The puzzle of plague transmission
Patricia Morison A and Ian Morison A BMicrobiology Australia 41(4) 172-174 https://doi.org/10.1071/MA20047
Published: 28 October 2020
Abstract
Bubonic plague is among the most feared diseases in human history, not only because of its death toll but also for its consequential impact on the way of life and economic endeavour of human society. Every few hundred years the advance of a pandemic has raised impotent fear, until the early 20th century when microbiological research solved the mystery of how it is transmitted to its victims, opening the way to protective measures.
History of bubonic plague
Recent archaeological evidence has pushed the existence of bubonic plague back to about 3000 BC, at a Neolithic burial site in Sweden1. More recent and well recorded evidence of the devastating impact of plague includes the sixth century pandemic that came from the East to hasten the end of the Roman Empire; the 14th century pandemic that travelled the Silk Road from China to devastate the people and economy of Europe in wave after wave of infection; Plague advanced again across Europe in the 17th century when infection was believed to come from foul air.
Of special interest today is the pandemic that came from China’s Yunnan province in the late 19th century, a time when modern scientific research was homing in on the bacterial source of many diseases. This pandemic reached Hong Kong in 1894 and spread to the world along shipping routes. However, the popular medieval belief that the disease was contagious and was carried in bad air had not changed much. A decade of scientific work had identified rats as carriers of plague, but bacteriologists held a variety of conflicting beliefs about how it might be carried to humans.
Alexandre Yersin of the Pasteur Institute discovered the plague bacillus in rats in 1894a. It was initially named Pasteurella pestis, and not renamed Yersinia pestis until 1944. Yersin demonstrated that plague bacilli were present in both rats and humans that had died from the disease, but the means of its transmission across the last link in the rat-flea-man chain remained a mystery. The idea of such a link was passionately rejected by advanced societies who could not accept that the insignificant flea, carried by animals, could be a carrier of plague to humans. Epidemiological studies had, however, noted that the infection of humans seemed to be related to the accessibility of dwellings to rats.
Sydney’s bubonic plague
John Ashburton Thompson, an epidemiologist and President of the NSW Board of Health, kept abreast of the southward movement from Hong Kong of plague outbreaks and prepared for its arrival in Sydney. On 19 January 1900 the first reported case, a wharf carter, caused panic because it was believed, even by many in the medical profession that bubonic plague, like the rare pneumonic variety, could pass from person to person and would therefore lead to widespread contagion. The terrified Sydney community readily accepted the strict public health measures arranged by Thompson: quarantine of exposed individuals; catching of rats and demolition of rat-infested areas; the disinfection of victims’ houses; doctors’ visits with kits to prepare cultures from the pus of the buboes on victims and to dispense an unproved vaccine of doubtful benefit. Public health measures seemed to have ended Sydney’s epidemic by September 1900, after it had infected 303 people, of whom 103 died2.
Thompson’s epidemiological examination of the circumstances of each case demonstrated the abundance of plague-affected fleas on rats, and confirmed that the disease was not transmitted by its human victims to others. He also confirmed, on epidemiological grounds, that the flea was the agent of transmission3. In the laboratory his assistant, Frank Tidswell, Australia’s first native-born microbiologist, identified the bacillus in plague rat fleas (Xenopsylla cheopis) and confirmed earlier work that showed that crushed fleas from a diseased rat transmitted plague when injected into mice4. In Sydney’s second but smaller outbreak of bubonic plague in 1902 he attempted to convey plague from rat to rat via fleas but the outbreak ended in June, cutting off the supply of specimens, before he could complete the task5.
Thompson and Tidswell had arrived at a plausible theory on the etiology of plague, but there was still no evidence as to how the flea transmitted the plague bacillus to its victims.
Lister Institute and plague in India
Since its arrival in 1896 plague in India had spread at an alarming rate, killing 80% of the people it infected. In September 1904 the British Government’s India Office asked the Lister Institute for help and Director Charles Martin accepted the challenge. A Commission for the Investigation of Plague in India was appointed, led by Martin, who had previously taught medical sciences at the Universities of Sydney and Melbourne.
Martin chose districts of Bombay (now Mumbai) for investigation, and arranged the organisation of the project with Indian officials. From October 1905 to September 1906 over 100 000 rats were caught and examined, 15% found to be infected with plague. The circumstances of over 10 000 humans who had died from plague in that period were written up. The Indian Government, having recognised the calibre of the Lister’s work, appointed its key scientists to take charge of the project in 1906. Laboratory experiments explored the transmission of plague from rats to domestic animals, and the infectivity of human housing conditions. Studies also extended to two isolated villages in the Punjab to find out how plague spread over distances. The Plague Commission’s reports provided a mass of data on the incidence and transmission of plague, with recommendations for its management. However, it was unable to reach a conclusion on the question of how plague was transmitted by fleas to its victims.
Back in London Martin’s first priority was to deal with the refusal of British doctors and plague experts to accept that fleas bite humans. Hundreds of experiments using Lister Institute volunteers, who exposed their arms to different species of healthy fleas, starved for up to 14 days, showed that they do. He concluded the doubters were ignorant of ‘the variety and distribution of fleas’ in the world so were relying on ‘conclusions drawn from too meagre experimentation’6.
Solving the puzzle of transmission
With Arthur Bacot, a self-educated entomologist, Martin set out to discover how infected fleas transmitted plague bacilli to humans. Of approximately six possible candidates (Chrystos Lynteris more recently investigated another theory7) there were two credible ways: by the rat rubbing flea-faeces into recent flea bites on the victim; or infection by the flea in the act of sucking blood from the victim. They rejected the first of these on the grounds that flea faeces ‘do not as a rule contain many bacilli, and soon dry up’, and bacilli that have passed through the flea gut do not have ‘a high degree of virulence’. They then turned to the remaining possibility8.
A series of experiments showed that plague can be conveyed to another animal during the act of an infected flea’s feeding, but only sometimes, even when many opportunities were made available for the flea to feed on its victim. However, careful observation through a hand lens showed that some feeding fleas had no pink streak of rat blood: despite sucking strongly and persistently, no blood was entering their stomachs.
This chance observation spurred them on, redoubling their efforts at delicate flea dissection. They needed to get a sequential understanding of the growth of plague bacilli in the flea, and how it impacted on the workings of the flea’s alimentary canal, in particular the pumping mechanism that sucked flea-blood along its oesophagus, pushing it through a one-way valve (proventriculus) into the stomach.
After many flea dissections Martin and Bacot acquired a succession of infected specimens. This showed, two days after feeding on an infected rat, blood in the flea’s stomach contained minute brown specks of plague bacilli, the first of four stages illustrated in Figure 1. At Stage 3 in Figure 1 the gelatinous mass of bacilli led to failure of the one-way valve, allowing a plug of bacilli culture to extend up the oesophagus, stopping fresh rat blood from entering the flea’s stomach despite continued efforts at sucking (Stage 4 in Figure 1). At any momentary pause in sucking the elastic recoil of the oesophageal wall regurgitates blood back into the wound, carrying with it plague bacilli8. They found that two species of rat flea, Xenopsylla cheopis and Ceratophyllus fasciitis could transmit plague during the act of sucking, and were probably responsible for all of the infections obtained by experiment.
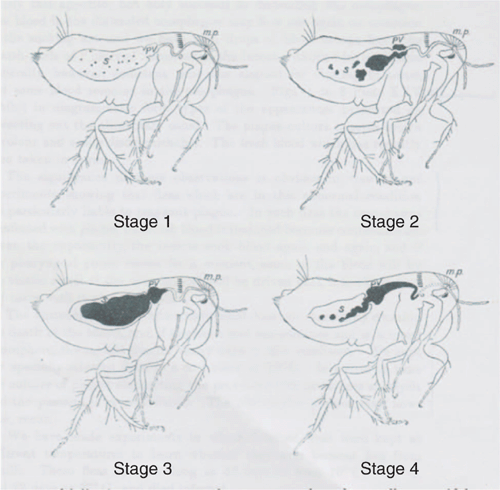
|
Conclusions
Martin and Bacot’s step by step persistence was typical of Martin’s research methodology, making him an ‘Unstoppable Plugger’ in the eyes of his students at Melbourne University. This, combined with his inspirational enjoyment of the ‘game’ of research, gave him an aura known in medical circles as ‘The Martin Spirit’ (reviewed in 9).
Martin and Bacot’s demonstration of how bubonic plague was transmitted to its victim encouraged public health authorities to promote the rat-proofing of houses. However, this was rarely possible for people in the developing world, where bubonic plague remains regionally endemic. Despite a century of work on vaccines, no long-lasting one seems yet to be available. However, medical protection, in support of intravenous fluids and respiratory aid, did come in mid-century in the form of antibiotics10.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
This research did not receive any specific funding.
References
[1] Starr, M. (2018) Scientists just found the oldest evidence of plague, in the bones of a neolithic woman. Science Alert 7 December. https://www.sciencealert.com/scientists-have-discovered-the-earliest-evidence-of-plague-in-a-neolithic-graveyard[2] Tidswell, F. (1901) Some practical aspects of the plague at Sydney. J. Sanitary Inst. xxi, 549.
[3] Thompson, A. (1900) Report of Chief Medical Officer on Outbreak of Plague at Sydney, N.S.W.
[4] Tidswell, F. (1900) Appendix A to Report on Outbreak of Plague at Sydney by A Thompson.
[5] Tidswell, F. (1903) Report to Board of Health on Second Outbreak of Plague at Sydney in 1902.
[6] Martin, C. (1911) Discussion on the spread of plague BMJ 2, 1249–1263.
[7] Lynteris, C. (2017) A ‘suitable soil’: plague’s urban breeding grounds at the dawn of the third pandemic. Med. Hist. 61, 343–357.
| A ‘suitable soil’: plague’s urban breeding grounds at the dawn of the third pandemic.Crossref | GoogleScholarGoogle Scholar | 28604289PubMed |
[8] Bacot, A.W. and Martin, C J (1914) Observations on the mechanism of the transmission of plague by fleas. J. Hygiene 13, 423–439.
[9] Barnard, R. and Power, C. (2020) The Martin Spirit: Charles Martin and the Foundation of Biological Science in Australia. [Book review.] Microbiol. Aust
[10] Jefferson, T. et al. (1998) Vaccines for preventing plague. Cochrane Database Syst. Rev. CD000976.
Biographies
Patricia Morison took a history major at the University of Western Australia. She acquired an interest in medical affairs when working in public health in Canberra and London. While tutoring at the Australian National University she contributed a number of entries to the Australian Dictionary of Biography in the field of medical science. As an independent scholar in this field her books are J T Wilson and the Fraternity of Duckmaloi (1997) and The Martin Spirit: Charles James Martin and the Foundation of Biological Science in Australia (2019).
Ian Morison, civil engineer and town planner, was transport planner for Canberra with the National Capital Development Commission, before entering the Commonwealth Public Service. In retirement he has acted as a research assistant to Patricia.
a Kitasato Shibasaburo’s discovery of the bacillus at about the same time led to a long dispute over who was first.


