Global, regional and local surveillance of antimicrobial resistance in Neisseria gonorrhoeae
Monica M. Lahra A B * , C. Robert R. George C and Sebastiaan J. van Hal D EA
B
C
D
E

Prof. Monica Lahra is a senior staff specialist in microbiology at New South Wales Health Pathology (NSWHP) at the Prince of Wales Hospital, and a conjoint professor at the University of New South Wales. She is the Director of the World Health Organization (WHO) Collaborating Centre for Sexually Transmitted Infections and Antimicrobial Resistance (WHO CC for STI and AMR), based at NSWHP in Randwick. She leads a team responsible for surveillance of pathogenic Neisseria species nationally and internationally and works with the WHO and partner WHO Collaborating Centres on the Enhanced Gonococcal Surveillance Programme (EGASP). |

Dr Robert George is a senior staff specialist in microbiology at NSWHP at the John Hunter Hospital, NSW. He has worked with the WHO CC for STI and AMR and with the WHO Antimicrobial Resistance (AMR) Network on a project mapping stakeholders and activities focusing on AMR and antimicrobial use (AMU) surveillance. He completed a PhD at The University of Queensland, where he worked on spatial modelling and, in 2017, was awarded a Churchill Fellowship to investigate keystone AMR surveillance initiatives of benefit to Australia. |

Prof. Sebastiaan van Hal is a senior staff specialist in infectious diseases and microbiology at the Royal Prince Alfred Hospital (RPAH), and a clinical professor at the University of Sydney. He is the current NSWHP microbiology clinical stream lead and is on the executive of Australian Group of Antimicrobial Resistance. He has been instrumental in setting up a National Association of Testing Authorities-accredited pathogen genomics service at RPAH and is the current chair of the Pathogen Genomics Committee. |
Abstract
Neisseria gonorrhoeae disease control strategies rely almost entirely on effective antibiotic treatment. Antimicrobial resistance (AMR) makes the future of gonococcal treatment uncertain. With the rapid evolution of gonococcal AMR, and a largely unmapped AMR landscape globally, it is very evident why concerns have been raised at the highest level. Urgent actions have been undertaken for better surveillance, new treatments and improved prevention. Most critical, however, is the need for a vaccine for disease prevention and control.
Keywords: AMR, antimicrobial resistance, ceftriaxone, extended-spectrum cephalosporin, Neisseria gonorrhoeae, surveillance.
Introduction
Current disease strategies for the control of Neisseria gonorrhoeae rely almost entirely on effective antibiotic treatment. Given the accelerating emergence of antimicrobial resistance, in the absence of an effective vaccine the future of gonococcal treatment is uncertain. Globally, the extended-spectrum cephalosporin, ceftriaxone, is near-universally relied upon for the treatment of gonococcal infections. However, ceftriaxone resistance continues to emerge, and has been recently reported in pockets within the Asia-Pacific region in very high proportions.1–4 New drugs are undergoing clinical trials, but it is anticipated their lifespan will likely be limited. Notably, with every new class of antimicrobial introduced to combat N. gonorrhoeae, resistance has developed within a matter of years.5 Concurrently, disease numbers continue to rise; the World Health Organization (WHO) estimates in 2020 alone, 82.4 million new cases of gonorrhoea occurred in adults and adolescents aged 15–49 years of age.6 Disease burden varies by geographic region, but the highest rates are estimated in the WHO regions of the Western Pacific and Africa. Complications of untreated or inadequately treated gonococcal infections disproportionately affect women; these have a significant effect on sexual, reproductive and perinatal health, and increase the risk of human immunodeficiency virus (HIV) acquisition.7 The WHO continues to develop strategies and systems to address these high-level concerns.
Global strategies
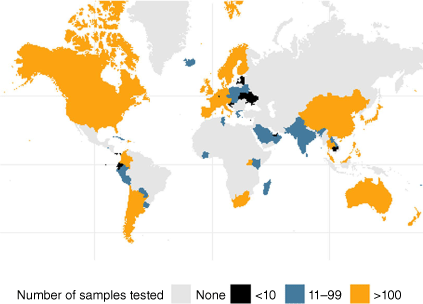
The WHO global health sector strategy for 2022–2030 on HIV, hepatitis and sexually transmitted infections (STIs) guides the health sector with regard to the implementation of strategically focused responses to these infections.8 The strategies recommend shared and disease-specific country actions, supported by actions by WHO and partners.8 Within this framework, the WHO is focussing work on developing global targets, norms and standards for STI prevention; testing and treatment; supporting the estimation and economic burden of STIs and the strengthening of STI surveillance; globally monitoring antimicrobial resistance (AMR) to gonorrhoea; and setting the global research agenda, including the development of diagnostic tests, vaccines and additional drugs for gonorrhoea. Globally, the monitoring of gonococcal AMR remains a particular challenge given regions with the highest rates of disease frequently have the least available surveillance data (Fig. 1).7
Global, regional and local gonococcal AMR surveillance systems
The WHO established the global Gonococcal Antimicrobial Surveillance Programme (GASP) in 1990.9 This worldwide laboratory network is coordinated by focal points and regional coordinating centres.10 Each designated regional focal point, in partnership with its WHO regional office, collates data on patterns of antimicrobial susceptibility in gonorrhoea in participating countries and reports these data to the WHO. The Western Pacific region GASP focal point is located in Sydney, NSW, Australia: WHO Collaborating Centre for Sexually Transmitted Infections and Antimicrobial Resistance (WHO CC for STI and AMR). The aims of the GASP include ensuring adequate sentinel surveillance of gonococcal AMR in order to inform treatment guidelines in all countries and to establish a strategy to rapidly detect patients who experience clinical or microbiological gonorrhoea treatment failure following recommended therapy.10
In 2009, the GASP was strengthened and expanded to all the WHO regions. The GASP works with international and national surveillance programmes for gonococcal AMR in Europe, the USA, Canada, Brazil, the UK and Australia.7 The most recent and largest gonococcal AMR study is the 2021 GASP report comprising data from 73 member states from 2017 to 2018; of these, 61 countries reported data to the GASP and 14 to the WHO’s Global Antimicrobial Resistance and Use Surveillance System (GLASS).7 The key findings regarding AMR globally were high rates of ciprofloxacin resistance, increasing azithromycin resistance, and that resistance to ceftriaxone and cefixime continues to emerge. However, although both the number of countries reporting data and the number of examined isolates have substantially increased compared with the GASP’s two earlier studies, the challenges of gonococcal AMR surveillance remain evident. Specific concerns include the low number of isolates reported by some member states, not meeting the WHO-recommended 100 per year.7 Based on these data, less than 1% infections have antimicrobial susceptibility testing, indicating the limits of the lens on the global situation.7 In May 2024, the WHO published a report on progress and gaps of implementing the global health sector strategies on HIV, viral hepatitis and STIs for 2022–2030.11 This report found that in high-income settings with strong surveillance systems, case notification rates for gonorrhoea have been increasing, and that nine countries reported decreased susceptibility to ceftriaxone at or greater than 5%.11
In 2015, the WHO launched the Enhanced Gonococcal Antimicrobial Surveillance Programme (EGASP) to address limitations and strengthen sentinel surveillance for gonococcal AMR in selected countries.12 Falling under the WHO’s GLASS umbrella, the EGASP has been and continues to be developed through collaboration between the WHO, the US Centers for Disease Control and Prevention (CDC) and WHO Collaborating Centres in Orebro, Sweden, and Sydney, Australia. Participant EGASP countries utilise standardised sampling and laboratory protocols to monitor gonococcal AMR. The EGASP approach facilitates countries to improve the quality, comparability and timeliness of gonococcal AMR data. The EGASP also enhances capacity for early detection and reporting of N. gonorrhoeae strains with pre-determined alert level minimum inhibitory concentration (MIC) values.9 In addition to strengthening AMR surveillance, implementing the EGASP will strengthen capacity for improving the epidemiological and surveillance data of sexually transmitted infections and AMR.9
In May 2015, the 68th World Health Assembly adopted the Global Action Plan on Antimicrobial Resistance (GAP-AMR).13 Then the WHO launched the GLASS in October,14 the first global collaborative effort to enable the collection of standardised, comparable and validated AMR data for analysis and global sharing. The aims of the GLASS included collating data to inform decision making, drive local, national and regional action, and provide the evidence base for both action and advocacy.14 For the first iteration of the GLASS, the WHO identified eight priority bacterial pathogens posing the greatest threat to human health, among these being antibiotic-resistant N. gonorrhoeae.15 Critically, gonococcal AMR data are limited globally, primarily due to lack of access to laboratory testing in low-middle income settings. The GLASS supports the WHO’s GAP-AMR. Because N. gonorrhoeae is a priority pathogen for the WHO’s GLASS, both GASP and GLASS methodology are aligned and data collection is synergised.9
The need for a national, representative gonococcal AMR surveillance was recognised in Australia in the 1970s, and, through collaboration in the scientific and medical community across the jurisdictions, a network of Neisseria reference laboratories was established. This network is known as the National Neisseria Network (NNN). The first programme of the NNN was the Australian Gonococcal Surveillance Programme (AGSP). Supported by the Australian Government, the AGSP was coordinated by the WHO CC for STI and AMR in Sydney and is also the regional focal point for the GASP. The AGSP has continuously provided national gonococcal AMR reports quarterly and annually since 1981. The NNN laboratories currently test and report on more than 10,000 gonococcal clinical samples per year. This equates to 25% of all infecting strains nationally, annually with the remainder diagnosed by nucleic acid amplification testing.
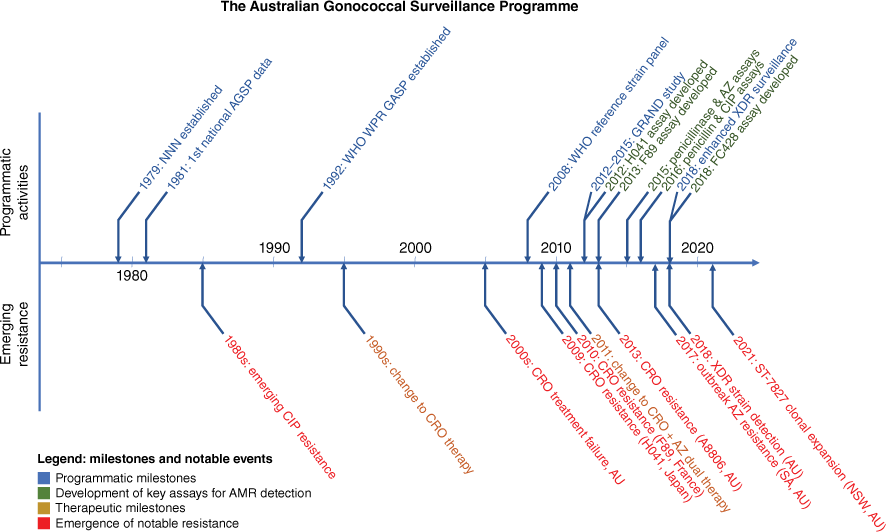
For quality practices, the NNN coordinating laboratory, the WHO CC for STI and AMR in Sydney, developed a panel of isolates for quality control and quality assurance that has been taken up by the WHO as the control strain panel, updated in 2016 and 2024.16,17 Since the establishment of the NNN, the WHO CC for STI and AMR in Sydney has provided a programme-specific quality-assurance panel annually to NNN laboratories as well as to GASP laboratories in a number of WHO regions. Since establishment, the AGSP has provided data to inform gonococcal treatment guidelines and worked to develop molecular detection strategies by documenting emergent AMR in circulating N. gonorrhoeae strains in the Australian setting, and monitoring national AMR trends.18 Fig. 2 summarises the NNN programmatic milestones, development of key assays for AMR detection by the NNN, therapeutic milestones and the emergence of notable resistances. The AGSP data have been submitted annually to the WHO for GASP since 1990, and were the first Australian data submitted to the WHO’s GLASS in 2019.18
The Australian Gonococcal Surveillance Programme 1979–2024, programmatic milestones, development of key assays for AMR detection by the NNN, therapeutic milestones, and the emergence of notable resistances. Abbreviations: AMR, antimicrobial resistance; AGSP, Australian Gonococcal Surveillance Programme; GASP, Gonococcal Antimicrobial Surveillance Programme; GRAND, Gonorrhoea Resistance Assessment by Nucleic Acid Detection; NNN, National Neisseria Network; WHO, World Health Organization; AZ, azithromycin; CIP, oprofloxacin; CRO, cefttiaxone; WPR, Western Pacific region; AU, Australia; NSW, New South Wales; SA, South Australia.
With the rapid evolution of gonococcal AMR and a largely unmapped AMR landscape globally, it is very evident why concerns have been raised at the highest level. Urgent actions have been undertaken for better surveillance, new treatments and improved prevention. Most critical, however, is the need for a vaccine, and emerging evidence suggests that the licensed meningococcal B vaccine, 4CMenB, may prevent N. gonorrhoeae infections.19 The efficacy of this vaccine is considered to be secondary to the production of cross-reacting antibodies, against shared antigenic epitopes between the pathogenic Neisseria spp.: N. meningitidis and N. gonorrhoeae. Studies to evaluate the long-term impact and effectiveness of vaccines against gonococcal infections are needed for the future of gonococcal disease control.
References
1 Ouk V et al. (2023) The Enhanced Gonococcal Surveillance Programme, Cambodia. Lancet Infect Dis 23, e332-e333.
| Crossref | Google Scholar | PubMed |
2 Ouk V et al. (2024) World Health Organization Enhanced Gonococcal Antimicrobial Surveillance Programme, Cambodia, 2023. Emerg Infect Dis 30, 1493-1495.
| Crossref | Google Scholar | PubMed |
3 Adamson PC et al. (2024) Ceftriaxone resistance in Neisseria gonorrhoeae associated with the penA-60.001 allele in Hanoi, Viet Nam. Lancet Infect Dis 24, e351-e352 PMCID: PMC11145020.
| Crossref | Google Scholar | PubMed |
4 Zhu X (2024) Ceftriaxone-resistant gonorrhea — China, 2022. MMWR Morb Mortal Wkly Rep 73, 255-259.
| Crossref | Google Scholar | PubMed |
5 Lahra MM et al. (2016) From zero to zero in 100 years: gonococcal antimicrobial resistance. Microbiol Aust 37, 173-176.
| Crossref | Google Scholar |
6 World Health Organization (2024) Multi-drug resistant gonorrhoea. WHO, Geneva, Switzerland. https://www.who.int/news-room/fact-sheets/detail/multi-drug-resistant-gonorrhoea
7 Unemo M et al. (2021) WHO global antimicrobial resistance surveillance for Neisseria gonorrhoeae 2017-18: a retrospective observational study. Lancet Microbe 2, e627-e636.
| Crossref | Google Scholar | PubMed |
8 World Health Organization (2022) Global health sector strategies on, respectively, HIV, viral hepatitis and sexually transmitted infections for the period 2022–2030. WHO, Geneva, Switzerland. https://iris.who.int/bitstream/handle/10665/360348/9789240053779-eng.pdf?sequence=1
9 World Health Organization (2021) Enhanced Gonococcal Antimicrobial Surveillance Programme (EGASP): general protocol. World Health Organization, Geneva, Switzerland. https://iris.who.int/bitstream/handle/10665/341333/9789240021341-eng.pdf?sequence=1
10 World Health Organization (2021) The Gonococcal Antimicrobial Surveillance Programme (GASP). WHO, Geneva, Switzerland. https://www.who.int/initiatives/gonococcal-antimicrobial-surveillance-programme
11 World Health Organization (2024) Implementing the global health sector strategies on HIV, viral hepatitis and sexually transmitted infections, 2022–2030: report on progress and gaps 2024. WHO, Geneva, Switzerland. https://iris.who.int/bitstream/handle/10665/376814/9789240094925-eng.pdf
12 Sirivongrangson P et al. (2018) The first year of the global Enhanced Gonococcal Antimicrobial Surveillance Programme (EGASP) in Bangkok, Thailand, 2015–2016. PLoS One 13, e0206419 PMCID: PMC6226150.
| Crossref | Google Scholar | PubMed |
13 World Health Organization (2015) Antimicrobial resistance. Draft global action plan on antimicrobial resistance. Report by the Secretariat, Sixty-Eighth World Health Assembly, A68/20, provisional agenda item 15.1. WHO, Geneva, Switzerland. https://apps.who.int/gb/ebwha/pdf_files/WHA68/A68_20-en.pdf?ua=1
14 World Health Organization (2015) Global antimicrobial resistance surveillance system: manual for early implementation. WHO, Geneva, Switzerland. https://iris.who.int/bitstream/handle/10665/188783/9789241549400_eng.pdf
15 World Health Organization (2017) WHO publishes list of bacteria for which new antibiotics are urgently needed. WHO, Geneva, Switzerland. https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed
16 Unemo M et al. (2016) The novel 2016 WHO Neisseria gonorrhoeae reference strains for global quality assurance of laboratory investigations: phenotypic, genetic and reference genome characterization. J Antimicrob Chemother 71, 3096-3108 PMCID: PMC5079299.
| Crossref | Google Scholar | PubMed |
17 Unemo M et al. (2024) The novel 2024 WHO Neisseria gonorrhoeae reference strains for global quality assurance of laboratory investigations and superseded WHO N. gonorrhoeae reference strains – phenotypic, genetic and reference genome characterization. J Antimicrob Chemother dkae176.
| Crossref | Google Scholar | PubMed |
18 Lahra MM et al. (2017) The Australian Gonococcal Surveillance Programme 1979–2017. Microbiol Aust 38, 175-179.
| Crossref | Google Scholar |
19 Marshall H et al. (2024) Comprehensive observational study evaluating the enduring effectiveness of 4CMenB, the meningococcal B vaccine against gonococcal infections in the Northern Territory and South Australia, Australia: study protocol. BMJ Open 14, e079144 PMCID: PMC11086485.
| Crossref | Google Scholar | PubMed |
 Prof. Monica Lahra is a senior staff specialist in microbiology at New South Wales Health Pathology (NSWHP) at the Prince of Wales Hospital, and a conjoint professor at the University of New South Wales. She is the Director of the World Health Organization (WHO) Collaborating Centre for Sexually Transmitted Infections and Antimicrobial Resistance (WHO CC for STI and AMR), based at NSWHP in Randwick. She leads a team responsible for surveillance of pathogenic Neisseria species nationally and internationally and works with the WHO and partner WHO Collaborating Centres on the Enhanced Gonococcal Surveillance Programme (EGASP). |
 Dr Robert George is a senior staff specialist in microbiology at NSWHP at the John Hunter Hospital, NSW. He has worked with the WHO CC for STI and AMR and with the WHO Antimicrobial Resistance (AMR) Network on a project mapping stakeholders and activities focusing on AMR and antimicrobial use (AMU) surveillance. He completed a PhD at The University of Queensland, where he worked on spatial modelling and, in 2017, was awarded a Churchill Fellowship to investigate keystone AMR surveillance initiatives of benefit to Australia. |
 Prof. Sebastiaan van Hal is a senior staff specialist in infectious diseases and microbiology at the Royal Prince Alfred Hospital (RPAH), and a clinical professor at the University of Sydney. He is the current NSWHP microbiology clinical stream lead and is on the executive of Australian Group of Antimicrobial Resistance. He has been instrumental in setting up a National Association of Testing Authorities-accredited pathogen genomics service at RPAH and is the current chair of the Pathogen Genomics Committee. |