Syphilis point-of-care tests: an Australian perspective
Gladymar Pérez Chacón A # , Amit Saha A # , Emily Phillips A , Rebecca Guy A , Tanya L. Applegate A , Louise Causer A , Skye McGregor A and Belinda Hengel A *A

Dr Gladymar Pérez Chacón is an early career researcher in public health and a medical epidemiologist based at The Kirby Institute, UNSW. Gladymar has professional experience in point-of-care (POC) testing and mass-treatment strategies for yaws in resource-constrained settings in the Asia–Pacific Region. Her research interests are broad and include pertussis and other vaccine preventable diseases as well as sexually and vertically transmitted infections. |

Dr Amit Saha is a medical epidemiologist and lecturer at The Kirby Institute, UNSW. His current work focuses on POC diagnostics for sexually transmitted infections (STIs) and respiratory infections and their impact in high-prevalence remote settings. He has professional experience in various fields of infectious disease epidemiology and large field-based clinical studies on enteric vaccines. |

Emily Phillips, BMedSci(Hons) is completing a Masters of Applied Epidemiology. Emily manages a large-scale research programme that aims to scale up infectious disease POC testing in remote Indigenous communities to enable person-centred care and improve health outcomes for Aboriginal and Torres Strait Islander peoples. Previously, Emily held the position of director of communicable diseases at the National Aboriginal Community Controlled Health Organisation, where she played a pivotal role in the Aboriginal and Torres Strait Islander response to the COVID-19 pandemic. |

Prof. Rebecca Guy is the head of the Surveillance Evaluation and Research Programme at The Kirby Institute, UNSW. She is a member of the Institute’s Executive and Aboriginal and Torres Strait Islander Committees. Prof. Guy specialises in epidemiology, implementation research, health services and translational research. Her public health research aims to reduce the impact of infectious diseases in vulnerable populations, evaluating the effectiveness, acceptability and cost effectiveness of novel strategies, including POC testing. |

Assoc. Prof. Tanya Applegate leads the Diagnostics Innovations Group at The Kirby Institute and manages the RAPID Point of Care Research Consortium for infectious diseases in the Asia–Pacific. She oversees a multidisciplinary diagnostics research program, successfully engaging industry partners. With over 20 years of experience in regulatory, hospital, private industry and academic environments, her research aims to provide equitable healthcare access for marginalised populations and people living in remote or resource limited settings. |

Dr Louise Causer is a medical epidemiologist and senior lecturer at The Kirby Institute, UNSW, leading the Decentralised Diagnostics Implementation Research Group. Her research focuses on evaluating and scaling up POC diagnostic tests for infectious diseases. She has worked on molecular POC testing in rural and remote Australian health services. She co-leads the national implementation of STI molecular POC testing with Flinders University and contributes to the First Nations Infectious Diseases POC Testing Programme and the National HCV POC Testing Programme. |

Dr Skye McGregor is an epidemiologist and lead of the Surveillance Innovation Group at The Kirby Institute, UNSW. Her research focuses on surveillance and prevention of sexually transmissible infections and blood borne viruses, and aims to understand and address persistent disparities in the burden of infection and disease among key populations in Australia. |

Dr Belinda Hengel is an infectious diseases researcher based with The Kirby Institute, UNSW. Her focus is on reducing the impact of infectious diseases, including STIs and respiratory infections in populations at risk. Belinda has experience in public health interventions, including molecular POC testing in remote communities and optimising health service delivery. |
Abstract
Syphilis is a sexually and vertically transmitted infection caused by Treponema pallidum. Although preventable and curable, syphilis remains a public health challenge worldwide, disproportionately affecting communities who experience marginalisation, stigma, and discrimination. Syphilis point-of-care (POC) tests may provide a tool to enhance patient access and timely treatment. In Australia, syphilis POC tests have been incorporated into various public health screening strategies and implemented across different jurisdictions as part of the nationwide response to an expanding syphilis epidemic. These programmes use a highly sensitive and specific POC lateral flow immunoassay test that detects antibodies to T. pallidum, and have largely focused on outbreaks areas, where the burden of infectious syphilis is highest and the distance to centralised laboratories remains significant. The COVID-19 pandemic has resulted in substantial advancements in diagnostic development capabilities with many companies now pivoting to development of POC tests for sexually transmitted infections (STIs). Here we summarise types of syphilis POC tests available, their role in clinical and public health strategies in Australia, and frameworks for selection of POC tests in screening programmes.
Keywords: Australia, nontreponemal test, point-of-care, POC, syphilis, Treponema pallidum, treponemal test.
Introduction – the Australian setting
Syphilis is a sexually and vertically transmitted infection caused by the spirochete Treponema pallidum.1 The natural history of untreated syphilis follows a course of primary, secondary and tertiary clinical manifestations, as well as periods of latency occurring within and following the first 12 months from infection.2 Untreated syphilis in pregnancy can lead to vertical transmission and may result in congenital infection, stillbirth or neonatal death.3
In Australia, notification rates of infectious syphilis have increased by 220% in the last decade, from 7.6 per 100,000 in 2013 to 24.3 per 100,000 in 2022, and remain highest among Aboriginal and Torres Strait Islander peoples living in remote areas, as well as in gay, bisexual and other men who have sex with men.4 Since 2016, notifications of infectious syphilis in women and vertically transmitted cases have also risen: among 69 cases of congenital syphilis reported between 2016 and 2022, 18 (26%) resulted in neonatal death or stillbirth – of these, 11 (61%) occurred among Aboriginal and Torres Strait Islander peoples.4,5 These reports highlight the need for strategies related to enhancing syphilis screening; however, inequitable access to culturally appropriate healthcare, compounded by a history of traumatic colonisation, Stolen Generations and social determinants of health, hinders public health efforts to revert the current epidemiologic syphilis trends.6
In Australia, syphilis POC tests have been used for over 1 decade as part of enhanced community screening programmes.7–9 In this paper, we summarise types of syphilis POC tests available, their role in public health strategies and frameworks for selecting POC tests in screening programmes.
Syphilis point-of-care testing: an opportunity to reach priority populations
Syphilis point-of-care (POC) tests are rapid diagnostic tools designed to facilitate same-day diagnosis, treatment and hasten contact tracing. POC testing for syphilis may support access to care in resource constrained or geographically isolated settings, among groups who face barriers to accessing health services or for highly mobile people where loss-to-follow-up is probable.10–13 In Australia, the settings and populations where POC tests have most value are influenced by the burden of disease and ability to improve clinical and public health management.
Syphilis POC tests
A range of syphilis POC tests are commercially available globally and have been described elsewhere.14,15 Briefly, these include treponemal-only tests that detect T. pallidum-specific antibodies in whole blood, plasma or serum (treponemal readout only), a POC test that simultaneously and separately detects treponemal and nontreponemal antibodies (separate treponemal and nontreponemal readouts), and dual human immunodeficiency virus (HIV)–syphilis test that detects HIV antibodies and treponemal-only antibodies (separate HIV and treponemal readouts).14
Australia’s syphilis POC programmes
The 2017 Federal Government’s Enhanced Syphilis Response (ESR) included a ‘Test and Treat’ model to address the ongoing syphilis epidemic in Aboriginal and Torres Strait Islander peoples living in remote areas.9,16 This decentralised model led by Aboriginal Community Controlled Health Services complements molecular POC testing strategies delivered in similar remote primary care settings.17,18 Before the ESR, syphilis POC tests had been used in some limited settings in Australia, including community-based screens and in diagnostic accuracy studies.8,19–21
The Test and Treat model uses the only syphilis POC test approved by the Australian Therapeutic Goods Administration (Determine Syphilis TP, Abbott), a lateral-flow immune-chromatographic assay that identifies treponemal antibodies in 15 min (sensitivity: 97.3%; 95% CI 95.8–98.3%; specificity: 96.4; 95% CI 94.1–97.8%).19 Since nontreponemal antibodies are not measured at the POC, this test is unable to distinguish between active (treponemal and nontreponemal test positive) and past treated infections (treponemal test positive and nontreponemal test negative). Interpretation of a positive test relies on access to previous syphilis serology, treatment history and traditional laboratory-based serological methods.22 In principle, the implementation of treponemal POC testing in outbreak affected areas aimed to provide enhanced access to testing in a population with low historical prevalence of infection. This facilitates timely treatment and reduces the time required to initiate treatment and contact tracing, minimising the number of individuals lost to follow-up and easing the follow-up burden on primary care.23 As a result of the ongoing epidemic, the benefits of using a treponemal-only POC test to guide management in a population with increased prevalence of treated syphilis has limitations. Also, to date, POC tests are not recognised as part of the national syphilis case definition,7 and there is no centralised mechanism to integrate results into national databases or syphilis registries.23 A POC test that simultaneously detects treponemal and quantifies nontreponemal antibodies would be ideal in this context. However, a qualitative readout of nontreponemal antibodies might still be valuable as it can be used to distinguish between current and past infection, enabling prompt and targeted treatment.24
Using a POC test that measures treponemal and qualitative nontreponemal antibodies, along with its electronic microreader has been shown to yield similar sensitivity and specificity compared to visual operator readings in a laboratory-based diagnostic accuracy study – in addition, the test has demonstrated good correlation between the quantitative electronic readout of nontreponemal antibodies and rapid plasma reagin (RPR) titres.25 However, further research is needed to examine the utility for active syphilis case detection and treatment monitoring. Visual and digital readouts show high sensitivity for the nontreponemal line in active syphilis cases with RPR titres ≥1:8, but lower sensitivity with RPR titres ≤1:4 – the latter could potentially result in missing very early and late latent syphilis cases.25 Moreover, conventional laboratory testing would still be necessary in the parent–child dyad as part of the follow-up for congenital syphilis.26 A field diagnostic accuracy study of the dual treponemal–nontreponemal test with its microreader demonstrated that sexual health nurses without laboratory training could accurately identify individuals with infectious syphilis in real-world POC settings.12 The microreader is now Clinical Laboratory Improvement Amendments (CLIA, 1988, USA)-waived for the dual HIV–syphilis POC test, but not currently approved for use for the dual treponemal–nontreponemal POC assay.27 The impact of the dual treponemal–nontreponemal test on antimicrobial stewardship requires further evaluation. One study suggested that those determined to have past infection were still treated, emphasising the need to enhance health system integration.28,29
To increase syphilis testing coverage among pregnant people, the World Health Organization recommends the implementation of dual HIV–syphilis POC testing as the first screening test in antenatal care,30 with test performance summarised elsewhere.31 The benefits of this technology in countries with overall HIV prevalence <5% is unclear, with modelling reporting cost savings compared to separate rapid HIV and laboratory-based syphilis tests.32 In Australia, for example, given the estimated national population prevalence of 0.14% and female population prevalence of 0.03%,4 a dual HIV–syphilis POC test is unlikely to be suitable for antenatal screening, as the false positives would possibly outweigh true HIV diagnoses.
Among the technological innovations to deliver a new diagnostic POC test for syphilis, a lateral-flow immunochromatographic assay for diagnosis of active syphilis developed by the Burnett Institute (Melbourne, Vic., Australia), which detects T. pallidum-specific immunoglobulin A (IgA) is intended to provide an alternative to laboratory-based RPR testing, in conjunction with a treponemal screening test. Diagnostic accuracy evaluations conducted using stored plasma and blood venous samples suggest that the sensitivity and specificity of this prototype for active syphilis diagnosis is greater than 95 and 80% respectively using a T. pallidum hemagglutination assay (TPHA) and RPR laboratory-based reference tests.33 Future strategies may involve the development of nucleic-acid amplification POC tests capable of detecting both herpes simplex virus (HSV) and T. pallidum in skin or mucosal lesions, as well as clustered regularly interspaced short palindromic repeats (CRISPR)-based POC test for detecting T. pallidum from genital ulcer exudates (M. Y. Chen, unpubl. data).
Selecting a POC test
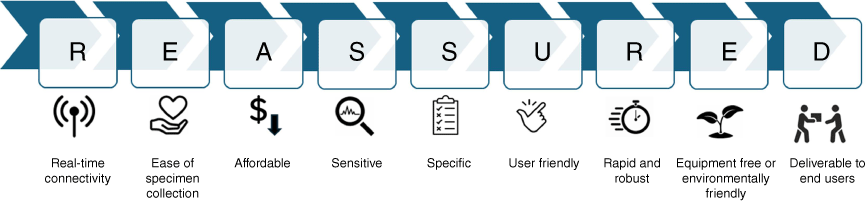
The selection of a POC test should be co-designed with communities. Although this process traditionally focuses on performance, cost and workflow integration, there are other attributes that are equally important.29 The REASSURED criteria (Fig. 1) provide a framework for considering new POC technologies34 – these are described below.
Real-time connectivity
The availability of readers to provide electronic outputs should be considered when selecting a test. Integrating these results with the patient’s medical records and syphilis registries where available (e.g. Queensland and Northern Territory), can facilitate data sharing with healthcare providers and public health units, including notifiable disease reporting. The recorded testing data can be used to monitor test uptake and target population (baseline and demographic characteristics of those tested), and support clinical management.
Ease of specimen collection
The test should have low fingerstick blood-volume requirements, as the amount of capillary blood needed can affect feasibility and uptake. This is particularly important if syphilis POC screening is integrated within existing services where access to venepuncture samples may be a barrier, or to complement existing testing strategies using fingerstick samples such as in drug and alcohol settings. Plasma and serum samples are used in laboratory-based accuracy studies, but they are not suitable for POC tests in real-world settings because they require venepuncture and centrifugation.
Affordable
Considerations include test costs, inclusive of quality management and other system processes.35 In Australia, the use of the POC tests should be cost effective compared to a laboratory test or no test at all, as determined by evaluation of clinical and public health benefits, and health resources saved.
Sensitive and specific
Although sensitivity and specificity of a test are important, evaluation of syphilis POC testing strategies should estimate disease probability in samples from the target population with the clinical syphilis stage also determined. In screening programmes, pre- and post-test probabilities of disease are crucial to ensure no cases are missed and to guide treatment. These probabilities can also inform the design of setting-specific clinical decision tools (e.g. drug and alcohol settings, prisons) to enhance the efficient use of healthcare resources.
User friendly
Assessments should be conducted in the hands of the users in real-world environments such as peer-outreach workers, non-laboratory-trained nurses and Aboriginal health practitioners, as ease of use influences uptake.12,18 A test with slightly lower sensitivity but easy to use, is likely to be used more by providers and, therefore, reach more individuals at risk of infection compared to a highly sensitive test that is difficult to use or read.
Rapid and robust
Ideally, test results should be available in less than 20 min, as the goal is to change the clinical management at the point of testing or near to. Also, the tests should be stable during prolonged transportation and in the environment in which they are intended to be used, such as remote areas with extreme heat and humidity, in health services, or in the community.
Equipment free
The test should either be equipment free or require simple devices (such as battery-operated readers) to enable outreach-based screening or use within clinics with limited space or unreliable power supply.
Deliverable to end users
Barriers to POC test registration can result in delays and additional complexity in accessing state-of-the-art technology. Simple, efficient and fit-for-purpose regulatory and sustainable financing structures, as well as approval for POC tests to be conducted by non-laboratory professionals are critical to support POC test uptake and sustained use by end users. This is exemplified by the Medicare rebate for molecular POC testing for STIs conducted in Aboriginal Medical Services or Aboriginal Community Controlled Heath Services located within remote communities.36
In Australia, a qualitative nontreponemal POC assay alongside a treponemal test may increase opportunities for immediate testing and treatment, enhance access to testing and treatment for priority populations, raise provider and community awareness, and be used in outreach and community screenings where immediate access to laboratory-based serological methods proves unfeasible or untimely. Community consultations to define POC tests optimal product profile, a landscape review of commercially POC tests, in addition to systematic reviews of their diagnostic accuracy, clinical, public health and economic impact may be beneficial in the Australian context. Future research is required to evaluate the operational performance, cost effectiveness and integration into supporting health systems within the intended settings and populations.
Conclusion
Syphilis POC tests are being used in Australia to increase access to syphilis testing and treatment in priority areas. Given the ongoing and developing nature of the syphilis epidemic, it is timely to re-evaluate the clinical utility of the treponemal-only POC test and assess if newer POC technologies could improve the uptake and effectiveness of the Test and Treat model. The REASSURED criteria guide the selection of POC tests, alongside simplified pathways to improve access and funding for state-of-the-art and quality assured technologies.
Data availability
Data sharing is not applicable as no new data were generated or analysed during this study.
Acknowledgements
We acknowledge the Traditional Owners of Country throughout Australia, and Aboriginal and Torres Strait Islander peoples’ continuing connection to culture, land, sea, waters and community. We pay our respects to Elders, both past and present. Prof. R. Guy and Dr S. McGregor are supported by National Health and Medical Research Council (NHMRC) Investigator Grants. Assoc. Prof. T. Applegate is supported by a NHMRC Ideas Grant.
References
1 Radolf JD et al. (2016) Treponema pallidum, the syphilis spirochete: making a living as a stealth pathogen. Nat Rev Microbiol 14, 744-759.
| Crossref | Google Scholar | PubMed |
2 Ghanem KG et al. (2020) The modern epidemic of syphilis. N Engl J Med 382, 845-854.
| Crossref | Google Scholar | PubMed |
3 Moseley P et al. (2024) Resurgence of congenital syphilis: new strategies against an old foe. Lancet Infect Dis 24, e24-e35.
| Crossref | Google Scholar | PubMed |
4 King J et al. (2023) HIV, viral hepatitis and sexually transmissible infections in Australia: annual surveillance report 2023. The Kirby Institute, UNSW Sydney, Sydney, NSW, Australia. 10.26190/f5ph-f972
5 Hengel B et al. (2024) Notification rates for syphilis in women of reproductive age and congenital syphilis in Australia, 2011–2021: a retrospective cohort analysis of national notifications data. Med J Aust [Published online early 15 July 2024].
| Crossref | Google Scholar | PubMed |
6 MacPhail C, McKay K (2018) Social determinants in the sexual health of adolescent Aboriginal Australians: a systematic review. Health Soc Care Community 26, 131-146.
| Crossref | Google Scholar | PubMed |
7 Australian Government Department of Health and Aged Care (2023) Syphilis – CDNA National Guidelines for Public Health Units. Commonwealth of Australia. https://www.health.gov.au/resources/publications/syphilis-cdna-national-guidelines-for-public-health-units?language=en
8 Su J-Y et al. (2015) 002.3 Are rapid point-of-care tests for syphilis useful in outbreak settings in remote Australia? An experience from the Northern Territory. Sex Transm Infect 91, A28 [Oral presentation abstract].
| Crossref | Google Scholar |
9 Flinders University (2024) FHMRI: International Centre for Point-of-Care Testing. Flinders University, Adelaide, SA, Australia. https://www.flinders.edu.au/international-centre-for-point-of-care-testing/infectious-syphilis-poct-program
10 Syphilis POC Testing (2024) Syphilis Point-of-Care Testing Program. https://www.syphilispoct.com.au/
11 Brett K, Askin N (2023) CADTH health technology review: point-of-care HIV and syphilis screening. Canada’s Drug Agency– L’Agence des médicaments du Canada. https://www.cadth.ca/sites/default/files/pdf/htis/2023/RC1490-POC-Syphilis-Screening.pdf
12 Caya C et al. (2023) Rapid diagnostic testing for syphilis in Arctic communities (the STAR study): a multisite prospective field diagnostic accuracy study in an intended-use setting. Clin Microbiol Infect 29, 1335.e1-1335.e7.
| Crossref | Google Scholar | PubMed |
13 Maliszewski KN et al. (2024) An evaluation of the performance, patient acceptability and feasibility of a point-of-care HIV–syphilis assay in an urban Emergency Department. Sex Transm Dis
| Crossref | Google Scholar | PubMed |
14 Global HIV, Hepatitis and STIs Programmes, Sexual and Reproductive Health and Research (2023) The diagnostics landscape for sexually transmitted infections. World Health Organization, Geneva, Switzerland. https://www.who.int/publications-detail-redirect/9789240077126
15 Angel-Müller E et al. (2021) Diagnostic accuracy of rapid point-of-care tests for detecting active syphilis: a systematic review and meta-analysis. Sex Transm Dis 48, e202.
| Crossref | Google Scholar | PubMed |
16 Australian Government Department of Health and Age Care (2024) National response to syphilis. Commonwealth of Australia. https://www.health.gov.au/our-work/national-response-to-syphilis (accessed 5 June 2024)
17 Hengel B et al. (2021) A decentralised point-of-care testing model to address inequities in the COVID-19 response. Lancet Infect Dis 21, e183-e190.
| Crossref | Google Scholar | PubMed |
18 Causer LM et al. (2024) Clinical effectiveness and analytical quality of a national point-of-care testing network for sexually transmitted infections integrated into rural and remote primary care clinics in Australia, 2016–2022: an observational program evaluation. Lancet Reg Health West Pac 48, 101110.
| Crossref | Google Scholar | PubMed |
19 Causer LM et al. (2014) A laboratory-based evaluation of four rapid point-of-care tests for syphilis. PLoS One 9, e91504.
| Crossref | Google Scholar | PubMed |
20 Causer LM et al. (2015) An evaluation of a novel dual treponemal/nontreponemal point-of-care test for syphilis as a tool to distinguish active from past treated infection. Clin Infect Dis 61, 184-191.
| Crossref | Google Scholar | PubMed |
21 Skinner L et al. (2015) Evaluation of the Dual Path Platform syphilis point of care test in north Queensland. Pathology 47, 718-720.
| Crossref | Google Scholar | PubMed |
22 Australasian Society for HIV, Viral Hepatitis and Sexual Health Medicine (2022) Syphilis: outbreak training website. Clinical management | Testing and treating. ASHM Health. https://syphilisoutbreaktraining.com.au/testing-and-management/
23 Government of Western Australia, Department of Health, Public and Aboriginal Health Division (2020) Guidelines for health service providers: use of syphilis point-of-care tests provided by the WA Department of Health. Government of Western Australia. https://www.health.wa.gov.au/~/media/Corp/Documents/Health-for/Sexual-health/SORG/Enhanced-Syphilis-Response-WA-PoCT-Program-Guidelines-Nov2020.pdf
24 Kay NS et al. (2014) State of the art syphilis diagnostics: rapid point-of-care tests. Expert Rev Anti Infect Ther 12, 63-73.
| Crossref | Google Scholar | PubMed |
25 Vargas SK et al. (2022) Laboratory evaluation of the DPP Syphilis Screen & Confirm assay. Microbiol Spectr 10, e0264221.
| Crossref | Google Scholar | PubMed |
26 Papp JR et al. (2024) CDC laboratory recommendations for syphilis testing, United States, 2024. MMWR Recomm Rep 73, 1-32.
| Crossref | Google Scholar | PubMed |
27 Chembio Diagnostics, Inc. (2024) DPP HIV–syphilis USA: the first & only FDA approved, CLIA waived* HIV–syphilis rapid test. Chembio Diagnostics, Inc. https://chembio.com/products/dpp-hiv-syphilis-usa/ (accessed: 28 July 2024)
28 Langendorf C et al. (2019) Dual screen and confirm rapid test does not reduce overtreatment of syphilis in pregnant women living in a non-venereal treponematoses endemic region: a field evaluation among antenatal care attendees in Burkina Faso. Sex Transm Infect 95, 402-404.
| Crossref | Google Scholar | PubMed |
29 Toskin I et al. (2020) Call to action for health systems integration of point-of-care testing to mitigate the transmission and burden of sexually transmitted infections. Sex Transm Infect 96, 342-347.
| Crossref | Google Scholar | PubMed |
30 World Health Organization, Global HIV, Hepatitis and STIs Programmes (2019) Dual HIV/syphilis rapid diagnostic tests can be used as the first test in antenatal care. 27 November 2019 | Policy brief. WHO reference number: WHO/CDS/HIV/19.38. WHO. https://www.who.int/publications/i/item/WHO-CDS-HIV-19.38
31 Pham MD et al. (2022) Point-of-care diagnostics for diagnosis of active syphilis infection: needs, challenges and the way forward. Int J Environ Res Public Health 19, 8172.
| Crossref | Google Scholar | PubMed |
32 Rodriguez PJ et al. (2021) Cost effectiveness of dual maternal HIV and syphilis testing strategies in high and low HIV prevalence countries: a modelling study. Lancet Glob Health 9, e61-e71.
| Crossref | Google Scholar | PubMed |
33 Pham MD et al. (2020) Improving the coverage and accuracy of syphilis testing: the development of a novel rapid, point-of-care test for confirmatory testing of active syphilis infection and its early evaluation in China and South Africa. EClinicalMedicine 24, 100440.
| Crossref | Google Scholar | PubMed |
34 Land KJ et al. (2019) REASSURED diagnostics to inform disease control strategies, strengthen health systems and improve patient outcomes. Nat Microbiol 4, 46-54.
| Crossref | Google Scholar | PubMed |
35 Saweri OPM et al. (2021) Economic evaluation of point-of-care testing and treatment for sexually transmitted and genital infections in pregnancy in low- and middle-income countries: a systematic review. PLoS ONE 16, e0253135.
| Crossref | Google Scholar | PubMed |
36 Medical Services Advisory Committee (2023) Medical Services Advisory Committee (MSAC) Public Summary Document. Application No. 1627.1 – Point-of-care testing for sexually transmitted infections provided by Aboriginal Medical Services or Aboriginal Community Controlled Heath Services in rural or remote areas. MSAC. https://www1.health.gov.au/internet/msac/publishing.nsf/Content/81953537EEDFB0EFCA258A570079B7E3/$File/1627.1%20Final%20PSD%20-%20Nov2023.docx
 Dr Gladymar Pérez Chacón is an early career researcher in public health and a medical epidemiologist based at The Kirby Institute, UNSW. Gladymar has professional experience in point-of-care (POC) testing and mass-treatment strategies for yaws in resource-constrained settings in the Asia–Pacific Region. Her research interests are broad and include pertussis and other vaccine preventable diseases as well as sexually and vertically transmitted infections. |
 Dr Amit Saha is a medical epidemiologist and lecturer at The Kirby Institute, UNSW. His current work focuses on POC diagnostics for sexually transmitted infections (STIs) and respiratory infections and their impact in high-prevalence remote settings. He has professional experience in various fields of infectious disease epidemiology and large field-based clinical studies on enteric vaccines. |
 Emily Phillips, BMedSci(Hons) is completing a Masters of Applied Epidemiology. Emily manages a large-scale research programme that aims to scale up infectious disease POC testing in remote Indigenous communities to enable person-centred care and improve health outcomes for Aboriginal and Torres Strait Islander peoples. Previously, Emily held the position of director of communicable diseases at the National Aboriginal Community Controlled Health Organisation, where she played a pivotal role in the Aboriginal and Torres Strait Islander response to the COVID-19 pandemic. |
 Prof. Rebecca Guy is the head of the Surveillance Evaluation and Research Programme at The Kirby Institute, UNSW. She is a member of the Institute’s Executive and Aboriginal and Torres Strait Islander Committees. Prof. Guy specialises in epidemiology, implementation research, health services and translational research. Her public health research aims to reduce the impact of infectious diseases in vulnerable populations, evaluating the effectiveness, acceptability and cost effectiveness of novel strategies, including POC testing. |
 Assoc. Prof. Tanya Applegate leads the Diagnostics Innovations Group at The Kirby Institute and manages the RAPID Point of Care Research Consortium for infectious diseases in the Asia–Pacific. She oversees a multidisciplinary diagnostics research program, successfully engaging industry partners. With over 20 years of experience in regulatory, hospital, private industry and academic environments, her research aims to provide equitable healthcare access for marginalised populations and people living in remote or resource limited settings. |
 Dr Louise Causer is a medical epidemiologist and senior lecturer at The Kirby Institute, UNSW, leading the Decentralised Diagnostics Implementation Research Group. Her research focuses on evaluating and scaling up POC diagnostic tests for infectious diseases. She has worked on molecular POC testing in rural and remote Australian health services. She co-leads the national implementation of STI molecular POC testing with Flinders University and contributes to the First Nations Infectious Diseases POC Testing Programme and the National HCV POC Testing Programme. |
 Dr Skye McGregor is an epidemiologist and lead of the Surveillance Innovation Group at The Kirby Institute, UNSW. Her research focuses on surveillance and prevention of sexually transmissible infections and blood borne viruses, and aims to understand and address persistent disparities in the burden of infection and disease among key populations in Australia. |
 Dr Belinda Hengel is an infectious diseases researcher based with The Kirby Institute, UNSW. Her focus is on reducing the impact of infectious diseases, including STIs and respiratory infections in populations at risk. Belinda has experience in public health interventions, including molecular POC testing in remote communities and optimising health service delivery. |