Vector-borne parasitic infections after the earthquake
Fadile Yıldız Zeyrek A , Salim Yakut A and Metin Korkmaz B *A
B

Fadile Yıldız Zeyrek is Professor and Head of the Department of Medical Microbiology, Faculty of Medicine, Harran University. She is a specialist in clinical microbiology and clinical parasitology. Her research interests include malaria, leishmaniasis, molecular microbiology, serology and immunology. She is also a member of the malaria and Leishmania science committee of the Ministry of Health. |

Salim Yakut is currently Assistant professor the Department of Medical Microbiology, Faculty of Medicine, Harran University. He is a specialist in clinical microbiology. His research interest includes multi-drug resistant bacteria and molecular microbiology. He is also a member of the Standardisation of Antibiotic Susceptibility Tests Working Group, Turkish Microbiology Society. |

Metin Korkmaz is a Professor of Medical Parasitology at Ege University and he is the responsible for serological diagnosis of human parasitic diseases at the university hospital. His research interests are development of new diagnostic methods, histopathological diagnosis of parasitic diseases and fermentation process. |
Abstract
The transmission of vector-borne infections after an earthquake is related to the changes in the environment caused by the earthquake. The displacement of thousands of people, especially in areas where vector-borne diseases are endemic, can significantly increase human exposure to mosquitoes and other vectors and the pathogens they may carry in overcrowded environments and inappropriate temporary shelters, leading to an increase in human infection cases. The devastating earthquakes in Türkiye on 6 February 2023 pose a risk of the spread and outbreaks of vector-borne infections such as cutaneous leishmaniasis (CL) and malaria, which are endemic in the region. Public health authorities should prioritise surveillance in all earthquake-affected areas. Immediate detection and identification of local vector species, monitor environmental conditions and potential breeding grounds, insecticide application and use of mosquito nets and development of interventions to prevent the emergence of vector-borne infections are essential. Case diagnosis and treatment follow-up, prophylaxis, training of the public and health personnel, improving temporary shelter conditions and facilitating access to clean drinking water and health services are essential to minimise the impact of vector-borne infections in post-earthquake situations.
Keywords: earthquake, malaria, parasites, Türkiye, vector-borne infection: leishmaniasis.
As stated by the WHO1 and Chala and Hamde,2 vector-borne infections, which are a significant public health issue worldwide, are infections that do not spread directly from person to person but are transmitted to humans through a vector. The most common vector-borne infections are transmitted by mosquitoes and ticks. Vector-borne infections can be caused by parasites, bacteria or viruses and annually, over 700 000 deaths are attributed to these infections, which represent more than 17% of all infectious diseases.1,2
Host–environment interactions are key in vector-borne disease emergence. Favourable conditions for vector–host–climate–pathogen–human interactions increase transmission risk. Social and demographic factors also significantly contribute to the emergence of vector-borne infections.2
Earthquakes, which constitute 8% of natural disasters, can cause an increase in the incidence of infectious diseases.3 The transmission of vector-borne infections after an earthquake is related to the changes in the environment caused by the earthquake. The displacement of thousands of people, especially in areas where vector-borne infections are endemic, can significantly increase human exposure to mosquitoes and other vectors and the pathogens they may carry in overcrowded environments and inappropriate temporary shelters, which can lead to an increase in human infection cases.4
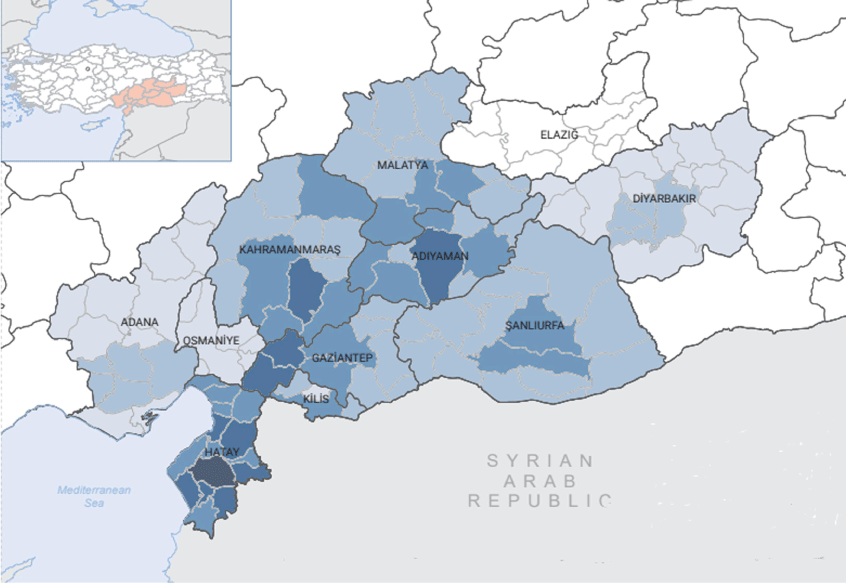
One of the most devastating earthquakes of the last century occurred in the south-eastern region of Türkiye on 6 February 2023. The first earthquake, centred in the Pazarcık district of Kahramanmaraş, had a magnitude of 7.7 and occurred at 04:17 hours local time. Subsequently, at 13:24 hours, another earthquake with a magnitude of 7.6, centred in the Elbistan district of Kahramanmaraş, occurred. These earthquakes were felt in 11 provinces, including Gaziantep, Şanlıurfa, Diyarbakır and Hatay, and caused widespread building collapses and severe structural damage. The earthquakes resulted in the loss of more than 50 000 lives, with over 107 000 people injured (Fig. 1). An area of ~500 km in diameter was directly affected by the earthquake, affecting over 14 million people residing in the region. It was reported that more than half (50.52%) of the Syrian migrant population in Türkiye, lived in this region and were affected by the earthquake. Due to the earthquakes, ~6 million people faced housing shortages, ~2 million people began living in temporary settlements, and ~4 million people migrated out of the earthquake-affected area. Access to clean drinking water and adequate sanitation facilities, including toilets and bathing, in tent camps was extremely limited.5
Additionally, on 15 March 2023, the earthquake-affected provinces of Şanlıurfa and Adıyaman experienced flooding. Twenty-one people lost their lives in this disaster. Because many of the temporary shelters were set up on unsuitable terrain, the living conditions of the local residents worsened after the flooding. Homeless earthquake victims were affected, and the floodwaters inundated many of the erected tents and containers, exacerbating the damage caused by the earthquake (see https://en.wikipedia.org/wiki/2023_Turkey_floods, accessed 29 October 2023).
Earthquakes and floods can affect vector-breeding sites and the transmission of diseases by these vectors. Although initial flooding can wash away existing mosquito breeding grounds, stagnant water caused by heavy rainfall, overflowing rivers or puddles created by earthquakes can create new breeding sites. This can lead to an increase in vector populations and disease transmission potential (typically with a delay of several weeks) depending on the local mosquito vector species and its preferred habitat.6
There are numerous examples demonstrating that earthquakes can lead to an increased incidence of vector-borne infections. In 1991, an earthquake in the Atlantic region of Costa Rica caused favourable changes in vector-breeding habitats and resulted in a significant increase in malaria cases.7 In 2003, in the city of Bam, Iran, 124 malaria cases were reported within 2 months.8 During the same period, the incidence of cutaneous leishmaniasis (CL) gradually and then exponentially increased from 2004 to 2008. This earthquake in 2003 resulted in the formation of 10 × 106 tonnes (~11 × 106 tons) of rubble, creating suitable conditions for the spread of sand-fly vectors. Since then, the epidemiology of CL has been changing, with the emergence of new foci and persistent clinical forms.9 After the 7.9-magnitude earthquake in China’s Sichuan province on 12 May 2008, it was reported that it contributed to the spread of vector-borne infections, leading to outbreaks.4 Following the earthquake in Haiti in 2010, 76 out of 255 patients (29.8%) presenting with an unknown fever were diagnosed with falciparum malaria. Among other vector-borne infections, there were 13 cases of filariasis and 6 cases of dengue fever.10 In China, after the 2014 earthquake, there was an increase in vector populations, coupled with homelessness, resulting in significantly increased exposure to mosquito bites.11
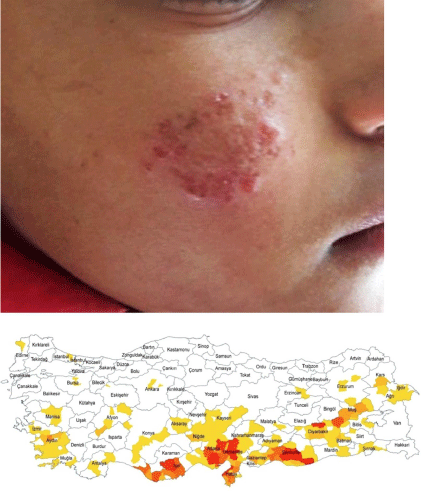
Leishmaniasis is a disease caused by protozoan parasites of the genus Leishmania. These parasites are transmitted to humans through the bite of infected female sandflies. The disease is still an important public health problem in Türkiye and is a notifiable disease (Fig. 2). CL is the most common form of the disease and exists in two main types in the Old World: zoonotic CL (ZCL), caused by L. major with rodents as the primary reservoir host, and anthroponotic CL (ACL), caused by L. tropica with humans as the reservoir host.9
A strong association between the number of earthquakes and an increased incidence of CL has been found and regions with frequent earthquakes have been considered as high-risk areas for CL.12 According to a 2022 meta-analysis, there was a 1.5-fold increase in infectious diseases, including skin, respiratory, gastrointestinal, and central nervous system infections, in the post-earthquake period.13 When we look at vector-borne infections expected to occur in the post-acute period after earthquakes (after the first month), it is observed that malaria is endemic in the region, and CL is one of the most common infections in the region.
ACL is endemic in the south-eastern Anatolia and Mediterranean regions. According to the Ministry of Health data, 96% of cases were reported from this region, with over half of them originating from Şanlıurfa. Although L. tropica remains the predominant causative agent in these cases, CL cases caused by L. major and L. infantum have also been reported in recent years.14–18 It is known that in the region, there are vectors such as Phlebotomus sergenti (the vector of L. tropica), P. papatasi (the vector of L. major) and P. perfiliewi (which could transmit L. infantum).19–21
Leishmaniasis is linked to human behavioral patterns, urbanisation, and environmental changes caused by both human activities and natural disasters. The disease is widespread in weak healthcare systems and can lead to large outbreaks, especially in crowded conditions.22 The major earthquake in Iran, for instance, created new breeding potentials for vectors and had the most significant impact on CL incidence.9
Malaria, caused by Plasmodium species and transmitted by female Anopheles mosquitoes, includes five human-infecting species: P. falciparum, P. vivax, P. ovale, P. malariae and P. knowlesi. In 2021, the World Health Organization reported 247 million new malaria cases in 84 endemic regions, with 619 000 malaria-related deaths.23
For many years, Türkiye faced a significant public health challenge with chloroquine-sensitive P. vivax malaria. After long-standing efforts, it entered the pre-elimination stage in 2009 and since 2013, no new indigenous cases have been reported. Although imported malaria cases are observed, the WHO has not declared Türkiye as a malaria-free region.23
In south and south-east Anatolia, malaria vectors, including 10 species such as Anopheles sacharovi and A. superpictus, are present.24–27 Identifying the vector species, environmental factors and breeding areas in the region is crucial for developing intervention activities and control measures against diseases transmitted by these vectors.
Factors such as an increase in infected and susceptible hosts following an earthquake, a weak public health infrastructure and interruptions in ongoing control programs are risk factors for the transmission of vector-borne infections. In public health emergencies like earthquakes, public health authorities should prioritise surveillance in all affected areas to reduce the emergence and spread of vector-borne infections. It is essential, especially in regions endemic to diseases such as malaria and CL, to promptly identify and characterise local vector species, environmental variables and breeding habitats. This is crucial for implementing control measures and preparing initiatives to prevent vector-borne infections. Key components of control measures include case diagnosis and treatment monitoring, prophylaxis, education of both the public and healthcare personnel, indoor application of insecticides for vector population control, the use of bed nets to reduce outdoor exposure, improvement of temporary housing conditions, and facilitating access to clean drinking water and healthcare services.
Considering the chaotic situation in the region, it is essential to begin implementing these measures as soon as possible. Following the earthquake in Haiti, the rapid diagnosis and treatment using fast tests played a vital role in controlling a potential malaria outbreak due to factors such as lack of coordination, logistical challenges, a mobile population, a constantly changing healthcare system and the absence of experienced microscopists.10 The use of pesticides has also been effective in preventing vector-borne infections from causing outbreaks after earthquakes.4
After an earthquake, the risk of transmission and spread of certain ectoparasitic infections may increase because of the environmental changes caused by the earthquake and the alteration of people’s living conditions.2 In areas with insufficient water, inadequate personal hygiene, and among those placed in temporary shelters or communal housing, scabies and pediculosis may pose significant problems.2
Scabies is a contagious skin infestation caused by the Sarcoptes scabiei. Infrequent bathing, not changing clothes regularly, sharing beds and incomplete treatment of entire families for scabies can increase the transmission risk. In the 2005 earthquake-affected Kaghan Valley of Pakistan, scabies was detected as the second most common infection among 22 122 patients, accounting for 17%.28
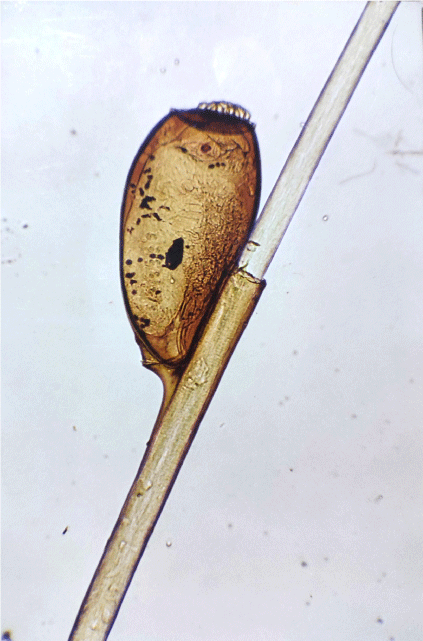
Pediculosis is an infestation caused by louse. The notable lice are the head louse (Pediculus humanus capitis; head louse nit shown in Fig. 3), the body louse (Pediculus humanus humanus) and the crab louse (Phthirus pubis). In overcrowded and poor hygiene conditions, there is an increased risk of lice transmission from person to person due to close physical contact. The prevention and control of the spread of pediculosis is aided by prompt diagnosis and effective treatment of infestations.29
The Ministry of Health has established a network of outbreak investigation laboratories by setting up field laboratories in earthquake-affected areas with both active and non-functional existing laboratories 1 week after the earthquake. Through this network, in which the authors participated, active and passive surveillance has been carried out, and potential outbreak agents have been closely monitored. Despite sporadic cases of malaria and CL observed in these surveillance activities, there has been no imminent threat of an outbreak thus far. Although an outbreak is not expected in the near future due to the earthquake occurring in the winter months, the number of cases may increase in October–November as the vector–human and environmental conditions in the region have become more favourable for the development of vector-borne infections due to the earthquake.
Conclusion
After natural disasters, the placement of disaster survivors should be well-planned. The location of the shelter camps to be established, their proximity to water sources, and the distance of mobile toilets and bathrooms to these water sources should be well planned. Additionally, it is crucial to ensure access to clean and safe water, establish proper sanitation conditions, deliver healthcare services, and collaborate with relevant institutions to ensure the collection and safe disposal of waste, garbage, toilets, and animal carcasses for environmental health protection. Measures such as rehabilitating vector-breeding sites, using appropriate pesticides, implementing interventions to reduce human–vector contact, and providing prophylaxis can reduce the risk of vector-borne infections.
In order to prevent the emergence of infectious diseases after an earthquake, it is crucial to assess potential outbreak risks and develop a scientific and reasonable monitoring and control plan that reduces economic and social damage.
References
1 World Health Organization (2023) Vector-borne diseases. https://www.who.int/news-room/fact-sheets/detail/vector-borne-diseases (accessed 7 September 2023)
2 Chala B, Hamde F (2021) Emerging and re-emerging vector-borne infectious diseases and the challenges for control: a review. Front Public Health 9, 715759.
| Crossref | Google Scholar | PubMed |
3 United Nations (2020) The human cost of disasters: an overview of the last 20 years (2000–2019). https://www.undrr.org/publication/humancost-disasters-overview-last-20-years-2000-2019 (accessed 7 September 2023)
4 Zhang S et al. (2013) Incidence of Japanese encephalitis, visceral leishmaniasis and malaria before and after the Wenchuan earthquake, in China. Acta Trop 128, 85-89.
| Crossref | Google Scholar | PubMed |
5 Turkish Medical Association (2023) Türk Tabipleri Birliği 6 Şubat 2023 Kahramanmaraş ve 20 Şubat 2023 Hatay Depremleri Birinci ay Raporu [The 2nd month report of Turkish Medical Association: 06–28 February 2023]. https://www.ttb.org.tr/userfiles/files/1ayraporu.pdf (in Turkish, accessed 7 September 2023)
6 Lifson AR (1996) Mosquitoes, models, and dengue. Lancet 347, 1201-1202.
| Crossref | Google Scholar | PubMed |
7 Sáenz R et al. (1995) Post-disaster malaria in Costa Rica. Prehosp Disaster Med 10, 154-160.
| Crossref | Google Scholar | PubMed |
8 Akbari ME et al. (2004) The devastation of Bam: an overview of health issues 1 month after the earthquake. Public Health 118, 403-408.
| Crossref | Google Scholar | PubMed |
9 Sharifi I et al. (2011) Emergence of a new focus of anthroponotic cutaneous leishmaniasis due to Leishmania tropica in rural communities of Bam district after the earthquake, Iran. Trop Med Int Health 16, 510-513.
| Crossref | Google Scholar | PubMed |
10 Townes D et al. (2012) Malaria survey in post-earthquake Haiti – 2010. Am J Trop Med Hyg 86, 29-31 PMCID: PMC3247104.
| Crossref | Google Scholar | PubMed |
11 Feng J et al. (2016) Risk assessment of malaria prevalence in Ludian, Yongshan, and Jinggu Counties, Yunnan Province, after 2014 earthquake disaster. Am J Trop Med Hyg 94, 674-678 PMCID: PMC4775906.
| Crossref | Google Scholar | PubMed |
12 Nikonahad A et al. (2017) A time series analysis of environmental and metrological factors impact on cutaneous leishmaniasis incidence in an endemic area of Dehloran, Iran. Environ Sci Pollut Res Int 24, 14117-14123.
| Crossref | Google Scholar | PubMed |
13 Najafi S et al. (2022) Incidence of infectious diseases after earthquakes: a systematic review and meta-analysis. Public Health 202, 131-138.
| Crossref | Google Scholar | PubMed |
14 Gürel MS et al. (2012) Türkiye’de Kutanöz Leishmaniasisin Durumu. [Cutaneous leishmaniasis in Turkey.]. Turkiye Parazitol Derg 36, 121-129 [In Turkish with English abstract].
| Crossref | Google Scholar | PubMed |
15 Zeyrek FY et al. (2014) Şanlıurfa’da Şark Çıbanı Etkeni Değişiyor Mu? İlk Leishmania major Vakaları [Is the agent of cutaneous leishmaniasis in Sanliurfa changing? First cases of Leishmania major]. Turkiye Parazitol Derg 38, 270-274 [In Turkish].
| Crossref | Google Scholar | PubMed |
16 Yıldız Zeyrek F et al. (2020) Şanlıurfa’da Leishmania infantum’un Etken Olduğu Kutanöz Leyşmanyazis (Şark Çıbanı) Olguları [Cutaneous leishmaniasis cases caused by Leishmania infantum in Şanlıurfa Province, Turkey]. Mikrobiyol Bul 54, 647-656 [In Turkish].
| Crossref | Google Scholar | PubMed |
17 Zeyrek FY et al. (2007) Serodiagnosis of anthroponotic cutaneous leishmaniasis (ACL) caused by Leishmania tropica in Sanliurfa Province, Turkey, where ACL Is highly endemic. Clin Vaccine Immunol 14, 1409-1415 PMCID: PMC2168175.
| Crossref | Google Scholar | PubMed |
18 Özbilgin A et al. (2016) Leishmaniasis in Turkey: first clinical isolation of Leishmania major from 18 autochthonous cases of cutaneous leishmaniasis in four geographical regions. Trop Med Int Health 21, 783-791 [Erratum in Trop Med Int Health 2016; 21(11): E1].
| Crossref | Google Scholar | PubMed |
19 Volf P et al. (2002) Sand flies (Diptera: Phlebotominae) in Sanliurfa, Turkey: relationship of Phlebotomus sergenti with the epidemic of anthroponotic cutaneous leishmaniasis. J Med Entomol 39, 12-15.
| Crossref | Google Scholar | PubMed |
20 Svobodová M et al. (2003) Shortreport: distribution and feding preference of the sandflies Phlebotomus sergenti and P. papatasi in a cutaneous leishmaniasis focus in Sanliurfa, Turkey. Am J Trop Med Hyg 68, 6-9.
| Google Scholar |
21 Toprak S, Ozer N (2005) Sandfly species of Sanliurfa province in Turkey. Med Vet Entomol 19, 107-110.
| Crossref | Google Scholar | PubMed |
22 Aflatoonian M et al. (2022) Fifty years of struggle to control cutaneous leishmaniasis in the highest endemic county in Iran: a longitudinal observation inferred with interrupted time series model. PLoS Negl Trop Dis 16, e0010271.
| Crossref | Google Scholar | PubMed |
23 World Health Organization (2022) World malaria report 2022. Tracking progress and gaps in the global response to malaria. https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2022
24 Zeyrek FY et al. (2006) Parasite density and serum cytokine levels in Plasmodium vivax malaria in Turkey. Parasite Immunol 28, 201-207.
| Crossref | Google Scholar | PubMed |
25 Çulha G et al. (2018) Hatay’da yurt dışı kaynaklı sıtma olgularının moleküler yöntem kullanarak değerlendirilmesi [Determination of imported malaria cases in Hatay by the use of molecular methods]. Mikrobiyol Bul 52, 206-213 [In Turkish].
| Crossref | Google Scholar | PubMed |
26 Zeyrek FY et al. (2008) Analysis of naturally acquired antibody responses to the 19-kD C-terminal region of merozoite surface protein-1 of Plasmodium vivax from individuals in Sanliurfa, Turkey. Am J Trop Med Hyg 78, 729-732.
| Google Scholar | PubMed |
27 Özbilgin A et al. (2011) Malaria in Turkey: successful control and strategies for achieving elimination. Acta Trop 120, 15-23.
| Crossref | Google Scholar | PubMed |
28 Shah N et al. (2010) Disease pattern in earthquake affected areas of Pakistan: data from Kaghan valley. J Ayub Med Coll Abbottabad 22, 81-86.
| Google Scholar | PubMed |
29 Kurt Ö et al. Treatment of head lice (Pediculus humanus capitis) infestation: is regular combing alone with a special detection comb effective at all levels? Parasitol Res 114, 1347-1353.
| Crossref | Google Scholar | PubMed |
 Fadile Yıldız Zeyrek is Professor and Head of the Department of Medical Microbiology, Faculty of Medicine, Harran University. She is a specialist in clinical microbiology and clinical parasitology. Her research interests include malaria, leishmaniasis, molecular microbiology, serology and immunology. She is also a member of the malaria and Leishmania science committee of the Ministry of Health. |
 Salim Yakut is currently Assistant professor the Department of Medical Microbiology, Faculty of Medicine, Harran University. He is a specialist in clinical microbiology. His research interest includes multi-drug resistant bacteria and molecular microbiology. He is also a member of the Standardisation of Antibiotic Susceptibility Tests Working Group, Turkish Microbiology Society. |
 Metin Korkmaz is a Professor of Medical Parasitology at Ege University and he is the responsible for serological diagnosis of human parasitic diseases at the university hospital. His research interests are development of new diagnostic methods, histopathological diagnosis of parasitic diseases and fermentation process. |