Viruses in corals: hidden drivers of coral bleaching and disease?
Patrick Buerger A B and Madeleine JH van Oppen B CA CSIRO Land and Water, Clunies Ross Street, Canberra, ACT 2601, Australia
Email: patrick.buerger@csiro.au
B School of BioSciences, University of Melbourne, Parkville, Vic. 3010, Australia
Email: madeleine.van@unimelb.edu.au
C Australian Institute of Marine Science, PMB #3, Townsville, Qld 4810, Australia
Microbiology Australia 39(1) 9-12 https://doi.org/10.1071/MA18004
Published: 27 February 2018
Marine viruses are the largest, but most poorly explored genetic reservoir on the planet. They occur ubiquitously in the ocean at an average density of 5–15 × 106 viruses per mL of seawater, which represents abundances an order of magnitude higher than those of bacteria. While viruses are known agents of a number of diseases in the marine environment, little is known about their beneficial function to corals. Herein, we briefly introduce the topic of viruses as potential drivers of coral bleaching and disease.
Increasing prevalence of coral bleaching and disease
Corals form a symbiosis with microscopic algae (Symbiodinium spp.), which are the primary carbon source of their host through translocation of photosynthates. The loss of these intracellular symbionts is referred to as coral bleaching, causing the coral tissue to pale and resulting in a vulnerable state of the coral animal1.
In recent years, coral bleaching and diseases have increasingly contributed to coral mortality for a number of reasons. First, warm seawater temperature anomalies that lead to mass bleaching events have increased in frequency and have left corals less time to recover2. Such temperature anomalies have been associated with higher disease incidence, possibly due to increased activity of pathogenic bacteria at elevated temperatures combined with reduced immunocompetence of stressed corals3. Second, the growing spatial scale of anthropogenic impacts on coral reefs such as reduced water quality4 and tourism activities5 have also been linked to higher disease prevalence. For example, up to 15-fold higher coral disease prevalence was reported on reefs in the Great Barrier Reef that had tourist platforms compared to those without5. Third, the frequency and severity of cyclones and crown-of-thorns starfish predation have increased; these disturbances cause breakages and injuries to corals and provide entry points for pathogenic microorganisms6,7. Despite the increase of coral disease occurrence, the tools required for rapid diagnostics are still lacking and management strategies to prevent and mitigate coral disease outbreaks are largely inadequate8. Of prime concern is that causative agents have not been identified for the majority of the described coral diseases. While a few known scleractinian coral pathogens are bacteria9, the role of viruses in coral health and disease has barely been examined.
Virus diversity in corals
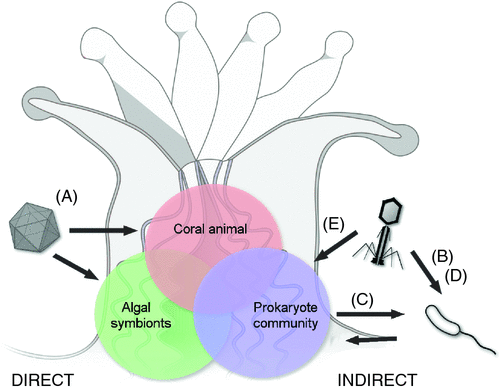
Coral-associated virus communities are highly diverse and comprise bacteriophages, archaeal and eukaryotic viruses10–12. Despite this diversity, only a smaller subset of taxonomic groups are commonly found in corals, including bacteriophages belonging to the order of the Caudovirales, and eukaryotic nucleocytoplasmic large DNA viruses (NCLDVs) belonging to the families Phycodnaviridae, Mimiviridae, Poxviridae and Iridoviridae, as well as Polydnavridae and Retroviridae10–12. The coral-associated viral diversity shows that viruses could infect all cellular members of the coral holobiont, i.e. the coral animal, algal symbionts and all of its other microscopic and macroscopic symbionts.
Eukaryotic viruses in coral disease and bleaching
Although over 20 coral diseases have been described, none of them are unequivocally shown to be caused by a eukaryotic virus that directly infects the coral animal or symbiotic algae (Figure 1A). For example, yellow band/blotch disease (YBD) causes degradation of Symbiodinium cells and has tentatively been linked to the abundance of virus-like particles (VLPs)13. Similarly, corals affected with white plague disease in the Caribbean have shown increased numbers of single-strand DNA viruses14. For both diseases, associated viruses still need to be isolated to investigate their causality using methods such as Koch’s postulates.
|
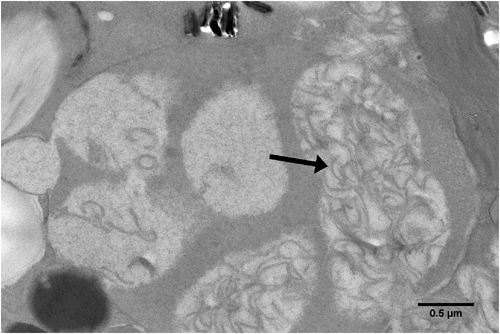
Viral lysis (disintegration of infected cells) of Symbiodinium may be responsible for some instances of coral bleaching (Figure 2). A distant cousin of the dinoflagellate Heterocapsa circularisquama RNA virus, first detected with metatranscriptomics16, has recently gained attention for its potential role in coral bleaching (reviewed in Thurber et al.17 and Sweet and Bythell18). Transcripts of the ssRNA virus were shown to be present at high abundance in a heat-sensitive Symbiodinium culture, while they were barely detectable in a conspecific heat-tolerant culture, suggesting Symbiodinium and perhaps coral thermal tolerance is linked to the presence of this virus19. In order to progress the research in the field, PCR primers have been designed to assess presence and diversity of the ssRNA virus; these primers can potentially be modified for virus quantification during in situ coral bleaching events20.
|
The potential roles of bacteriophages in coral disease
The mechanisms by which lysogenic and lytic bacteriophages interfere with or contribute to coral disease pathogenesis are primarily indirect, i.e. bacteriophages on their own do not influence the coral animal or Symbiodinium, but infect bacteria, which then secondarily influence coral health.
After infection of a target bacterium, the lysogenic stage refers to the integration of the bacteriophage genome into the bacterial host genome as a prophage. Bacteriophages may increase the virulence of a bacterial pathogen after establishing lysogeny and transferring new genetic material into the host bacterium. For example, the pathogenicity of the bacterium Vibrio cholerae primarily depends on infection by a lysogenic bacteriophage (CTXphi). The bacteriophage transfers genes that encode for one of the primary virulence factors, in this case the cholera toxin (CT), and converts V. cholerae from a non-pathogenic to a pathogenic strain21. Lysogenic conversion has been suggested to also increase the virulence of Vibrio coralliilyticus (Figure 1B), because parts of the bacterium’s virulence factors that are linked to the coral disease white syndrome and coral bleaching are arranged similarly to the pathogenicity islands of the V. cholerae prophage22.
Other lysogenic bacteriophages persist over extended periods of time until a trigger induces a lytic cycle, e.g., an increase in temperature or UV radiation. The lytic stage is characterised by the replication of bacteriophages within the bacterial host, which results in lysis of the host cell and release of newly produced bacteriophages23. For instance, traces of bacteriophages were detected in the CRISPR arrays within the genomes of cyanobacteria, Roseofilum reptotaenium and Geitlerinema sp., two species dominating the black band disease mat in terms of biomass24. These findings suggest the cyanobacteria are regularly infected by bacteriophages and that phages may play a role in the disease development24.
Viruses may also have positive effects on coral health, such as purely lytic bacteriophages25. Specific bacteriophages that target pathogenic bacteria may form part of the natural coral microbiome and confer some disease resistance by preventing bacteria from excessive proliferation (Figure 1C)26. Lytic bacteriophages have been applied successfully in lab-based phage therapies for the treatment of several bacterial coral diseases, e.g., white syndrome caused by Vibrio coralliilyticus strains27,28. The promising potential of phage therapy to treat a coral disease has been showcased, for instance, through the effective mitigation of white plague-like progression and transmission to other corals, during both a seven-week field experiment29 and a 21-day laboratory experiment27.
Conclusion and progress
Although viruses might contribute key aspects to coral bleaching and diseases, our understanding of this field of research is still scant. Even less is known about the functional contribution of viruses to coral health18. The current scarcity of coral virus-related studies can be linked to scientific challenges associated with environmental virus research and the difficulty to distinguish between causality and correlation of viruses with a coral disease. In order to overcome these issues, future research should establish coral host-virus model systems and consider versatile research approaches. For instance, relevant hosts for establishing virus cultures are Symbiodinium to investigate coral bleaching, R. reptotaenium for black band disease virulence models, and V. coralliilyticus for white syndrome virulence models. Multifaceted research approaches should include (1) viral metagenomics to characterise and describe virus communities in field-collected corals30, (2) flow cytometry for virus enumeration31, (3) liquid and plaque assays to isolate bacteriophages32, and (4) bioinformatic pipelines that are designed for virus sequence data33. Research over the next decade will likely solve some of these issues and shed more light on the ecological importance of viruses in coral holobiont functioning. This will hopefully provide new ways to manage coral diseases on the reef.
References
[1] Glynn, P.W. (1996) Coral reef bleaching: facts, hypothesis, and implications. Glob. Change Biol. 2, 495–509.| Coral reef bleaching: facts, hypothesis, and implications.Crossref | GoogleScholarGoogle Scholar |
[2] Hughes, T.P. et al. (2018) Spatial and temporal patterns of mass bleaching of corals in the Anthropocene. Science 359, 80–83.
| Spatial and temporal patterns of mass bleaching of corals in the Anthropocene.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC1cXjslOkug%3D%3D&md5=c303583d036329eed2d5b90ab2b99cfbCAS |
[3] Ruiz-Moreno, D. et al. (2012) Global coral disease prevalence associated with sea temperature anomalies and local factors. Dis. Aquat. Organ. 100, 249–261.
| Global coral disease prevalence associated with sea temperature anomalies and local factors.Crossref | GoogleScholarGoogle Scholar |
[4] Sutherland, K.P. et al. (2004) Disease and immunity in Caribbean and Indo-Pacific zooxanthellate corals. Mar. Ecol. Prog. Ser. 266, 273–302.
| Disease and immunity in Caribbean and Indo-Pacific zooxanthellate corals.Crossref | GoogleScholarGoogle Scholar |
[5] Lamb, J.B. and Willis, B.L. (2011) Using coral disease prevalence to assess the effects of concentrating tourism activities on offshore reefs in a tropical marine park. Conserv. Biol. 25, 1044–1052.
| Using coral disease prevalence to assess the effects of concentrating tourism activities on offshore reefs in a tropical marine park.Crossref | GoogleScholarGoogle Scholar |
[6] Katz, S.M. et al. (2014) Crown-of-thorns starfish predation and physical injuries promote brown band disease on corals. Coral Reefs 33, 705–716.
| Crown-of-thorns starfish predation and physical injuries promote brown band disease on corals.Crossref | GoogleScholarGoogle Scholar |
[7] De’ath, G. et al. (2012) The 27-year decline of coral cover on the Great Barrier Reef and its causes. Proc. Natl. Acad. Sci. USA 109, 17995–17999.
| The 27-year decline of coral cover on the Great Barrier Reef and its causes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xhsl2ktbjF&md5=204861e51a5e95c381fbb773f6f57e55CAS |
[8] Pollock, F.J. et al. (2011) The urgent need for robust coral disease diagnostics. PLoS Pathog. 7, e1002183.
| The urgent need for robust coral disease diagnostics.Crossref | GoogleScholarGoogle Scholar |
[9] Harvell, D. et al. (2007) Coral disease, environmental drivers, and the balance between coral and microbial associates. Oceanography (Wash. D.C.) 20, 172–195.
| Coral disease, environmental drivers, and the balance between coral and microbial associates.Crossref | GoogleScholarGoogle Scholar |
[10] Vega Thurber, R.L. et al. (2008) Metagenomic analysis indicates that stressors induce production of herpes-like viruses in the coral Porites compressa. Proc. Natl. Acad. Sci. USA 105, 18413–18418.
| Metagenomic analysis indicates that stressors induce production of herpes-like viruses in the coral Porites compressa.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhsVyhsrvF&md5=da1280d6fa9aad485d8f7000773c5f79CAS |
[11] Wood-Charlson, E.M. et al. (2015) Metagenomic characterization of viral communities in corals: mining biological signal from methodological noise. Environ. Microbiol. 17, 3440–3449.
| Metagenomic characterization of viral communities in corals: mining biological signal from methodological noise.Crossref | GoogleScholarGoogle Scholar |
[12] Correa, A.M.S. et al. (2016) Viral outbreak in corals associated with an in situ bleaching event: atypical herpes-like viruses and a new megavirus infecting Symbiodinium. Front. Microbiol. 7, 127.
| Viral outbreak in corals associated with an in situ bleaching event: atypical herpes-like viruses and a new megavirus infecting Symbiodinium.Crossref | GoogleScholarGoogle Scholar |
[13] Cervino, J.M. et al. (2004) Zooxanthellae regulation in yellow blotch/band and other coral diseases contrasted with temperature related bleaching: In situ destruction vs expulsion. Symbiosis 37, 63–85.
[14] Soffer, N. et al. (2014) Potential role of viruses in white plague coral disease. ISME J. 8, 271–283.
| Potential role of viruses in white plague coral disease.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXht1ektbc%3D&md5=cd6a142fc419fa70e4dfc3dcce0972b2CAS |
[15] Weynberg, K.D. et al. (2017) Prevalent and persistent viral infection in cultures of the coral algal endosymbiont Symbiodinium. Coral Reefs 36, 773–784.
| Prevalent and persistent viral infection in cultures of the coral algal endosymbiont Symbiodinium.Crossref | GoogleScholarGoogle Scholar |
[16] Correa, A.M.S. et al. (2013) Unique nucleocytoplasmic dsDNA and +ssRNA viruses are associated with the dinoflagellate endosymbionts of corals. ISME J. 7, 13–27.
| Unique nucleocytoplasmic dsDNA and +ssRNA viruses are associated with the dinoflagellate endosymbionts of corals.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhvVOrtr%2FN&md5=0e38fee8ba9647a354b3a792152766a1CAS |
[17] Thurber, R.V. et al. (2017) Virus–host interactions and their roles in coral reef health and disease. Nat. Rev. Microbiol. 15, 205–216.
| Virus–host interactions and their roles in coral reef health and disease.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2sXpvVWgtw%3D%3D&md5=dbc43a553d7cc571d41900c4cf20b3a8CAS |
[18] Sweet, M. and Bythell, J. (2017) The role of viruses in coral health and disease. J. Invertebr. Pathol. 147, 136–144.
| The role of viruses in coral health and disease.Crossref | GoogleScholarGoogle Scholar |
[19] Levin, R.A. et al. (2017) Evidence for a role of viruses in the thermal sensitivity of coral photosymbionts. ISME J. 11, 808–812.
| Evidence for a role of viruses in the thermal sensitivity of coral photosymbionts.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2sXivFeksb8%3D&md5=d5006de4c4510338404525e71f001538CAS |
[20] Montalvo-Proaño, J. et al. (2017) A PCR-based assay targeting the major capsid protein gene of a dinorna-like ssRNA virus that infects coral photosymbionts. Front. Microbiol. 8, 1665.
| A PCR-based assay targeting the major capsid protein gene of a dinorna-like ssRNA virus that infects coral photosymbionts.Crossref | GoogleScholarGoogle Scholar |
[21] Waldor, M.K. and Mekalanos, J.J. (1996) Lysogenic conversion by a filamentous phage encoding cholera toxin. Science 272, 1910–1914.
| Lysogenic conversion by a filamentous phage encoding cholera toxin.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28XjvVOis78%3D&md5=18bb85dfb6680b16751afab1df406c61CAS |
[22] Weynberg, K.D. et al. (2015) From cholera to corals: viruses as drivers of virulence in a major coral bacterial pathogen. Sci. Rep. 5, 17889.
| 1:CAS:528:DC%2BC2MXhvFyks7nK&md5=3d3d80198372c2eed5b508d3e93ca393CAS |
[23] Adams, M. (1959) Bacteriophages. Bacteriophages bacteriophages’. Interscience Publishers Inc., New York. http://archive.org/details/bacteriophages00adam
[24] Buerger, P. et al. (2016) CRISPR-Cas defense system and potential prophages in cyanobacteria associated with the coral black band disease. Front. Microbiol. 7, 2077.
| CRISPR-Cas defense system and potential prophages in cyanobacteria associated with the coral black band disease.Crossref | GoogleScholarGoogle Scholar |
[25] van Oppen, M.J.H. et al. (2009) Coral-virus interactions: a double-edged sword? Symbiosis 47, 1–8.
| Coral-virus interactions: a double-edged sword?Crossref | GoogleScholarGoogle Scholar |
[26] Bourne, D.G. et al. (2009) Microbial disease and the coral holobiont. Trends Microbiol. 17, 554–562.
| Microbial disease and the coral holobiont.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhsV2gsrnN&md5=bdd1aaebc1c4e2dd5faaa1705457f5eaCAS |
[27] Efrony, R. et al. (2007) Phage therapy of coral disease. Coral Reefs 26, 7–13.
| Phage therapy of coral disease.Crossref | GoogleScholarGoogle Scholar |
[28] Efrony, R. et al. (2009) Phage therapy of coral white plague disease: properties of phage BA3. Curr. Microbiol. 58, 139–145.
| Phage therapy of coral white plague disease: properties of phage BA3.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXksVShtQ%3D%3D&md5=12d92d84368e65afdfdb845ef268fe73CAS |
[29] Atad, I. et al. (2012) Phage therapy of the white plague-like disease of Favia favus in the Red Sea. Coral Reefs 31, 665–670.
| Phage therapy of the white plague-like disease of Favia favus in the Red Sea.Crossref | GoogleScholarGoogle Scholar |
[30] Weynberg, K.D. et al. (2014) Generating viral metagenomes from the coral holobiont. Front. Microbiol 5, 206.
| Generating viral metagenomes from the coral holobiont.Crossref | GoogleScholarGoogle Scholar |
[31] Brussaard, C.P.D. (2004) Optimization of procedures for counting viruses by flow cytometry. Appl. Environ. Microbiol. 70, 1506–1513.
| Optimization of procedures for counting viruses by flow cytometry.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXisVKju7k%3D&md5=75c6d2225db83f176700c24c6fd296a8CAS |
[32] Middelboe, M. et al. (2010) Isolation and life cycle characterization of lytic viruses infecting heterotrophic bacteria and cyanobacteria. Man. Aquat. Viral Ecol. , 118–133.
| Isolation and life cycle characterization of lytic viruses infecting heterotrophic bacteria and cyanobacteria.Crossref | GoogleScholarGoogle Scholar |
[33] Laffy, P.W. et al. (2016) HoloVir: a workflow for investigating the diversity and function of viruses in invertebrate holobionts. Front. Microbiol. 7, 822.
| HoloVir: a workflow for investigating the diversity and function of viruses in invertebrate holobionts.Crossref | GoogleScholarGoogle Scholar |
Biographies
Dr Patrick Buerger is a postdoctoral researcher with CSIRO’s Synthetic Biology Future Science Platform and The University of Melbourne, Australia. He completed his PhD research on bacteriophages in the coral black band disease at the Australian Institute of Marine Science in Townsville, Australia.
Professor Madeleine van Oppen holds positions at The University of Melbourne and the Australian Institute of Marine Science (Townsville, Australia). Her research focuses on assisted evolution, coral reef restoration, and microbial symbionts of corals.


