Otolith δ13C values as a metabolic proxy: approaches and mechanical underpinnings
Ming-Tsung Chung A D , Clive N. Trueman B , Jane Aanestad Godiksen C and Peter Grønkjær AA Department of Bioscience, Section for Aquatic Biology, Aarhus University, DK-8000 Aarhus C, Denmark.
B Ocean and Earth Science, University of Southampton Waterfront Campus, European Way, Southampton, SO14 3ZH, UK.
C Institute of Marine Research, Postbox 1870 Nordnes, NO-5817 Bergen, Norway.
D Corresponding author. Email: mingtsungchung@bios.au.dk
Marine and Freshwater Research 70(12) 1747-1756 https://doi.org/10.1071/MF18317
Submitted: 28 August 2018 Accepted: 15 January 2019 Published: 26 March 2019
Journal Compilation © CSIRO 2019 Open Access CC BY-NC-ND
Abstract
Knowledge of metabolic costs associated with maintenance, foraging, activity and growth under natural conditions is important for understanding fish behaviours and the bioenergetic consequences of a changing environment. Fish performance in the wild and within a complex environment can be investigated by analysing individual-level field metabolic rate and, at present, the natural stable carbon isotope tracer in otoliths offers the possibility to reconstruct field metabolic rate. The isotopic composition of carbon in fish otoliths is linked to oxygen consumption through metabolic oxidation of dietary carbon. The proportion of metabolically derived carbon can be estimated with knowledge of δ13C values of diet and dissolved inorganic carbon in the water. Over the past 10 years, new techniques to study fish ecology have been developed, and these can be used to strengthen the application of otolith δ13C values as a metabolic proxy. Here, we illustrate the great potential of the otolith δ13C metabolic proxy in combination with other valuable and well-established approaches. The novel approach of the otolith δ13C metabolic proxy allows us to track the effects of ontogenetic and environmental drivers on individual fish physiology, and removes a major obstacle to understanding and predicting the performance of free-ranging wild fish.
Additional keywords : bioenergetics, field metabolic rate, isotopic mixing models, oxygen consumption.
Introduction
Metabolic rate is a proxy of the energy used by individual animals and provides a physiological perspective to interpret behavioural ecology. Laboratory-based measurement of fish standard and maximum metabolic rate (SMR and MMR respectively) is a common approach to investigating fish physiology in response to environmental changes (Killen et al. 2007, 2010; Chabot et al. 2016; Metcalfe et al. 2016), but has limited value for explaining fish behaviours in the field. The relevant trait to measure is the field metabolic rate (FMR). Unfortunately, FMR is challenging to measure with conventional methods for estimating metabolic rates in free-living fish (Treberg et al. 2016). A method of in situ oxygen consumption measurement termed field-based respirometry has been tried to investigate the metabolic rate and swimming activity of wild fish in natural environment (Bailey et al. 2002; Farrell et al. 2003). However, the methods do not allow for monitoring realistic energy demands of, for example, prey–predator interactions or recording a time-integrated total metabolic rate in a wild individual. The stable carbon isotope composition of otolith aragonite (expressed as δ13Coto values) may provide an answer to this problem. δ13Coto values have been studied for decades and show potential as a metabolic proxy because the carbon used to precipitate otolith aragonite is drawn from both metabolic (dietary) and ambient water sources. Therefore, the isotopic composition of carbon in otolith aragonite is a weighted average between the isotope compositions of metabolic carbon released from respiration and the dissolved inorganic carbon from ambient water (Kalish 1991a, 1991b; Iacumin et al. 1992; Gauldie et al. 1994; Gauldie 1996; Thorrold et al. 1997; Wurster and Patterson 2003; Wurster et al. 2005; Solomon et al. 2006; Dufour et al. 2007; Tohse and Mugiya 2008). However, despite a clear theoretical basis backed up by consistent observational data, relating variations in δ13Coto values directly to alternative measurements of metabolic rate have proven challenging, partly due to the difficulty of estimating FMR in aquatic organisms. Over the past 10 years, new knowledge and enhanced analytical techniques have been developed, and the potential for using δ13Coto values as a metabolic proxy should be updated. This short paper briefly overviews the use of δ13Coto values in relation to metabolism and illustrates a way forward to improve the methodology, and thereby provide fish ecologists and physiologists with a strong tool to explore some of the current challenges in fish and fisheries ecology. First, we outline the mechanism underpinning carbon incorporation into otolith aragonite and describe analytical approaches to quantify the otolith metabolic proxy. Second, we summarise efforts to describe the relationship between the otolith metabolic proxy and oxygen consumption. Finally, we show the great potential of using otolith metabolic proxy in combination with other otolith-based analyses to answer physiological questions.
δ13Coto metabolic proxy expressed as a two-component mixing model
Previously, δ13Coto values have often been used as natural tracers for differences in water or diet source between or within individuals (Nonogaki et al. 2006; Ashford and Jones 2007; Schloesser et al. 2009; Elsdon et al. 2010; von Biela et al. 2015; Fraile et al. 2016) to answer various fishery and ecological questions, such as stock identification (Gao and Beamish 1999; Gao et al. 2001; Bastow et al. 2002; Correia et al. 2011; Shen and Gao 2012) and fish movements and migration (Augley et al. 2007; Kimirei et al. 2013; Currey et al. 2014; Javor and Dorval 2014; Gerard et al. 2015). Other applications have associated δ13Coto values with metabolic rate to reveal variation in fish physiological performance and the factors affecting it (Wurster and Patterson 2003; Wurster et al. 2005; Shephard et al. 2007; Hanson et al. 2013). However, we need to carefully evaluate the drivers behind variation in δ13C values of dissolved inorganic carbon (DIC) in the water (δ13CDIC) and δ13C values of the diet (δ13Cdiet) in order to use δ13Coto as an accurate estimate of fish FMR.
The δ13Coto value is the weighted average of the isotopic composition of carbon in two main carbon sources, DIC and diet, which are typically very distinct (~1 and –16‰ respectively; Sherwood and Rose 2005; Tagliabue and Bopp 2008). Therefore, the δ13Coto value can be described as the outcome of a two-component mixing model (Schwarcz et al. 1998; Solomon et al. 2006):
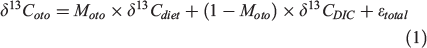
where Moto is the proportion of metabolically derived carbon (from the diet) in otolith carbonate and ϵtotal is the total net isotopic fractionation during carbon exchange between DIC and blood, as well as between the blood and endolymph in which the otolith is formed. DIC uptake is primarily across the gut and gills (Solomon et al. 2006). In contrast, metabolic carbon is released into the blood through the respiration and oxidisation processes, and the rate of oxidation of dietary carbon by definition reflects metabolic rate. Therefore, the weighted average of the isotopic values between DIC and diet is controlled by fish metabolism, and Moto is viewed and named as a metabolic proxy in the following discussion. Below we review sources of uncertainty in evaluating Eqn 1 and therefore Moto values.
δ13CDIC values
δ13CDIC values vary between water masses, geographic locations and time, influenced by the release of carbon from the lithosphere, carbon flux exchange within the atmosphere and respiration and photosynthesis from the biosphere. Fresh waters have a wide range of δ13CDIC values among rivers and lakes (for a review, see Bade et al. 2004). For example, δ13CDIC values range from –16 to –8‰ in the Ottawa River basin (Canada; Telmer and Veizer 1999) and from 2.6 to –31‰ among 104 lakes on four different continents (for details, see Bade et al. 2004). The variation of freshwater δ13CDIC values is strongly dependent on geological chemistry, water metabolism and biogeochemical process. By contrast, δ13CDIC values are relatively constant in marine systems, with values that generally vary between 0 and 3‰ on the horizontal spatial scale, and ~1‰ in the vertical gradient (Kroopnick 1985; Tagliabue and Bopp 2008; Schmittner et al. 2013; Becker et al. 2016). In addition to spatial variations, temporal differences in the δ13CDIC values, such as seasonal or annual changes, have been noticed. In areas with strong phytoplankton booms, rates of removal of CO2 from DIC may exceed diffusion rates of atmospheric CO2 into surface waters, and preferential uptake of 12C into algal cells can cause a temporary increase in δ13CDIC values. Over the past 100 years, oceanic δ13CDIC values have declined continuously because anthropogenic carbon decreases the oceanic δ13CDIC values by CO2 exchanges between the atmosphere and the ocean. This has been termed the Suess effect and, interestingly, the Suess effect has been recorded in otoliths from Atlantic bluefin tuna (Fraile et al. 2016). According to a recent biogeochemical model, the oceanic δ13CDIC value decreased 0.07‰ per decade from 1860 to 2000, whereas in the recent period from 1970 to 2000 it decreased at a rate of –0.18‰ per decade (Tagliabue and Bopp 2008). The decreasing rate speeds up with time. If we want to use δ13Coto metabolic proxy to compare fish metabolism between decades with an assumed δ13CDIC value but without calibrating the Suess effect, it will overestimate the metabolic rate of fish caught in a recent year. Therefore, we suggest using a model calibration to predict δ13CDIC values or reconstructing δ13CDIC values with given oceanographic parameters for the specific year.
It is possible to acquire δ13CDIC values from the direct measurement of water samples, but it is not always feasible, particularly where studies are based on historical otolith collections or from remote oceanic locations. Nevertheless, there are several ways to acquire δ13CDIC values through modelling predictions. δ13CDIC values can be predicted with a given value of apparent oxygen utilisation (AOU) in the world’s ocean (Kroopnick 1985), and Filipsson et al. (2017) presented a regional relationship between δ13CDIC values and AOU with salinity revision in the Baltic–Skagerrak region at water depths below the halocline:
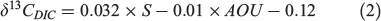
where S is salinity and AOU is measured in micromoles per kilogram. In addition, a regional multiple linear regression model predicting δ13CDIC values from salinity, temperature and DIC concentrations was used by Becker et al. (2016) to model δ13CDIC values at a depth of more than 1500 m in the North Atlantic Ocean:
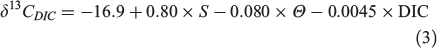
where Θ is potential temperature (°C) and DIC is measured in micromoles per kilogram. δ13CDIC values can also be extracted from interpolated spatial models (McMahon et al. 2013) or biogeochemical models (Tagliabue and Bopp 2008; Schmittner et al. 2013). Biogeochemical models take into account both the spatial and temporal factors and yield a global pattern that is necessary for studies on large-scale fish migration and movement.
δ13Cdiet values
Distinguishing and measuring the isotopic values of metabolically derived carbon from DIC in blood and endolymph is difficult. Hence, using the δ13C values in various tissues, such as muscle, liver and heart, is an alternative approach to estimating the δ13C values of metabolically derived carbon, δ13Cdiet. Tissue δ13C values represent a weekly to monthly average of diet signals, depending on the tissue turnover rates among species, the types of tissue and diet preferences (Ankjærø et al. 2012). Isotopic enrichment from diets to tissues is also influenced by various biological and environmental factors, such as growth rate, metabolism and temperature, with typical isotopic offsets between diet and muscle tissue ranging from –1.75 to 3.7‰ (Sweeting et al. 2007). Post (2002) reported an average value of 0.4‰ in carbon isotope enrichment, but Sweeting et al. (2007) suggested out that 1.5‰ is a more appropriate value. These reported values are species averages, but in reality tissue–diet isotopic spacing is a dynamic variable rather than a fixed trait, varying within and among individuals and species depending on physiological status, life history traits and feeding histories. Despite the variation found between species and studies, muscle δ13C values provide a reasonable approximation of δ13Cdiet in the Moto estimation, because a 1‰ variation of δ13Cdiet values only contributes a maximum of ~0.005 to the uncertainty in the Moto term (see details in the following sections). A drawback of using soft tissue is that individual trophic history cannot be reconstructed from these tissues because their δ13C values are continuously changing due to variable diet and their metabolic turnover.
δ13C values recorded in otolith organic matters have been recently used to indicate diet signals and trophic information in wild fish (Sirot et al. 2017). Compared with muscle tissue, otolith organic materials have the advantage that their δ13C values appear close to those of the diet (i.e. show little trophic enrichment; Grønkjær et al. 2013). Moreover, otoliths grow continuously and record ontogenetic information and, in theory, if we can extract the organic materials from otolith aragonite formed at different periods or life stages of an individual, it would be possible to reconstruct that individual’s trophic history. This would allow estimates of FMR through the life of a single individual. However, the proportion of organic material in otoliths is extremely small (<10%), and analysis of individual trophic history is at present only feasible with fish species possessing large otoliths.
The ϵtotal term
Physiology controls carbon isotope incorporation into otoliths and it directly affects the isotopic fractionation factor, ϵtotal. There are three different settings of ϵtotal that have been used in previous studies. Schwarcz et al. (1998) used a value of 2, which was based on the findings of carbon isotope enrichment from ambient fluids (HCO3–) to biogenic aragonite carbonates at 5°C (Grossman and Ku 1986). Høie et al. (2003) and Wurster and Patterson (2003) adopted a value of 2.7, which was derived from the inorganic precipitation of aragonite carbonate where the enrichment factor was temperature independent (Romanek et al. 1992). Solomon et al. (2006) used rainbow trout (Oncorhynchus mykiss) and conducted a controlled laboratory experiment with 13C-enriched diets and a 13C bicarbonate spike in water, finding that ϵtotal was slightly negative (–1.8), but not significantly different from zero. The determination of ϵtotal is still unresolved and remains a source of uncertainty in Moto measurements (Dufour et al. 2007). Further research is needed to investigate the specific ϵtotal values among species and minimise the bias of Moto estimations.
Moto estimations
Two notable studies have conducted controlled laboratory experiments to estimate the proportion of metabolic carbon in fish otoliths. Solomon et al. (2006) reared juvenile rainbow trout (O. mykiss) in water with different δ13CDIC values and fed them food with different δ13Cdiet values, and reported a Moto value of 0.17. Tohse and Mugiya (2008) used the isotope labelling technique on goldfish (Carassius auratus) to estimate the proportion of metabolically derived carbon, which they found to account for 25% of overall otolith carbon composition (Moto value of 0.25). The percentage of metabolically derived carbon was higher (28%; Moto value of 0.28) during the day and lower (13–20%; Moto value of 0.13–0.20) during the night. In most other previous studies, Moto values estimated from the two-component mixing model fell in the range 0–0.5 (Table 1). High values over 0.5 suggested by Wurster and Patterson (2003) and Hanson et al. (2013) reflect consideration of a range of possible δ13CDIC and δ13Cdiet values and associated the uncertainty in Moto estimations.

|
Uncertainty in the δ13CDIC and δ13Cdiet values determine the precision of Moto estimations. As an example, we performed a sensitivity test considering the effect on estimates of Moto rising from the sources of variation in Eqn 1.
We calculated Moto values corresponding to simulated values of δ13Coto ranging between 0 and –6‰. We allowed δ13CDIC values to vary by 1‰, capturing the likely uncertainty in most marine applications (Kroopnick 1985; Tagliabue and Bopp 2008; Schmittner et al. 2013; Becker et al. 2016). We varied δ13Cdiet values in a range from –16 to –22‰, reflecting typical isotope values of dietary items for benthic to pelagic fish species in temperate latitudes. The ϵtotal term was assumed to be 0 based on the observations by Solomon et al. (2006). Varying the δ13CDIC term across a range of 1‰ resulted in Moto values ranging between ~0.05 and 0.35, depending on the δ13Cdiet and δ13Coto values used in the calculation (Fig. 1a). The s.d. of the Moto term varied between 0.01 and 0.02, and systematically changed with δ13Coto and δ13Cdiet values. This suggests variation in the precision of Moto within the fish functional groups. Fish with more positive δ13Cdiet values, such as benthic fishes, usually also have higher δ13Coto values (Sherwood and Rose 2003). Higher δ13Coto values mean that the difference between δ13Coto and δ13CDIC values is smaller, and therefore the uncertainty associated with the Moto term will increase. This is seen in our sensitivity tests, because benthic fish have a systematically higher uncertainty (s.d.) in the Moto estimations than pelagic fish (with more negative δ13Cdiet and δ13Coto values; Fig. 1b). Therefore, uncertainty in δ13CDIC values contributes more to estimated Moto values of benthic or low metabolic rate fishes than to pelagic or higher metabolic rate fishes.
Similarly, we conducted sensitivity tests on the effect of δ13Cdiet uncertainty, which was set as a 1‰ variation between –18 and –19‰. δ13CDIC values were set to range between 0 and 3‰, which basically covers δ13CDIC values in the surface ocean around the world (Tagliabue and Bopp 2008). δ13Coto values ranged from 0 to –6‰. As expected, higher Moto values were accompanied by a higher uncertainty (Fig. 1c, d). However, compared with DIC, a smaller s.d. was observed from the diet sensitivity test even with the same setting of 1‰ variation. The range of δ13Cdiet values is comparable to those commonly seen in temperate and subtropical marine environments, but in coastal or freshwater ecosystems the uncertainty will be amplified according to a wider range of δ13C baseline changes. Moreover, higher uncertainty is expected in the migratory species with habitat changes (δ13CDIC variation), especially if these habitat changes infer diet shifts (δ13Cdiet variation).
To deal with the variation in both δ13CDIC and δ13Cdiet values, as well as uncertainty in ϵtotal, a Bayesian framework for isotopic mixing models offers an attractive statistical solution. This method provides the likelihood of a given Moto term determined using Bayesian methods and considering the uncertainty of the two sources in terms of δ13CDIC and δ13Cdiet variations. It also facilitates comparing metabolic performance (Moto term) between fish populations, and is easy to conduct within the well-established R software package MixSIAR (see https://github.com/brianstock/MixSIAR, accessed 21 March 2019; Stock et al. 2018).
Relationship between Moto and oxygen consumption
Although a δ13Coto metabolic proxy corresponding to fish mass-specific metabolism has been described (Dufour et al. 2007; Trueman et al. 2013, 2016; Chung 2015), there are limited studies describing the scaling of Moto values with mass-specific oxygen consumption. Here, we introduce a standard bioenergetics model to evaluate the likely relationship between Moto values and oxygen consumption. The model allocates energy intake into three compartments: metabolism, growth and waste (Treberg et al. 2016; Deslauriers et al. 2017):
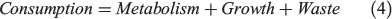
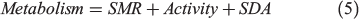
where SDA is specific dynamic action. SMR can be predicted by measuring experienced temperature and body mass of the fish according to the metabolic theory of ecology (MTE; Brown et al. 2004):
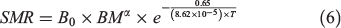
where the B0 is the normalised constant, BM is the body mass and T is temperature in kelvin; α is the allometric scaling exponent of body mass, which follows the three-quarters power law in MTE (as –0.25 for mass-specific metabolism; Brown et al. 2004) but was found to be 0.79 for teleost fishes (Clarke and Johnston 1999; Clarke 2006).
For wild-caught fishes, experienced temperature can be estimated from otolith δ18O values (e.g. Shirai et al. 2018 and references therein). Second, otolith increment analysis provides a chronological record of body mass. A lifelong history of body mass can be reconstructed from von Bertalanffy growth curves with given age inferred by the otolith increment numbers. Otherwise, it is possible to back-calculate fish body mass from fish length, obtained from otolith back-calculations (Campana 1990). Using these methods, several previous studies present expected relationships between δ13Coto or Moto values and temperature (Kalish 1991a; Høie et al. 2004a; Gao et al. 2010) and body mass (Trueman et al. 2013; Chung 2015).
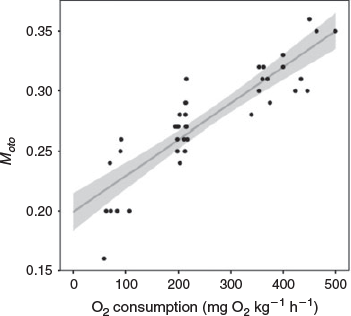
The Moto value is regarded as a proxy of FMR, corresponding to the sum of SMR, activity and SDA. To examine the relationship, we obtained Moto values as well as fish length data and the otolith δ18O values of Atlantic cod (Gadus morhua) extracted from Jamieson (2001) and Jamieson et al. (2004). Fish lengths and otolith δ18O values were used to reconstruct body mass and experienced temperature of fish, which are critical for metabolic rate estimations (Table 2). The three metabolic compartments (i.e. SMR, activity and SDA) are estimated theoretically with the body mass and temperature by Fish Bioenergetics (ver. 4.0, see http://fishbioenergetics.org, accessed 21 March 2019), a package in R programming software (Deslauriers et al. 2017). The metabolic rate of the sum of the three metabolic compartments is expressed as the mass-specific oxygen consumption rate. The Moto term increased significantly with mass-specific oxygen consumption (Fig. 2). Our regression trend indicated a positive and linear relationship between the Moto term and oxygen consumption, but gave an unrealistic Moto value (0.20) when the oxygen consumption was close to zero (Fig. 2). Considering that the Moto term is constrained by both upper (~0.5) (Table 1) and lower boundaries (0), this may imply that the relationship is not a simple linear regression (Kalish 1991a), but an exponential decay model in increasing form (Chung et al. 2019). It is critical that the relationship between Moto values and oxygen consumption should be widely investigated, especially across species. The functional form, including the upper limit of Moto values, may vary between species according to their life history traits and physiological regulations. Nevertheless, it is believed that the relationship between Moto and oxygen consumption rate among species will provide valuable information that will enhance progress in the research field of fish physiological ecology.

|
|
Further development based on the δ13Coto metabolic proxy
Knowledge of fish energy allocation between metabolic compartments (SMR, SDA and activity) may increase our understanding of their behavioural adaptation to environmental changes. The use of the otolith metabolic proxy could be instrumental in gaining this knowledge. For example, Sherwood and Rose (2003) found that δ13Coto values relate to the aspect ratios of the caudal fin of fish, which is associated with swimming form and activity. Solomon et al. (2006) further analysed these data to provide a regressed trend of Moto values with the aspect ratios of the caudal fin of fish:
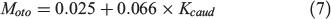
where Kcaud is the aspect ratio of the caudal fin. The relationship revealed the potential of using Moto values to evaluate activity but without a link to fish swimming speeds and oxygen consumption rate. Thus, an experimental design in which the activity level of fish is manipulated (e.g. by enforcing different swimming speeds) may give direct evidence of the effect of activity metabolism on Moto variations.
Otolith accretion and opacity are regulated by metabolic processes. The otolith annual pattern with alternating opaque and translucent bands is likely synchronised with energy acquisition and usage (Grønkjær 2016). At the microstructural level, increment widths have been found to relate linearly to SDA (Armstrong et al. 2004). As a general assumption, SDA is proportional to energy intake, and corresponds to 0.1- to 0.4-fold the total assimilated energy (Jobling 1981; Soofiani and Hawkins 1982; Kiørboe et al. 1987; Wieser and Medgyesy 1990). However, in wild fishes, it is difficult to determine SDA owing to uncertainties in meal size, feeding frequencies and postprandial durations. As an alternative, otolith increment analysis combined with the otolith metabolic proxy may make SDA determination possible. Furthermore, a modelling framework based on Dynamic Energy Budget (DEB) theory can be used to try to reconstruct individual and otolith growth history with known temperature and otolith opacity patterns (Fablet et al. 2011; Pecquerie et al. 2012). In this modelling framework, otolith growth and opacity are defined by two energy fluxes in the metabolism (i.e. maintenance and fish growth; Fablet et al. 2011). This means that the metabolic information of SMR and SDA, which is associated with maintenance and growth energy fluxes in the DEB model, can be acquired by analysing the optical properties of the otolith microstructure. Multiple approaches combining the δ13Coto metabolic proxy, otolith δ18O analyses, microstructure analyses and the DEB model hold great potential when it comes to investigating and reconstructing individual life history in response to environmental changes.
Conclusion
In this paper we have illustrated three perspectives on otolith δ13C metabolic proxy: (1) how to obtain the parameters used to estimate Moto values according to a two-component mixing model; (2) the several unanswered questions that should be considered when using the otolith metabolic proxy; and (3) the great potential of using the otolith δ13C metabolic proxy to study fish physiological ecology in combination with other valuable and well-established approaches. Despite the considerable efforts needed to acquire the necessary parameter values across species, the novel approach of the δ13Coto metabolic proxy shows great promise with regard to allowing us to track the ontogenetic and environmental effects on individual fish physiology, and thereby removes a major obstacle to understanding and predicting the performance of free-ranging wild fish.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Declaration of funding
This review paper is supported by the European Commission Marie Skłodowska-Curie Individual Fellowships (‘OTOLOG’ project, 707481) and the European Regional Development Fund (Interreg IVa, ‘MarGen’ project).
Acknowledgement
The authors thank Prof. Chia-Hui Wang, the convener of the 6th International Otolith Symposium held 15–20 April 2018, Keelung, Taiwan, for inviting us to present these ideas at the symposium.
References
Ankjærø, T., Christensen, J. T., and Grønkjær, P. (2012). Tissue-specific turnover rates and trophic enrichment of stable N and C isotopes in juvenile Atlantic cod Gadus morhua fed three different diets. Marine Ecology Progress Series 461, 197–209.| Tissue-specific turnover rates and trophic enrichment of stable N and C isotopes in juvenile Atlantic cod Gadus morhua fed three different diets.Crossref | GoogleScholarGoogle Scholar |
Armstrong, J. D., Fallon Cousins, P. S., and Wright, P. J. (2004). The relationship between specific dynamic action and otolith growth in pike. Journal of Fish Biology 64, 739–749.
| The relationship between specific dynamic action and otolith growth in pike.Crossref | GoogleScholarGoogle Scholar |
Ashford, J., and Jones, C. (2007). Oxygen and carbon stable isotopes in otoliths record spatial isolation of Patagonian toothfish (Dissostichus eleginoides). Geochimica et Cosmochimica Acta 71, 87–94.
| Oxygen and carbon stable isotopes in otoliths record spatial isolation of Patagonian toothfish (Dissostichus eleginoides).Crossref | GoogleScholarGoogle Scholar |
Augley, J., Huxham, M., Fernandes, T. F., Lyndon, A. R., and Bury, S. (2007). Carbon stable isotopes in estuarine sediments and their utility as migration markers for nursery studies in the Firth of Forth and Forth Estuary, Scotland. Estuarine, Coastal and Shelf Science 72, 648–656.
| Carbon stable isotopes in estuarine sediments and their utility as migration markers for nursery studies in the Firth of Forth and Forth Estuary, Scotland.Crossref | GoogleScholarGoogle Scholar |
Bade, D. L., Carpenter, S. R., Cole, J. J., Hanson, P. C., and Hesslein, R. H. (2004). Controls of δ13C‐DIC in lakes: geochemistry, lake metabolism, and morphometry. Limnology and Oceanography 49, 1160–1172.
| Controls of δ13C‐DIC in lakes: geochemistry, lake metabolism, and morphometry.Crossref | GoogleScholarGoogle Scholar |
Bailey, D. M., Jamieson, A. J., Bagley, P. M., Collins, M. A., and Priede, I. G. (2002). Measurement of in situ oxygen consumption of deep-sea fish using an autonomous lander vehicle. Deep-sea Research – I. Oceanographic Research Papers 49, 1519–1529.
| Measurement of in situ oxygen consumption of deep-sea fish using an autonomous lander vehicle.Crossref | GoogleScholarGoogle Scholar |
Bastow, T. P., Jackson, G., and Edmonds, J. S. (2002). Elevated salinity and isotopic composition of fish otolith carbonate: stock delineation of pink snapper, Pagrus auratus, in Shark Bay, Western Australia. Marine Biology 141, 801–806.
| Elevated salinity and isotopic composition of fish otolith carbonate: stock delineation of pink snapper, Pagrus auratus, in Shark Bay, Western Australia.Crossref | GoogleScholarGoogle Scholar |
Becker, M., Andersen, N., Erlenkeuser, H., Humphreys, M. P., Tanhua, T., and Körtzinger, A. (2016). An internally consistent dataset of δ13C-DIC in the North Atlantic Ocean – NAC13v1. Earth System Science Data 8, 559–570.
| An internally consistent dataset of δ13C-DIC in the North Atlantic Ocean – NAC13v1.Crossref | GoogleScholarGoogle Scholar |
Brown, J. H., Gillooly, J. F., Allen, A. P., Savage, V. M., and West, G. B. (2004). Toward a metabolic theory of ecology. Ecology 85, 1771–1789.
| Toward a metabolic theory of ecology.Crossref | GoogleScholarGoogle Scholar |
Campana, S. E. (1990). How reliable are growth back-calculations based on otoliths? Canadian Journal of Fisheries and Aquatic Sciences 47, 2219–2227.
| How reliable are growth back-calculations based on otoliths?Crossref | GoogleScholarGoogle Scholar |
Chabot, D., Steffensen, J. F., and Farrell, A. P. (2016). The determination of standard metabolic rate in fishes. Journal of Fish Biology 88, 81–121.
| The determination of standard metabolic rate in fishes.Crossref | GoogleScholarGoogle Scholar | 26768973PubMed |
Chung, M.-T. (2015). Functional and life-history traits in deep-sea fishes. Ph.D. Thesis, University of Southampton, Southampton, UK.
Chung, M.-T., Trueman, C. N., Godiksen, J. A., Holmstrup, M. E., and Grønkjær, P. (2019). Field metabolic rates of teleost fishes are recorded in otolith carbonate. Communications Biology 2, 24.
| Field metabolic rates of teleost fishes are recorded in otolith carbonate.Crossref | GoogleScholarGoogle Scholar | 30675522PubMed |
Clarke, A. (2006). Temperature and the metabolic theory of ecology. Functional Ecology 20, 405–412.
| Temperature and the metabolic theory of ecology.Crossref | GoogleScholarGoogle Scholar |
Clarke, A., and Johnston, N. M. (1999). Scaling of metabolic rate with body mass and temperature in teleost fish. Journal of Animal Ecology 68, 893–905.
| Scaling of metabolic rate with body mass and temperature in teleost fish.Crossref | GoogleScholarGoogle Scholar |
Correia, A. T., Barros, F., and Sial, A. N. (2011). Stock discrimination of European conger eel (Conger conger L.) using otolith stable isotope ratios. Fisheries Research 108, 88–94.
| Stock discrimination of European conger eel (Conger conger L.) using otolith stable isotope ratios.Crossref | GoogleScholarGoogle Scholar |
Currey, L. M., Heupel, M. R., Simpfendorfer, C. A., and Williams, A. J. (2014). Inferring movement patterns of a coral reef fish using oxygen and carbon isotopes in otolith carbonate. Journal of Experimental Marine Biology and Ecology 456, 18–25.
| Inferring movement patterns of a coral reef fish using oxygen and carbon isotopes in otolith carbonate.Crossref | GoogleScholarGoogle Scholar |
Deslauriers, D., Chipps, S. R., Breck, J. E., Rice, J. A., and Madenjian, C. P. (2017). Fish Bioenergetics 4.0: an R‐based modeling application. Fisheries 42, 586–596.
| Fish Bioenergetics 4.0: an R‐based modeling application.Crossref | GoogleScholarGoogle Scholar |
Dufour, E., Gerdeaux, D., and Wurster, C. M. (2007). Whitefish (Coregonus lavaretus) respiration rate governs intra-otolith variation of δ13C values in Lake Annecy. Canadian Journal of Fisheries and Aquatic Sciences 64, 1736–1746.
| Whitefish (Coregonus lavaretus) respiration rate governs intra-otolith variation of δ13C values in Lake Annecy.Crossref | GoogleScholarGoogle Scholar |
Elsdon, T. S., Ayvazian, S., McMahon, K. W., and Thorrold, S. R. (2010). Experimental evaluation of stable isotope fractionation in fish muscle and otoliths. Marine Ecology Progress Series 408, 195–205.
| Experimental evaluation of stable isotope fractionation in fish muscle and otoliths.Crossref | GoogleScholarGoogle Scholar |
Fablet, R., Pecquerie, L., de Pontual, H., Høie, H., Millner, R., Mosegaard, H., and Kooijman, S. A. L. M. (2011). Shedding light on fish otolith biomineralization using a bioenergetic approach. PLoS One 6, e27055.
| Shedding light on fish otolith biomineralization using a bioenergetic approach.Crossref | GoogleScholarGoogle Scholar | 22110601PubMed |
Farrell, A. P., Lee, C. G., Tierney, K., Hodaly, A., Clutterham, S., Healey, M., Hinch, S., and Lotto, A. (2003). Field-based measurements of oxygen uptake and swimming performance with adult Pacific salmon using a mobile respirometer swim tunnel. Journal of Fish Biology 62, 64–84.
| Field-based measurements of oxygen uptake and swimming performance with adult Pacific salmon using a mobile respirometer swim tunnel.Crossref | GoogleScholarGoogle Scholar |
Filipsson, H. L., McCorkle, D. C., Mackensen, A., Bernhard, J. M., Andersson, L. S., Naustvoll, L.-J., Caballero-Alfonso, A. M., Nordberg, K., and Danielssen, D. S. (2017). Seasonal variability of stable carbon isotopes (δ13CDIC) in the Skagerrak and the Baltic Sea: distinguishing between mixing and biological productivity. Palaeogeography, Palaeoclimatology, Palaeoecology 483, 15–30.
| Seasonal variability of stable carbon isotopes (δ13CDIC) in the Skagerrak and the Baltic Sea: distinguishing between mixing and biological productivity.Crossref | GoogleScholarGoogle Scholar |
Fraile, I., Arrizabalaga, H., Groeneveld, J., Kölling, M., Santos, M. N., Macías, D., Addis, P., Dettman, D. L., Karakulak, S., Deguara, S., and Rooker, J. R. (2016). The imprint of anthropogenic CO2 emissions on Atlantic bluefin tuna otoliths. Journal of Marine Systems 158, 26–33.
| The imprint of anthropogenic CO2 emissions on Atlantic bluefin tuna otoliths.Crossref | GoogleScholarGoogle Scholar |
Gao, Y. W., and Beamish, R. J. (1999). Isotopic composition of otoliths as a chemical tracer in population identification of sockeye salmon (Oncorhynchus nerka). Canadian Journal of Fisheries and Aquatic Sciences 56, 2062–2068.
| Isotopic composition of otoliths as a chemical tracer in population identification of sockeye salmon (Oncorhynchus nerka).Crossref | GoogleScholarGoogle Scholar |
Gao, Y. W., Joner, S. H., and Bargmann, G. G. (2001). Stable isotopic composition of otoliths in identification of spawning stocks of Pacific herring (Clupea pallasi) in Puget Sound. Canadian Journal of Fisheries and Aquatic Sciences 58, 2113–2120.
| Stable isotopic composition of otoliths in identification of spawning stocks of Pacific herring (Clupea pallasi) in Puget Sound.Crossref | GoogleScholarGoogle Scholar |
Gao, Y., Dettman, D. L., Piner, K. R., and Wallace, F. R. (2010). Isotopic correlation (δ18O versus δ13C) of otoliths in identification of groundfish stocks. Transactions of the American Fisheries Society 139, 491–501.
| Isotopic correlation (δ18O versus δ13C) of otoliths in identification of groundfish stocks.Crossref | GoogleScholarGoogle Scholar |
Gauldie, R. W. (1996). Biological factors controlling the carbon isotope record in fish otoliths: principles and evidence. Comparative Biochemistry and Physiology – B. Biochemistry & Molecular Biology 115, 201–208.
| Biological factors controlling the carbon isotope record in fish otoliths: principles and evidence.Crossref | GoogleScholarGoogle Scholar |
Gauldie, R. W., Thacker, C. E., and Merrett, N. R. (1994). Oxygen and carbon isotope variation in the otoliths of Beryx splendens and Coryphaenoides profundicolus. Comparative Biochemistry and Physiology – A. Physiology 108, 153–159.
| Oxygen and carbon isotope variation in the otoliths of Beryx splendens and Coryphaenoides profundicolus.Crossref | GoogleScholarGoogle Scholar |
Gerard, T., Malca, E., Muhling, B. A., Mateo, I., and Lamkin, J. T. (2015). Isotopic signatures in the otoliths of reef-associated fishes of southern Florida: Linkages between nursery grounds and coral reefs. Regional Studies in Marine Science 2, 95–104.
| Isotopic signatures in the otoliths of reef-associated fishes of southern Florida: Linkages between nursery grounds and coral reefs.Crossref | GoogleScholarGoogle Scholar |
Gerdeaux, D., and Dufour, E. (2015). Life history traits of the fish community in Lake Annecy: evidence from the stable isotope composition of otoliths. Knowledge and Management of Aquatic Ecosystems 416, 35.
| Life history traits of the fish community in Lake Annecy: evidence from the stable isotope composition of otoliths.Crossref | GoogleScholarGoogle Scholar |
Grønkjær, P. (2016). Otoliths as individual indicators: a reappraisal of the link between fish physiology and otolith characteristics. Marine and Freshwater Research 67, 881–888.
| Otoliths as individual indicators: a reappraisal of the link between fish physiology and otolith characteristics.Crossref | GoogleScholarGoogle Scholar |
Grønkjær, P., Pedersen, J. B., Ankjærø, T. T., Kjeldsen, H., Heinemeier, J., Steingrund, P., Nielsen, J. M., and Christensen, J. T. (2013). Stable N and C isotopes in the organic matrix of fish otoliths: validation of a new approach for studying spatial and temporal changes in the trophic structure of aquatic ecosystems. Canadian Journal of Fisheries and Aquatic Sciences 70, 143–146.
| Stable N and C isotopes in the organic matrix of fish otoliths: validation of a new approach for studying spatial and temporal changes in the trophic structure of aquatic ecosystems.Crossref | GoogleScholarGoogle Scholar |
Grossman, E. L., and Ku, T.-L. (1986). Oxygen and carbon isotope fractionation in biogenic aragonite: temperature effects. Chemical Geology. Isotope Geoscience Section 59, 59–74.
| Oxygen and carbon isotope fractionation in biogenic aragonite: temperature effects.Crossref | GoogleScholarGoogle Scholar |
Guiguer, K. R. R. A., Drimmie, R., and Power, M. (2003). Validating methods for measuring δ18O and δ13C in otoliths from freshwater fish. Rapid Communications in Mass Spectrometry 17, 463–471.
| Validating methods for measuring δ18O and δ13C in otoliths from freshwater fish.Crossref | GoogleScholarGoogle Scholar |
Hanson, N. N., Wurster, C. M., EIMF Todd, C. D. (2013). Reconstructing marine life-history strategies of wild Atlantic salmon from the stable isotope composition of otoliths. Marine Ecology Progress Series 475, 249–266.
| Reconstructing marine life-history strategies of wild Atlantic salmon from the stable isotope composition of otoliths.Crossref | GoogleScholarGoogle Scholar |
Høie, H., Folkvord, A., and Otterlei, E. (2003). Effect of somatic and otolith growth rate on stable isotopic composition of early juvenile cod (Gadus morhua L.) otoliths. Journal of Experimental Marine Biology and Ecology 289, 41–58.
| Effect of somatic and otolith growth rate on stable isotopic composition of early juvenile cod (Gadus morhua L.) otoliths.Crossref | GoogleScholarGoogle Scholar |
Høie, H., Andersson, C., Folkvord, A., and Karlsen, O. (2004a). Precision and accuracy of stable isotope signals in otoliths of pen-reared cod (Gadus morhua) when sampled with a high-resolution micromill. Marine Biology 144, 1039–1049.
| Precision and accuracy of stable isotope signals in otoliths of pen-reared cod (Gadus morhua) when sampled with a high-resolution micromill.Crossref | GoogleScholarGoogle Scholar |
Høie, H., Otterlei, E., and Folkvord, A. (2004b). Temperature-dependent fractionation of stable oxygen isotopes in otoliths of juvenile cod (Gadus morhua L.). ICES Journal of Marine Science 61, 243–251.
| Temperature-dependent fractionation of stable oxygen isotopes in otoliths of juvenile cod (Gadus morhua L.).Crossref | GoogleScholarGoogle Scholar |
Iacumin, P., Bianucci, G., and Longinelli, A. (1992). Oxygen and carbon isotopic composition of fish otoliths. Marine Biology 113, 537–542.
| Oxygen and carbon isotopic composition of fish otoliths.Crossref | GoogleScholarGoogle Scholar |
Jamieson, R. E. (2001). Environmental history of northern cod from otolith isotopic analysis. Ph.D. Thesis, McMaster University, Hamilton, ON, Canada.
Jamieson, R. E., Schwarcz, H. P., and Brattey, J. (2004). Carbon isotopic records from the otoliths of Atlantic cod (Gadus morhua) from eastern Newfoundland, Canada. Fisheries Research 68, 83–97.
| Carbon isotopic records from the otoliths of Atlantic cod (Gadus morhua) from eastern Newfoundland, Canada.Crossref | GoogleScholarGoogle Scholar |
Javor, B., and Dorval, E. (2014). Geography and ontogeny influence the stable oxygen and carbon isotopes of otoliths of Pacific sardine in the California Current. Fisheries Research 154, 1–10.
| Geography and ontogeny influence the stable oxygen and carbon isotopes of otoliths of Pacific sardine in the California Current.Crossref | GoogleScholarGoogle Scholar |
Jobling, M. (1981). The influences of feeding on the metabolic rate of fishes: a short review. Journal of Fish Biology 18, 385–400.
| The influences of feeding on the metabolic rate of fishes: a short review.Crossref | GoogleScholarGoogle Scholar |
Kalish, J. M. (1991a). 13C and 18O isotopic disequilibria in fish otoliths: metabolic and kinetic effects. Marine Ecology Progress Series 75, 191–203.
| 13C and 18O isotopic disequilibria in fish otoliths: metabolic and kinetic effects.Crossref | GoogleScholarGoogle Scholar |
Kalish, J. M. (1991b). Oxygen and carbon stable isotopes in the otoliths of wild and laboratory-reared Australian salmon (Arripis trutta). Marine Biology 110, 37–47.
| Oxygen and carbon stable isotopes in the otoliths of wild and laboratory-reared Australian salmon (Arripis trutta).Crossref | GoogleScholarGoogle Scholar |
Killen, S. S., Costa, I., Brown, J. A., and Gamperl, A. K. (2007). Little left in the tank: metabolic scaling in marine teleosts and its implications for aerobic scope. Proceedings of the Royal Society of London – B. Biological Sciences 274, 431–438.
| Little left in the tank: metabolic scaling in marine teleosts and its implications for aerobic scope.Crossref | GoogleScholarGoogle Scholar |
Killen, S. S., Atkinson, D., and Glazier, D. S. (2010). The intraspecific scaling of metabolic rate with body mass in fishes depends on lifestyle and temperature. Ecology Letters 13, 184–193.
| The intraspecific scaling of metabolic rate with body mass in fishes depends on lifestyle and temperature.Crossref | GoogleScholarGoogle Scholar | 20059525PubMed |
Kimirei, I. A., Nagelkerken, I., Mgaya, Y. D., and Huijbers, C. M. (2013). The mangrove nursery paradigm revisited: otolith stable isotopes support nursery-to-reef movements by Indo-Pacific fishes. PLoS One 8, e66320.
| The mangrove nursery paradigm revisited: otolith stable isotopes support nursery-to-reef movements by Indo-Pacific fishes.Crossref | GoogleScholarGoogle Scholar | 23776658PubMed |
Kiørboe, T., Munk, P., and Richardson, K. (1987). Respiration and growth of larval herring Clupea harengus: relation between specific dynamic action and growth efficiency. Marine Ecology Progress Series 40, 1–10.
| Respiration and growth of larval herring Clupea harengus: relation between specific dynamic action and growth efficiency.Crossref | GoogleScholarGoogle Scholar |
Kroopnick, P. M. (1985). The distribution of 13C of ΣCO2 in the world oceans. Deep-Sea Research – A. Oceanographic Research Papers 32, 57–84.
| The distribution of 13C of ΣCO2 in the world oceans.Crossref | GoogleScholarGoogle Scholar |
Martino, J. C., Doubleday, Z. A., and Gillanders, B. M. (2019). Metabolic effects on carbon isotope biomarkers in fish. Ecological Indicators 97, 10–16.
| Metabolic effects on carbon isotope biomarkers in fish.Crossref | GoogleScholarGoogle Scholar |
McMahon, K. W., Hamady, L. L., and Thorrold, S. R. (2013). Ocean ecogeochemistry: a review. Oceanography and Marine Biology – an Annual Review 51, 321–373.
Metcalfe, N. B., Van Leeuwen, T. E., and Killen, S. S. (2016). Does individual variation in metabolic phenotype predict fish behaviour and performance? Journal of Fish Biology 88, 298–321.
| Does individual variation in metabolic phenotype predict fish behaviour and performance?Crossref | GoogleScholarGoogle Scholar | 26577442PubMed |
Nelson, J., Hanson, C. W., Koenig, C., and Chanton, J. (2011). Influence of diet on stable carbon isotope composition in otoliths of juvenile red drum Sciaenops ocellatus. Aquatic Biology 13, 89–95.
| Influence of diet on stable carbon isotope composition in otoliths of juvenile red drum Sciaenops ocellatus.Crossref | GoogleScholarGoogle Scholar |
Nonogaki, H., Nelson, J. A., and Patterson, W. P. (2006). Dietary histories of herbivorous loricariid catfishes: evidence from δ13C values of otoliths. Environmental Biology of Fishes 78, 13–21.
| Dietary histories of herbivorous loricariid catfishes: evidence from δ13C values of otoliths.Crossref | GoogleScholarGoogle Scholar |
Pecquerie, L., Fablet, R., De Pontual, H., Bonhommeau, S., Alunno-Bruscia, M., Petitgas, P., and Kooijman, S. (2012). Reconstructing individual food and growth histories from biogenic carbonates. Marine Ecology Progress Series 447, 151–164.
| Reconstructing individual food and growth histories from biogenic carbonates.Crossref | GoogleScholarGoogle Scholar |
Post, D. M. (2002). Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology 83, 703–718.
| Using stable isotopes to estimate trophic position: models, methods, and assumptions.Crossref | GoogleScholarGoogle Scholar |
Radtke, R. L. (1984). Formation and structural composition of larval striped mullet otoliths. Transactions of the American Fisheries Society 113, 186–191.
| Formation and structural composition of larval striped mullet otoliths.Crossref | GoogleScholarGoogle Scholar |
Romanek, C. S., Grossman, E. L., and Morse, J. W. (1992). Carbon isotopic fractionation in synthetic aragonite and calcite: effects of temperature and precipitation rate. Geochimica et Cosmochimica Acta 56, 419–430.
| Carbon isotopic fractionation in synthetic aragonite and calcite: effects of temperature and precipitation rate.Crossref | GoogleScholarGoogle Scholar |
Schloesser, R. W., Rooker, J. R., Louchuoarn, P., Neilson, J. D., and Secord, D. H. (2009). Interdecadal variation in seawater δ13C and δ18O recorded in fish otoliths. Limnology and Oceanography 54, 1665–1668.
| Interdecadal variation in seawater δ13C and δ18O recorded in fish otoliths.Crossref | GoogleScholarGoogle Scholar |
Schmittner, A., Gruber, N., Mix, A. C., Key, R. M., Tagliabue, A., and Westberry, T. K. (2013). Biology and air–sea gas exchange controls on the distribution of carbon isotope ratios (δ13C) in the ocean. Biogeosciences 10, 5793–5816.
| Biology and air–sea gas exchange controls on the distribution of carbon isotope ratios (δ13C) in the ocean.Crossref | GoogleScholarGoogle Scholar |
Schwarcz, H. P., Gao, Y., Campana, S., Browne, D., Knyf, M., and Brand, U. (1998). Stable carbon isotope variations in otoliths of Atlantic cod (Gadus morhua). Canadian Journal of Fisheries and Aquatic Sciences 55, 1798–1806.
| Stable carbon isotope variations in otoliths of Atlantic cod (Gadus morhua).Crossref | GoogleScholarGoogle Scholar |
Shen, J., and Gao, Y. (2012). Stable isotope analyses in otoliths of silver carp: a pilot study in identification of natal sources and stock differences. Environmental Biology of Fishes 95, 445–453.
| Stable isotope analyses in otoliths of silver carp: a pilot study in identification of natal sources and stock differences.Crossref | GoogleScholarGoogle Scholar |
Shephard, S., Trueman, C., Rickaby, R., and Rogan, E. (2007). Juvenile life history of NE Atlantic orange roughy from otolith stable isotopes. Deep-sea Research – I. Oceanographic Research Papers 54, 1221–1230.
| Juvenile life history of NE Atlantic orange roughy from otolith stable isotopes.Crossref | GoogleScholarGoogle Scholar |
Sherwood, G. D., and Rose, G. A. (2003). Influence of swimming form on otolith δ13C in marine fish. Marine Ecology Progress Series 258, 283–289.
| Influence of swimming form on otolith δ13C in marine fish.Crossref | GoogleScholarGoogle Scholar |
Sherwood, G. D., and Rose, G. A. (2005). Stable isotope analysis of some representative fish and invertebrates of the Newfoundland and Labrador continental shelf food web. Estuarine, Coastal and Shelf Science 63, 537–549.
| Stable isotope analysis of some representative fish and invertebrates of the Newfoundland and Labrador continental shelf food web.Crossref | GoogleScholarGoogle Scholar |
Shirai, K., Otake, T., Amano, Y., Kuroki, M., Ushikubo, T., Kita, N. T., Murayama, M., Tsukamoto, K., and Valley, J. W. (2018). Temperature and depth distribution of Japanese eel eggs estimated using otolith oxygen stable isotopes. Geochimica et Cosmochimica Acta 236, 373–383.
| Temperature and depth distribution of Japanese eel eggs estimated using otolith oxygen stable isotopes.Crossref | GoogleScholarGoogle Scholar |
Sirot, C., Grønkjær, P., Pedersen, J. B., Panfili, J., Zetina-Rejon, M., Tripp-Valdez, A., Ramos-Miranda, J., Flores-Hernandez, D., Sosa-Lopez, A., and Darnaude, A. M. (2017). Using otolith organic matter to detect diet shifts in Bardiella chrysoura, during a period of environmental changes. Marine Ecology Progress Series 575, 137–152.
| Using otolith organic matter to detect diet shifts in Bardiella chrysoura, during a period of environmental changes.Crossref | GoogleScholarGoogle Scholar |
Solomon, C. T., Weber, P. K., Cech, J. J., Ingram, B. L., Conrad, M. E., Machavaram, M. V., Pogodina, A. R., and Franklin, R. L. (2006). Experimental determination of the sources of otolith carbon and associated isotopic fractionation. Canadian Journal of Fisheries and Aquatic Sciences 63, 79–89.
| Experimental determination of the sources of otolith carbon and associated isotopic fractionation.Crossref | GoogleScholarGoogle Scholar |
Soofiani, N. M., and Hawkins, A. D. (1982). Energetic costs at different levels of feeding in juvenile cod, Gadus morhua L. Journal of Fish Biology 21, 577–592.
| Energetic costs at different levels of feeding in juvenile cod, Gadus morhua L.Crossref | GoogleScholarGoogle Scholar |
Stock, B. C., Jackson, A. L., Ward, E. J., Parnell, A. C., Phillips, D. L., and Semmens, B. X. (2018). Analyzing mixing systems using a new generation of Bayesian tracer mixing models. PeerJ 6, e5096.
| Analyzing mixing systems using a new generation of Bayesian tracer mixing models.Crossref | GoogleScholarGoogle Scholar | 29942712PubMed |
Sweeting, C. J., Barry, J. T., Polunin, N. V. C., and Jennings, S. (2007). Effects of body size and environment on diet–tissue δ13C fractionation in fishes. Journal of Experimental Marine Biology and Ecology 352, 165–176.
| Effects of body size and environment on diet–tissue δ13C fractionation in fishes.Crossref | GoogleScholarGoogle Scholar |
Tagliabue, A., and Bopp, L. (2008). Towards understanding global variability in ocean carbon‐13. Global Biogeochemical Cycles 22, GB1025.
| Towards understanding global variability in ocean carbon‐13.Crossref | GoogleScholarGoogle Scholar |
Telmer, K., and Veizer, J. (1999). Carbon fluxes, pCO2 and substrate weathering in a large northern river basin, Canada: carbon isotope perspectives. Chemical Geology 159, 61–86.
| Carbon fluxes, pCO2 and substrate weathering in a large northern river basin, Canada: carbon isotope perspectives.Crossref | GoogleScholarGoogle Scholar |
Thorrold, S. R., Campana, S. E., Jones, C. M., and Swart, P. K. (1997). Factors determining δ13C and δ18O fractionation in aragonitic otoliths of marine fish. Geochimica et Cosmochimica Acta 61, 2909–2919.
| Factors determining δ13C and δ18O fractionation in aragonitic otoliths of marine fish.Crossref | GoogleScholarGoogle Scholar |
Tohse, H., and Mugiya, Y. (2008). Sources of otolith carbonate: experimental determination of carbon incorporation rates from water and metabolic CO2, and their diel variations. Aquatic Biology 1, 259–268.
| Sources of otolith carbonate: experimental determination of carbon incorporation rates from water and metabolic CO2, and their diel variations.Crossref | GoogleScholarGoogle Scholar |
Treberg, J. R., Killen, S. S., MacCormack, T. J., Lamarre, S. G., and Enders, E. C. (2016). Estimates of metabolic rate and major constituents of metabolic demand in fishes under field conditions: methods, proxies, and new perspectives. Comparative Biochemistry and Physiology – A. Molecular & Integrative Physiology 202, 10–22.
| Estimates of metabolic rate and major constituents of metabolic demand in fishes under field conditions: methods, proxies, and new perspectives.Crossref | GoogleScholarGoogle Scholar |
Trueman, C. N., Rickaby, R., and Shephard, S. (2013). Thermal, trophic and metabolic life histories of inaccessible fishes revealed from stable-isotope analyses: a case study using orange roughy Hoplostethus atlanticus. Journal of Fish Biology 83, 1613–1636.
| Thermal, trophic and metabolic life histories of inaccessible fishes revealed from stable-isotope analyses: a case study using orange roughy Hoplostethus atlanticus.Crossref | GoogleScholarGoogle Scholar | 24298954PubMed |
Trueman, C. N., Chung, M.-T., and Shores, D. (2016). Ecogeochemistry potential in deep time biodiversity illustrated using a modern deep-water case study. Philosophical Transactions of the Royal Society of London – B. Biological Sciences 371, 20150223.
| Ecogeochemistry potential in deep time biodiversity illustrated using a modern deep-water case study.Crossref | GoogleScholarGoogle Scholar | 26977063PubMed |
von Biela, V. R., Newsome, S. D., and Zimmerman, C. E. (2015). Examining the utility of bulk otolith δ13C to describe diet in wild-caught black rockfish Sebastes melanops. Aquatic Biology 23, 201–208.
| Examining the utility of bulk otolith δ13C to describe diet in wild-caught black rockfish Sebastes melanops.Crossref | GoogleScholarGoogle Scholar |
Weidel, B. C., Ushikubo, T., Carpenter, S. R., Kita, N. T., Cole, J. J., Kitchell, J. F., Pace, M. L., and Valley, J. W. (2007). Diary of a bluegill (Lepomis macrochirus): daily δ13C and δ18O records in otoliths by ion microprobe. Canadian Journal of Fisheries and Aquatic Sciences 64, 1641–1645.
| Diary of a bluegill (Lepomis macrochirus): daily δ13C and δ18O records in otoliths by ion microprobe.Crossref | GoogleScholarGoogle Scholar |
Weidman, C. R., and Millner, R. (2000). High-resolution stable isotope records from North Atlantic cod. Fisheries Research 46, 327–342.
| High-resolution stable isotope records from North Atlantic cod.Crossref | GoogleScholarGoogle Scholar |
Wieser, W., and Medgyesy, N. (1990). Cost and efficiency of growth in the larvae of two species of fish with widely differing metabolic rates. Proceedings of the Royal Society of London – B. Biological Sciences 242, 51–56.
| Cost and efficiency of growth in the larvae of two species of fish with widely differing metabolic rates.Crossref | GoogleScholarGoogle Scholar |
Wurster, C. M., and Patterson, W. P. (2003). Metabolic rate of late Holocene freshwater fish: evidence from δ13C values of otoliths. Paleobiology 29, 492–505.
| Metabolic rate of late Holocene freshwater fish: evidence from δ13C values of otoliths.Crossref | GoogleScholarGoogle Scholar |
Wurster, C. M., Patterson, W. P., Stewart, D. J., Bowlby, J. N., and Stewart, T. J. (2005). Thermal histories, stress, and metabolic rates of Chinook salmon (Oncorhynchus tshawytscha) in Lake Ontario: evidence from intra-otolith stable isotope analyses. Canadian Journal of Fisheries and Aquatic Sciences 62, 700–713.
| Thermal histories, stress, and metabolic rates of Chinook salmon (Oncorhynchus tshawytscha) in Lake Ontario: evidence from intra-otolith stable isotope analyses.Crossref | GoogleScholarGoogle Scholar |



