Clinical application of bacteriophages in Europe
Jean-Paul Pirnay A B , Daniel De Vos A and Gilbert Verbeken AA Laboratory for Molecular and Cellular Technology, Queen Astrid Military Hospital, Bruynstraat 1, B-1120 Brussels, Belgium
B Tel: +32 2 2644844, Email: jean-paul.pirnay@mil.be
Microbiology Australia 40(1) 8-15 https://doi.org/10.1071/MA19010
Published: 28 February 2019
Bacteriophages could help address the antibiotic resistance crisis that impacts health systems all over the world. In 2011, the European Commission formally confirmed that phage products used as therapeutics are medicinal products and thus manufacturers need to navigate the extremely arduous and enormously expensive medicine development and marketing pathway. However, up until now, not one therapeutic phage product has made it to the European market, and yet clinicians are under increasing pressure to use phages in the treatment of multidrug-resistant bacterial infections. While a handful of small European enterprises are struggling to squeeze therapeutic phage products through the conventional and centralised European medicinal products funnel, some clinicians and academics are exploring (European) national solutions to accelerate the availability of phages for the treatment of an increasing number of desperate patients. This mini-review summarises the actual status and perspectives of clinical phage application in Europe.
Two decades before the advent of antibiotics, bacteriophages (or phages) were sporadically used to treat bacterial infection across the world. By 1940, therapeutic phage preparations were commercialised by renowned pharmaceutical companies such as Eli Lilly. After World War II, broad spectrum antibiotics were established as the antibacterial agents of choice, but isolated from Western advances in antibiotic production, scientists in the Soviet Union continued to develop phage therapy, with the George Eliava Institute in Tbilisi, Georgia, as the epicentre of these activities. Global pandemics of antibiotic resistant bacteria, causing the death of hundreds of thousands of patients, demonstrate the need for a sea change in antibacterial treatment policy. Today, in the Western world, phage therapy is brought out of mothballs to come to the aid of patients being increasingly failed by antibiotics. The renewed interest in phage therapy research is illustrated by an exponential increase in phage therapy-related papers in medical literature (Figure 1). Unfortunately, the reintroduction of phage therapy in Western medicine is not running smoothly1–3. In this paper we summarise the state of affairs in Europe.
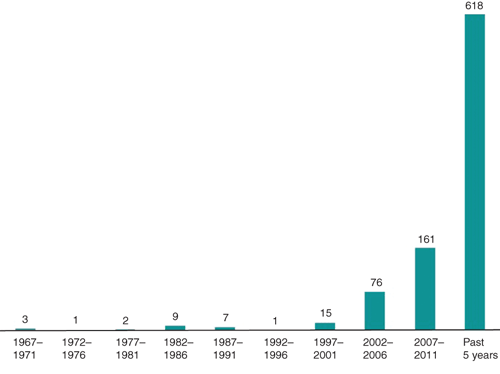
|
Phages are medicinal products
In the European Union (EU), the rediscovered phage preparations were classified as ‘Medicinal Products’ – ‘Drugs’ in the United States (US)4. In February 2011, two Members of the European Parliament, Ivo Belet (Belgium/PPE) and Catherine Trautmann (France/S&D) raised the question to the European Commission and Council of how bacteriophage therapy should be regulated in Europe and whether the Commission would consider creating an extra ‘Bacteriophage Therapy’ section in the EU Medicinal Product framework4. On 29 March 2011, the European Commissioner for Health and Consumer Policy, Mr Dalli, answered on behalf of the European Commission: ‘The EU’s legislation on medicinal products does not define specific requirements related to bacteriophage therapy or medicines composed of bacteriophages.’ He added, ‘the Commission considers that the existing regulatory framework is adequate for bacteriophage therapy without the need for an extra set of documentation’. The EU phage classification was based on a blinkered application of the Medicinal Product definition:
-
any substance or combination of substances presented as having properties for treating or preventing disease in human beings; or
-
any substance or combination of substances which may be used in or administered to human beings either with a view to restoring, correcting or modifying physiological functions by exerting a pharmacological, immunological or metabolic action, or to making a medical diagnosis.
The EU legal framework for medicinal products was primarily laid down in 2001 in Directive 2001/83/EC and Regulation (EC) No 726/2004, and was developed to guarantee high standards of quality and safety, but also to promote the EU internal market with measures that encourage innovation and competitiveness in Europe. A large body of Medicinal Product requirements were implemented and progressively harmonised across the whole European Economic Area, roughly and generally (there are exceptions) consisting of:
-
Manufacturing according to good manufacturing procedures (GMP)
-
Preclinical studies
-
Phase I, II and III clinical trials
-
Centralised marketing authorisation (granted by the European Medicines Agency or for certain types of medicines by the National competent authorities)
As a result, the large body of costly and time-consuming requirements and procedures for manufacturing and for obtaining marketing authorisation for conventional medicinal products for human use were also imposed on phage therapy medicinal products (PTMPs). These requirements were developed to cater for widely used and industrially produced static (immutable) drugs such as aspirin and antibiotics, but are less suitable for sustainable, customised, phage therapy approaches5,6. Technically speaking, pre-defined PTMPs, produced on an industrial scale, could make it through the medicinal product funnel – minding some adaptations, but it is unlikely that such preparations will be able to timely deal with changes in the incidences of infecting bacterial species in certain settings or geographical areas and with the inevitable emergence of phage-resistant clones7. The efficacy of PTMPs is therefore likely to decrease over time, requiring regular adaptions and re-approval (of the new PTMP) for extended use. Multiple phage types are usually needed to treat the different clinically relevant strains of one bacterial species. Furthermore, several bacterial strains are often present in an infection. Therefore, to acquire a more or less broad level of activity, phage cocktails harbouring many different phages will be required. Ideally, therapeutic phages need to be tested for effectiveness against the patients’ pathogens (a ‘phagogram’) and individually prepared. Intermediate or combined (industrially prepared and personalised phage preparations) approaches might be feasible5. Unfortunately, it turns out that the established pharmaceutical industry is not interested in PTMPs, mainly because of limitations in intellectual property protection of a technique that is in the public domain since the 1920s and uses ‘products of nature’ such as phages, and because of the above-mentioned phage specificity and bacterial resistance issues, which compromise widespread and long-term use of immutable pre-defined PTMPs. In the absence of government initiatives, it is left to a handful of small and medium enterprises (SMEs) to develop these PTMPs, using venture capital and/or public funding2.
Randomised controlled trials
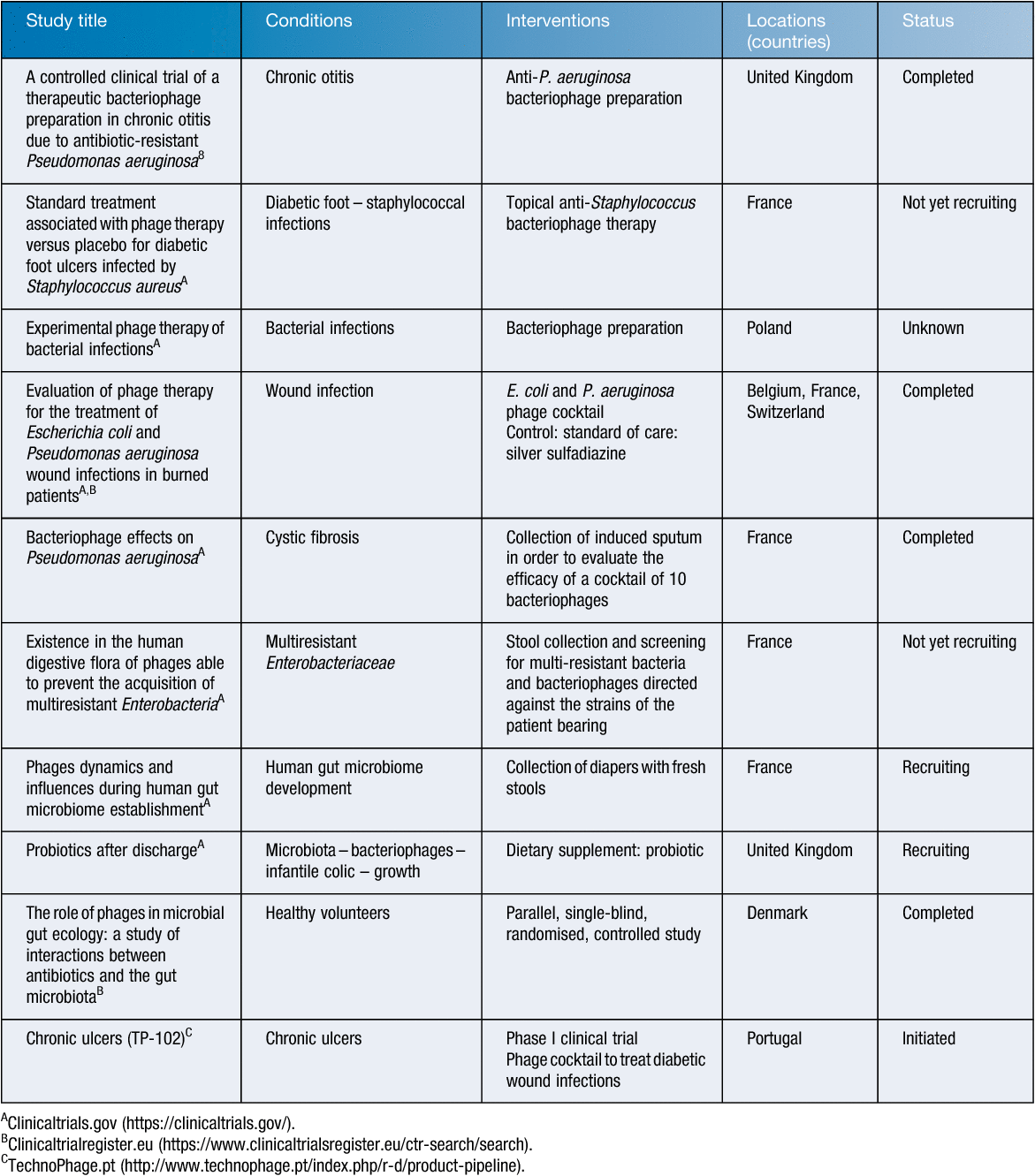
The European Medicines Agency (EMA), based in Amsterdam, underpins the centralised authorisation procedure. The Agency also guarantees a constant exchange and flow of information regarding the scientific assessment of medicinal products in the EU. It is in this context that the EMA organised a workshop on the therapeutic use of bacteriophages in London, on 8 June 2015. At the end of the workshop, EMA emphasised that a medicine cannot be recommended for approval before its efficacy and safety have been proven on the basis of appropriately designed clinical trials8. Several formal clinical trials were launched in Europe (Table 1), but none of them managed to avowedly prove a sufficient efficacy of PTMPs. Note that, by default (EU classification), most of these clinical trials evaluated PTMPs using conventional clinical trial designs, but did NOT evaluate long-established flexible and often personalised phage therapy approaches using regularly updated phage preparations. Different phase I, I/II and II clinical trials did however demonstrate the safety of PTMPs, which is consistent with the safety data provided by numerous preclinical animal studies. To date, two European randomised controlled phase I/II clinical trials showing some phage treatment efficacy have been reported in literature. In the first one, phage therapy against chronic Pseudomonas aeruginosa otitis was investigated. The bacterial load was significantly lower in 12 phage-treated patients as compared to the 12 placebo-treated patients and no adverse effects were observed9. The second one is the clinical phase II Phagoburn trial (http://www.phagoburn.eu), designed to evaluate the treatment of P. aeruginosa and Escherichia coli infected burn wounds using two dedicated phage cocktails10. Cocktails of no less than 12 and 13 phages were needed to ensure a certain activity against pre-defined collections of P. aeruginosa and E. coli isolates, respectively. Manufacturing of one batch (according to GMP) of the investigational PTMPs took 20 months and the largest part of the study budget and only a very small number of phages (10–100 PFU/mL instead of the anticipated 106 PFU/mL) was actually applied due to stability problems of the phage cocktails. In addition, phage specificity issues hampered the recruitment of patients. Because each of the two study products, which couldn’t be applied simultaneously, targeted only one of the multiple bacterial species that are known to (simultaneously) infect or colonise burn wounds, physicians were reluctant to include patients11. Only 27 patients were enrolled in the P. aeruginosa arm of the study and the E. coli arm was stopped (only one patient was enrolled). At very low concentrations, the P. aeruginosa phage cocktail was shown to decrease the bacterial load in burn wounds, but at a slower pace than the standard of care (silver sulfadiazine cream). Further studies using higher phage concentrations and selected phage preparations, taking into account the results of ‘phagograms’, in a larger sample of participants are warranted. Regardless of the final clinical outcome of the PhagoBurn study, it showed that dedicated and realistic production and documentation requirements and treatment protocols are urgently needed.
|
Article 37 of the Declaration of Helsinki
The bottom line is that today there are no PTMPs on the EU market and that the pressure on clinicians to apply phage therapy in desperate cases is increasing, fuelled by an increasing promotion of phage therapy in the media (e.g. eulogising documentaries in prime time). However, even when confronted to serious public health threats, such as the 2011 E. coli O104:H4 outbreak in Germany12, competent authorities are reluctant to authorise the use of non-licensed phage therapy preparations. During this lethal foodborne epidemic, which took the life of 54 patients, Nestlé Research Centre offered a lytic phage to the German public health sector, but this phage was ultimately not used13. Awaiting commercially available licensed PTMPs, some European patients suffering from chronic, extremely resistant or difficult to treat bacterial infections are travelling to phage therapy centres abroad, such as the Eliava Phage Therapy Center in Tbilisi, Georgia.
In addition, sporadic ‘non-PTMP’ phage applications were carried out in Europe, often under the umbrella of Article 37 (Unproven Interventions in Clinical Practice) of the Declaration of Helsinki (www.wma.net)14,15:
In the treatment of a patient, where proven prophylactic, diagnostic and therapeutic methods do not exist or have been ineffective, the physician, with informed consent from the patient, must be free to use unproven or new prophylactic, diagnostic and therapeutic measures, if in the physician’s judgment it offers hope of saving life, re-establishing health or alleviating suffering. This intervention should subsequently be made the object of research, designed to evaluate its safety and efficacy. In all cases, new information must be recorded and, where appropriate, made publically available.
Even though no safety issues were reported and most targeted infections seemed to have been resolved, the low number and diversity of these ‘Helsinki’ phage therapy cases does not allow one to unambiguously demonstrate that the positive clinical outcome was related to the use of phages. For instance, with the exception of a reported use of phages in the treatment of P. aeruginosa septicaemia in a patient with acute kidney injury15, most patients were given other potent anti-bacterials together with the phage preparations.
Poland: experimental treatment
In Poland, a member of the EU, phage therapy is considered an ‘Experimental Treatment’, covered by the adapted Act of 5 December 1996 on the Medical Profession (Polish Law Gazette, 2011, No. 277 item 1634) and Article 37 of the Declaration of Helsinki16,17.
Experimental phage treatments are possible in Poland minding the following requirements:
-
Written informed consent of the patient (or legal representative)
-
Approval by an institutional review board (bioethics commission)
-
Phages can only be applied by a qualified doctor
-
Phages can only be applied when other available treatments have failed
In June 2005, the Ethical Committee of the Medical Academy in Wroclaw authorised a study named ‘Experimental Phage Therapy in Bacterial Infections’ (Table 1). Neither the EU Medicinal Product Regulation nor its Polish National translation was applied and the EU did not oppose. As such, the Phage Therapy Unit of the Ludwik Hirszfeld Institute of Immunology and Experimental Therapy in Wrocław (an institute of the Polish Academy of Sciences) offers phage therapy to treat patients infected with drug-resistant bacteria. In 2012, Międzybrodzki et al.18 presented a detailed retrospective analysis of the results of phage therapy of 153 patients with a wide range of infections, resistant to antibiotic therapy, admitted for treatment at the Wrocław Phage Therapy Unit between January 2008 and December 2010. They suggested that phage therapy had provided good clinical results in a significant cohort of patients with otherwise untreatable chronic bacterial infections.
France: compassionate use
Compassionate use is a treatment option that allows the use of an unauthorised medicine. Under strict conditions, products in development can be made available to groups of patients who have a disease with no satisfactory authorised therapies and who cannot enter clinical trials (https://www.ema.europa.eu/en/human-regulatory/research-development/compassionate-use). The EMA provides recommendations, but the practice of ‘compassionate use’ is actually coordinated and implemented by the Member States, and there is some variation in national rules and procedures. Much like article 37 of the Declaration of Helsinki, the compassionate use treatment option or programmes can only be put in place if the medicine, the phage in-casu, is expected to be of help in life-threatening, long-lasting (chronic) and/or seriously debilitating illnesses that are not treatable using the current armamentarium. In principle, the compassionate approach can only apply to medicinal products that are being tested or have entered the marketing authorisation application process after early study results have shown efficacy and safety, but have not yet been approved. In France, phages have been used under the umbrella of the compassionate use setting. A recent publication describes a number of cases of patients treated compassionately with phages with a focus on osteoarticular infections during the past 10 years19. In practice, a multidisciplinary team (surgeons, infectiologists, microbiologists and pharmacists) discussed the potential compassionate phage application and compiled a medical dossier and specific treatment protocol, in consultation with the patient (or his legal representative) and the treating hospital’s ethical committee. However, since 2016 the ‘Agence Nationale de Sécurité du Medicament et les Produits de Santé (ANSM),’ the French competent authority, is also tightly involved. ANSM created a specific committee ‘comité scientifique spécialisé temporaire (CSST)’ for phage therapy, which is composed of (external) experts in different fields. Their task is to specifically evaluate and guide the phage therapy requests sent to the ANSM. They meet on a regular basis and will remain active as long as the problem exists. The requests for phage applications are discussed in dialogue with the treating physicians and a consensus advice is transmitted to the ANSM, who will or will not authorise the request. From 2006 to 2018, 15 patients were treated compassionately with phages in France. Eleven were immediately cured19. These compassionate phage treatments, under supervision of the competent authorities, allow for the analysis, evaluation and correction, if necessary, of the clinical phage application protocols. A clinical report is compiled for each application, which helps to optimise the phage therapy approaches without the existence of an adapted regulatory frame.
The Czech and Slovak Republics: Stafal®
In the Czech and Slovak Republics, EU Member States, a (publicly reimbursed) anti-staphylococcal bacteriophage product (a phage lysate) is available on the market under the trade name Stafal®20. It was approved for market placement by the Czech National Competent Authority, the State Institute for Drug Control. The product is an anti-staphylococcal phage lysate intended for topical treatment of Staphylococcus skin infections (registration number 59/0149/89-CS).
Belgium: magistral phage
Faecal transplantation is an established practice in several European countries. Even though faecal matter for transplantation unquestionably meets the Medicinal Product definition, it was not classified as a Medicinal Product in the EU. As such, stool transplants do not need to comply with costly and lengthy development and marketing requirements, such as GMP production and marketing authorisation, and physicians were able to show efficacy in controlled trials21. In Belgium, the ‘Superior Health Council (SHC)’ elaborated and published pragmatic recommendations regarding the therapeutic indications, the procedures, safety and quality of the transplantation of faecal material (Opinion 22 of the SHC). Seen that faecal microbiota contain billions of uncharacterised phages, a Belgian pragmatic solution for phage preparations should logically be possible too.
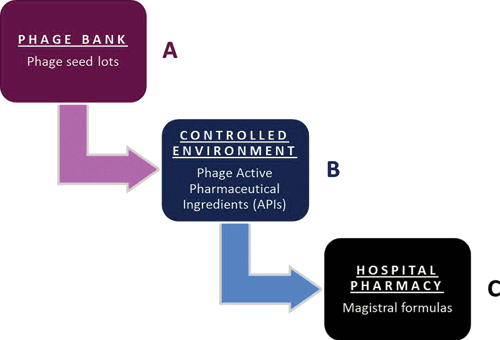
On 5 July 2016, in response to two parliamentary questions related to the waning implementation of phage therapy, the Minister of Social Affairs and Public Health acknowledged that it is indeed not obvious to deal with phages as industrially prepared medicinal products and therefore suggested exploring the option of magistral phage preparations22. The former is subject to constraints related to their production and marketing authorisation, unlike the latter, which was created to offer a practical way to medical doctors to personalise patient treatments to specific needs and to make medicines available that are not (yet) on the market. For instance, allergens and natural hormone combination products, which often lack patent protection, are not produced by commercial manufacturers, but are typically delivered as magistral preparations. In European and Belgian law, a magistral preparation (compounded prescription drugs in the US) is defined as ‘any medicinal product prepared in a pharmacy in accordance with a medical prescription for an individual patient’ (Article 3 of Directive 2001/83 of the European Parliament and Article 6 quater, § 3 of the Belgian Medicines Law of 25 March 1964). Magistral preparations are compounded by a pharmacist from their constituent ingredients (or under his/her supervision), following the technical and scientific standards of the pharmaceutical art, for a given patient according to a physician’s prescription. As a general rule, active ingredients of magistral preparations must meet the requirements of a monograph (describing their preparation) in an official pharmacopoeia such as the European or the Belgian Pharmacopoeia. However, if no such monograph exists, the Minister of Public Health can still authorise the active ingredients, following a favourable opinion of the national Pharmacopoeia Commission. In addition, magistral preparations may also harbour non-authorised ingredients, providing that they are accompanied by a certificate of analysis. This certificate must be issued by a ‘Belgian Approved Laboratory (BAL)’, quality control laboratories that have been granted an accreditation by the Belgian regulatory authorities to perform batch release testing of medicinal products. Some BALs belong to the European Official Medicines Control Laboratories (OMCL) network, which groups independent public laboratories that have been appointed by their national authorities. Since there is no ‘phage monograph’ in any official pharmacopoeia and because of the almost endless variety of phages that could be used as active ingredients and should then each obtain an authorisation issued by the Minister of Public Health, the option of the ‘non-authorised ingredient’ was chosen. The standard procedure for unauthorised active ingredients only involves the medical doctor, his patient, the manufacturer of the active ingredients, the approved laboratory and the pharmacist, but because of the innovative and very specific character of phage therapy it was decided to also involve the Federal Agency for Medicines and Health Products (FAMHP), the Belgian competent authority for medicines, in the elaboration of the Belgian magistral phage medicine framework. Experts of the Queen Astrid Military Hospital in Brussels, the FAMHP and the Belgian Scientific Institute of Public Health developed a supplier monograph, which describes how phage Active Pharmaceutical Ingredients (APIs) should be produced and tested. It was conceived as an evolving document, applicable to most phages. The phage API monograph received a formal positive advice by the FAMHP on 10 January 2018. As from that date, in Belgium, phages can be delivered in the form of magistral preparations to specific (nominal) patients under the direct responsibility of medical doctors and pharmacists. The general concept of the Belgian magistral phage medicine strategy, depicted in Figure 2, was recently published, including the phage API monograph22. Next, standard clinical protocols describing – amongst others – medical indications, formulations and posology for phage applications need to be drafted at the Belgian and at the European level. Ultimately, magistral phage preparations should be listed as products eligible for reimbursement, keeping in mind that their cost price will likely influence the patients’ access to phage therapy. Belgian phage preparations have sporadically been ‘exported’ to France for application in desperate cases, with competent authority approval. Germany is currently investigating the magistral phage pathway and The Netherlands are considering the ‘importation’ of Belgian phage products for clinical trials (personal communication).
|
Biological Master File
As a European solution to the phage therapy regulatory issues, novel EU regulations based on the ‘Biological Master File’ (BMF) principle, similar to procedures already existing for chemical drugs, has been suggested23. Phage preparations compounded by a pharmacist for an individual patient are not industrially produced and can be regarded as magistral preparations. However, the production of phage APIs, ingredients of these magistral preparations, often fulfils the characteristics of an industrial process. Customised PTMPs are thus somewhere in between magistral formulas and industrially produced medicinal products. This uncomfortable situation should best be addressed within the current EU regulatory framework. The licensing of customised PTMPs could rely on the concept of a BMF. However, the European regulation does not allow an extension of this concept to biological active substances such as phages. Instead, the current registration procedure of biologicals (a Medicinal Product subclass) requires the approval of the Medicinal Product as a whole, not of its active ingredients alone. The BMF concept would thus cover only a part of a biological Medicinal Product application, submitted as a stand-alone package. In the case of customised PTMPs, one BMF for each individual phage, or for a homologous group of phages, could be submitted for licensing by the competent authorities. The BMF would cover the industrial aspects of the manufacturing process of the phage API and requirements such as a quality module and batch release by a qualified person. In addition, the BMF could also include safety profiling performed for individual phage suspensions. The finished product could be prepared as a magistral formula and would not require approval by competent authorities. This BMF concept could solve a number of regulatory issues with regard to personalised healthcare products in Europe, but unfortunately, extension of this concept to biologicals is not on the agenda of the Commission.
Phages in food and agriculture
Commercial phage products are already used as antimicrobial agents in plant and animal agriculture and food decontamination24. These phage-based products navigated easier commercial and regulatory paths than their human therapeutic counterparts. Even though it is expected that these non-human phage applications will pave the way for human applications, we wonder what will be the impact of this type of massive and unlimited environmental use of phages on the emergence of bacterial phage resistance (how fast will it emerge and will it persist or spread?) and on the composition of bacterial populations in the environment? Shouldn’t we study this first? Shouldn’t we learn from our (antibiotic) mistakes?
P-H-A-G-E.org
Phages for Human Applications Group Europe (http://www.P-H-A-G-E.org) is an international non-profit organisation aiming to support phage research and therapy and to develop a specific regulatory framework for phage therapy in Europe. The organisation was founded in 2009 as a vehicle to explore and help develop a sustainable alternative to the threatening appearance of antibiotic-resistant bacteria. Its members are typically scientists, physicians and specialists in the fields of health economics and legal, regulatory or quality control matters.
Concluding remarks
Awaiting an adapted EU regulatory framework, Member States are exploring national solutions to make phages available to physicians, which are in desperate need of additional tools (in combination with other antibacterials) to fight multidrug resistant infections. Politicians need to support these efforts, preferably at the European level25. Phage banks containing well characterised phage stocks (including genome sequences and host specificity) should be set up and this information should be made available to physicians. These phage banks should be able to supply phages for fast amplification and treatment. Even though (European) regulatory approval for use of genetically modified phages is currently non-existent, bio-engineered phages are now gaining interest as future anti-bacterial agent26.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
This research did not receive any specific funding.
References
[1] Abedon, S.T. et al. (2011) Phage treatment of human infections. Bacteriophage 1, 66–85.| Phage treatment of human infections.Crossref | GoogleScholarGoogle Scholar | 22334863PubMed |
[2] Pirnay, J.P. et al. (2012) Introducing yesterday’s phage therapy in tomorrow’s medicine. Future Virol. 7, 379–390.
| Introducing yesterday’s phage therapy in tomorrow’s medicine.Crossref | GoogleScholarGoogle Scholar |
[3] Huys, I. et al. (2013) Paving a regulatory pathway for phage therapy. Europe should muster the resources to financially, technically and legally support the introduction of phage therapy. EMBO Rep. 14, 951–954.
| Paving a regulatory pathway for phage therapy. Europe should muster the resources to financially, technically and legally support the introduction of phage therapy.Crossref | GoogleScholarGoogle Scholar | 24136414PubMed |
[4] Verbeken, G. et al. (2012) Optimizing the European regulatory framework for sustainable bacteriophage therapy in human medicine. Arch. Immunol. Ther. Exp. (Warsz.) 60, 161–172.
| Optimizing the European regulatory framework for sustainable bacteriophage therapy in human medicine.Crossref | GoogleScholarGoogle Scholar | 22527355PubMed |
[5] Pirnay, J.P. et al. (2011) The phage therapy paradigm: prêt-à-porter or sur-mesure? Pharm. Res. 28, 934–937.
| The phage therapy paradigm: prêt-à-porter or sur-mesure?Crossref | GoogleScholarGoogle Scholar | 21063753PubMed |
[6] Verbeken, G. et al. (2014) Call for a dedicated European legal framework for bacteriophage therapy. Arch. Immunol. Ther. Exp. (Warsz.) 62, 117–129.
| Call for a dedicated European legal framework for bacteriophage therapy.Crossref | GoogleScholarGoogle Scholar | 24500660PubMed |
[7] Rohde, C. et al. (2018) Expert opinion on three phage therapy related topics: bacterial phage resistance, phage training and prophages in bacterial production strains. Viruses 10, 178.
| Expert opinion on three phage therapy related topics: bacterial phage resistance, phage training and prophages in bacterial production strains.Crossref | GoogleScholarGoogle Scholar |
[8] Debarbieux, L. et al. (2016) A bacteriophage journey at the European Medicines Agency. FEMS Microbiol. Lett. 363, fnv225.
| A bacteriophage journey at the European Medicines Agency.Crossref | GoogleScholarGoogle Scholar | 26656541PubMed |
[9] Wright, A. et al. (2009) A controlled clinical trial of a therapeutic bacteriophage preparation in chronic otitis due to antibiotic-resistant Pseudomonas aeruginosa; a preliminary report of efficacy. Clin. Otolaryngol. 34, 349–357.
| A controlled clinical trial of a therapeutic bacteriophage preparation in chronic otitis due to antibiotic-resistant Pseudomonas aeruginosa; a preliminary report of efficacy.Crossref | GoogleScholarGoogle Scholar | 19673983PubMed |
[10] Jault, P. et al. (2019) Efficacy and tolerability of a cocktail of bacteriophages to treat burn wounds infected by Pseudomonas aeruginosa (PhagoBurn): a randomised, controlled, double-blind phase 1/2 trial. Lancet Infect. Dis. 19, 35–45.
| Efficacy and tolerability of a cocktail of bacteriophages to treat burn wounds infected by Pseudomonas aeruginosa (PhagoBurn): a randomised, controlled, double-blind phase 1/2 trial.Crossref | GoogleScholarGoogle Scholar | 30292481PubMed |
[11] Servick, K. (2016) Beleaguered phage therapy trial presses on. Science 352, 1506.
| Beleaguered phage therapy trial presses on.Crossref | GoogleScholarGoogle Scholar | 27339963PubMed |
[12] Frank, C. et al. (2011) Epidemic profile of Shiga-toxin-producing Escherichia coli O104:H4 outbreak in Germany. N. Engl. J. Med. 365, 1771–1780.
| Epidemic profile of Shiga-toxin-producing Escherichia coli O104:H4 outbreak in Germany.Crossref | GoogleScholarGoogle Scholar | 21696328PubMed |
[13] Brüssow, H. (2012) What is needed for phage therapy to become a reality in Western medicine? Virology 434, 138–142.
| What is needed for phage therapy to become a reality in Western medicine?Crossref | GoogleScholarGoogle Scholar | 23059181PubMed |
[14] Vogt, D. et al. (2017) Beyond antibiotic therapy – future antiinfective strategies – update 2017. Unfallchirurg 120, 573–584.
| Beyond antibiotic therapy – future antiinfective strategies – update 2017.Crossref | GoogleScholarGoogle Scholar | 28643099PubMed |
[15] Jennes, S. et al. (2017) Use of bacteriophages in the treatment of colistin-only-sensitive Pseudomonas aeruginosa septicaemia in a patient with acute kidney injury – a case report. Crit. Care 21, 129.
| Use of bacteriophages in the treatment of colistin-only-sensitive Pseudomonas aeruginosa septicaemia in a patient with acute kidney injury – a case report.Crossref | GoogleScholarGoogle Scholar | 28583189PubMed |
[16] Górski, A. et al. (2009) Bacteriophage therapy for the treatment of infections. Curr. Opin. Investig. Drugs 10, 766–774.
| 19649921PubMed |
[17] Verbeken, G. et al. (2007) European regulatory conundrum of phage therapy. Future Microbiol. 2, 485–491.
| European regulatory conundrum of phage therapy.Crossref | GoogleScholarGoogle Scholar | 17927471PubMed |
[18] Międzybrodzki, R. et al. (2012) Clinical aspects of phage therapy. Adv. Virus Res. 83, 73–121.
| Clinical aspects of phage therapy.Crossref | GoogleScholarGoogle Scholar | 22748809PubMed |
[19] Patey, O. et al. (2019) Clinical indications and compassionate use of phage therapy: personal experience and literature review with a focus on osteoarticular infections. Viruses 11, 18.
| Clinical indications and compassionate use of phage therapy: personal experience and literature review with a focus on osteoarticular infections.Crossref | GoogleScholarGoogle Scholar |
[20] Dvořáčková, M. et al. (2019) Antimicrobial effect of commercial phage preparation Stafal® on biofilm and planktonic forms of methicillin-resistant Staphylococcus aureus. Folia Microbiol. (Praha) 64, 121–126.
| Antimicrobial effect of commercial phage preparation Stafal® on biofilm and planktonic forms of methicillin-resistant Staphylococcus aureus.Crossref | GoogleScholarGoogle Scholar | 29923129PubMed |
[21] van Nood, E. et al. (2013) Duodenal infusion of donor feces for recurrent Clostridium difficile. N. Engl. J. Med. 368, 407–415.
| Duodenal infusion of donor feces for recurrent Clostridium difficile.Crossref | GoogleScholarGoogle Scholar | 23323867PubMed |
[22] Pirnay, J.P. et al. (2018) The magistral phage. Viruses 10, 64.
| The magistral phage.Crossref | GoogleScholarGoogle Scholar |
[23] Fauconnier, A. (2017) Regulating phage therapy: the biological master file concept could help to overcome regulatory challenge of personalized medicines. EMBO Rep. 18, 198–200.
| Regulating phage therapy: the biological master file concept could help to overcome regulatory challenge of personalized medicines.Crossref | GoogleScholarGoogle Scholar | 28082313PubMed |
[24] Svircev, A. et al. (2018) Framing the future with bacteriophages in agriculture. Viruses 10, 218.
| Framing the future with bacteriophages in agriculture.Crossref | GoogleScholarGoogle Scholar |
[25] Moelling, K. et al. (2018) A wake-up call: we need phage therapy now. Viruses 10, 688.
| A wake-up call: we need phage therapy now.Crossref | GoogleScholarGoogle Scholar |
[26] Pires, D.P. et al. (2016) Genetically engineered phages: a review of advances over the last decade. Microbiol. Mol. Biol. Rev. 80, 523–543.
| Genetically engineered phages: a review of advances over the last decade.Crossref | GoogleScholarGoogle Scholar | 27250768PubMed |
Biographies
Dr Jean-Paul Pirnay graduated as Industrial Engineer in Biotechnology at the University College Ghent and obtained a Scientific Degree in Agriculture Development at the University of Ghent. He received his PhD in Medical Sciences from the Vrije Universiteit Brussel. In 1993 he performed his military service and served since then as a researcher in the Queen Astrid Military Hospital (QAMH) in Brussels. He is currently head of the Laboratory for Molecular and Cellular Technology (LabMCT) of the QAMH and research collaborator at the Royal Military Academy. His research interests include human cell and tissue banking, cell therapy, tissue engineering, molecular microbiology and bacteriophage therapy. He was appointed as an Expert to the Belgian Superior Health Council and participated in several National, EU and NATO research task groups and evaluation/advisory committees.
Dr Daniel De Vos obtained a PhD in Sciences (molecular-microbiology), at the Vrije Universiteit Brussel. Professionally he was active in the biomedical field in the tropics (Central Africa, South East Asia) and Europe. He spent 10 years in Central Africa as head of an interdisciplinary team active in the fields of infection, nutrition and epidemiology, with an emphasis on diarrheal diseases and sepsis in malnourished children and antibiotic resistance. He is a visiting professor in microbiology at the Université Catholique de Bukavu (Democratic Republic of the Congo). During 8 years he was project leader for the development of rapid molecular diagnostics, in an industrial setting (Innogenetics/Roche). Since 2006 he is Research Manager at the Laboratory for Molecular and Cellular Technology of the Queen Astrid Military Hospital in Brussels. His actual focus is on infectiology and phage therapy.
Dr Gilbert Verbeken studied at the Ghent University, the KU Leuven and the Royal Military Academy (Belgium). As a biologist, he is more than 30 years active in the field of human cell- and tissue-engineering and tissue banking. Gilbert built up, among other things, significant ‘Advanced Therapy Medicinal Products’ (ATMP) regulatory experience and is now also involved in the creation of a Bacteriophage Therapy Centre at the QAMH. He obtained his PhD degrees studying the regulatory hurdles and ethical issues related to the (re-) introduction of (natural) bacteriophage therapy in Europe.


