A project to validate the GLU test for preterm birth prediction in First Nations women
Kiarna Brown A B * , Holger W. Unger A B C , Margaret Peel D , Dorota A. Doherty E , Martin Lee F , Agatha Kujawa G , Sarah Holder B , Gilda Tachedjian H I J , Lindi Masson H I K L , Jane C. Thorn B , John P. Newnham E and Matthew S. Payne EA Menzies School of Health Research, Charles Darwin University, Darwin, NT, Australia.
B Department of Obstetrics and Gynaecology, Royal Darwin Hospital, Darwin, NT, Australia.
C Department of Clinical Sciences, Liverpool School of Tropical Medicine, Liverpool, UK.
D Geraldton Regional Aboriginal Medical Service, Rifle Range Road, Rangeway, WA, Australia.
E Division of Obstetrics and Gynaecology, The University of Western Australia, Perth, WA, Australia.
F Rural Clinical School, The University of Western Australia, Perth, WA, Australia.
G Gove District Hospital, Nhulunbuy, NT, Australia.
H Life Sciences Discipline, Burnet Institute, Melbourne, Vic., Australia.
I Department of Microbiology, Monash University, Clayton, Vic., Australia.
J Department of Microbiology and Immunology, The Peter Doherty Institute for Infection and Immunity, University of Melbourne, Melbourne, Vic., Australia.
K Department of Pathology, Institute of Infectious Disease and Molecular Medicine (IDM), University of Cape Town, Cape Town 7925, South Africa.
L Centre for the AIDS Programme of Research in South Africa, Durban, South Africa.
|
Dr Kiarna Brown is a First Nations Specialist Obstetrician and Gynaecologist and Clinical Research Fellow. Her research interests are in reducing preterm birth for First Nations women. |
|
Dr Holger Unger is an Obstetrician and Gynaecologist at the Royal Darwin Hospital and a Senior Research Fellow at the Menzies School of Health Research. He conducts clinical research that focusses on the prevention of adverse birth outcomes in low-resource and remote populations. |
|
Maggie Peel is an experienced midwife and the Practice Manager at the Geraldton Regional Aboriginal Medical Service. She prides herself on empowering Aboriginal women to be able to receive and access the best antenatal care possible to assist them in gaining better health outcomes during their antenatal, intrapartum, and post-partum periods. |
|
Professor Dorota Doherty is Head of the Biostatistics and Research Design Unit at the Women and Infants Research Foundation at King Edward Memorial Hospital (KEMH), and an Adjunct Professor at the Division of Obstetrics and Gynaecology, the University of Western Australia (UWA). Her expertise in biostatistical techniques has been pivotal in translational research in clinical medicine with complex epidemiological models that explore associations between subject phenotypes and health outcomes. |
|
Dr Martin Lee is the Lead Medical Coordinator of the Rural Clinical School of Western Australia in Geraldton, maintains clinical work at Headspace Youth Focus, provides anaesthesia at St John of God Hospital, conducts sessional work at WA Cardiology, and is the current Chair of the Western Australian Primary Health Alliance – Midwest Gascoyne region. He has a keen rural community focus, and is passionate about quality workforce development and retention. |
|
Dr Agatha Kujawa is a Rural Generalist and GP-Obstetrician working in Nhulunbuy, East Arnhem Land. She is passionate about the provision of equitable healthcare for those living in rural and remote areas in Australia. |
|
Sarah Holder is a senior midwife and former Maternity Unit Manager at the Gove District Hospital located in Nhulunbuy, Arnhem Land. She has many years of experience in working with and caring for First Nations pregnant women in the NT. Sarah currently coordinates care for women with complex medical and psychosocial needs with Darwin’s Midwifery Group Practice. |
|
Professor Gilda Tachedjian is Head of Life Sciences at The Burnet Institute. She is a microbiologist with >25 years of experience in identifying and developing HIV antiviral strategies, and is recognised as an expert on HIV prevention in women and the role of the vaginal microbiome and their metabolites in HIV acquisition. She has a growing interest in its role in adverse reproductive health outcomes. |
|
Dr Lindi Masson is a Senior Research Fellow at the Burnet Institute, Honorary Research Associate at the University of Cape Town (UCT), Associate Member of the Institute of Infectious Disease and Molecular Medicine at UCT, Honorary Research Associate of the Centre for the AIDS Programme of Research in South Africa, and Adjunct Senior Lecturer at Monash University. She has been involved in genital microbiome and immunology research for >14 years. |
|
Dr Jane Thorn is a Senior Obstetrician and Gynaecologist, and current Head of the Department of Obstetrics and Gynaecology at the Royal Darwin Hospital. She has provided antenatal and obstetric care for First Nations women in remote and urban communities at the Top End for over a decade. |
|
Professor John Newnham is a Professor of Obstetrics at The University of Western Australia, and a sub-specialist in Maternal Fetal Medicine at KEMH. His enduring research interest has been to discover strategies to safely reduce the rate of preterm birth. He has published widely on this subject and is recognised as one of the world’s leading authorities. |
|
Dr Matt Payne is a Senior Research Fellow at UWA with expertise in perinatal molecular microbiology. His major research interest is the role of the perinatal microbiome in preterm birth. Dr Payne is the lead investigator on the current study and designed the GLU test. |
Microbiology Australia 43(3) 130-134 https://doi.org/10.1071/MA22032
Submitted: 24 June 2022 Accepted: 15 August 2022 Published: 13 September 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing on behalf of the ASM. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
The protocol described in the present article aims to validate the GLU test, a test of mid-pregnancy vaginal microbiome, for PTB risk prediction in pregnant First Nations women. Preterm birth (PTB; birth before 37 completed weeks gestation) is associated with a higher risk of adverse neonatal outcomes. First Nations communities are affected by increasing PTB rates, highest in remote communities, reaching 23%. Being able to predict women at high risk of PTB is one of the greatest challenges of our time. No reliable clinical predictors of PTB risk currently exist, beyond a previous history. Spontaneous PTB (sPTB) is highly associated with microbial infection. Recently, a Western Australian research team developed an innovative mid-pregnancy vaginal microbial DNA test, the ‘Gardnerella, Lactobacillus, Ureaplasma’ (GLU) test, capable of predicting up to 45% of sPTB cases. However, this test has only been validated in predominantly Caucasian pregnant women. The protocol described aims to validate the GLU test in pregnant First Nations women and where applicable, make modifications to this test to improve sensitivity and specificity within this population.
Keywords: Australian First Nations, diagnostic test, genotype, microbiome, pregnancy, preterm labour, preterm premature rupture of membranes, real-time polymerase chain reaction, vagina.
Introduction
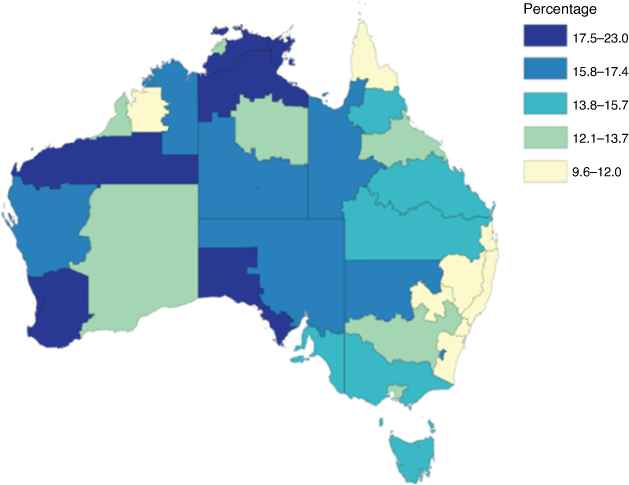
Preterm birth (PTB; delivery before 37 gestational weeks) is the leading cause of death and disability in children under 5 years of age. Globally, approximately 10% of infants are born too early.1 Australian First Nations (the Aboriginal and Torres Strait Islander people of Australia) women are at far greater risk of this major complication of pregnancy. Nationally, in 2019, 13% of babies born to First Nations mothers were preterm, compared with 8.3% of babies from non-First Nations mothers.2 The PTB incidence is even greater for First Nations women in regional and remote parts of the Northern Territory (NT) and Western Australia (WA) with national data reporting rates up to 23%3 (Fig. 1). The risk factors for PTB in First Nations women are multifactorial and not completely understood. Much of the discrepancy is likely the result of disparities in social determinants of health, fuelled by poverty, racism, and intergenerational trauma.4 However, at least one-quarter of all PTBs, but especially those that occur due to spontaneous preterm labour (sPTB), are attributed to intrauterine bacterial infection, especially at earlier gestational ages (GA).5
|
Numerous bacterial taxa have been implicated, with the bulk of these represented by Ureaplasma spp. and anaerobic organisms associated with a dysbiotic vaginal state.6 Conversely, dominance of certain Lactobacillus spp. in the vagina during pregnancy may offer some level of protection from sPTB.7 However, the vaginal bacterial microbiome varies substantially between ethnic cohorts,8 and little is known about its composition in First Nations women; the only study completed to date recruited 23 pregnant Australian First Nations women and reported a vaginal microbiome composition not dissimilar to that observed amongst African–American women, characterised by Lactobacillus spp.-depletion and presence of diverse anaerobic genera.9
Although infection may result in sPTB,10 it is the mother’s inflammatory response to infection that triggers preterm labour.11 Whereas data exist on uterine inflammation during infectious and non-infectious scenarios,12 very little is known about the pregnant vaginal environment relative to the microbial profile.13 Based on studies in non-pregnant women, McKinnon et al. have defined that a non-optimal vaginal microbiome elicits a pro-inflammatory milieu in the cervicovagina in contrast to an optimal vaginal microbiota that is non-inflammatory.14 Hence, an understanding of the vaginal pro- and anti-inflammatory state in pregnancy, alongside its microbial composition and function, may identify additional host and microbial biomarkers for sPTB prediction, as well as furthering our knowledge of the mechanistic nature of infection-related sPTB, which remains poorly understood.
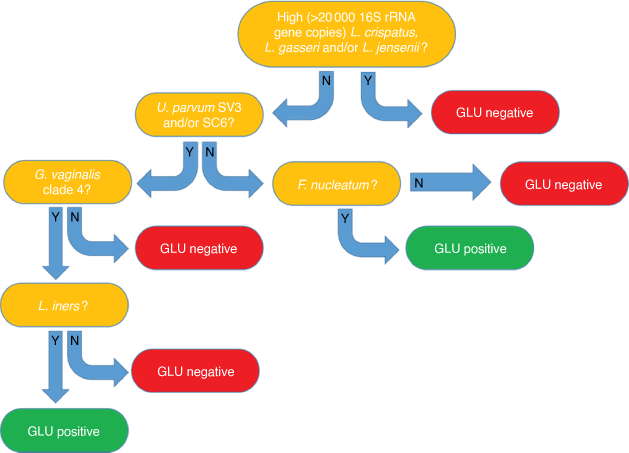
Payne et al. recently conducted the largest mid-pregnancy vaginal microbiology study to date, the Predict1000 study, which resulted in the development of a novel mid-gestation vaginal microbial DNA test for prediction of Australian women at high sPTB risk, the GLU test (Fig. 2).15 In a cohort of 936 women, this test was able to detect women at risk of sPTB <37 weeks’ GA and ≤34 weeks’ GA, with sensitivities of 37.9 and 44.4%, respectively. More importantly, women who had a prior history of sPTB, our current best clinical predictor of sPTB risk, were almost as likely to deliver via sPTB as women who were GLU-positive in mid-gestation (adjusted odds ratio 3.61 vs 3.28). This test now underpins a National Health and Medical Research Council-funded randomised controlled trial (RCT) (ACTRN12617001593325) to assess the efficacy of a novel antimicrobial and probiotic treatment regimen in GLU-positive women to reduce sPTB risk. However, data on performance of the GLU test came from a predominantly Australian Caucasian cohort; only 22 women recruited to the Predict1000 study identified as First Nation Australians (2.3%).
|
Further research is needed to document the vaginal microbiome and associated inflammatory state in pregnant First Nations women. This includes an assessment of whether the GLU-test in its current form can identify First Nations women at increased risk of sPTB, and if not, ascertain if different host and microbial biomarkers can be identified that may be useful for this purpose.
Very few tools are currently available to identify women at high risk of sPTB during pregnancy. Identification of vaginal microbial biomarkers during mid-pregnancy has high potential for translation into clinically relevant tools that, importantly, can be acted on with suitable interventions to either prevent sPTB from occurring or delay the onset of preterm labour.
Methods
Aims and objectives
This research aims to develop approaches to identify pregnant First Nations women at risk of sPTB based on vaginal host and microbial biomarkers evaluated in early to mid-gestation. The specific research objectives are to:
Assess performance of the GLU test for prediction of sPTB in First Nations women.
Characterise the early to mid-gestation vaginal microbiome, inflammatory state, and host/microbial protein profile and document associations between these and maternal factors (such as smoking and diabetes).
Identify microbial and host biomarkers that will enable modification of the existing GLU test to enhance sPTB prediction in First Nations women.
Study design
To address the aims and objectives we will conduct a prospective cohort study.
Study setting and population
In the Northern Territory, recruitment will occur from Royal Darwin Hospital (RDH), and Gove District Hospital (GDH). Both hospitals service towns and communities across the Top End. Approximately 600 First Nations mothers have babies across these two hospitals per annum.16
In Western Australia, recruitment will occur from the Geraldton Regional Aboriginal Medical Service (GRAMS), a community health service for First Nations peoples. GRAMS midwives provide antenatal care for over 100 pregnancies per year.
Pregnant First Nations women aged 16+ years attending antenatal clinics at ≤24 weeks’ GA will be invited to participate. Exclusion criteria include multiple pregnancies, current symptomatic vaginal infections, current or recent (preceding 2 weeks) antibiotic/antimycotic use, cervical sutures, high dependence on medical care, illicit drug use, and lack of capacity to provide written informed consent.
Sample size
The anticipated recruitment number is 750 women, 500 at RDH/GDH and 250 at GRAMS. Assuming a baseline sPTB rate in Aboriginal women of ~7%, a cohort of 750 will attain ≥90% power to detect an increase in sPTB probability associated with a GLU (or modified version) positive test by an odds ratio ≥2.25 (increase from 7 to 14.5% sPTB risk), while simultaneously adjusting for other relevant microbial and clinical risk factors with partial r2 = 0.1.
Sample collection
Participating women will first complete a medical/lifestyle questionnaire enquiring about key clinical and environmental factors that may impact the vaginal microbiome and pregnancy outcome. These include diabetes (type 1, type 2, or gestational), smoking and alcohol intake (assessed semi-quantitatively), and area of primary residence (urban vs rural). Women will then provide two self-collected vaginal swabs, one in liquid Amies media (COPAN e-Swab) for vaginal microbiome profiling and GLU analysis and one in QIAGEN Allprotect media for cytokine, chemokine, and host and microbial protein content analyses. A vaginal pH reading will also be obtained using a self-test kit (Canesten).
Laboratory methods
DNA will be extracted from swab 1 and GLU-status assessed via qPCR.15 The vaginal microbiome will then be characterised using full-length 16S rRNA gene sequencing on the PacBio Sequel II as per Goldenberg et al.10 Sequences will be analysed using Mothur (v1.47).17
Swab 2 (n = 180) will undergo cytokine and chemokine quantification and metaproteomic analyses to define the host inflammatory state and microbial functional properties. Samples will be selected based on pregnancy outcome, with a 60:120 split between sPTB (all cases in the cohort) and term births. This subgroup will attain ≥80% power to detect medium effect sizes for binary descriptors and ≥95% power to detect a difference of 0.5 s.d. for continuous descriptors between groups. Cytokines and chemokines will be analysed using a multiplex Luminex assay; targets include IL-1α, IL-1β, IL-6, IL-8, TNF-α, IL-1RA, RANTES, CXCL10, MCP-1, MIP-1α, MIP-1β and MIP-3α. Metaproteomic analyses will involve shotgun liquid chromatography tandem mass spectrometry using a Q-Exactive Quadrupole-Orbitrap mass spectrometer as per Alisoltani et al.18
End-points
The primary clinical end-point in this study is sPTB, defined as the spontaneous commencement of labour before 37 weeks’ gestation. Secondary end-points include established PTB research Core Outcome Measures,19 including sPTB ≤34- and 28-weeks GA. All end-points will be measured by obtaining delivery outcome data from NT and WA hospital pregnancy databases and patient medical records.
Statistical analysis
Evaluations of the effects of microorganisms, cytokines, host and microbial proteins, and maternal characteristics on the timing of birth will be conducted using linear, logistic and Cox proportional hazards regressions, as appropriate for gestational age at birth or sPTB. These regressions will be supplemented with recursive partitioning models, such as binary, regression and survival trees, designed to explore the non-linear relationships within the laboratory data alone and when combined with other obstetrics risk factors.
Ethics
Ethical approval to conduct this study was granted by the Human Research Ethics Committee of the Northern Territory Department of Health and Menzies School of Health Research (HREC-2020-3659), and the Western Australian Aboriginal Health Ethics Committee (HREC-937). The NT study was approved by the Menzies School of Health Research Child Health First Nations Reference Group.
Conclusion
PTB has devastating impacts on First Nations families and communities, with significant long- and short-term complications. There are substantial emotional, psychological, and financial costs to families and communities. The cost to health services remains high. The continued poor rates are unacceptable. Further understanding of the pathophysiology is required. The presence and abundance of some bacterial pathogens in the mid-trimester vaginal microbiome has been shown to increase the risk of PTB in Caucasian women but this information is yet to be validated in First Nations women. Data generated from this study will confirm whether the GLU test in its current form is suitable for prediction of First Nations women at increased sPTB risk. In the case that the test is unsuitable for this cohort, additional data from 16S rRNA gene and inflammatory marker/protein profiling may allow cohort-specific microbial DNA and host and microbial protein signatures to be identified that predict First Nations women at high sPTB risk and who may benefit from mid-gestation treatment with specific antimicrobials/probiotics. Additionally, identifying protein biomarkers could be used to develop true point-of-care tests that are low cost and convenient to use in very remote settings where PTB rates are highest. This is likely to prolong pregnancy for many women and ultimately reduce mortality and morbidity for hundreds of infants each year.
Data availability
Data sharing is not applicable as as no new data were generated or analysed during this study.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
This is a National Health and Medical Research Council (NHMRC) funded project.
References
[1] Chawanpaiboon, S (2019) Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob Health 7, e37–e46.| Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis.Crossref | GoogleScholarGoogle Scholar |
[2] AIHW (2021) Australia’s mothers and babies. AIWH, Canberra.
[3] AIHW (2020) Antenatal care use and outcomes for Aboriginal and Torres Strait Islander mothers and their babies 2016-2017. AIHW, Canberra.
[4] Brown, K (2021) A better start to life: risk factors for, and prevention of, preterm birth in Australian First Nations women – a narrative review. Int J Gynaecol Obstet 155, 260–267.
| A better start to life: risk factors for, and prevention of, preterm birth in Australian First Nations women – a narrative review.Crossref | GoogleScholarGoogle Scholar |
[5] Goldenberg, RL (2008) Epidemiology and causes of preterm birth. Lancet 371, 75–84.
| Epidemiology and causes of preterm birth.Crossref | GoogleScholarGoogle Scholar |
[6] Mendz, GL (2013) Bacterial aetiological agents of intra-amniotic infections and preterm birth in pregnant women. Front Cell Infect Microbiol 3, 58.
| Bacterial aetiological agents of intra-amniotic infections and preterm birth in pregnant women.Crossref | GoogleScholarGoogle Scholar |
[7] Kindinger, LM (2017) The interaction between vaginal microbiota, cervical length, and vaginal progesterone treatment for preterm birth risk. Microbiome 5, 6.
| The interaction between vaginal microbiota, cervical length, and vaginal progesterone treatment for preterm birth risk.Crossref | GoogleScholarGoogle Scholar |
[8] Ravel, J (2011) Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci U S A 108, 4680–4687.
| Vaginal microbiome of reproductive-age women.Crossref | GoogleScholarGoogle Scholar |
[9] Dinsdale, NK (2020) Comparison of the genital microbiomes of pregnant Aboriginal and non-aboriginal women. Front Cell Infect Microbiol 10, 523764.
| Comparison of the genital microbiomes of pregnant Aboriginal and non-aboriginal women.Crossref | GoogleScholarGoogle Scholar |
[10] Goldenberg, RL (2000) Intrauterine infection and preterm delivery. N Engl J Med 342, 1500–1507.
| Intrauterine infection and preterm delivery.Crossref | GoogleScholarGoogle Scholar |
[11] Romero, R (2007) Inflammation in pregnancy: its roles in reproductive physiology, obstetrical complications, and fetal injury. Nutr Rev 65, S194–S202.
| Inflammation in pregnancy: its roles in reproductive physiology, obstetrical complications, and fetal injury.Crossref | GoogleScholarGoogle Scholar |
[12] Romero, R (2014) Prevalence and clinical significance of sterile intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Reprod Immunol 72, 458–474.
| Prevalence and clinical significance of sterile intra-amniotic inflammation in patients with preterm labor and intact membranes.Crossref | GoogleScholarGoogle Scholar |
[13] Fettweis, JM (2019) The vaginal microbiome and preterm birth. Nat Med 25, 1012–1021.
| The vaginal microbiome and preterm birth.Crossref | GoogleScholarGoogle Scholar |
[14] McKinnon, LR (2019) The evolving facets of bacterial vaginosis: implications for HIV Transmission. AIDS Res Hum Retroviruses 35, 219–228.
| The evolving facets of bacterial vaginosis: implications for HIV Transmission.Crossref | GoogleScholarGoogle Scholar |
[15] Payne, MS (2021) A specific bacterial DNA signature in the vagina of Australian women in midpregnancy predicts high risk of spontaneous preterm birth (the Predict1000 study). Am J Obstet Gynecol 224, 206.e1–206.e23.
| A specific bacterial DNA signature in the vagina of Australian women in midpregnancy predicts high risk of spontaneous preterm birth (the Predict1000 study).Crossref | GoogleScholarGoogle Scholar |
[16] Li L, O’Neil L (2021) Mothers and Babies 2018: Northern Territory Midwives’ Collection. Department of Health, Darwin, Northern Territory.
[17] Schloss, PD (2020) Reintroducing mothur: 10 years later. Appl Environ Microbiol 86, e02343-19.
| Reintroducing mothur: 10 years later.Crossref | GoogleScholarGoogle Scholar |
[18] Alisoltani, A (2020) Microbial function and genital inflammation in young South African women at high risk of HIV infection. Microbiome 8, 165.
| Microbial function and genital inflammation in young South African women at high risk of HIV infection.Crossref | GoogleScholarGoogle Scholar |
[19] van ‘t Hooft, J (2016) A core outcome set for evaluation of interventions to prevent preterm birth. Obstet Gynecol 127, 49–58.
| A core outcome set for evaluation of interventions to prevent preterm birth.Crossref | GoogleScholarGoogle Scholar |


