Managing emerging pathogen risks in recycled water
M. D. Short A , B. van den Akker A B , P. Monis B and E. Donner A *A Future Industries Institute, University of South Australia, Mawson Lakes, SA 5095, Australia.
B Research and Development, South Australian Water Corporation, Adelaide, SA 5000, Australia.

Michael Short is a Senior Research Fellow at the University of South Australia’s Future Industries Institute with research expertise in urban water systems including wastewater treatment and water recycling, public health microbiology and microbial ecology, sustainability and life cycle assessment, and the intersection of environmental science and policy. |

Ben van den Akker is a Lead Wastewater Research Scientist at SA Water and an Adjunct Associate Professor at the University of South Australia. His research is focused on enhancing the environmental and public health performance of wastewater treatment and water reclamation schemes. |

Paul Monis is a Senior Research molecular microbiologist at SA Water who specialises in developing techniques and knowledge to assess and understand water quality microbial risks to support public health risk management. Most recently Paul was part of the team undertaking the SARS-CoV-2 wastewater monitoring program on behalf of the South Australian Department of Health. |

Erica Donner is Research Professor at the University of South Australia’s Future Industries Institute where she leads industry-partnered research focussed on food, soil, and water security; and antimicrobial resistance in the environment. She is Research Director of the Cooperative Research Centre for Solving Antimicrobial Resistance in Agribusiness, Food, and Environments (CRC SAAFE). |
Microbiology Australia 43(4) 177-182 https://doi.org/10.1071/MA22058
Submitted: 10 October 2022 Accepted: 25 November 2022 Published: 9 December 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing on behalf of the ASM. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
The COVID-19 pandemic raised the public profile of wastewater-based infectious disease monitoring. General media coverage about wastewater detection of SARS-CoV-2 (the COVID-19 coronavirus) increased community awareness of the potential use of wastewater for the detection and surveillance of emerging diseases and also heightened recognition of the potential for wastewater to harbour and convey a variety of pathogens. This has also generated questions about the potential public health impacts of emerging pathogens, such as SARS-CoV-2 and mpox, in sewage and recycled water. To ensure water security in an era of climate change, water recycling is increasingly important in Australia and other water-stressed nations and managing disease risks in integrated water management is thus of critical importance. This paper demonstrates the existing risk management provisions for recycled water and explores potential issues posed by novel and emerging pathogens. First, a synopsis of some key emerging and re-emerging human pathogens is presented and the risks associated with these pathogens in the context of recycled water provision is considered. Then, an overview of the engineered treatment systems and regulatory framework used to manage these emerging risks in Australia is presented, together with a discusion of how emerging pathogen risks can be managed to ensure safe recycled water supply now and into the future.
Keywords: COVID‐19, emerging pathogens, microbial risk assessment, mpox, quantitative risk-based management, recycled water supply, water recycling.
Introduction
Access to clean drinking water and sanitation services is critical for maintaining public health. The absence of these services places a large burden on developing countries, causing an estimated 829 000 deaths annually, of which 432 000 are linked to poor sanitation.1 Sanitation services, such as sewer networks and wastewater treatment facilities, break faecal–oral disease transmission by reducing levels of faecal contamination in the environment and thereby reducing subsequent exposure to pathogens via contaminated food and water. Reduced access to fresh water, due to population growth, increased industrial and agricultural use, and climate change, has driven increased wastewater recycling as jurisdictions seek out secure ‘climate-independent’ supplies. Direct use of untreated wastewater for irrigation is practiced in some countries;2 however in Australia, the treatment and use of recycled water is highly regulated.
In Australia, major wastewater recycling initiatives commenced during the 1990s, with Class A recycled water supplied for horticultural irrigation (e.g. the Virginia Pipeline Scheme in South Australia) and residential use via dual reticulation systems (e.g. toilet flushing and outdoor use at Mawson Lakes in Adelaide and Rouse Hill in Sydney).3 Water recycling initiatives were significantly expanded in Australia during the so-called ‘millennium drought’ of 2000–20104 and recycled water is now a key component of diverse water supply portfolios for many Australian water authorities nationwide5 (Fig. 1).

|
Compared with treated wastewater, which is diluted when released into environmental waters, recycled wastewater poses increased risks to human health due to the types of end uses and exposure pathways. Consequently, recycled wastewater is more highly treated than discharged effluent and Australian Guidelines adopt a quantitative risk-based management approach to protect public health.6 A key element of the Guidelines is the characterisation of microbial hazards in sewage to ensure that sufficient treatment is used to ensure the safety of recycled water; this includes consideration of emerging pathogens. This paper introduces some emerging pathogens and describes how these emerging microbial human health risks are effectively managed in recycled water supplies for public health protection.
Emerging pathogens but existing risks
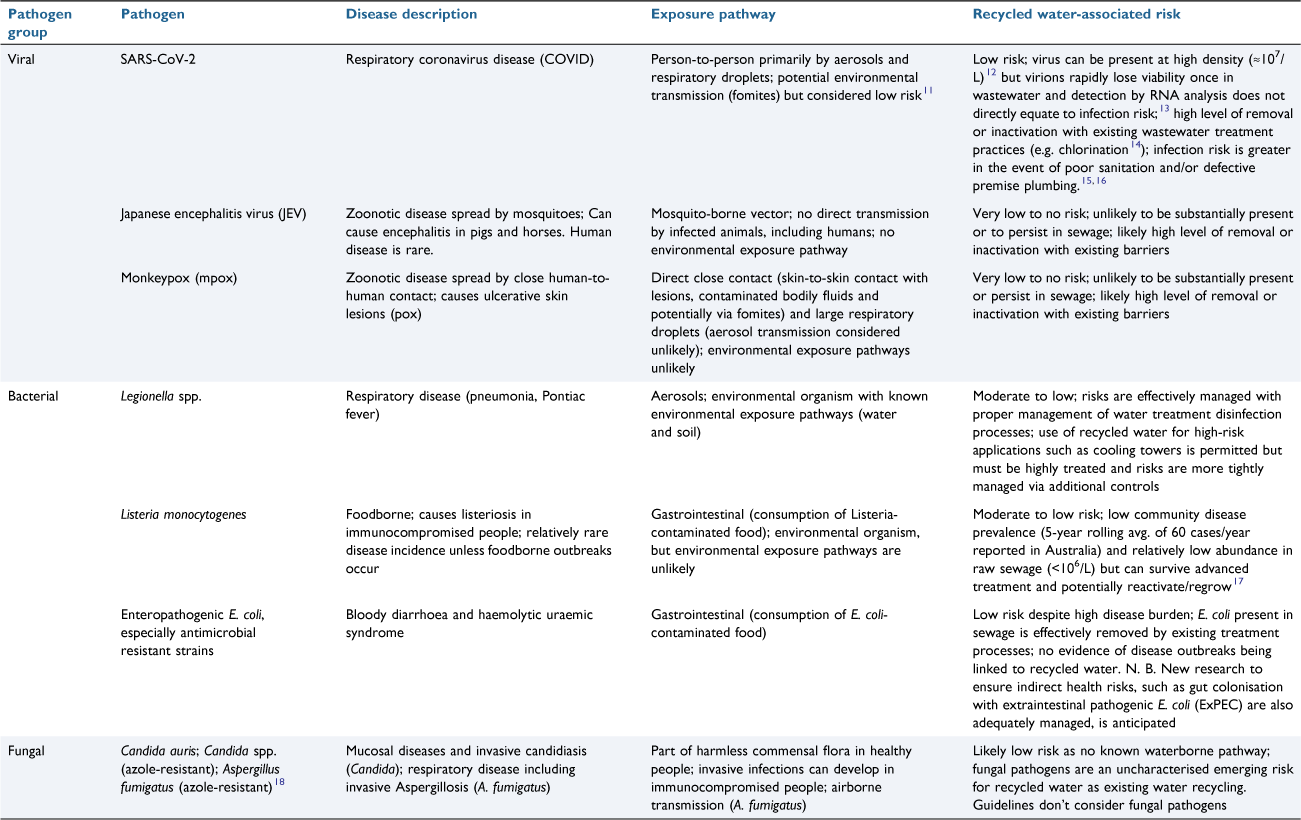
The COVID-19 pandemic has heightened the focus on emerging pathogens internationally. During the early days of the pandemic, it was unclear to what extent the SARS-CoV-2 virus posed human health risks from environmental exposure pathways, including exposure to potentially contaminated water and wastewater.7 As more information emerged, it was clear that human health risks from these exposure pathways were low and effectively managed by existing water industry protocols and public health protection measures.8 Since the genesis of COVID, other novel pathogens continue to emerge and wastewater monitoring has confirmed their presence in sewage (e.g. mpox9), which raises questions about potential human health risks from wastewater and recycled water. While much of the focus is on viral and bacterial pathogens, fungal pathogens including those resistant to antimicrobials also impose a substantial disease burden internationally, with cumulative global mortality rates for serious fungal disease now surpassing those of malaria, tuberculosis, breast or prostate cancer.10 Table 1 provides a synopsis of current high-profile emerging bacterial, fungal and viral diseases, and the likely level of associated risk posed by recycled water use.
|
Risk-based approach to pathogens in recycled water
Across Australia, health risks associated with the use of recycled wastewater are managed using the national Australian Guidelines for Water Recycling.6 A key feature of the Guidelines is a framework that promotes the application of quantitative microbial risk assessment principles using disability adjusted life years (DALYs). This marks a change from past management frameworks that were based on ‘too little, too late’ end-point monitoring of faecal indicators (e.g. E. coli). First introduced in the 2004 World Health Organization Guidelines for Drinking Water Quality,19 DALYs convert the likelihood of infection or illness into burdens of disease and set a tolerable risk benchmark of one micro-DALY (10−6 DALYs) per person per year. When applied to water recycling schemes, this benchmark is used to set microbial health-based targets (HBTs) as water treatment system performance targets, expressed as log reductions, for reference organisms that represent the major sewage pathogen groups (bacteria, viruses and protozoa). These HBTs can be met using multiple treatment barriers and by the adoption of on-site preventive measures that lower human exposure to recycled water at point of use.
The adoption of a risk-based approach inherently safeguards against emerging pathogen risks in the following ways:
Flexible framework capable of accommodating change: Risk management plans are an ongoing and iterative process, whereby risk is continually evaluated and updated based on new information including that pertaining to emerging pathogens (e.g. densities in source waters, exposure pathways, pathogen fate/response to treatment processes, dose–response and disease burden).
Use of conservative assumptions: In identifying hazards, it is impractical to set HBTs for all pathogens in sewage, therefore, the guidelines specify the use of reference pathogens which include Campylobacter for bacteria, rotavirus and adenovirus for viruses, and Cryptosporidium parvum for protozoa. These have been selected based on their high incidence within the community, high abundances in sewage, low infectious dose, high disease severity and resistance/resilience to treatment barriers. Hence, if these indicators are adequately controlled, the scheme should ensure protection against all pathogens of concern within the same class. Furthermore, conservative assumptions are also applied to estimate human exposure for many end-uses and both intended and unintended uses are considered.
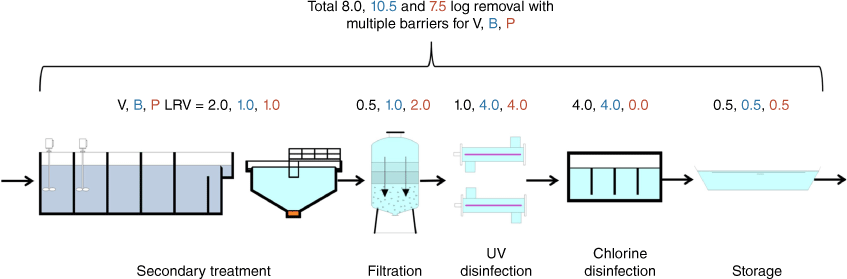
Redundancy in water treatment: Reuse schemes that supply water for high exposure uses (e.g. dual reticulation for unrestricted municipal use) require the use of multiple barriers to meet HBTs such as secondary treatment, filtration, UV and chlorine disinfection (Fig. 2). In the Fig. 2 example, the multiple barrier system-wide organism LRVs for virus, bacteria and protozoa (8.0, 10.5 and 7.5-log10 reductions respectively) exceed the stipulated guideline requirements for the highest risk recycled water end uses like dual reticulation (i.e. 6.5, 5.0 and 5.0-log10 reductions respectively). Validation of individual treatment barriers is also assessed, often through challenge testing, whereby verified pathogen log reduction credits are assigned using worse case 5th percentile performance values. Additionally, some treatment barriers have considerable flexibility in terms of being able to respond quickly to emerging risks or hazardous events. For example, disinfection processes such as chlorination and to some extent UV can be rapidly adjusted to increase disinfection performance (UV and chlorine dose) to deliver enhanced microbial inactivation as required.
Looking forward
Our experience with COVID has demonstrated that even with the emergence of new pathogens, the risks posed from recycled water are similar to those for known pathogens in terms of how these organisms respond to treatment barriers. Effectively, these emerging risks are managed by existing processes and management frameworks. Increased emphasis on wastewater monitoring20 combined with detection methods that are increasingly rapid, informative, sensitive and low cost21 will continue to shine the spotlight on microbial contaminants in wastewater. However, an increased capacity to detect known and emerging pathogens in wastewater does not necessarily translate to elevated risk because presence measured by molecular methods does not equate to infectivity and risk.22 Additionally, recycled water exposure pathways for emerging viruses in particular are not the highest risk pathways (Table 1) and so risks are likely to be substantially reduced prima facie. Of course the water industry must remain alert to novel and emerging microbial hazards and the continued adoption of risk-based management of recycled water is the best way of ensuring ongoing public safety and acceptance of water recycling operations.
Although current approaches are effective in managing risk, knowledge gaps do exist in some aspects of recycled water supply and these should be the focus of future research. For example, better understanding water quality in recycled water distribution networks, especially under a future warming climate and how this might impact potential regrowth of opportunistic pathogens.17 Another area of uncertainty is the emerging risk posed by antimicrobial resistance (AMR), where there is potential for complex but as-yet uncharacterised risks (acute and diffuse) arising from both AMR genes and organisms at various exposure points across wastewater treatment and water recycling systems.23–26 Some of these knowledge gaps will be the subject of future research to be undertaken by the newly established Cooperative Research Centre for Solving Antimicrobial Resistance in Agribusiness, Food, and Environments (CRC SAAFE; https://www.crcsaafe.com.au) (Fig. 3). There is also a level of uncertainty regarding non-enteric pathogens (bacterial and fungal) and the risks posed by opportunistic pathogens (e.g. Pseudomonas or Candida) for which dose-response models and exposure pathways are not well defined.17,27,28
Conclusion
There will continue to be new risks and uncertainties from emerging pathogens, but existing water industry processes and water recycling guidelines are able to manage these risks effectively as a result of in-built flexibility and conservatism in how water recycling systems are operated and how health risks are managed. As we move towards a future with increased climate variability and water supply insecurity, water recycling will play an increasingly important role in ensuring water security for our liveable urban environments and for future food production. Existing approaches to managing human health risks in recycled water supplies will be revised and updated in response to new risks to ensure that future risks from new and emerging pathogens continue to be managed appropriately.
Data availability
Data sharing is not applicable as no new data were generated or analysed during this study.
Conflicts of interest
Paul Monis and Ben van den Akker are employees of the South Australian Water Corporation, which is a supplier of recycled water, and both hold adjunct positions at the University of South Australia. The other authors declare no conflicts of interest.
Declaration of funding
This research did not receive any specific funding.
References
[1] World Health Organization (2022) WHO Sanitation. https://www.who.int/news-room/fact-sheets/detail/sanitation (accessed 15 September 2022)[2] Jiménez B, Asano T (2008) Water Reuse: An International Survey of Current Practices, Issues and Needs. 650 p. IWA Publishing, London, UK.
[3] Radcliffe JC (2004) Water Recycling in Australia. A review undertaken by the Australian Academy of Technological Sciences and Engineering (ATSE).
[4] Radcliffe, JC (2015) Water recycling in Australia – during and after the drought. Environ Sci Water Res Technol 1, 554–562.
| Water recycling in Australia – during and after the drought.Crossref | GoogleScholarGoogle Scholar |
[5] Radcliffe, JC and Page, D (2020) Water reuse and recycling in Australia — history, current situation and future perspectives. Water Cycle 1, 19–40.
| Water reuse and recycling in Australia — history, current situation and future perspectives.Crossref | GoogleScholarGoogle Scholar |
[6] NRMMC et al. (2006) Australian Guidelines for Water Recycling. Managing health and environmental risks (phase 1). In Natural Resource Management Ministerial Council, Environment Protection and Heritage Council, Australian Health Ministers Conference. National Water Quality Management Strategy, November 2006.
[7] Nghiem, LD et al.. (2020) The COVID-19 pandemic: considerations for the waste and wastewater services sector. Case Stud Chem Environ Eng 1, 100006.
| The COVID-19 pandemic: considerations for the waste and wastewater services sector.Crossref | GoogleScholarGoogle Scholar |
[8] Water RA (2020) Factsheet: SARS-CoV-2 - Water and Sanitation. Water Research Australia.
[9] Nelson, B (2022) What poo tells us: wastewater surveillance comes of age amid covid, monkeypox, and polio. BMJ 378, o1869.
| What poo tells us: wastewater surveillance comes of age amid covid, monkeypox, and polio.Crossref | GoogleScholarGoogle Scholar |
[10] Stevenson E et al. (2022) Scoping review into environmental selection for antifungal resistance and testing methodology. Environment Agency, Bristol, UK. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1091307/Scoping_review_into_environmental_selection_for_antifungal_resistance_and_testing_methodology_-_report.pdf (accessed 14 September 2022)
[11] Goldman, E (2020) Exaggerated risk of transmission of COVID-19 by fomites. Lancet Infect Dis 20, 892–893.
| Exaggerated risk of transmission of COVID-19 by fomites.Crossref | GoogleScholarGoogle Scholar |
[12] Wu, F et al.. (2021) Wastewater surveillance of SARS-CoV-2 across 40 U.S. states from February to June 2020. Water Res 202, 117400.
| Wastewater surveillance of SARS-CoV-2 across 40 U.S. states from February to June 2020.Crossref | GoogleScholarGoogle Scholar |
[13] Bivins, A et al.. (2020) Persistence of SARS-CoV-2 in water and wastewater. Environ Sci Technol Lett 7, 937–942.
| Persistence of SARS-CoV-2 in water and wastewater.Crossref | GoogleScholarGoogle Scholar |
[14] Greaves, J et al.. (2022) Sodium hypochlorite disinfection of SARS-CoV-2 spiked in water and municipal wastewater. Sci Total Environ 807, 150766.
| Sodium hypochlorite disinfection of SARS-CoV-2 spiked in water and municipal wastewater.Crossref | GoogleScholarGoogle Scholar |
[15] McKinney, KR et al.. (2006) Environmental transmission of SARS at Amoy Gardens. J Environ Health 68, 26–30.
[16] Yu, IT-S et al.. (2014) Severe acute respiratory syndrome beyond Amoy Gardens: completing the incomplete legacy. Clin Infect Dis 58, 683–686.
| Severe acute respiratory syndrome beyond Amoy Gardens: completing the incomplete legacy.Crossref | GoogleScholarGoogle Scholar |
[17] Drigo, B et al.. (2021) Inactivation, removal, and regrowth potential of opportunistic pathogens and antimicrobial resistance genes in recycled water systems. Water Res 201, 117324.
| Inactivation, removal, and regrowth potential of opportunistic pathogens and antimicrobial resistance genes in recycled water systems.Crossref | GoogleScholarGoogle Scholar |
[18] WHO (2020) First meeting of the WHO antifungal expert group on identifying priority fungal pathogens: Meeting Report. World Health Organization, Geneva, Switzerland.
[19] WHO (2004) Guidelines for Drinking-water Quality, Vol. 1, 3rd edn. World Health Organization, Geneva, Switzerland.
[20] Donner, E et al.. (2021) Wastewater monitoring for SARS-CoV-2. Microbiol Aust 42, 18–22.
| Wastewater monitoring for SARS-CoV-2.Crossref | GoogleScholarGoogle Scholar |
[21] Nnachi, RC et al.. (2022) Biosensors for rapid detection of bacterial pathogens in water, food and environment. Environ Int 166, 107357.
| Biosensors for rapid detection of bacterial pathogens in water, food and environment.Crossref | GoogleScholarGoogle Scholar |
[22] Sobsey, MD (2021) Absence of virological and epidemiological evidence that SARS-CoV-2 poses COVID-19 risks from environmental fecal waste, wastewater and water exposures. J Water Health 20, 126–138.
| Absence of virological and epidemiological evidence that SARS-CoV-2 poses COVID-19 risks from environmental fecal waste, wastewater and water exposures.Crossref | GoogleScholarGoogle Scholar |
[23] Christou, A et al.. (2017) The potential implications of reclaimed wastewater reuse for irrigation on the agricultural environment: the knowns and unknowns of the fate of antibiotics and antibiotic resistant bacteria and resistance genes – a review. Water Res 123, 448–467.
| The potential implications of reclaimed wastewater reuse for irrigation on the agricultural environment: the knowns and unknowns of the fate of antibiotics and antibiotic resistant bacteria and resistance genes – a review.Crossref | GoogleScholarGoogle Scholar |
[24] Garner, E et al.. (2021) Towards risk assessment for antibiotic resistant pathogens in recycled water: a systematic review and summary of research needs. Environ Microbiol 23, 7355–7372.
| Towards risk assessment for antibiotic resistant pathogens in recycled water: a systematic review and summary of research needs.Crossref | GoogleScholarGoogle Scholar |
[25] Rodríguez-Molina, D et al.. (2021) Carriage of ESBL-producing Enterobacterales in wastewater treatment plant workers and surrounding residents — the AWARE Study. Eur J Clin Microbiol Infect Dis. , .
| Carriage of ESBL-producing Enterobacterales in wastewater treatment plant workers and surrounding residents — the AWARE Study.Crossref | GoogleScholarGoogle Scholar |
[26] Zhang, A-N et al.. (2021) An omics-based framework for assessing the health risk of antimicrobial resistance genes. Nat Commun 12, 4765.
| An omics-based framework for assessing the health risk of antimicrobial resistance genes.Crossref | GoogleScholarGoogle Scholar |
[27] Roser, DJ et al.. (2014) Pseudomonas aeruginosa dose response and bathing water infection. Epidemiol Infect 142, 449–462.
| Pseudomonas aeruginosa dose response and bathing water infection.Crossref | GoogleScholarGoogle Scholar |
[28] Roser, DJ et al.. (2015) Dose-response algorithms for water-borne Pseudomonas aeruginosa folliculitis. Epidemiol Infect 143, 1524–1537.
| Dose-response algorithms for water-borne Pseudomonas aeruginosa folliculitis.Crossref | GoogleScholarGoogle Scholar |