Gonococcal antimicrobial resistance: 80 years in the making
David SpeersUWA Medical School, The University of Western Australia, Perth, WA 6000, Australia, Email: David.Speers@health.wa.gov.au
Microbiology Australia 40(2) 57-62 https://doi.org/10.1071/MA19017
Published: 18 April 2019
Antimicrobial resistance has been a problem for the treatment of gonorrhoea since the introduction of sulfa drugs in the 1930s. The gonococcus has a remarkable ability to obtain the genetic elements required to develop resistance and for these resistant strains to then widely disseminate. Many decades of antibiotic monotherapy have seen the introduction of a number of antibiotic classes herald a promising new era of treatment only to subsequently fail due to resistance development. The world is now faced with the prospect of extensively resistant Neisseria gonorrhoea and requires a coordinated action plan to detect and treat these resistant strains.
The pre-chemotherapy era of gonorrhoea treatment
Gonorrhoea has been known since biblical times, and has been given names such as ‘the clap’, presumably from the medieval Parisian prostitute district Les Clapiers. Following the fatal attempt by the 18th century English surgeon John Hunter to ‘prove’ syphilis and gonorrhoea were the same disease (he subsequently died of co-transmitted syphilis), the German microbiologist Albert Neisser discovered the gonococcus in 1879. It was first cultivated in 1882 by Leistikow and Loeffler but it was the introduction of a new bacterial staining method by Hans Gram in 1884 that greatly simplified its identification1.
The recommended treatment of gonorrhoea at this time was botanicals, such as Indonesian pepper and South American balsam of copaiba2. Neisser produced a vaccine from killed gonococci in the 1890s which benefitted the arthritis, possibly from the fever it induced, but not the urethritis. Heat therapy of joints and genitalia was initiated in 1913 and the Mayo Clinic extended this to a fever cabinet, which included heating elements in the vagina and rectum. The view of Osler in his 1920 edition of ‘Principles and Practice of Medicine’ was that drugs were of little value3. Intravenous mercurochrome, a complex of fluorescein, bromine and mercury, was later introduced, which was then combined with urethral irrigations of potassium permanganate.
The chemotherapy era of gonorrhoea treatment
The chemotherapy era of gonorrhoea treatment was heralded by the 1937 introduction of the much more effective sulfanilamide at the Johns Hopkins University Clinic and then in London where a three-week course achieved an 80% cure rate. Sulfanilamide was then superseded by the analogue sulfathiazole, which was better tolerated with a shorter treatment course. However, by the 1940s resistance to sulfonamides was found and heat treatment was reintroduced with the sulfonamide for better cure rates.
A significant advance in treatment occurred with the introduction of penicillin, which was almost universally curative, even with low doses of relatively impure product. Such was the euphoria at the time that penicillin was advertised as curative in four hours and was provided free by health departments. However, in a repeat of the sulfonamide experience cases resistance to penicillin became more frequent through the 1950s. The response was to increase the penicillin dose from 1.5 g to 2.4 g, replace penicillin with amoxicillin which had a better oral bioavailability and pharmacodynamics, and finally in 1969 to combine amoxicillin with probenecid to return the cure rates to 95% or better.
The demise of penicillins as a reliable treatment for gonorrhoea was due to the 1976 appearance of plasmid-mediated penicillinase production resulting in high level resistance2. These penicillinase-producing Neisseria gonorrhoeae (PPNG) rapidly spread to most parts of the world, and together with the discovery in 1980 of a new chromosomal mutation also causing high level resistance, led to the abandonment of penicillin and amoxicillin for gonococcal therapy. Only in several remote populations, such as northern Western Australia and the Northern Territory did antimicrobial susceptible gonococcal strains remain predominant. Tetracycline was initially effective but resistance developed within a few years when the R-plasmid in PPNG acquired the genes for high level tetracycline resistance, resulting in its withdrawal from empiric treatment in 1987. Spectinomycin, introduced in 1967, replaced tetracycline as the penicillin alternative but reports of high level spectinomycin resistance in PPNG emerged in 19814.
The modern era of gonorrhoea treatment
The recommendation for monotherapy in the treatment of gonorrhoea persisted following the downfall of penicillins, tetracycline, and spectinomycin in most parts of the world. The fluoroquinolone antibiotics ciprofloxacin and ofloxacin became available in the 1990s but by 2004 resistance was such that their recommendation for empiric gonococcal treatment was withdrawn. Extended spectrum cephalosporins (ESCs) were introduced in the 1980s and quickly became the treatments of choice, with some countries, such as Japan, using the oral drug cefixime while other countries used parenteral ceftriaxone. Despite monotherapy with ceftriaxone as empiric and directed anti-gonococcal therapy for several decades resistance did not seem to develop readily. This was possibly due to its very favourable pharmacodynamic profile facilitating clearance of pharyngeal N. gonorrhoeae, a property that was variably lacking in the preceding monotherapy regimens. However, this optimism came to an end in 2007 when ESC-resistant N. gonorrhoeae was discovered in Japan and Australia and in 2008 when reduced susceptibility of N. gonorrhoeae to cefixime was detected in the USA. This was followed by the detection of ceftriaxone resistance in Japan, France and Spain in 20115–7. Therefore the era of sequential monotherapy for treatment of gonorrhoea was witnessing the potential demise of a fourth antibiotic class, the ESCs, with few other single dose antibiotic options available.
Gonococcal resistance to ceftriaxone is primarily due to alterations in the penA gene encoding penicillin binding protein 2 (PBP2). The alteration is most frequently due to acquisition of a mosaic gene by transformation following recombination with penA genes from commensal oral Neisseria species. Specific amino acid alterations to PBP2 near the beta-lactam active site result in the decreased susceptibility of gonococci to cephalosporins whereas high level resistance is due to amino acid changes in the αβ2 helix and β3 strand of PBP2 interfering with cephalosporin binding and acylation2.
Combination therapy for gonorrhoea
To respond to the looming threat of widespread ESC resistance a review of current guidelines was warranted, with the options being to increase the dose of the cephalosporin, combine it with another antibiotic with a different mechanism of action, or move to another antibiotic class.
The British Association for Sexual Health and HIV recommended ceftriaxone and azithromycin combination therapy in 2011 but this year has revised this to recommend an increased ceftriaxone dose of 1 g and removal of azithromycin8. This was due to a number of reasons; the increased modal ceftriaxone minimum inhibitory concentration (MIC) distribution in Britain, the emergence of ceftriaxone resistance from other regions of the world where gonococcal ceftriaxone reduced susceptibility is more frequent, the lack of evidence that combination therapy lowers the potential to promote antimicrobial resistance, and the risk of azithromycin resistance increasing when used as part of combination therapy thereby diminishing its use as an alternative therapy for ESC-resistant gonorrhoea.
Ceftriaxone at a dose of 500 mg is recommended for uncomplicated gonorrhoea in the Therapeutic guidelines – Antibiotic Version 15 and the Australian STI management Guidelines whereas the Centers for Disease Control (CDC), USA and the World Health Organization (WHO) treatment guidelines recommended a dose of 250 mg. Pharmacodynamic analysis has shown that for a gonococcal strain with an MIC of 0.5 μg/mL the time above the MIC increases from 15.6 h for a 500 mg dose to 24.3 h for a 1 g dose of ceftriaxone9 but almost all gonococcal isolates in Australia remain susceptible (MIC <0.125 μg/mL) to ceftriaxone. Therefore the current recommended Australian dose should achieve sufficient time above the MIC to remain highly effective for uncomplicated gonorrhoea for all stains with an MIC of 0.25 μg/mL or less. Also, the prevalence of isolates with decreased susceptibility to ceftriaxone has actually fallen since 2013 with the introduction of combination therapy with azithromycin10.
Combination therapy is not a new concept in microbiology, where it is employed to either produce a synergistic effect by reducing the MIC of the organism below that compared to when the antibiotic is used alone, to target specific biochemical pathways in bacteria such as toxin production to reduce symptoms, or to broaden the spectrum of cover to a greater range of pathogens when the microbial aetiology is unknown. Combination therapy for gonorrhoea has been used for some time in the remote regions of Australia to treat the high rate of coincident Chlamydia trachomatis infection. In contrast, the more recent rationale for the addition of azithromycin to ceftriaxone for the treatment of gonorrhoea was to improve treatment outcomes and to prevent or retard the development of ceftriaxone resistance, as borne out of clinical experience with other bacteria that acquire resistance quickly. The theory is that it is more difficult for a bacterium to simultaneously develop resistance to two antibiotics with different mechanisms of action, but this rationale has been questioned11. Moreover, this approach has added cost, the risk of adverse effects from two drugs, and the possible acceleration of azithromycin resistance development in gonococci as well as other sexually transmitted pathogens, such as Mycoplasma genitalium and Treponema pallidum.
The WHO and the CDC revised their treatment guidelines by adding a second antibiotic to ceftriaxone. The CDC in 2010 recommended the addition of either azithromycin or doxycycline but in 2015 dropped doxycycline due to growing resistance in the USA. In 2016 the WHO similarly revised their 2003 guidelines to also recommend dual therapy. There is no microbiological evidence to support the concept that either increasing the dose of ceftriaxone or moving to combination antibiotic therapy will retard the ESC ‘MIC creep’ that has been observed in gonococcal isolates. Unfortunately no viable long-term single dose alternative for empiric anti-gonococcal therapy is readily available.
Azithromycin has activity against N. gonorrhoeae12,13 and may improve treatment efficacy for pharyngeal gonorrhoea14 although the evidence for synergy between azithromycin and cephalosporins is limited15. Two grams of azithromycin given orally as a single dose has been demonstrated to be up to 99.2% effective against uncomplicated urogenital gonorrhoea16 but is not recommended as monotherapy due to concerns over the development of resistance and reported treatment failures. Resistance to azithromycin may be encouraged by moving to higher dose therapy as this extends the duration of sub-MIC levels for up to one month. Azithromycin at a dose of 2 g is also recommended as part of combination therapy with either gentamicin or gemifloxacin for uncomplicated gonorrhoea in those allergic to cephalosporins by the CDC in their updated 2015 guidelines. In 2017, most Australian gonococcal isolates (>90%) remain susceptible to azithromycin10 although increasing prevalence of low level resistance has been reported from around the world, including Australia, since combination anti-gonococcal therapy was recommended.
Azithromycin resistance in clinical gonococcal isolates appears to be due to target modification from 23S rRNA mutations rather than erm genes that encode rRNA methylases. The C2611T modification results in low level resistance with the number of 23S rRNA alleles affected (up to four) determining the MIC from 1–64 μg/mL. Gonococci with azithromycin MICs just above the breakpoint would be more likely to respond to a 2 g dose rather than a 1 g dose. High level azithromycin resistance (MIC ≥256 μg/mL) is due to three or four alleles containing the 23S rRNA gene A2059G mutation and would be expected to fail azithromycin therapy regardless of the dose. Outbreaks of gonococcal strains with high level azithromycin resistance have been reported from the UK but not from Australia.
The multidrug-resistant and extensively drug- resistant era of gonorrhoea treatment
A new scheme to define multidrug-resistant N. gonorrhoeae (MDR NG) and extensively drug-resistant N. gonorrhoeae (XDR NG) was proposed by Tapsall et al.17 in 2009 to focus attention on gonococcal strains with resistance to antibiotics in current use rather than on those antibiotics superseded or not used for treatment of gonorrhoea. These definitions (Table 1) were soon put into practice. Until 2017, cases of MDR NG resistant to penicillin, ceftriaxone (MIC range 0.5–2 μg/mL), cefixime, and ciprofloxacin were reported from Japan, France, Spain, Australia and Canada. These isolates contained the same mosaic penA allele suggesting international spread but with varying additional genetic modifications. In 2017 there was evidence that there was sustained international transmission of one of these strains (FC428) containing the mosaic penA-allele designated as PenA-60.001 with isolates found in Canada, Denmark and Australia18. Of some comfort was the fact that, for those cases with follow up and tests of cure, combination cephalosporin and azithromycin therapy still appeared effective19. However, this reassurance was short-lived when in 2018 two XDR NG infections acquired in South-East Asia were reported from the UK and Australia together with a second Australian case without a travel history20,21. These strains were resistant to ceftriaxone (MIC 0.25-0.5 μg/mL) and possessed high level azithromycin resistance (MIC >256 μg/mL). Perhaps as expected, the English case failed to clear the pharynx with 1 g ceftriaxone plus 2g spectinomycin, but was subsequently cured with intravenous ertapenem for three days. Reduced susceptibility of N. gonorrhoeae to antimicrobial treatments in men who have sex with men (MSM) compared to heterosexual men and women has been observed for some years for a number of classes of antibiotics. Persistent pharyngeal infection in MSM is therefore a particular area of concern for MDR NG and XDR NG.

|
The future of gonorrhoea treatment
Resurrecting or repurposing antibiotics may have a role in the management of MDR NG and XDR NG infections. Spectinomycin has regained activity since its widespread use ceased; however, it requires SAS approval for use in Australia, is only 50% effective for pharyngeal gonorrhoea and resistance is likely to rapidly redevelop if use again becomes widespread. Gentamicin has been used in Africa for many years but is also inferior for clearance of pharyngeal gonorrhoea. Ertapenem, a carbapenem antibiotic, which appeared effective for treatment of the English XDR NG case has good in vitro activity against gonococcal isolates but there is no treatment trial data available as yet to confirm its clinical efficacy. Almost all the reported MDR NG and XDR NG strains to date were fluoroquinolone resistant making ciprofloxacin unlikely to have a role in managing such cases.
Rifampicin has some limited clinical efficacy data when used in multiple doses but has a less favourable side-effect profile and interacts with a number of other commonly used medications. Fosfomycin, aztreonam and tigecycline could be administered as part of an antibiotic combination to treat MDR NG and XDR NG but again, there is little data to support their clinical efficacy. Solithromycin and delafloxacin were recently trialled against ceftriaxone and azithromycin and ceftriaxone monotherapy, respectively, in uncomplicated gonorrhoea but failed to meet their noninferiority margins. Zoliflodacin, a piropyrimidinetrione antimicrobial agent that inhibits DNA biosynthesis, has shown activity against fluoroquinolone and ESC-resistant gonococci. Gepotidacin, a triazaacenaphthylene antibiotic that inhibits bacterial DNA gyrase and type II topoisomerase to block bacterial DNA replication, has shown activity against ciprofloxacin and azithromycin resistant strains. Both have completed Phase 2 trials and, if Phase 3 trials are successful, could provide future treatment options.
International surveillance for antimicrobial resistant N. gonorrhoeae
A number of countries have surveillance programs for the monitoring of antibiotic resistance in N. gonorrhoeae strains, including the Gonococcal Isolate Surveillance Project in the United States, the Gonococcal Resistance to Antimicrobials Surveillance Programme in the UK, the European Gonococcal Antimicrobial Surveillance Programme and the National Neisseria Network in Australia. More countries, especially in the Asia-Pacific region should be encouraged to initiate surveillance programs due to the recent emergence of XDR NG from this region. The WHO and the CDC have been supporting such efforts in the South-East Asian and Western Pacific regions through initiatives such as the Enhanced Gonococcal Antimicrobial Surveillance Program, which began in Thailand in 2015.
The Australian response to MDR NG and XDR NG
The long established National Neisseria Network will continue to survey referred N. gonorrhoeae isolates for MDR NG and XDR NG along with monitoring trends of decreased susceptibility to the commonly used anti-gonococcal agents. The Communicable Diseases Network of Australia currently has a working group reviewing empiric N. gonorrhoeae treatment and producing guidelines for treatment of MDR NG and XDR NG in Australia, with the recommendations soon to be released. These guidelines will require regular review of the latest available antimicrobial resistance data to ensure they remain effective. When reviewing this data, the recommended breakpoint proposed in the early 1990s22 of 95% susceptibility for use of an antibiotic for treatment of gonorrhoea is likely far too low to prevent the spread of ESC-resistant N. gonorrhoeae.
A more effective response may be the rapid deployment of alternative empiric therapies to the area or sexual network where an ESC-resistant gonococcal strain is detected, akin to a ring vaccination strategy for vaccine preventable diseases. However, the current lack of available gonococcal clinical isolates for antimicrobial susceptibility testing would hamper the effectiveness of this response. The reality is that the vast majority of gonorrhoea diagnoses in Australia will continue to be molecular based and the only way to resolve this information gap is to introduce molecular-based antimicrobial resistance detection. Until now, only in-house molecular tests for detection of antibiotic resistance in gonorrhoea specimens have been used in reference laboratories23 but commercial assays that include molecular markers for antibiotic resistance as well as N. gonorrhoeae DNA detection are entering the Australian market. Earlier diagnosis can also help combat the spread of MDR NG and XDR NG. Collaboration with primary clinical services for point of care testing is encouraged for N. gonorrhoeae to assist with earlier treatment and contact tracing.
Microbiology laboratories are centrally placed to contribute to the response against the spread of gonococcal antimicrobial resistance because of their primary isolate detection and susceptibility testing roles and their liaison and advice roles with the treating clinicians (Table 2).
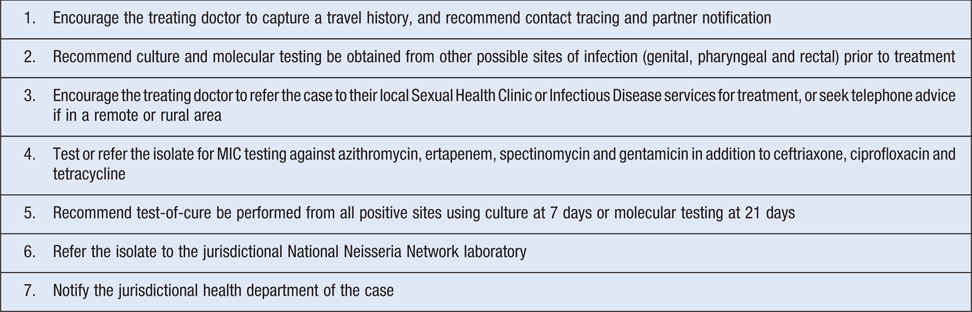
|
Conflicts of interest
The author declares no conflicts of interest.
Acknowledgements
This research did not receive any specific funding.
References
[1] (1955) Albert Neisser and the gonococcus. Am. J. Public Health 45, 95–97.[2] Unemo, M. and Shafer, W.M. (2014) Antimicrobial resistance in Neisseria gonorrhoeae in the 21st century: past, evolution, and future. Clin. Microbiol. Rev. 27, 587–613.
| Antimicrobial resistance in Neisseria gonorrhoeae in the 21st century: past, evolution, and future.Crossref | GoogleScholarGoogle Scholar | 24982323PubMed |
[3] Osler, W. (1921) The Principles and Practice of Medicine, 9th edition. New York: Appleton & Co.
[4] Buono, S.A. et al. (2015) Stemming the tide of drug-resistant Neisseria gonorrhoeae: the need for an individualized approach to treatment. J. Antimicrob. Chemother. 70, 374–381.
| Stemming the tide of drug-resistant Neisseria gonorrhoeae: the need for an individualized approach to treatment.Crossref | GoogleScholarGoogle Scholar | 25331059PubMed |
[5] Ohnishi, M. et al. (2011) Is Neisseria gonorrhoeae initiating a future era of untreatable gonorrhea? Detailed characterization of the first high-level ceftriaxone resistant strain. Antimicrob. Agents Chemother. 55, 3538–3545.
| Is Neisseria gonorrhoeae initiating a future era of untreatable gonorrhea? Detailed characterization of the first high-level ceftriaxone resistant strain.Crossref | GoogleScholarGoogle Scholar | 21576437PubMed |
[6] Unemo, M. et al. (2012) High-level cefixime- and ceftriaxone-resistant Neisseria gonorrhoeae in France: novel penA mosaic allele in a successful international clone causes treatment failure. Antimicrob. Agents Chemother. 56, 1273–1280.
| High-level cefixime- and ceftriaxone-resistant Neisseria gonorrhoeae in France: novel penA mosaic allele in a successful international clone causes treatment failure.Crossref | GoogleScholarGoogle Scholar | 22155830PubMed |
[7] Camara, J. et al. (2012) Molecular characterization of two high-level ceftriaxone-resistant Neisseria gonorrhoeae isolates detected in Catalonia, Spain. J. Antimicrob. Chemother. 67, 1858–1860.
| Molecular characterization of two high-level ceftriaxone-resistant Neisseria gonorrhoeae isolates detected in Catalonia, Spain.Crossref | GoogleScholarGoogle Scholar | 22566592PubMed |
[8] British Association for Sexual Health and HIV (2019) BASHH guidelines for the management of infection with Neisseria gonorrhoeae. https://www.bashh.org/guidelines (accessed 6 March 2019).
[9] Chisholm, S.A. et al. (2010) Cephalosporin MIC creep among gonococci: time for a pharmacodynamics rethink? J. Antimicrob. Chemother. 65, 2141–2148.
| Cephalosporin MIC creep among gonococci: time for a pharmacodynamics rethink?Crossref | GoogleScholarGoogle Scholar | 20693173PubMed |
[10] Lahra, M.M. et al. (2017) Australian Gonococcal Surveillance Programme Annual Report. http://www.health.gov.au/internet/main/publishing.nsf/Content/BE39C1C702DBA06FCA25829700839388/$File/Annual-Report-2017.pdf (accessed 6 March 2019).
[11] Rice, L.B. (2015) Will use of combination cephalosporin/azithromycin therapy forestall resistance to cephalosporins in Neisseria gonorrhoeae? Sex. Transm. Infect. 91, 238–240.
| Will use of combination cephalosporin/azithromycin therapy forestall resistance to cephalosporins in Neisseria gonorrhoeae?Crossref | GoogleScholarGoogle Scholar | 25926405PubMed |
[12] Steingrimsson, O. et al. (1990) Azithromycin in the treatment of sexually transmitted disease. J. Antimicrob. Chemother. 25, 109–114.
| Azithromycin in the treatment of sexually transmitted disease.Crossref | GoogleScholarGoogle Scholar | 2154428PubMed |
[13] Waugh, M.A. (1993) Open study of the safety and efficacy of a single oral dose of azithromycin for the treatment of uncomplicated gonorrhoea in men and women. J. Antimicrob. Chemother. 31, 193–198.
| Open study of the safety and efficacy of a single oral dose of azithromycin for the treatment of uncomplicated gonorrhoea in men and women.Crossref | GoogleScholarGoogle Scholar | 8396093PubMed |
[14] Barbee, L.A. et al. (2013) A retrospective comparative study of 2-drug oral and intramuscular cephalosporin treatment regimens for pharyngeal gonorrhea. Clin. Infect. Dis. 56, 1539–1545.
| A retrospective comparative study of 2-drug oral and intramuscular cephalosporin treatment regimens for pharyngeal gonorrhea.Crossref | GoogleScholarGoogle Scholar | 23408680PubMed |
[15] Barbee, L.A. (2014) Preparing for an era of untreatable gonorrhea. Curr. Opin. Infect. Dis. 27, 282–287.
| Preparing for an era of untreatable gonorrhea.Crossref | GoogleScholarGoogle Scholar | 24685549PubMed |
[16] Newman, L.M. et al. (2007) Update on the management of gonorrhea in adults in the United States. Clin. Infect. Dis. 44, S84–S101.
| Update on the management of gonorrhea in adults in the United States.Crossref | GoogleScholarGoogle Scholar | 17342672PubMed |
[17] Tapsall, J.W. et al. (2009) Meeting the public health challenge of multi-drug and extensively-drug resistant Neisseria gonorrhoeae. Expert Rev. Anti Infect. Ther. 7, 821–834.
| Meeting the public health challenge of multi-drug and extensively-drug resistant Neisseria gonorrhoeae.Crossref | GoogleScholarGoogle Scholar | 19735224PubMed |
[18] Whiley, D.M. et al. (2018) Direct detection of penA gene associated with ceftriaxone-resistant Neisseria gonorrhoeae FC428 strain by using PCR. Emerg. Infect. Dis. 24, 1573–1575.
| Direct detection of penA gene associated with ceftriaxone-resistant Neisseria gonorrhoeae FC428 strain by using PCR.Crossref | GoogleScholarGoogle Scholar | 30016236PubMed |
[19] Lefebvre, B. et al. (2018) Ceftriaxone-resistant Neisseria gonorrhoeae, Canada, 2017. Emerg. Infect. Dis. 24, 381–383.
| Ceftriaxone-resistant Neisseria gonorrhoeae, Canada, 2017.Crossref | GoogleScholarGoogle Scholar |
[20] European Centre for Disease Prevention and Control (2018) Rapid risk assessment: extensively drug-resistant (XDR) Neisseria gonorrhoeae in the United Kingdom and Australia. https://ecdc.europa.eu/sites/portal/files/documents/RRA-Gonorrhoea%2C%20Antimicrobial%20resistance-United%20Kingdom%2C%20Australia.pdf (accessed 7 March 2019).
[21] Whiley, D.M. et al. (2018) Genetic characterisation of Neisseria gonorrhoeae resistant to both ceftriaxone and azithromycin, Australia 2018. Lancet Infect. Dis. 18, 717–718.
| Genetic characterisation of Neisseria gonorrhoeae resistant to both ceftriaxone and azithromycin, Australia 2018.Crossref | GoogleScholarGoogle Scholar | 29976521PubMed |
[22] Handsfield, H.H. et al. (1992) Evaluation of new anti-infective drugs for the treatment of uncomplicated gonorrhea in adults and adolescents. Clin. Infect. Dis. 15, S123–S130.
| Evaluation of new anti-infective drugs for the treatment of uncomplicated gonorrhea in adults and adolescents.Crossref | GoogleScholarGoogle Scholar | 1477219PubMed |
[23] Whiley, D.M. et al. (2017) Molecular antimicrobial resistance surveillance for Neisseria gonorrhoeae, Northern Territory, Australia. Emerg. Infect. Dis. 23, 1478–1485.
| Molecular antimicrobial resistance surveillance for Neisseria gonorrhoeae, Northern Territory, Australia.Crossref | GoogleScholarGoogle Scholar | 28820128PubMed |
Biography
Dr David Speers is an Infectious Diseases Physician at Sir Charles Gairdner Hospital, Head of Microbiology, QEII Network at PathWest Laboratory Medicine WA and a Clinical Associate Professor for the Department of Medicine and Pharmacology, University of WA. He is a fellow of the Royal Australasian College of Physicians, the Royal College of Pathologists of Australasia, and the Australasian College of Tropical Medicine.


