New approaches to VLP-based vaccines
Alekhya Penumarthi A B and Peter M Smooker A CA School of Science, RMIT University
Bundoora, Vic. 3083, Australia
B Email: alekhya.penumarthi@rmit.edu.au
C Email: peter.smooker@rmit.edu.au
Microbiology Australia 38(2) 93-94 https://doi.org/10.1071/MA17038
Published: 24 March 2017
Vaccination is a long and established field of research, and outputs from the research have saved countless millions of lives. The early vaccines were developed with scant regard for the immunological mechanisms at play, largely because they were unknown. We are now in a position to use our knowledge of immunology to rationally design vaccines. This article focusses on the use of virus-like particles (VLPs) as vaccines.
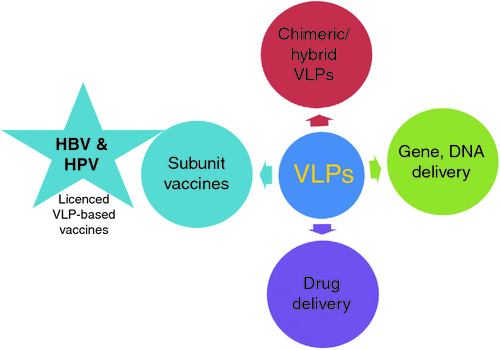
VLPs successfully prevent human papilloma (HPV) and hepatitis B virus (HBV) infections, but they may be exploited for novel uses as shown in Figure 1. VLPs are empty protein capsids formed by the self-assembly of viral proteins without any viral nucleic acids: therefore there is little chance of pathogenicity1. The primary prerequisite for the production of VLPs is to produce viral proteins which have the capacity to self-assemble into VLPs2. Bacterial, yeast, insect and plant expression systems are used depending on the nature of protein being produced. Proteins which do not require any post translational modifications can be expressed in a prokaryotic host like E. coli, and those requiring these modifications can be expressed in eukaryotic hosts such as yeasts (e.g. S. cerevisiae and P. pastoris). Two commercially available VLP-based vaccines are those for HBV and HPV. Despite WHO recommendations to incorporate HBV vaccine in the childhood immunisation schedule, only 76% of countries implemented it by 20033. This percentage was increased to 90% by 2013 due to its displayed success in HBV prevention4. The first quadrivalent HPV vaccine, Gardasil was licensed in 2006 with considerable success in prevention, despite some ongoing debate on its side-effects5. The possible reason for the success of the HPV vaccine is that its prolonged infectious cycle enables the induction of strong inhibitory humoral responses6. VLPs are also being trialled in gene therapy and DNA vaccine delivery. Yeast transposon VLPs (Ty-VLPs) are native transposition intermediates in various species of Saccharomyces and are similar to that of retroviral cores7. Their application for HIV-1 p24 antigen delivery as a subunit vaccine was successful in vivo, eliciting specific immune responses8. Ty-VLPs are capable of holding 5.7 kb Ty1-RNA9, hence they might have gene delivery ability also. This application was not explored previously. Therefore we investigated the efficiency of these Ty-VLPs to deliver plasmid DNA to dendritic cells10, and an increased transfection efficiency was observed compared to naked plasmid (Figure 2). Dendritic cells were chosen for in vitro study as they are the main antigen presenting cells in the mammalian immune system11. They preferentially take up nanoparticles within the viral size range of 20–200 nm12. Some other types of VLPs such as polyoma13 and various types of papilloma VLPs14,15 were reported for their gene and DNA delivery abilities.
|
Error1: Incorrect filename or format (MA17038_F2.gif). Please check out Figure 2.
Chimeric or hybrid VLPs produced by the engineering of surface exposed amino acids on VLPs are also under study, as well as chimeric VLPs loaded with drugs16, and multiple antigens17.
The main criteria for a successful VLP-based vaccine is the ease of efficient scale up and approval by the FDA for use in humans. A ‘selective flocculation and precipitation method’ for scaling up of virus yield at the commercial level was developed with a significant improved yield18. The effects of sparging, agitation and bioreactor scale on baculovirus-insect cell line growth, infection kinetics and productivity of Porcine parvovirus VLP production at the commercial scale was studied and highlighted their importance19.
Many VLP-based vaccines are at various stages of clinical trials and the chances of their success seems high judging from their laboratory level efficiency. Interestingly a VLP vaccine designed for hypersensitivity20 was successful in pre-clinical and phase-I trials and is under further clinical trials. The practical utility of many VLP-based vaccines is being restricted due to their limitation to incorporate large antigens and difficulties in commercial scale production21. Therefore, more research needs to be undertaken in terms of developing commercial scale-up methods and choosing suitable protein subunits of the virus to produce effective VLPs, which may elicit robust immunity.
References
[1] Crisci, E. et al. (2012) Virus-like particles: the new frontier of vaccines for animal viral infections. Vet. Immunol. Immunopathol. 148, 211–225.| Virus-like particles: the new frontier of vaccines for animal viral infections.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XovVahsbs%3D&md5=6febcaced09c1c5de96d4a18a1e23925CAS |
[2] Kushnir, N. et al. (2012) Virus-like particles as a highly efficient vaccine platform: diversity of targets and production systems and advances in clinical development. Vaccine 31, 58–83.
| Virus-like particles as a highly efficient vaccine platform: diversity of targets and production systems and advances in clinical development.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhslSlsr7F&md5=da83b9c088d654f5522dae2f13b65d0cCAS |
[3] Roldão, A. et al. (2010) Virus-like particles in vaccine development. Expert Rev. Vaccines 9, 1149–1176.
| Virus-like particles in vaccine development.Crossref | GoogleScholarGoogle Scholar |
[4] Meireles, L.C. et al. (2015) Three decades of hepatitis B control with vaccination. World J. Hepatol. 7, 2127–2132.
| Three decades of hepatitis B control with vaccination.Crossref | GoogleScholarGoogle Scholar |
[5] Macartney, K.K. et al. (2013) Safety of human papillomavirus vaccines: a review. Drug Saf. 36, 393–412.
| Safety of human papillomavirus vaccines: a review.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhsl2nsL7E&md5=a708f477ff1b5fc308946f179d39ce72CAS |
[6] Schiller, J.T. and Lowy, D.R. (2012) Understanding and learning from the success of prophylactic human papillomavirus vaccines. Nat. Rev. Microbiol. 10, 681–692.
| Understanding and learning from the success of prophylactic human papillomavirus vaccines.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xhtlajs7zK&md5=f0e7339c724bde710423d02559f7d30dCAS |
[7] Burns, N.R. et al. (1992) Symmetry, flexibility and permeability in the structure of yeast retrotransposon virus-like particles. EMBO J. 11, 1155–1164.
| 1:CAS:528:DyaK38XhvFSktr4%3D&md5=e8821d1a3cf7bc2137e00b063215ed14CAS |
[8] Gilmour, J.E.M. et al. (1989) A novel method for the purification of HIV-1 p24 protein from hybrid Ty virus-like particles (Ty-VLPs). AIDS 3, 717–723.
| A novel method for the purification of HIV-1 p24 protein from hybrid Ty virus-like particles (Ty-VLPs).Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK3c7kvVGntw%3D%3D&md5=c27750700d9b1c7a6da1fbadbea62ce2CAS |
[9] Roth, J.F. (2000) The yeast Ty virus-like particles. Yeast 16, 785–795.
| The yeast Ty virus-like particles.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXlt1Gitrg%3D&md5=be43f7bc5b19c0414697c067e5532352CAS |
[10] Penumarthi, A. et al. (2016) Utilising noval nanoparticles for DNA vaccine delivery. Nanotech, Dubai.
[11] Steinman, R.M. (2007) Dendritic cells: versatile controllers of the immune system. Nat. Med. 13, 1155–1159.
| Dendritic cells: versatile controllers of the immune system.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtFagsb3P&md5=5eed900a5bbae9a0887d4212b9abcc09CAS |
[12] Fifis, T. et al. (2004) Size-dependent immunogenicity: therapeutic and protective properties of nano-vaccines against tumors. J. Immunol. 173, 3148–3154.
| Size-dependent immunogenicity: therapeutic and protective properties of nano-vaccines against tumors.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXmvVCqtrs%3D&md5=52a1fdd94f648c34c185ce9dd8b76caaCAS |
[13] Braun, H. et al. (1999) Oligonucleotide and plasmid DNA packaging into polyoma VPI virus-like particles expressed in Escherichia coli. Biotechnol. Appl. Biochem. 29, 31–43.
| 1:CAS:528:DyaK1MXhsFyqtb8%3D&md5=efdc976f0dfc60c24386bed3383c8cc1CAS |
[14] Liu, Y. et al. (2001) Efficiency of delivery of DNA to cells by bovine papillomavirus type-1 L1/L2 pseudovirions. Appl. Microbiol. Biotechnol. 56, 150–156.
| Efficiency of delivery of DNA to cells by bovine papillomavirus type-1 L1/L2 pseudovirions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXls1ShtL4%3D&md5=ea90353a7c54113efd5bdf97f8c3f2e6CAS |
[15] Touze, A. and Coursaget, P. (1998) In vitro gene transfer using human papillomavirus-like particles. Nucleic Acids Res. 26, 1317–1323.
| In vitro gene transfer using human papillomavirus-like particles.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXhvFejs7k%3D&md5=cf4d4757f5dcb6af779945b3900d7823CAS |
[16] Hoque, M. et al. (1999) Chimeric virus-like particle formation of adeno-associated virus. Biochem. Biophys. Res. Commun. 266, 371–376.
| Chimeric virus-like particle formation of adeno-associated virus.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXnvFersL4%3D&md5=0e5a4092e829f581fbf3545fd11e4140CAS |
[17] Gedvilaite, A. et al. (2000) Formation of immunogenic virus-like particles by inserting epitopes into surface-exposed regions of hamster polyomavirus major capsid protein. Virology 273, 21–35.
| Formation of immunogenic virus-like particles by inserting epitopes into surface-exposed regions of hamster polyomavirus major capsid protein.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXks1Omtr0%3D&md5=2ebe8e62e11d67156b288d42336a90b8CAS |
[18] Tsoka, S. et al. (2000) Selective flocculation and precipitation for the improvement of virus-like particle recovery from yeast homogenate. Biotechnol. Prog. 16, 661–667.
| Selective flocculation and precipitation for the improvement of virus-like particle recovery from yeast homogenate.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXjtlygsbo%3D&md5=383177d80d229abe0536df5b31f9bda8CAS |
[19] Maranga, L. et al. (2004) Scale-up of virus-like particles production: effects of sparging, agitation and bioreactor scale on cell growth, infection kinetics and productivity. J. Biotechnol. 107, 55–64.
| Scale-up of virus-like particles production: effects of sparging, agitation and bioreactor scale on cell growth, infection kinetics and productivity.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXpvF2htrY%3D&md5=9b44a22f178bd27cce5b3eb936108c31CAS |
[20] Ambühl, P.M. et al. (2007) A vaccine for hypertension based on virus-like particles: preclinical efficacy and phase I safety and immunogenicity. J. Hypertens. 25, 63–72.
| A vaccine for hypertension based on virus-like particles: preclinical efficacy and phase I safety and immunogenicity.Crossref | GoogleScholarGoogle Scholar |
[21] Grgacic, E.V.L. and Anderson, D.A. (2006) Virus-like particles: passport to immune recognition. Methods 40, 60–65.
| Virus-like particles: passport to immune recognition.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XpvFGhsro%3D&md5=c0e5e8b066568674e45f4fadf523180cCAS |
Biographies
Alekhya Penumarthi is a PhD candidate in Professor Peter Smookers’ Biotechnology lab in RMIT University, who is currently writing her thesis. She completed her Master’s degree in Virology from Sri Venkateswara University, Tirupati, India. Her research interest is in utilising virus like particles and nanoparticles in vaccine development, particularly for DNA vaccine delivery.
Peter Smooker is a Professor of Biotechnology and head of the Biotechnology laboratory at RMIT University. The laboratory’s main activities are in vaccine design, including antigen identification and engineering, and vector development.


