing of gonad maturation in all species (Vazzoler 1996; Gogola et al. 2010).
Studies have reported the effects of seasonal flooding characteristics (e.g. duration, intensity and delay) on the reproduction and recruitment of fishes, especially migratory species (Bailly et al. 2008; King et al. 2009; Oliveira et al. 2015). Rising water levels cause local environments to flood, and these flood waters remain for a certain period, increasing the area covered by the river and reducing the land area. Thus, the forest extends to the riverside and the contact with marginal lagoons increases, providing the larvae and juveniles of migratory and sedentary species with protection and food (Suzuki et al. 2009; Reynalte-Tataje et al. 2013). During the seven reproductive periods in the present study, the intensity and duration of flooding in the region varied, and no significant relationship was found between the hydrological attributes and the occurrence of S. brasiliensis larvae. However, we believe that the hydrological attributes (intensity and duration) of floods are key to the recruitment of S. brasiliensis because they enable the early developmental stages to reach the lagoons and other microhabitats of the flood plain that are ideal for their growth and refuge (Oliveira et al. 2015). According to our results, the possible dominant factor influencing the spawning of the species is the increase in water level, regardless of whether flooding occurs during the reproductive period.
In the conceptual model, the reproductive migrations of S. brasiliensis occurred over ~210 km from the spawning areas in the tributaries of the Ivinheima River. However, this model has some deficiencies because it only considers the current velocity of the river and the duration of the embryonic and larval development of the species, but other environmental variables also can affect this process (e.g. temperature, dissolved oxygen and pH). Therefore, future studies should include these predictors in models of fish migration. However, the results of this study reinforce the observations reported by Bonetto et al. (1971) and Petrere (1985) of S. brasiliensis in the Paraná River; specifically, mark–recapture methods indicated that the species can migrate ~250 km upstream to spawn in tributaries. In another study using radiotelemetry in the basin of the upper Uruguay River, Hahn et al. (2011) found that during their reproductive migration, dourado can reach possible spawning sites 450 km upstream of the Itá Reservoir.
In conclusion, S. brasiliensis reproduces annually, primarily at the beginning of each reproductive period in October and January, and uses the Ivinheima River and its tributaries as spawning grounds. These environments are free of dams and provide favourable environmental conditions for the migration and reproduction of the species. It is estimated that increased water levels and high temperatures act as spawning triggers in both the river and its tributaries, but the dissolved oxygen concentration also sets an important threshold for the reproduction of the species because it is correlated with the incubation of eggs and the development of larvae. Therefore, considering that S. brasiliensis is a key target species of conservation actions, knowledge of its reproductive biology and specific ecological requirements is extremely valuable. Ensuring the reproductive success of this species will benefit all regional fish fauna, both migratory and sedentary, by promoting the maintenance of natural stocks and perpetuation of the species. However, the reproductive success of S. brasiliensis depends on the conservation of dam-free stretches in the basin (i.e. any anthropogenic change may result in further population decline or even extinction).
Conflicts of interest
The authors declare that they have no conflicts of interest.
Declaration of funding
This study was supported by grants from Programa Integrado de Ecologia (PIE), Pesquisa Ecológica de Longa Duração (PELD), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (520026/1998-5, 558118/2009-7 and 403686/2012-1) and CNPq (485159/2007-4, 480804/2010-9 and 483324/2012-4). The authors thank Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for the award of a study grant.
Acknowledgements
The authors thank the Núcleo de Pesquisas em Limnologia, Ictiologia e Aquicultura (Nupélia) for logistical support during field work and Programa de Pós-Graduação em Ecologia de Ambientes Aquáticos Continentais (PEA) for the opportunity to perform the study. The authors also thank Jaime Luiz Lopes Pereira for developing the map and conceptual model, and our friends from the Ichthyoplankton Laboratory (Nupélia, Universidade Estadual de Maringá) for assisting with the fieldwork and laboratory analyses.
References
Abilhoa, V., and Duboc, L. F. (2004). Peixes. In ‘Livro vermelho da fauna ameaçada do Estado do Paraná’. (Eds S. B. Mikichs and R. S. Bérnils.) pp. 581–677. (Instituto Ambiental do Paraná: Curitiba, Brazil.)Agostinho, A. A., Gomes, L. C., Veríssimo, S., and Okada, E. K. (2004). Flood regime, dam regulation and fish in the Upper Paraná River: effects on assemblage attributes, reproduction and recruitment. Reviews in Fish Biology and Fisheries 14, 11–19.
| Flood regime, dam regulation and fish in the Upper Paraná River: effects on assemblage attributes, reproduction and recruitment.Crossref | GoogleScholarGoogle Scholar |
Agostinho, A. A., Gomes, L. C., and Pelicice, F. M. (2007). ‘Ecologia e Manejo de Recursos Pesqueiros em Reservatórios do Brasil’. (Editora da Universidade Estadual de Maringá, EDUEM: Maringá, Brazil.)
Ahlstrom, E. H., and Moser, H. G. (1976). Eggs and larvae of fishes and their role in systematic investigations and in fisheries. Revue des Travaux de l’Institut des Pêches Maritimes 40, 379–398.
Ávila-Simas, S., Reynalte-Tataje, D. A., and Zaniboni Filho, E. (2014). Pools and rapids as spawning and nursery areas for fish in a river stretch without floodplains. Neotropical Ichthyology 12, 611–622.
| Pools and rapids as spawning and nursery areas for fish in a river stretch without floodplains.Crossref | GoogleScholarGoogle Scholar |
Bailly, D., Agostinho, A. A., and Suzuki, H. I. (2008). Influence of the flood regime on the reproduction of fish species with different reproductive strategies in the Cuiabá River, Upper Pantanal, Brazil. River Research and Applications 24, 1218–1229.
| Influence of the flood regime on the reproduction of fish species with different reproductive strategies in the Cuiabá River, Upper Pantanal, Brazil.Crossref | GoogleScholarGoogle Scholar |
Barzotto, E., and Mateus, L. (2017). Reproductive biology of the migratory freshwater fish Salminus brasiliensis (Cuvier, 1816) in the Cuiabá River basin, Brazil. Journal of Applied Ichthyology 33, 415–422.
| Reproductive biology of the migratory freshwater fish Salminus brasiliensis (Cuvier, 1816) in the Cuiabá River basin, Brazil.Crossref | GoogleScholarGoogle Scholar |
Barzotto, E., Sanches, P. V., Bialetzki, A., Orvati, L., and Gomes, L. C. (2015). Larvae of migratory fish (Teleostei: Ostariophysi) in the lotic remant of the Paraná River in Brazil. Zoologia 32, 270–280.
| Larvae of migratory fish (Teleostei: Ostariophysi) in the lotic remant of the Paraná River in Brazil.Crossref | GoogleScholarGoogle Scholar |
Baumgartner, G., Nakatani, K., Gomes, L. C., Bialetzki, A., Sanches, P. V., and Makrakis, M. C. (2004). Identification of spawning sites and natural nurseries of fishes in the upper Paraná River, Brazil. Environmental Biology of Fishes 71, 115–125.
| Identification of spawning sites and natural nurseries of fishes in the upper Paraná River, Brazil.Crossref | GoogleScholarGoogle Scholar |
Baumgartner, G., Nakatani, K., Gomes, L. C., Bialetzki, A., Sanches, P. V., and Makrakis, M. C. (2008). Fish larvae from the upper Paraná River: do abiotic factors affect larval density? Neotropical Ichthyology 6, 551–558.
| Fish larvae from the upper Paraná River: do abiotic factors affect larval density?Crossref | GoogleScholarGoogle Scholar |
Bialetzki, A., Nakatani, K., Sanches, P. V., Baumgartner, G., and Gomes, L. C. (2005). Larval fish assemblage in the Baía River (Mato Grosso do Sul State, Brazil): temporal and spatial patterns. Environmental Biology of Fishes 73, 37–47.
| Larval fish assemblage in the Baía River (Mato Grosso do Sul State, Brazil): temporal and spatial patterns.Crossref | GoogleScholarGoogle Scholar |
Björnsson, B., Steinarsson, A., and Oddgeirsson, M. (2001). Optimal temperature for growth and feed conversion of immature cod (Gadus morhua L.). ICES Journal of Marine Science 58, 29–38.
| Optimal temperature for growth and feed conversion of immature cod (Gadus morhua L.).Crossref | GoogleScholarGoogle Scholar |
Bonetto, A. A., Pignalberi, C., Cordiviola, E., and Oliveros, O. (1971). Informaciones complementarias sobre migracion de peces en la cuenca de la Plata. Physis (Rio de Janeiro, Brazil) 30, 505–520.
Carolsfeld, J., Harvey, B., Ross, C., and Baer, A. (2003). ‘Migratory Fishes of South America: Biology, Fisheries, and Conservation Status’. (World Fisheries Trust, the World Bank and the International Development Research Centre: Victoria, BC, Canada.)
Comrey, A. L., and Lee, H. B. (1992). ‘A First Course in Factor Analysis’, 2nd edn. (Lawrence Erlbaum Associates: Hillsdale, NJ, USA.)
Comunello, E., Souza Filho, E. E., Rocha, P. C., and Nanni, M. R. (2003). Dinâmica de inundação de áreas sazonalmente alagáveis na Planície Aluvial do Alto Rio Paraná: Estudo preliminar. In ‘Anais do 11° Simpósio brasileiro de sensoriamento remoto’. pp. 2459–2466. (Instituto Nacional de Pesquisas Espaciais, INPE: São José Dos Campos, Brazil.)
Copp, G. H. (1990). Shifts in the microhabitat of larval and juvenile roach, Rutilis rutilus (L.), in a floodplain channel. Journal of Fish Biology 36, 683–692.
| Shifts in the microhabitat of larval and juvenile roach, Rutilis rutilus (L.), in a floodplain channel.Crossref | GoogleScholarGoogle Scholar |
Daga, V. S., Gogola, T. M., Sanches, P. V., Baumgartner, G., Baumgartner, D., Piana, P. A., Gubiani, E. A., and Delariva, R. L. (2009). Fish larvae assemblages in two floodplain lakes with different degrees of connection to the Paraná River, Brazil. Neotropical Ichthyology 7, 429–438.
| Fish larvae assemblages in two floodplain lakes with different degrees of connection to the Paraná River, Brazil.Crossref | GoogleScholarGoogle Scholar |
de Castro, R. J., Nakatani, K., Bialetzki, A., Sanches, P. V., and Baumgartner, G. (2002). Temporal distribution and composition of the ichthyoplankton from Leopoldo’s Inlet on the upper Paraná River floodplain (Brazil). Journal of Zoology 256, 437–443.
| Temporal distribution and composition of the ichthyoplankton from Leopoldo’s Inlet on the upper Paraná River floodplain (Brazil).Crossref | GoogleScholarGoogle Scholar |
De Leão Serafini, R., Zaniboni-Filho, E., and Baldisserotto, B. (2009). Effect of combined non-ionized ammonia and dissolved oxygen levels on the survival of juvenile dourado, Salminus brasiliensis (Cuvier). Journal of the World Aquaculture Society 40, 695–701.
| Effect of combined non-ionized ammonia and dissolved oxygen levels on the survival of juvenile dourado, Salminus brasiliensis (Cuvier).Crossref | GoogleScholarGoogle Scholar |
Godoy, M. P. (1975). ‘Peixes do Brasil, subordem Characoidei: bacia do rio Mogi Guassu’. (Franciscana: Piracicaba, Brazil.)
Gogola, T. M., Daga, V. S., Silva, P. R. L., Sanches, P. V., Gubiani, E. A., Baumgartner, G., and Delariva, R. L. (2010). Spatial and temporal distribution patterns of ichthyoplankton in a region affected by water regulation by dams. Neotropical Ichthyology 8, 341–349.
| Spatial and temporal distribution patterns of ichthyoplankton in a region affected by water regulation by dams.Crossref | GoogleScholarGoogle Scholar |
Hahn, L., Agostinho, A. A., English, K. K., Carosfeld, J., da Câmara, L. F., and Cooke, S. J. (2011). Use of radiotelemetry to track threatened dorados Salminus brasiliensis in the upper Uruguay River, Brazil. Endangered Species Research 15, 103–114.
| Use of radiotelemetry to track threatened dorados Salminus brasiliensis in the upper Uruguay River, Brazil.Crossref | GoogleScholarGoogle Scholar |
Hermes-Silva, S., Reynalte-Tataje, D. A., and Zaniboni-Filho, E. (2009). Spatial and temporal distribution of ichthyoplankton in the upper Uruguay River, Brazil. Brazilian Archives of Biology and Technology 52, 933–944.
| Spatial and temporal distribution of ichthyoplankton in the upper Uruguay River, Brazil.Crossref | GoogleScholarGoogle Scholar |
Humphries, P., Serafini, L., and King, A. J. (2002). River regulation and fish larvae: variations through space and time. Freshwater Biology 47, 1307–1331.
| River regulation and fish larvae: variations through space and time.Crossref | GoogleScholarGoogle Scholar |
Jackson, D. A. (1993). Stopping rules in principal components analysis: a comparison of heuristical and statistical approaches. Ecology 74, 2204–2214.
| Stopping rules in principal components analysis: a comparison of heuristical and statistical approaches.Crossref | GoogleScholarGoogle Scholar |
Jiang, W., Liu, H. Z., Duan, Z. H., and Cao, W. X. (2010). Seasonal variation in drifting eggs and larvae in the Upper Yangtze, China. Zoological Science 27, 402–409.
| Seasonal variation in drifting eggs and larvae in the Upper Yangtze, China.Crossref | GoogleScholarGoogle Scholar | 20443687PubMed |
Jiménez-Segura, L. F., Palacio, J., and Leite, R. (2010). River flooding and reproduction of migratory fish species in the Magdalena River basin, Colombia. Ecology Freshwater Fish 19, 178–186.
| River flooding and reproduction of migratory fish species in the Magdalena River basin, Colombia.Crossref | GoogleScholarGoogle Scholar |
Junk, W. J., Bayley, P. B., and Sparks, R. E. (1989). The flood pulse concept in river–floodplain systems. Canadian Special Publication of Fisheries and Aquatic Sciences 106, 110–127.
Kerguelén-Durango, E., and Atencio-García, V. (2015). Environmental characterization of the reproductive season of migratory fish of the Sinú River (Córdoba, Colombia). Revista Mvz Cordoba 20, 4766–4778.
| Environmental characterization of the reproductive season of migratory fish of the Sinú River (Córdoba, Colombia).Crossref | GoogleScholarGoogle Scholar |
King, A. J. (2004). Ontogenetic patterns of habitat use by fishes within the main channel of an Australian floodplain river. Journal of Fish Biology 65, 1582–1603.
| Ontogenetic patterns of habitat use by fishes within the main channel of an Australian floodplain river.Crossref | GoogleScholarGoogle Scholar |
King, A. J., Tonkin, Z., and Mahoney, J. (2009). Environmental flows enhance native fish spawning and recruitment in the Murray River, Australia. River Research and Applications 25, 1205–1218.
| Environmental flows enhance native fish spawning and recruitment in the Murray River, Australia.Crossref | GoogleScholarGoogle Scholar |
King, A. J., Gwinn, D. C., Tonkin, Z., Mahoney, J., Raymond, S., and Beesley, L. (2016). Using abiotic drivers of fish spawning to inform environmental flow management. Journal of Applied Ecology 53, 34–43.
| Using abiotic drivers of fish spawning to inform environmental flow management.Crossref | GoogleScholarGoogle Scholar |
Lenth, R. V. (2016). Least-squares means: the R package lsmeans. Journal of Statistical Software 69, 1–33.
| Least-squares means: the R packageCrossref | GoogleScholarGoogle Scholar |
Lopes, C. A., Garcia, V., Reynalte-Tataje, D. A., Zaniboni-Filho, E., and Nuñer, A. P. O. (2014). Temporal distribution of ichthyoplankton in the Forquilha river, upper Uruguay River Brazil: relationship with environmental factors. Acta Scientiarum. Biological Sciences 36, 59–65.
| Temporal distribution of ichthyoplankton in the Forquilha river, upper Uruguay River Brazil: relationship with environmental factors.Crossref | GoogleScholarGoogle Scholar |
López-Casas, S., Jiménez-Segura, L. F., Agostinho, A. A., and Pérez, C. M. (2016). Potamodromous migrations in the Magdalena River basin: bimodal reproductive patterns in Neotropical rivers. Journal of Fish Biology 89, 157–171.
| Potamodromous migrations in the Magdalena River basin: bimodal reproductive patterns in Neotropical rivers.Crossref | GoogleScholarGoogle Scholar | 27073186PubMed |
Marques, A. A. B., Fontana, C. S., Vélez, E., Bencke, G. A., Schneider, M., and Reis, R. E. (2002). ‘Livro Vermelho da fauna ameaçada de extinção no Rio Grande do Sul.’ (Fundação Zoobotânica (FZB), Museu de Ciências e Tecnologia Pontifícia Universidade Católica do Rio Grande do Sul (MCTPUCRS), Associação Ambientalista Internacional (PANGEA): Porto Alegre, Brazil.)
Martin, F. D., and Paller, M. H. (2008). Ichthyoplankton transport in relation to floodplain width and inundation and tributary creek discharge in the lower Savannah River of Georgia and South Carolina. Hydrobiologia 598, 139–148.
| Ichthyoplankton transport in relation to floodplain width and inundation and tributary creek discharge in the lower Savannah River of Georgia and South Carolina.Crossref | GoogleScholarGoogle Scholar |
Morais-Filho, M. B., and Schubart, O. (1955). ‘Contribuição ao estudo do dourado (Salminus maxillosus Val.) do Rio Mogi Guassú (Pisces, Characidae).’ (Ministério da Agricultura, Divisão de Caça e Pesca: São Paulo, Brazil.)
Nakatani, K., Baumgartner, G., and Cavicchioli, M. (1997). Ecologia de ovos e larvas de peixes. In ‘A planície de inundação do alto rio Paraná: aspectos físicos, biológicos e socioeconômicos’. (Eds A. E. A. Vazzoler, A. A. Agostinho, and S. H. Hahn.) pp. 281–306. (Editora da Universidade Estadual de Maringá, Editora da Universidade Estadual de Maringá, EDUEM : Maringá, Brazil.)
Nakatani, K., Agostinho, A. A., Baumgartner, G., Bialetzki, A., Sanches, P. V., Makrakis, M. C., and Pavanelli, C. S. (2001). ‘Ovos e larvas de peixes de água doce: desenvolvimento e manual de identificação.’ (Editora da Universidade Estadual de Maringá, EDUEM: Maringá, Brazil.)
Nakatani, K., Bialetzki, A., Baumgartner, G., Sanches, P. V., and Makrakis, M. C. (2004). Temporal and spatial dynamics of fish eggs and larvae. In ‘The Upper Paraná River and its Floodplain: Physical Aspects, Ecology and Conservation’. (Eds S. M. Thomaz, A. A. Agostinho, and N. S. Hahn.) pp. 293–308. (Backuys Publishers: Leiden, Netherlands.)
Oliveira, A. G., Suzuki, H. I., Gomes, L. C., and Agostinho, A. A. (2015). Interspecific variation in migratory fish recruitment in the upper Paraná River: effects of the duration and timing of floods. Environmental Biology of Fishes 98, 1327–1337.
| Interspecific variation in migratory fish recruitment in the upper Paraná River: effects of the duration and timing of floods.Crossref | GoogleScholarGoogle Scholar |
Petrere, M. Jr (1985). Migraciones de peces de agua dulce en America Latina: algunos comentarios. Documento Ocasional 1. (Comision de Pesca Continental para América Latina (COPESCAL), Fisheries and Aquaculture Department, Food and Agriculture Organization of the United Nations.) Available at http://www.fao.org/tempref/FI/CDrom/aquaculture/a0844t/docrep/008/R4125S/R4125S00.htm [Verified 8 August 2019].
Reinfelds, I. V., Walsh, C. T., van der Meulen, D. E., Growns, I. O., and Gray, C. A. (2013). Magnitude, frequency and duration of instream flows to stimulate and facilitate catadromous fish migrations: Australian bass (Macquaria novemaculeata Perciformes, Percichthyidae). River Research and Applications 29, 512–527.
| Magnitude, frequency and duration of instream flows to stimulate and facilitate catadromous fish migrations: Australian bass (Macquaria novemaculeata Perciformes, Percichthyidae).Crossref | GoogleScholarGoogle Scholar |
Reynalte-Tataje, D. A., Nakatani, K., Fernandes, R., Agostinho, A. A., and Bialetzki, A. (2011). Temporal distribution of ichthyoplankton in the Ivinhema River (Mato Grosso do Sul State/ Brazil): influence of environmental variables. Neotropical Ichthyology 9, 427–436.
| Temporal distribution of ichthyoplankton in the Ivinhema River (Mato Grosso do Sul State/ Brazil): influence of environmental variables.Crossref | GoogleScholarGoogle Scholar |
Reynalte-Tataje, D. A., Agostinho, A. A., and Bialetzki, A. (2013). Temporal and spatial distributions of the fish larval assemblages of the Ivinheima River sub-basin (Brazil). Environmental Biology of Fishes 96, 811–822.
| Temporal and spatial distributions of the fish larval assemblages of the Ivinheima River sub-basin (Brazil).Crossref | GoogleScholarGoogle Scholar |
Rombough, P. J. (1997). The effects of temperature on embryonic and larval development. In ‘Global Warming. Implications for Freshwater and Marine Fish’. (Eds C. M. Wood and D. G. McDonald.) pp. 177–223. (Cambridge University Press: Cambridge, UK.)
Rosa, G. R., Salvador, G. N., Bialetzki, A., and Santos, G. B. (2018). Spatial and temporal distribution of ichthyoplankton during an unusual period of low flow in a tributary of the São Francisco River, Brazil. River Research and Applications 34, 69–82.
| Spatial and temporal distribution of ichthyoplankton during an unusual period of low flow in a tributary of the São Francisco River, Brazil.Crossref | GoogleScholarGoogle Scholar |
Sanches, P. V., Nakatani, K., Bialetzki, A., Baumgartner, G., Gomes, L. C., and Luiz, E. A. (2006). Flow regulation by dams affecting ichthyoplankton: the case of the Porto Primavera Dam, Paraná River, Brazil. River Research and Applications 22, 555–565.
| Flow regulation by dams affecting ichthyoplankton: the case of the Porto Primavera Dam, Paraná River, Brazil.Crossref | GoogleScholarGoogle Scholar |
Shuai, F., Xinhui, L., Yuefei, L., Jie, L., Jiping, Y., and Sovan, L. (2016). Temporal patterns of larval fish occurrence in a large subtropical river. PLoS One 11, 1–20.
| Temporal patterns of larval fish occurrence in a large subtropical river.Crossref | GoogleScholarGoogle Scholar |
Silva, J. C., Rosa, R. R., Galdioli, E. M., Soares, C. M., Domingues, W. M., Veríssimo, S., and Bialetzki, A. (2017). Importance of dam-free stretches for fish reproduction: the last remnant in the upper Paraná River. Acta Limnologica Brasiliensia 29, e106.
| Importance of dam-free stretches for fish reproduction: the last remnant in the upper Paraná River.Crossref | GoogleScholarGoogle Scholar |
Souza Filho, E. E., and Stevaux, J. C. (1997). Geologia e geomorfologia do complexo rio Baía, Corutuba, Ivinhema. In: ‘A planície de inundação do alto rio Paraná: aspectos físicos, biológicos e socioeconômicos’. (Eds A. E. A. M. Vazzoler, A. A. Agostinho, and N. S. Hahn.) pp. 3–46. (Editora da Universidade Estadual de Maringá, EDUEM: Maringá, Brazil.)
Statsoft (2010). ‘STATISTICA for Windows.’ (Statsoft: Tulsa, OK, USA.)
Suzuki, F. M., and Pompeu, P. S. (2016). Influence of abiotic factors on ichthyoplankton occurrence in stretches with and without dams in the upper Grande River basin, south-eastern Brazil. Fisheries Management and Ecology 23, 99–108.
| Influence of abiotic factors on ichthyoplankton occurrence in stretches with and without dams in the upper Grande River basin, south-eastern Brazil.Crossref | GoogleScholarGoogle Scholar |
Suzuki, H. I., Agostinho, A. A., Bailly, D., Gimenes, M. F., Julio, H. F., and Gomes, L. C. (2009). Inter-annual variations in the abundance of young-of-the-year of migratory fishes in the upper Paraná River floodplain: relations with hydrographic attributes. Brazilian Journal of Biology 69, 649–660.
| Inter-annual variations in the abundance of young-of-the-year of migratory fishes in the upper Paraná River floodplain: relations with hydrographic attributes.Crossref | GoogleScholarGoogle Scholar |
Thomaz, S. M., Roberto, M. C., Lansac-Tôha, F. A., Lima, A. F., and Esteves, F. A. (1992). Características limnológicas de uma estação de amostragem do alto rio Paraná e outra do baixo rio Ivinheima (PR, MS-Brasil). Acta Limnologica Brasiliensia IV, 32–51.
Thomaz, S. M., Roberto, M. C., and Bini, L. M. (1997). Caracterização limnológica dos ambientes aquáticos e influência dos níveis fluviométricos. In ‘A planície de inundação do alto rio Paraná. Aspectos físicos, biológicos e socioeconômicos’. (Eds A. E. A. M. Vazzoler, A. A. Agostinho, and N. S. Hahn.) pp. 73–102. (Editora da Universidade Estadual de Maringá, EDUEM : Maringá, Brazil.)
Vazzoler, A. E. A. M. (1996). ‘Biologia da reprodução de peixes teleósteos: teoria e prática.’ (Editora da Universidade Estadual de Maringá, EDUEM : Maringá, Brazil.)
Vega‐Orellana, O. M., Fracalossi, D. M., and Sugai, J. K. (2006). Dourado (Salminus brasiliensis) larviculture: weaning and ontogenetic development of digestive proteinases. Aquaculture 252, 484–493.
| Dourado (Salminus brasiliensis) larviculture: weaning and ontogenetic development of digestive proteinases.Crossref | GoogleScholarGoogle Scholar |
Venables, W. N., and Ripley, B. D. (2002). ‘Modern Applied Statistics with S.’ (Springer: New York, NY, USA.)
Vilizzi, L. (2012). Abundance trends in floodplain fish larvae: the role of annual flow characteristics in the absence of overbank flooding. Fundamental and Applied Limnology 181, 215–227.
| Abundance trends in floodplain fish larvae: the role of annual flow characteristics in the absence of overbank flooding.Crossref | GoogleScholarGoogle Scholar |
Welcomme, R., and Halls, A. (2005). Dependence of tropical river fisheries on flow. In ‘Proceedings of the Second International Symposium on the Management of Large Rivers for Fisheries’. (Eds R. Welcomme and T. Petr.) pp. 267–283. (FAO Regional Office for Asia and the Pacific: Bangkok, Thailand.)
Werner, R. G. (2002). Habitat requirements. In ‘Fishery Science: the Unique Contributions of Early Life Stages’. (Eds L. A. Fuiman and R. G. Werner.) pp. 161–182. (Iowa State Press: Ames, IO, USA.)
Winemiller, K. O. (1989). Patterns of variation in life history among South American fishes in seasonal environments. Oecologia 81, 225–241.
| Patterns of variation in life history among South American fishes in seasonal environments.Crossref | GoogleScholarGoogle Scholar | 28312542PubMed |
Zacardi, D. M., Bittencourt, S. C. S., Nakayama, L., and Queiroz, H. L. (2017). Distribution of economically important fish larvae (Characiformes, Prochilodontidae) in the Central Amazonia, Brazil. Fisheries Management and Ecology 24, 283–291.
| Distribution of economically important fish larvae (Characiformes, Prochilodontidae) in the Central Amazonia, Brazil.Crossref | GoogleScholarGoogle Scholar |
Ziober, S. M., Reynalte-Tataje, D. A., and Zaniboni-Filho, E. (2015). The importance of a conservation unit in a subtropical basin for fish spawning and growth. Environmental Biology of Fishes 98, 725–737.
| The importance of a conservation unit in a subtropical basin for fish spawning and growth.Crossref | GoogleScholarGoogle Scholar |